Abstract
The promoter is the center for regulation of gene transcription due to containing numerous transcription factor binding sites. The aim of the study was to determine whether genetic variations at excision repair cross complementation group 5 (ERCC5) promoter could affect transcription factor binding and whether such single nucleotide polymorphism (SNP)-dependent binding could affect gene expression, drug response, and clinical outcome.
A total of 170 patients who were cytologically or histologically confirmed with advanced colorectal cancer (CRC), at least 1 measurable lesion, and underwent oxaliplatin-based chemotherapy were studied. The polymerase chain reaction–ligation detection reaction (PCR-LDR) was used to analyze SNPs. The reporter gene assay system and electrophoretic mobility shift assays (EMSA) were performed to investigate the effect of SNPs on the ERCC5 promoter activity and DNA-binding activity, respectively. The mRNA and protein expression of ERCC5 in tumor tissues of colorectal cancer patients with different genotypes were detected by real-time PCR and western blot, respectively.
Both −763A and −763G allele had nuclear protein-binding ability. +25A allele did not show any nuclear protein-binding ability, whereas +25G allele did. The relative luciferase activity of the −763A/+25G haplotype was significantly higher than other 3 haplotypes (P < 0.05). The expression level of ERCC5 mRNA and protein was significantly higher in tumor tissues with −763AA+25GG genotype combination than that with −763GG+25AA genotype combination (P < 0.05, respectively). Allelic variants (−763AA vs −763AG or –763GG, +25GG versus +25AG or +25AA) were significantly associated with shorter progression-free survival (PFS) and overall survival (OS) (P < 0.05, respectively). At multivariate analysis, patients with risk genotypes (−763AA or +25GG genotype) demonstrated a significantly increasing risk of progression (P = 0.01) or worse OS (P = 0.001).
The ERCC5 promoter polymorphisms at −763 and +25 may be important functional variants and predictors of clinical outcome of CRC patients who received oxaliplatin chemotherapy.
INTRODUCTION
Colorectal cancer (CRC) is one of the most common malignancies with high death rate worldwide. Although screening methods have been improved, about one-third of cases are found in the advanced stages at the time of initial diagnosis when adjuvant chemotherapy or palliative setting is necessary. Oxaliplatin-based chemotherapy has been proved to be effective in treating advanced CRC.1,2 However, individual efficacy of oxaliplatin demonstrates significant variations. It is known that individual difference in drug sensitivity is mainly determined by genetic factors such as single nucleotide polymorphisms (SNPs) of genes which are related to drug mechanism and drug metabolism.3,4 As one of the major pathways of DNA repair system and the only known mechanism for the removal of DNA adducts produced by platinum agents in mammalian cells, the nucleotide excision repair pathway is closely related to platinum-based drug resistance.5 Although oxaliplatin compounds has become the first-line regimen in advanced colorectal cancer treatment, only limited studies reported the relationship between SNPs in the NER pathway.6–8 Therefore, it is significant to find a predictor to evaluate the response to oxaliplatin chemotherapy. The excision repair cross-complementation group 5 (ERCC5) gene, also known as Xeroderma pigmentosum group G (XPG), is one of the essential DNA repair enzymes of the NER pathway. ERCC5 gene expression has been proved to exist in various tumor cell lines or tissues, and its expression level is correlated with the response to platinum-based chemotherapy.9–12
The promoter is the center for regulation of gene transcription due to containing numerous transcription factor binding sites. There are evidences indicating that genetic variations in this region such as SNPs might affect transcription factor binding and gene expression, and thus contribute to complex phenotypes such as disease association and response to drugs.13,14 It is early to make a conclusion, but some studies implied that SNPs in the promoter region might play a functional role in ERCC5 transcription and/or function.14–16 In our previous study, ERCC5 promoter polymorphisms at −763 and +25 showed to be associated with the response to oxaliplatin-based chemotherapy in patients with advanced CRC.17 As the −763A>G and +25A>G polymorphisms are located at the gene promoter region, it is necessary to further investigate the function of these SNPs and reveal the relationship of SNPs, gene expression, and clinical outcome.
In this study, we set out to determine whether genetic variations at ERCC5 promoter could affect transcription factor binding and whether such SNP-dependent binding could affect gene expression, drug response, and clinical outcome. For this purpose, we investigated the effect of SNPs on the ERCC5 promoter activity and DNA-binding activity, respectively; then the expression of ERCC5 in tumor tissues of colorectal cancer patients with different genotypes was also detected. Furthermore, we investigated the association between the ERCC5 polymorphisms at the promoter region in Chinese Han population and the clinical outcome in patients with advanced CRC treated with oxaliplatin-based chemotherapy.
MATERIALS AND METHODS
Subjects
Patients who were histologically or cytologically confirmed with advanced CRC and underwent oxaliplatin-based chemotherapy either at the Southwest Hospital or at Daping Hospital, Third Military Medical University, Chongqing, China, between April 2004 and December 2009, were eligible for this study. The patients were at least 18 years old, had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2, presented at least 1 measurable lesion, previous cytotoxic chemotherapy was not permitted, and adjuvant treatment (without oxaliplatin) was completed at least 6 months before study. This study was reviewed and approved by the Ethical and Protocol Review Committee of the Third Military Medical University, and written consent form was obtained from all patients. A complete medical and clinical-physical examination, ECOG evaluation, baseline measurement of tumor size based on computed tomography (CT) scan or other radiographic means were obtained within 7 days before initial treatment. All eligible patients received oxaliplatin-based regimen chemotherapy every 3 weeks. Each cycle consisted of oxaliplatin (130 mg/m2/day) as a continuous intravenous infusion on the first day, simultaneously with a continuous infusion of fluorouracil (300 mg/m2/day), and leucovorin (200 mg/m2/day) for 5 days. Patients must undergo chemotherapy cycles until severe toxicity or disease progression appears. Chemotherapy response was evaluated by CT scan or other radiographic means after 2 cycles of treatment and every 2 cycles thereafter, adopting the Response Evaluation Criteria in Solid Tumors Group (RECIST) criteria.18 Progression-free survival (PFS) was defined as the time from the start of chemotherapy to the first occurrence of disease progression or death. Overall survival (OS) was the time from the start of chemotherapy to death from any cause. Patients without progression or death at the time of analysis were censored at their last available follow-up assessment.
Sample Collection and Genotyping of SNPs
Peripheral blood samples were collected at the time of enrollment in EDTA-containing tubes. Genomic DNA was extracted using a DNA isolation kit (BioFlux, Tokyo, Japan). Genotyping was performed using the PCR–LDR method.19 The design of primers for amplification and probes for LDR were performed as described previously.17 The PCR reactions were carried out on the ABI 9600 (Applied Biosystems, Foster City, CA) in a total volume of 15 μL, including 10 ng genomic DNA, 1.5 μL 10 × PCR buffer, 0.3 mM dNTPs, 2 μM each primer, and 1 U Taq DNA polymerase (TaKaRa, Otsu, Japan). Cycling parameters were as follows: 94°C for 1 minutes; 35 cycles of 94°C for 10 seconds; 56°C for 20 seconds; 72°C for 40 seconds; and a final extension step at 72°C for 10 minutes. The probes for LDR were also derived from published genomic sequences. Two specific probes which discriminated specific base and 1 common probe were synthesized for each polymorphism. The common probe was labeled at the 3’ end with 6-carboxyxuorescein (FAM) and phosphorylated at the 5’ end. The multiplex ligation reaction for each PCR product was carried out with a reaction volume of 10 μl containing 2 μL of PCR product, 1 μL 10 × Taq DNA ligase buffer, 1 μM of each discriminating probe, 5 U Taq DNA ligase (New England Biolabs, Beverly, MA). The LDR parameters were as follows: 94°C for 2 minutes, 25 cycles of 94°C for 30 seconds, and 56°C for 4 minutes. After the LDR reaction, 1 μL LDR reaction product was mixed with 1 μL ROX passive reference and 1 μL loading buffer. The mixture was then denatured at 95°C for 3 minutes, chilled rapidly in ice water, and analyzed by the ABI Prism 377 DNA Sequencer (Applied Biosystems). In addition, the representative PCR products were subjected to direct DNA sequencing in an ABI Prism 310 Sequencer (Applied Biosystems) to confirm accuracy of this method.
Promoter Activity
The possible effect of both −763A>G and +25A>G SNPs on the promoter activity was investigated using a dual reporter gene assay system (Promega, Madison, WI). A total of 4 plasmid constructs were prepared by inserting a 1203-bp sequence (−910∼+292) of the ERCC5 gene into a promoterless pGL3-Basic vector (Promega), which contained a combined wild genotype of −763A and +25G, or a combined mutant genotype of −763G and+25A, or a single mutant genotype of either −763G or +25A. These 4 constructed vectors (0.8 μg DNA) and control plasmid pRL-SV40 (0.02 μg DNA) were transiently cotransfected into Human LOVO cells, respectively, using the Lipofectamine2000 reagent (Invitrogen, Carlsbad, CA). Forty-eight hours later, cells were collected and luciferase activities were measured with a model GloMax 20/20 Luminometer (Promega). Luminescence experiments were performed at least 3 times, with each transfection in triplicate, using 6 separate DNA preparations. Results were expressed as fold increase in relative luciferase activity (RLA) of the ERCC5 promoter construct vectors compared with the RLA of pGL3-Basic.
Electrophoretic Mobility Shift Assay
To assess the DNA-binding activity of ERCC5 −763A>G and +25A>G in vitro, we performed electrophoretic mobility shift assays (EMSA) as described previously.20 Nuclear extracts were prepared from the LOVO cell line. Biotin-labeled double-stranded oligonucleotides corresponding to ERCC5 −763A, −763G, +25A and +25G alleles were obtained by annealing 5′-BIOTIN-CAAAAGGCTACATACGAGTTTCTGATAAG-3′, 5′-BIOTIN-CAAAAGGCTGCATACGAGTTTCTGATAAG-3′, 5′-BIOTIN-GCCCATTTTTCATGGGTTTGCGGACCCAC-3′, and 5′-BIOTIN-GCCCATTTTTCGTGGGTTTGCGGACCCAC-3′ (synthesized by Shanghai Sangon Co, Shanghai, China) with their respective complementary oligonucleotides. EMSA was performed by using the LightShift EMSA kit (Pierce, Rockford). For binding reaction, 10 fmol biotin-labeled, double-stranded oligonucleotides were incubated with nuclear extract (2–5 μg) in 1 × binding buffer, 1 μg poly (dI:dC) for 20 min at room temperature. For competition studies, unlabeled double-stranded oligonucleotides (200-fold molar excess) were incubated during preincubation. Reaction products were separated in 4% nondenaturing polyacrylamide gels in 0.5 × TBE buffer and visualized by chemiluminescent detection after electric transfer of the products onto nylon membrane (Roche, Nutley, NJ). The results were scanned using a ChemDoc CCD camera system (BioRad, Hercules, CA), and band intensity was quantified by using the Image Quantity One 1.5.4 software (BioRad).
Tissue Sample Collection and DNA, RNA, Protein Extraction
A total of 33 tumor samples were obtained by surgical resection from corresponding patients with primary colorectal adenocarcinoma. The patients were all Chinese. Approximately 2 g of the surgically removed tissues were stored immediately at liquid nitrogen for genomic DNA, total RNA, and nuclear protein extraction. The remaining section of the sample was fixed with formalin and used for additional histological examination to confirm the diagnosis.
Real-Time Quantitative PCR
Expression of ERCC5 mRNA was quantified by real-time quantitative PCR with the SYBR gene expression assay (Takara). Primes were 5′GAGTCAACGGATTTGGTCGTATTG3′, 5′ CCTGGAAGATGGTGATGGGATT3′ for the internal control, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and 5′TCGCTCCATGAATGGCAAGATA 3′, 5′ TTCCATGGGAGCCTGGATGTA3′ for ERCC5. Reverse transcription parameter was 15 minutes at 37°C, then an inactivation step for reverse transcription was 10 minutes at 85°C. Every cDNA target was amplified in duplicate in separate wells. 95°C for 10 s; 40 cycles of 95°C for 5 s; 62°C for 34 seconds; 72°C for 1 minutes; and a final extension step at 72°C for 10 minutes were performed using QPCR (ABI prism 7500, Applied Biosystems). Relative expression intensity of ERCC5 mRNA was normalized as the ratio of ERCC5 to GAPDH and calculated with the formula of 2 power (−ΔΔCt). Data was expressed as fold increase (2−ΔΔCt) compared with the value of −763GG+25AA genotype.
Western Blot
About 40 μg nuclear proteins was loaded in each well and separated in SDS-polyacrylamide electrophoresis gels. After being transferred to polyvinylidene fluoride microporous membranes (BioRad), the membranes were saturated and blocked with 5% fat-free milk at 37°C for 1 hour and were incubated with mouse antihuman ERCC5 monoclonal antibody (1:300, Santa Cruz, CA) overnight at 4°C, After extensive washing, the second antibody (goat antimouse HRP [1:6000, Santa Cruz]) was added and the membranes were incubated for 45 minutes followed by extensive washes. β-Tubulin was used as internal control. Specific antibody–antigen complexes were detected by using the supersignal west femto detection kit (Pierce). The results were scanned using a ChemDoc CCD camera system (BioRad) and, band intensity was quantified by using the image analysis software Image Quantity One 1.5.4 (BioRad). Relative expression intensity of ERCC5 protein was normalized as the ratio of ERCC5 to β-Tubulin.
Statistical Analysis
Genotype distribution was analyzed using the χ2 test for Hardy–Weinberg equilibrium. Linkage disequilibrium was assessed via Linkage disequilibrium analyzer software (LDA).21 Hazard ratio (HR) and 95% confidence interval (CI) were calculated as an estimate of relative risk. The Kaplan–Meier method was adopted to estimate survival curves, and the log-rank test was used to compare patients’ PFS or OS between genotype groups. Univariate and multivariate Cox proportional hazards regression models were used to assess the importance of genotypes with adjustment for clinical features. Student's t-tests were used to analyze the result of luciferase activities, EMSA, and gene expression. Results were considered to be statistically significant if bilateral P values were <0.05. Statistical analyses were carried out using SPSS Version 13.0 software package for Windows (SPSS Inc, Chicago, IL).
RESULTS
Effect of the −763A>G and +25A>G Polymorphism on DNA-Binding Activity
As the −763A>G and +25A>G polymorphisms were located at the gene promoter region, which contains numerous transcription factor binding sites, we investigated whether the allele changes at these SNPs might alter transcription factor binding. For this purpose, TRANSFAC, PROMO3.0 software were used, and we found that at −763A>G polymorphism, the A allele might create a binding site for the C/EBP beta factor, but G allele did not show any binding effect. At +25A>G polymorphism, the G allele might create a binding site for the C/EBP alpha factor and A allele did not show any binding effect. In view of the limitations of transcription factor prediction, we detected the binding capacity of these 2 locus and nuclear protein by EMSA to further identify the function of SNPs. The results showed that both −763A and −763G allele had nuclear protein binding ability, but the intensity of binding band corresponding to the −763A allele was more intense than that corresponding to the −763G allele, but there was no significant difference between the 2 alleles (P > 0.05) (Figure 1A and B). +25A allele did not show any nuclear protein binding ability, whereas +25G allele did (P < 0.05) (Figure 1C and D).
FIGURE 1.
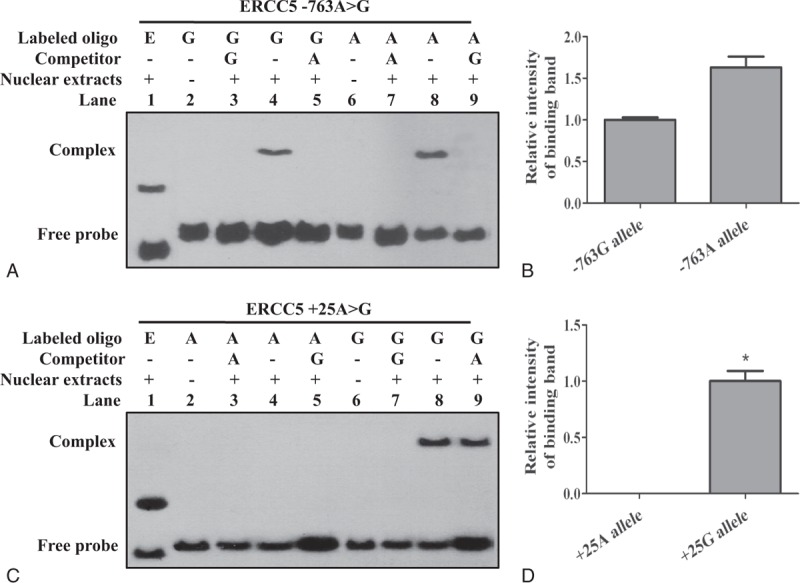
Effect of the −763A>G and +25A>G polymorphism on DNA-binding activity. Competitors, 200-fold unlabeled probes (corresponding to cold probes); Lane 1, Epstein–Barr virus nuclear antigen-positive control from LightShift EMSA kit; lanes 3, 5, 7, and 9, the binding complex was specifically competed by excess unlabelled corresponding oligonucleotide. (A, B) Binding affinity of nuclear proteins to biotin-labeled double-stranded oligonucleotides flanking the –763A>G locus. Unknown nuclear protein of LOVO cells nuclear extracts formed a stronger complex with the –763A oligonucleotide compared with the –763G oligonucleotide, but there was no significant difference between the 2 alleles (lane 8 vs lane 4, P>0.05). (C, D) Binding affinity of nuclear proteins to biotin-labeled double-stranded oligonucleotides flanking the +25A>G locus. Unknown nuclear protein of LOVO cells nuclear extracts formed a complex with the +25G oligonucleotide, and the binding band was not competitive inhibited by excess unlabelled +25A oligonucleotide. +25A allele did not show any nuclear protein binding ability, whereas +25G allele did (lane 4 vs lane 8, ∗P < 0.05).
Effect of the −763A>G and +25A>G Polymorphism on Transcriptional Activity
As allele changes at −763A>G and +25A>G polymorphism may alter transcription factor binding, we further investigate the impact of the 2 SNPs upon translation of the downstream cistron in a cell culture-based system. We conducted linkage disequilibrium analysis among −763A>G and +25A>G, and found that they were in tight linkage disequilibrium (r2 = 0.77, D′ = 0.88, P < 0.0001). Four haplotype DNA which contain ERCC5 −763A>G and +25A>G sites were cloned into a promoterless pGL3-Basic vector respectively and the plasmids were transfected into LOVO cells, and the luciferase activity was determined. As the sequences of the 4 plasmids which contain ERCC5 −763A>G and +25A>G sites were consistent with each other except the mutational side, the dual luciferase reporter system could reflect the impact of polymorphism site on promoter activity. The data showed that promoter activities were observed at −910 to +292 bp fragment of ERCC5 gene, and the promoter activity was different in each haplotype (Figure 2A). The relative luciferase activity of the −763A/+25G haplotype was significantly higher than other 3 haplotypes (P < 0.05), and it indicated that −763A allele and +25G allele might enhance the transcriptional activity of ERCC5 gene promoter.
FIGURE 2.

(A) Effect of the ERCC5 -763A>G and +25A>G polymorphisms on transcription activity. Results are expressed as fold increase in relative luciferase activity (RLA) of the ERCC5 promoter construct vectors as compared with pGL3-Basic. ∗P < 0.05 compared with −763A/+25G haplotype. (B) Expression of ERCC5 mRNA in tumor tissues of colorectal cancer patients with different genotypes detected by real-time PCR. Data are expressed as fold increase (2–ΔΔCt) compared with the value of –763GG/+25AA genotype. GAPDH was used as an internal control. ∗P < 0.05 compared with –763GG/+25AA genotype. (C, D) Expression of ERCC5 protein (200KD) in tumor tissues of colorectal cancer patients with different genotypes was detected by Western blot. β-Tubulin (55 kd) was used as an internal control. ∗P < 0.05 compared with −763GG/+25AA genotype.
Expression of ERCC5 Gene at Colorectal Cancer Tissues
To test the hypothesis that the polymorphism could be associated with altered expression of endogenous ERCC5, we measured ERCC5 expression in tumor samples from 33 CRC patients. The data showed that the expression level of ERCC5 mRNA and protein were variant in tumor tissues with different genotypes. The expression of ERCC5 mRNA was significantly higher in tumor tissues with −763AG+25GG, −763AA+25GG, or −763AA+25AG genotype combination than that in −763GG+25AA genotype combination (P < 0.05). However, the expression level of mRNA in tumor tissues with −763AG+25AG genotype combination was significantly lower than that in −763GG+25AA genotype combination (P < 0.05) (Figure 2B). Similarly, the expression of ERCC5 protein was significantly higher in tumor tissues with −763AA+25GG, −763AG+25GG genotype combination than that in −763GG+25AA genotype combination (P < 0.05) (Figure 2C and 2D). However, the expression level of protein in tumor tissues with −763AG+25AG genotype combination was significantly lower than that in −763GG+25AA genotype combination (P < 0.05).
Genotype and Clinical Outcome
As the above data showed that −763A>G and +25A>G polymorphisms were associated with ERCC5 expression, we hypothesize that the 2 SNPs would affect clinical outcome of CRC patients who received oxaliplatin-based chemotherapy due to the fact that the expression level of ERCC5 was correlated with response to platinum-based chemotherapy.
Patient Characteristics
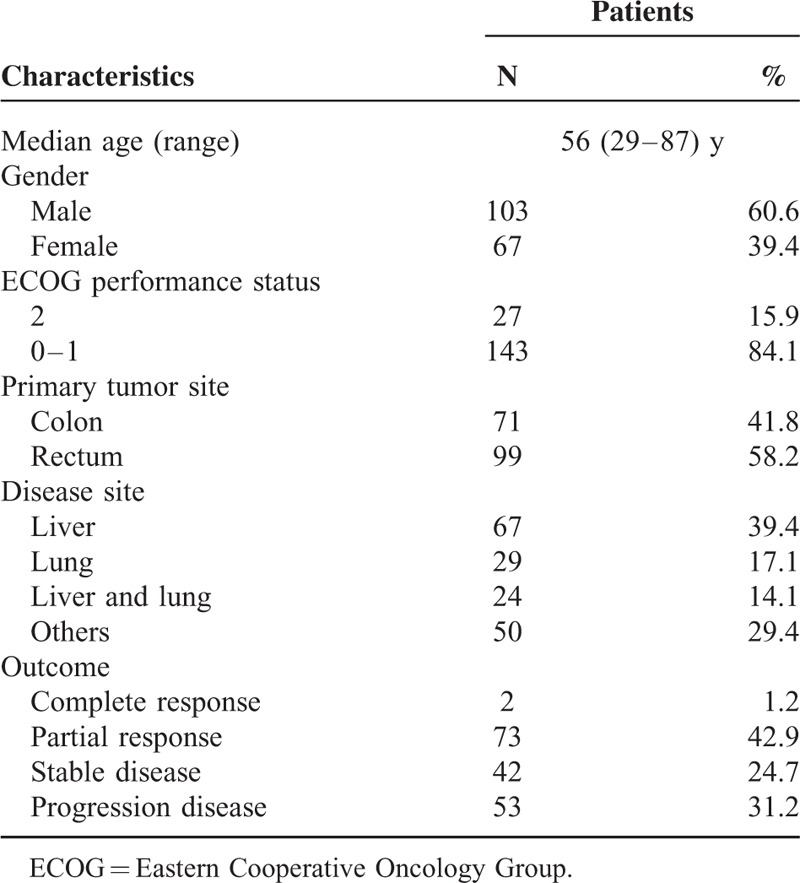
A total of 170 valuable patients were studied. All the patients are Chinese Han population. Baseline data are presented in Table 1. The response rate (complete response or partial response) was 44.1% (75 of 170 patients), with 2 complete responders (1.2%), 73 partial responders (42.9%), 42 patients with stable disease (24.7%), and 53 patients with progression disease (31.2%). No significant association between polymorphisms and baseline data such as demographic, clinical, or pathological characteristics was observed (data not shown). The genotype distribution for each polymorphism was found to be in Hardy–Weinberg equilibrium (P = 0.384 for −763A>G, P = 0.945 for +25A>G). The median follow-up period was 40 months. The median PFS and OS were 8.4 and 17 months, respectively.
TABLE 1.
Characteristics of the 170 Patients
Genotype and PFS/OS
We found that ERCC5 −763A>G and +25A>G polymorphisms were significantly associated with the response to chemotherapy (data not showed), which was consistent with our previous study.17 Here we further investigated the relationship of the polymorphisms and clinical outcome. The median PFS among patients with the −763AA genotype (57/170 cases, 6.8 months) was significantly lower than that with other genotypes (78/170 case, 8.4 months for −763AG genotype and 35/170 cases, 10.0 months for −763GG genotype, P = 0.001). Similarly, the median PFS among patients with the +25GG genotype (55/170 cases, 6.8 months) was significantly lower than that with other genotypes (83/170 case, 8.1 months for +25AG genotype and 32/170 cases, 11.0 months for +25AA genotype, P = 0.004). The median OS among patients with the −763AA genotype (14.5 months) was significantly lower than that with other genotypes (16.0 months for −763AG genotype and 20.5 months for −763GG genotype, P = 0.002). Similarly, the median OS among patients with the +25GG genotype (14.5 months) was significantly lower than that with other genotypes (16.4 months for +25AG genotype and 20.5 months for +25AA genotype, P = 0.005).
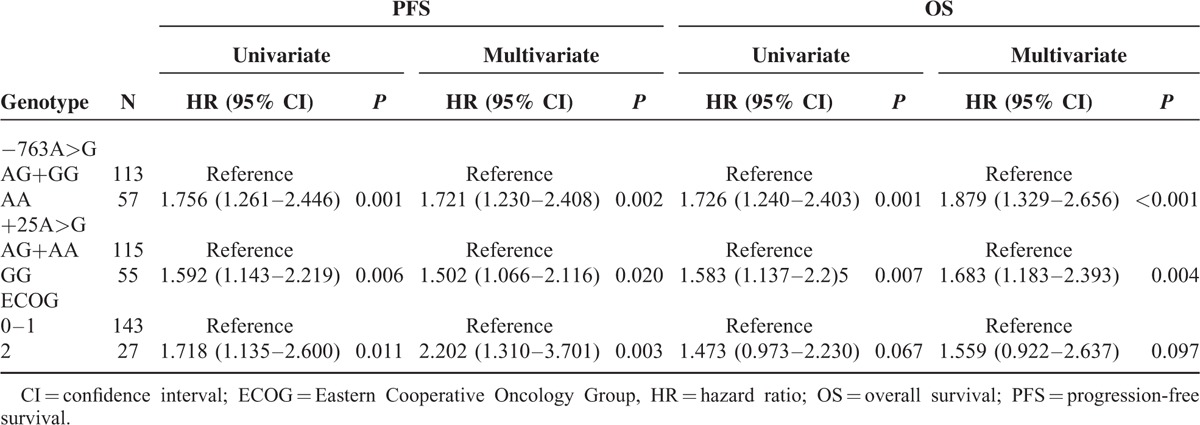
To test the hypothesis that −763A>G or +25A>G polymorphism is an independent prognostic factor in our population, we carried out a Cox proportional hazards regression including all variables known to possibly affect PFS or OS in patients. At univariate analysis (Table 2), a significantly higher risk of progression or worse OS was associated with ERCC5 −763A>G and +25A>G variants and poor ECOG performance status was associated with worse PFS. In the multivariate model including age, gender, ECOG, disease site, primary tumor site, ERCC5 −763AA genotype retained its significant association with worse PFS (HR = 1.721, 95% CI 1.230–2.408; P = 0.002) or OS (HR = 1.879, 95% CI 1.329–2.656; P < 0.001) (Table 2). +25GG genotype also retained its significant association with worse PFS (HR = 1.502, 95% CI 1.066–2.116; P = 0.020) or OS (HR = 1.683, 95% CI 1.183–2.393; P = 0.004). Another factor found to be associated with worse PFS in our series was ECOG.
TABLE 2.
Univariate and Multivariate Cox Proportional Hazards Regression Models for Association of Polymorphism With PFS and OS
As the 2 genotypes, −763A>G and +25A>G, were in tight linkage disequilibrium, combination of the risk genotypes (ERCC5 −763AA genotype and +25GG genotype) was analyzed. Of the 170 patients, 110 (64.7%) did not show any risk genotype (group 0), 9 (5.3%) were carriers of either the −763AA genotype or the +25GG genotype, and 51 (30.0%) were carriers of both risk genotypes. Median PFS was 9 months in group 0 and 6.5 months in group 1 (patients with 1 or more risk genotypes) (P = 0.001). Median OS was 20.0 months in group 0 and 14.2 months in group 1 (P = 0.001). Kaplan–Meier plots with log-rank comparisons among the 2 groups are shown in Figure 3. In the multivariate analyses, a significantly higher risk of progression was associated with −763AA genotype and +25GG genotype, and the patient with these risk genotypes demonstrated a significantly increasing risk of progression (HR 1.564, 95% CI 1.115–2.193, P = 0.01) or worse OS (HR 1.840, 95% CI 1.298–2.608, P = 0.001). Another factor found to be associated with worse PFS in our series was ECOG (HR 1.993, 95% CI 1.168–3.201, P = 0.01).
FIGURE 3.
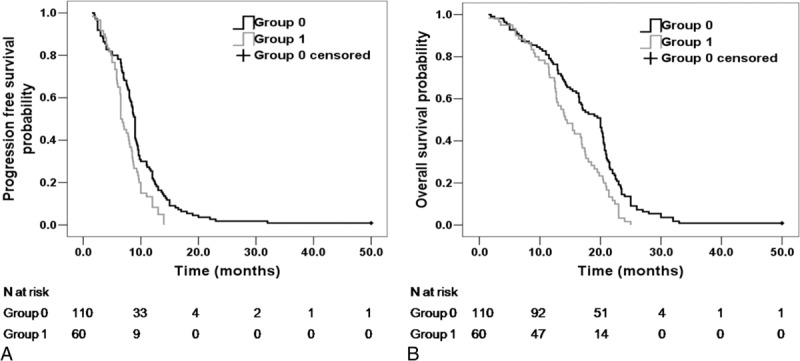
(A) Progression-free survival (PFS) curves of patients without risk genotypes (group 0) and patients with 1 or more risk genotypes (group 1) (P = 0.001). (B) Overall survival (OS) curves of patients without risk genotypes (group 0) and patients with 1 or more risk genotypes (group 1) (P = 0.001).
DISCUSSION
Due to the fact that the anticancer efficiency of oxaliplatin is mediated by the formation of interstrand and intrastrand DNA adducts, which block replication and inhibit transcription, more and more studies pay close attention to the relationship between SNPs of DNA repair genes and chemotherapy response to oxaliplatin in recent years.22–24 In our previous study, we found that ERCC5 −763A>G and +25A>G polymorphisms were significantly associated with the response to chemotherapy. As the −763A>G and +25A>G polymorphisms were located at gene promoter region, we further investigated the function of these SNPs and reveal the relationship of SNPs, gene expression, and clinical outcome in this study.
The promoter is the center for regulation of gene transcription due to containing numerous transcription factor binding sites. SNPs present in the promoter region may involve within common and genetically complex diseases as well as drug response because it may regulate the transcriptional activity of target genes by altering promoter activity, cause the changes in gene expression, and further influence its biological function.25 Some studies suggested that the SNPs at the ERCC5 gene promoter region might be related to the transcriptional regulation of genes.15,26 As the −763A>G and +25A>G polymorphisms were located at gene promoter region, we used bioinformatics analysis and found that allele changes of these polymorphisms might affect the binding capacity of the nucleic acid sequence containing the allele and the potential transcription factor. In view of the limitations of transcription factor prediction, we further identify the function of SNPs. As standard techniques widely used in the functional study of regulatory SNPs, EMSA and luciferase reporter assay were used to assess effects of −763A>G and +25A>G on binding and gene transcription in the study. Because the sequences of the 4 plasmids, which contain ERCC5 −763A>G and +25A>G sites are consistent with each other except the mutational side, dual luciferase reporter system can reflect the impact of polymorphism site on promoter activity. We found that the promoter activity was different in each haplotype, and the relative luciferase activity of the −763A/+25G haplotype was significantly higher than other 3 haplotypes (P < 0.05). From the result, we can infer that 1 single base change of a regulatory SNP could lead to variation of promoter activity. Similarly, the corresponding probes such as –763A allele and −763G allele are consistent with each other except the mutational side, so the EMSA assay can reflect the nuclear protein-binding ability of different alleles. And the result shows that the nuclear protein binding ability between −763A and −763G allele, or +25A and +25G allele is different. It means that −763A>G and +25A>G polymorphisms may alter the binding affinity with nuclear protein. Still, supershift EMSA or Chromatin immunoprecipitation analysis is necessary to validate the correlated transcription factor. From the results of EMSA and luciferase reporter assay, we presume that −763A allele and +25G allele might increase the binding affinity with transcription factors, and increase the promoter activity, and then regulate ERCC5 gene transcription positively.
So far, the expression of ERCC5 gene was well studied either at normal tissues or at tumors and the results showed that its expression level was correlated with tumor genesis and response to platinum-based chemotherapy. Cheng et al27 measured the expression of 5 NER genes by multiple RT-PCR and found that the expression level of ERCC5 and ERCC6 was statistically significant different in lung cancer patients and controls. The study also inferred that individuals whose expression level of ERCC5 and ERCC6 was reduced might be at higher risk of lung cancer. In addition, a study28 showed that reduced expression of ERCC1, XPB/ERCC3, XPG/ERCC5, and CSB/ ERCC6 was associated with a more than 2-fold increased risk of squamous cell carcinoma of the head and neck. The ERCC5 gene was lost or under-expressed at testicular germ cell tumors by comparative genomic hybridization microarrays.29 The above data indicated that reduced expression of ERCC5 gene is one of risk factors of cancer. Moreover, the expression of ERCC5 was found to be reduced in cell lines, which were sensitive to platinum drugs.30 Steven et al9 reported that the high expression of ERCC5 in ovarian cancer and colon cancer was correlated with cisplatin resistance. In this study, we showed that the expression level of ERCC5 mRNA and protein were variant in colorectal cancer tissues with different genotypes. The expression level of mRNA and protein was discrepant at some genotypes. The phenomenon might be related to protein folding, termination resection, or chemical modification. The expression level of ERCC5 mRNA and protein was significantly higher in tumor tissues with −763AA+25GG genotype combination than that with −763GG+25AA genotype combination (P < 0.05, respectively). The results are coincident with that of the EMSA and dual luciferase reporter assay: different alleles of −763A>G and +25A>G play different role on transcriptional regulation. The haplotype −763A/+25G can increase the ERCC5 promoter activity more significantly than haplotype −763G/+25A, so the expression level of ERCC5 is higher at corresponding genotype combination (−763AG+25GG, −763AA+25GG, −763AA+25AG) than at −763GG+25AA genotype combination.
As one of the essential DNA repair enzymes of NER pathway, the ERCC5 gene was showed to be related to genetic susceptibility or prognosis to many cancers, such as lung cancer31–34 and breast cancer.35,36 Otherwise, more studies tried to link ERCC5 gene polymorphisms to response to platinum-based chemotherapy in recent years. For example, a report which detected XP gene polymorphisms in 146 patients of advanced epithelial ovarian cancer who received platinum-based chemotherapy found that carriers of at least 1 variant allele of Asp1104His SNP showed significantly increased risk of death compared to carriers of the wild-type allele, and individuals with a homozygous variant XPG/ERCC5 allele had a significantly shorter median survival when compared with individuals with the homozygous XPG/ERCC5 wild-type allele.37 Furthermore, Monzo et al analyzed SNPs at XPA 5′ UTR T/C, XPD Lys751Gln, ERCC1 Lys259Thr and ERCC5 Hys46Hys in 42 advanced colorectal cancer patients treated with first-line oxaliplatin/fluoropyrimidine chemotherapy and the results showed that patients with ERCC5 CC genotype had longer time to progression than those with other genotypes.6 A study from Sun et al38 investigated polymorphisms of DNA repair genes in 82 advanced nonsmall cell lung cancer patients treated with platinum-based chemotherapy and polymorphisms of XRCC1 194Arg/Trp and XPG 46His/His were showed to be associated with clinical response. The above studies indicated that the ERCC5 gene might play an important role in tumorgenesis and therapeutic effect of platinum drugs. In the present study, we found that allelic variants (−763AA versus −763AG or −763GG, +25GG versus +25AG or +25AA) were significantly associated with shorter PFS and OS (P < 0.05, respectively). Furthermore, patient with risk genotypes (−763AA or +25GG genotype) demonstrated a significantly increasing risk of progression (HR 1.564, 95% CI 1.115–2.193, P = 0.01) or worse OS (HR 1.840, 95% CI 1.298–2.608, P = 0.001). Though a larger sample size study is needed to assess effect of oxaliplatin-based chemotherapy with these polymorphisms, we could imply that a significantly higher risk of progression or death was associated with −763AA genotype and +25GG genotype.
In conclusion, the ERCC5 −763A allele and +25G allele might increase the binding affinity with transcription factors and gene transcription activity, and thus increase the expression level of ERCC5 in related genotypes and lead to poor clinical outcome to platinum-based chemotherapy. The 2 polymorphisms might be important predictors of clinical outcome of CRC patients who received oxaliplatin chemotherapy. Although further studies with larger number of patients are warranted to confirm these results, selecting optimal drug strategy on the basis of predictive genotypes may represent an innovative strategy.
Acknowledgments
The authors thank Dr. Yusheng Ma and the technical staff of the Generay Biotech Co., Ltd, for their technical support on genotyping.
Footnotes
Abbreviations: CI = confidence interval, CRC = colorectal cancer, CT = computed tomography, ECOG = Eastern Cooperative Oncology Group, EMSA = electrophoretic mobility shift assays, ERCC5 = excision repair cross complementation group 5, HR = hazard ratio, OS = overall survival, PCR-LDR = polymerase chain reaction-ligation detection reaction, PFS = progression-free survival, RLA = relative luciferase activity, SNPs = single nucleotide polymorphisms.
This work was supported by the National Natural Science Foundation of China (No.81101629 and No.81172114). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Kelly H, Goldberg RM. Systemic therapy for metastatic colorectal cancer: current options, current evidence. J Clin Oncol 2005; 23:4553–4560. [DOI] [PubMed] [Google Scholar]
- 2.Petrelli F, Coinu A, Ghilardi M, et al. Efficacy of oxaliplatin-based chemotherapy + bevacizumab as first-line treatment for advanced colorectal cancer: a systematic review and pooled analysis of published trials. Am J Clin Oncol 2015; 38:227–233. [DOI] [PubMed] [Google Scholar]
- 3.Hertz DL, McLeod HL. Use of pharmacogenetics for predicting cancer prognosis and treatment exposure, response and toxicity. J Human Genet 2013; 58:346–352. [DOI] [PubMed] [Google Scholar]
- 4.Hertz DL, Rae J. Pharmacogenetics of cancer drugs. Ann Rev Med 2015; 66:65–81. [DOI] [PubMed] [Google Scholar]
- 5.Shuck SC, Short EA, Turchi JJ. Eukaryotic nucleotide excision repair: from understanding mechanisms to influencing biology. Cell Res 2008; 18:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monzo M, Moreno I, Navarro A, et al. Single nucleotide polymorphisms in nucleotide excision repair genes XPA, XPD, XPG and ERCC1 in advanced colorectal cancer patients treated with first-line oxaliplatin/fluoropyrimidine. Oncology 2007; 72:364–370. [DOI] [PubMed] [Google Scholar]
- 7.Ruzzo A, Graziano F, Loupakis F, et al. Pharmacogenetic profiling in patients with advanced colorectal cancer treated with first-line FOLFOX-4 chemotherapy. J Clin Oncol 2007; 25:1247–1254. [DOI] [PubMed] [Google Scholar]
- 8.Pare L, Marcuello E, Altes A, et al. Pharmacogenetic prediction of clinical outcome in advanced colorectal cancer patients receiving oxaliplatin/5-fluorouracil as first-line chemotherapy. Brit J Cancer 2008; 99:1050–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stevens EV, Nishizuka S, Antony S, et al. Predicting cisplatin and trabectedin drug sensitivity in ovarian and colon cancers. Mol Cancer Ther 2008; 7:10–18. [DOI] [PubMed] [Google Scholar]
- 10.Koeppel F, Poindessous V, Lazar V, et al. Irofulven cytotoxicity depends on transcription-coupled nucleotide excision repair and is correlated with XPG expression in solid tumor cells. Clin Cancer Res 2004; 10:5604–5613. [DOI] [PubMed] [Google Scholar]
- 11.Walsh CS, Ogawa S, Karahashi H, et al. ERCC5 is a novel biomarker of ovarian cancer prognosis. J Clin Oncol 2008; 26:2952–2958. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton SR. Targeted therapy of cancer: new roles for pathologists in colorectal cancer. Modern Pathol 2008; 21:S23–S30. [DOI] [PubMed] [Google Scholar]
- 13.Heinz S, Romanoski CE, Benner C, et al. Effect of natural genetic variation on enhancer selection and function. Nature 2013; 503:487–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards SL, Beesley J, French JD, et al. Beyond GWASs: illuminating the dark road from association to function. Am J Human Genet 2013; 93:779–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohrenweiser H. Survey of polymorphic sequence variation in the immediate 5′ region of human DNA repair genes. Mutat Res 2007; 616:221–226. [DOI] [PubMed] [Google Scholar]
- 16.Soccio RE, Chen ER, Rajapurkar SR, et al. Genetic variation determines PPAR gamma function and anti-diabetic drug response in vivo. Cell 2015; 162:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen JF, Xie FW, Chen K, et al. ERCC5 promoter polymorphisms at-763 and+25 predict the response to oxaliplatin-based chemotherapy in patients with advanced colorectal cancer. Cancer Biol Ther 2009; 8:1424–1430. [DOI] [PubMed] [Google Scholar]
- 18.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92:205–216. [DOI] [PubMed] [Google Scholar]
- 19.Khanna M, Park P, Zirvi M, et al. Multiplex PCR/LDR for detection of K-ras mutations in primary colon tumors. Oncogene 1999; 18:27–38. [DOI] [PubMed] [Google Scholar]
- 20.Fonseca C, Lindahl GE, Ponticos M, et al. A polymorphism in the CTGF promoter region associated with systemic sclerosis. N Engl J Med 2007; 357:1210–1220. [DOI] [PubMed] [Google Scholar]
- 21.Ding K, Zhou K, He F, et al. LDA–a java-based linkage disequilibrium analyzer. Bioinformatics 2003; 19:2147–2148. [DOI] [PubMed] [Google Scholar]
- 22.Koopman M, Venderbosch S, Nagtegaal ID, et al. A review on the use of molecular markers of cytotoxic therapy for colorectal cancer, what have we learned? Eur J Cancer 2009; 45:1935–1949. [DOI] [PubMed] [Google Scholar]
- 23.Braun MS, Richman SD, Thompson L, et al. Association of molecular markers with toxicity outcomes in a randomized trial of chemotherapy for advanced colorectal cancer: the FOCUS trial. J Clin Oncol 2009; 27:5519–5528. [DOI] [PubMed] [Google Scholar]
- 24.Zaanan A, Dalban C, Emile JF, et al. ERCC1, XRCC1 and GSTP1 single nucleotide polymorphisms and survival of patients with colon cancer receiving oxaliplatin-based adjuvant chemotherapy. J Cancer 2014; 5:425–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maurano MT, Wang H, Kutyavin T, et al. Widespread site-dependent buffering of human regulatory polymorphism. PLoS Genet 2012; 8:e1002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Somers J, Wilson LA, Kilday JP, et al. A common polymorphism in the 5′ UTR of ERCC5 creates an upstream ORF that confers resistance to platinum-based chemotherapy. Genes Dev 2015; 29:1891–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng L, Spitz MR, Hong WK, et al. Reduced expression levels of nucleotide excision repair genes in lung cancer: a case-control analysis. Carcinogenesis 2000; 21:1527–1530. [PubMed] [Google Scholar]
- 28.Cheng L, Sturgis EM, Eicher SA, et al. Expression of nucleotide excision repair genes and the risk for squamous cell carcinoma of the head and neck. Cancer 2002; 94:393–397. [DOI] [PubMed] [Google Scholar]
- 29.Skotheim RI, Autio R, Lind GE, et al. Novel genomic aberrations in testicular germ cell tumors by array-CGH, and associated gene expression changes. Cell Oncol 2006; 28:315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furuta T, Ueda T, Aune G, et al. Transcription-coupled nucleotide excision repair as a determinant of cisplatin sensitivity of human cells. Cancer Res 2002; 62:4899–4902. [PubMed] [Google Scholar]
- 31.Zienolddiny S, Campa D, Lind H, et al. Polymorphisms of DNA repair genes and risk of non-small cell lung cancer. Carcinogenesis 2006; 27:560–567. [DOI] [PubMed] [Google Scholar]
- 32.Chang JS, Wrensch MR, Hansen HM, et al. Nucleotide excision repair genes and risk of lung cancer among San Francisco Bay Area Latinos and African Americans. Int J Cancer 2008; 123:2095–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matakidou A, el Galta R, Webb EL, et al. Genetic variation in the DNA repair genes is predictive of outcome in lung cancer. Hum Mol Genet 2007; 16:2333–2340. [DOI] [PubMed] [Google Scholar]
- 34.Liang Y, Deng J, Xiong Y, et al. Genetic association between ERCC5 rs17655 polymorphism and lung cancer risk: evidence based on a meta-analysis. Tumour Biol 2014; 35:5613–5618. [DOI] [PubMed] [Google Scholar]
- 35.Rajaraman P, Bhatti P, Doody MM, et al. Nucleotide excision repair polymorphisms may modify ionizing radiation-related breast cancer risk in US radiologic technologists. Int J Cancer 2008; 123:2713–2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H, Wang T, Guo H, et al. Association analysis of ERCC5 gene polymorphisms with risk of breast cancer in Han women of northwest China. Breast Cancer 2016; 23:479–485. [DOI] [PubMed] [Google Scholar]
- 37.Saldivar JS, Lu KH, Liang D, et al. Moving toward individualized therapy based on NER polymorphisms that predict platinum sensitivity in ovarian cancer patients. Gynecol Oncol 2007; 107:S223–S229. [DOI] [PubMed] [Google Scholar]
- 38.Sun X, Li F, Sun N, et al. Polymorphisms in XRCC1 and XPG and response to platinum-based chemotherapy in advanced non-small cell lung cancer patients. Lung Cancer 2009; 65:230–236. [DOI] [PubMed] [Google Scholar]


