Abstract
The objective of this study was to investigate spatiotemporal characteristics with gait variability in patients with freezing of gait (FOG) after hypoxic-ischemic brain injury (HIBI).
Eleven patients showing FOG after HIBI and 15 normal controls were consecutively enrolled. We performed gait analysis using a computerized gait system (VICON MX-T10 Motion Analysis System) and compared spatiotemporal characteristics and gait variability in both groups. Additionally, we performed correlation analysis to identify the gait parameters associated with severity of freezing, which we measured based on unified Parkinson disease Rating Scale subscore.
Spatiotemporal characteristic of FOG patients showed increased stance time and double support phase and decreased swing time, single support phase, stride length, step length, and gait velocity compared with normal controls (P < 0.05). Besides baseline spatiotemporal characteristics, step time asymmetry and step length asymmetry were significantly increased in HIBI patients with FOG (P < 0.05). The coefficient of variation, which reflects the variability of each parameter, demonstrated increased cadence, stride time, swing time, single support phase, stride length, step length, and gait velocity variability in HIBI patients with FOG compared with normal controls (P < 0.05). Correlation analysis between FOG severity and spatiotemporal parameters revealed gait velocity, step length, and single support phase to be spatiotemporal parameters related to FOG severity (P < 0.05).
Our findings suggest that bilateral gait coordination deterioration plays a considerable role for pathophysiology of FOG in HIBI patients. Additional studies with a larger number of subjects are needed to further investigate the neural mechanism of FOG after HIBI.
INTRODUCTION
Freezing of gait (FOG) is a unique gait disorder in which patients are unable to initiate or continue locomotion, and it is 1 of the most disabling and least understood symptoms in advanced Parkinson disease (PD).1 In FOG, patients are unable to lift a foot to step forward, making the patients feel as if their foot is glued or magnetized to the ground. FOG is very troublesome because it increases the risk of falling, and it has a significant impact on quality of life in PD patients.2
Hypoxic-ischemic brain injury (HIBI) is a well-known consequence of cardiac arrest or respiratory failure, and there are 6 subtypes, which include ischemic, oligemic, anoxic, hypoxic, anemic, and histotoxic.3 The exact prevalence of HIBI remains unknown, but those who survive HIBI often experience several types of movement disorders such as Parkinsonism, myoclonus, dystonia, and chorea.4 However, FOG after HIBI has rarely been reported. Two previous studies on gait disturbance following HIBI suggested the causative pathological mechanism to be damage to the basal ganglia (BG) inducing a loss of functional integrity of the BG-thalamus-frontal cortex5,6; however, the pathophysiology of FOG in PD and HIBI patients still remains poorly understood.
Previous research about gait characteristics of FOG in PD patients reported increased cadence and double support phase; decreased stride length, gait velocity, and single support phase; higher stride time variability; and greater gait asymmetry.7–10 However, no study has evaluated the gait characteristics of FOG after HIBI. Therefore, we performed this study to investigate the basic spatiotemporal characteristics and gait variability of FOG in patients after HIBI compared with those of normal controls. Additionally we sought to determine the relationships between various spatiotemporal parameters and severity of FOG in HIBI patients.
METHODS
Participants
From March 2009 to February 2015, 13 patients with FOG after HIBI were consecutively enrolled in this study. For normal controls, we recruited 15 age- and sex-matched healthy people in stable medical condition without any previous neurologic, orthopedic, or visual problems affecting locomotion. The etiologies of HIBI in the 13 FOG patients included 7 cases of cardiac arrest, 2 cases of respiratory failure, 2 cases of hypovolemia, and 2 cases of intoxication. All subjects underwent neurological and physical examination, which included Mini Mental Status Exam and Functional Independence Measure (FIM). Also we evaluated the Complex Figure test (copy) for visuospatial perception, the Block Design test for visuospatial processing of motor task, and muscle power using medical research council grading. Since an objective method for grading FOG severity in HIBI patients has not yet been established, we evaluated severity using FOG subscore of unified Parkinson disease Rating Scale (UPDRS) ranging from 0, indicating no freezing, to 4, indicating frequent falls due to freezing. All participants provided written informed consent, and procedures were performed with the approval of the Institutional Review Board for Clinical Studies in our institution.
Protocol11
All participants walked barefoot on an 8-m pathway at their preferred gait speed. Gait analysis data from 5 trials performed were collected for all participants who were asked to walk naturally at self-selected gait speed while looking forward ahead. Every data were gathered in the motion analysis laboratory. During the gait cycle, we measured the spatiotemporal parameters of gait cycle using computerized motion analysis system (VICON MX-T10 Motion Analysis System, Oxford Metrics Inc., Oxford, UK). All participants were equipped with reflective infrared makers of the VICON Plug-in-Gait model for motion analysis. An expert investigator who had clinical experience of motion analysis over 20 years placed a total of 16 infrared makers on the posterior superior iliac spine, anterior superior iliac spine, mid points of the lateral femur, lateral knee joint axis, mid points of the lateral tibia, lateral malleolus, heel, and dorsal foot between metatarsal heads 2 and 3. Also, we captured simultaneous gait motion with 6 digital videos from the front, rear, and side during gait cycle. Two force plates (AMTI OR 6-5, Advanced Mechanical Technology, Newton, MA) were embedded under the pathway but were not used for data analysis.
Data Analysis
Basic spatiotemporal parameters of gait were determined from the walking trial. Outcome variables included cadence, stride time, step time, stance time, swing time, single support phase, double support phase, stride length, step length, and gait velocity. Measures of asymmetry for step length and step time were calculated manually, as each variable can yield different information about gait asymmetry.9,12,13 Asymmetry measures were calculated using the following equation: asymmetry = |right measure − left measure|. The absolute asymmetry value was quantified using the average right-left difference for each trial of each patient.
Variability of each baseline spatiotemporal parameter during the gait cycle was calculated using coefficient of variation (CV = standard deviation/mean × 100%).8,14,15 The data from each trial for each participant were averaged to calculate an individual mean and standard deviation for each variable.
Statistical Analysis
Statistical analysis was performed using SPSS, 20.0 for windows. Chi-squared and Mann–Whiney tests were used to evaluate categorical (gender) and continuous (age, cognition, FIM) baseline characteristics between FOG patients and normal controls. The Mann–Whitney test was used to compare spatiotemporal gait parameters between the 2 groups. Multiple linear regression analysis was used to analyze relationships between spatiotemporal parameters and FOG severity. A P value of 0.05 or less was considered statistically significant.
RESULTS
We initially recruited 13 FOG patients, but 2 were excluded because they did not undergo gait analysis. The FOG patients group consisted of 9 men and 2 women with a mean age of 37.36 ± 15.78 years (range, 22–53 years), whereas the normal controls consisted of 11 men and 4 women with a mean age of 40.27 ± 15.48 years (range, 25–56 years). There was no significant difference between the 2 groups with respect to age or gender (P > 0.05). The mean duration of disease from onset was 14.27 ± 15.44 months, and the mean freezing UPDRS subscore, which reflects the severity of freezing, was 3.18 ± 1.17 at the time of evaluation. The mean Hoehn and Yahr scale score for the FOG patient group was 3.18 ± 0.72. The Mini Mental Status Exam (FOG group: 20.45 ± 7.50, control group: 29.27 ± 1.49, P < 0.05) and FIM scores (FOG group: 58.45 ± 24.85, control group: 125.60 ± 0.91, P < 0.05) were significantly different between 2 groups, and they revealed severely impaired cognitive function and functional independence in the FOG group. Table 1 lists the individual baseline characteristics, the subtypes of FOG by phase of locomotion, the muscle power, the Rey Complex Figure test (copy) score, and the Block Design Test score. The brain magnetic resonance imaging (MRI) findings in FOG patients after HIBI showed heterogeneous cerebral injuries to the bilateral deep gray matter (patients 1 and 11), diffuse cortices (patient 5), and diffuse deep white matter (patients 7 and 9) (Figure 1).
TABLE 1.
General Characteristics of Hypoxic-Ischemic Brain Injured Patients With FOG
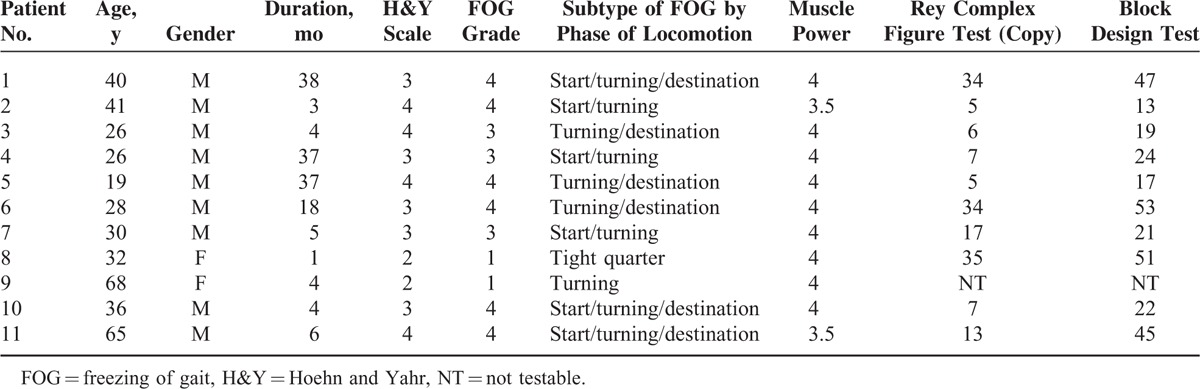
FIGURE 1.
Brain magnetic resonance imaging findings of patients with freezing of gait after hypoxic-ischemic brain injury showed heterogeneous cerebral injuries to the bilateral deep gray matter (patients 1 and 11), diffuse cortices (patient 5), and diffuse deep white matter (patients 7 and 9).
Spatiotemporal Characteristics of FOG After HIBI Compared With Those of Normal Controls
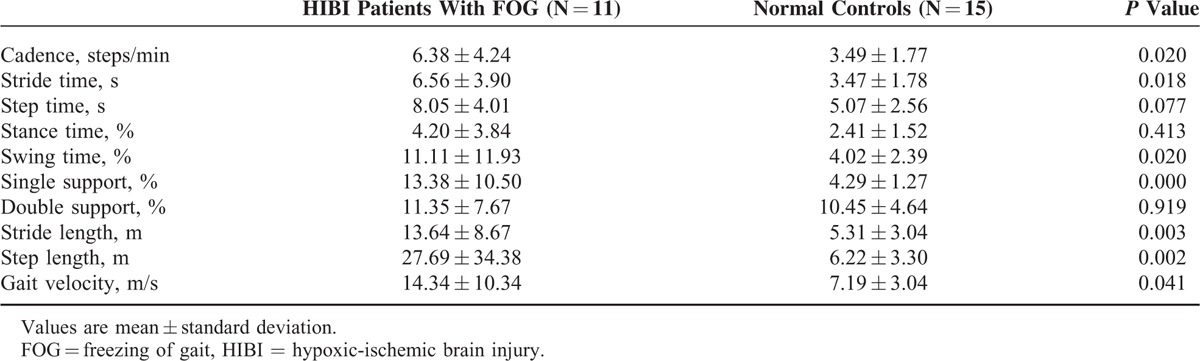
The FOG group showed significantly decreased swing time (28.14 vs 36.63, P < 0.001), single support phase (27.80 vs 37.00, P < 0.001), stride length (0.53 vs 1.19, P < 0.001), step length (0.27 vs 0.59, P < 0.001), and gait velocity (0.44 vs 1.09, P < 0.001) compared with normal controls. Additionally, the FOG group showed significantly increased stance time (71.86 vs 63.37, P < 0.001) and double support phase (44.06 vs 26.37, P < 0.001) compared with normal controls; however, there was no significant difference in cadence, stride time, or step time between the 2 groups (P > 0.05). On asymmetry analysis, step length asymmetry (0.10 vs 0.02, P = 0.001) and step time asymmetry (0.09 vs 0.02, P = 0.047) were significantly increased in FOG patients compared with normal controls. Table 2 summarizes the spatiotemporal characteristics between the 2 groups. Based on CV, there was higher variability of cadence, stride time, swing time, single support phase, stride length, step length, and velocity in HIBI patients with FOG compared with normal controls (Table 3).
TABLE 2.
Comparison of Spatiotemporal Characteristics Between HIBI Patients With FOG and Normal Controls
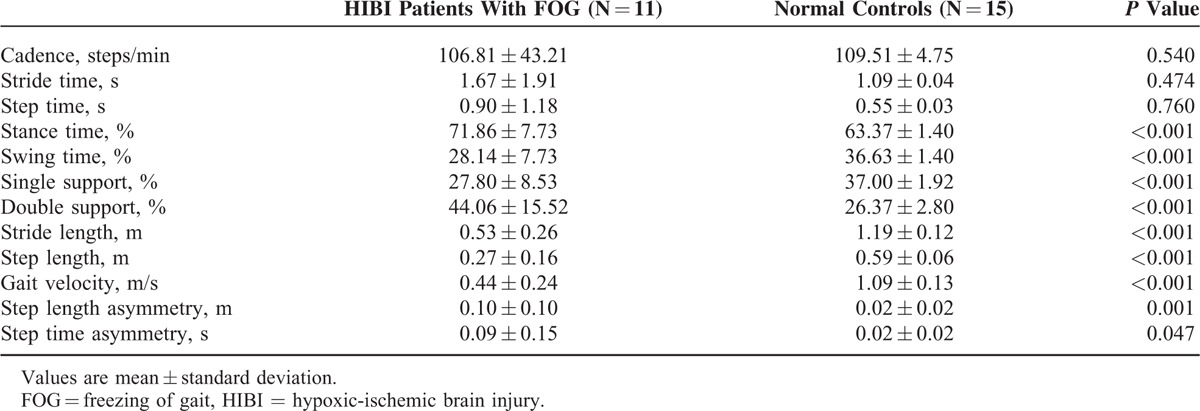
TABLE 3.
Coefficients of Variation of Spatiotemporal Gait Patterns Between HIBI Patients With FOG and Normal Controls
Correlation Analysis Between Spatiotemporal Parameters and FOG Severity
Multiple linear regression analysis revealed that gait velocity was significantly correlated with FOG severity (r2 = 0.408, P = 0.034). Also, gait velocity + step length (r2 = 0.659, P = 0.014) and gait velocity + step length + single support phase (r2 = 938, P < 0.001) showed significant correlation with FOG severity (Table 4). Other spatiotemporal parameters, including asymmetry factors, were not significantly associated with FOG severity.
TABLE 4.
Correlations Between Spatiotemporal Parameters of Gait Analysis and Freezing of Gait Severity Score in Hypoxic-Ischemic Brain Injured Patients
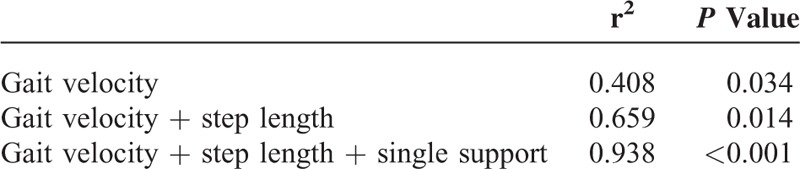
DISCUSSION
To our knowledge, this is the first study to investigate spatiotemporal characteristics of FOG in patients after HIBI. Our results demonstrate that spatiotemporal parameters of swing time, single support phase, stride length, step length, and gait velocity were significantly decreased in the FOG group, while other parameters such as stance time and double support phase were significantly increased. Additionally, increased step time and step length asymmetries were found in the FOG group, and CV of the spatiotemporal parameters of cadence, stride time, swing time, single support phase, stride length, step length, and gait velocity were increased in the FOG group. These results suggest that deterioration of gait coordination is 1 of the pathophysiological mechanisms of FOG in patients after HIBI.
FOG is a common symptom in advanced PD, and several studies on gait analysis in PD patients with FOG have been reported. Compared with normal controls, PD patients with FOG in previous studies demonstrated decreased gait velocity, step length, and single support phase and increased cadence and double support phase.10,16 These results are similar to our findings in HIBI patients with FOG with the exception that cadence was not statistically different compared with normal controls. In addition to basic spatiotemporal characteristics, PD patients with FOG revealed increase in stride length asymmetry, stride time variability,8,15 step time, and step length asymmetry.9 Among the many hypotheses on the pathophysiology of FOG in PD, increased stride-to-stride variability and marked gait asymmetry denote impairment of gait cycle coordination.17,18 From our CV results, which indicate single-subject gait parameter variability, HIBI patients with FOG showed an increase in step length asymmetry, step time asymmetry, cadence, stride time, swing time, single support phase, stride length, step length, and gait velocity. These findings suggest that the disturbance of bilateral gait coordination is an important factor in the pathophysiology of FOG in HIBI patients, similar to that of FOG in PD patients.
The pathophysiology of FOG is still unknown; however, we postulate that dysfunction of gait-related cerebral structures such as the BG circuit could provoke FOG. The BG is an important neural correlate for locomotion control and is susceptible to hypoxic-ischemic injury. Feve et al5 reported 4 cases of axial motor disturbance and FOG accompanied by bilateral BG lesions after HIBI. Severe HIBI primarily affects gray matter structures including the BG, thalami, and cerebral cortices (in particular, the sensorimotor and visual cortices).19 In PD patients, disturbance of the BG circuit connecting the thalamus and frontal lobe was shown to be a neural correlate in the pathophysiology of FOG.20 However, in our study, MRI findings in patients with FOG after HIBI showed diffuse neural damage in the bilateral cerebral cortices, BG, thalami, and deep white matter. These finding suggest that the neural pathophysiology of FOG in HIBI patients is different from that of FOG in PD patients.
The association between FOG and cognitive dysfunction has long been recognized in PD patients. Such cognitive dysfunction includes frontal executive dysfunction, inattention, and anxiety.21,22 Also, Amboni et al23 reported that the presence of FOG was considered to be a marker of early executive dysfunction in a 2-year follow-up study. In addition, it is well documented that PD subjects rely more on visual feedback to control balance and locomotion than healthy subjects.24 PD freezers demonstrated impaired visual information processing,25 and impairment of visuospatial processing is correlated with the severity of FOG in PD patients.26 In our study, HIBI patients with FOG exhibited significant impairment of cognitive function, visuospatial perception and processing. The brain damage including parieto-occipital and frontal areas which is associated with cognitive and visuospatial dysfunction might affect the development of FOG in HIBI patients. In the future, functional neuroimaging studies about the relationship between FOG and cognitive, visuospatial dysfunction are recommended. Because we did not compare HIBI patients with FOG to those without FOG, we could not conclusively determine the relationship between cognitive, visuospatial dysfunction and FOG in HIBI patients. However, it could be a potential pathophysiology of FOG, and further research comparing these 2 groups is warranted.
Falls are common and troublesome complications in elderly populations and advanced PD. There have been some reports that gait variability including the stride time and length variability was a predictor of falling.27,28 Nakamura et al29 proposed that a CV of stride length of 7% puts the walker at a high risk of falling. In the present study, the CV of step length in normal control was 5.31%. On the other hand, subjects with FOG after HIBI was 13.64% which surpassed the suggested 7% threshold, and it implies that HIBI patients with FOG have high risk of falling. Considering the finding that subjects with FOG exceeded the proposed fall-related variability threshold, further validation is needed to determine the thresholds more sensitive for predicting future falls. In addition, the clinical application of preventive and compensatory rehabilitative program is needed to reduce the risk of future falling.
Our study has several limitations. The primary limitation is the small sample size. In a period of 6 years, we enrolled only 11 HIBI patients with FOG due to a low incidence of FOG after HIBI. In the future, studies with a larger sample size should be performed. The second limitation is that we used the mean values of spatiotemporal parameters after 5 gait trials. These mean values could represent general spatiotemporal characteristics of patients with FOG after HIBI, but they do not reflect spatiotemporal data just before gait block. Several previous studies focusing on FOG used gate analysis data just before gate block, and this method seemed to better reflect FOG.10,14 However, ours is the first study to investigate the gait characteristics of HIBI patients with FOG; hence, the general spatiotemporal characteristics of FOG in HIBI patients might have a significant meaning. Finally, we compared HIBI patients with FOG to normal controls. This makes it difficult to definitively conclude whether different characteristics between the 2 groups are attributable to FOG, HIBI, or both. Therefore, further studies performing a 3-group comparison between HIBI patients with FOG, without FOG, and normal control group are warranted.
In conclusion, patients with FOG after HIBI showed deterioration of bilateral gait coordination compared with normal controls. We assume that neural networks related to coordination contribute to the pathophysiology of FOG. Further research with a larger sample size and a more detailed group comparison are needed. Additionally, neuroimaging studies are recommended to determine the pathophysiology of FOG after HIBI.
Footnotes
Abbreviations: BG = basal ganglia, CV = coefficient of variation, FOG = freezing of gait, HIBI = hypoxic ischemic brain injury, PD = Parkinson's disease.
This work was supported by a faculty research grant from Yonsei University College of Medicine, Seoul, Republic of Korea, for 2013 (6-2013-0054).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Bartels AL, Balash Y, Gurevich T, et al. Relationship between freezing of gait (FOG) and other features of Parkinson's: FOG is not correlated with bradykinesia. J Clin Neurosci 2003; 10:584–588. [DOI] [PubMed] [Google Scholar]
- 2.Bloem BR, Hausdorff JM, Visser JE, et al. Falls and freezing of gait in Parkinson's disease: a review of two interconnected, episodic phenomena. Mov Disord 2004; 19:871–884. [DOI] [PubMed] [Google Scholar]
- 3.Brierly J, Graham D. Hypoxia and vascular disorders of the nervous system. In: Adams JH, Corsellis JAN, Duchen LW, eds. Greenfield's Neuropathology. New York: Wiley; 1984:125–207. [Google Scholar]
- 4.Venkatesan A, Frucht S. Movement disorders after resuscitation from cardiac arrest. Neurol Clin 2006; 24:123–132. [DOI] [PubMed] [Google Scholar]
- 5.Feve AP, Fenelon G, Wallays C, et al. Axial motor disturbances after hypoxic lesions of the globus pallidus. Mov Disord 1993; 8:321–326. [DOI] [PubMed] [Google Scholar]
- 6.Hawker K, Lang AE. Hypoxic-ischemic damage of the basal ganglia. Case reports and a review of the literature. Mov Disord 1990; 5:219–224. [DOI] [PubMed] [Google Scholar]
- 7.Alice N, Fabienne C, Anne-Marie W, et al. Does freezing in Parkinson's disease change limb coordination? A kinematic analysis. J Neurol 2007; 254:1268–1277. [DOI] [PubMed] [Google Scholar]
- 8.Hausdorff JM, Schaafsma JD, Balash Y, et al. Impaired regulation of stride variability in Parkinson's disease subjects with freezing of gait. Exp Brain Res 2003; 149:187–194. [DOI] [PubMed] [Google Scholar]
- 9.Danoudis M, Iansek R, Simpson P. Freezing of gait in Parkinson's disease: further insights into pathophysiological mechanisms. Parkinsonism Relat Disord 2012; 18:543–547. [DOI] [PubMed] [Google Scholar]
- 10.Nieuwboer A, Dom R, De Weerdt W, et al. Abnormalities of the spatiotemporal characteristics of gait at the onset of freezing in Parkinson's disease. Mov Disord 2001; 16:1066–1075. [DOI] [PubMed] [Google Scholar]
- 11.Choi JY, Jung S, Rha DW, et al. Botulinum toxin type A injection for spastic equinovarus foot in children with spastic cerebral palsy: effects on gait and foot pressure distribution. Yonsei Med J 2016; 57:496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lythgo N, Wilson C, Galea M. Basic gait and symmetry measures for primary school-aged children and young adults whilst walking barefoot and with shoes. Gait Posture 2009; 30:502–506. [DOI] [PubMed] [Google Scholar]
- 13.Patterson KK, Gage WH, Brooks D, et al. Evaluation of gait symmetry after stroke: a comparison of current methods and recommendations for standardization. Gait Posture 2010; 31:241–246. [DOI] [PubMed] [Google Scholar]
- 14.Roemmich RT, Nocera JR, Vallabhajosula S, et al. Spatiotemporal variability during gait initiation in Parkinson's disease. Gait Posture 2012; 36:340–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nanhoe-Mahabier W, Snijders AH, Delval A, et al. Split-belt locomotion in Parkinson's disease with and without freezing of gait. Neuroscience 2013; 236:110–116. [DOI] [PubMed] [Google Scholar]
- 16.Nanhoe-Mahabier W, Snijders AH, Delval A, et al. Walking patterns in Parkinson's disease with and without freezing of gait. Neuroscience 2011; 182:217–224. [DOI] [PubMed] [Google Scholar]
- 17.Okuma Y. Practical approach to freezing of gait in Parkinson's disease. Pract Neurol 2014; 14:222–230. [DOI] [PubMed] [Google Scholar]
- 18.Okuma Y. Freezing of gait in Parkinson's disease. J Neurol 2006; 253 Suppl 7:VII27–VII32. [DOI] [PubMed] [Google Scholar]
- 19.Huang BY, Castillo M. Hypoxic-ischemic brain injury: imaging findings from birth to adulthood. Radiographics 2008; 28:417–439.quiz 617. [DOI] [PubMed] [Google Scholar]
- 20.Panisset M. Freezing of gait in Parkinson's disease. Neurol Clin 2004; 22 (Suppl):S53–S62. [DOI] [PubMed] [Google Scholar]
- 21.Giladi N, Hausdorff JM. The role of mental function in the pathogenesis of freezing of gait in Parkinson's disease. J Neurol Sci 2006; 248:173–176. [DOI] [PubMed] [Google Scholar]
- 22.Amboni M, Cozzolino A, Longo K, et al. Freezing of gait and executive functions in patients with Parkinson's disease. Mov Disord 2008; 23:395–400. [DOI] [PubMed] [Google Scholar]
- 23.Amboni M, Barone P, Picillo M, et al. A two-year follow-up study of executive dysfunctions in Parkinsonian patients with freezing of gait at on-state. Mov Disord 2010; 25:800–802. [DOI] [PubMed] [Google Scholar]
- 24.Cowie D, Limousin P, Peters A, et al. Insights into the neural control of locomotion from walking through doorways in Parkinson's disease. Neuropsychologia 2010; 48:2750–2757. [DOI] [PubMed] [Google Scholar]
- 25.Almeida QJ, Lebold CA. Freezing of gait in Parkinson's disease: a perceptual cause for a motor impairment? J Neurol Neurosurg Psychiatry 2010; 81:513–518. [DOI] [PubMed] [Google Scholar]
- 26.Kelly VE, Johnson CO, McGough EL, et al. Association of cognitive domains with postural instability/gait disturbance in Parkinson's disease. Parkinsonism Relat Disord 2015; 21:692–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brach JS, Berlin JE, Van Swearingen JM, et al. Too much or too little step width variability is associated with a fall history in older persons who walk at or near normal gait speed. J Neuroeng Rehabil 2005; 2:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: a 1-year prospective study. Arch Phys Med Rehabil 2001; 82:1050–1056. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura T, Meguro K, Sasaki H. Relationship between falls and stride length variability in senile dementia of the Alzheimer type. Gerontology 1996; 42:108–113. [DOI] [PubMed] [Google Scholar]


