Abstract
This study investigated whether migraine influences the risk of primary open-angle glaucoma (POAG) and primary angle-closure glaucoma (PACG) in Taiwan.
We retrieved the data analyzed in this study from the National Health Insurance Research Database in Taiwan. We included 17,606 newly diagnosed migraine patients without preexisting glaucoma and randomly selected and matched 70,423 subjects without migraine as the comparison cohort. The same exclusion criteria were also applied to comparison subjects. Multivariate Cox proportion-hazards regression model was used to assess the effects of migraines on the risk of glaucoma after adjusting for demographic characteristics and comorbidities.
The cumulative incidence of POAG was higher in the migraine cohort than that in the comparison cohort (log-rank P = 0.04). The overall incidence of POAG (per 10,000 person-years) was 9.62 and 7.69, respectively, for migraine cohort and nonmigraine cohort (crude hazard ratio [HR] = 1.24, 95% confidence interval [CI] = 1.01–1.54). After adjusting the covariates, the risk of POAG was not significantly higher in the migraine cohort than in the comparison cohort (adjusted HR [aHR] = 1.15, 95% CI = 0.93–1.42). The cumulative incidence of PACG did not differ between the migraine cohort and the comparison cohort (log-rank test P = 0.53). The overall incidence of PACG was not significantly higher in the migraine cohort than that in the comparison cohort (7.42 vs 6.84 per 10,000 person-years), with an aHR of 1.04 (95% CI = 0.82–1.32).
This study shows that migraines are not associated with a higher risk either in POAG or in PACG.
INTRODUCTION
Migraine is a primary debilitating headache disorder affecting 10% to 15% of people worldwide.1 Epidemiological data show that an increased prevalence of migraine in patients with glaucoma, particularly in patients with normal tension glaucoma (NTG).1 Furthermore, the headache in migraine patients might mimic acute angle closure glaucoma attack, which is an important problem in differential diagnosis.2,3 Glaucoma comprises a set of ocular disorders that lead to optic nerve damage that is often associated with increased intraocular pressure,4 which is the leading cause of irreversible blindness worldwide.5 Primary glaucoma can be divided into 2 major types, primary open-angle glaucoma (POAG), and primary angle-closure glaucoma (PACG), which are the 2 most common types in the Chinese ethnic population of Taiwan.6,7 Therefore, to identify whither migraine would influence the risk of glaucoma in our population, we conducted this study by using a population-based data set from the National Health Insurance (NHI) program of Taiwan. According to a review of relevant literature, this study is the first to address this crucial problem by using a large claims database.
METHODS
Data Source
The NHI program is a nationwide single-payer insurance system launched in 1995 provides a universal and comprehensive healthcare system for all citizens in Taiwan. Registration to the NHI is obligatory for every citizen and hence its coverage rate is nearly 100%.8 The NHI program includes coverage for outpatient, inpatient, emergency, traditional Chinese medicine services, dental care, and prescriptions. The National Health Research Institutes (NHRI) acquired the original claims data for reimbursement from the Administration of National Health Insurance, Taiwan. After de-identification and some encryption processing, the data were released under the name of National Health Insurance Research Database (NHIRD) for research purposes. This retrospective cohort study used data from the Longitudinal Health Insurance Database 2000 (LHID2000), contains all the original claim data of 1,000,000 individuals randomly sampled from the NHIRD. This study was approved to fulfill the condition for exemption by the Institutional Review Board (IRB) of China Medical University (CMUH104-REC2-115). The IRB also specifically waived the consent requirement. The diagnoses and procedures coded in the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) format.
Sampled Participants
We enrolled 20 years of age and older patients with diagnosed migraine (ICD-9 codes 346), based on claims data between January 1, 2000 and December 31, 2010 as the study cohort. The index date was the date of an initial diagnosis of migraine. We excluded patients diagnosed with glaucoma (ICD-9 code 365) before index date and <20 years old and those without information on medical. A nonmigraine comparison cohort was randomly selected among patients without diagnosis of migraine during 2000 to 2011, frequency matched on age group (every 5 years), sex, and index year to the migraine cohort from the registry of beneficiaries based on a 1:4 ratio. The same exclusion criteria were also applied to nonmigraine subjects. In addition, almost all of the sampled participants are the Chinese ethnic population in this study.
Outcome and Comorbidities
We identified the first diagnoses of POAG (ICD-9-CM code 365.1x) or PACG (ICD-9-CM code 365.2x) from claim records as the study end point. All of the study population were followed from then index date to occurrence of end point, withdrawal from the database, or December 31, 2011, whichever date came first. We also incorporated outpatient and inpatient diagnosis file to ascertain the baseline comorbidities, including diabetes mellitus (ICD-9-CM code 250), hypertension (ICD-9-CM codes 401–405), hyperlipidemia (ICD-9-CM code 272), and coronary artery disease (CAD) (ICD-9-CM codes 410–414).
Statistical Analysis
The distributions of baseline characteristics were compared between the cohorts with and without migraine by chi-squared test for categorical variables and Student t test for continuous variables. For estimating the cumulative incidence of POAG or PACG in migraine cohort and nonmigraine cohort, we performed survival analysis using the Kaplan–Meier method, with significance based on the log-rank test. Incidence densities of POAG and PACG by demographic variables and comorbidity were calculated in each cohort. The univariable and multivariable Cox proportion hazard regression models were used to examine the effect of migraine on the risk of POAG or PACG, as shown by hazard ratio (HR) with a 95% confidence interval (CI). In the multivariable Cox model, only hypertension and hyperlipidemia attained significance. Further data analysis was performed to evaluate the joint effect between migraine, hypertension and hyperlipidemia. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). The significance level was set at <0.05 for the 2-sided P value.
RESULTS
Table 1 shows the demographic characteristics in this study. Both cohorts exhibited similar distributions regarding gender and age, and the patients were predominantly women (73.5%) and <49 years (66.4%). The mean ages in the migraine cohort and nonmigraine cohort were 45.1 (standard deviation [SD] = 14.8) and 45.1 (SD = 14.9) years, respectively. Compared with the nonmigraine cohort, the migraine cohort had a significantly higher proportion of hypertension (26.8% vs 18.7%), hyperlipidemia (18.8% vs 12.5%), and CAD (14.6% vs 8.31%). During the mean follow-up period of 6.73 years for the migraine cohort and 6.58 years for the nonmigraine cohort, the overall incidence of POAG (per 10,000 person-years) was 9.62 and 7.69, respectively (crude HR = 1.24, 95% CI = 1.01–1.54; Table 2). Figure 1A shows the cumulative incidence curve of POAG for the 2 cohorts, the incidence of POAG was higher in the migraine cohort than in the nonmigraine cohort (log-rank P = 0.04). After mutual adjustment for all relevant confounding factors, the risk of POAG was not significantly higher in the migraine cohort than in the nonmigraine cohort (adjusted HR [aHR] = 1.15, 95% CI = 0.93–1.42). The incidence of POAG was higher in men than in women and increased with age and with comorbidity in both cohorts. During the mean follow-up period of 6.74 years for the migraine cohort and 6.58 years for the nonmigraine cohort, the overall incidence of PACG was not significantly higher in the migraine cohort than in the nonmigraine cohort (7.42 vs 6.84 per 10,000 person-years), with an aHR of 1.04 (95% CI = 0.82–1.32). The Kaplan–Meier curve showed that the cumulative incidence of PACG did not differ between the migraine cohort and the nonmigraine cohorts (Figure 1B, log-rank test P = 0.53).
TABLE 1.
Comparisons in Demographic Characteristics and Comorbidities in Patient With and Without Migraine
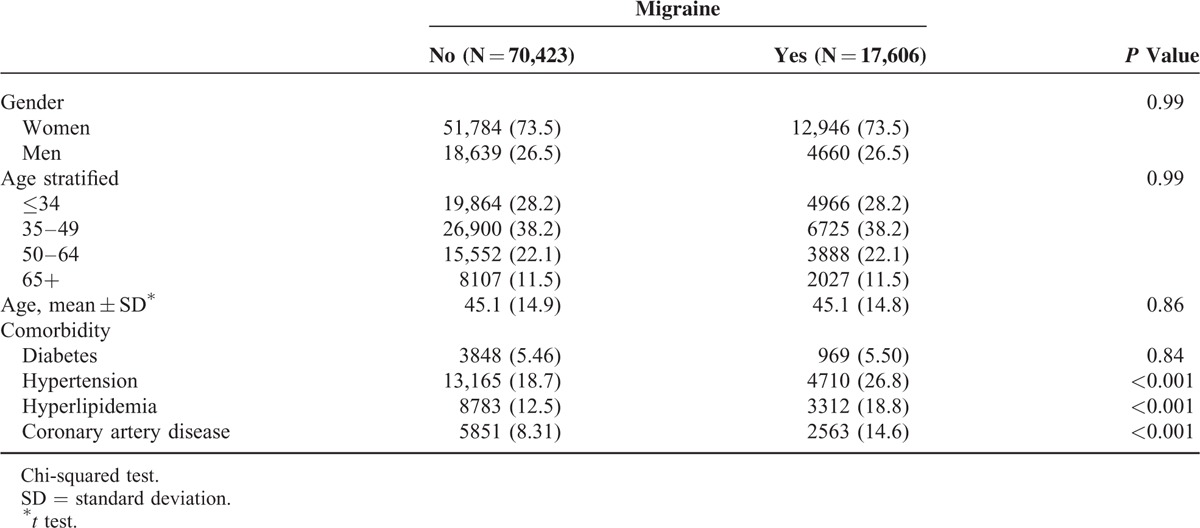
TABLE 2.
Comparison of Incidence Densities of POAG and PACG HR Between With and Without Migraine by Demographic Characteristics and Comorbidity
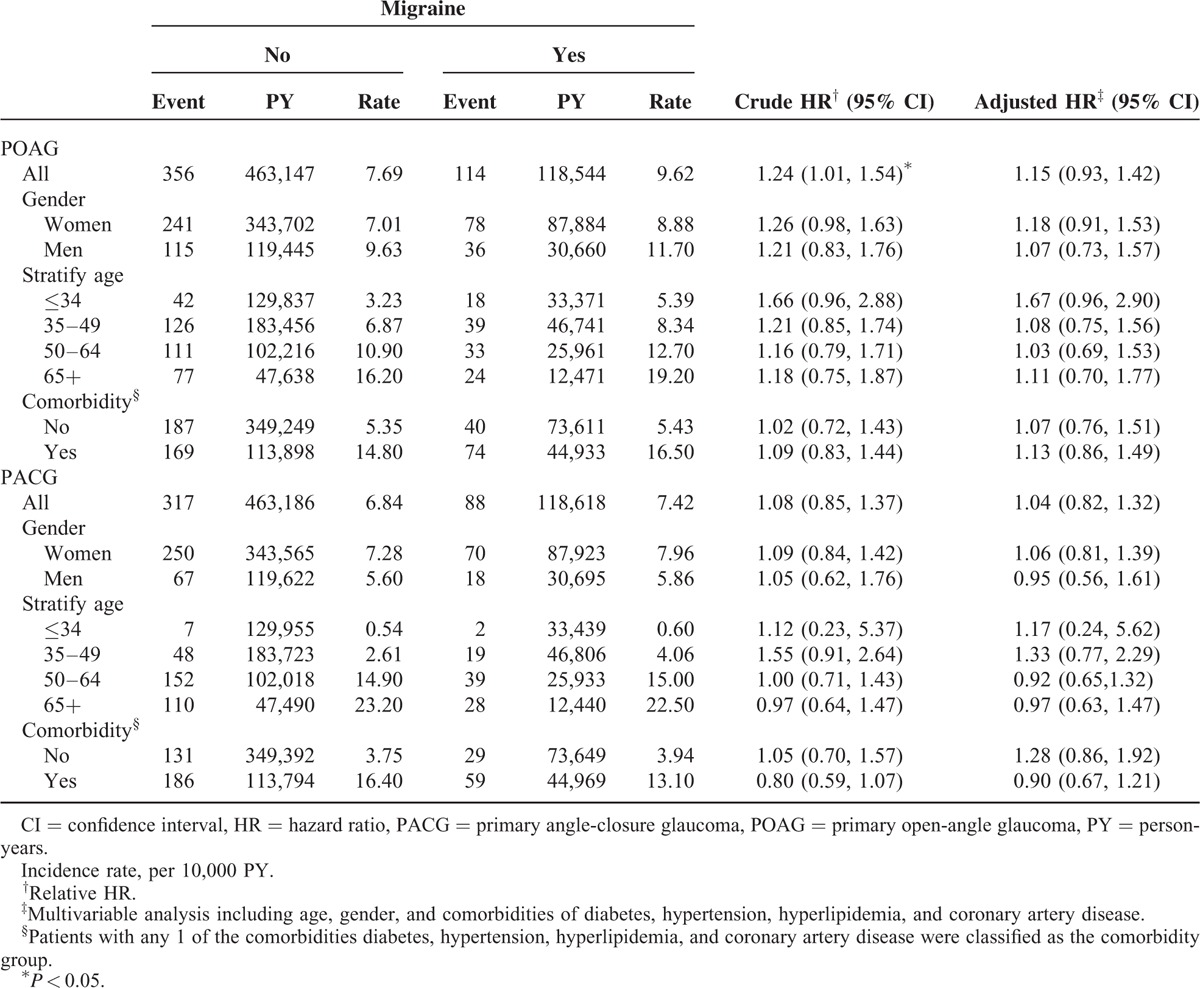
FIGURE 1.
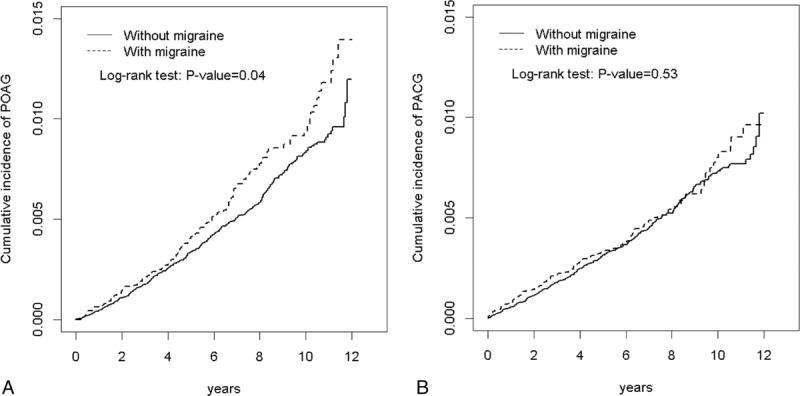
A shows that the cumulative incidence of POAG was higher in the migraine cohort than that in the nonmigraine cohort (log-rank P = 0.04). B shows that the cumulative incidence of PACG did not differ between the migraine cohort and the nonmigraine cohorts (log-rank test P = 0.53). PACG = primary angle-closure glaucoma, POAG = primary open-angle glaucoma.
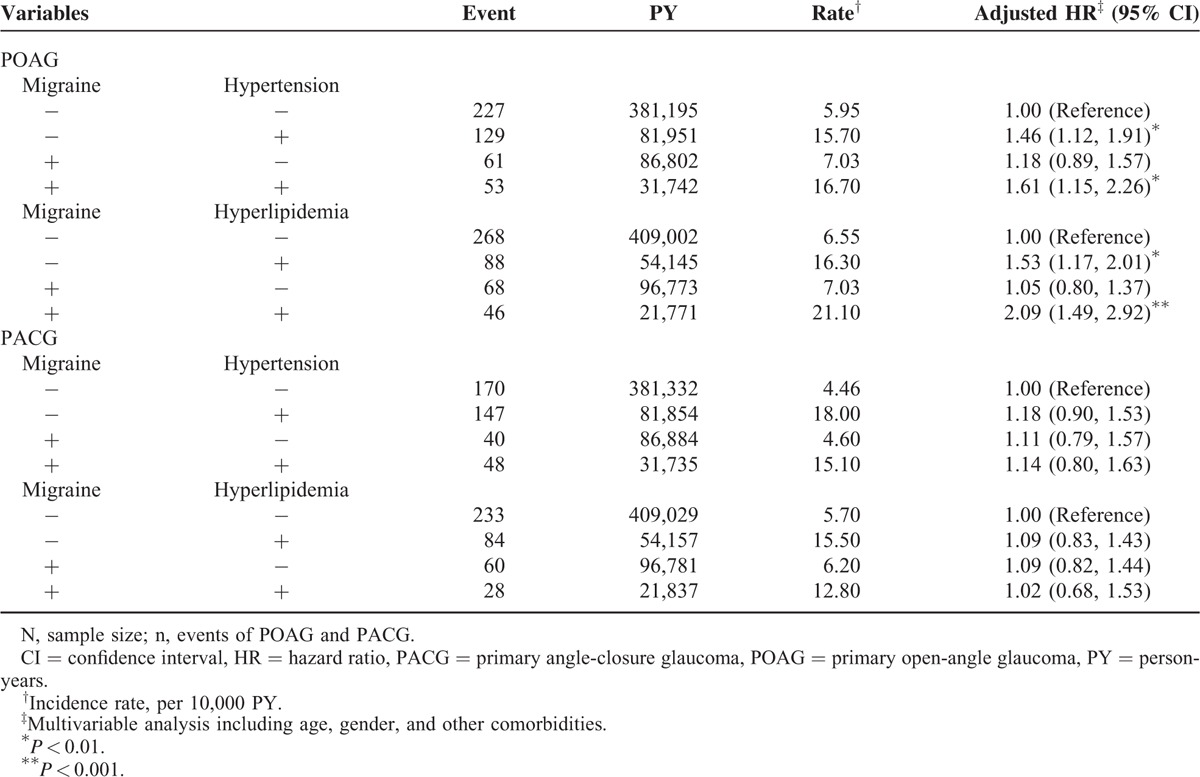
Table 3 illustrates the joint effects of migraine with hypertension and migraine with hyperlipidemia on the risks of POAG and PACG. A significantly higher POAG risk was observed in patients with both migraine and hypertension (aHR = 1.61, 95% CI = 1.15–2.26) and with both migraine and hyperlipidemia (aHR = 2.09, 95% CI = 1.49–2.92) than those without migraine, hypertension, and hyperlipidemia. Compared to subjects without migraine and hypertension, patients only with hypertension had a significantly higher risk of POAG (aHR = 1.46, 95% CI = 1.12–1.91). We also observed significantly higher risk of POAG in patients without migraine and with hyperlipidemia (aHR = 1.53, 95% CI = 1.17–2.01), compared with in subjects without migraine and hyperlipidemia.
TABLE 3.
Cox Proportional Model Measured HRs for the Patients With Migraine-Associated POAG and PACG With Joint Effect of Migraine, Hypertension, and Hyperlipidemia
DISCUSSION
Potential pathophysiological link between migraine and glaucoma has been proposed.9,10 Migraine is a disorder of the central nervous system, with both neural and vascular involvement.11,12 Vascular factors have been well noted to play an important role in the pathophysiology of glaucoma.13 Among the glaucoma types, NTG, a subtype of POAG, is associated with retinal vascular dysregulation14 and poor blood flow over the optic nerve head.15 The common vasospastic mechanism between NTG and migraine has been proposed.16 This hypothesis is also supported by some epidemiological studies that higher prevalence of migraine was among NTG patients in Asian population.17,18 However, contradictory reported was still noted.19 Very few studies evaluate the risk of migraine in PACG patients. PACG is very common type in Chinese ethnic population.6 PACG patients used to present with headache, which is another challenging issue for clinicians.2,20,21 Even delayed diagnosis of PACG in migraine or headache patients was not uncommon in daily clinical practice.2,22 Furthermore, many medical illnesses are reported to be comorbid with migraine and glaucoma, including hypertension, hyperlipidemia, diabetes, CAD, and others.23,24 The co-occurrence of migraine and glaucoma can complicate the diagnosis and treatment because of different focal signs of headache in migraine and nonstandard clinical features.
In the present study, we first report that migraine does not influence the risk of POAG in the Taiwan population based on the claims database. Conflicting reports regarding the association of migraine and POAG were found in the literatures. Some studies find no significant difference in migraine prevalence between glaucoma and nonglaucoma control groups.25 However, in Blue Mountains Eye study,26 a greater likelihood of developing POAG in elderly patients age 70 to 79 years with a self-reported history of migraine, compared to people without migraine episode in their lives. Further studies show that migraine is more common (∼30%) in glaucoma patients than general population (10–15%),26 which suggests migraine is a risk factor for glaucoma. The Glaucoma Inheritance Study found that migraine history was significantly associated with familial POAG compared to those with sporadic POAG, implying that migraine is an important heritable risk factor for glaucoma.27 We think the potential reasons for different results across studies are coming from different study designs and different study ethnic population. It is not really appropriate to directly compare the findings among the all studies.
Few studies evaluate the relationship between migraine and PACG. Some important clinical points should be mentioned. The diagnosis of migraine is purely based on its symptomatology, as detailed in the International Classification of Headache Disorders.28 The duration of the headache phase of both 2 common types of migraine (without aura, with aura) is 4 to 72 h and is characterized by photophobia, and nausea and/or vomiting.28 PACG patients, especially acute or subacute angle closure attacks generally experience ocular pain, headache, nausea, vomiting, and conjunctival injection, which is accompanied by blurred vision and halos around lights. Therefore, these attacks may be mistaken for migraine.29 We believe that there should exist some PACG cases who might be mistaken as migraine patients in present study design. However, the subsequent PACG risk in migraine is still not higher compared to nonmigraine cohort. Plausible reasons for this finding remain unclear, and further observation is warranted to address this finding.29
Glaucoma and migraine patients are significantly more likely to have comorbidities.23,24 Therefore, we considered the joint effects of hypertension and hyperlipidemia on the risks of POAG and PACG in migraine. Our results show that the risk of POAG increases significantly when migraine occurs with hypertension and hyperlipidemia. However, there is no such joint effect in PACG. In recent 1 work also from Taiwan health insurance database, the authors report that more than half (50.5%) of the OAG patients had hypertension, and more than 30.5% had hyperlipidemia.30 Our work again confirms that hypertension and hyperlipidemia are important risk factors for POAG in our population. On the contrary, PACG is caused by angle closure itself and trabecular meshwork will be obstructed by the iris, which can result in impaired aqueous flow and intraocular pressure elevation. To date, no evidence show that PACG has strong medical risk factors. We believe that this is the potential reason why there is no joint effect of comorbid disease for migraine in the risk of PACG.
Despite obtaining promising results, our study had the following limitations. First, we defined glaucoma by relying entirely on claims data (ICD-9-CM coding from clinicians), an approach that may be less accurate than determining diagnoses individually through a standardized procedure. In this type of claims database study, clinical information such as that on intraocular pressure (IOP), central corneal thickness, visual field findings, and optic nerve evaluations is unavailable. Second, this study had a selection bias. Because the NHI database is composed only of data from patients who underwent treatment, patients who had not received treatment for headache might have been recruited in the comparison cohort. Third, because of the lack of laboratory and imaging data in individual chart records, the NHIRD is used primarily for insurance purposes and has not been validated entirely for research; thus, uncontrolled confounding factors such as visual field severity, IOP reading, and potential biases may have affected our retrospective case–control study. Fourth, despite the large sample, the study cohort consisted of Taiwanese patients, and thus, these findings are not easily generalizable to other population groups.
Our study had the following strengths. First, the strength of the database is excellent because of the large sample randomization, and we could follow patient cases over time to assess the relationship between migraine and the subsequent onset of POAG or PACG. Second, the database includes data on a broad range of people with different sociodemographic profiles, unlike those used in smaller studies, in which patients are recruited from specific regions that might not be representative of the entire population. Third, our study is the first to evaluate the relationship between migraine and glaucoma risk in a purely Chinese population by using a large claims database. Our findings can provide a strong foundation for further longitudinal research.
In conclusion, migraine does not influence the risk of POAG or PACG in Chinese ethnic population. However, clinicians should still consider the potential risk of glaucoma in certain high-risk groups.
Footnotes
Abbreviations: aHR = adjusted hazard ratio, BNHI = Bureau of National Health Insurance, CI = confidence interval, LHID 2000 = Longitudinal Health Insurance Database 2000, NHI = National Health Insurance, NHIA = National Health Insurance Administration, NHIRD = National Health Insurance Research Database, NHRI = National Health Research Institutes, PACG = primary angle-closure glaucoma, POAGprimary = open-angle glaucoma.
All authors have contributed significantly, and that all authors are in agreement with the content of the manuscript: Conception/design: H-YC, C-HK; Provision of study materials: C-HK; Collection and/or assembly of data: all authors; Data analysis and interpretation: all authors; Manuscript writing: all authors; Final approval of manuscript: all authors.
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW105-TDU-B-212-133019), China Medical University Hospital, Academia Sinica Taiwan Biobank Stroke Biosignature Project (BM10501010037), NRPB Stroke Clinical Trial Consortium (MOST 104-2325-B-039-005), Tseng-Lien Lin Foundation, Taichung, Taiwan, Taiwan Brain Disease Foundation, Taipei, Taiwan, and Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Stovner L, Hagen K, Jensen R, et al. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia 2007; 27:193–210. [DOI] [PubMed] [Google Scholar]
- 2.Friedman DI. The eye and headache. Continuum (Minneap Minn) 2015; 21:1109–1117. [DOI] [PubMed] [Google Scholar]
- 3.Friedman DI, Gordon LK, Quiros PA. Headache attributable to disorders of the eye. Curr Pain Headache Rep 2010; 14:62–72. [DOI] [PubMed] [Google Scholar]
- 4.Casson RJ, Chidlow G, Wood JP, et al. Definition of glaucoma: clinical and experimental concepts. Clin Experiment Ophthalmol 2012; 40:341–349. [DOI] [PubMed] [Google Scholar]
- 5.Tham YC, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 2014; 121:2081–2090. [DOI] [PubMed] [Google Scholar]
- 6.Chen HY, Chang YC, Wang IJ, et al. Comparison of glaucoma diagnoses using stratus and cirrus optical coherence tomography in different glaucoma types in a Chinese population. J Glaucoma 2013; 22:638–646. [DOI] [PubMed] [Google Scholar]
- 7.Chen HY, Chang YC, Chen WC, et al. Association between plasma endothelin-1 and severity of different types of glaucoma. J Glaucoma 2013; 22:117–122. [DOI] [PubMed] [Google Scholar]
- 8.Database NHIR. Taiwan. http://nhird.nhri.org.tw/en/Background.html Accessed 2015. [Google Scholar]
- 9.Nguyen BN, Lek JJ, Vingrys AJ, et al. Clinical impact of migraine for the management of glaucoma patients. Prog Retin Eye Res 2016; 51:107–124. [DOI] [PubMed] [Google Scholar]
- 10.Demircan S, Ataş M, Yüksel SA, et al. The impact of migraine on posterior ocular structures. J Ophthalmol 2015; 2015:868967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charles AC, Baca SM. Cortical spreading depression and migraine. Nat Rev Neurol 2013; 9:637–644. [DOI] [PubMed] [Google Scholar]
- 12.Pietrobon D, Moskowitz MA. Pathophysiology of migraine. Annu Rev Physiol 2013; 75:365–391. [DOI] [PubMed] [Google Scholar]
- 13.Flammer J, Orgul S, Costa VP, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res 2002; 21:359–393. [DOI] [PubMed] [Google Scholar]
- 14.Broadway DC, Drance SM. Glaucoma and vasospasm. Br J Ophthalmol 1998; 82:862–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plange N, Remky A, Arend O. Color Doppler imaging and fluoresce in filling defects of the optic disc in normal tension glaucoma. Br J Ophthalmol 2003; 87:731–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flammer J, Konieczka K, Flammer AJ. The primary vascular dysregulation syndrome: implications for eye diseases. EPMA J 2013; 4:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cursiefen C, Wisse M, Cursiefen S, et al. Migraine and tension headache in high-pressure and normal-pressure glaucoma. Am J Ophthalmol 2000; 129:102–104. [DOI] [PubMed] [Google Scholar]
- 18.Usui T, Iwata K, Shirakashi M, et al. Prevalence of migraine in low-tension glaucoma and primary open-angle glaucoma in Japanese. Br J Ophthalmol 1991; 75:224–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pradalier A, Hamard P, Sellem E, et al. Migraine and glaucoma: an epidemiologic survey of French ophthalmologists. Cephalalgia 1998; 18:74–76. [DOI] [PubMed] [Google Scholar]
- 20.Ringeisen AL, Harrison AR, Lee MS. Ocular and orbital pain for the headache specialist. Curr Neurol Neurosci Rep 2011; 11:156–163. [DOI] [PubMed] [Google Scholar]
- 21.Lee AG, Al-Zubidi N, Beaver HA, et al. An update on eye pain for the neurologist. Neurol Clin 2014; 32:489–505. [DOI] [PubMed] [Google Scholar]
- 22.Nesher R, Mimouni MD, Khoury S, et al. Delayed diagnosis of subacute angle closure glaucoma in patients presenting with headaches. Acta Neurol Belg 2014; 114:269–272. [DOI] [PubMed] [Google Scholar]
- 23.Chuang CS, Lin CL, Lin MC, et al. Migraine and risk of dementia: a nationwide retrospective cohort study. Neuroepidemiology 2013; 41:139–145. [DOI] [PubMed] [Google Scholar]
- 24.Wang SJ, Chen PK, Fuh JL. Comorbidities of migraine. Front Neurol 2010; 1:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orgul S, Flammer J. Headache in normal-tension glaucoma patients. J Glaucoma 1994; 3:292–295. [PubMed] [Google Scholar]
- 26.Wang JJ, Mitchell P, Smith W. Is there an association between migraine headache and open-angle glaucoma? Findings from the Blue Mountains Eye Study. Ophthalmology 1997; 104:1714–1719. [DOI] [PubMed] [Google Scholar]
- 27.Hewitt AW, Wu J, Green CM, et al. Systemic disease associations of familial and sporadic glaucoma: the Glaucoma inheritance study in Tasmania. Acta Ophthalmol 2010; 88:70–74. [DOI] [PubMed] [Google Scholar]
- 28.International Headache Society. The International Classification of Headache Disorders. In: Cephalalgia, vol. 33. 3rd ed.; 2013:629–808. [DOI] [PubMed] [Google Scholar]
- 29.Friedman DI, Gordon LK, Quiros PA. Headache attributable to disorders of the eye. Curr Pain Headache Rep 2010; 14:62–72. [DOI] [PubMed] [Google Scholar]
- 30.Lin HC, Chien CW, Hu CC, et al. Comparison of comorbid conditions between open-angle glaucoma patients and a control cohort: a case–control study. Ophthalmology 2010; 117:2088–2095. [DOI] [PubMed] [Google Scholar]


