Abstract
The objective of this study was to evaluate the association between maternal subclinical thyroid dysfunction and autoimmunity with the risk for intrauterine growth restriction (IUGR).
Design is a systematic review and meta-analysis.
A literature search was conducted using PubMed, Embase, and Cochrane database. A combination of 2 key words was used to search for the eligible studies: one indexed thyroid dysfunction or antithyroid antibodies; and the other one indexed the adverse neonatal outcomes of pregnancy, such as IUGR, small for gestational age, fetal growth restriction, or low birth weight.
Two reviewers selected the studies, and eligible studies met the following criteria: prospective cohort studies or case control studies, studies of maternal thyroid dysfunction and positive antithyroid antibodies as the exposure of interest, and studies of IUGR or small for gestational age as the outcome of interest.
Data were recorded, including data from maternal thyroid disorders and IUGR, and compared with a reference group.
There were 22 individual data from the 13 cohort articles. Among these, 7 were focused on subclinical hypothyroidism (SCH), 4 on subclinical hyperthyroidism, 7 on positivity for thyroid peroxidase antibody (TPOAb), and 4 on isolated hypothyroxinemia. Meta-analysis showed that there was no effect of subclinical hyperthyroidism (odds ratio (OR) = 0.98; 95% confidence interval (CI), 0.40–2.41), TPOAb positivity (OR = 1.57; 95% CI, 0.77–3.18), or isolated hypothyroxinemia (OR = 1.05, 95% CI: 0.37–2.92) on IUGR. However, SCH is associated with IUGR (OR = 1.54; 95% CI, 1.06–2.25).
SCH is associated with IUGR; however, subclinical hyperthyroidism, TPOAb positivity, or isolated hypothyroxinemia do not affect the risk of IUGR.
INTRODUCTION
The term intrauterine growth restriction (IUGR), or fetal growth restriction, is used to describe a fetus that cannot reach its growth potential. It is usually diagnosed by fetal biometry and Doppler flow.1 Small for gestational age (SGA) is used to describe those infants who are smaller in size than normal for their gestational age, defined as a weight below the 10th percentile or 2 standard deviations (SD) for the gestational age.2 Although the 2 terms are different, SGA is widely considered to be a proxy for IUGR, and weights below 2 SD would capture the majority of fetuses with IUGR.3
Retarded development of the infants results in short-term adverse outcomes, such as increased mortality and morbidity, prematurity, and hypoglycemia, as well as some long-term outcomes such as delayed growth during childhood, short stature, obesity, higher thyroid-stimulating hormone (TSH) levels, hypertension, and type 2 diabetes.4–6
Thyroid diseases are relatively common in women during their reproductive period. Normal maternal thyroid function is currently considered crucial for fetal growth and neurocognitive development. Many epidemiological studies also indicate a possible effect of thyroid dysfunction or antithyroid antibodies (ATA) on increased risks for pregnancy complications such as IUGR or SGA. However, the results vary between studies, and drawing conclusions remains controversial, especially with respect to subclinical thyroid dysfunction or positive ATA with euthyroid status. It is widely accepted that overt hypothyroidism (OH) and overt hyperthyroidism increase the risk for deleterious outcomes. Therefore, the aim of this meta-analysis was to review all of the eligible studies to evaluate the association between thyroid disorders, including subclinical hypothyroidism (SCH), subclinical hyperthyroidism, thyroid peroxidase antibody (TPOAb) positivity, and isolated hypothyroxinemia, and the risk for IUGR.
METHODS
Search Strategy and Study Selection
A literature search was conducted using PubMed, Embase, and Cochrane database in October, 2015 with a combination of 2 key words: one key word was related to thyroid dysfunction, including thyroid disease, thyroid function, thyroid dysfunction, hyperthyroidism, hypothyroidism, hypothyroxinemia, subclinical hyperthyroidism, subclinical hypothyroidism, thyroid peroxidase antibody, anti-TPO; autoantibodies to thyroid peroxidase, thyroperoxidase, and TPO; the other term was related to restricted development of fetus or infants, including SGA, small for gestational age, IUGR, intrauterine growth retardation, intrauterine growth restriction, fetal growth restriction, fetal growth retardation, fetal growth restriction, and low birth weight. All terms were searched without setting limits. We then performed citation tracking of selected studies and recent reviews. Studies were considered qualified if they met the following criteria: the research was a prospective cohort study or a case control study; the interested exposures were maternal mild thyroid disorders, including SCH, subclinical hyperthyroidism, isolated hypothyroxinemia, and TPO positivity; the outcome of the selected studies was IUGR or SGA; and the data concerning numbers for IUGR in each study were reported.
Data Extraction
The exposure was thyroid disorders and the outcome of interest was IUGR, defined as fetal weight below the 10th percentile of gestational age; SGA, defined as birth weight <2500 g at full-term delivery, or fetal weight below the 10th percentile or 2 SD of the gestational age. Diagnosis of thyroid disease in all selected articles was definitive. Data were recorded, including any incidence data for maternal thyroid disorders, and IUGR or SGA compared with a reference group. We extracted information from each selected article as to author, publication year, quality, and results.
Quality Assessment of the Included Studies
The Newcastle–Ottawa scale was used to assess the quality of the selected studies. Information on selection, comparability, and outcomes was evaluated for selected studies. A study scored a maximum of 4 for selection, 2 for comparability, and 3 for assessment of outcome or ascertainment of exposure.
Statistical Analysis
Stata (version 11) was used to analyze the data. The combined odds ratio (OR) was calculated with its 95% confidence interval (CI) to evaluate the strength of the relationships between thyroid disorders and IUGR risk. The significance of the combined OR calculated using the Mantel–Haenszel statistical method was determined by the Z test. Two models of meta-analysis were considered: the random-effects model and the fixed effects model. P value less than 0.05 was considered significant.
To assess the between-study heterogeneity, both the X2-based Q statistic test and the I2 statistic were calculated. I2 values of 25%, 50%, and 75% were used as evidence of low, moderate, or high heterogeneity, respectively. Random-effects model was used to pool the results if high heterogeneity existed; fixed-effects model was used to pool the results if heterogeneity is not high. The influence of each study on the overall risk estimate was evaluated by sequential omission of each study to validate the credibility of outcomes in this meta-analysis. Begg funnel plots and Egger linear regression test were used to assess potential publication bias.
Ethics Committee Approval
This meta-analysis was based on published data; thus no ethical approval and patient consent were required.
RESULTS
Study Selection and Study Characteristics
Our search strategy initially retrieved 769 articles from PubMed, Embase, and Cochrane databases for critical appraisal. Six hundred seventy-three articles were excluded by reading their titles and abstracts. After full-text review of the remaining 96 articles, 13 prospective cohort studies were identified to be eligible articles. There were 22 individual data from the 13 articles. They were assessed as at a low risk of bias based on the Newcastle–Ottawa System. Among these studies, 7 were focused on SCH,7–13 4 on subclinical hyperthyroidism,8–11 7 on TPO positivity,7,10,13–17 and 4 on isolated hypothyroxinemia.11,13,18,19Figure 1 shows the study selection.
FIGURE 1.
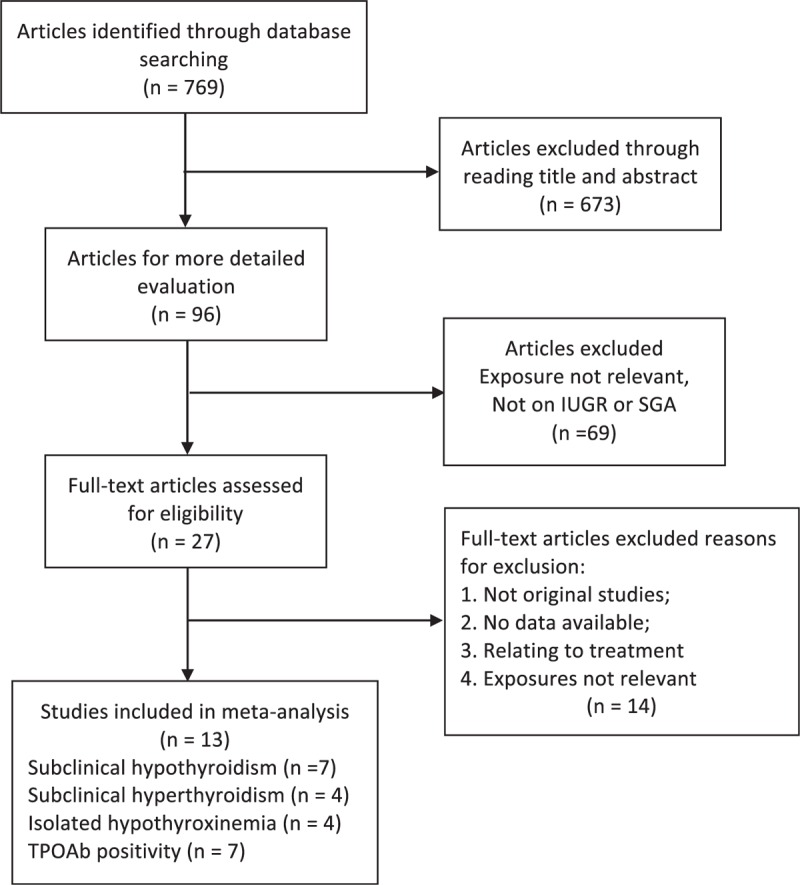
Flow chart of literature search and article selection.
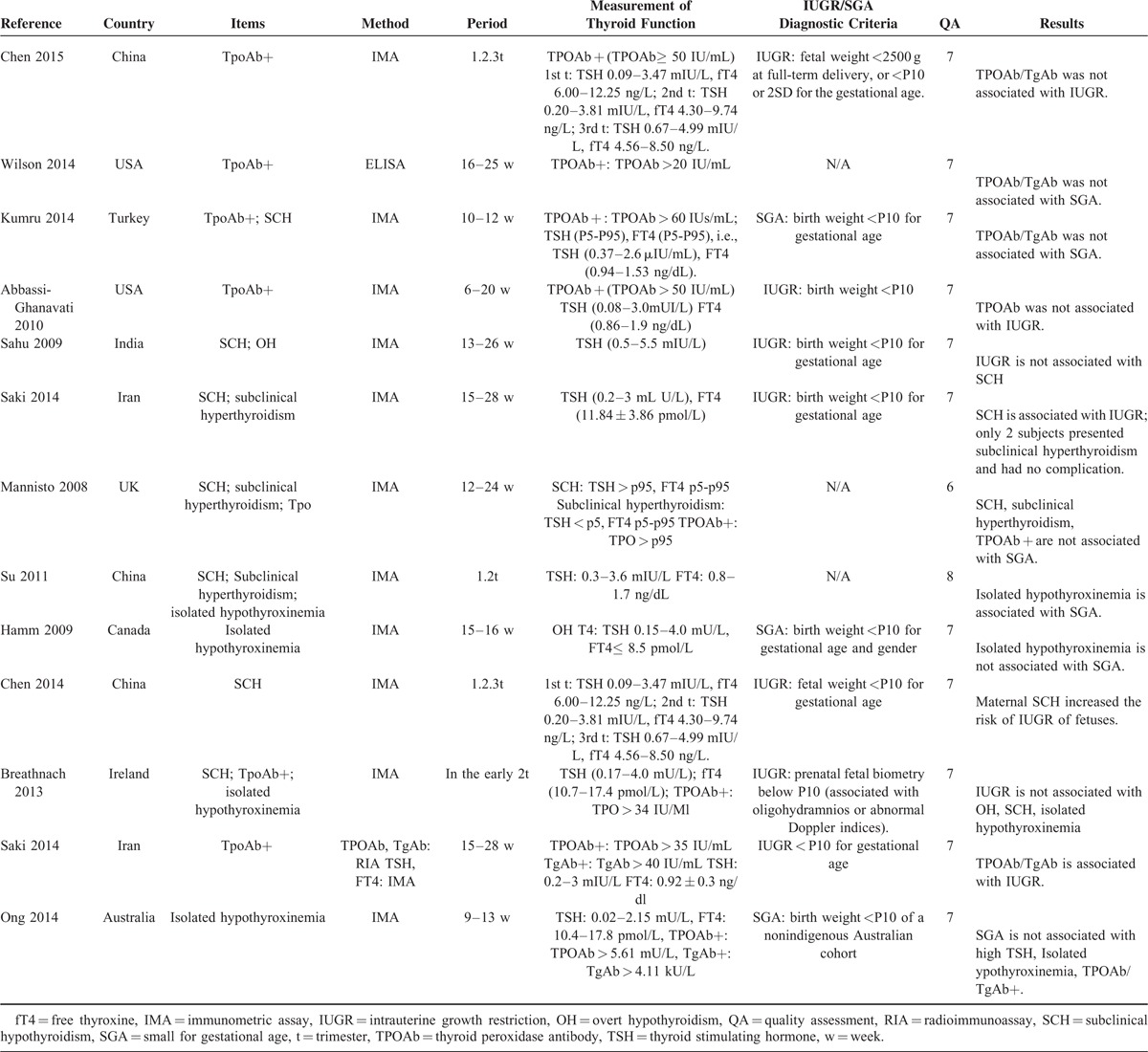
Table 1 shows the characteristics of the 13 studies. These studies were published between 2009 and 2015. Most of the selected studies focused on the 1st and 2nd trimesters. A fixed-effects model was used to calculate the overall combined OR with its corresponding CI to evaluate the relationship between subclinical hyperthyroidism/SCH and IUGR. A random-effects model was used to evaluate the relationship between TPOAb+/isolated hypothyroxinemia and IUGR.
TABLE 1.
The Characteristics of Selected Studies
IUGR and SCH
Meta-analysis of these 7 studies that reported relevant data on the association between SCH and IUGR showed that the combined OR of IUGR for SCH pregnant women was 1.54 (95% CI, 1.06–2.25), indicating that SCH was associated with IUGR (Figure 2). Among the 7 selected studies, 2 showed an association between IUGR and SCH.9,12 Antibodies were not mentioned or measured in 5 studies;9–13 therefore, we used the number of SCH cases without considering the effect of antibodies, even though 1 study divided the subjects based upon both thyroid function and antibodies.7 In 1 study, TPOAb and free thyroxine (fT4) were measured only when TSH levels were aberrant.8 Therefore, it was possible to miss patients with isolated hypothyroxinemia and those who were antibody positive but euthyroid. Both the Q statistic test (P = 0.16) and I2 statistic (I2 = 35.1%) showed low heterogeneity. Sensitivity analyses by sequential omission of each study did not change the overall OR. Begg test (P = 0.55) and Egger test (P = 0.28) did not identify any publication bias.
FIGURE 2.
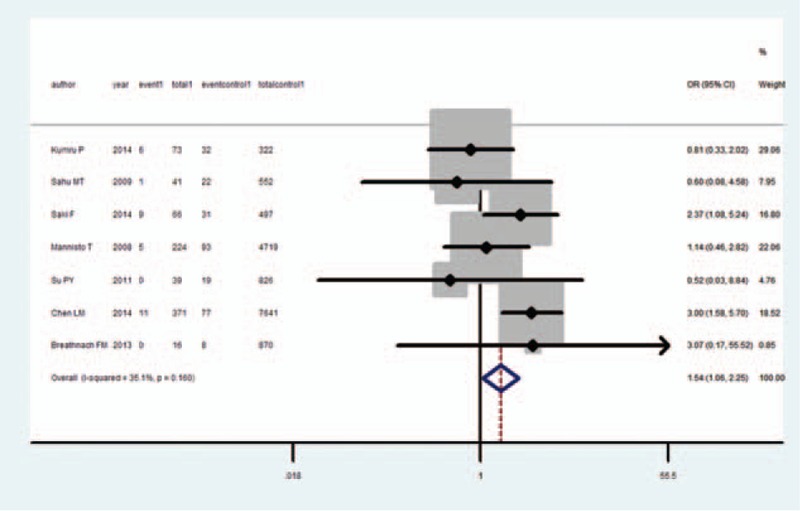
Forest plots of studies comparing intrauterine growth restriction (IUGR) rates between subclinical hypothyroidism (SCH) and euthyroid pregnant women. Event 1: IUGR infant from SCH women; total 1: SCH women; event control 1: IUGR infant from euthyroid women; total 1: euthyroid women.
IUGR and Subclinical Hyperthyroidism
Meta-analysis of these 4 studies that reported relevant data on the association between subclinical hyperthyroidism and IUGR showed that the combined OR of IUGR for subclinical hyperthyroidism pregnant women was 0.98 (95% CI, 0.40–2.41), indicating that subclinical hyperthyroidism was not associated with IUGR (Figure 3). None of the 4 studies showed an association between IUGR and subclinical hyperthyroidism, and none considered the ATA of the subjects; in 1 study, the author measured TPOAb only when TSH was abnormal.8 Both the Q statistic test (P= 0.26) and I2 statistic (I2 = 20.8%) showed low heterogeneity. Sensitivity analyses by sequential omission of each study did not change the overall OR. Begg test (P= 0.73) and Egger test (P = 0.63) did not identify any publication bias.
FIGURE 3.
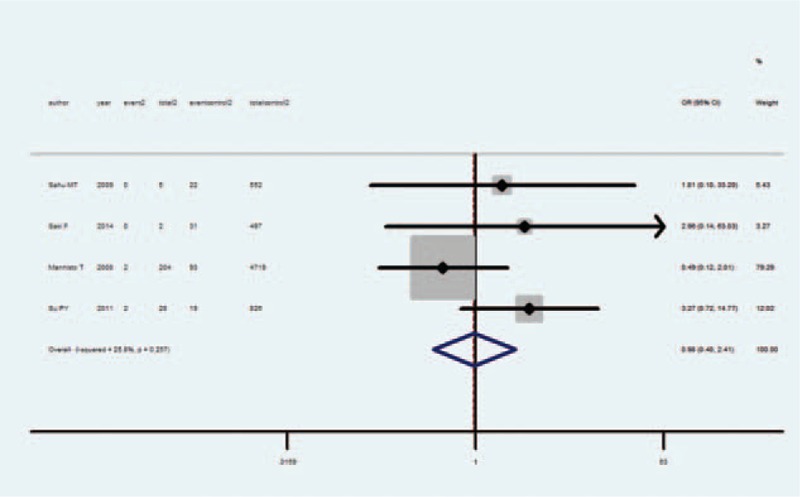
Forest plots of studies comparing intrauterine growth restriction (IUGR) rates between subclinical hyperthyroidism and euthyroid pregnant women. Event 2: IUGR infant from subclinical hyperthyroidism women; total 2: subclinical hyperthyroidism women; event control 2: IUGR infant from euthyroid women; total 2: euthyroid women.
IUGR and TPO-Antibody Positivity
Meta-analysis of these 7 studies that reported relevant data on the association between TPO positivity and IUGR showed that the combined OR of IUGR for TPOAb positive pregnant women was 1.57 (95% CI, 0.77–3.18), indicating that TPOAb positivity is not associated with IUGR (Figure 4). Among these 7 studies, only 1 showed an association between IUGR and TPOAb + status. The subjects of 4 studies were isolated TPO+ and euthyroid patients.7,14,16,17 Both the Q statistic test (P < 0.001) and I2 statistic (I2 = 82.9%) showed high heterogeneity. Therefore, random-effect model was used. One study17 was the main cause of high heterogeneity; when this study was deleted, the heterogeneity decreased (Q statistic test, P = 0.36, I2 = 8.7%), and the OR was 0.96 (95% CI, 0.79–1.17). Begg test (P = 0.76) and Egger test (P = 0.33) did not identify any publication bias.
FIGURE 4.
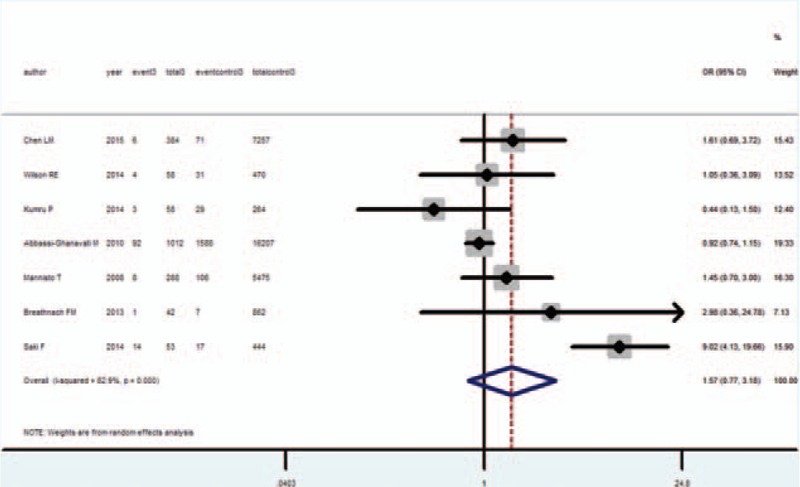
Forest plots of studies comparing intrauterine growth restriction (IUGR) rates between thyroid peroxidase antibody (TPOAb) positive and negative pregnant women. Event 3: IUGR infant from TPOAb positive pregnant women; total 3: TPOAb positive pregnant women; event control 1: IUGR infant from TPOAb negative pregnant women; total 1: TPOAb negative pregnant women.
IUGR and Isolated Hypothyroxinemia
Meta-analysis of these 4 studies that reported relevant data on the association between isolated hypothyroxinemia and IUGR showed that the combined OR of IUGR for isolated hypothyroxinemia pregnant women was 1.05 (95% CI 0.37–2.92), indicating that isolated hypothyroxinemia was not associated with IUGR (Figure 5). Both the Q statistic test (P = 0.06) and I2 statistic (I2 = 60.0%) showed high heterogeneity, and therefore, the random-effects model was used. Sensitivity analyses by sequential omission of each study did not change the overall OR. Begg test (P = 1) and Egger test (P > 0.78) did not identify any publication bias.
FIGURE 5.
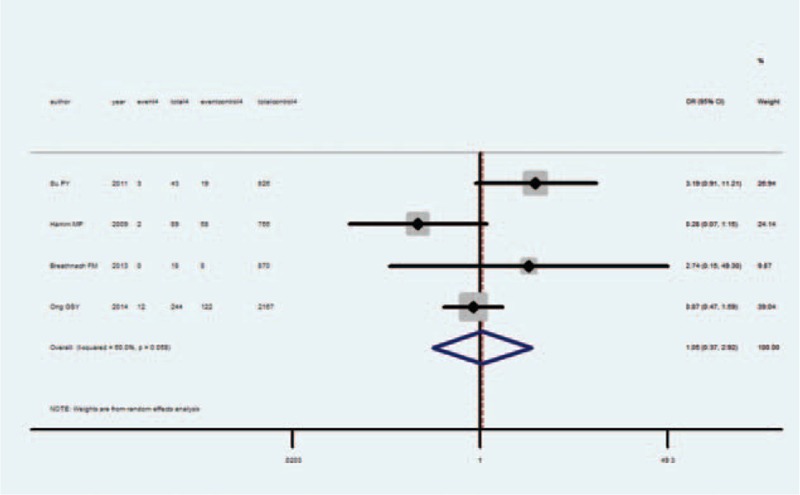
Forest plots of studies comparing intrauterine growth restriction (IUGR) rates between isolated hypothyroxinemia and euthyroid pregnant women. Event 4: IUGR infant from isolated hypothyroxinemia women; total 4: isolated hypothyroxinemia women; event control 4: IUGR infant from euthyroid women; total 4: euthyroid women.
DISCUSSION
The current meta-analysis was aimed specifically at assessing an association between mild maternal thyroid disorders and IUGR. The OR of SCH, subclinical hyperthyroidism, TPOAb positivity, and isolated hypothyroxinemia indicated that SCH is a risk factor for IUGR; while subclinical hyperthyroidism, positive TPOAb status, and isolated hypothyroxinemia are not. Since in most studies, SGA is used as a proxy for IUGR, and the diagnosis criteria of SGA and IUGR are identical, we also searched for studies related to SGA. To our knowledge, this is the first meta-analysis to evaluate the association between mild thyroid disorders and fetal development.
Maternal thyroid function can influence fetal development in 2 different ways: thyroid hormone impacts the regulation of both proliferation and differentiated function of human trophoblast cells;20 and maternal thyroid hormone is important for the development of the infant. Therefore, theoretically, maternal thyroid dysfunction can cause IUGR via both maternal and placental mechanisms.
OH and overt hyperthyroidism are relatively easy to diagnose and the treatments are not controversial.21 Most studies demonstrate that OH and overt hyperthyroidism can increase the risk of IUGR or low birth weight.22–25 Although 2 studies also observed a higher prevalence of large for gestational age infants with hyperthyroid pregnant women,10,26 these results were at borderline significance or lost its significance after adjusting for parity.
Subclinical disorders and euthyroid autoimmune thyroid disease are often missed since they are asymptomatic. However, SCH, subclinical hyperthyroidism, positivity for TPOAb, and isolated hypothyroxinemia during pregnancy are proven to be associated with many adverse outcomes. SCH was proven to be related to spontaneous abortion, abruptio placentae, premature delivery, and impaired fetal neurological development.27–29 Subclinical hyperthyroidism during pregnancy is not thought to be associated with adverse outcomes and does not require treatment.30,31 About 5% to 20% of women of childbearing age are autoimmune thyroid disease, but most of them are euthyroid. Pregnant women who are positive for TPO antibodies are reported to be at increased risk for spontaneous abortion, preterm delivery, and postpartum thyroiditis.21,32–35 Authors of a meta-analysis reviewed these studies and demonstrated a significant relationship between ATA and pregnancy loss.36 Isolated hypothyroxinemia is defined as normal TSH concentration but fT4 concentrations in the lower 5th or 10th percentile;37 even transient hypothyroxinemia was proven to exert an adverse effect.38 Isolated hypothyroxinemia has been shown to affect the process of neuronal migration in the cortex and hippocampus during early pregnancy.39
IUGR, as one of these adverse outcomes, is not clearly understood as to its association with subclinical thyroid dysfunction or autoimmunity. IUGR is a heterogeneous condition, and the reasons for its manifestation usually arise from maternal, placental, or fetal mechanisms. Investigator using meta-analysis attempted to evaluate biomarkers for IUGR and divided them into angiogenesis-related, endothelial function/oxidative stress-related, placental protein/hormone-related, and other biomarkers.40
In the present analysis, we found that SCH, rather than subclinical hyperthyroidism, was associated with IUGR. Although adult hypothyroid patients increase their weight, and hyperthyroid patients decrease their weight since thyroid hormones increase metabolic rates, thyroid hormone in early life is an important hormone for development. Therefore, it is plausible that thyroid hypofunction, even subclinical, would impair the development of infants.
It is a confusing finding that isolated hypothyroxinemia showed a negative result. Compared with SCH, isolated hypothyroxinemia is a clinical disorder. Theoretically, if SCH, which shows normal thyroid hormone levels, exerts impact on fetal development, isolated hypothyroxinemia should also have an impact on it. The reasons for the contrasting results may be that: there are only 4 studies that focus on the relationship between isolated hypothyroxinemia and IUGR, and none of them considered the impact of antibodies or other confounding factors; the type 2 and 3 deiodinases and transporters expressed on the placenta would change with maternal thyroid dysfunction, which would compensate for the impairment caused by maternal thyroid disorders; and adverse pregnant outcomes, such as child loss and neonatal death, have been observed to be related to higher TSH levels, rather than FT4 concentrations.41 Therefore, TSH levels would play a role in these conflicting result. However, the mechanism is unclear and this hypothesis still needs further investigation.
One of the major limitations of this review is that IUGR cannot be measured directly, and SGA is used as a substitute. In most of the selected studies, IUGR was defined as birth weight below the 10th percentile for gestational age, which is the same as the definition for SGA; only 1 study defined IUGR by Doppler ultrasound. However, some children diagnosed as SGA are constitutionally small and healthy. Therefore SGA is not a perfect indicator for restricted fetal development within the uterus.
In conclusion, among these mild thyroid disorders, only SCH exhibited a statistically significant association with IUGR, while others showed none. However, evidence was limited because only a few studies were available for each analysis. Further studies are certainly required to update supporting the evidence in this area.
Footnotes
Abbreviations: AITD = autoimmune thyroid disease, ATA = antithyroid antibodies, CI = confidence interval, FGR = fetal growth restriction, fT4 = free thyroxine, IUGR = intrauterine growth restriction, LBW = low birth weight, OH = overt hypothyroidism, OR = odds ratio, SCH = subclinical hypothyroidism, SD = standard deviation, SGA = small for gestational age, TH = thyroid hormone, TPOAb = thyroid peroxidase antibody, TSH = thyroid-stimulating hormone.
ZT and ZX contributed equally to this work.
This work was supported by grants from Research Foundation, Department of Science and Technology, Liaoning Province Government, China (Grant no. 2011225023) and Important Platform of Science and Technology for Universities in Liaoning Province (16010).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Figueras F, Gardosi J. Intrauterine growth restriction: new concepts in antenatal surveillance, diagnosis, and management. Am J Obstet Gynecol 2011; 204:288–300. [DOI] [PubMed] [Google Scholar]
- 2.Battaglia FC, Lubchenco LO. A practical classification of newborn infants by weight and gestational age. J Pediatr 1967; 71:159–163. [DOI] [PubMed] [Google Scholar]
- 3.Franco B, Laura F, Sara N, Salvatore G. Thyroid function in small for gestational age newborns: a review. J Clin Res Pediatr Endocrinol 2013; 5 Suppl 1:2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McIntire DD, Bloom SL, Casey BM, Leveno KJ. Birth weight in relation to morbidity and mortality among newborn infants. N Engl J Med 1999; 340:1234–1238. [DOI] [PubMed] [Google Scholar]
- 5.Cianfarani S, Maiorana A, Geremia C, et al. Blood glucose concentrations are reduced in children born small for gestational age (SGA), and thyroid-stimulating hormone levels are increased in SGA with blunted postnatal catch-up growth. J Clin Endocrinol Metab 2003; 88:2699–2705. [DOI] [PubMed] [Google Scholar]
- 6.Barker DJ, Hales CN, Fall CH, et al. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia 1993; 36:62–67. [DOI] [PubMed] [Google Scholar]
- 7.Kumru P, Erdogdu E, Arisoy R, et al. Effect of thyroid dysfunction and autoimmunity on pregnancy outcomes in low risk population. Arch Gynecol Obstet 2014; 291:1047–1054. [DOI] [PubMed] [Google Scholar]
- 8.Sahu MT, Das V, Mittal S, et al. Overt and subclinical thyroid dysfunction among Indian pregnant women and its effect on maternal and fetal outcome. Arch Gynecol Obstet 2010; 281:215–220. [DOI] [PubMed] [Google Scholar]
- 9.Saki F, Dabbaghmanesh MH, Ghaemi SZ, et al. Thyroid function in pregnancy and its influences on maternal and fetal outcomes. Int J Endocrinol Metab 2014; 12:e19378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mannisto T, Vaarasmaki M, Pouta A, et al. Perinatal outcome of children born to mothers with thyroid dysfunction or antibodies: a prospective population-based cohort study. J Clin Endocrinol Metab 2009; 94:772–779. [DOI] [PubMed] [Google Scholar]
- 11.Su PY, Huang K, Hao JH, et al. Maternal thyroid function in the first twenty weeks of pregnancy and subsequent fetal and infant development: a prospective population-based cohort study in China. J Clin Endocrinol Metab 2011; 96:3234–3241. [DOI] [PubMed] [Google Scholar]
- 12.Chen LM, Du WJ, Dai J, et al. Effects of subclinical hypothyroidism on maternal and perinatal outcomes during pregnancy: a single-center cohort study of a Chinese population. PLoS One 2014; 9:e109364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breathnach FM, Donnelly J, Cooley SM, et al. Subclinical hypothyroidism as a risk factor for placental abruption: evidence from a low-risk primigravid population. Aust N Z J Obstet Gynaecol 2013; 53:553–560. [DOI] [PubMed] [Google Scholar]
- 14.Chen L-M, Zhang Q, Si G-X, et al. Associations between thyroid autoantibody status and abnormal pregnancy outcomes in euthyroid women. Endocrine 2014; 48:924–928. [DOI] [PubMed] [Google Scholar]
- 15.Wilson RE, Salihu HM, Groer MW, et al. Impact of maternal thyroperoxidase status on fetal body and brain size. J Thyroid Res 2014; 2014:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abbassi-Ghanavati M, Casey BM, Spong CY, et al. Pregnancy outcomes in women with thyroid peroxidase antibodies. Obstetr Gynecol 2010; 116 (2 Pt 1):381–386. [DOI] [PubMed] [Google Scholar]
- 17.Saki F, Dabbaghmanesh MH, Ghaemi SZ, et al. Thyroid autoimmunity in pregnancy and its influences on maternal and fetal outcome in Iran (a prospective study). Endocr Res 2015; 40:139–145. [DOI] [PubMed] [Google Scholar]
- 18.Hamm MP, Cherry NM, Martin JW, et al. The impact of isolated maternal hypothyroxinemia on perinatal morbidity. J Obstet Gynaecol Can 2009; 31:1015–1021. [DOI] [PubMed] [Google Scholar]
- 19.Ong GS, Hadlow NC, Brown SJ, et al. Does the thyroid-stimulating hormone measured concurrently with first trimester biochemical screening tests predict adverse pregnancy outcomes occurring after 20 weeks gestation? J Clin Endocrinol Metab 2014; 99:E2668–2672. [DOI] [PubMed] [Google Scholar]
- 20.Barber KJ, Franklyn JA, McCabe CJ, et al. The in vitro effects of triiodothyronine on epidermal growth factor-induced trophoblast function. J Clin Endocrinol Metab 2005; 90:1655–1661. [DOI] [PubMed] [Google Scholar]
- 21.LaFranchi SH, Haddow JE, Hollowell JG. Is thyroid inadequacy during gestation a risk factor for adverse pregnancy and developmental outcomes? Thyroid 2005; 15:60–71. [DOI] [PubMed] [Google Scholar]
- 22.Chang DLF, Pearce EN. Screening for maternal thyroid dysfunction in pregnancy: a review of the clinical evidence and current guidelines. J Thyroid Res 2013; 2013:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mannisto T, Mendola P, Reddy U, Laughon SK. Neonatal outcomes and birth weight in pregnancies complicated by maternal thyroid disease. Am J Epidemiol 2013; 178:731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Millar LK, Wing DA, Leung AS, et al. Low birth weight and preeclampsia in pregnancies complicated by hyperthyroidism. Obstetr Gynecol 1994; 84:946–949. [PubMed] [Google Scholar]
- 25.Mitsuda N, Tamaki H, Amino N, et al. Risk factors for developmental disorders in infants born to women with Graves disease. Obstetr Gynecol 1992; 80 (3 Pt 1):359–364. [PubMed] [Google Scholar]
- 26.Leon G, Murcia M, Rebagliato M, et al. Maternal thyroid dysfunction during gestation, preterm delivery, and birthweight. The Infancia y Medio Ambiente Cohort, Spain. Paediatr Perinat Epidemiol 2015; 29:113–122. [DOI] [PubMed] [Google Scholar]
- 27.Mannisto T, Vaarasmaki M, Pouta A, et al. Thyroid dysfunction and autoantibodies during pregnancy as predictive factors of pregnancy complications and maternal morbidity in later life. J Clin Endocrinol Metab 2010; 95:1084–1094. [DOI] [PubMed] [Google Scholar]
- 28.Nelson DB, Casey BM, McIntire DD, Cunningham FG. Subsequent pregnancy outcomes in women previously diagnosed with subclinical hypothyroidism. Am J Perinatol 2014; 31:77–84. [DOI] [PubMed] [Google Scholar]
- 29.Casey BM, Dashe JS, Wells CE, et al. Subclinical hypothyroidism and pregnancy outcomes. Obstetr Gynecol 2005; 105:239–245. [DOI] [PubMed] [Google Scholar]
- 30.Casey BM, Dashe JS, Wells CE, et al. Subclinical hyperthyroidism and pregnancy outcomes. Obstetr Gynecol 2006; 107 (2 Pt 1):337–341. [DOI] [PubMed] [Google Scholar]
- 31.Stagnaro-Green A, Pearce E. Thyroid disorders in pregnancy. Nat Rev Endocrinol 2012; 8:650–658. [DOI] [PubMed] [Google Scholar]
- 32.Glinoer D, Soto MF, Bourdoux P, et al. Pregnancy in patients with mild thyroid abnormalities: maternal and neonatal repercussions. J Clin Endocrinol Metab 1991; 73:421–427. [DOI] [PubMed] [Google Scholar]
- 33.Glinoer D, Riahi M, Grun JP, Kinthaert J. Risk of subclinical hypothyroidism in pregnant women with asymptomatic autoimmune thyroid disorders. J Clin Endocrinol Metab 1994; 79:197–204. [DOI] [PubMed] [Google Scholar]
- 34.Stagnaro-Green A, Roman SH, Cobin RH, et al. Detection of at-risk pregnancy by means of highly sensitive assays for thyroid autoantibodies. JAMA 1990; 264:1422–1425. [PubMed] [Google Scholar]
- 35.Stagnaro-Green A. Maternal thyroid disease and preterm delivery. J Clin Endocrinol Metab 2009; 94:21–25. [DOI] [PubMed] [Google Scholar]
- 36.Thangaratinam S, Tan A, Knox E, et al. Association between thyroid autoantibodies and miscarriage and preterm birth: meta-analysis of evidence. BMJ 2011; 342:d2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stagnaro-Green A, Abalovich M, Alexander E, et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 2011; 21:1081–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glinoer D. Management of hypo- and hyperthyroidism during pregnancy. Growth Horm IGF Res 2003; 13 (Suppl A):S45–54. [DOI] [PubMed] [Google Scholar]
- 39.Calvo RM, Jauniaux E, Gulbis B, et al. Fetal tissues are exposed to biologically relevant free thyroxine concentrations during early phases of development. J Clin Endocrinol Metab 2002; 87:1768–1777. [DOI] [PubMed] [Google Scholar]
- 40.Conde-Agudelo A, Papageorghiou AT, Kennedy SH, Villar J. Novel biomarkers for predicting intrauterine growth restriction: a systematic review and meta-analysis. BJOG 2013; 120:681–694. [DOI] [PubMed] [Google Scholar]
- 41.Benhadi N, Wiersinga WM, Reitsma JB, et al. Higher maternal TSH levels in pregnancy are associated with increased risk for miscarriage, fetal or neonatal death. Eur J Endocrinol 2009; 160:985–991. [DOI] [PubMed] [Google Scholar]


