Abstract
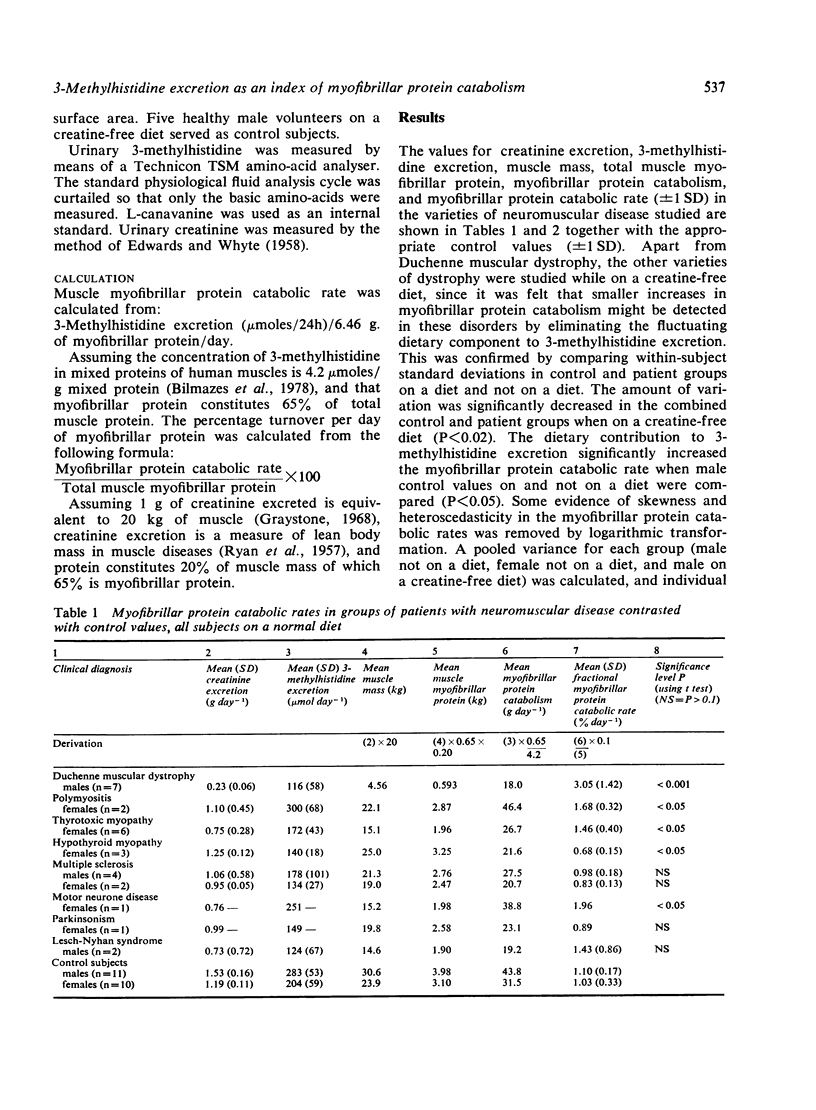
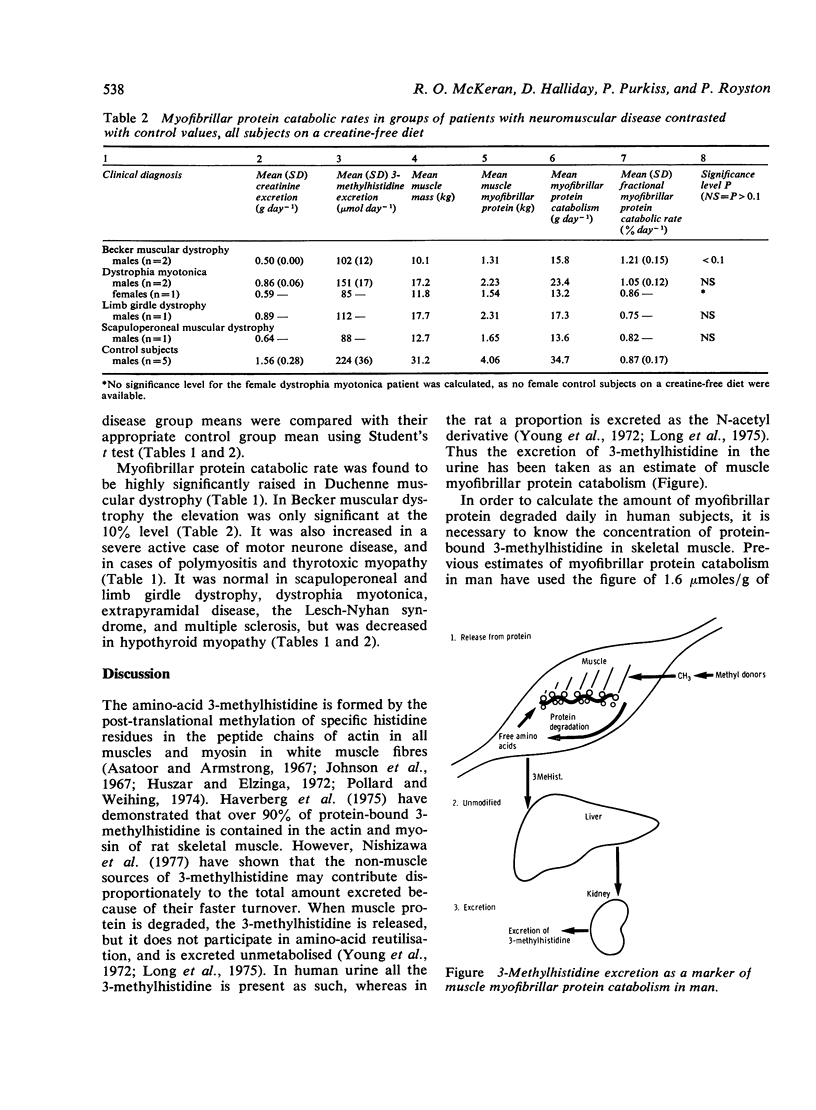
Myofibrillar protein catabolism has been calculated in a variety of neuromuscular diseases from the amount of 3-methylhistidine excreted in the urine. It was found to be significantly raised in Duchenne type muscular dystrophy, motor neurone disease, polymyositis, and thyrotoxic myopathy. In Becker type muscular dystrophy the level was slightly raised. It was normal in scapuloperoneal and limb girdle dystrophy, dystrophia myotonica, extrapyramidal disease, and multiple sclerosis. It was significantly decreased in hypothyroid myopathy.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asatoor A. M., Armstrong M. D. 3-methylhistidine, a component of actin. Biochem Biophys Res Commun. 1967 Jan 23;26(2):168–174. doi: 10.1016/0006-291x(67)90229-x. [DOI] [PubMed] [Google Scholar]
- Bilmazes C., Uauy R., Haverberg L. N., Munro H. N., Young V. R. Musle protein breakdown rates in humans based on Ntau-methylhistidine (3-methylhistidine) content of mixed proteins in skeletal muscle and urinary output of Ntau-methylhistidine. Metabolism. 1978 May;27(5):525–530. doi: 10.1016/0026-0495(78)90018-5. [DOI] [PubMed] [Google Scholar]
- Buttery P. J., Beckerton A., Mitchell R. M., Davies K., Annison E. F. The turnover rate of muscle and liver protein in sheep. Proc Nutr Soc. 1975 Sep;34(2):91A–92A. [PubMed] [Google Scholar]
- DICKERSON J. W., WIDDOWSON E. M. Chemical changes in skeletal muscle during development. Biochem J. 1960 Feb;74:247–257. doi: 10.1042/bj0740247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS K. D., WHYTE H. M. The measurement of creatinine in plasma and urine. Aust J Exp Biol Med Sci. 1958 Aug;36(4):383–394. doi: 10.1038/icb.1958.41. [DOI] [PubMed] [Google Scholar]
- Edwards R. H. Percutaneous needle-biopsy of skeletal muscle in diagnosis and research. Lancet. 1971 Sep 11;2(7724):593–595. doi: 10.1016/s0140-6736(71)92165-9. [DOI] [PubMed] [Google Scholar]
- Enomoto A., Bradley W. G. Therapeutic trials in muscular dystrophy. 1. Gold in murine dystrophy. J Neurol Neurosurg Psychiatry. 1978 May;41(5):404–407. doi: 10.1136/jnnp.41.5.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes G. B., Bruining G. J. Urinary creatinine excretion and lean body mass. Am J Clin Nutr. 1976 Dec;29(12):1359–1366. doi: 10.1093/ajcn/29.12.1359. [DOI] [PubMed] [Google Scholar]
- Halliday D., McKeran R. O. Measurement of muscle protein synthetic rate from serial muscle biopsies and total body protein turnover in man by continuous intravenous infusion of L-(alpha-15N)lysine. Clin Sci Mol Med. 1975 Dec;49(6):581–590. doi: 10.1042/cs0490581. [DOI] [PubMed] [Google Scholar]
- Haverberg L. N., Omstedt P. T., Munro H. N., Young V. R. Ntau-methylhistidine content of mixed proteins in various rat tissues. Biochim Biophys Acta. 1975 Sep 9;405(1):67–71. doi: 10.1016/0005-2795(75)90315-3. [DOI] [PubMed] [Google Scholar]
- Huszar G., Elzinga M. Homologous methylated and nonmethylated histidine peptides in skeletal and cardiac myosins. J Biol Chem. 1972 Feb 10;247(3):745–753. [PubMed] [Google Scholar]
- Ionasescu V. Distinction between Duchenne and other muscular dystrophies by ribosomal protein synthesis. J Med Genet. 1975 Mar;12(1):49–54. doi: 10.1136/jmg.12.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P., Harris C. I., Perry S. V. 3-methylhistidine in actin and other muscle proteins. Biochem J. 1967 Oct;105(1):361–370. doi: 10.1042/bj1050361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar N. C., Pearson C. M. Acid, neutral and alkaline cathepsins in normal and diseased human muscle. Enzyme. 1972;13(4):188–196. doi: 10.1159/000459660. [DOI] [PubMed] [Google Scholar]
- Kuehl W. M., Adelstein R. S. The absence of 3-methylhistidine in red, cardiac and fetal myosins. Biochem Biophys Res Commun. 1970 Jun 5;39(5):956–964. doi: 10.1016/0006-291x(70)90417-1. [DOI] [PubMed] [Google Scholar]
- Long C. L., Haverberg L. N., Young V. R., Kinney J. M., Munro H. N., Geiger J. W. Metabolism of 3-methylhistidine in man. Metabolism. 1975 Aug;24(8):929–935. doi: 10.1016/0026-0495(75)90084-0. [DOI] [PubMed] [Google Scholar]
- McKeran R. O., Halliday D., Purkiss P. Comparison of human myofibrillar protein catabolic rate derived from 3-methylhistidine excretion with synthetic rate from muscle biopsies during L-[alpha-15N]lysine infusion. Clin Sci Mol Med. 1978 May;54(5):471–475. doi: 10.1042/cs0540471. [DOI] [PubMed] [Google Scholar]
- McKeran R. O., Halliday D., Purkiss P. Increased myofibrillar protein catabolism in Duchenne muscular dystrophy measured by 3-methylhistidine excretion in the urine. J Neurol Neurosurg Psychiatry. 1977 Oct;40(10):979–981. doi: 10.1136/jnnp.40.10.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeran R. O., Slavin G., Andrews T. M., Ward P., Mair W. G. Muscle fibre type changes in hypothyroid myopathy. J Clin Pathol. 1975 Aug;28(8):659–663. doi: 10.1136/jcp.28.8.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millward D. J., Garlick P. J., Nnanyelugo D. O., Waterlow J. C. The relative importance of muscle protein synthesis and breakdown in the regulation of muscle mass. Biochem J. 1976 Apr 15;156(1):185–188. doi: 10.1042/bj1560185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa N., Noguchi T., Hareyama S., Funabiki R. Fractional flux rates of Nt-methylhistidine in skin and gastrointestine: the contribution of these tissues to urinary excretion of Nt-methylhistidine in the rat. Br J Nutr. 1977 Jul;38(1):149–151. doi: 10.1079/bjn19770072. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Weihing R. R. Actin and myosin and cell movement. CRC Crit Rev Biochem. 1974 Jan;2(1):1–65. doi: 10.3109/10409237409105443. [DOI] [PubMed] [Google Scholar]
- RYAN R. J., WILLIAMS J. D., ANSELL B. M., BERNSTEIN L. M. The relationship of body composition to oxygen consumption and creatinine excretion in healthy and wasted men. Metabolism. 1957 Jul;6(4):365–377. [PubMed] [Google Scholar]
- Sokoloff L., Roberts P. A., Januska M. M., Kline J. E. Mechanisms of stimulation of protein synthesis by thyroid hormones in vivo. Proc Natl Acad Sci U S A. 1968 Jun;60(2):652–659. doi: 10.1073/pnas.60.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayer I. P., Harris C. I., Perry S. V. 3-Methyl histidine and adult and foetal forms of skeletal muscle myosin. Nature. 1968 Feb 3;217(5127):452–453. doi: 10.1038/217452a0. [DOI] [PubMed] [Google Scholar]
- Wannemacher R. W., Jr, Dinterman R. E., Pekarek R. S., Bartelloni P. J., Beisel W. R. Urinary amino acid excretion during experimentally induced sandfly fever in man. Am J Clin Nutr. 1975 Feb;28(2):110–118. doi: 10.1093/ajcn/28.2.110. [DOI] [PubMed] [Google Scholar]
- Young V. R., Alexis S. D., Baliga B. S., Munro H. N., Muecke W. Metabolism of administered 3-methylhistidine. Lack of muscle transfer ribonucleic acid charging and quantitative excretion as 3-methylhistidine and its N-acetyl derivative. J Biol Chem. 1972 Jun 10;247(11):3592–3600. [PubMed] [Google Scholar]


