NH4+ increased production of NO causes an increment of pectin and PME activity, which results in increased soluble P, and the concomitant up-regulation of OsPT2 facilitates the translocation of P.
Abstract
NH4+ is a major source of inorganic nitrogen for rice (Oryza sativa), and NH4+ is known to stimulate the uptake of phosphorus (P). However, it is unclear whether NH4+ can also stimulate P remobilization when rice is grown under P-deficient conditions. In this study, we use the two rice cultivars ‘Nipponbare’ and ‘Kasalath’ that differ in their cell wall P reutilization, to demonstrate that NH4+ positively regulates the pectin content and activity of pectin methylesterase in root cell walls under −P conditions, thereby remobilizing more P from the cell wall and increasing soluble P in roots and shoots. Interestingly, our results show that more NO (nitric oxide) was produced in the rice root when NH4+ was applied as the sole nitrogen source compared with the NO3−. The effect of NO on the reutilization of P from the cell walls was further demonstrated through the application of the NO donor SNP (sodium nitroprusside) and c-PTIO (NO scavenger 2-(4-carboxyphenyl)-4, 4, 5, 5-tetramethylimidazoline-1-oxyl-3-oxide). What’s more, the P-transporter gene OsPT2 is up-regulated under NH4+ supplementation and is therefore involved in the stimulated P remobilization. In conclusion, our data provide novel (to our knowledge) insight into the regulatory mechanism by which NH4+ stimulates Pi reutilization in cell walls of rice.
Phosphorous (P) is an important inorganic nutrient that plays pivotal roles in plant growth and development. It is part of cellular components such as membranes and nucleic acids and is vital for both vegetative and reproductive growth (Marschner, 1995). However, inorganic phosphorous (Pi) is the least available resource, as it is easily leached out from soil. Furthermore, the Pi retained in the soil could be bound with cations such as Fe3+ and Al3+, or converted to organic matters through microorganisms, thereby becoming immobile and hardly to be utilized by plants (Marschner and Rimmington, 1988; Raghothama, 1999; Tiessen, 2008). For instance, 80% to 90% of applied P-fertilizer is fixed in soil particles (Gerke et al., 1994), decreasing the availability of P for plants and resulting in lower primary crop productivity. To ensure crop production, one of the most common agricultural practices is to apply chemical Pi-fertilizer at high concentrations. However, such measures are harmful to the environment and economic development (Bennett et al., 2001). Therefore, it is desirable to develop crops that are able to use P more efficiently under conditions of low P availability.
To maintain cellular Pi homeostasis under Pi-deficient conditions, plants have developed two main adaptive processes: one is to facilitate Pi acquisition from the external environment, while the other is to reutilize the Pi already inside the plant (Lin et al., 2009; Zhu et al., 2014). One of the most valuable mechanisms to improve P uptake is to remodel the architecture of the root system, such as to increase their root-to-shoot ratio, root branching, or root hair number (Wu et al., 2003; Misson et al., 2005; Lynch and Brown, 2008; Vance, 2008). In addition to changes in root architecture, roots can induce chemical and biological changes in the soil. For instance, under P-deficient conditions, roots will secrete protons (Hinsinger, 2001), organic acids (Noriharu et al., 1990), phosphatases (Vance et al., 2003; Vance, 2008), and other substances (Ae et al., 1996), thereby increasing the uptake of Pi from external environment.
In addition, the content and activity of phosphohydrolases, acid phosphatase (APase), and RNase within the plant are increased under P-deficient conditions. These increases stimulate the reutilization of the internal P (Yun and Kaeppler, 2001; Gong et al., 2011). Kuga et al. (2008)speculated that the vacuole may play a role in storage of P in plant cells such that under P-sufficient conditions, vacuoles would store phosphate, and under P-deficient conditions, vacuoles would release Pi as needed (Kuga et al., 2008). However, other studies demonstrated that the Pi released from the vacuole under P starvation is insufficient (Pratt et al., 2009). Recently, Zhu et al. (2014) found that nearly 50% of total root P is stored in the cell walls of two rice (Oryza sativa) cultivars (‘Nip’ and ‘Kas’) and that cell wall pectin can facilitate the reutilization of the cell wall P due to its high affinity for Al3+, Fe3+, and Cd2+, which firmly combine with Pi (Blamey et al., 1990; Chang et al., 1999; Zhu et al., 2012). However, whether other factors also affect the P remobilization capacity of pectin is still unclear.
Accumulating evidence has demonstrated that nitrogen (N) can induce the uptake of P by plants (Grunes, 1959; Miller, 1974), such as in the seedlings of maize (Zea mays; Smith and Jackson, 1987). Recently, Jin et al., (2014) demonstrated that nitrogen in the forms of urea and nitrate affect plant P uptake differently (Jin et al., 2014). NH4+ and NO3− are the two major N sources that are taken up by plant roots (Marschner, 1995; Falkengren-Grerup et al., 2000). In general, with the absorption of NH4+ by plants, the related proton release decreases the pH of the rhizosphere (Wang et al., 1993; Mistrik and Ullrich, 1996; Schubert and Yan, 1997; Zhao et al., 2009), which leads to increased solubility and uptake of P by the plants (Sarkar and Jones, 1982; Hoffmann et al., 1994). The opposite appears to be true with the uptake of N in the form of NO3− (Smiley, 1974; Marschner and Römheld, 1983; Moorby et al., 1985; Watanabe et al., 1998). Furthermore, Zeng et al. (2012) demonstrated that in rice the increased P uptake in the presence of NH4+ instead of NO3− is due to increased activity of the plasma membrane H+-ATPase. However, whether these two different forms of nitrogen under P-deprivation conditions affect the P remobilization in rice is still unclear.
Accumulating evidence has pointed to NO (nitric oxide) as a signaling molecule involved in physiological and developmental processes in higher plants, such as root growth (Pagnussat et al., 2002), leaf expansion (An et al., 2005), and the cytokinin signaling pathway (Stöhr and Stremlau, 2006), as well as in responses to stresses, including to drought in wheat (Triticum spp.; García-Mata and Lamattina, 2001), high temperature in Lucerne, Switzerland, low temperature in tomato (Solanum lycopersicum), wheat, and maize (Cueto et al., 1996); Al toxicity in rice bean (Vigna umbellata; Zhou et al., 2012); Fe deficiency in Arabidopsis (Chen et al., 2010b); and P deficiency in white lupin (Lupinus albus; Meng et al., 2012). NO improves the growth of white lupin under P deficiency through inducing cluster-root development and citrate exudation (Wang et al., 2010). Two pathways for NO production have be identified in plants: one is to reduce nitrite through the activity of NR (nitrate reductase), and the other is to oxidate Arg to form citrulline through NOS (nitric oxide synthase) activity (Wendehenne et al., 2001; Stöhr and Ullrich, 2002; García-Mata and Lamattina, 2003; Lamattina et al., 2003). However, the exact mechanism underlying NO accumulation in response to P deficiency in rice remains elusive.
Rice is one of the most important cereal crops and previous studies have demonstrated that different rice cultivars use P with different efficiencies. For example, due to the specific PHOSPHORUS STARVATION TOLERANCE1 gene, the root system of Kas exhibits greater uptake of P in P-limited soils and is therefore more vigorous than that of Nip (Gamuyao et al., 2012). However, when grown in P-deficient solutions, Nip plants display increased P reutilization compared to Kas plants. This phenomenon can be explained by the higher pectin content in the cell walls of Nip plants compared with Kas plants (Zhu et al., 2014). In this study, we use these two rice cultivars with different P reutilization efficiencies to study the correlation between nitrogen forms and cell wall P reutilization. This correlation was then further verified by studying the pectin content as an indicator of the cell wall P reutilization efficiency. This study is the first, to our knowledge, to propose a mechanism for reutilization of cell wall P in the presence of different nitrogen forms and under P-deficient conditions.
RESULTS
Effect of Different N Sources on the Concentration of Soluble P in Rice
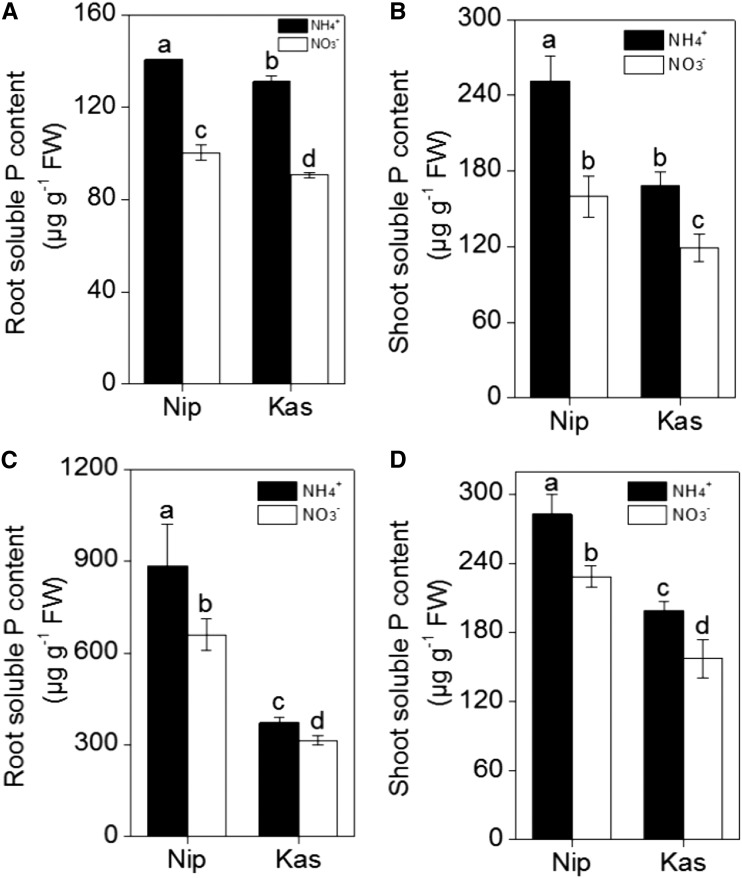
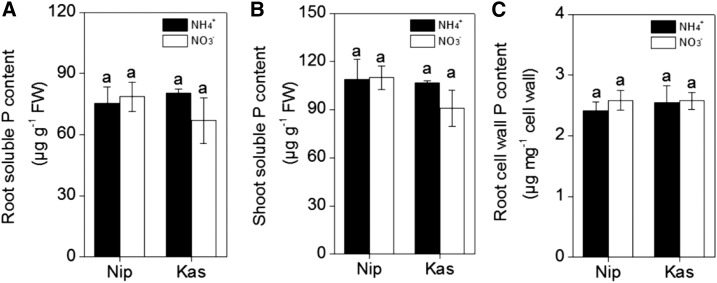
To investigate the effects of different nitrogen forms on the reutilization of P in rice roots, we tested the japonica variety ‘Nipponbare’ (‘Nip’) and the indica variety ‘Kasalath’ (‘Kas’), which showed different P reutilization efficiencies in previous research (Zhu et al., 2014). To analyze the soluble Pi content, roots and shoots were collected separately after seven days of growth in nutrient solution with NH4+ or NO3− as the sole N source under P-sufficient (+P) or P-deficient (−P) conditions. The results clearly showed that there was more soluble P in ‘Nip’ roots and shoots compared with those of ‘Kas’ (irrespective of P status and nitrogen form; Fig. 1), which is in agreement with the previous study (Zhu et al., 2014). However, both ‘Nip’ and ‘Kas’ showed greater soluble P concentrations under −P conditions when grown with NH4+ as the N source, compared with NO3− as the N source (Fig. 1). These results imply that NH4+ as an N source may stimulate P reutilization in both rice cultivars.
Figure 1.
Soluble Pi in the two rice cultivars ‘Nip’ and ‘Kas’. Seedlings were grown in P-deficient (A, B) or P-sufficient (C, D) solution containing different nitrogen forms (NH4+ or NO3−) for 1 week and the root (A, C)- and shoot (B, D)-soluble Pi contents were measured. Data are means ± sd (n = 4). Columns with different letters are significantly different at P < 0.05.
Effect of Different N Sources on the Cell Wall Soluble P Content, Pectin Content, and Pectin Methylesterase Activity in Rice Roots
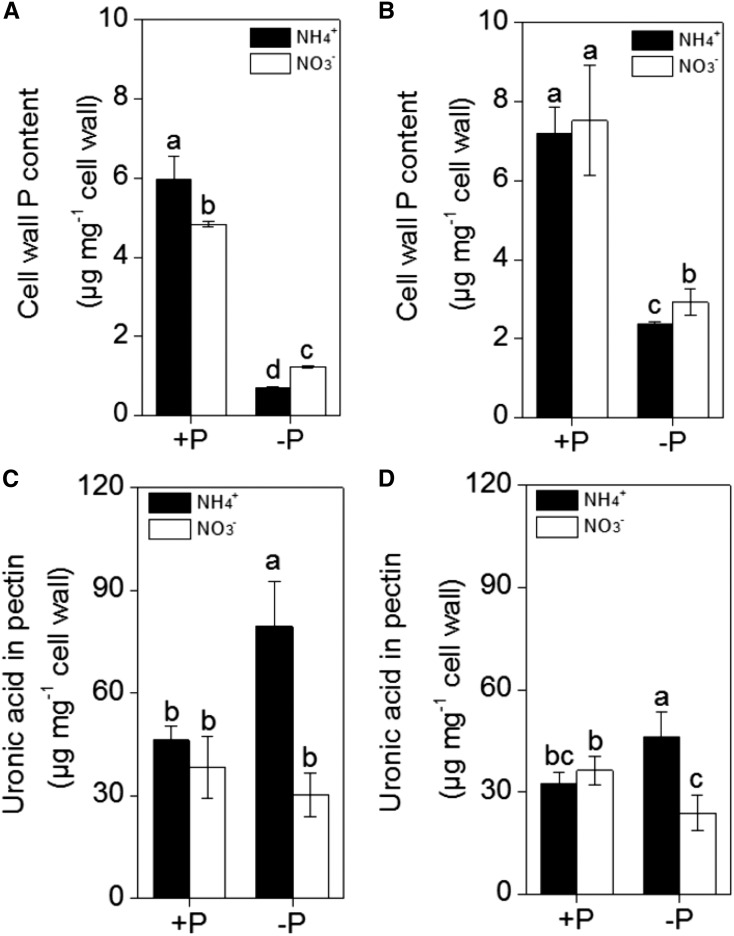
Because approximately 50% of the total P is accumulated in the cell walls of rice, and pectin contributes to the P remobilization differences in ‘Kas’ and ‘Nip’ (Zhu et al., 2014), we extracted root cell walls and measured the P retained in the cell walls. As shown in Figure 2, A and B, less P accumulated in the cell walls of both rice cultivars when NH4+ was applied as the sole N source when compared with NO3− as the sole N source under P-deficient conditions. In addition, under −P conditions, the P content in the Nip cultivar cell walls was lower than the P content in ‘Kas’ cultivar cell walls, which is consistent with previous results (Zhu et al., 2014).
Figure 2.
Effect of different nitrogen form (NH4+ or NO3−) on the retention of the P in the cell wall in ‘Nip’ (A) and ‘Kas’ (B) and the pectin content in ‘Nip’ (C) and ‘Kas’ (D). Seedlings were transferred to NH4+ or NO3− nutrient solution in the presence or absence of P for 1 week. Pectin content is reported by uronic acid content in the cell wall. Data are means ± sd (n = 4). Columns with different letters are significantly different at P < 0.05.
Although the cell wall is composed of a polysaccharide matrix mainly composed of cellulose, hemicellulose, and pectin, only pectin contributes significantly to the release of P from cell walls (Zhu et al., 2014). Under P-deficient conditions, there was a significant increase in cell wall pectin content (as reported by uronic acid content) in both rice cultivars when NH4+ was the sole N source, while such an increase in pectin content was not observed with NO3− (Fig. 2, C and D). This difference indicates that the decrease of P in the NH4+-treated cell wall may be related to the increase of the cell wall pectin content.
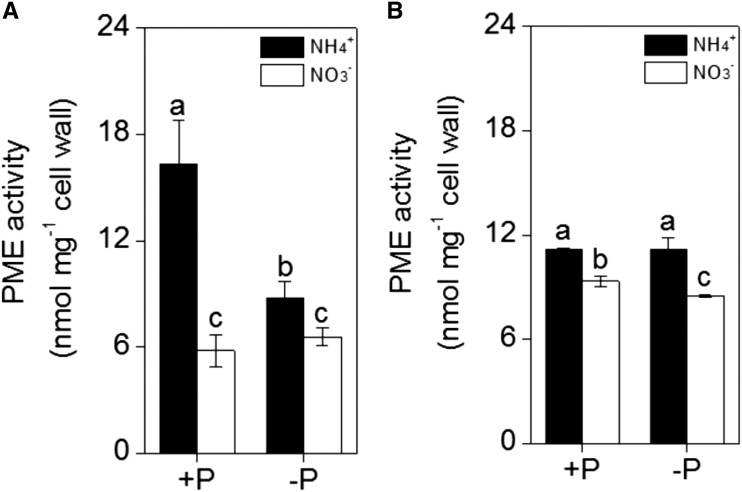
We measured PME (pectin methylesterase) activity because the negative charges of the cell wall are caused by the demethylation of pectin, which is catalyzed by PME. As shown in Figure 3, the PME activity was significantly higher after NH4+ treatment than after NO3− treatment under different P concentrations in both rice cultivars. This finding indicates that NH4+ treatment may enhance negative charges in the cell wall.
Figure 3.
Effect of nitrogen form (NH4+ or NO3−) on the activity of PME in the cell wall of A, ‘Nip’ and B, ‘Kas’ roots. Seedlings were transferred to NH4+ or NO3− nutrient solution in the presence or absence of P for 1 week. Data are means ± sd (n = 4). Columns with different letters are significantly different at P < 0.05.
Effect of Different N Sources on Nitric Oxide Accumulation in Rice Roots
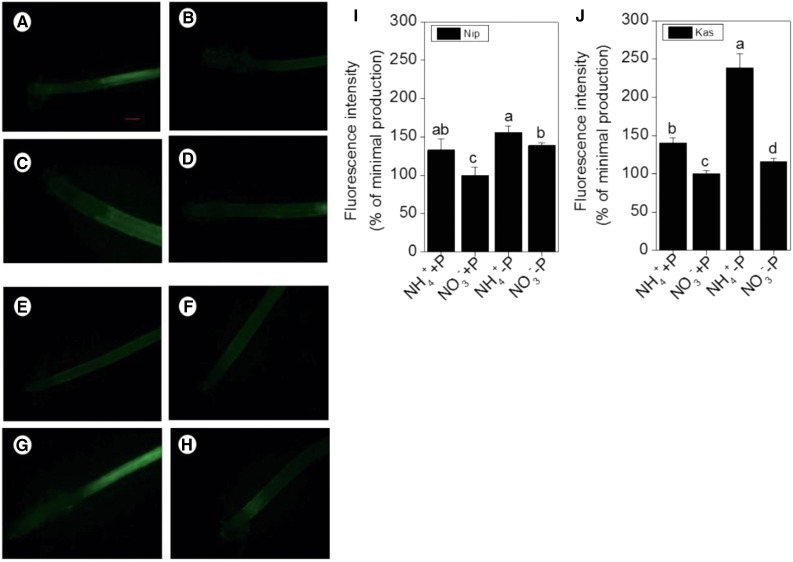
Because the nitrogen form can affect the endogenous NO content (Chen et al., 2010a) and NO is involved in P deficiency (Wang et al., 2010), we hypothesized a direct relationship among nitrogen form (NH4+ or NO3−), P condition (+P or −P), and NO production. The NO content increased in both rice cultivars under P-deficient conditions, and treatment with NH4+ significantly increased the NO content compared with NO3− treatment, independent of P conditions (Fig. 4). To determine whether this increase of NO was involved in the NH4+-stimulated cell-wall P reutilization, a NO scavenger (c-PTIO) was added to the nutrient solution. As expected, the fluorescence associated with the presence of NO was decreased (Fig. 5). The presence of c-PTIO eliminated the NH4+-induced increase in soluble P concentration in the root and shoot in both rice cultivars (Fig. 6, A and B) and eliminated the difference of root cell-wall P content between NH4+ and NO3− treatment (Fig. 6C) under −P conditions. Then, a question arose whether there is a direct relationship between NO and cell wall P reutilization. Thus, we applied NO donor (SNP) exogenously, and found that with the increment of NO accumulation in rice root tip (Supplemental Figs. S1 and S2), the content of cell wall pectin and activity of cell wall PME were both increased whenever put under NH4+ or NO3− treatment, irrespective of P conditions (Supplemental Figs. S5 and S6). As a result, more root- and shoot-soluble P content was detected (Supplemental Figs. S3 and S4). This finding further indicates that NO plays an important role in the NH4+-regulated reutilization of cell-wall P in rice root.
Figure 4.
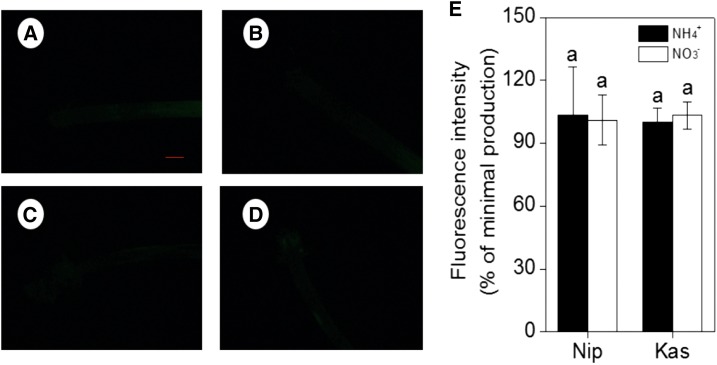
Effect of nitrogen form (NH4+ or NO3−) on NO production, as indicated by green fluorescence. A, B, Representative roots of ‘Nip’ cultivar in the full nutrition solution. C, D, ‘Nip’ cultivar in P-deficiency solution. E, F, ‘Kas’ cultivar in the full nutrition solution. G, H, ‘Kas’ cultivar in the P-deficiency solution. (A, C, E, G) Culture in the NH4+ solution. (B, D, F, H) Culture in the NO3− solution. I, J, NO production expressed as relative fluorescence intensity (% of minimal production). Data are means ± sd (n = 10). Scale bar = 1 mm.
Figure 5.
Effect of NO scavenger c-PTIO on NO accumulation, as indicated by green fluorescence. Representative roots are shown. A, B, ‘Nip’ and C, D, ‘Kas’ cultivar in P-deficiency solution. (A, C) Culture in the NH4+ solution. (B, D) Culture in the NO3− nutrition solution. (E) NO production expressed as relative fluorescence intensity (% of minimal production). Data are means ± sd (n = 10). Scale bar = 1 mm.
Figure 6.
Effect of NO scavenger c-PTIO on (A) root- and (B) shoot-soluble Pi and (C) root cell wall P content in the two rice cultivars ‘Nip’ and ‘Kas’ under P-deficient conditions. Seedlings were subjected to P-deficient conditions in the presence of 10 μM c-PTIO with different nitrogen forms (NH4+ or NO3−) for 1 week and the root- and shoot-soluble Pi content was measured. Data are means ± sd (n = 4). Columns with different letters are significantly different at P < 0.05.
Effect of Different N Sources on the Expression of P-Transporter Genes in Rice Roots
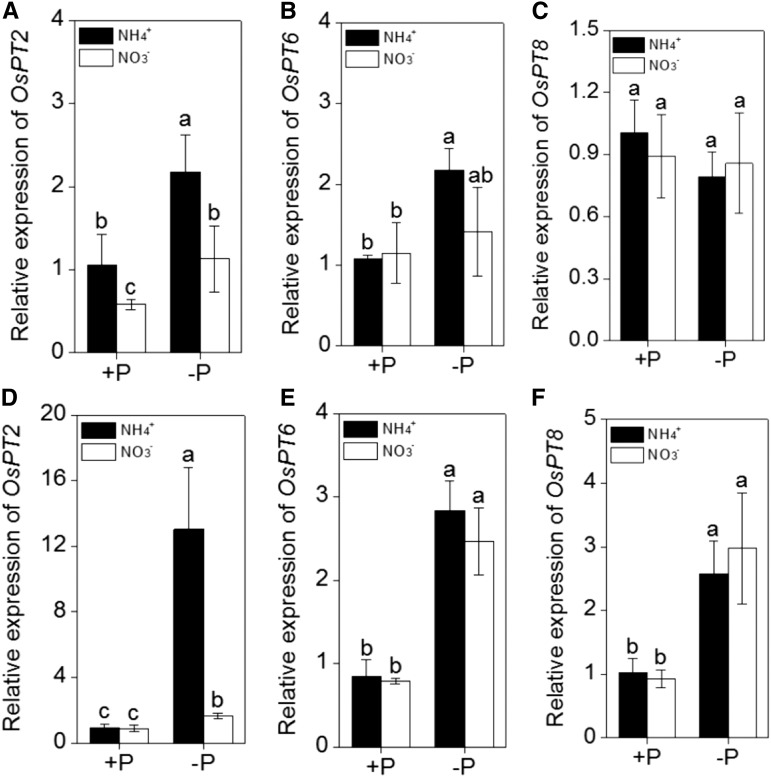
To determine whether the different N forms influence the translocation of P from roots to shoots under P-deficient conditions, the expression of genes that are typically induced in response to P deficiency and that are involved in P translocation from roots to shoots was analyzed by quantitative RT-PCR. Roots from both cultivars were grown in normal or P-deficient medium, supplemented with NH4+ or NO3− as the sole nitrogen source. Under P-sufficient conditions, there were no significant differences between NH4+ and NO3− treatment, except for OsPT2 in the ‘Nip’ cultivar, which showed higher expression in NH4+ treatment than NO3− treatment (Fig. 7). Interestingly, under −P conditions, NH4+ as a nitrogen source strongly induced OsPT2 expression in both ‘Nip’ and ‘Kas’ when compared with NO3− as a nitrogen source (Fig. 7, A and D). This result is in agreement with the increased shoot-soluble P content, indicating that OsPT2 may be involved in the NH4+ alleviated P deficiency.
Figure 7.
Effect of different nitrogen form (NH4+ or NO3−) on the expression of Pi translocation-related genes. A, B and C, ‘Nip’ cultivar; D, E and F, ‘Kas’ cultivar. Seedlings were transferred to P-deficient (−P) or P-sufficient (+P) nutrient solution in the presence of NH4+ or NO3− for 1 week. After treatment, total root RNA was extracted and used to synthesize cDNA. Quantitative RT-PCR was performed using gene-specific primers. Data are means ± sd (n = 4). Columns with different letters are significantly different at P < 0.05.
Effect of Different N Sources on Exudates from Rice Roots
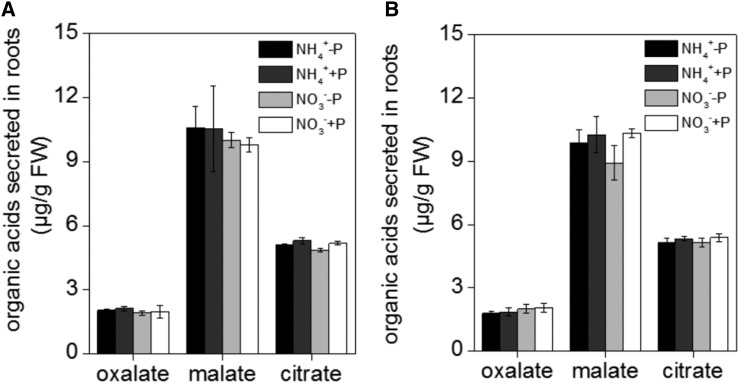
It has previously been reported that the secretion of organic acids may affect the capacity of the cell wall to bind cations such as Al (Li et al., 2009). However, we found no significant difference in either the pH (because the nutrient solution was buffered by 5 mm MES, pH 5.5) or organic acid (malate, citrate, and oxalate) secretion between ‘Nip’ and ‘Kas’ (independent of P and nitrogen form status; Fig. 8). This suggests that organic acid efflux and acidification are unlikely to promote root P mobilization during P starvation in this study.
Figure 8.
Effect of different nitrogen form (NH4+ or NO3−) on the secretion of organic acids. A, ‘Nip’ cultivar; B, ‘Kas’ cultivar. Seedlings were transferred to P-deficient (−P) or P-sufficient (+P) nutrient solution in the presence of NH4+ or NO3− for 1 week. After treatment, total root exudates were collected and measured by HPLC. Data are means ± sd (n = 4). Columns with different letters are significantly different at P < 0.05.
DISCUSSION
Nitrogen, specifically NH4+, has a stimulating effect on P uptake by plants. In this study, we found that NH4+ also stimulates the reutilization of P from the cell wall. NH4+ likely promotes the uptake of P from the soil via changes in the acidity and chemical composition of the rhizosphere (Blair et al., 1971; Riley and Barber, 1971; Jing et al., 2010). When plants absorb NH4+, their roots secrete protons, thereby acidifying their rhizosphere and causing a release of P from the soil. However, in this study, we buffered the nutrient solution with 5 mm MES at pH 5.5, and at a stable pH, the hydrolytic activity or pumping activity of the H+-ATPase should be the same under both NH4+ and NO3− treatment (Schubert and Yan, 1997; Zhu et al., 2009). Furthermore, because of the absence of both soluble and insoluble P in the −P hydroponic solution, the involvement of the pH is excluded. In addition, secretion of organic acids was not involved in the NH4+-specific stimulation of P reutilization in the two rice cultivars (Fig. 8).
Because nearly half of the total P content is present in the cell walls of rice (Zhu et al., 2014), we speculate that both rice cultivars contain more soluble P when grown in NH4+ nutrient solution under P-deprivation conditions and the differences between cultivars may therefore result from differences in reutilization of P in the cell walls. The cell wall is composed of a matrix of polysaccharides such as cellulose, hemicellulose, and pectin. Pectin is a main source of negative charges in the cell wall that facilitate the binding of cations, such as Al (Eticha et al., 2005; Yang et al., 2008), Fe (Chang et al., 1999), and Cd (Zhu et al., 2012). In addition, previous studies have demonstrated that pectin in the root cell walls is involved in reutilization of insoluble P during P starvation (Nagarajah et al., 1970; Ae et al., 1996; Gessa et al., 1997; Zhu et al., 2014). What’s more, it has been demonstrated that exposure of Arabidopsis and rice (‘Kas’) to a P deficiency condition led to a decrement of pectin content while this effect was diminished on another rice cultivar (‘Nip’), and pectin contributed to the cell wall P reutilization in Arabidopsis and rice when suffering from P deficiency, which means, the more pectin content, the more cell wall P reutilization (Zhu et al., 2014).
Previous studies have demonstrated that compared with NO3−, there is lower root cell-wall pectin content under NH4+ treatment when two rice cultivars (YD6 and WYJ7) grow in the CaCl2 solution without control pH (Wang et al., 2015), however, the change in pectin content might depend on plant cultivars, culture conditions, and physiological stresses. In this study, both rice cultivars showed an increase in cell wall pectin content when grown in the NH4+ nutrient solution under −P conditions, which means that they had an increased ability to reutilize the cell wall P. This is in agreement with the decrease in cell-wall P content found in plants grown in NH4+ nutrient solution. What is more, pectin is synthesized and methylesterified in the Golgi apparatus and secreted into the cell wall in a highly methylesterified state (Micheli, 2001) that can only weakly to absorb cations. To increase its binding capacity for cations, free carboxylic groups of pectin must be exposed through demethylation. This process is catalyzed by the PME enzyme and renders pectin as the main binding site for cations in the cell wall. Once the amount of carboxylate group (–COO–) in root cell wall pectin is increased, the pectin will have higher capacity to bind Fe, thus facilitating the release of cell wall P. Under NH4+ nutrition solution, although there is no difference of the pectin content when compared with NO3− treatment under P sufficient condition, the PME activity is significantly higher. However, when under a P-deficient condition, the pectin content and the PME activity are both higher in the NH4+ treatment than in the NO3− treatment. So there may be higher cell-wall negative charges under NH4+ nutrition than NO3− nutrition, independent of P status. Therefore, the decreased retention of P in the cell wall and the increase in root-soluble P may further be due to the higher demethylation state of pectin under −P+NH4+ treatment (Fig. 7). However, it is strange that under NH4+ and −P condition, PME activity in ‘Kas’ was higher than that in ‘Nip’ (Fig. 3), while the soluble P content in Nip was higher than that in Kas (Fig. 1). This is mainly because, in addition to PME activity, the content of pectin (which is the substrate catalyzed by PME enzymes) is another important factor that attributes to the cell wall P release. Maybe there is a threshold of the PME activity under P-deficient condition, and this needs our further study.
There may be another signal that acts downstream of this NH4+-mediated increased pectin content under P-deficient conditions. Increased NO production in various plant species has been widely observed in response to nutrient deficiency in general (Chen et al., 2010b) and P deficiency in particular (Wang et al., 2010). Interestingly, in this study, we found that NO is involved in the P deficiency response in rice. An increase in NO production under NH4+ combined with −P treatment was associated with increased reutilization of cell wall P. Together, this increased root- and shoot-soluble P content (Fig. 1) and decreased the P retention in the cell wall (Fig. 2, A and B), indicating that NO may indeed be involved in the cell wall P reutilization process. This was further demonstrated by effect of the NO scavenger c-PTIO and the NO donor SNP. After the addition of c-PTIO, the stimulating effects of NH4+ on cell wall P reutilization under P deprivation were inhibited: no difference in root- and shoot-soluble P content and root cell-wall P content between NH4+ and NO3− treatment could be observed (Fig. 6). However, after being treated with SNP for 24 h, it was found that the concentration of rice root- and shoot-soluble P (Supplemental Figs. S3 and S4), the content of cell wall pectin, and the activity of cell wall PME were all increased (Supplemental Figs. S5 and S6), in company with the increment of NO content (Supplemental Figs. S1 and S2). It is noteworthy that the content of signal molecular NO was increased under NH4+ nutrition, which can stimulate the production of pectin and the increment of PME activity (Supplemental Figs. S5 and S6), thus more carboxylate group (–COO–) in pectin was produced (Supplemental Figs. S2 and S3). All the above results emphasize that NO plays an important role in rice cell wall P reutilization in response to different nitrogen forms.
In addition, up-regulation of the expression of multiple genes that mediate Pi translocation would be another effective way for plants to cope with P deficiency. As expected, NH4+ treatment in the absence of P significantly enhanced the expression of OsPT2 (Fig. 8), which expressed mainly in the stele of primary and lateral roots under Pi-deficient conditions (Ai et al., 2009), indicating NH4+ may be involved in the transportation of P from root to shoot by regulating the expression of OsPT2. P deficiency also induced the expression of OsPT6 (expressed in the epidermis, cortex, and stelar tissue under Pi-deficient conditions) in both rice cultivars and of OsPT8 (expressed constitutively and functioning in P hemeostasis) in the ‘Kas’ cultivar (Fig. 7); however, there were no differences in responses to NH4+ and NO3− treatments, thus ruling out the possibility that OsPT6 and OsPT8 are transcriptionally regulated by NH4+ under P-deficient conditions.
In conclusion, we identified a novel (to our knowledge) physiological and molecular pathway of NH4+-induced cell wall P remobilization under P-deficient conditions. In the presence of NH4+, increased production of NO causes an increase of pectin and PME activity in the cell wall, which results in increased release of soluble P, and the concomitant up-regulation of OsPT2 facilitates the translocation of P to the shoot.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Rice (Oryza sativa) spp. japonica ‘Nipponbare’ (‘Nip’) and indica ‘Kasalath’ (‘Kas’) were used in this study. Seeds were surface-sterilized with 1% NaClO for 10 min, washed with deionized water three times, and allowed to germinate on filter paper with deionized water for 24 h. Subsequently, seedlings were cultivated in 0.5 mm CaCl2 (pH 5.5) solution for 2 d, and then transferred to full-strength nutrient solution containing 0.5 mm NH4NO3, 0.18 mm NaH2PO4·2H2O, 0.18 mm KCl, 0.36 mm CaCl2, 0.6 mm MgSO4·7H2O, 9 μm MnCl2·4H2O, 0.1 μm Na2MoO4·4H2O, 10 μm H3BO3, 0.7 μm ZnSO4·7H2O, 0.3 μm CuSO4 and 20 μm FeSO4·7H2O-EDTA. All experiments were conducted in a growth chamber with a 14-h/26°C day and a 10-h/23°C night regime, a light intensity of 400 µmol m−2 S−1, and a relative humidity of 60%.
After 7 d, uniform seedlings were planted in 1.5-l pots (10 seedlings per pot) with the following treatments: +P+NH4+, −P+NH4+, +P+NO3−, −P+NO3−, −P+NH4++c-PTIO, −P+NO3−, and +c-PTIO. For NH4+ and NO3− treatments, 1 mm NH4Cl and 1 mm NaNO3 were applied, respectively. The final concentration of c-PTIO was 10 μM. The pH was adjusted to 5.5 with 5 mm MES. The solution was renewed every 3 d.
For the NO (nitric oxide) donor SNP application experiment, eight treatments, named +P+NH4+, −P+NH4+, +P+NO3−, −P+NO3−, +P+NH4++SNP, +P+NO3−+SNP, −P+NH4++SNP, and −P+NO3−+SNP were performed. Seedlings with unanimous growth were treated with or without 2.5 μm SNP for 24 h under four respective treatments (+P+NH4+, −P+NH4+, +P+NO3−, and −P+NO3−). Afterward, the nutrient solution was totally renewed and the treated seedlings were still grown in P-deficient or P-sufficient solution containing different nitrogen forms (NH4+ or NO3−) for another 6 d. The pH was adjusted to 5.5 with 5 mm MES. The solution was renewed every 3 d.
Determination of Soluble Inorganic Phosphorous Concentrations
The soluble inorganic phosphorous (Pi) concentration was determined according to Zhu et al. (2014). Briefly, after washing three times with deionized water, roots, and shoots were weighed and homogenized in 5-m sulfuric acid. After centrifugation at 4000 rpm for 5 min, 400 μl supernatant was transferred to an 1.5-ml Eppendorf tube, and 200 μl ammonium molybdate containing 15% (w/v) fresh ascorbic acid (pH 5.0) was added. The mixture was incubated at 37°C for 30 min and the absorbance was determined at 650 nm. And the Pi concentration was calculated by normalization of the fresh weight (Zheng et al., 2009).
Cell Wall Extraction and Fractionation
The extraction of cell wall materials were carried out according to Zhong and Läuchli (1993). Briefly, roots were homogenized in 8 ml 75% ethanol, incubated on ice for 20 min, and centrifuged at 4000 rpm for 10 min. Then, the pellets were homogenized in 8 ml acetone, a 1:1 ratio of methanol/chloroform, and methanol, respectively, for 20 min each. These steps were carried out at 4°C. The remaining pellets were freeze-dried and stored at 4°C until use.
The extraction of pectin was carried out by washing approximately 2 mg of isolated cell walls three times with 1 ml water at 100°C for 1 h. The supernatants containing pectin were collected in a 5-ml tube after centrifugation at 13,200 rpm for 10 min (Zhong and Läuchli, 1993; Yang et al., 2011).
Uronic Acid Measurement
The uronic acid concentration was used as an indicator for the pectin concentration and assayed according to Blumenkrantz and Asboe-Hansen (1973). Briefly, 200 μl pectin that was extracted as described above was incubated with 1 mL 98% H2SO4 combined with 0.0125 m Na2B4O7·10H2O at 100°C for 5 min, and chilled immediately. Subsequently, 20 μl 0.15% M-hydro-diphenyl was applied to the solution and incubated at 25°C for 20 min. Finally, the absorbance was measured spectrophotometrically at 520 nm, using GalUA (Sigma-Aldrich, St. Louis, MO) as a standard (Blumenkrantz and Asboe-Hansen, 1973).
P Retention in Cell Wall Materials
The cell wall P content was extracted by shaking approximately 2 mg of isolated cell walls with 1 ml HCl (2 M) in 1.5-ml Eppendorf tube. After the solution was shaken on a rotary shaker for at least 24 h, samples were centrifuged at 13,200 rpm at room temperature for 5 min to collect the supernatant. The P retained in the cell wall was determined by inductively coupled plasma atomic emission spectroscopy (Fisons ARL Accuris; Écublens, Vaud, Switzerland; Zhu et al., 2014).
Pectin Methylesterase Extraction and Activity Assay
For the extraction of crude PME (pectin methylesterase), 5 mg cell wall material was suspended in 1 m NaCl solution (pH 6.0) at 4°C for 1 h with repeated vortexing (for 20 s every 10 min). After centrifugation at 15,000 rpm for 10 min, the supernatant was retained as the PME sample.
The reaction solution used to analyze the PME activity consisted of 100 μl 200 mm PBS (containing 0.64 mg ml−1 pectin), 10 μl AO (alcohol oxidase), and 50 μl PME sample. After incubation at 30°C for 10 min, 200 μl NaOH (0.5 N) containing 5 mg ml−1 Purpald (Sigma-Aldrich) was added and the sample was incubated at 30°C for 30 min. Subsequently, 550 μl water was added to a final volume of 1.0 ml and that was used to measure the A550 spectrophotometrically (Zhu et al., 2012).
Determination of NO Content in Roots
The probe DAF-FM DA (4-amino-5-methylamino-2,7-difluorofluorescein diacetate) was used to determine the accumulation of endogenous NO in rice roots. The root tips were incubated with 10 μm DAF-FM DA in the dark for 30 min after being washed with HEPES-KOH (pH 7.4) for 15 min. After the incubation, the root tips were washed three times with HEPES-KOH (pH 7.4) to remove excess fluorescence. The epifluorescence images were captured by an Eclipse 80i, EX 460-500, DM 505, and BA 510-560 (Nikon, Melville, NY). The fluorescence intensity was measured using Photoshop 7.0 (Adobe Systems, Mountain View, CA) according to Besson-Bard et al. (2009).
Measurement of Organic Acid Efflux
To collect root exudates, plants were placed in 1.25 l 0.5 mm CaCl2 (pH 5.5) for 12 h after treatment for 6 d. Subsequently, the root exudates were passed through a cation exchange column (16 mm × 14 cm) filled with 5-g Amerlite IR-120B resin (H+ form; Muromachi Chemical, Tokyo, Japan) and an anion-exchange column filled with 1.5 g Dowex 1 × 8 resin (100–200 mesh, formate form; Dow Chemical, Midland, MI) successively. Then, 15 ml, 1 m HCl was used to elute the organic acid anions that were retained in the anion-exchange resin and the eluent was concentrated using a rotary evaporator at 40°C. The residue was redissolved in 1 mL H2O and filtered (0.2 μm) before analysis. Organic acid anions were detected by ion chromatography (ICS 3000; Dionex, Sunnyvale, CA) equipped with an AS11 anion-exchange analytical column (4 × 250 mm) and a guard column (4 × 50 mm; Dionex). The mobile phase was 1200 mg l−1 NaOH at a flow rate of 0.6 ml min−1 (Zhu et al., 2014).
Gene Expression Analysis
To determine gene expression, roots were harvested after 7 d treatment and immediately frozen and ground in liquid nitrogen. Total RNA was isolated with TRIZOL (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions, and the RNA integrity and quality was confirmed by 1% agarose gel electrophoresis and spectroscopy. A PrimeScript RT reagent kit (Takara Bio, Kyoto, Japan) was used to reverse transcribe 1 μg total RNA into cDNA. A 10-μl real-time PCR mixture contained the following: 1 μl 10-fold-dilution of cDNA, 0.6 µl forward and reverse primers, 5 μl SYBR Premix ExTaq (Takara Bio), and 2.8 μl sterile distilled water. The sequences of the gene-specific primers are as follows: OsPT2 (forward: 5′-GACGAGACCGCCCAAGAAG-3′; reverse: 5′-TTTTCAGTCACTCACGTCGAGAC-3′); OsPT6 (forward: 5′-TATAACTGATCGATCGAGACCAGAG-3′; reverse: 5′-TGGATAGCCAGGCCAGTTATATATC-3′); OsPT8 (forward: 5′-AGAAGGCAAAAGAAATGTGTGTTAAAT-3′; reverse: 5′-AAAATGTATTCGTGCCAAATTGCT-3′). Each cDNA sample was run in triplicate. Expression data were normalized to the expression level of the actin gene (forward: 5′-AAGTTCTGGGAAGTGGTT-3′; reverse: 5′-CTCCCAATGAGTGACAAA-3′; Jia et al., 2011; Wu et al., 2011).
Statistical Analysis
Each experiment was repeated at least three times, and one set of data are shown in the Results. Data were analyzed by one-way ANOVA and the mean values were compared by Duncan’s multiple range test. Different letters indicate that the mean values were statistically different at the P < 0.05 level.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers OsPT2 (AF536962), OsPT6 (AF536966), OsPT8 (AF536968), actin (AB047313).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Effect of Exogenous NO Addition on NO Accumulation in ‘Nip’ Root
Supplemental Figure S2. Effect of Exogenous NO Addition on NO Accumulation in ‘Kas’ Root
Supplemental Figure S3. Soluble Pi in the Rice Cultivar ‘Nip’
Supplemental Figure S4. Soluble Pi in the Rice Cultivar ‘Kas’
Supplemental Figure S5. Effect of Exogenous NO Addition on the Pectin Content in ‘Nip’ (A, B) and ‘Kas’ (C, D) Roots under P-Sufficient (A, C) or P-Deficient (B, D) Conditions
Supplemental Figure S6. Effect of Exogenous NO Addition on the Activity of PME in the Cell Wall of ‘Nip’ (A, B) and ‘Kas’ (C, D) Roots under P-Sufficient (A, C) or P-Deficient (B, D) Conditions
Supplementary Material
Footnotes
1This work was funded by the National Key Basic Research Program of China (grant no. 2014CB441000), Natural Science Foundation of China (grant no. 31501825), the ‘Strategic Priority Research Program’ of the Chinese Academy of Sciences (grant nos. XDB15030302 and XDB15030202), and the 135 Program of Institute of Soil Science (grant no. Y613400000).
2These authors contributed equally to the article.
Article can be viewed without a subscription.
References
- Ae N, Arihara J, Okada K, Yoshihara T, Johansen C (1990) Phosphorus uptake by pigeon pea and its role in cropping systems of the Indian subcontinent. Science 248: 477–480 [DOI] [PubMed] [Google Scholar]
- Ae N, Otani T, Makino T, Tazawa J (1996) Role of cell wall of groundnut roots in solubilizing sparingly soluble phosphorus in soil. Plant Soil 186: 197–204 [Google Scholar]
- Ai P, Sun S, Zhao J, Fan X, Xin W, Guo Q, Yu L, Shen Q, Wu P, Miller AJ, Xu G (2009) Two rice phosphate transporters, OsPht1;2 and OsPht1;6, have different functions and kinetic properties in uptake and translocation. Plant J 57: 798–809 [DOI] [PubMed] [Google Scholar]
- An L, Liu Y, Zhang M, Chen T, Wang X (2005) Effects of nitric oxide on growth of maize seedling leaves in the presence or absence of ultraviolet-B radiation. J Plant Physiol 162: 317–326 [DOI] [PubMed] [Google Scholar]
- Bennett EM, Carpenter SR, Caraco NF (2001) Human impact on erodable phosphorus and eutrophication: a global perspective increasing accumulation of phosphorus in soil threatens rivers, lakes, and coastal oceans with eutrophication. Bioscience 51: 227–234 [Google Scholar]
- Besson-Bard A, Gravot A, Richaud P, Auroy P, Duc C, Gaymard F, Taconnat L, Renou J-P, Pugin A, Wendehenne D (2009) Nitric oxide contributes to cadmium toxicity in Arabidopsis by promoting cadmium accumulation in roots and by up-regulating genes related to iron uptake. Plant Physiol 149: 1302–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair GJ, Mamaril C, Miller M (1971) Influence of nitrogen source on phosphorus uptake by corn from soils differing in pH. Agron J 63: 235–238 [Google Scholar]
- Blamey F, Edmeades D, Wheeler D (1990) Role of root cation‐exchange capacity in differential aluminum tolerance of Lotus species. J Plant Nutr 13: 729–744 [Google Scholar]
- Blumenkrantz N, Asboe-Hansen G (1973) New method for quantitative determination of uronic acids. Anal Biochem 54: 484–489 [DOI] [PubMed] [Google Scholar]
- Chang YC, Yamamoto Y, Matsumoto H (1999) Accumulation of aluminium in the cell wall pectin in cultured tobacco (Nicotiana tabacum L.) cells treated with a combination of aluminium and iron. Plant Cell Environ 22: 1009–1017 [Google Scholar]
- Chen J, Xiao Q, Wu F, Pei Z, Wang J, Wu Y, Zheng H (2010a) Nitric oxide emission from barley seedlings and detached leaves and roots treated with nitrate and nitrite. Plant Soil Environ-UZEI (Czech Republic) (April 2010) 194–199 [Google Scholar]
- Chen WW, Yang JL, Qin C, Jin CW, Mo JH, Ye T, Zheng SJ (2010b) Nitric oxide acts downstream of auxin to trigger root ferric-chelate reductase activity in response to iron deficiency in Arabidopsis. Plant Physiol 154: 810–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cueto M, Hernández-Perera O, Martín R, Bentura ML, Rodrigo J, Lamas S, Golvano MP (1996) Presence of nitric oxide synthase activity in roots and nodules of Lupinus albus. FEBS Lett 398: 159–164 [DOI] [PubMed] [Google Scholar]
- Eticha D, Stass A, Horst WJ (2005) Cell‐wall pectin and its degree of methylation in the maize root‐apex: significance for genotypic differences in aluminium resistance. Plant Cell Environ 28: 1410–1420 [Google Scholar]
- Falkengren-Grerup U, Månsson KF, Olsson MO (2000) Uptake capacity of amino acids by ten grasses and forbs in relation to soil acidity and nitrogen availability. Environ Exp Bot 44: 207–219 [DOI] [PubMed] [Google Scholar]
- Gamuyao R, Chin JH, Pariasca-Tanaka J, Pesaresi P, Catausan S, Dalid C, Slamet-Loedin I, Tecson-Mendoza EM, Wissuwa M, Heuer S (2012) The protein kinase Pstol1 from traditional rice confers tolerance of phosphorus deficiency. Nature 488: 535–539 [DOI] [PubMed] [Google Scholar]
- García-Mata C, Lamattina L (2003) Abscisic acid, nitric oxide and stomatal closure—is nitrate reductase one of the missing links? Trends Plant Sci 8: 20–26 [DOI] [PubMed] [Google Scholar]
- García-Mata C, Lamattina L (2001) Nitric oxide induces stomatal closure and enhances the adaptive plant responses against drought stress. Plant Physiol 126: 1196–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerke J, Roerner W, Jungk A (1994) The excretion of citric and malic acid by proteoid roots of Lupinus albus L.; effects on soil solution concentrations of phosphate, iron, and aluminum in the proteoid rhizosphere in samples of an oxisol and a luvisol. Zeitschrift für Pflanzenernahrung und Bodenkunde 157: 289 [Google Scholar]
- Gessa C, Deiana S, Premoli A, Ciurli A (1997) Redox activity of caffeic acid towards iron (III) complexed in a polygalacturonate network. Plant Soil 190: 289–299 [Google Scholar]
- Gong Y, Guo Z, He L, Li J (2011) Identification of maize genotypes with high tolerance or sensitivity to phosphorus deficiency. J Plant Nutr 34: 1290–1302 [Google Scholar]
- Grunes D. (1959) Effect of nitrogen on the availability of soil and fertilizer phosphorous to plants. Adv Agron 11: 369–396 [Google Scholar]
- Hinsinger P. (2001) Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant Soil 237: 173–195 [Google Scholar]
- Hoffmann C, Ladewig E, Claassen N, Jungk A (1994) Phosphorus uptake of maize as affected by ammonium and nitrate nitrogen‐Measurements and model calculations‐. Zeitschrift für Pflanzenernährung und Bodenkunde 157: 225–232 [Google Scholar]
- Jia H, Ren H, Gu M, Zhao J, Sun S, Zhang X, Chen J, Wu P, Xu G (2011) The phosphate transporter gene OsPht1;8 is involved in phosphate homeostasis in rice. Plant Physiol 156: 1164–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Tang C, Hogarth TW, Armstrong R, Sale P (2014) Nitrogen form but not elevated CO2 alters plant phosphorus acquisition from sparingly soluble phosphorus sources. Plant Soil 374: 109–119 [Google Scholar]
- Jing J, Rui Y, Zhang F, Rengel Z, Shen J (2010) Localized application of phosphorus and ammonium improves growth of maize seedlings by stimulating root proliferation and rhizosphere acidification. Field Crops Res 119: 355–364 [Google Scholar]
- Kuga Y, Saito K, Nayuki K, Peterson RL, Saito M (2008) Ultrastructure of rapidly frozen and freeze-substituted germ tubes of an arbuscular mycorrhizal fungus and localization of polyphosphate. New Phytol 178: 189–200 [DOI] [PubMed] [Google Scholar]
- Lamattina L, García-Mata C, Graziano M, Pagnussat G (2003) Nitric oxide: the versatility of an extensive signal molecule. Annu Rev Plant Biol 54: 109–136 [DOI] [PubMed] [Google Scholar]
- Li Y Y, Zhang Y J, Zhou Y, Yang J L, Zheng S J (2009) Protecting cell walls from binding aluminum by organic acids contributes to Al resistance. J Integr Plant Biol 51:574–580 [DOI] [PubMed] [Google Scholar]
- Lin W-Y, Lin S-I, Chiou T-J (2009) Molecular regulators of phosphate homeostasis in plants. J Exp Bot 60: 1427–1438 [DOI] [PubMed] [Google Scholar]
- Lynch JP, Brown KM. 2008. Root strategies for phosphorus acquisition. In The Ecophysiology of Plant-Phosphorus Interactions. Springer, New York [Google Scholar]
- Marschner H. 1995. Mineral Nutrition of Higher Plants, 2nd Ed Academic Press, London, UK [Google Scholar]
- Marschner H, Rimmington G (1988) Mineral nutrition of higher plants. Plant Cell Environ 11: 147–148 [Google Scholar]
- Marschner H, Römheld V (1983) In vivo measurement of root-induced pH changes at the soil-root interface: effect of plant species and nitrogen source. Z Pflanzenphysiol 111: 241–251 [Google Scholar]
- Meng ZB, Chen LQ, Suo D, Li GX, Tang CX, Zheng SJ (2012) Nitric oxide is the shared signalling molecule in phosphorus- and iron-deficiency-induced formation of cluster roots in white lupin (Lupinus albus). Ann Bot (Lond) 109: 1055–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheli F. (2001) Pectin methylesterases: cell wall enzymes with important roles in plant physiology. Trends Plant Sci 6: 414–419 [DOI] [PubMed] [Google Scholar]
- Miller M. 1974. Effect of nitrogen on phosphorus absorption by plants. In Carson W. (ed), The Plant Root and its Environment, University of Virginia Press, Charlottesville, VA, pp 643–668. [Google Scholar]
- Misson J, Raghothama KG, Jain A, Jouhet J, Block MA, Bligny R, Ortet P, Creff A, Somerville S, Rolland N, Doumas P, Nacry P, et al. (2005) A genome-wide transcriptional analysis using Arabidopsis thaliana Affymetrix gene chips determined plant responses to phosphate deprivation. Proc Natl Acad Sci USA 102: 11934–11939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistrik I, Ullrich C (1996) Mechanism of anion uptake in plant roots: quantitative evaluation of H+/NO3− and H+/H2PO4− stoichiometries. Plant Physiol Biochem 34: 629–636 [Google Scholar]
- Moorby H, Nye P, White R (1985) The influence of nitrate nutrition on H+ efflux by young rape plants (Brassica napus cv. emerald). Plant Soil 84: 403–415 [Google Scholar]
- Nagarajah S, Posner AM, Quirk JP (1970) Competitive adsorption of phosphate with polygalacturonate and other organic anions on kaolinite and oxide surfaces. Nature 228: 83–85 [DOI] [PubMed] [Google Scholar]
- Noriharu A, Arihara J, Okada K, Yoshihara T, Johansen C (1990) Phosphorus Uptake by Pigeon Pea and Its Role in Cropping Systems of the Indian Subcontinent. Science 248: 477–480 [DOI] [PubMed] [Google Scholar]
- Pagnussat GC, Simontacchi M, Puntarulo S, Lamattina L (2002) Nitric oxide is required for root organogenesis. Plant Physiol 129: 954–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt J, Boisson A-M, Gout E, Bligny R, Douce R, Aubert S (2009) Phosphate (Pi) starvation effect on the cytosolic Pi concentration and Pi exchanges across the tonoplast in plant cells: an in vivo 31P-nuclear magnetic resonance study using methylphosphonate as a Pi analog. Plant Physiol 151: 1646–1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghothama KG. (1999) Phosphate acquisition. Annu Rev Plant Physiol Plant Mol Biol 50: 665–693 [DOI] [PubMed] [Google Scholar]
- Riley D, Barber S (1971) Effect of ammonium and nitrate fertilization on phosphorus uptake as related to root-induced pH changes at the root-soil interface. Soil Sci Soc Am J 35: 301–306 [Google Scholar]
- Sarkar A, Jones RW (1982) Influence of rhizosphere on the nutrient status of dwarf French beans. Plant Soil 64: 369–380 [Google Scholar]
- Schubert S, Yan F (1997) Nitrate and ammonium nutrition of plants: effects on acid/base balance and adaptation of root cell plasmalemma H+ ATPase. Zeitschrift für Pflanzenernährung und Bodenkunde 160: 275–281 [Google Scholar]
- Smiley R. (1974) Rhizosphere pH as influenced by plants, soils, and nitrogen fertilizers. Soil Sci Soc Am J 38: 795–799 [Google Scholar]
- Smith FW, Jackson WA (1987) Nitrogen enhancement of phosphate transport in roots of Zea mays L: I. Effects of ammonium and nitrate pretreatment. Plant Physiol 84: 1314–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöhr C, Stremlau S (2006) Formation and possible roles of nitric oxide in plant roots. J Exp Bot 57: 463–470 [DOI] [PubMed] [Google Scholar]
- Stöhr C, Ullrich WR (2002) Generation and possible roles of NO in plant roots and their apoplastic space. J Exp Bot 53: 2293–2303 [DOI] [PubMed] [Google Scholar]
- Tiessen H. 2008. Phosphorus in the global environment. In The Ecophysiology of Plant-Phosphorus Interactions. Springer, New York [Google Scholar]
- Vance CP. 2008. Plants without arbuscular mycorrhizae. In The Ecophysiology of Plant-Phosphorus Interactions. Springer, New York [Google Scholar]
- Vance CP, Uhde-Stone C, Allan DL (2003) Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol 157: 423–447 [DOI] [PubMed] [Google Scholar]
- Wang BL, Tang XY, Cheng LY, Zhang AZ, Zhang WH, Zhang FS, Liu JQ, Cao Y, Allan DL, Vance CP, Shen JB (2010) Nitric oxide is involved in phosphorus deficiency-induced cluster-root development and citrate exudation in white lupin. New Phytol 187: 1112–1123 [DOI] [PubMed] [Google Scholar]
- Wang MY, Siddiqi MY, Ruth TJ, Glass A (1993) Ammonium uptake by rice roots (II. Kinetics of 13NH4+ influx across the plasmalemma). Plant Physiol 103: 1259–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Zhao XQ, Chen RF, Dong XY, Lan P, Ma JF, Shen RF (2015) Altered cell wall properties are responsible for ammonium-reduced aluminium accumulation in rice roots. Plant Cell Environ 38: 1382–1390 [DOI] [PubMed] [Google Scholar]
- Watanabe T, Osaki M, Tadano T (1998) Effects of nitrogen source and aluminum on growth of tropical tree seedlings adapted to low pH soils. Soil Sci Plant Nutr 44: 655–666 [Google Scholar]
- Wendehenne D, Pugin A, Klessig DF, Durner J (2001) Nitric oxide: comparative synthesis and signaling in animal and plant cells. Trends Plant Sci 6: 177–183 [DOI] [PubMed] [Google Scholar]
- Wu J, Wang C, Zheng L, Wang L, Chen Y, Whelan J, Shou H (2011) Ethylene is involved in the regulation of iron homeostasis by regulating the expression of iron-acquisition-related genes in Oryza sativa. J Exp Bot 62:667–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Ma L, Hou X, Wang M, Wu Y, Liu F, Deng XW (2003) Phosphate starvation triggers distinct alterations of genome expression in Arabidopsis roots and leaves. Plant Physiol 132: 1260–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JL, Li YY, Zhang YJ, Zhang SS, Wu YR, Wu P, Zheng SJ (2008) Cell wall polysaccharides are specifically involved in the exclusion of aluminum from the rice root apex. Plant Physiol 146: 602–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JL, Zhu XF, Peng YX, Zheng C, Li GX, Liu Y, Shi YZ, Zheng SJ (2011) Cell wall hemicellulose contributes significantly to aluminum adsorption and root growth in Arabidopsis. Plant Physiol 155: 1885–1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun SJ, Kaeppler SM (2001) Induction of maize acid phosphatase activities under phosphorus starvation. Plant Soil 237: 109–115 [Google Scholar]
- Zeng H, Liu G, Kinoshita T, Zhang R, Zhu Y, Shen Q, Xu G (2012) Stimulation of phosphorus uptake by ammonium nutrition involves plasma membrane H+ ATPase in rice roots. Plant Soil 357: 205–214 [Google Scholar]
- Zhao XQ, Shen RF, Sun QB (2009) Ammonium under solution culture alleviates aluminum toxicity in rice and reduces aluminum accumulation in roots compared with nitrate. Plant Soil 315: 107–121 [Google Scholar]
- Zheng L, Huang F, Narsai R, Wu J, Giraud E, He F, Cheng L, Wang F, Wu P, Whelan J, Shou H (2009) Physiological and transcriptome analysis of iron and phosphorus interaction in rice seedlings. Plant Physiol 151: 262–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Läuchli A (1993) Changes of cell wall composition and polymer size in primary roots of cotton seedlings under high salinity. J Exp Bot 44: 773–778 [Google Scholar]
- Zhou Y, Xu XY, Chen LQ, Yang JL, Zheng SJ (2012) Nitric oxide exacerbates Al-induced inhibition of root elongation in rice bean by affecting cell wall and plasma membrane properties. Phytochemistry 76: 46–51 [DOI] [PubMed] [Google Scholar]
- Zhu XF, Lei GJ, Jiang T, Liu Y, Li GX, Zheng SJ (2012) Cell wall polysaccharides are involved in P-deficiency-induced Cd exclusion in Arabidopsis thaliana. Planta 236: 989–997 [DOI] [PubMed] [Google Scholar]
- Zhu XF, Wang ZW, Wan JX, Sun Y, Wu YR, Li GX, Shen RF, Zheng SJ (2014) Pectin enhances rice (Oryza sativa) root phosphorus remobilization. J Exp Bot 66: 1017–1024 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Di T, Xu G, Chen X, Zeng H, Yan F, Shen Q (2009) Adaptation of plasma membrane H+-ATPase of rice roots to low pH as related to ammonium nutrition. Plant Cell Environ 32: 1428–1440 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.