SWR1-C is required for miRNA-mediated developmental controls through transcriptional activation and generates proper balances between miRNAs and target mRNAs for plant development in Arabidopsis thaliana.
Abstract
The ATP-dependent SWR1 chromatin remodeling complex (SWR1-C) exchanges the histone H2A-H2B dimer with the H2A.Z-H2B dimer, producing variant nucleosomes. Arabidopsis thaliana SWR1-C contributes to the active transcription of many genes, but also to the repression of genes that respond to environmental and developmental stimuli. Unlike other higher eukaryotic H2A.Z deposition mutants (which are embryonically lethal), Arabidopsis SWR1-C component mutants, including arp6, survive and display a pleiotropic developmental phenotype. However, the molecular mechanisms of early flowering, leaf serration, and the production of extra petals in arp6 have not been completely elucidated. We report here that SWR1-C is required for miRNA-mediated developmental control via transcriptional regulation. In the mutants of the components of SWR1-C such as arp6, sef, and pie1, miR156 and miR164 levels are reduced at the transcriptional level, which results in the accumulation of target mRNAs and associated morphological changes. Sequencing of small RNA libraries confirmed that many miRNAs including miR156 decreased in arp6, though some miRNAs increased. The arp6 mutation suppresses the accumulation of not only unprocessed primary miRNAs, but also miRNA-regulated mRNAs in miRNA processing mutants, hyl1 and serrate, which suggests that arp6 has a transcriptional effect on both miRNAs and their targets. We consistently detected that the arp6 mutant exhibits increased nucleosome occupancy at the tested MIR gene promoters, indicating that SWR1-C contributes to transcriptional activation via nucleosome dynamics. Our findings suggest that SWR1-C contributes to the fine control of plant development by generating a balance between miRNAs and target mRNAs at the transcriptional level.
Chromatin structure is closely associated with the regulation of transcription. ATP-dependent chromatin remodeling complexes contribute to precise spatiotemporal transcription through distinct combinations of regulatory DNA sequences, DNA-binding transcription regulators, and chromatin-modifying enzymes (Cosma, 2002; Li et al., 2007). The ATP-dependent SWR1 chromatin remodeling complex (SWR1-C) catalyzes the replacement of H2A-H2B dimers with H2A.Z-H2B dimers in nucleosome structures, thus producing variant nucleosomes with dynamic properties (Mizuguchi et al., 2004; Luk et al., 2010). The H2A.Z-containing nucleosomes preferentially localize around transcription start sites and in the vicinity of the genes where SWR1-C is recruited, through the direct DNA binding of the SWR1 and SWC2, components of the complex (Raisner et al., 2005; Deal et al., 2007; Zilberman et al., 2008; Jin et al., 2009; Ranjan et al., 2013; Yen et al., 2013).
This SWR1-C-mediated histone exchange can have both positive and negative effects on transcription (Noh and Amasino, 2003; Choi et al., 2005; Deal et al., 2005; Choi et al., 2007; Deal et al., 2007; March-Diaz et al., 2008; Kumar and Wigge, 2010; Smith et al., 2011; Coleman-Derr and Zilberman, 2012; Jarillo and Pineiro, 2015). For example, a mutation in a component of SWR1-C reduces the transcription rate of floral repressor genes FLC, MAF4, and MAF5, thus contributing to early flowering. This shows the positive role played by H2A.Z deposition in transcription (Noh and Amasino, 2003; Choi et al., 2005; Deal et al., 2005; Martin-Trillo et al., 2006; Choi et al., 2007; Deal et al., 2007). In contrast, negative effects of SWR1-C on transcription have been reported in genes involved in systemic-acquired resistance (SAR), jasmonate (JA)-mediated immunity, the P-starvation response (PSR), and genes that respond to high temperatures (March-Diaz et al., 2008; Kumar and Wigge, 2010; Smith et al., 2011; Coleman-Derr and Zilberman, 2012). Such genes are de-repressed in the arp6 mutant under non-inductive conditions. Thus, the increased expression of SAR and PSR genes leads to a respective increase in pathogen resistance and root hair development in arp6 (March-Diaz et al., 2008; Smith et al., 2011). The dual transcriptional roles of SWR1-C may be a result of its cooperative activities with different transcription regulators such as chromatin modifiers, post-translational modifications of H2A.Z and other histone variants, and DNA methylation, which generates broad and distinct ranges of nucleosome stability (Santisteban et al., 2000; Millar et al., 2006; Albert et al., 2007; Jin and Felsenfeld, 2007; Sarcinella et al., 2007; Venkatasubrahmanyam et al., 2007; Zilberman et al., 2008; Hardy et al., 2009; Jin et al., 2009; Marques et al., 2010; Choi et al., 2011; Coleman-Derr and Zilberman, 2012; Stroud et al., 2012; Wollmann et al., 2012).
It has been reported that H2A.Z is required for embryonic stem cell differentiation and gene activation through nucleosome depletion in mice (Li et al., 2012). However, how SWR1-C in plants influences transcriptional regulation and development remains to be established. H2A.Z deposition mutants were embryonically lethal in tested metazoans (Vandaal and Elgin, 1992; Iouzalen et al., 1996; Faast et al., 2001; Whittle et al., 2008), while Arabidopsis SWR1-C mutants such as arp6 and sef/atswc6 survive embryogenesis, but develop pleiotropic developmental phenotypes such as early flowering, serrated leaf shape, reduced fertility, decreased organ size, spontaneous necrosis, longer petioles, increased root hairs, and extra floral organs (Noh and Amasino, 2003; Choi et al., 2005; Deal et al., 2005; Martin-Trillo et al., 2006; Choi et al., 2007; Deal et al., 2007; March-Diaz et al., 2008; Kumar and Wigge, 2010; Smith et al., 2011). The developmental defects seen in SWR1-C mutants are largely mirrored in Arabidopsis h2a.z triple mutants and knock-down plants, suggesting that SWR1-C is required for H2A.Z deposition (Choi et al., 2007; March-Diaz et al., 2008; Kumar and Wigge, 2010; Coleman-Derr and Zilberman, 2012). Thus, understanding the molecular basis of the developmental phenotypes in SWR1-C mutants may provide insight into how H2A.Z influences chromatin structure and plant development. However, the developmental phenotypes of Arabidopsis SWR1-C mutants have not been fully investigated. For example, the arp6 flc, arp6 ft, and sef ft double mutants undergo earlier flowering than flc and ft, respectively. This indicates that arp6 and sef influence FLC- and FT-independent flowering pathways (Choi et al., 2005; Choi et al., 2007). The molecular mechanisms behind the FLC/FT-independent effect on flowering and other developmental defects, including leaf serration and extra petal formation, remain unknown in arp6.
MicroRNAs (miRs) repress protein production post-transcriptionally (Chen, 2009). In flowering plants, miRNAs control diverse developmental processes including phase transitions, leaf shape, and floral organ identity. Plant miRNAs are spatiotemporally transcribed by RNA polymerase II, similar to miRNA-regulated target genes (Chen, 2009). Modules of miRNAs and their target transcription factors, such as the miR156-SPLs/miR172-AP2 LIKEs and miR319-TCPs/miR164-CUCs modules, regulate each other via feedback loops in Arabidopsis (Baker et al., 2005; Nikovics et al., 2006; Sieber et al., 2007; Chen, 2009; Wang et al., 2009; Wu et al., 2009; Koyama et al., 2010; Hasson et al., 2011; Huijser and Schmid, 2011). The miR156-SPLs/miR172-AP2 LIKEs module regulates age-dependent floral transitions: the miR156 level decreases gradually with age, and the transcript levels of the target genes, the SPLs, are subsequently increased. Sequentially, SPL genes increase the transcript level of miR172, which suppresses the flowering repressors, AP2 LIKEs, post-transcriptionally. SPLs also induce the transcription of SOC1, FUL, AP1, and LFY for floral induction (Wu and Poethig, 2006; Wang et al., 2009; Wu et al., 2009; Yamaguchi et al., 2009; Huijser and Schmid, 2011). The development of leaf margin serration is regulated by miR319-TCPs/miR164-CUCs modules, in which a transcription factor, TCP3, regulated by miR319, induces the transcription of MIR164A (Koyama et al., 2010). Subsequently, mature miR164a suppresses the expression of CUC2, a gene required to prevent the development of leaf serration (Nikovics et al., 2006). In a similar manner, floral organ number, and lateral root formation are regulated by the miR164-CUC/NAC1 module (Guo et al., 2005; Sieber et al., 2007).
Although DNA-specific transcription factors and the subunits of an Arabidopsis mediator complex are known to regulate the transcription of MIR genes (Chen, 2009; Wang et al., 2009; Kim et al., 2011), the correlation between chromatin structure and MIR gene transcription has not been fully explored. Here, we demonstrate that Arabidopsis SWR1-C is required to maintain the active transcription of several MIR genes, including MIR156 and MIR164, in addition to heat shock or JA-responsive genes. We also demonstrate that the transcriptional misregulation of miR156 and miR164 in the mutations of the components of SWR1-C such as arp6, sef, and pie1 may contribute to early flowering, leaf serration, and the formation of extra petals. Genome-wide small RNAs analysis revealed that many miRNAs including miR156 are decreased in arp6, although we found that some miRNAs, miR397, miR398, and miR408 were increased in arp6. In addition, we observed that the transcription of many other MIR genes is decreased by arp6, and that nucleosome occupancy is higher at the promoters of the MIR genes in arp6 mutants than in the wild type, which suggests that SWR1-C directly regulates them at transcriptional level. Finally, we show that the transcription of both MIRs and their target genes is attenuated in arp6 mutants, such that the transcription level of both primary transcripts (pri-miRNA) and the miRNA-target genes failed to be elevated in hyl1 and serrate miRNA processing mutants. We propose that such fine-tuning allows SWR1-C mutants in Arabidopsis to survive, while exhibiting a distinct developmental phenotype.
RESULTS
ARP6 Is Required for Transcriptional Activation After Environmental Induction
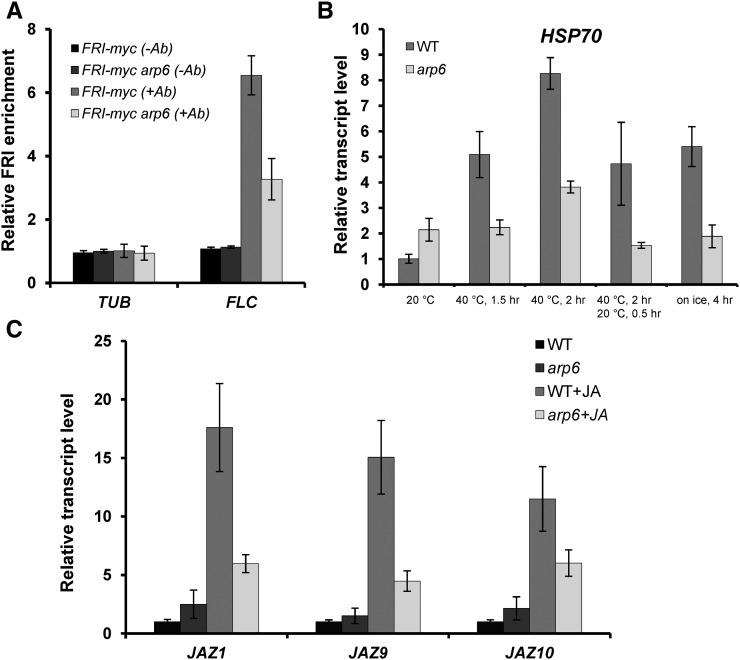
There are many reports showing that SWR1-C-mediated histone exchange can have both positive and negative effects on transcription (Choi et al., 2005; Deal et al., 2005; March-Diaz et al., 2008; Kumar and Wigge, 2010; Smith et al., 2011; Coleman-Derr and Zilberman, 2012; Jarillo and Pineiro, 2015). To investigate the molecular mechanism behind the mediation of transcriptional activation by Arabidopsis SWR1-C, we checked whether arp6 mutation affects the binding of transcription factors to the promoter. To achieve this, we exploited the FRIGIDA transcriptional activation complex’s (FRI-C) accessibility to the FLC promoter, because the transcriptional activation of FLC by FRI-C is well established (Choi et al., 2011). We performed a chromatin immunoprecipitation (ChIP) assay to detect the presence of FRI binding on the FLC promoter using a FRI-myc transgenic line, which possesses a transgene containing the myc-tagged FRI genomic sequence under the control of an endogenous promoter in a Columbia background. The enrichment of FRI-myc was reduced by the arp6 mutation (Fig. 1A). This indicates that ARP6 is required for the recruitment of FRI-C, a transcriptional activator, on the FLC promoter, and is consistent with the previous reports showing that arp6 exhibits reduced FLC transcription and decreased enrichment of RNA polymerase II (Choi et al., 2011).
Figure 1.
Positive transcriptional role of ARP6 for FLC-, HSP70-, and JA-responsive genes. A, Reduced binding of FRI to the FLC promoter in arp6. The 10-d-old whole plants expressing FRI-myc in wild type and arp6 were used for the ChIP-qPCR assay. (−Ab) Control experiments of ChIP without antibody; (+Ab) with antibody. Tubulin 2 (TUB; +1556 approximately +2038 bp from the TSS) was used as a negative control for FRI binding while FLC promoter (−366 to approximately −492 for TSS) was used as a positive control. ChIP-qPCRs for (+Ab) were normalized by that of (−Ab) at TUB and FLC. B, Relative transcript level of HSP70 in arp6 under inductive conditions. For RT-qPCR, the 10-d-old plants were treated with inductive conditions consisting of high temperature (40°C for 90, 120, or 120 min followed by 20°C for 15 min) or on ice for 4 h. C, Transcript level of JAZ1, JAZ9, and JAZ10 in arp6 treated with methyl JA. Adult leaves of 30-d-old wild type and arp6 were sampled at 1.5 h after methyl JA treatment (300 µM) for RT-qPCR. UBQ was used as a reference gene for RT-qPCR. Error bars show sd of the three biological replicates of the ChIP and RT-qPCR analyses. WT, wild type.
Since we observed that ARP6 promotes the recruitment of the transcription factor complex, FRI-C, and RNA polymerase II to the FLC promoter for strong activation, we checked whether ARP6 is also required for strong induction of the environmentally inductive heat shock protein gene, HSP70. In the previous report, it was shown that at room temperature, HSP70 is de-repressed by the arp6 mutation, and thus the function of SWR1-C was proposed to negatively impact the transcription of heat shock factor genes (Kumar and Wigge, 2010). We confirmed that HSP70 transcripts were de-repressed by the arp6 mutation at 20°C, as previously reported (Fig. 1B). However, after heat shock treatment at 40°C, HSP70 transcripts increased approximately 5-fold in the wild type, but barely increased in arp6. Thus, the wild type exhibited a much higher transcript level than arp6. Such reductions in the HSP70 transcript level in arp6 compared to that of the wild type were observed consistently at 1.5 h and 2 h after heat shock and even after conditions were returned to room temperature for 30 min (Fig. 1B). Interestingly, the HSP70 transcript level is reduced in arp6 at low temperature (0°C on ice), which also induces HSP70 expression. Such results clearly show that ARP6 has a positive effect on the transcription of HSP70 under inductive conditions, but has a negative effect on basal expression. To determine whether ARP6 has the opposite effect on the transcription of environmentally inductive genes for induction and basal expression, we analyzed the effect of arp6 on the expression of the genes induced by JA. Similar to HSP70, under the non-inductive conditions, without JA treatment, three JA-responsive genes, JAZ1, JAZ9, and JAZ10, exhibited slightly stronger transcript levels in arp6 than in the wild type. This suggests that ARP6 has a negative effect on the transcription of these genes (Fig. 1C). However, when JA is treated, arp6 exhibited lower transcript levels of JAZ1, JAZ9, and JAZ10 than the wild type. This result also indicates that SWR1-C has a positive effect on the transcription of inductive genes. Thus, it seems that SWR1-C has a negative effect on the basal-level transcription, but a positive effect on the inductive transcription.
Regulation of Age-Dependent Flowering by ARP6 Mediating Transcriptional Activation
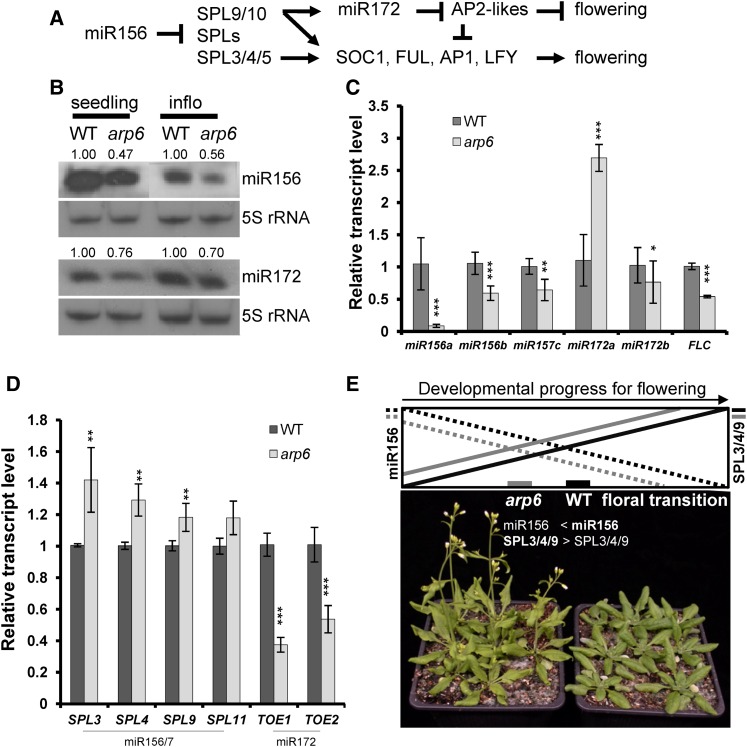
To search for additional evidence of SWR1-C-mediating gene activation in Arabidopsis, we explored the molecular links between arp6 phenotypes and gene expressions. We previously speculated on the presence of another down-regulated floral repressor gene(s) in arp6, in addition to FLC, MAF4, and MAF5, which contributes to early flowering in a photoperiod-, FLC-, and FT-independent manner (Choi et al., 2005; Deal et al., 2005; Martin-Trillo et al., 2006; Choi et al., 2007; Deal et al., 2007). We tested whether miR156-SPLs and miR172-AP2 LIKEs pathways were affected in arp6 (Fig. 2), because these miRNAs pathways regulate age-dependent flowering (Wang et al., 2009; Wu et al., 2009; Yamaguchi et al., 2009; Huijser and Schmid, 2011). We observed via small RNA blot analysis that levels of mature miR156 were reduced in arp6 (Fig. 2B). miR156 is an upstream regulator of sequential phase transition pathways, and acts as a floral repressor, inhibiting SPL-family floral activators (Wang et al., 2009; Huijser and Schmid, 2011). Therefore, we investigated whether MIR156 might be another floral repressor gene positively regulated by ARP6 at the transcription level. The pri-miRNAs of MIR156A, B, and MIR157C genes, which are all processed to mature miR156, were reduced in arp6, similar to FLC (Fig. 2C), indicating that H2A.Z deposition is necessary for the transcriptional activation of these MIR156/7 genes. As a result of the reduced miR156, the levels of the mRNAs of miR156-regulated genes SPL3, -4, and -9 (but not SPL11) were found to be higher in arp6 than in wild-type plants (Fig. 2D). The similar transcriptional changes to miR156 and its targets were observed in arp6 grown under short-day conditions, wherein the age-dependent pathway mainly contributes to flowering induction (Supplemental Fig. S1). This may contribute to the early flowering of arp6, arp6 ft, and sef ft via the age-dependent pathway (Fig. 2, A, D, and E), because SPL3, -4 and -9 proteins directly increase the transcription of SOC1, FUL, AP1, and LFY (Wang et al., 2009; Yamaguchi et al., 2009; Huijser and Schmid, 2011).
Figure 2.
miR-mediated age-dependent flowering in arp6. A, Genetic hierarchy among modules of miR156-SPLs, miR172-AP2 LIKEs, and floral activators for flowering-time control. Arrows indicate a positive effect, and T bars indicate a negative effect. B, Small RNA blots of miR156 and miR172. The 20-d-old whole seedlings and inflorescences of wild type and arp6 were used for the small RNA blot. C, Transcript levels of primary miRNAs, pri-miR156, pri-miR172, and FLC in wild type and arp6. D, Transcript level of miR156- and miR172-regulated genes in arp6. E, Early flowering in arp6 caused by a lower level of miR156 and higher SPL activity. In the top diagram, black continuous and dotted lines represent the wild type, and gray lines represent the arp6. The dotted lines show the miR156 level, and continuous lines represent SPL3, 4, and 9 levels. Photos show an arp6 mutant (left) and wild type (right) grown at the same time under long-day conditions. Total RNAs from plants grown for 20 d under long-day conditions were extracted for RT-qPCR analysis. TUB was used as a reference gene for RT-qPCR. Error bars indicate sd of three biological replicates, with two technical replicates per sample. Three asterisks indicate a p-value of Student’s t-test lower than 0.01 among the means of three biological replicates in arp6, compared to wild type; two asterisks indicate the value is lower than 0.05; one asterisk indicates that the value is lower than 0.1. WT, wild type; inflo, influorescence.
We also observed that mature miR172 and pri-miR172b levels were moderately decreased, while the level of pri-miR172a was increased, in arp6 (Fig. 2, B and C). This is unexpected because the miR172 level is inversely correlated with the miR156 level, and miR156-regulated SPL9 directly induces the transcription of MIR172B, which mainly contributes to the levels of mature miR172 in Arabidopsis (Fig. 2A; Wang et al., 2009). Therefore, the accumulation of SPL9 transcripts in arp6 due to lower miR156 levels may not be sufficient to increase the transcription of MIR172B under long-day conditions. This suggests that not only SPL9 protein, but also SWR1-C, may be required for MIR172B transcription—a finding that is consistent with previous observations of H2A.Z, which found that H2A.Z regulates transcription via other transcription factors and chromatin factors (Santisteban et al., 2000; Choi et al., 2011; Li et al., 2012). However, the arp6 mutant exhibits a higher SPL9 transcript level under short-day conditions than under long-day conditions, which may contribute to the increase in MIR172B transcription under short-day conditions (Supplemental Fig. S1). Because SPL9 directly activates SOC1, AP1, and LFY transcription (Wang et al., 2009; Yamaguchi et al., 2009; Huijser and Schmid, 2011), highly expressed SPL9 may offset the effect of floral repressors, AP2-Like, thus causing early flowering in arp6 in long days. In addition, in short days, both higher increase of SPL9 and increased level of miR172 seem to cause much earlier flowering in short days (approximately 20 leaves in arp6 versus 60 leaves in wild type) than long days (approximately 6 versus approximately 10 leaves).
MicroRNA172 suppresses five members of the AP2 family: AP2, TOE1, TOE2, SMZ, and SNZ, all of which act as floral repressors in a redundant manner (Huijser and Schmid, 2011). Despite the lower miR172 level, we observed that among the mRNAs of AP2 family genes, TOE1 and TOE2 levels were significantly lower in arp6, suggesting that SWR1-C may be required for the transcription of both MIR genes and miRNA-regulated genes. However, because the miR172-TOE1/2 module involves feedback-loop regulation, wherein TOE1 and TOE2 increase MIR172 transcription while miR172 inhibits TOE1/2 post-transcriptionally (Wu et al., 2009), we cannot dismiss the possibility that the reduced transcription of TOE1 and TOE2 in arp6 mutants may lead to decreased MIR172 transcription and miR172 levels. Thus, we show that three MIR156 genes and two AP2-LIKE genes involved in floral repression are down-regulated at the transcriptional level in arp6, leading to early flowering. This indicates that ARP6 has a positive effect on the transcription of the genes involved in the miR156/172-mediated flowering pathways (Fig. 2).
Control of miR164-Mediated Developments by ARP6
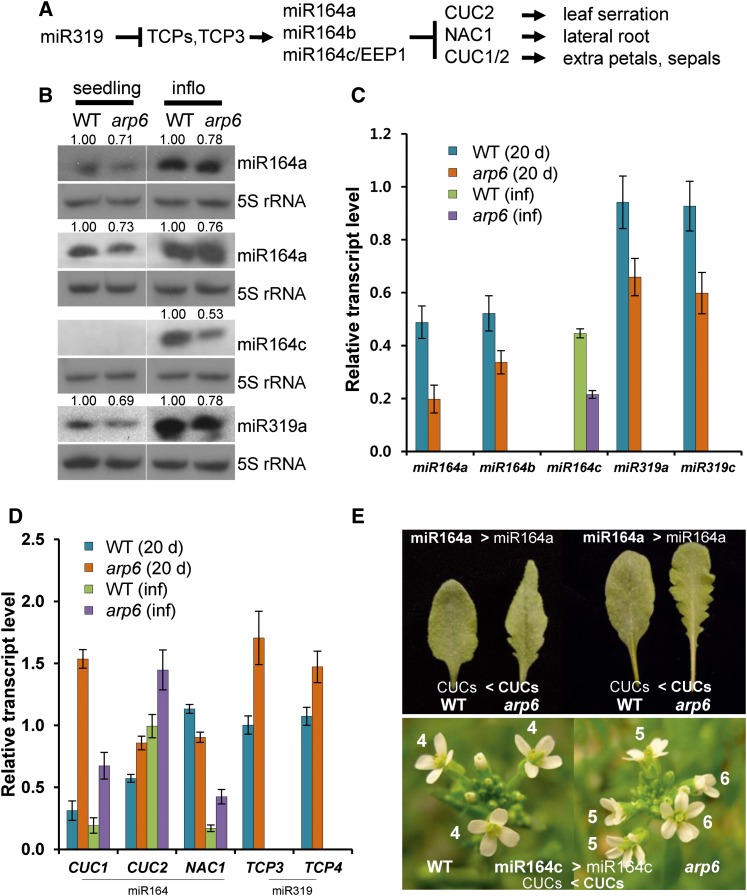
In addition to the early flowering phenotype, arp6 mutants show leaf serration and extra petals (Figs. 2 and 3, A and E; Supplemental Fig. S2; Choi et al., 2005; Deal et al., 2005; Martin-Trillo et al., 2006; Choi et al., 2007). We investigated whether these phenotypes are associated with the misregulation of the miR164 family, because miR164a controls leaf margin formation (Nikovics et al., 2006), and miR164c from MIR164C, also known as EARLY EXTRA PETAL1, prevents the production of extra petals by repressing several NAC-like family transcription factors, including CUP-SHAPED COTYLEDON1 (CUC1) and CUC2 (Fig. 3A; Baker et al., 2005; Sieber et al., 2007). In the miR164a-CUC2 module, we observed that both mature miR164a and pri-miR164a levels were decreased in arp6 (Fig. 3, B and C). Consequently, the mRNA levels of miR164a-target genes CUC1 and CUC2 (but not NAC1) were increased in 20-d-old whole plants (Fig. 3D), which may cause leaf serration in arp6 (Fig. 3E; Nikovics et al., 2006). Similarly, both mature miR164c and pri-miR164c levels, which are high in wild-type inflorescences but not in seedlings, were reduced in arp6 inflorescences. Correspondingly, the transcription levels of the miR164c targets, CUC1 and CUC2, were significantly increased, which may lead to the development of extra petals in arp6 (Fig. 3, B–E; Baker et al., 2005; Sieber et al., 2007). Our results suggest that the arp6 mutation may result in both the development of leaf serration and extra petals, due to the transcriptional attenuation of MIR164 genes and the subsequent accumulation of CUC transcripts.
Figure 3.
Effects of arp6 on the miR-mediated organ boundary formation. A, Diagram of genetic hierarchy between miR319-TCPs and miR164-CUCs modules. B, Small RNA blots of miR164a, miR164c, and miR319 from seedlings and inflorescences. C, Transcript levels of the primary miRNAs pri-miR164 and pri-miR319. D, Transcript levels of miR164- and miR319-regulated genes. E, Photos of serrated leaves and extra petals in arp6. Leaves of wild type and arp6 grown at long days (left) and short days (right) are displayed. Numbers of petals from early arising flowers in wild type (right) and arp6 (left) grown at short days were denoted (bottom panels). Error bars indicate sd of three technical replicates. The same RNAs from seedlings and inflorescences in Fig. 1 were used for RT-qPCR. WT, wild type; inflo, influorescence.
It was revealed that in the determination of leaf margin shape, miR319-regulated-TCP3 acts as an activator of MIR164A (Koyama et al., 2010). We measured the levels of miR319 and TCP3/4 transcripts in arp6, which could influence the miR164-CUC2 module (Hasson et al., 2011). Interestingly, the amount of both miR319 and pri-miR319 was reduced in the arp6 mutant (Fig. 3, B and C). As a consequence of lower miR319 levels, mRNAs of TCP3 and TCP4 accumulated; however, this was not sufficient to increase the transcription of MIR164A in arp6, as with MIR172B regulated by SPL9 and ARP6 (Figs. 2B and 3, B and C). It appears that SWR1-C is necessary for the transcription of both MIR319 and MIR164 (Fig. 3, B and C).
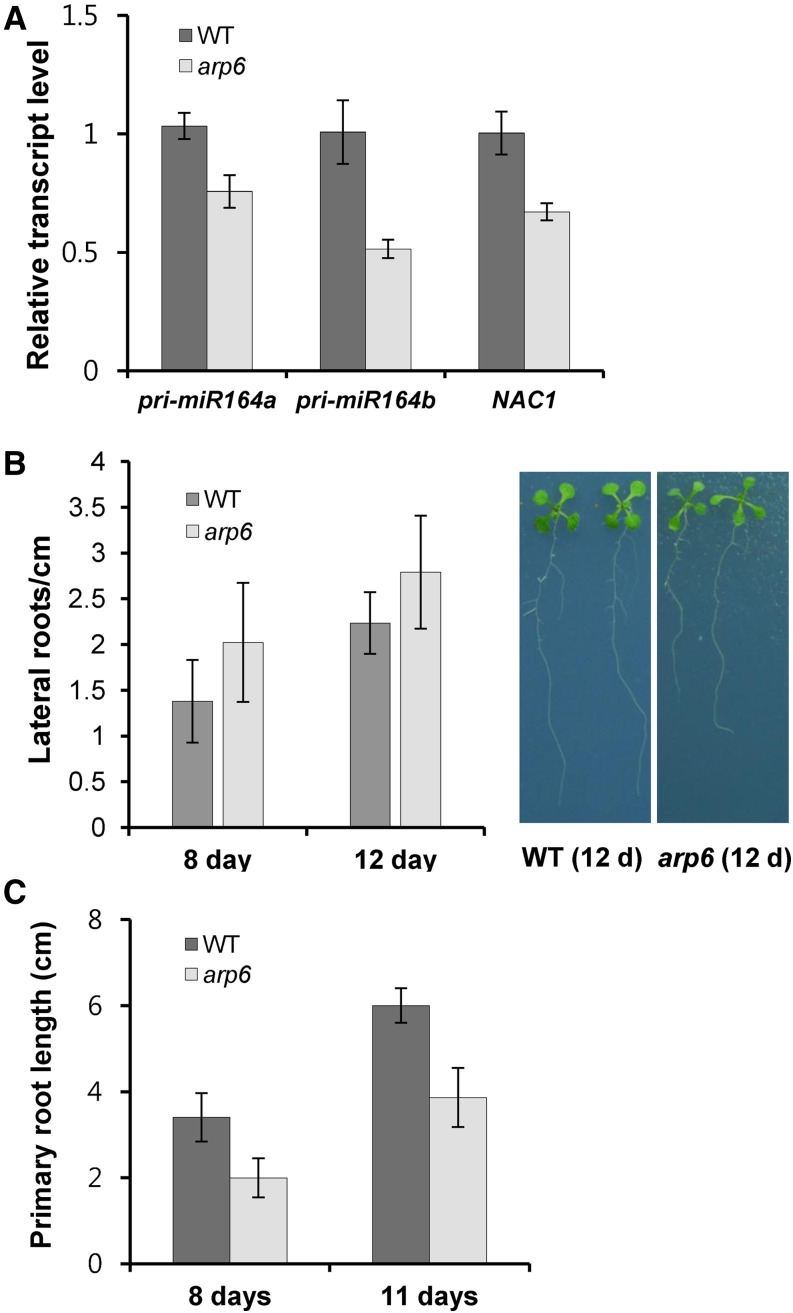
Despite the lower levels of miR164 observed in whole seedlings and reduced pri-miR164 levels in roots (Figs. 3D and 4A), the transcript level of NAC1, a miR164-target gene promoting root branching, was decreased. This caused arp6 to display lateral root formation comparable to that of wild-type plants, although the root length was shorter (Fig. 4, B and C, Supplemental Tables S2 and S3; and Guo et al., 2005). Therefore, the loss of H2A.Z deposition has a differential effect on the expression of each miR164-regulated gene. Unlike miR156-SPLs and miR164-CUC modules in which target mRNAs were accumulated in arp6, the transcriptional attenuation of both miR164 and its target NAC1 in arp6 might lead to wild-type-level root branching (Figs. 3D and 4; Supplemental Table S2). The miR164-NAC1 module in arp6 shows an example indicating why the reduced miRNA cannot produce all miRNA-associated phenotypes, because of the diverse effects of arp6 on the transcription of each miRNA-regulated gene, as shown below (Figs. 5–9).
Figure 4.
miR164-NAC1 module-mediated root branching in arp6. A, Relative transcript level of pri-miR164a, pri-miR164b, and NAC1 in roots. RNAs were isolated from roots of plants grown in MS media for 30 d. B, Numbers of lateral roots. Plants were grown on plates of MS medium containing 1% Suc for 8 d and 12 d. The roots of 10 plants per genotype were harvested to count the lateral roots per centimeter (Supplemental Table S2). C, Primary root length in 8- and 11-d-old wild type and arp6. Nine to 15 plants were used (Supplemental Table S3). WT, wild type.
Figure 5.
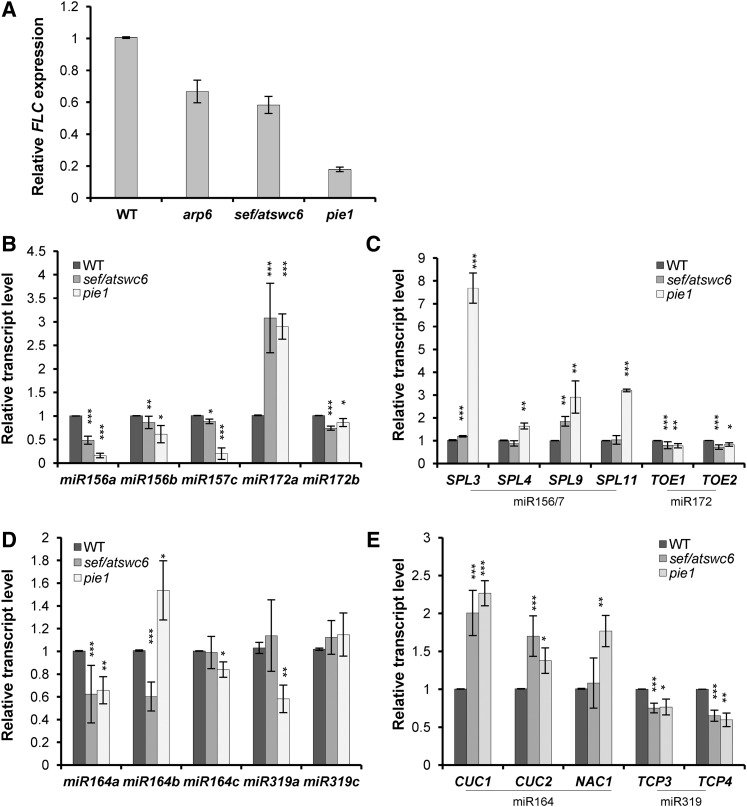
Effects of pie1 and sef/atswc6 on the transcription of miR156/SPLs and miR164/CUCs modules. A, Relative expression of FLC in wild type, arp6, sef, and pie1. B, Transcript levels of pri-miR156 and pri-miR172. C, Transcript levels of miR156- and miR172-regulated genes. D, Transcript levels of pri-miR164 and pri-miR319. E, Transcript levels of miR164 and miR319-regulated genes. TUB was used as a reference for normalization of qPCR. WT, wild type.
Figure 9.
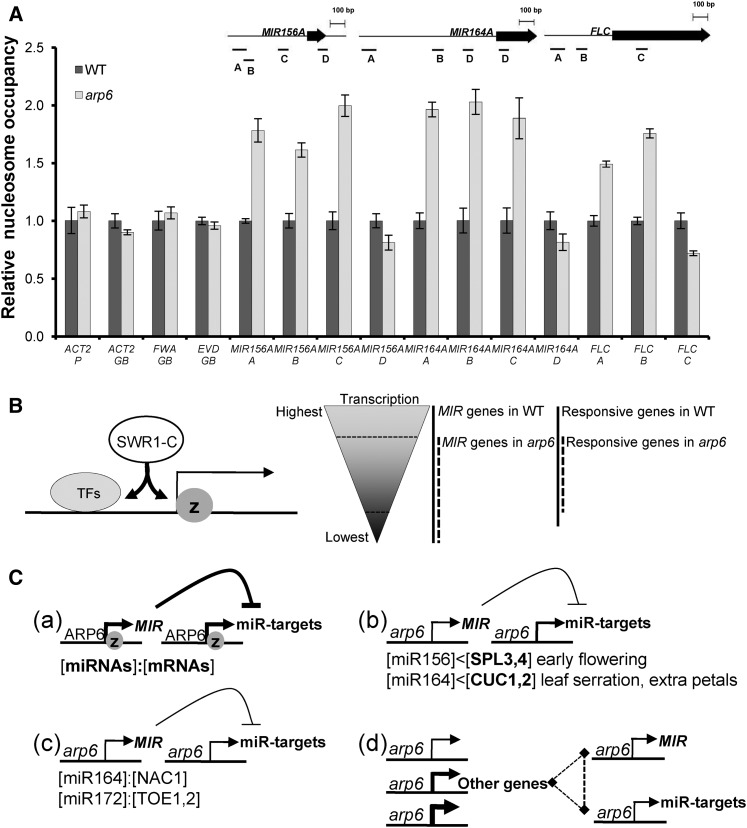
Altered nucleosome occupancy around the promoters of MIR156, MIR164, and FLC by arp6. A, MNase-qPCR assay to analyze relative nucleosomal occupancy at the promoter and 5ʹ end of MIR156, MIR164, and FLC in wild type and arp6. Locations of the amplified PCR products are based on TSS as below: MIR156A A (−496 to approximately −402), B (−421 to approximately −351), C (−192 to approximately −123), and D (+69 to approximately +134, +1 nuc); MIR164A A (−899 to approximately −800), B (−430 to approximately −352), C (−22 to approximately −154), and D (+17 to approximately +85, +1 nuc); and FLC A (−317 to approximately −414), B (−168 to approximately −239), and C (+157 to approximately +234, +1 nuc). The input chromatin without MNase treatment was used for normalization and fold enrichment at each site. Error bars indicate sd of three biological replicates. B, Model of the role of Arabidopsis SWR1-C in transcription. SWR1-C may maintain both the transcription state and the accessibility of transcription regulators to the promoter. C, Control of miRNA-mediated plant developments by SWR1-C. The proper quantitative balance between transcript levels of miRNAs and their target mRNAs is regulated by SWR1-C in wild type (a) but that is disturbed by the arp6 mutation (b, c, d). The stronger effect of arp6 on the transcription of MIR genes than that of target genes causes the increased level of target mRNAs, and thus the phenotypes of early flowering, serrated leaves, and extra floral organs are prominent (b). Similar effect of arp6 on the transcription of both miRNA and target mRNA may not lead to the development of specific phenotypes (c). Multiple influences of arp6 on the expression of miRNA pathway genes, other developmental genes, and environmentally responsive genes may cause other developmental defects such as longer hypocotyls and increased root hair density (d). WT, wild type.
pie1 and sef Show Similar Transcriptional Changes in miR156/SPLs and miR164/CUCs Modules to arp6
Because arp6 shows similar phenotypes to pie1 and sef/atswc6 (Noh and Amasino, 2003; Choi et al., 2005; Deal et al., 2005; Martin-Trillo et al., 2006; Choi et al., 2007; March-Diaz et al., 2007), we investigated whether pie1 and sef mutants also show similar transcriptional changes in the genes involved in miR156 and miR164 modules. As shown previously, arp6, sef, and pie1 have reduced FLC expressions (Fig. 5A). We observed that like arp6, sef and pie1 mutants show reduced transcription of MIR156/7 and increased expression of SPLs, compared to wild type in fold-change analyses using RT-quantitative (q)PCRs (Figs. 2, C and D; 5, B and C). Expressional fold changes of MIR172A/B and TOE1/2 in sef and pie1 were similar to arp6 (Figs. 2, C and D; 5, B and C). We also observed that transcript level of MIR164A, a major MIR gene related to leaf serration, was reduced, and subsequently CUC1/2 expressions were increased in sef and pie1, similar to arp6 (Figs. 3, C and D; 5, D and E). These similar developmental phenotypes and transcriptional misregulation of miR156 and miR164 modules in arp6, sef, and pie1 suggest that Arabidopsis SWR1-C including ARP6, SEF, and PIE1 is required for the transcription of genes involved in the miR156 and miR164 modules.
Transcriptional Activation of Some MIR Genes and miRNA-Target Genes by ARP6
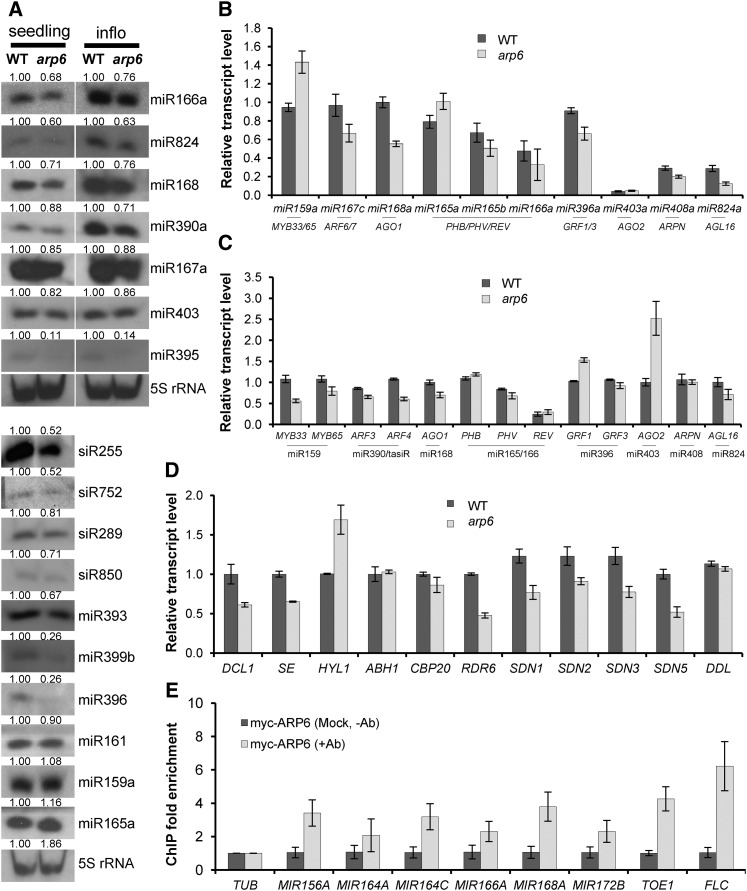
Since we observed that the arp6 mutation led to the misregulation of miR156 and miR164 pathway genes, we explored whether ARP6 could also be involved in the transcriptional regulation of other MIR genes (Fig. 6). Indeed, the levels of some miRNAs and their precursors decreased to approximately 30% to 80% of those observed in wild-type plants, but miR165 and miR159 levels either were not changed or were increased slightly, and the precursors of miR165 and miR159 were also moderately elevated (Fig. 6, A and B). This suggests that changes in the mature miRNAs may be determined mainly at the transcriptional level in arp6. Additionally, the transcription of several miRNA/transacting (ta)siRNA biogenesis genes was also affected (Fig. 6D). Therefore, the miRNA level seems to be regulated by both transcription and miRNA processing. The significant reduction of RDR6 transcription may lead to lower levels of tasiRNAs in arp6 (Fig. 6, A and D).
Figure 6.
Effect of arp6 on the transcript levels of genes involved in miRNA processing and miRNA target genes. A, Small RNA blots of miRNAs and siRNAs in arp6 and wild type. B, Transcript levels of pri-miRNAs in arp6 and wild type, detected by RT-qPCR. The miRNA-target genes are shown below the corresponding pri-miRNAs. C, Transcript levels of the genes regulated by miRNAs in arp6 and wild type. The miRNAs are shown below their target genes. D, Transcript levels of miRNA-biogenesis genes in arp6 and wild type. E, ChIP-qPCR analysis of myc-tagged ARP6 enrichment at the MIR genes miR172-target TOE1 and FLC. Primer pairs were used for amplification in the promoter region (−560 to approximately −700) for MIR156A; (−738 to approximately −840) for MIR164A; (−594 to approximately −613) for MIR164C; (−447 to approximately −591) for MIR166A; (−847 to approximately −948) for MIR168A; (−649 to approximately −761) for MIR172B; (−452 to approximately −521) for TOE1; and (−366 to approximately −492) for FLC. Dark gray bars (−Ab) indicate control experiments performed under the same conditions as sample experiments (light gray bars, +Ab), with the exception of the addition of antibody. Primer pairs amplifying the TUB gene body (+1556 to approximately +2038) were used for normalization and fold enrichment. WT, wild type; Ab, antibody.
Next, we measured the transcript levels of miRNA-regulated genes in arp6 (Fig. 6C). We observed that the transcripts of some miRNA-target genes, including AGO2 and GRF1, were increased, similar to SPL3/4/9 and CUC1 and 2, while the expression of most miRNA-target genes, including MYB33, ARF4, AGO1, and AGL16, was reduced, much like NAC1, TOE1, and TOE2 (Fig. 6, A and C). The reduction in the expression of these miRNA-regulated genes occurs regardless of the decrease in the levels of miRNAs targeting them. These results imply that miRNA-regulated genes whose transcripts were reduced in arp6 may require SWR1-C for active transcription, and thus the SWR1-C defect overwhelms the effect of the decrease in miRNA. It suggests that the influence of the arp6 mutation on gene regulation may vary among miRNAs-regulated genes, as seen in miR156- or miR164-regulated genes (Figs. 2–4). This probably depends on the transcriptional potential of each gene promoter. To test whether SWR1-C regulates MIR genes directly, we performed a ChIP analysis using 35S-myc:ARP6 transgenic plants, which fully complements the phenotypes of arp6 (Choi et al., 2007). We observed the enrichment of myc-tagged recombinant ARP6 around the promoters of the tested MIR156A, MIR164A, MIR164C, MIR166A, MIR168A, and MIR172B, as well as TOE1 and FLC, compared to the gene body of tubulin (TUB; Fig. 6E).
Genome-Wide Change in the Levels of miRNAs in arp6
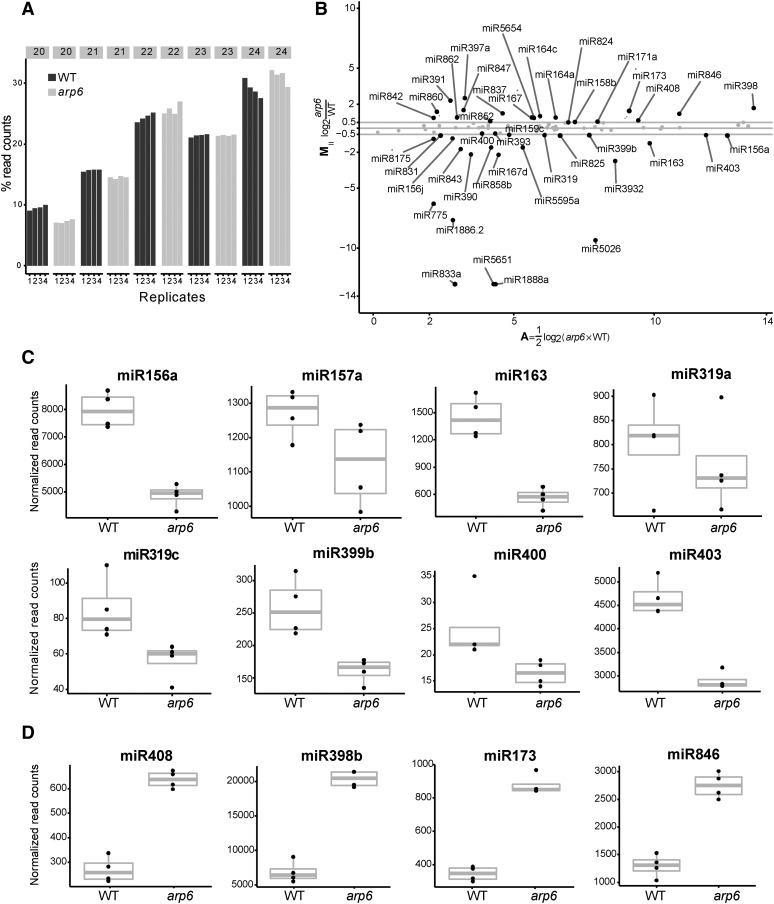
To examine how arp6 affects abundance of miRNAs at genome level, we generated and analyzed four biological replicates of small RNA libraries from 7-d whole seedlings. We found that arp6 has fewer small RNAs with the sizes of 20 and 21 nt than wild type, indicating that arp6 may reduce transcription or production of miRNAs that are mostly 20 to 21 nt. On the other hand, the levels of 23 to 24 nt small RNAs, which are mainly associated with repetitive sequences, were comparable in wild type and arp6 (Fig. 7A). We confirmed that the abundance of miR156 (which is a 20-nt small RNA) in the library was significantly reduced in arp6, consistent with the results from small RNA-blot analysis (Figs. 2, B and C; 7, B and C). Some miRNAs including miR157, miR163, miR399, miR319, and miR403 were also reduced in arp6, supporting a positive transcriptional role of ARP6 in the MIR genes (Fig. 7, B and C; Supplemental Table S4). Additionally, we observed that some miRNAs such as miR398 and miR408 were increased in arp6 while some were not changed (Fig. 7, B and D; Supplemental Table S4). It is noteworthy that miR398 and miR408 are small RNAs that are induced by copper deficiency and heat stresses (Abdel-Ghany and Pilon, 2008; Guan et al., 2013). Therefore, it may indicate that ARP6 is required for the basal repression of some miRNAs, which are environmentally inducible, similar to the heat inducible gene HSP70.
Figure 7.
Genome-wide analysis of miRNAs in wild type and arp6. A, Proportion of small RNAs from 20 to 24 nt size in wild type and arp6. Bars indicate proportion of small RNAs in size at each library. Replicates (1–4) represent four biological replicates of small RNA libraries per genotype. B, MA plot showing fold changes of miRNAs in arp6. The y axis (M) represents fold change. The x axis (A) indicates mean miRNA read counts of wild type and arp6. Black dots indicate miRNAs with greater than ±0.5 of fold changes; gray represent unchanged miRNAs. C, Boxplots of decreased miRNAs in arp6. D, Boxplots of increased miRNAs in arp6. (C and D) The y axis ticks indicate normalized miRNA read counts (see Materials and Methods). Black dots show miRNA reads from wild-type and arp6 small RNA libraries. The bold vertical gray lines indicate the median of four libraries. WT, wild type.
In summary, genome-wide miRNA analysis showed that approximately 37% of miRNAs were reduced but approximately 23% increased by arp6 mutation, suggesting that ARP6 is required for transcriptional regulation of approximately 60% of miRNA. In contrast, approximately 40% of miRNAs were not affected by arp6 mutation.
arp6 Mutation Attenuates Both the pri-miRNAs and mRNAs of miRNA-Target Genes of miRNA Biogenesis Mutants
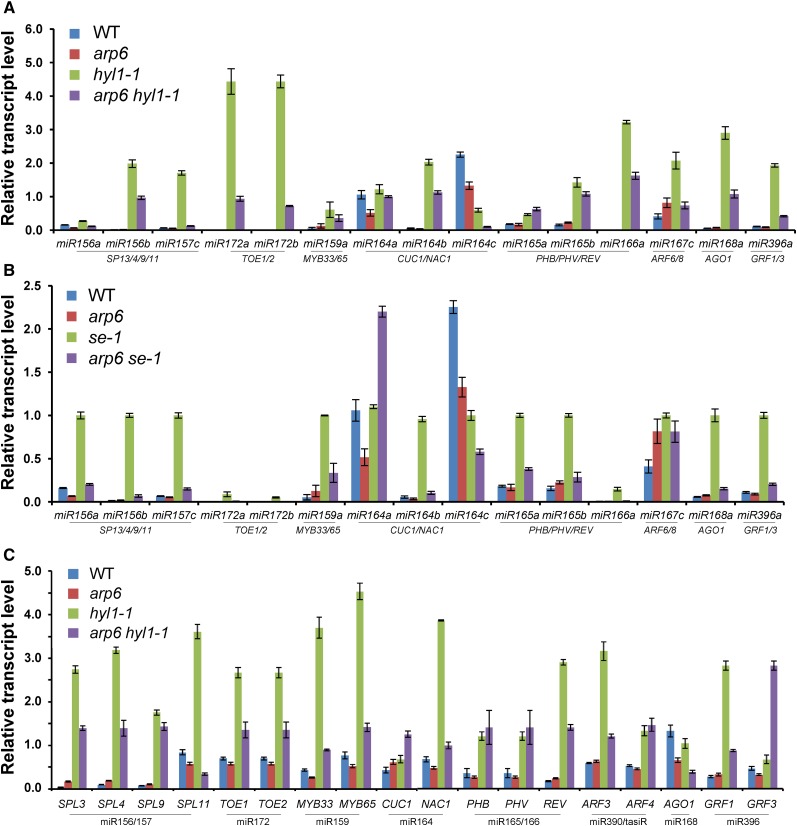
To check whether ARP6 is required for the direct transcriptional regulation of MIR genes and miRNA-regulated genes (Figs. 2–6), we measured the levels of pri-miRNAs and miRNA-regulated genes present in the double mutants of arp6 hyl1-1 or arp6 se-1 (Fig. 8). As expected, higher levels of pri-miRNAs were detected in hyl1 and serrate mutants in which there is inefficient processing of pri-miRNAs into mature miRNAs, leading to fewer miRNAs and abundant mRNAs of miRNA-regulated genes (Fig. 8, A and B; Lu and Fedoroff, 2000; Lobbes et al., 2006). Strikingly, the arp6 mutation significantly suppressed the accumulation of most unprocessed pri-miRNAs in hyl1-1 and se-1, supporting the positive role of ARP6 in the transcription of MIR genes (Fig. 8, A and B). In addition, we observed that the uncut and accumulated transcripts of many miRNA-target genes in hyl1-1 were reduced by arp6 mutation, indicating that SWR1-C positively regulates the transcription of miRNA-regulated genes (Fig. 6C). This data rules out the effect of arp6 on the misregulation of MIR and miRNA processing genes that cause post-transcriptional changes in miRNA-regulated gene expression (Figs. 2–5, and 6, A–D), and strongly suggests that SWR1-C mediates gene activation in miRNA-mediated plant developmental pathways.
Figure 8.
Effects of arp6 mutation on the transcript levels of pri-miRNAs and miRNA-target genes in miRNA biogenesis mutants. A, Transcript levels of pri-miRNAs in wild type, arp6, hyl1-1, and arp6 hyl1-1. Total RNAs from whole seedlings grown under long-day conditions for 20 d were used for RT-qPCR. B, Quantification of pri-miRNAs in wild type, arp6, se-1, and arp6 se-1 RT-qPCR. C, Transcript levels of miRNA-regulated genes in wild type, arp6, hyl1, and arp6 hyl1. The relationships between miRNAs and their regulated genes are shown between underlines. TUB was used as a reference for normalization of qPCR. WT, wild type.
ARP6 Is Required for Distinct Nucleosome Occupancy Around the Transcription Start Sites of MIR156, MIR164, and FLC
As it has been revealed that transcription is controlled by the alteration of nucleosomal occupancy around promoters, transcription start sites (TSSs), and the genes downstream of TSSs (Workman and Kingston, 1998; Albert et al., 2007; Li et al., 2007; Hu et al., 2013; Soboleva et al., 2014), we examined whether arp6 mutation could affect nucleosomal occupancy in the promoter regions and TSSs of MIR156A, MIR164A, and FLC as effective sites of SWR1-C (Jin et al., 2009; Hu et al., 2012; Li et al., 2012; Ranjan et al., 2013; Yen et al., 2013; Fig. 9A). We performed a micrococcal nuclease (MNase)-qPCR assay during which the greater enrichment of nucleosomal DNAs reflects more nucleosome occupancy and less DNA accessibility (Supplemental Fig. S3). The arp6 mutants exhibited higher nucleosome occupancy than did wild-type plants at the MIR156, MIR164, and FLC promoters, but it was unchanged at the ACTIN2 (ACT2) promoter and the gene bodies of ACT2, FWA, and EVADE (EVD). The control, ACT2, is expressed similarly in wild type and arp6, while FWA and EVD are the DNA-hypermethylated gene and transposon, respectively, with low transcription and H2A.Z deposition (Zilberman et al., 2008). Nucleosome occupancy in arp6 was slightly reduced at the 5ʹ end region of the +1 nucleosome (+1 nuc) position but highly increased at the promoters of MIR156, MIR164, and FLC genes (Fig. 9A). This suggests that the arp6 mutation, which is defective in H2A removal and H2A.Z deposition, may affect the dynamics of nucleosomes in the promoters of the MIR genes and FLC—potentially leading to the decreased accessibility of positive transcription regulators or transcriptional machineries, as shown in the promoter of FLC (Fig. 1A).
DISCUSSION
Transcriptional Regulation by SWR1-C in Arabidopsis
In Arabidopsis, the negative effect of SWR1-C on the transcription of responsive genes is well understood (March-Diaz et al., 2008; Kumar and Wigge, 2010; Smith et al., 2011; Jarillo and Pineiro, 2015). In addition, we demonstrate here that SWR1-C is required to maintain the active transcription of many MIR genes, miRNA-regulated genes, and heat shock- and JA-responsive genes. How Arabidopsis SWR1-C maintains both the repressive and activating transcription states of inductive genes such like HSP70 and JAZs remains unclear, although the dual effects of H2A.Z on transcription have been demonstrated in different species (Thambirajah et al., 2009; Marques et al., 2010; Talbert and Henikoff, 2010; Hu et al., 2012; Li et al., 2012; Soboleva et al., 2014; Jarillo and Pineiro, 2015). One possibility is that H2A.Z triggers the production of more or less stable nucleosomes with other histones, depending on the transcription state (Jin and Felsenfeld, 2007; Henikoff, 2009; Jin et al., 2009; Thambirajah et al., 2009). Different transcription states may lead to specific combinations of active or repressive histone modifications such as H2A.Z and H3 acetylation, thereby affecting SWR1-C activity and specificity for diverse nucleosome stability (Keogh et al., 2006; Millar et al., 2006; Thambirajah et al., 2009; Altaf et al., 2010; Draker et al., 2012; Watanabe et al., 2013).
In repressive transcription states, Arabidopsis H2A.Z deposition may result in more stable nucleosomes that act as barriers to the access of RNA polymerase II. Indeed, Arabidopsis H2A.Z is highly detected in the environment-responsive genes under non-inductive conditions, suggesting that H2A.Z plays a direct negative transcriptional role of them (Kumar and Wigge, 2010; Coleman-Derr and Zilberman, 2012). However, in highly active transcription states, our results suggest that Arabidopsis SWR1-C makes nucleosomes less stable, thus promoting transcription (Fig. 1, B and C). Consequently, H2A.Z is rapidly evicted from the nucleosome during transcription, so that the detected H2A.Z level displays an anticorrelation with the transcript level (Deal et al., 2007; Kumar and Wigge, 2010). It is also possible that SWR1-C acts in concert with other active chromatin modifiers such as EFS and COMPASS to promote active transcription as seen in FLC gene (Kim et al., 2005; Choi et al., 2011; Jiang et al., 2011). Another possibility is that Arabidopsis SWR1-C may help promote or maintain the accessibility of active or repressive transcription regulators to the chromatin (Fig. 9B). The results of our ChIP assay using FRI support this hypothesis, as FRI, a specific activator, is less accessible to the FLC promoter in arp6 than in wild type (Fig. 1A). Additionally, our MNase-qPCR analysis showed that arp6 has higher nucleosome occupancy at the promoter of FLC, MIR156A, and MIR164A, indicating reduced chromatin accessibility (Fig. 9A). This is similar to the proposed function of mouse H2A.Z that mediates nucleosome depletion to promote the accessibility of active or repressive regulators during embryonic stem cell differentiation (Hu et al., 2012; Li et al., 2012).
Although Arabidopsis SWR1-C is required to maintain the active transcription states of developmentally regulated genes, such as FLC and MIR genes, it is also required for the repressive transcription states especially of environmentally responsive genes (Figs. 2–7). Not only the protein-coding genes, such as HSP70 and JAZs, but also some MIR genes, such as miR398 and miR408, may require SWR1-C for their basal repression prior to environmental induction (Fig. 7, B and D; Kumar and Wigge, 2010; Coleman-Derr and Zilberman, 2012; Qin et al., 2014). Thus, SWR1-C may contribute to maintain the repressive transcription states for the environmentally induced genes. It is likely that Arabidopsis SWR1-C contributes to an expansion of the range of transcription plasticity by modulating nucleosome stability and/or the accessibility of transcription regulators to chromatin (Fig. 9B).
However, not all the genes show changes in the expression in arp6, sef, and pie1 mutants compared to wild type, although H2A.Z localizes at almost all the genes in Arabidopsis (Noh and Amasino, 2003; Choi et al., 2005; Deal et al., 2005; Martin-Trillo et al., 2006; Choi et al., 2007; Deal et al., 2007; March-Diaz et al., 2007; Jarillo and Pineiro, 2015). Approximately 5% to 10% protein coding genes and 40% MIR genes show differential expression in arp6. It indicates that arp6 does not significantly effect on the accessibility of transcription regulators or nucleosome stability/accessibility in most of the genes (Figs 6 and 7). Thus, the transcription of many genes and MIR genes seems not to be changed in mutants of the components of SWR1-C. However, FLC, and some MIR genes and the environmentally responsive genes affected by arp6 are regulated by SWR1-C, probably in concert with other transcription factors and chromatin-modifying factors. It would be intriguing to explore further how Arabidopsis SWR1-C affects the accessibility of transcription regulators or nucleosome stability in responsive and developmental genes under different conditions.
How Does Arabidopsis SWR1-C Contribute to miRNA-Mediated Development?
Our results suggest that SWR1-C contributes to the transcription of some miRNA-regulated genes as well as MIR genes, thereby affecting plant development (Figs. 2–9). Here, we propose that SWR1-C is required to fine-tune quantitative gradients between miRNAs and their target mRNAs through transcriptional activation during plant development. In our model (Fig. 9C), the wild-type plant exhibits proper spatiotemporal transcription of MIR genes and miRNA-regulated genes by SWR1-C (Fig. 9C[a]), while the arp6 mutant exhibits the miR156 and miR164-associated phenotype, with reduced miRNA levels and increased miRNA-target mRNAs (Figs. 2, 3, and 9C[b]). However, in most cases, the arp6 mutant has a phenotype similar to that of the wild-type plants due to the effect of arp6 on the transcription of both miRNA and miRNA target genes. For example, root branching in arp6 is almost normal, because arp6 effects on the transcription of both MIR164 and the miR164-regulated NAC1 gene (Figs. 4, 6, and 9C[c]). It is also important to note that SWR1-C regulates the transcription of many genes affecting miRNA pathways both positively and negatively (Fig. 9C[d]).
Although single Arabidopsis H2A.Z deposition mutants such as pie1, arp6, and sef are viable and exhibit phenotype similar to h2a.z double (hta9 hta11) and triple (hta8/9/11) mutants, the pie1;hta8/9/11 quadruple mutation resulted in a lethal phenotype at the early seedling stage. This suggests that Arabidopsis H2A.Z may still be incorporated in the nucleosome by other chromatin remodelers in the absence of SWR1-C, or that PIE1 may have a H2A.Z-independent function (Coleman-Derr and Zilberman, 2012). It could be a reason why the Arabidopsis SWR1-C mutants are viable, even with the moderate transcriptional changes observed in this study and in the previous reports (Noh and Amasino, 2003; Choi et al., 2005; Deal et al., 2005; Martin-Trillo et al., 2006; Choi et al., 2007; Deal et al., 2007; March-Diaz et al., 2007; Coleman-Derr and Zilberman, 2012).
Here our findings show that MIR and miRNA-target genes that are positively regulated by SWR1-C provide a platform to elucidate the transcription mechanism via which SWR1-C modulates chromatin features by itself (cis) or cross talks with other factors (trans) to regulate plant development and responses to environmental changes.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis Columbia (Col-0) plants were used as the wild type. The arp6/suf3-1 mutant allele in a Col-0 background was used as the experimental plant and all plants were grown under long-day (16 h of light/8 h of darkness at 22°C) or short-day conditions (8 h of light/16 h of darkness at 22°C) as previously described in Choi et al. (2005, 2007). The hy11-1 and se-1 alleles in the Col-0 background were crossed with arp6 to obtain arp6 hyl1-1 and arp6 se-1 double mutants (Lu and Fedoroff, 2000; Lobbes et al., 2006). The T-DNA insertion sef/atswc6 (SAIL_1142_C03) and pie1 (SALK_003776) lines in Col-0 background from ABRC were used for gene expression analyses (Noh and Amasino, 2003; Choi et al., 2007).
Small RNA Blot
All small RNA blot procedures followed the method including chemical cross linking as described in Pall and Hamilton (2008). Ten micrograms of total RNAs were loaded per lane, after which the RNAs were transferred to the nylon membrane. The 5S rRNA probe was used to check that equal amounts of each sample were loaded, and for normalization. The intensity of the small RNA signals was analyzed using the Image J program (National Institutes of Health, Bethesda, MD). Information on the oligonucleotides used for probes of small RNA blots is provided in Supplemental Table S1.
Small RNA Library Construction
Total RNA was extracted from 7-d-old whole seedlings of Arabidopsis Col-0 and arp6. Ten μg of total RNA was used to construct a single small RNA library. Total RNA was mixed with the same volume of 2× RNA loading buffer (de-ionized formamide, 0.5 mm EDTA, pH 8.0, 0.1% w/v bromophenol blue, 0.1% w/v xylene cyanol), incubated at 65°C for 10 min and cooled on ice. Preloading-treated RNA and RNA ladder were loaded to 15% TBE-urea gel. Gel was run in a 1× TBE running buffer at 150 V until the bromophenol blue reached the bottom of the gel. After SYBR Gold (Life Technologies, Carlsbad, CA) staining for 3 min, gel containing the small RNA band was excised and placed into a shredder (a 0.5-ml microcentrifuge tube with three 21-G needle holes punctured into the bottom within a 2-ml nuclease-free microcentrifuge tube) and spun at 10,000g. Three volumes of 0.3 m NaCl (pH 7.0) were added and rotated at 4°C overnight. The solution and gel pieces were transferred into a Costar SpinX column (Dow Corning, Midland, MI) and spun at 17,000g for 5 min at 4°C. Flowthrough was transferred to a new tube and ethanol-precipitated. Small RNA was dissolved in nuclease free water. Four biological replicate libraries per genotype were constructed according to the manual of NEBNext small RNA library prep set (cat. no. E7300S; New England BioLabs, Ipswich, MA) and then pooled, sequenced in NextSeq500 instrument (Illumina, San Diego, CA).
Computational Analyses of Small RNA Libraries
Small-RNA reads samples were pooled since all samples were split up into four lanes on the NextSeq 500 desktop sequencer (Illumina), trimmed using Trim Galore! 0.4.1 (Babraham Bioinformatics, http://www.bioinformatics.babraham.ac.uk/projects/trim_galore/) and aligned against the Arabidopsis genome (TAIR10; https://www.arabidopsis.org/download/index-auto.jsp?dir=%2Fdownload_files%2FGenes%2FTAIR10_genome_release) using Bowtie v. 1.1.1 (http://bowtie-bio.sourceforge.net/index.shtml) requiring perfect matches. Non-mapping reads were discarded and individual small-RNA species were subsequently counted and imported into R, ver. 3.2 (https://cran.r-project.org/bin/windows/base/old/3.2.0/) for downstream analysis. Small RNA species were normalized using the TMM method implemented in edgeR (Bioconductor; https://bioconductor.org/packages/release/bioc/html/edgeR.html) using default parameters; however, since a shift of 21/22 nt small-RNAs could not be excluded in arp6, only 23/24-nt small-RNAs were used to determine the TMM library sizes for normalization. MicroRNAs were identified by matching small-RNA species against mature miRNAs derived from miRBase rel. 21 (http://www.mirbase.org/). Boxplots of microRNA quantity were created using ggplot2 (http://ggplot2.org/) based on normalized read count.
MA Plots
Normalized reads from arp6 and wild-type libraries were plotted as an MA-plot using ggplot2; y axis (M) shows log2 fold change [log2(arp6/wild type)] and x axis (A) shows average abundance [0.5*log2 (arp6*wild type); bioinformatic scripts are available on request from S.Y.M.]. Libraries are available for download at ArrayExpress E-MTAB-4498 (ArrayExpress, EMBL-EBI; https://www.ebi.ac.uk/arrayexpress/).
Differential Expression
The Bioconductor package baySeq v. 1.17.3 (https://github.com/Bioconductor-mirror/segmentSeq/commits/master) was used to test for differential expression between wild-type and arp6 libraries for each miRNA species, based on counts of individual miRNA species in the respective libraries.
Reverse Transcription-Quantitative PCR
For reverse transcription-quantitative PCR (RT-qPCR), the total RNA was isolated using an RNeasy Plant Mini Kit (cat. no. 74904; Sigma-Aldrich). For the RT-qPCR of JAZ genes, total RNA was isolated from the adult leaves of 30-d-old plants. The leaves were treated with 300 µM of methyl jasmonate (cat. no. 392707; Sigma-Aldrich) for 1.5 h. The cDNA was generated using 5 µg of total RNA, reverse transcriptase (no. EP0441; Fermentas/Thermo Fisher Scientific, Guilford, CT), and oligo dT. qPCR was performed as described previously in Choi et al. (2007). The relative transcript levels were calculated using the 2ΔΔCt method. Sequences of oligonucleotides for the RT-qPCR used in this study are provided in the Supplemental Table S1.
Chromatin Immunoprecipitation Analysis
All chromatin immunoprecipitation (ChIP) analysis procedures were followed as reported previously in Choi et al. (2007). Two grams of 10-day-old 35S-myc:ARP6 or FRIp::myc:FRI grown under long-day conditions were used. To quantify the enrichment of 6 myc-tagged ARP6 or FRI in chromatin, monoclonal antic-myc (cat. no. sc-40; Santa Cruz Biotechnology, Santa Cruz, CA) was used for immunoprecipitation, and a negative control experiment was performed that was identical to the sample experiment, with the exception of the addition of antibody. The information on the primer pairs for ChIP-qPCR is presented in Supplemental Table S1.
MNase-qPCR Assay
Nuclei were isolated from 2 g of 10-day-old wild-type and arp6 plants, as described previously in Kumar and Wigge (2010). The isolated chromatin was digested in the buffer (0.05 units micrococcal nuclease (New England BioLabs), 4 mm CaCl2, 10 mm Tris-HCl, pH 8.0, 10 mm NaCl, and 1 mm EDTA) at 37°C for 10 min followed by vortexing at 1000g. The digested mononucleosomal DNA of the supernatant was collected by centrifuging at 14,000g for 5 min, and genomic DNAs were purified using a PCR purification kit (Qiagen, Hilden, Germany) after digestion with protease K and a phenol/chloroform extraction. Undigested genomic DNA was prepared and used as the input control and for normalization of the qPCR. Relative nucleosome occupancies were calculated using the 2ΔΔCt method. The procedure and information on the primer pairs for MNase-qPCR are provided in Supplemental Fig. S3 and Supplemental Table S1, respectively.
Accession Numbers
Sequence data from this article can be found in the EMBL data libraries (ArrayExpress E-MTAB-4498, EMBL-EBI; https://www.ebi.ac.uk/arrayexpress/).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Transcription levels of pri-miR156 and pri-miR172 in arp6 and wild type grown for 2 d under short day conditions (A) and transcription levels of miR156- and miR172-regulated genes in arp6 and wild type under short-day conditions (B).
Supplemental Figure S2. Comparison of leaf shape in arp6 and wild-type.
Supplemental Figure S3. Effects of sef and pie1 on transcription of miRNA gene and miRNA-regulated genes.
Supplemental Figure S4. MNase-qPCR assay in arp6.
Supplemental Table S1. Information about oligonucleotides used for the small RNA blot and qPCR.
Supplemental Table S2. Numbers of lateral root in wild type and arp6.
Supplemental Table S3. Root lengths of wild type and arp6.
Supplemental Table S4. Comparison of individual miRNA abundance in small RNA libraries between wild type and arp6.
Supplementary Material
Acknowledgments
We thank James C. Carrington (Donald Danforth Plant Science Center) for providing us hyl1-1 and rdr6-11 seeds.
Glossary
- ChIP
chromatin immunoprecipitation
- TSS
transcription start site
Footnotes
This work was supported by a National Research Foundation of Korea grant (no. 2014023132) from the Korean Government; S.Y.M. was supported by a European Research Council Advanced Investigator Grant (no. ERC-2013-AdG 340642); and I.R. was funded by a Biotechnology and Biological Sciences Research Council grant (no. BB/L006847/1).
References
- Abdel-Ghany SE, Pilon M (2008) MicroRNA-mediated systemic down-regulation of copper protein expression in response to low copper availability in Arabidopsis. J Biol Chem 283: 15932–15945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert I, Mavrich TN, Tomsho LP, Qi J, Zanton SJ, Schuster SC, Pugh BF (2007) Translational and rotational settings of H2A.Z nucleosomes across the Saccharomyces cerevisiae genome. Nature 446: 572–576 [DOI] [PubMed] [Google Scholar]
- Altaf M, Auger A, Monnet-Saksouk J, Brodeur J, Piquet S, Cramet M, Bouchard N, Lacoste N, Utley RT, Gaudreau L, Cote J (2010) NuA4-dependent acetylation of nucleosomal histones H4 and H2A directly stimulates incorporation of H2A.Z by the SWR1 complex. J Biol Chem 285: 15966–15977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CC, Sieber P, Wellmer F, Meyerowitz EM (2005) The early extra petals1 mutant uncovers a role for MicroRNA miR164c in regulating petal number in Arabidopsis. Curr Biol 15: 303–315 [DOI] [PubMed] [Google Scholar]
- Chen XM. (2009) Small RNAs and their roles in plant development. Annu Rev Cell Dev Biol 25: 21–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K, Kim J, Hwang H-J, Kim S, Park C, Kim SY, Lee I (2011) The FRIGIDA complex activates transcription of FLC, a strong flowering repressor in Arabidopsis, by recruiting chromatin modification factors. Plant Cell 23: 289–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K, Kim S, Kim SY, Kim M, Hyun Y, Lee H, Choe S, Kim SG, Michaels S, Lee I (2005) SUPPRESSOR OFFRIGIDA3 encodes a nuclear ACTIN-RELATED PROTEIN6 required for floral repression in Arabidopsis. Plant Cell 17: 2647–2660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K, Park C, Lee J, Oh M, Noh B, Lee I (2007) Arabidopsis homologs of components of the SWR1 complex regulate flowering and plant development. Development 134: 1931–1941 [DOI] [PubMed] [Google Scholar]
- Coleman-Derr D, Zilberman D (2012) Deposition of histone variant H2A.Z within gene bodies regulates responsive genes. PLoS Genet 8: e1002988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosma MP. (2002) Ordered recruitment: gene-specific mechanism of transcription activation. Mol Cell 10: 227–236 [DOI] [PubMed] [Google Scholar]
- Deal RB, Kandasamy MK, McKinney EC, Meagher RB (2005) The nuclear actin-related protein ARP6 is a pleiotropic developmental regulator required for the maintenance of FLOWERING LOCUS C expression and repression of flowering in Arabidopsis. Plant Cell 17: 2633–2646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deal RB, Topp CN, McKinney EC, Meagher RB (2007) Repression of flowering in Arabidopsis requires activation of FLOWERING LOCUS C expression by the histone variant H2A.Z. Plant Cell 19: 74–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draker R, Ng MK, Sarcinella E, Ignatchenko V, Kislinger T, Cheung P (2012) A Combination of H2A.Z and H4 acetylation recruits Brd2 to chromatin during transcriptional activation. PLoS Genet 8: e1003047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faast R, Thonglairoam V, Schulz TC, Beall J, Wells JRE, Taylor H, Matthaei K, Rathjen PD, Tremethick DJ, Lyons I (2001) Histone variant H2A.Z is required for early mammalian development. Curr Biol 11: 1183–1187 [DOI] [PubMed] [Google Scholar]
- Guan Q, Lu X, Zeng H, Zhang Y, Zhu J (2013) Heat stress induction of miR398 triggers a regulatory loop that is critical for thermotolerance in Arabidopsis. Plant J 74: 840–851 [DOI] [PubMed] [Google Scholar]
- Guo HS, Xie Q, Fei JF, Chua NH (2005) MicroRNA directs mRNA cleavage of the transcription factor NAC1 to downregulate auxin signals for Arabidopsis lateral root development. Plant Cell 17: 1376–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy S, Jacques PE, Gevry N, Forest A, Fortin ME, Laflamme L, Gaudreau L, Robert F (2009) The euchromatic and heterochromatic landscapes are shaped by antagonizing effects of transcription on H2A.Z deposition. PLoS Genet 5: e1000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson A, Plessis A, Blein T, Adroher B, Grigg S, Tsiantis M, Boudaoud A, Damerval C, Laufs P (2011) Evolution and diverse roles of the CUP-SHAPED COTYLEDON genes in Arabidopsis leaf development. Plant Cell 23: 54–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. (2009) Labile H3.3+H2A.Z nucleosomes mark ‘nucleosome-free regions’. Nat Genet 41: 865–866 [DOI] [PubMed] [Google Scholar]
- Hu G, Cui K, Northrup D, Liu C, Wang C, Tang Q, Ge K, Levens D, Crane-Robinson C, Zhao K (2013) H2A.Z facilitates access of active and repressive complexes to chromatin in embryonic stem cell self-renewal and differentiation. Cell Stem Cell 12: 180–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu GQ, Cui KR, Northrup D, Liu CY, Wang CC, Tang QS, Ge K, Levens D, Crane-Robinson C, Zhao KJ (2012) H2A.Z facilitates access of active and repressive complexes to chromatin in embryonic stem cell self-renewal and differentiation. Cell Stem Cell 12: 180–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijser P, Schmid M (2011) The control of developmental phase transitions in plants. Development 138: 4117–4129 [DOI] [PubMed] [Google Scholar]
- Iouzalen N, Moreau J, Mechali M (1996) H2A.ZI, a new variant histone expressed during Xenopus early development exhibits several distinct features from the core histone H2A. Nucleic Acids Res 24: 3947–3952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarillo JA, Pineiro MA (2015) H2A.Z mediates different aspects of chromatin function and modulates flowering responses in Arabidopsis. Plant J 10.1111/tpj.12873 [DOI] [PubMed] [Google Scholar]
- Jiang D, Kong NC, Gu X, Li Z, He Y (2011) Arabidopsis COMPASS-like complexes mediate histone H3 Lysine-4 trimethylation to control floral transition and plant development. PLoS Genet 2011 Mar;7(3):e1001330. doi: 10.1371/journal.pgen.1001330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin CY, Felsenfeld G (2007) Nucleosome stability mediated by histone variants H3.3 and H2A.Z. Genes Dev 21: 1519–1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin CY, Zang CZ, Wei G, Cui KR, Peng WQ, Zhao KJ, Felsenfeld G (2009) H3.3/H2A.Z double variant-containing nucleosomes mark ‘nucleosome-free regions’ of active promoters and other regulatory regions. Nat Genet 41: 941–U112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keogh MC, Mennella TA, Sawa C, Berthelet S, Krogan NJ, Wolek A, Podolny V, Carpenter LR, Greenblatt JF, Baetz K, Buratowski S (2006) The Saccharomyces cerevisiae histone H2A variant Htz1 is acetylated by NuA4. Genes Dev 20: 660–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, He YH, Jacob Y, Noh YS, Michaels S, Amasino R (2005) Establishment of the vernalization-responsive, winter-annual habit in Arabidopsis requires a putative histone H3 methyl transferase. Plant Cell 17: 3301–3310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Zheng B, Yu Y, Won SY, Mo B, Chen X (2011) The role of Mediator in small and long noncoding RNA production in Arabidopsis thaliana. EMBO J 30: 814–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama T, Mitsuda N, Seki M, Shinozaki K, Ohme-Takagi M (2010) TCP transcription factors regulate the activities of ASYMMETRIC LEAVES1 and miR164, as well as the auxin response, during differentiation of leaves in Arabidopsis. Plant Cell 22: 3574–3588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SV, Wigge PA (2010) H2A.Z-containing nucleosomes mediate the thermosensory response in Arabidopsis. Cell 140: 136–147 [DOI] [PubMed] [Google Scholar]
- Li B, Carey M, Workman JL (2007) The role of chromatin during transcription. Cell 128: 707–719 [DOI] [PubMed] [Google Scholar]
- Li ZY, Gadue P, Chen KF, Jiao Y, Tuteja G, Schug J, Li W, Kaestner KH (2012) Foxa2 and H2A.Z mediate nucleosome depletion during embryonic stem cell differentiation. Cell 151: 1608–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobbes D, Rallapalli G, Schmidt DD, Martin C, Clarke J (2006) SERRATE: a new player on the plant microRNA scene. EMBO Rep 7: 1052–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Fedoroff N (2000) A mutation in the Arabidopsis HYL1 gene encoding a dsRNA binding protein affects responses to abscisic acid, auxin, and cytokinin. Plant Cell 12: 2351–2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk E, Ranjan A, FitzGerald PC, Mizuguchi G, Huang Y, Wei D, Wu C (2010) Stepwise histone replacement by SWR1 requires dual activation with histone H2A.Z and canonical nucleosome. Cell 143: 725–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- March-Diaz R, Garcia-Dominguez M, Florencio FJ, Reyes JC (2007) SEF, a new protein required for flowering repression in Arabidopsis, interacts with PIE1 and ARP6. Plant Physiol 143: 893–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- March-Diaz R, Garcia-Dominguez M, Lozano-Juste J, Leon J, Florencio FJ, Reyes JC (2008) Histone H2A.Z and homologues of components of the SWR1 complex are required to control immunity in Arabidopsis. Plant J 53: 475–487 [DOI] [PubMed] [Google Scholar]
- Marques M, Laflamme L, Gervais AL, Gaudreau L (2010) Reconciling the positive and negative roles of histone H2A.Z in gene transcription. Epigenetics 5: 267–272 [DOI] [PubMed] [Google Scholar]
- Martin-Trillo M, Larazo A, Poethig RS, Gomez-Mena C, Pineiro MA, Martinez-Zapater JM, Jarillo JA (2006) EARLY IN SHORT DAYS 1 (ESD1) encodes ACTIN-RELATED PROTEIN 6 (AtARP6), a putative component of chromatin remodelling complexes that positively regulates FLC accumulation in Arabidopsis. Development 133: 1241–1252 [DOI] [PubMed] [Google Scholar]
- Millar CB, Xu F, Zhang KL, Grunstein M (2006) Acetylation of H2AZ Lys 14 is associated with genome-wide gene activity in yeast. Genes Dev 20: 711–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi G, Shen XT, Landry J, Wu WH, Sen S, Wu C (2004) ATP-driven exchange of histone H2AZ variant catalyzed by SWR1 chromatin remodeling complex. Science 303: 343–348 [DOI] [PubMed] [Google Scholar]
- Nikovics K, Blein T, Peaucelle A, Ishida T, Morin H, Aida M, Laufs P (2006) The balance between the MIR164A and CUC2 genes controls leaf margin serration in Arabidopsis. Plant Cell 18: 2929–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh YS, Amasino RM (2003) PIE1, an ISWI family gene, is required for FLC activation and floral repression in Arabidopsis. Plant Cell 15: 1671–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pall GS, Hamilton AJ (2008) Improved northern blot method for enhanced detection of small RNA. Nat Protoc 3: 1077–1084 [DOI] [PubMed] [Google Scholar]
- Qin Y, Zhao LH, Skaggs MI, Andreuzza S, Tsukamoto T, Panoli A, Wallace KN, Smith S, Siddiqi I, Yang ZB, Yadegari R, Palanivelu R (2014) ACTIN-RELATED PROTEIN6 regulates female meiosis by modulating meiotic gene expression in Arabidopsis. Plant Cell 26: 1612–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisner RM, Hartley PD, Meneghini MD, Bao MZ, Liu CL, Schreiber SL, Rando OJ, Madhani HD (2005) Histone variant H2A.Z marks the 5′ ends of both active and inactive genes in euchromatin. Cell 123: 233–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjan A, Mizuguchi G, FitzGerald PC, Wei D, Wang F, Huang YZ, Luk E, Woodcock CL, Wu C (2013) Nucleosome-free region dominates histone acetylation in targeting SWR1 to promoters for H2A.Z replacement. Cell 154: 1232–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santisteban MS, Kalashnikova T, Smith MM (2000) Histone H2A.Z regulates transcription and is partially redundant with nucleosome remodeling complexes. Cell 103: 411–422 [DOI] [PubMed] [Google Scholar]
- Sarcinella E, Zuzarte PC, Lau PNI, Draker R, Cheung P (2007) Monoubiquitylation of H2A.Z distinguishes its association with euchromatin or facultative heterochromatin. Mol Cell Biol 27: 6457–6468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber P, Wellmer F, Gheyselinck J, Riechmann JL, Meyerowitz EM (2007) Redundancy and specialization among plant microRNAs: role of the MIR164 family in developmental robustness. Development 134: 1051–1060 [DOI] [PubMed] [Google Scholar]
- Smith AP, Jain A, Deal RB, Nagarajan VK, Poling MD, Raghothama KG, Meagher RB (2011) Histone H2A.Z regulates the expression of several classes of phosphate starvation response genes but not as a transcriptional activator. Plant Physiol 152: 217–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soboleva TA, Nekrasov M, Ryan DP, Tremethick DJ (2014) Histone variants at the transcription start-site. Trends Genet 30: 199–209 [DOI] [PubMed] [Google Scholar]
- Stroud H, Otero S, Desvoyes B, Ramirez-Parra E, Jacobsen SE, Gutierrez C (2012) Genome-wide analysis of histone H3.1 and H3.3 variants in Arabidopsis thaliana. Proc Natl Acad Sci USA 109: 5370–5375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbert PB, Henikoff S (2010) Histone variants—ancient wrap artists of the epigenome. Nat Rev Mol Cell Biol 11: 264–275 [DOI] [PubMed] [Google Scholar]
- Thambirajah AA, Li A, Ishibashi T, Ausio J (2009) New developments in post-translational modifications and functions of histone H2A variants. Biochem Cell Biol 87: 7–17 [DOI] [PubMed] [Google Scholar]
- Vandaal A, Elgin SCR (1992) A histone variant, H2AVD, is essential in Drosophila melanogaster. Mol Biol Cell 3: 593–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatasubrahmanyam S, Hwang WW, Meneghini MD, Tong AHY, Madhani HD (2007) Genome-wide, as opposed to local, antisilencing is mediated redundantly by the euchromatic factors Set1 and H2A.Z. Proc Natl Acad Sci USA 104: 16609–16614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JW, Czech B, Weigel D (2009) miR156-regulated SPL transcription factors define an endogenous flowering pathway in Arabidopsis thaliana. Cell 138: 738–749 [DOI] [PubMed] [Google Scholar]
- Watanabe S, Radman-Livaja M, Rando OJ, Peterson CL (2013) A histone acetylation switch regulates H2A.Z deposition by the SWR-C remodeling enzyme. Science 340: 195–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle CM, McClinic KN, Ercan S, Zhang XM, Green RD, Kelly WG, Lieb JD (2008) The genomic distribution and function of histone variant HTZ-1 during C-elegans embryogenesis. PLoS Genet 4: e1000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollmann H, Holec S, Alden K, Clarke ND, Jacques PE, Berger F (2012) Dynamic deposition of histone variant H3.3 accompanies developmental remodeling of the Arabidopsis transcriptome. PLoS Genet 8: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman JL, Kingston RE (1998) Alteration of nucleosome structure as a mechanism of transcriptional regulation. Annu Rev Biochem 67: 545–579 [DOI] [PubMed] [Google Scholar]
- Wu G, Park MY, Conway SR, Wang JW, Weigel D, Poethig RS (2009) The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell 138: 750–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Poethig RS (2006) Temporal regulation of shoot development in Arabidopsis thaliana by miR156 and its target SPL3. Development 133: 3539–3547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi A, Wu M-F, Yang L, Wu G, Poethig RS, Wagner D (2009) The MicroRNA-regulated SBP-box transcription factor SPL3 is a direct upstream activator of LEAFY, FRUITFULL, and APETALA1. Dev Cell 17: 268–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen KY, Vinayachandran V, Pugh BF (2013) SWR-C and INO80 chromatin remodelers recognize nucleosome-free regions near+1 nucleosomes. Cell 154: 1246–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberman D, Coleman-Derr D, Ballinger T, Henikoff S (2008) Histone H2A.Z and DNA methylation are mutually antagonistic chromatin marks. Nature 456: 125–U114 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.