Plastids are multifunctional, pleomorphic organelles of purported endosymbiotic origin that in plants and green algae display a characteristic double membrane envelope (Wise, 2007). All plastids originate from colorless proplastids, and a simple pigmentation-based classification distinguishes chloroplasts from other plastids by the presence of chlorophyll, chromoplasts by the predominance of other pigments, and leucoplasts by the absence of all pigmentation (Schimper, 1883, 1885). Plastids are able to interconvert according to tissue and developmental requirements (Schimper, 1883, 1885).
In higher plants the majority of chloroplasts are found in the leaf mesophyll tissue. The presence of chloroplasts in the epidermis of some higher plant species, including tobacco (Nicotiana tabacum), is also generally accepted (Shaw and MacLachlan, 1954; Dupree et al., 1991; Brunkard et al., 2015). However, several modern textbooks and primary publications categorically state that the epidermis of higher plants contains chloroplasts only in the guard cells, while pavement and trichome cells have leucoplasts (MacDonald, 2003; Smith, 2005; Bowes and Mauseth, 2008; Solomon et al., 2010; Vaughan, 2013). In the model plant Arabidopsis (Arabidopsis thaliana), observations of leucoplasts in the unicellular trichomes are consistent, but there is considerable ambiguity regarding the presence or absence of chloroplasts in pavement cells (Table I).
Table I. Noncomprehensive list of publications reflecting on the status of chloroplasts in pavement cells in Arabidopsis.
| Suggested | Basis | Reference |
|---|---|---|
| Absent | Chlorophyll autofluorescence in guard cells only | Brunkard et al. (2015) |
| Absent | Chlorophyll-containing plastids not observed | Haseloff et al. (1997) |
| Absent | Reported as being nongreen; chlorophyll signal not observed | Haswell and Meyerowitz (2006) |
| Absent | Chlorophyll autofluorescence in guard cells only | Chiang et al. (2012) |
| Absent | Reported; chlorophyll signal not shown | Bergmann et al. (2004) |
| Absent | Expression of 35S-PAC-GFP construct only in guard cells; chlorophyll signal not shown | Meurer et al. (1998) |
| Absent | Stated in discussion, no citation | Kagawa and Wada (2000) |
| Ambiguous | Chlorophyll fluorescence; typical chloroplast internal structure in embryo | Tejos et al. (2010) |
| Ambiguous | Chlorophyll autofluorescence in leaf primordia; indicate loss of chlorophyll later | Charuvi et al. (2012) |
| Ambiguous | Chlorophyll autofluorescence | Higa et al. (2014) |
| Present | Citation only | Pyke and Page (1998) |
| Present | Internal thylakoid ultrastructure observed | Robertson et al. (1996) |
| Present | Chlorophyll autofluorescence observed | Kojo et al. (2009) |
| Present | Chlorophyll autofluorescence observed | Fujiwara et al. (2015) |
| Present | Chlorophyll autofluorescence observed | Holzinger et al. (2008) |
| Present | Pale chloroplasts reported | Pyke and Leech (1994) |
| Present | Chlorophyll autofluorescence observed | Joo et al. (2005) |
| Present | Acknowledged as chloroplasts | Vitha et al. (2001) |
Several publications clearly show chloroplasts in the pavement cells of Arabidopsis, and a precise, observation-based statement that contradicts the common textbook knowledge has been made by Pyke (2009): “In a leaf, the chloroplasts in the epidermal cells covering the leaf surface are significantly smaller and poorly developed compared with mesophyll chloroplasts, but do contain low levels of chlorophyll and should be considered as chloroplasts” (p. 15). Nevertheless, a degree of uncertainty has remained since other investigators who have observed chlorophyll fluorescence in pavement cells have either dismissed it as artifactual or have described such chloroplasts as not being fully developed (Haseloff et al., 1997; Chiang et al., 2012; Higa et al., 2014). Still others report an absence of chlorophyll fluorescence in the pavement cells (Table I). The significance of this issue is highlighted by a recent publication that uses the purported absence of chloroplasts in the pavement cells to explain differences in plastid behavior between cotyledon pavement and guard cells in response to chemically induced redox stress (Brunkard et al., 2015).
The plastid type identified in a tissue creates an association with specific attributes. The name influences our comprehension of its internal biochemistry, its response and susceptibility to environmental stimuli such as redox imbalances, and its overall behavior and interactions with other cytoplasmic components and compartments. For example, photosynthesis in chloroplasts suggests a primary source of sugars, whereas leucoplasts are recognized as sink plastids that receive already synthesized sugar molecules. For models that rely on identifying a plastid type to explain plastid behavior, a changed label can suggest a different but perhaps experimentally unsubstantiated interpretation.
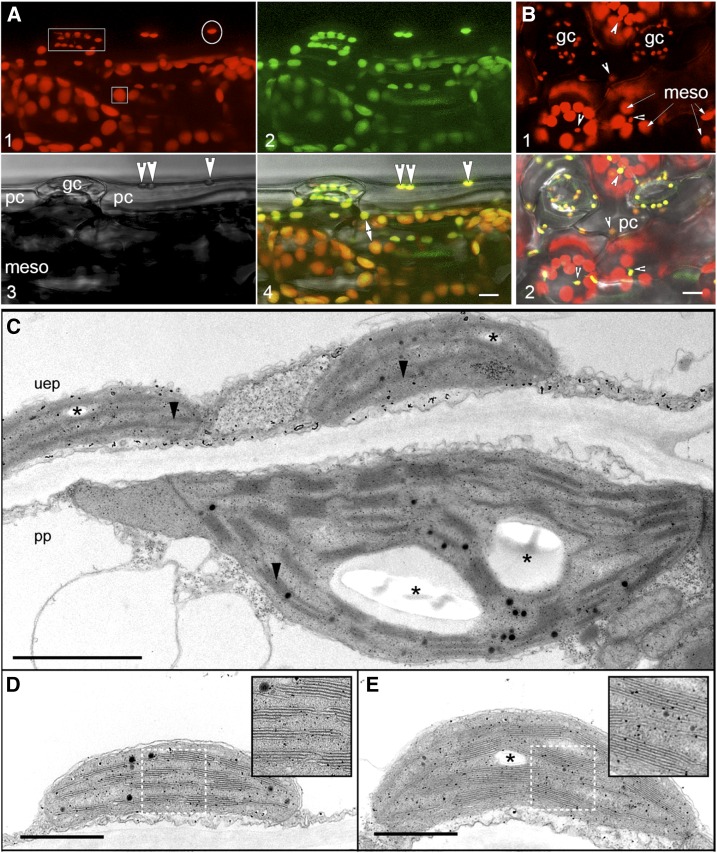
After recognizing the present ambiguity on the subject, we investigated the presence of chloroplasts in the pavement cells of Arabidopsis. Representative images and observations obtained independently in several different labs are presented (Fig. 1). Chlorophyll autofluorescence (emission peak 485 nm) is routinely detected using epifluorescent microscopy (B-3A long-pass filter set) as well as confocal laser scanning microscopy (excitation 488 nm; emission collected 650–750 nm) in pavement cell plastids. The observations remain consistent for plants in different stages of development, grown on soil or on Suc-containing medium under varying light conditions (Fig. 1, A and B). In accordance with an earlier report by Pyke and Leech (1994), the number of chloroplasts in a pavement cell is one-tenth (10 ± 3) of that observed in mesophyll cells (110 ± 10). In comparison to the clustered chloroplasts in mesophyll cells, pavement cell chloroplasts appear very dispersed, often located near the edges of the jigsaw puzzle-shaped cells. The average size of pavement cell chloroplasts is approximately one-half the size of a mesophyll chloroplasts but slightly larger than guard cell chloroplasts. The average chlorophyll a fluorescence values of pavement cell chloroplasts lies between that of guard cell and mesophyll cell chloroplasts. Observations of low chlorophyll signal are matched by ultrastructural details that show a small number of clearly defined grana (Fig. 1, C–E). Moreover, under actinic illumination pavement cell chloroplasts exhibit a fluorescence transient comparable to that shown by mesophyll chloroplasts, suggesting that they do have an active photosystem II and can utilize light energy for carbon fixation.
Figure 1.
Representative images illustrating the presence of small chloroplasts in epidermal pavement cells of Arabidopsis thaliana. A, Lateral view of the upper epidermal surface of a soil grown Arabidopsis plant expressing tpFNR:GFP shows the clear fluorescence of chlorophyll (red; panel 1) and the stroma-targeted probe (green; panel 2) in guard cells (gc) and pavement cell (pc) plastids (arrowheads in panel 3 and 4). The bright field image (panel 3) provides the spatial relationship between the epidermis and the mesophyll layer (meso) with the latter displaying larger chloroplasts (square box in panel 1) as compared to guard cell and pavement cell chloroplasts (rectangle and and circle, respectively, in panel 1; also compartive size shown by double headed arrow in panel 4). Non-trangenic plants provide a similar image for chlorophyll fluorscence (collected 650–750 nm) upon illumination with the 488 nm laser. B, Top-down view of the adaxial surface of a leaf from an Arabidopsis plant expressing tpFNR:GFP highlights the chlorophyll (red) in guard cell chloroplasts (gc), pavement cell chloroplasts (e.g. small arrowheads) and the underlying layer of the relatively large mesophyll chloroplasts. Note that the size and fluorescence exhibited by plastids in pavement cells (e.g. arrowheads) is very similar to that of the guard cell chloroplasts. However, gc (panel 1) exhibit a typical arc-shaped arrangement while pc are scattered and often not detectable against the large, more fluorescent mesophyll (meso) chloroplasts. When targeted by a stromalocalized probe (e.g. panel 2) pc are brightly highlighted due to a high stroma to grana ratio. C, An overview showing plastids from an upper epidermis pavement (uep) cell and the subtending mesophyll (palisade parenchyma, pp) layer. Despite the difference in their size, plastids from both layers contain grana (arrow heads) and starch granules (*). D, General ultrastructure of a plastid in the pavement cell of the upper epidermis. Single thylakoids within grana are shown in a magnified view of the white outlined box. E, A plastid in a pavement cell of the lower epidermis exhibits clear grana (boxed region has been magnified). Scale bars: A and B = 10 μm; C, = 2 μm; D and E = 1 μm.
Whereas each observation presented here supports earlier publications (referenced in Table I) and the presence of chloroplasts in Arabidopsis pavement cells, there is some basis for their perceived absence too. One reason that they may be overlooked lies in their low number and sparse distribution in pavement cells. Further, like chloroplasts in the mesophyll, pavement cell chloroplasts exhibit light avoidance responses (Higa et al., 2014) and relocate to the lower lateral regions of the cells in tissue exposed to light. This location places them very close to the mesophyll layer so that when imaged from above, as is the usual practice, they appear positioned alongside the mesophyll chloroplasts even when using confocal microscopy. Their location in the lower region of pavement cells also removes them from the focal plane for guard cell chloroplasts and conveys an impression of their absence from this plane. However, a comparison of chloroplast size and the use of a stroma-targeted probe clearly demonstrate their presence (Fig. 1B). As shown in Figure 1A, imaging a tissue from a lateral perspective in addition to the top-down view (Fig. 1B) allows all autofluorescent plastids to be detected and helps dispel the illusion of absence. We also note that a prevalent practice during multichannel confocal imaging is to minimize the fluorescence detection levels to obtain clear images of the strongly autofluorescent mesophyll chloroplasts. Since pavement cell chloroplasts display considerably lower autofluorescence, their fluorescent signal may fall below the detection range in this circumstance.
Another factor requiring consideration in the context of pavement cell chloroplasts is the intrinsic ability of plastids to interconvert from one kind to another. Chlorophyll, the distinguishing feature of a chloroplast, is lost quite rapidly in senescing as well as wounded tissue. This would allow a plastid to be classified as a leucoplast. As cotyledons of varying ages have been used in some studies (Chiang et al., 2012; Brunkard et al., 2015), we observed this tissue carefully and found that pavement cells in older cotyledons and senescing leaves do contain a mixture of chloroplasts and leucoplasts. Whether observations made on these tissues can be taken as representative of normally functioning leaves and be used to promote the view that pavement cells in Arabidopsis plants have only leucoplasts is questionable.
It appears that categorizing plastids in pavement cells in Arabidopsis as leucoplasts is largely due to limited information to the contrary rather than evidence in favor of this conclusion. While Arabidopsis becomes another lab plant like tobacco (Dupree et al., 1991), in which chloroplasts in pavement cells can be observed, it is noteworthy that independent surveys by Moore (1887) and Stohr (1879) had already indicated that between 85% and 95% of dicotyledonous species contain chlorophyll in the lower epidermis, while at least one-half of the 120 species investigated by Moore (1887) had chloroplasts in the upper epidermis. Perhaps the presence of chloroplasts in pavement cells occurs more widely than acknowledged hitherto. Recognition of a population of small chloroplasts with a high stroma to grana ratio in the pavement cells should open new avenues for research on their actual contribution to the general upkeep and functioning of the aerial plant epidermis.
References
- Bergmann DC, Lukowitz W, Somerville CR (2004) Stomatal development and pattern controlled by a MAPKK kinase. Science 304: 1494–1497 [DOI] [PubMed] [Google Scholar]
- Bowes BG, Mauseth JD (2008) Plant Structure—A Colour Guide, Ed 2 Manson Publishing, London, p 123 [Google Scholar]
- Brunkard JO, Runkel AM, Zambryski PC (2015) Chloroplasts extend stromules independently and in response to internal redox signals. Proc Natl Acad Sci USA 112: 10044–10049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charuvi D, Kiss V, Nevo R, Shimoni E, Adam Z, Reich Z (2012) Gain and loss of photosynthetic membranes during plastid differentiation in the shoot apex of Arabidopsis. Plant Cell 24: 1143–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang YH, Zubo YO, Tapken W, Kim HJ, Lavanway AM, Howard L, Pilon M, Kieber JJ, Schaller GE (2012) Functional characterization of the GATA transcription factors GNC and CGA1 reveals their key role in chloroplast development, growth, and division in Arabidopsis. Plant Physiol 160: 332–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupree P, Pwee KH, Gray JC (1991) Expression of photosynthesis gene-promoter fusions in leaf epidermal cells of transgenic tobacco plants. Plant J 1: 115–120 [Google Scholar]
- Fujiwara MT, Kojo KH, Kazama Y, Sasaki S, Abe T, Itoh RD (2015) The Arabidopsis minE mutation causes new plastid and FtsZ1 localization phenotypes in the leaf epidermis. Front Plant Sci 6: 823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseloff J, Siemering KR, Prasher DC, Hodge S (1997) Removal of a cryptic intron and subcellular localization of green fluorescent protein are required to mark transgenic Arabidopsis plants brightly. Proc Natl Acad Sci USA 94: 2122–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haswell ES, Meyerowitz EM (2006) MscS-like proteins control plastid size and shape in Arabidopsis thaliana. Curr Biol 16: 1–11 [DOI] [PubMed] [Google Scholar]
- Higa T, Suetsugu N, Kong SG, Wada M (2014) Actin-dependent plastid movement is required for motive force generation in directional nuclear movement in plants. Proc Natl Acad Sci USA 111: 4327–4331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzinger A, Kwok EY, Hanson MR (2008) Effects of arc3, arc5 and arc6 mutations on plastid morphology and stromule formation in green and nongreen tissues of Arabidopsis thaliana. Photochem Photobiol 84: 1324–1335 [DOI] [PubMed] [Google Scholar]
- Joo JH, Wang S, Chen JG, Jones AM, Fedoroff NV (2005) Different signaling and cell death roles of heterotrimeric G protein α and β subunits in the Arabidopsis oxidative stress response to ozone. Plant Cell 17: 957–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa T, Wada M (2000) Blue light-induced chloroplast relocation in Arabidopsis thaliana as analyzed by microbeam irradiation. Plant Cell Physiol 41: 84–93 [DOI] [PubMed] [Google Scholar]
- Kojo KH, Fujiwara MT, Itoh RD (2009) Involvement of AtMinE1 in plastid morphogenesis in various tissues of Arabidopsis thaliana. Biosci Biotechnol Biochem 73: 2632–2639 [DOI] [PubMed] [Google Scholar]
- MacDonald MS. (2003) Selected photobiological responses. In McDonald MS, ed, Photobiology of Higher Plants. John Wiley and Sons, Chichester, UK, pp 274–301 [Google Scholar]
- Meurer J, Grevelding C, Westhoff P, Reiss B (1998) The PAC protein affects the maturation of specific chloroplast mRNAs in Arabidopsis thaliana. Mol Gen Genet 258: 342–351 [DOI] [PubMed] [Google Scholar]
- Moore SLM. (1887) On epidermal chlorophyll. J Bot 25: 358–363 [Google Scholar]
- Pyke KA. (2009) Plastid Biology. Cambridge University Press, New York, pp 13–18 [Google Scholar]
- Pyke KA, Leech RM (1994) A genetic analysis of chloroplast division and expansion in Arabidopsis thaliana. Plant Physiol 104: 201–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyke KA, Page AM (1998) Plastid ontogeny during petal development in Arabidopsis. Plant Physiol 116: 797–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson EJ, Rutherford SM, Leech RM (1996) Characterization of chloroplast division using the Arabidopsis mutant arc5. Plant Physiol 112: 149–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimper AFW. (1883) Über die entwickelung der chlorophyllköerner und farbköerper. Bot Zeit 41: 105–113 [Google Scholar]
- Schimper AFW. (1885) Die entwickelung und gliederung des chromatophorensystems. In Fitting H, Pfeffer W, Pringsheim N, Strasburger E, eds, Jahrbücher Für Wissenschaftliche Botanik. G. Borntraeger, Berlin, pp 1–246 [Google Scholar]
- Shaw M, MacLachlan GA (1954) The physiology of stomata: carbon dioxide fixation in guard cells. Can J Bot 32: 784–794 [Google Scholar]
- Smith BN. (2005) Photosynthesis, respiration, and growth. In Pessarakli M, ed, Handbook of Photosynthesis, Ed 2 Taylor and Francis Group, Boca Raton, FL, pp 671–676 [Google Scholar]
- Solomon EP, Berg LR, Martin DW (2010) Biology, Ed 9 Brooks/Cole, Belmont, CA, p 732 [Google Scholar]
- Stohr A. (1879) Uber vorkommen von chlorophyll in der epidermis der phanerogamen-laubblatter. Sitzb der K Akad Wien 79: 87–118 [Google Scholar]
- Tejos RI, Mercado AV, Meisel LA (2010) Analysis of chlorophyll fluorescence reveals stage specific patterns of chloroplast-containing cells during Arabidopsis embryogenesis. Biol Res 43: 99–111 [PubMed] [Google Scholar]
- Vaughan K. (2013) Immunocytochemistry of Plant Cells. Springer, Dordrecht, The Netherlands, pp 1–129 [Google Scholar]
- Vitha S, McAndrew RS, Osteryoung KW (2001) FtsZ ring formation at the chloroplast division site in plants. J Cell Biol 153: 111–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RR. (2007) The diversity of plastid form and function. In Wise R, Hoober J, eds, Advances in Photosynthesis and Respiration: The Structure and Function of Plastids, Vol 23. Springer, Dordrecht, The Netherlands, pp 3–26 [Google Scholar]