Abstract
Purpose
To report the prevalence of postoperative retinal tear or rhegmatogenous retinal detachment secondary to intravitreal injections.
Methods
Surgical and medical records of patients who received intravitreal injections at the practice of a single retina specialist from January 2004 to May 2013 and who were followed for at least 6 months were investigated retrospectively.
Results
During the study period, a total of 3,907 intravitreal injections were performed in 1,049 eyes of 784 patients (416 males [47%]). The mean number of injections per eye was 3.72 ± 3.43 (range, 1–22). The mean age of the participants was 67.03 ± 13.56 (range, 5–94 years). The mean follow-up time was 31.98 ± 22.86 months (range, 6–144 months). Retinal break or rhegmatogenous retinal detachment occurred in 3 injections of 3 eyes, yielding an overall prevalence of 0.077% per injection and 0.29% per eye.
Conclusions
Retinal tear and rhegmatogenous detachment are rare complications of intravitreal injection. Precautions should be taken especially in patients having predisposing conditions, such as high myopia, or any other vitreoretinal disorders.
Introduction
Intravitreal injection is considered to be relatively safe; however, most patients require consecutive injections for durability of treatment effect, and serious complications can occur; for example, endophthalmitis, retinal tear (RT), rhegmatogenous retinal detachment (RRD), cataract formation, and retinal artery occlusion.1 The aim of this study was to investigate the prevalence of RT and RRD following the intravitreal injections performed over the last decade from the practice of a single retina specialist at a tertiary health care center.
Methods
Surgical and medical records of patients who received intravitreal injections at the Ophthalmology Department of Dokuz Eylul Medical School from January 2004 to May 2013 were retrospectively reviewed. The following data were recorded: age, sex, retinal disease, number of injections, injected agent(s), and postoperative complications. Patients with follow-up of <6 months after the first given injection were excluded. Patients treated for endophthalmitis with vitreous tap and subsequent intravitreal antibiotic injections were also excluded.
Every patient provided informed consent in writing before each injection. Mydriasis was achieved with phenylephrine (25g/L), tropicamide (5g/L) and cyclopentolate (10g/L) eyedrops. All injections were performed in an operating theater with the patient in a supine position. Periocular skin and eyelids were scrubbed with 100g/L topical povidone-iodine. After topical anesthesia with proparacaine hydrochloride (5g/L), povidone iodine (50g/L) was instilled into the cul-de-sac 5 minutes prior to injection. A sterile drape and eyelid speculum were used in each injection. The injections were performed through the inferotemporal quadrant, either 3.5 or 4 mm posterior to the limbus for pseudophakic and phakic individuals, respectively. For triamcinolone acetonide (TA; Sinacort-A, 40 mg/ml triamcinolone acetonide, İbrahim Ethem Ulagay, Istanbul, Turkey), 26-gauge needles were used; for ranibizumab (Lucentis, Genentech, South San Francisco, CA), bevacizumab (Altuzan, Roche, Istanbul, Turkey), and ganciclovir (Cymevene, Roche, Istanbul, Turkey) injections, 30-gauge needles; and for pegaptanib sodium (Macugen, Pfizer, NY), the original 27-gauge syringe system. The needle was directed into the middle of the vitreous cavity, and the drug was slowly injected. After the intravitreal injection, a sterile cotton applicator was applied over the injection site to prevent reflux of medication or vitreous. The vision was checked briefly to confirm at least perception of hand movement after the injection. Topical antibiotics were administered 6 times daily for 6 days postoperatively.
The prevalence of RT and RRD was calculated on the basis of the number of RT and RRD cases that occurred during the postoperative period. Every patient was examined prior to each injection following pupillary dilation. Patients with alarming symptoms for retinal break formation (eg, floaters and flashes) were examined immediately. All statistical calculations were performed using SPSS 15.0 for Windows (Statistical Package for Scientific Studies for Windows, SPSS Inc, Chicago, IL).
Results
A total of 1,049 eyes of 784 patients (416 males [46.9%]) received 3,907 intravitreal injections. Mean patient age was 67.3±13.56 years (range, 5–94 years). The mean follow-up period was 31.98 ± 22.86 months (range, 6–144 months).
The mean number of injections per eye was 3.72 ± 3.43 (range, 1–22). The agents/doses and total number of injections administered were as follows: ranibizumab, 930 injections (23.8%); bevacizumab, 789 (20.2%); pegaptanib sodium, 358 (9.2%); 2 mg TA, 831 (21.3%); 4 mg TA, 908 (23.2%); 1mg TA, 48 (1.2%); ganciclovir, 40 (1%); and dexamethasone, 3 (0.07%). The frequency and distribution of intravitreal agents used are summarized in Figure 1.
Figure 1.
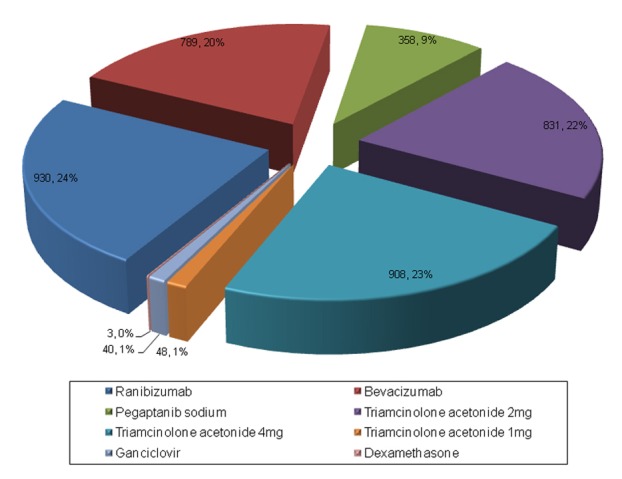
Distribution of intravitreal drugs and their frequencies in the study eyes (number of eyes; %).
The most common indications for intravitreal injections were diabetic macular edema (DME), 472 eyes (45%); and age-related macular degeneration (AMD), 402 eyes (38.3%). Other indications and their frequencies are noted in Table 1.
Table 1.
Indications for intravitreal therapy
In our study, RRD was noted in 2 eyes and a retinal tear without detachment was observed in one eye (3/1049 [0.29%]). The overall prevalence of RRD and RT was 3 in 3,907 injections (0.077%).
Report of Cases
Case 1
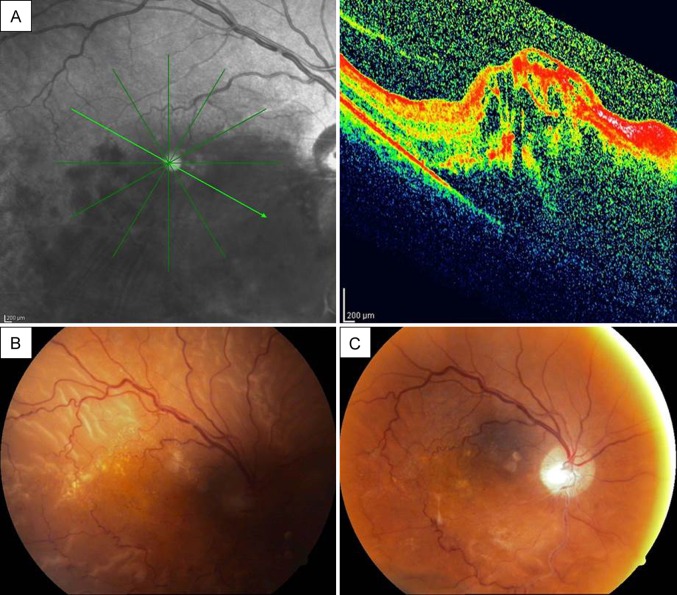
A 65-year-old man diagnosed with inferior hemorrhagic hemispheric vein occlusion in his right eye initially received 4 consecutive uneventful 2 mg triamcinolone acetonide injections (Figure 2A). During the course of treatment, a cataract developed and phacoemulsification with posterior chamber IOL implantation was planned. At the time of surgery, the posterior capsule was ruptured and vitreous loss occurred because of tamsulosin-related floppy iris syndrome. Anterior vitrectomy was performed, and a 3-piece intraocular lens was implanted into the ciliary sulcus. During the follow-up, the macular edema increased and another 4 mg TA injection was administered 4 weeks after the cataract surgery. One month after this injection, visual acuity decreased and fundus examination showed an RRD (Figure 2B) and a retinal tear in the inferior quadrant (not pictured). The axial length was measured as 23.08 mm by laser interference biometer. The patient underwent a successful vitrectomy procedure with an encircling band (Figure 2C).
Figure 2.
Case 1, right eye. A, Optical coherence tomograph (OCT) showing immense retinal edema and hemorrhagic type, inferior hemispheric vein occlusion at the initial presentation. B, Color fundus photograph showing near total retinal detachment, with residual macular hard exudates. C, Postoperative color fundus photograph showing the attached retina.
Case 2
A 64-year-old mild myopic man (axial length, 24.35 mm) was examined after presenting with vision loss in his right eye. He was diagnosed as having neovascular AMD with sub-foveal hemorrhage in the right eye (Figure 3A). His best-corrected visual acuity was hand movements in the right eye. One month after a single intravitreal ranibizumab injection, visual acuity increased to counting fingers at 1 meter. However, fundus examination revealed an RRD, with a small retinal tear in the inferior quadrant in the right eye. The patient was treated with an encircling band, pars plana vitrectomy, 360° endolaser, and silicone oil (Figure 3B). One year after the vitreoretinal surgery the patient complained of floaters in his fellow eye. On fundus examination, a retinal tear was noticed in the nasal quadrant, which was successfully treated with laser photocoagulation. During the follow-up, neovascular AMD developed in the left eye, which was treated with 3 consecutive intravitreal ranibizumab injections without any complications at latest follow-up, 15 months after final treatment.
Figure 3.
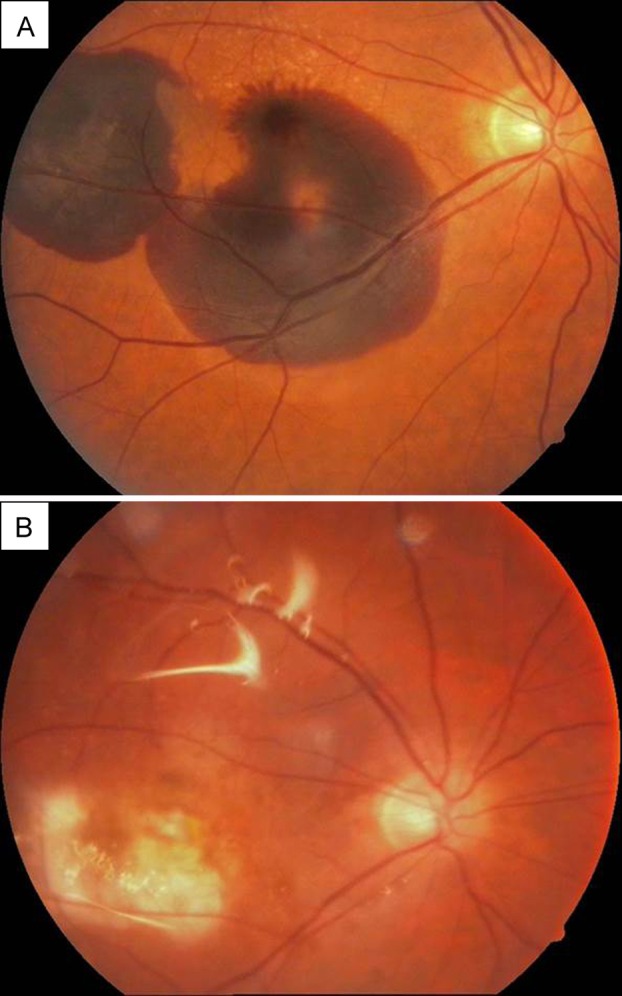
Case 2, right eye. A, Wide subretinal hemorrhage in two lobules. B, Color fundus photograph, 18 months after the silicone oil surgery, showing the atrophic macular area.
Case 3
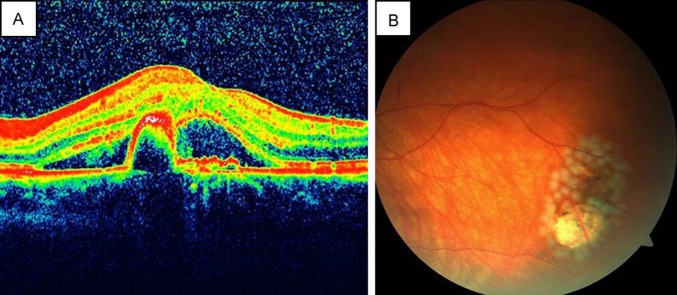
A 57-year-old woman with exudative AMD in the left eye (Figure 4A) was treated with 8 consecutive intravitreal ranibizumab injections. Her visual acuity increased from 20/200 to 20/25. Seven weeks after the last injection she complained of floaters in the treated eye. On fundus examination, a tiny retinal tear was observed in the temporal quadrant right next to a pigmented area. The tear and the pigmented area were surrounded with laser spots (Figure 4B). Two additional intravitreal ranibizumab injections were performed during the follow-up, without further complication. The axial length was measured as 22.90 mm on laser interference biometry.
Figure 4.
Case 3, left eye. A, OCT showing retinal pigment epithelium detachment and intraretinal fluid. B, Color fundus photograph immediately after laser photocoagulation for symptomatic small retinal tear adjacent to a pigmented retinal area.
Discussion
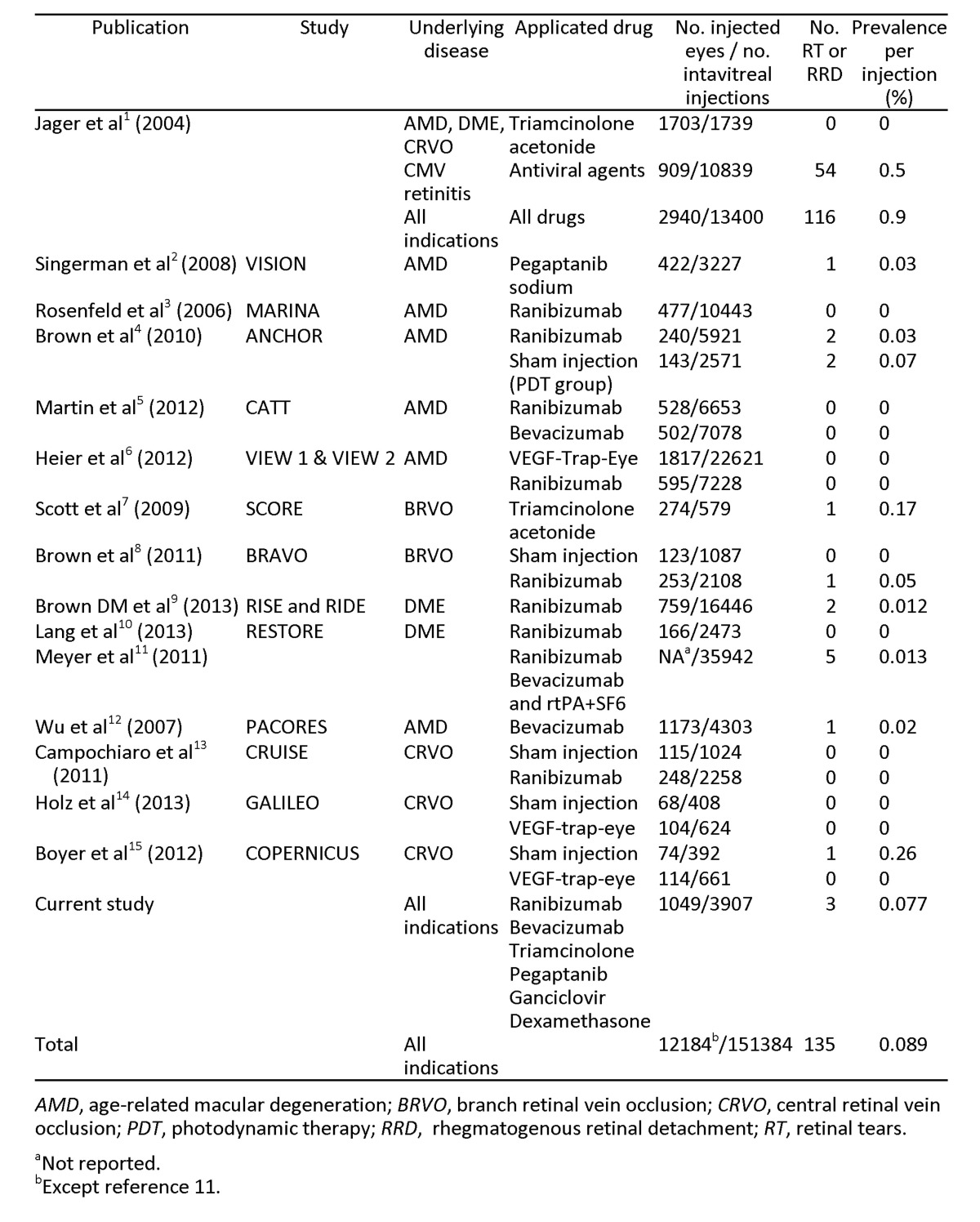
In the last decade, the incidence of RT and RRD has been reported to be from 0.0% to 0.9% per injection (Table 2).1–15
Table 2.
Major clinical trials and prevalence of retinal tears or rhegmatogenous retinal detachment after intravitreal injection
In our study, 2 RD and 1 RT were observed in a total of 3,907 intravitreal injections. In the first case, RRD developed 6 weeks after an intravitreal 4 mg TA injection. Four weeks before, the last injection the patient underwent a complicated cataract surgery with a posterior capsule rupture and vitreous loss due to floppy iris syndrome. In this case, the eventful cataract surgery could have been a major contributing factor for RRD. Stein et al16 reported the incidence of RRD after cataract surgery as 0.26%. Furthermore, in a large consecutive case series of 63,298 cataract surgery procedures, posterior capsule rupture was determined as the major risk factor for RRD (odds ratio, 19.9).17 The cumulative risk for RRD of the eventful cataract surgery is far more than the previously reported RRD incidence after intravitreal injections.
The second case was a myopic male who was only treated with one intravitreal ranibizumab injection before RRD developed. A symptomatic posterior vitreous detachment with a retinal tear occurred 1 year later in his untreated fellow eye perhaps indicating the patient’s predisposition to retinal tear formation. Meyer et al11 reported in their retrospective multicenter case series that 5 RRDs developed after 35,942 intravitreal injections (0.013%). They pointed out the predisposition of myopia because 4 of these 5 affected eyes were myopic, ranging from −1.75 D to −5.5 D and with an axial length >25 mm. They recommended a careful fundus examination in myopic eyes, and the possible treatment of peripheral degenerations or asymptomatic holes.
In our third case, who was not myopic, we noticed a symptomatic, small retinal tear next to a pigmented, atrophic area in the retinal periphery following 8 consecutive ranibizumab injections. The pigmented area could also be predisposed to retinal tear formation.
The underlying retinal conditions or previous surgeries seem to be an important factor in RRD development after intravitreal injections. Patients with CMV retinitis are prone to RRD development following intravitreal ganciclovir injections. In our study, a single HIV-positive patient was treated with intravitreal ganciclovir for bilateral CMV retinitis. Despite multiple injections (right eye, 11; left eye, 19), RRD did not occur. Incidence of RRD was much higher and ranged between 0.64% and 16.6% after intravitreal therapy for CMV retinitis.18–20
While performing intravitreal injections anatomic structures should be handled delicately. The injection should be precisely made 3.5–4 mm posterior to the limbus, according to the lens status, avoiding the ora serrata and thereby the vitreous base. A more posterior or too steep insertion can cause a transretinal penetration. The needle should be inserted in an oblique fashion in order to avoid vitreous incarceration, which is also a risk factor.21
The needle gauge and injection volume seems not to be related with the post-operative RD incidence. In 2 of our cases, 0.05ml ranibizumab was injected with a 30-gauge needle, whereas a 26-gauge needle was used for 4mg/1ml triamcinolone acetonide. To our knowledge, no study has evaluated the role of needle thickness and injection volume in the development of RRD.
The present study is limited by the relatively small total number of injections. On the other hand, all patients were from the practice of a single retina specialist; thus, our injection-related retinal tear and RRD prevalence probably reflects the real life scenario more when compared to multicenter studies.
Literature Search
A PubMed search for English- and German-language articles was conducted in July 2013 using the following keywords: intravitreal injection, retinal tear, retinal break, retinal detachment, and the names of pharmacological agents.
References
- 1.Jager RD, Aiello LP, Patel SC, Cunningham ET., Jr Risks of intravitreous injection: a comprehensive review. Retina. 2004;24:676–98. doi: 10.1097/00006982-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Singerman LJ, Masonson H, Patel M, et al. Pegaptanib sodium for neovascular age-related macular degeneration: third-year safety results of the VEGF Inhibition Study in Ocular Neovascularisation (VISION) trial. Br J Ophthalmol. 2008;92:1606–11. doi: 10.1136/bjo.2007.132597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenfeld PJ, Brown DM, Heier JS, et al. (MARINA Study Group) Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 4.Brown DM, Michels M, Kaiser PK, et al. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR study. Ophthalmology. 2009;116:57–65. doi: 10.1016/j.ophtha.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 5.Martin DF, Maguire MG, Fine SL, et al. (Comparison of age-related macular degeneration treatments trials (CATT) research group). Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119:1388–98. doi: 10.1016/j.ophtha.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heier JS, Brown DM, Chong V, et al. Intravitreal Aflibercept (VEGF Trap-Eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–48. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Scott IU, Ip MS, VanVeldhuisen PC, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular edema secondary to branch retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 6. Arch Ophthalmol. 2009;127:1115–28. doi: 10.1001/archophthalmol.2009.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown DM, Campochiaro PA, Bhisitkul RB, et al. Sustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology. 2011;118:1594–602. doi: 10.1016/j.ophtha.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 9.Brown DM, Nguyen QD, Marcus DM, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III Trials: RISE and RIDE. Ophthalmology. 2013;120:2013–22. doi: 10.1016/j.ophtha.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 10.Lang GE, Berta A, Eldem BM, et al. Two-year safety and efficacy of ranibizumab 0.5 mg in diabetic macular edema: Interim analysis of the RESTORE extension study. Ophthalmology. 2013;120:2004–12. doi: 10.1016/j.ophtha.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 11.Meyer CH, Michels S, Rodrigues EB, et al. Incidence of rhegmatogenous retinal detachments after intravitreal antivascular endothelial factor injections. Acta Ophthalmol. 2011;89:70–5. doi: 10.1111/j.1755-3768.2010.02064.x. [DOI] [PubMed] [Google Scholar]
- 12.Wu L, Martínez-Castellanos MA, Quiroz-Mercado H, et al. Twelve-month safety of intravitreal injections of bevacizumab (Avastin): results of the Pan-American Collaborative Retina Study Group (PACORES) Graefes Arch Clin Exp Ophthalmol. 2008;246:81–7. doi: 10.1007/s00417-007-0660-z. [DOI] [PubMed] [Google Scholar]
- 13.Campochiaro PA, Brown DM, Awh CC, et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: twelve-month outcomes of a phase III study. Ophthalmology. 2011;118:2041–9. doi: 10.1016/j.ophtha.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 14.Holz FG, Roider J, Ogura Y, et al. VEGF Trap-Eye for macular oedema secondary to central retinal vein occlusion: 6-month results of the phase III GALILEO study. Br J Ophthalmol. 2013;97:278–84. doi: 10.1136/bjophthalmol-2012-301504. [DOI] [PubMed] [Google Scholar]
- 15.Boyer D, Heier J, Brown DM, et al. Vascular endothelial growth factor trap-eye for macular edema secondary to central retinal vein occlusion: six-month results of the phase 3 COPERNICUS study. Ophthalmology. 2012;119:1024–32. doi: 10.1016/j.ophtha.2012.01.042. [DOI] [PubMed] [Google Scholar]
- 16.Stein JD, Grossman DS, Mundy KM, Sugar A, Sloan FA. Severe adverse events after cataract surgery among medicare beneficiaries. Ophthalmology. 2011;118:1716–23. doi: 10.1016/j.ophtha.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tuft SJ, Minassian D, Sullivan P. Risk factors for retinal detachment after cataract surgery: a case-control study. Ophthalmology. 2006;113:650–6. doi: 10.1016/j.ophtha.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Baudouin C, Chassain C, Caujolle C, Gastaud P. Treatment of cytomegalovirus retinitis in AIDS patients using intravitreal injections of highly concentrated ganciclovir. Ophthalmologica. 1996;210:329–35. doi: 10.1159/000310734. [DOI] [PubMed] [Google Scholar]
- 19.Arevalo JF, Garcia RA, Mendoza AJ. High-dose (5000-microg) intravitreal ganciclovir combined with highly active antiretroviral therapy for cytomegalovirus retinitis in HIV-infected patients in Venezuela. Eur J Ophthalmol. 2005;15:610–8. doi: 10.1177/112067210501500512. [DOI] [PubMed] [Google Scholar]
- 20.Hodge WG, Lalonde RG, Sampalis J, Deschênes J. Once-weekly intraocular injections of ganciclovir for maintenance therapy of cytomegalovirus retinitis: clinical and ocular outcome. J Infect Dis. 1996;174:393–6. doi: 10.1093/infdis/174.2.393. [DOI] [PubMed] [Google Scholar]
- 21.Knecht PB, Michels S, Sturm V, et al. Tunnelled versus straight intravitreal injection: intraocular pressure changes, vitreous reflux, and patient discomfort. Retina. 2009;29:1175–81. doi: 10.1097/IAE.0b013e3181aade74. [DOI] [PubMed] [Google Scholar]