Abstract
We identified two female siblings, derived from healthy first cousin parents, with congenital unilateral cerebral ventriculomegaly detected prenatally. Patient 1 underwent ventriculoperitoneal shunt operation at 1 week old, while Patient 2 was followed without surgical intervention. Both patients presented with mild developmental delay and hemiparesis contralateral to the involved hemisphere. Focal seizures were observed in Patient 1, whose neuroimaging revealed posterior insular polymicrogyria in the normal sized ventricle hemisphere and retrocerebellar cyst. Both siblings displayed near absence of white matter with marked thinning of the overlying cortex in the affected hemisphere and very thin corpus callosum. Investigations revealed no other system involvement and karyotyping was normal. Normal TORCH screening in subsequent pregnancies, normal parental coagulation profile and undetectable maternal autoantibodies suggested against the possible role of extrinsic factors as an etiological factor for unilateral ventriculomegaly. Parents had normal brain imaging findings. We suggest delineation of a distinct developmental brain defect, most likely of autosomal recessive inheritance.
Keywords: unilateral ventriculomegaly, familial, polymicrogyria, autosomal recessive, developmental brain defects
INTRODUCTION
Cerebral ventriculomegaly is one of the common anomalies recognized prenatally. Unilateral ventriculomegaly occurs much less frequently than bilateral form of disease. It is often referred to occlusion of the foramen of Monro due to congenital anomaly or inflammation leading to scarring [Oi and Matsumoto, 1985; Gaston and Jones, 1989; Sherer et al., 1995]. Several authors suggested using the term ventriculomegaly in preference to hydrocephalus, because it seems to describe the abnormalities found in prenatal ultrasound more accurately [Chervenak et al., 1984; Senat et al., 1999]. The accepted standard for ventricle size is the measurement of the ventricular atrial width at the level of the choroids plexus. Normally, it ranges between 5.4 and 7.6 mm and measurements exceeding 10 mm are considered enlarged [Cardoza et al., 1988; Farrell et al., 1994]. Mild ventriculomegaly is assumed when the width of the atria of lateral ventricle is from 10 to 12 mm, moderate from 12.1 to 14.9, while severe when equal or exceeding 15 mm, which is often associated with an unfavorable outcome [Romero et al., 1988; Gaglioti et al., 2005]. Congenital unilateral severe ventriculomegaly or hydrocephalus is rarely described, with only few reports in the literature [Husag et al., 1976; Wilberger et al., 1983; Oi et al., 1985; Dorwling-Carter et al., 1987; Gaston and Jones, 1989; Nakamura et al., 1989; Anderson et al., 1993; Tsao et al., 1996; Chudley et al., 1997; Schulman et al., 2000; Durfee et al., 2001]. Congenital unilateral ventriculomegaly associated with sensorineural hearing loss was assigned by Chudley et al. [1997] who delineated the new autosomal recessive disorder “Chudley–McCullough syndrome” and further similar cases were described (OMIM 604213). Moreover, borderline unilateral ventriculomegaly had been diagnosed prenatally in two sibs whose surprisingly same finding was detected in their mother [Muhler et al., 2008]. Authors postulated an inherited anatomical variant most likely in autosomal dominant fashion. Autosomal dominant hemiparesis with retinal tortuosity was reported as a dominant condition with full penetrance affecting one parent [Vahedi et al., 2003]. To our knowledge, there have been no other reports of familial cases with congenital unilateral hydrocephalus or ventriculomegaly.
Herein, we describe sibs with congenital unilateral ventriculomegaly. We discuss the etiology and pathogenesis of this anatomical developmental defect. Since they are born to healthy consanguineous parents, we suggest autosomal recessive as a possible mode of inheritance.
CLINICAL REPORTS
Family History
The present Egyptian family was referred to the Genetic Clinic for counseling, as their only two children had unexplained prenatal onset unilateral ventriculomegaly. Parents were first cousins from Upper Egypt (Fig. 1). Maternal and paternal age was 25 and 29 years old, respectively. No history of repeated abortions or early infant deaths was recorded. Maternal pregnancies were uncomplicated, during which repeated TORCH screening was negative. Ventriculomegaly versus hydrocephaly was suspected intrauterine by fetal ultrasound in both sibs and delivery was by caesarian section. Parents were phenotypically normal without history of any neurological problems. Brain MRI for both was entirely normal. Complete blood picture, prothrombin time, concentration, partial thromboplastin time, antithrombin III, protein C and protein S were normal for both parents. Detection of autoantibodies in maternal blood as lupus anticoagulant, anticardiolipin and anti-nuclear antibodies were negative.
FIG. 1.
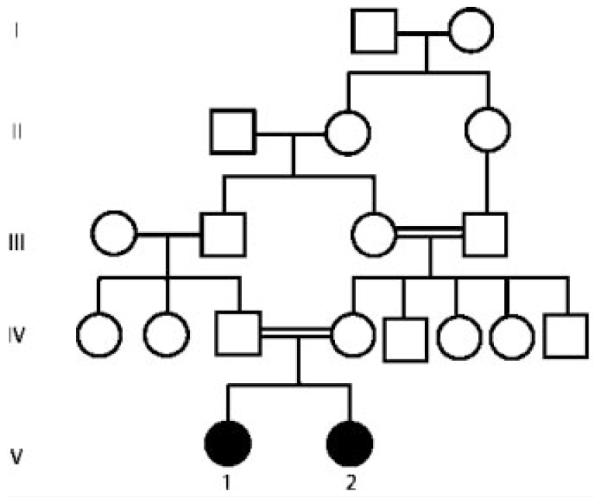
Pedigree of the assigned family.
Patient 1
Patient 1 was a 310/12-year-old female presenting with developmental delay and weakness of the left side of her body. She was the product of a full-term gestation. At birth the head circumference was 39 cm (+3.13 SD), while the weight was 3.200 kg (−0.5 SD) and length was 50 cm (+0.2 SD). On the second day of her life, CT brain examination revealed unilateral right ventriculomegaly and suspicion of hydrocephalus; ventriculoperitoneal shunt operation was done at the age of 1 week. The parents noticed left sided weakness in the first few years of life, but there was no sudden onset of symptoms. Developmental milestones were mildly delayed; she supported her head at 6 months, sat unsupported at 14 months, and walked unsupported at 30 months with mild unsteady gait, and attained bowel and bladder control by 3 years old. The patient pronounced several double syllable words. She had history of several attacks of partial seizures, controlled with carbamazepine.
On examination at 310/12 years the height on −1.1 SD and head circumference on −1.4 SD with closed fontanels. The patient had mild asymmetric facial features, thick vermillion to the lips (Fig. 2A) and unilateral single palmar creases. Neurological evaluation revealed wide based gait and mild limping on left side. There was facial weakness of the left during smiling (Fig. 2A). The left upper and lower limbs showed weakness, hypotonia and brisk reflexes, with pathological reflexes positive Babinski and ankle clonus. There was mild intention tremor but no detectable nystagmoid eye movement.
FIG. 2.
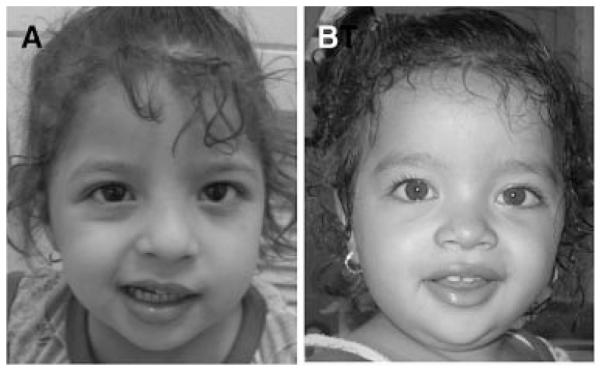
Both patients have specific facial features. A: Patient 1: Note, squint, apparent hypertelorism, mild downslanting of the eyes, thick lips. There is prominent asymmetry of the face when attempting to smile. Note inability of the patient’s left lip to elevate. B: Patient 2: Note similar facies as older sister, together with prominent forehead and broad nose. There is less pronounced asymmetry with smiling, most notable is the failure to elevate the patient’s lip, and to partially close the eye on the right.
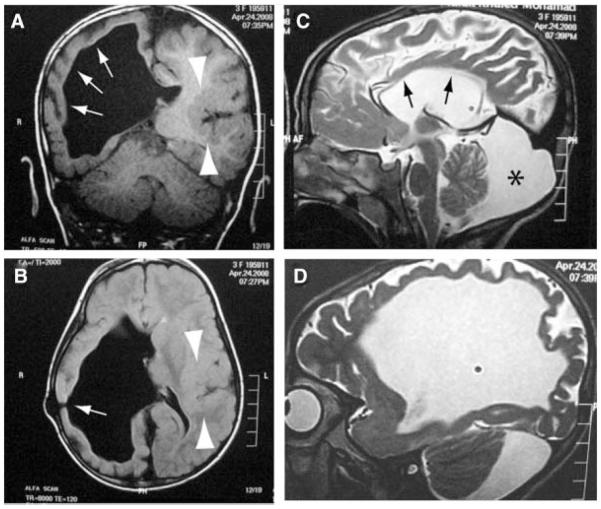
Complete audiometry, EMG, and NCV were normal. Ophthalmological examination revealed normal fundi, however there was visual reduction of left eye with asymmetric evoked potential. EEG showed abnormal potentials in the form of focal spike and slow waves. Chromosomal analysis was normal, 46,XX in all examined metaphases. Developmental milestones were evaluated by Stanford–Binet test [Thorndike et al., 1986] and revealed IQ score of 71 (low borderline range). Serial neuroimaging revealed severe right ventriculomegaly and intact shunt (Fig. 3). Near absence of overlying white matter, marked thinning of the cerebral cortex, absent thalami and basal ganglia were striking in the affected hemisphere. Furthermore, Wallerian degeneration of the brain stem was noted, with the affected side having small cerebellar peduncles, pons and medullary pyramids. Cerebellum was normal or nearly normal in size. Patient had thin corpus callosum and retrocerebellar cyst. The left lateral ventricle was normal while the overlying cerebral cortex showed posterior opercular polymicrogyria that seemed to extend into the posterior temporal lobe (Fig. 3).
FIG. 3.
Brain MRI of Patient 1. A,B: Right severe ventriculomegaly with near absence of overlying white matter and marked thinning of the cortex on the right (arrows) together with absence of thalamus and basal ganglion on the right. Normal left lateral ventricle and overlying cerebral cortex showed posterior opercular polymicrogyria (arrowheads). Site of shunt indicated by arrow in (B). C: Thin corpus callosum (arrows), retrocerebellar cyst (*). D: Right parasagittal showing ventriculomegaly and thin cortical mantle.
Patient 2
Patient 2 was a 23/12 years old female. She was the second child of the family and the younger sib of Patient 1 (Fig. 1). Pregnancy history was noncontributory. Meticulous serial ultrasound during gestation detected congenital unilateral left hydrocephalus by the 8th month. At birth the head circumference was 36.8 cm (+1.7 SD). Neurosurgery consultants opted to avoid surgical intervention. No signs or symptoms of increased intracranial tension were present and patient had no history of seizures. The developmental milestones were mildly delayed: she was able to support her head at 6 months, sat unsupported at 13 months and stood with support at 16 months.
On examination her weight was on the mean, height on +0.6 SD, and head circumference on +1 SD with patent anterior fontanel. She had similar facies as her sister together with prominent forehead, broad nose (Fig. 2B) and bilateral single palmar creases. Neurological evaluation showed only mild weakness of motor power in right side, including the right face during smiling (Fig. 2B) although both sides showed similar hypotonia and brisk reflexes. All investigations were normal including EEG, fundus examination, VEP, complete audiometry, EMG, NCV, and karyotyping. Patient’s IQ score was 80 (borderline range) using Stanford–Binet test [Thorndike et al., 1986].
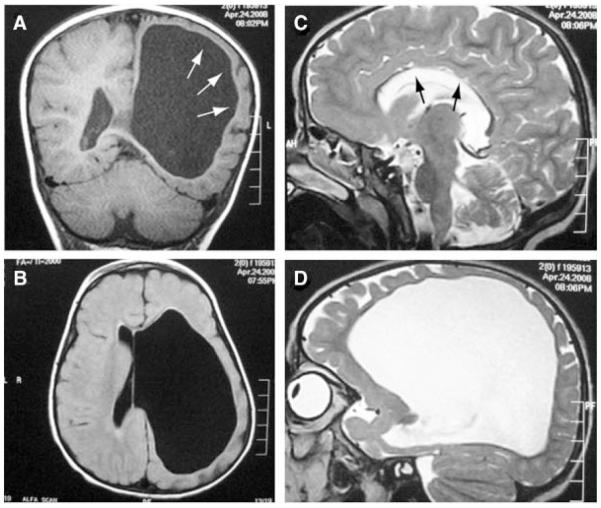
Serial Brain MRI scans were done at 4 weeks, 13 months, and 2 years old, revealing left ventriculomegaly. Recent MRI (Fig. 4) showed nearly identical findings as was found in her older sister regarding cerebrum and brain stem affection, but on the opposite side of the brain. No retrocerebellar cyst or contralateral opercular polymicrogyria were detected, although the posterior aspect of the sylvian fissure was slightly irregular.
FIG. 4.
Brain MRI of Patient 2. A,B: Left severe ventriculomegaly with near absence of overlying white matter and marked thinning of the cortex and absence of thalamus and basal ganglion on the left. C: Thin corpus callosum (arrows) was also evident. Retroretrocerebellar cyst was not evident. D: Left parasagittal image showing ventriculomegaly and thin cortical mantle.
DISCUSSION
The pathophysiology of congenital unilateral ventriculomegaly has been categorized by Oi and Matsumoto [1985]. They proposed four different potential mechanisms: congenital atresia of the foramen of Monro, morphological obstruction as space occupying lesion, patent foramen of Monro with unilateral hemispheric degenerative parenchymal changes or unbalanced intracranial compliance. In this study, we present two female sibs with unilateral congenital severe ventriculomegaly. The older sibling underwent shunt operation early in life; however no obvious decompression of the affected ventricle was present in subsequent scans. Patient 2 did not require surgical intervention. We postulate that the severe unilateral ventriculomegaly in these two sibs was attributed to developmental defect or vascular lesion of the affected hemisphere during embryogenesis rather than obstruction of foramen of Monro, based on absence of the expected marked asymmetry of the hemicalvaria with obstruction. Moreover, the lack of improvement in the ventriculomegaly following the placement of the shunt suggests brain dysplasia or early vascular insult rather than obstruction [Tsao et al., 1996].
Based on several published studies, congenital unilateral ventriculomegaly has a lower risk of mortality and morbidity compared with bilateral hydrocephalus, with only few cases having associated central nervous system malformations or other system anomalies [Senat et al., 1999; Schulman et al., 2000; Durfee et al., 2001; Gaglioti et al., 2005; Ouahba et al., 2006]. Extracranial malformations were not observed in our patients. Patient 1 had focal polymicrogyria (PMG) that surprisingly affected the posterior opercular region in the normal ventricular sized hemisphere and a large retrocerebellar cyst, again suggesting a developmental origin of the defects. The etiology of the PMG on the side opposite of the ventriculomegaly could be primarily related to the developmental defect. Robin et al. [2006] suggested that del 22q11 is a relatively common cause of perisylvian PMG, especially when present asymmetrically. This patient had normal chromosome 22 based upon FISH analysis. Nevertheless, authors hypothesized an intriguing hypothesis that asymmetric PMG may be a sequelae of abnormal hypoperfusion of the embryonic brain due to haploinsufficiency of a gene acting extrinsically, perhaps affecting the vasculature or growth factors. Alternatively, this PMG could be a result of a “bystander” effect, similar to the finding that an early injury to one cerebral hemisphere can lead to abnormal sulcation in the contralateral hemisphere [Goldman-Rakic, 1980]. The corpus callosum was well formed in these patients, but appeared thin due to the severity of white matter injury in the affected hemisphere.
Prognosis of unilateral ventriculomegaly is uncertain. However, it depends on the severity of dilatation, therapeutic options as well as absence of associated anomalies [Benacerraf, 2001; Sadan et al., 2007]. Patients with mild unilateral ventriculomegaly have favorable outcome with adequate cognitive function and normal to mild physical impairment [Patten et al., 1991]. Mild to moderate developmental delay, squint strabismus, hemianopia, hemiparesis, mild speech difficulties and learning problems have been ascertained in the few reports that assessed developmental outcome of unilateral ventriculomegaly [Patten et al., 1991; Tsao et al., 1996; Durfee et al., 2001; Benacerraf, 2001; Sadan et al., 2007]. Both patients had mild developmental delay, hemiparesis contralateral to the involved hemisphere, and mild intellectual impairment. Patient 1 experienced focal seizures that were probably attributed to the presence of polymicrogyria, although seizures were reported in few patients with isolated unilateral hydrocephaly [Wilberger et al., 1983]. Interestingly, the present cases had very thin cerebral mantle on the affected hemisphere with low borderline and borderline intelligence in patients 1 and 2, respectively. This agrees with previous reports that documented poor correlation between thinning of the cortical mantle and subsequent intelligence in unilateral ventriculomegaly [Chervenak et al., 1985; Patten et al., 1991].
The pathogenesis of congenital hydrocephalus appears to be more complicated than mere disorder of CSF circulation, and is not yet well understood. Growing evidence indicates that genetic factors play a major role in the pathogenesis of hydrocephalus. It is proposed, that it may develop at a specific embryonic time period of neural stem proliferation and differentiation in the brain caused by abnormal cellular signal and functioning through cytokines, growth factors or related molecules produced by hydrocephalic gene products [Zhang et al., 2006]. The present patients had hydrocephalus ex vacuo secondary to parenchymal loss rather than impaired CSF reabsorption, as seen in the more common cases of pediatric hydrocephaly. We suggested local vascular insults as possible explanation for severe ventriculomegaly in both sibs, as well as the unilateral polymicrogyria in the contralateral hemisphere in the older affected. However, the presence of normal parental hematological profile and absence of detectable autoanti-bodies in maternal serum argues against parental vascular or alloimmune disorders in the etiology of this condition. Pilu and Hobbins [2002] stressed on the importance of TORCH screening when ventriculomegaly was diagnosed prenatally. Congenital infections seem unlikely in both sibs as the mother had serial normal TORCH results. Gaglioti et al. [2005] mentioned that the rate of chromosomal anomalies in isolated ventriculomegaly is fairly low, and these were normal in this family. Isolated ventriculomegaly has been associated with chromosomal aneuploidy in few reports [Terry et al., 2000; Benacerraf, 2001; Ouahba et al., 2006]. Nevertheless, the presence of a closely similar picture in the sibship is highly suggestive of a genetic basis. This might point to a responsible gene(s) that may play a putative role in development of the hemisphere as well as cortical folding, possibly by determining vascular supply, as has been suggested [Vahedi et al., 2003]. Recently, mutations of collagen IV A1, a major component of vascular basement membrane, have been incriminated in autosomal dominant porencephaly [Gould et al., 2005; Breedveld et al., 2006; van der Knaap et al., 2006]. Authors clarified that COLIVA1 mutations compromise the structure integrity of the vascular basement membrane rendering vessels more susceptible to disruption especially during stress as partition leading to perinatal intracerebral hemorrhage and porencephaly. It would be interesting in the future to test this family for a mutation in the COLIVA1 gene, but we would expect it to be negative due to the differences in both neuroradiological presentation and inheritance pattern.
To our knowledge, familial congenital unilateral severe ventriculomegaly was previously reported rarely: in the family described by Muhler et al. [2008] and in association with sensorineural hearing loss in Chudley–McCullough syndrome (OMIM 604213) and with retinal arteriolar tortuosity [Vahedi et al., 2003], both of which were absent in this family. The present patients had normal hearing and fundus exams and were derived from healthy first consanguineous parents from Upper Egypt, where the consanguinity rate among couples in this area is approximately 40% [Hafez et al., 1983], suggesting recessive inheritance. In conclusion, we suggest that this family represents a form of brain dysplasia of genetic basis, most likely inherited as autosomal recessive trait.
REFERENCES
- Anderson NN, Malpas T, Davison M. Prenatal diagnosis of unilateral hydrocephalus. Pediatr Radiol. 1993;23:69–70. doi: 10.1007/BF02020232. [DOI] [PubMed] [Google Scholar]
- Benacerraf BR. Unilateral cerebral ventriculomegaly. Is one better than two? J Ultrasound Med. 2001;20:179–181. doi: 10.7863/jum.2001.20.3.179. [DOI] [PubMed] [Google Scholar]
- Breedveld G, de Coo IF, Lequin MH, Arts WFM, Heutink P, Gould DB, John SWM, Oostra B, Mancini GMS. Novel mutations in three families confirm a major role of COL4A1 in hereditary porencephaly. J Med Genet. 2006;43:490–495. doi: 10.1136/jmg.2005.035584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoza JD, Goldstein RB, Filly RA. Exclusion of fetal with a single measurement: The width of the lateral ventricular atrium. Radiology. 1988;169:711–714. doi: 10.1148/radiology.169.3.3055034. [DOI] [PubMed] [Google Scholar]
- Chervenak FA, Ment LR, McClure M, Duncan C, Hobbins JC, Scott D. Outcome of fetal ventriculomegaly. Lancet. 1984;2:179–181. doi: 10.1016/s0140-6736(84)90477-x. [DOI] [PubMed] [Google Scholar]
- Chervenak FA, Berkowitz RL, Tortora M, Hobbins JC. The management of fetal hydrocephalus. Am J Obstet Gynecol. 1985;151:933–942. doi: 10.1016/0002-9378(85)90672-6. [DOI] [PubMed] [Google Scholar]
- Chudley AE, McCullough C, McCullough DW. Bilateral sensorineural deafness and hydrocephalus due to foramen of Monro obstruction in sibs: A newly described autosomal recessive disorder. Am J Med Genet. 1997;68:350–356. doi: 10.1002/(sici)1096-8628(19970131)68:3<350::aid-ajmg19>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Scherpereel B, Baudrillart JC, Omez F, Lejeune JP, Rousseaux P, Motte J. Unilateral non-tumor hydrocephalus in children. Atresia of the foramen of Monro? Neurochirurgie. 1987;33:129–134. [PubMed] [Google Scholar]
- Durfee SM, Kim FM, Benson CB. Postnatal outcome of fetuses with the prenatal diagnosis of asymmetric hydrocephalus. Ultrasound Med. 2001;20:263–268. doi: 10.7863/jum.2001.20.3.263. [DOI] [PubMed] [Google Scholar]
- Farrell TA, Hertzberg BS, Kliewer MA, Harris L, Paine SS. Fetal lateral ventricles: Reassessment of normal values for atrial diameter at US. Radiology. 1994;193:409–411. doi: 10.1148/radiology.193.2.7972754. [DOI] [PubMed] [Google Scholar]
- Gaglioti P, Danelon D, Bontempo S, Mombro M, Cardaropoli S, Todros T. Fetal cerebral ventriculomegaly: Outcome in 176 cases. Ultrasound Obstet Gynecol. 2005;25:372–377. doi: 10.1002/uog.1857. [DOI] [PubMed] [Google Scholar]
- Gaston BM, Jones BE. Perinatal unilateral hydrocephalus. Atresia of the foramen of Monro. Pediatr Radiol. 1989;19:328–329. doi: 10.1007/BF02467306. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Morphological consequences of prenatal injury to the primate brain. Prog Brain Res. 1980;53:1–19. [PubMed] [Google Scholar]
- Gould DB, Campbell Phalan F, Breedveld GJ, van Mil SE, Smith RS, Schimenti JC, Aguglia U, van der Knaap MS, Heutink P, John SWM. Mutations in Cola1 cause perinatal cerebral hemorrhage and porencephaly. Science. 2005;308:1167–1171. doi: 10.1126/science.1109418. [DOI] [PubMed] [Google Scholar]
- Hafez M, El-Tahan H, Awadalla M, El-Khayat H, Abdel-Gafar A, Ghoneim M. Consanguineous matings in the Egyptian population. J Med Genet. 1983;20:58–60. doi: 10.1136/jmg.20.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husag L, Wieser HG, Probst C. Unilateral hydrocephalus due to membranous occlusions of the foramen of Monro. Acta Neurochir (Wien) 1976;33:183–212. doi: 10.1007/BF01886669. [DOI] [PubMed] [Google Scholar]
- Muhler MR, Rake A, Heling K-S, Klingebeil R, Chaoui R. Unilateral borderline fetal ventriculomegaly as inherited anatomical variant depicted by fetal and maternal magnetic resonance imaging. Ultrasound Obster Gynecol. 2008;31:358–360. doi: 10.1002/uog.4090. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Makiyama H, Miyagi A, Tsubokawa T, Ushinohama H. Congenital unilateral hydrocephalus. Childs Nerv Syst. 1989;5:367–370. doi: 10.1007/BF00271895. [DOI] [PubMed] [Google Scholar]
- Oi S, Matsumoto S. Pathophysiology of nonneoplastic obstruction of the foramen of Monro and progressive unilateral hydrocephalus. Neurosurgery. 1985;17:891–896. doi: 10.1227/00006123-198512000-00003. [DOI] [PubMed] [Google Scholar]
- Oi S, Yamada H, Sasaki K, Matsumoto S. Atresia of the foramen of Monro resulting in severe unilateral hydrocephalus with subfalcial herniation and infratentorial diverticulum. Neurosurgery. 1985;16:103–106. [PubMed] [Google Scholar]
- Ouahba J, Luton D, Vuillard E, Garel C, Gressens P, Blanc N, Elmaleh M, Evrard P, Oury J. Prenatal isolated mild ventriculomegaly: Outcome in 167 cases. BJOG. 2006;113:1072–1079. doi: 10.1111/j.1471-0528.2006.01050.x. [DOI] [PubMed] [Google Scholar]
- Patten RM, Mack LA, Finberg HJ. Unilateral hydrocephalus: Prenatal sonographic diagnosis. Am J Roentgenol. 1991;156:359–363. doi: 10.2214/ajr.156.2.1898814. [DOI] [PubMed] [Google Scholar]
- Pilu G, Hobbins JC. Sonography of fetal cerebrospinal anomalies. Prenat Diagn. 2002;22:321–330. doi: 10.1002/pd.310. [DOI] [PubMed] [Google Scholar]
- Robin NH, Taylor CJ, McDonald-McGinn DM, Zackai EH, Bingham P, Collins KJ, Earl D, Gill D, Granata T, Guerrini R, Katz N, Kimonis V, Lin J-P, Lynch DR, Mohammed SN, Massey RF, McDonald M, Rogers RC, Splitt M, Stevens CA, Tischkowitz MD, Stoodley N, Leventer RJ, Pilz DT, Dobyns WB. Polymicrogyria and deletion 22q11.2 syndrome: Window to the etiology of a common cortical malformation. Am J Med Genet Part A. 2006;140A:2416–2425. doi: 10.1002/ajmg.a.31443. [DOI] [PubMed] [Google Scholar]
- Romero R, Pilu G, Jeanty P, Ghindini A, Hobbins JC, editors. Prenatal diagnosis of congenital anomalies. Appleton & Lange.; East Norwalk, Connecticut: 1988. The central nervous system; pp. 1–79. [Google Scholar]
- Sadan S, Malinger G, Schweiger A, Lev D, Lerman-Sagie T. Neuropsychological outcome of children with asymmetric ventricles or unilateral mild ventriculomegaly identified in utero. BJOG. 2007;114:596–602. doi: 10.1111/j.1471-0528.2007.01301.x. [DOI] [PubMed] [Google Scholar]
- Schulman H, Landau D, Schulman P, Hertzanu Y. Congenital unilateral hydrocephalus-CT findings. Eur J Radiol. 2000;36:161–164. doi: 10.1016/s0720-048x(00)00181-9. [DOI] [PubMed] [Google Scholar]
- Senat MV, Bernard JP, Scharzler P, Britten J, Ville Y. Prenatal diagnosis and follow up of 14 cases of unilateral ventriculomegaly. Ultrasound Obstet Gynecol. 1999;14:327–332. doi: 10.1046/j.1469-0705.1999.14050327.x. [DOI] [PubMed] [Google Scholar]
- Sherer DM, Allen TA, Ghezzi F, Epstein LG. Prenatal diagnosis of moderate unilateral hydrocephalus subsequently not requiring neonatal decompression. Am J Perinatol. 1995;12:50–52. doi: 10.1055/s-2007-994400. [DOI] [PubMed] [Google Scholar]
- Terry M, Calhoun BC, Walker W, Apodaca C, Martin L, Pierce B, Hume RF, Emans MI. Aneuploidy and isolated mild ventriculomegaly. Attributable risk for isolated fetal marker. Fetal Diagn Ther. 2000;15:331–334. doi: 10.1159/000021031. [DOI] [PubMed] [Google Scholar]
- Thorndike RL, Hagen EP, Sattler JM. Guide for Administering and scoring, the Stanford-Binet Intelligence Scale. 4th Riverside Publishing; Chicago: 1986. [Google Scholar]
- Tsao PN, Teng RJ, Wu TJ, Yau KI, Wang PJ. Non progressive congenital unilateral ventriculomegaly. Pediatr Neurol. 1996;14:66–68. doi: 10.1016/0887-8994(95)00256-1. [DOI] [PubMed] [Google Scholar]
- Vahedi K, Massin P, guichard J-P, Miocque S, Polivka M, Goutieres F, Dress D, Chapon F, Ruchoux M-M, Riant F, Joutel A, Gaudric A, Bousser MG, Tournier-Lasserve E. Hereditary infantile hemiparesis, retinal arteriolar tortuosity, and leukoencephalopathy. Neurology. 2003;60:57–63. doi: 10.1212/wnl.60.1.57. [DOI] [PubMed] [Google Scholar]
- van der Knaap MS, Smit LME, Barkhof F, Pijnenburg YAL, Zweegman S, Niessen HWM, Imhof S, Heutink P. Neonatal porencephaly and adult stroke related to mutations in collagen IV A1. Ann Neurol. 2006;59:e504–511. doi: 10.1002/ana.20715. [DOI] [PubMed] [Google Scholar]
- Wilberger JE, Jr, Vertosick FT, Jr, Vries JK. Unilateral hydrocephalus secondary to congenital atresia of the foramen of Monro. Case report. J Neurosurg. 1983;59:899–901. doi: 10.3171/jns.1983.59.5.0899. [DOI] [PubMed] [Google Scholar]
- Zhang J, Williams MA, Rigamonti D. Genetics of human hydrocephalus. J Neurol. 2006;253:1255–1266. doi: 10.1007/s00415-006-0245-5. [DOI] [PMC free article] [PubMed] [Google Scholar]