Abstract
The effects of evolutionary pressure on human immunodeficiency virus-1 (HIV) have resulted in a variety of clades and recombinants. The functional implications of HIV clades on disease onset and progression of HIV-associated neurocognitive disorders (HAND) have been suggested by clinical and basic science studies, which will be reviewed in detail. Some clinical studies suggest that patients infected with clade D show the greatest propensity for developing HIV-associated dementia (HAD) followed by clades B, C, and A respectively. However, there are conflicting reports. This review summarizes clinical studies that have assessed behavioral abnormalities and HIV clade type in HAND patients, focusing on the clades stated above. The limitations include variations in testing used to define the cohorts, patient sample size, lack of HIV clade characterization, combination antiretroviral therapy (cART) availability, and other factors, which are highlighted and compared between clinical studies performed primarily in Africa and India. Basic science studies provide substantial evidence that HIV clade differences can result in varying degrees of neuropathology and are also reviewed in some detail. These studies indicate that there are a number of clade differences, most notably in Tat, that result in different degrees of neurovirulence or neuropathological effects in vitro and in a mouse model of HAND. In order to confirm the hypothesis that HIV clade differences are important determinants of HAND pathogenesis, larger, longitudinal studies that employ standard definitions of HAND and HIV clade testing must be performed. In a larger sense, HAND continues to be highly prevalent despite the advent of cART and, therefore, further studies into HAND pathogenesis are critical to develop better therapies.
Keywords: HIV encephalitis, encephalitis, HIV clades, HIV associated neurocognitive disorders
Introduction
Despite the development of effective antiretroviral therapies (ART) HIV-1 infection remains a significant worldwide problem. It is estimated that there are over 34 million persons living with HIV (PLHIV) and as many as half of these individuals will develop HIV associated neurocognitive disorders (HAND), with or without ART (Liner, Ro et al. 2010). While combined ART (cART) has reduced the incidence of HIV-1 associated dementia (HAD), milder forms of HAND such Asymptomatic Cognitive Impairment (ACI) and Mild Neurocognitive Disorder (MND) remain highly prevalent. Importantly, HAND is probably the most common cause of cognitive dysfunction in young adults in the world (Sacktor, Nakasujja et al. 2007). Other than cART, specific therapies for HAND have not been developed. Therefore it is paramount to gain a better understanding of the pathogenesis of HAND so that better treatments can be devised (Tyor 2009). HIV-2 appears to be less virulent compared to HIV-1 and neurocognitive dysfunction maybe less common, but has not been well studied in this population (Choi, Townend et al. 2011). Therefore this discussion will be limited to HAND caused by HIV-1 (HIV).
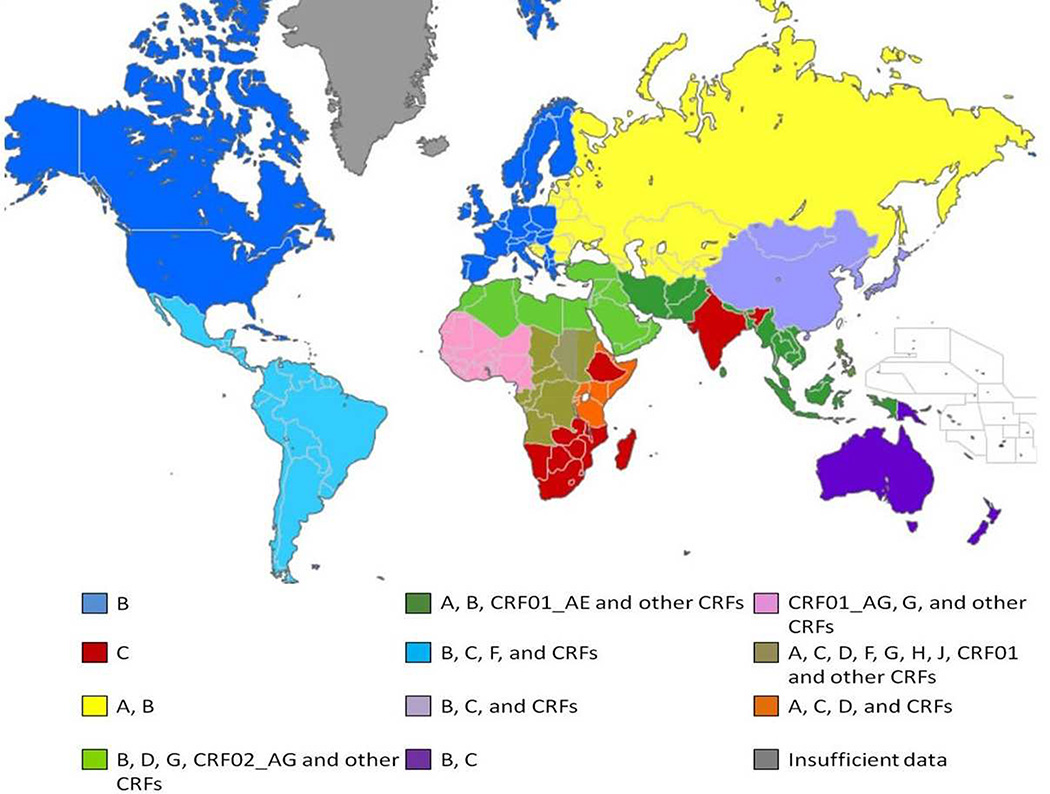
One aspect of HAND pathogenesis that has received recent attention is the possible effect of HIV clade or subtype on the development and/or course of HAND (McArthur, Steiner et al. 2010). Most infections are caused by group M and within this group there are 9 clades. By far, the predominant clade in North America and Europe is B, whereas worldwide it is C (Fig 1). Clade C is particularly prevalent in sub-Saharan Africa and India, where there is a large number of PLHIV (Hemelaar, Gouws et al. 2006). A, D, and F are also common clades seen in Africa. This review focuses on clades A, B, C and D, where there is the most published information on their relationship to HAND.
Figure 1. Geographical distribution of HIV-1 subtypes and recombinants 2004–2007.
The map represents an overview of the distribution of HIV-1 subtypes based on data adapted from Hemelaar et. al. AIDS 2007 (Table 2b). Clade C continues to be the predominant clade found in HIV-infected individuals. Clade B is primarily found in North America and Europe and is the focus of the majority of research. The most diverse HIV-1 subtypes are found in Central Africa. Circulating recombinant forms (CRF) and unique recombinant forms (URF) are increasing in prevalence. The variety of colors in the map reflects the growing complexity and diversity in the HIV-1 and HAND epidemic.
Clinical Studies
Studies in Africa and India have suggested that HIV-1 clade differences lead to disparate frequencies of HAND. Wong et al. recently found that 31% of HIV+ patients in Kampala, Uganda met criteria for HAD (Wong, Robertson et al. 2007). The authors compared their findings to pre-cART rates in the US, which were similar (McArthur, Hoover et al. 1993), although in distinction to pre-cART US rates 28% of the Ugandan PLHIV were on or had been on ART. In addition, an older study of sub-Saharan PLHIV found only 5.9% to 6.9% with HAD (Maj, Satz et al. 1994). The reasons for these discrepancies may have been related to recruitment differences, but largely appear unexplained. An additional 47% in the more recent Ugandan study had MND (Sacktor, Nakasujja et al. 2007), suggesting that approximately 80% of PLHIV in this area have HAND. The Ugandan studies used excellent methods for determining cognitive impairment, though, both the older sub-Saharan study (Maj, Satz et al. 1994) and the more recent Ugandan studies suffer in that they do not provide a more widely based and representative population analysis. Nevertheless, a follow-up study in a subset of this cohort reported HIV clade determination in 60 subjects (Sacktor, Nakasujja et al. 2009). Of the 9 clade D infected individuals, 8 had HAD, compared with 7 of 33 clade A infected individuals with HAD. These results suggest that HAD is more common in clade D infected than in clade A infected PLHIV. However, the numbers of subjects are low, the area sampled is limited, and clearly the results need to be replicated in a larger study. In fact, somewhat conflicting results have been reported for clade A and D influences on HAND development. Boivin et al. studied 54 Ugandan children who were HIV+, not on ART, using cognitive tests and determining their HIV-1 clade status (Boivin, Ruel et al. 2010). Children with clade A performed more poorly than those with clade D, especially on memory and learning tasks. The Boivin study also potentially suffers from the same flaws as the study performed in adults. Nevertheless, the different findings of the two studies could reflect a fundamental distinction between adult and pediatric populations in how clades affect these populations with respect to the development of HAND. Boivin et al. endorse this possibility and also suggest the differences in results could be due to the relatively advanced disease state of the adults compared to the children.
The studies above focused on clades A and D. The association of HAND with HIV-1 clade C infection has also been investigated. In Ethiopia where clade C is predominant 73 PLHIV and 87 HIV negative individuals were investigated in the context of an ongoing community based longitudinal study (Clifford, Mitike et al. 2007). These patients were less immunosuppressed with less functional impairment than those in the Ugandan studies reported above. They underwent neuropsychological testing, among other assessments, which showed only poor finger tapping in PLHIV versus controls. The results of this cross-sectional study suggested low prevalence of HAND in this population. A strength was that testing in PLHIV was compared with age and education matched controls recruited locally. However, there are possible ascertainment biases inherent in all of the studies discussed thus far. For example, PLHIV with more advanced disease may not have been able to be recruited. Another weakness in the Ethiopian study is that clade was not determined and assumptions were therefore made about clade C positivity. Nevertheless, clade C HIV-1 is known to be highly prevalent in Ethiopia.
Clade C is also known to prevalent in Zambia. Holguin et al. recruited 83 HIV+ and 57 negative individuals from collaborating physicians as well as various community services (Holguin, Banda et al. 2011). Of the PLHIV, 35% were on ART. In addition to standard neurological and psychological assessments, a brief neuropsychological test battery including the International HIV Dementia Scale (IHDS) (Sacktor, Wong et al. 2005) was administered. Of the 54 PLHIV not taking ART, 22% had “neurocognitive impairment.” Lawler et al. studied HAND in 120 randomly selected PLHIV in Botswana, another southern Africa country also with a purported clade C predominance (Lawler, Mosepele et al. 2010). In addition to the IHDS, a limited neuropsychological test battery was administered. Almost all of these patients were taking cART and 38% met criteria for “neurocognitive impairment.” In the Zambia study recruitment was somewhat selective and could have biased the outcome, although one might think it would bias it in favor of higher prevalence of HAND. In the Botswana study there was no HIV control group. Both studies do not define “neurocognitive impairment” in the context of the HAND defined categories mentioned above. The reason for the divergence of frequencies of neurocognitive impairment between the studies is unclear. It is particularly confusing when one considers that most of the Botswana PLHIV were taking ART. This discrepancy highlights the possibility that relatively minor differences, at face value, in study design and methods used may result in significant differences in estimating the prevalence of HAND. In South Africa clade C accounts for approximately 90% of HIV-1 infections (Robertson, Liner et al. 2010). Joska et al. have studied HAND in Cape Town, although, as with most of the studies, they did not determine clade status (Joska, Fincham et al. 2010, Joska, Westgarth-Taylor et al. 2011). In their initial study, HAND was defined by using the HIV dementia scale, a brief screening tool (Power, Selnes et al. 1995). While the HIV dementia scale probably detects HAD well, it is relatively insensitive to the more common forms of HAND, ACI and MND, that are defined by neuropsychological testing (Joska, Westgarth-Taylor et al. 2011). In over 500 PLHIV attending HIV clinics 23.5% were cognitively impaired (Joska, Fincham et al. 2010). In an attempt to better define this population Joska et al studied 283 (170 completed the full assessment) PLHIV (Joska, Westgarth-Taylor et al. 2011). Although ascertainment of this cohort was not completely defined, the investigators used a neuropsychological test battery to characterize these PLHIV and importantly they were compared to an HIV-negative (n=51) group. MND was prevalent in 42.4% and HAD in 25.4%. Heaps et al. examined brain MRI features of 28 PLHIV (23 HIV negative) (Heaps, Joska et al. 2012). They found lower volumes in white matter, thalamus and total gray matter in PLHIV compared to HIV negative controls, thus lending support to the concept that clade C causes neuronal damage and HAND.
Royal et al. recently reported a study examining HAND prevalence and HIV clade status in Nigeria (Royal, Cherner et al. 2012). Sixty PLHIV, who were ART naïve and not overtly demented, and 56 HIV-negative were recruited from several local hospitals/clinics. Testing included the IHDS and a brief neuropsychological battery. In general the HIV+ group performed more poorly on the IHDS than controls. In a smaller subset of patients who were given a larger battery of neuropsychological tests the HIV+ group performed worse on action fluency and recall. Of seven PLHIV who had clade status determined, 3 had neurocognitive impairment and none had clade types emphasized in this review. The overall frequency of HAND in Nigeria could not be determined from this study.
In most of these African studies, HAND is highly prevalent. However, there is significant disagreement between studies, which to some degree obscures the potential role of clade status in the development and course of HAND. The basis for the discrepancies between the prevalence of HAND in largely clade C populations in Ethiopia, Zambia, Botswana, and South Africa remains unexplained, but ascertainment issues, which have been discussed above, or other undefined factors such as actual clade status, methods of defining HAND and undetected confounding disease risk factors could be important. Rao et al. recently reported that in southern Africa countries clade C isolates appear to retain the Tat dicysteine motif (CC) commonly found in clade B isolates (Rao, Neogi et al. 2013). The importance of this Tat motif to the development of HAND is explained in more detail below. The finding of clade C HIV in South Africa with a Tat motif, which purportedly is associated with greater likelihood of HAD, may, at least in part, explain the possible, relatively high prevalence of HAD (25%) in South Africa (14).
Aside from African studies examining clade C in relation to HAND, India has also been a source of investigations since most PLHIV there have clade C. Gupta et al. studied 119 adults in South India who were infected with clade C and not on ART (Gupta, Satishchandra et al. 2007). They used neuropsychological testing in 119 PLHIV that was compared to 126 HIV-negative, healthy, age, gender and sex matched subjects. Of the HIV+ individuals, 60.5% had mild to moderate cognitive deficits, but none of them had HAD despite 16.4% with CD4 counts < 200. The percentage of PLHIV with HAND is comparable to the 56% found in a previous study of clade C infected individuals in India (Yepthomi, Paul et al. 2006). In both clade C studies the percentage of PLHIV with HAND is similar to rates in the United States (Sacktor, Skolasky et al. 2007). However, one must keep in mind that this South India cohort was not receiving ART and there were no patients with HAD. Previous studies in India suggested that the percentage of PLHIV with HAD was 2 to 4% (Satishchandra, Nalini et al. 2000, Wadia, Pujari et al. 2001). Satishchandra et al. evaluated 100 PLHIV in Bangalore, India referred for neurological problems from 1989 through 1996 (Satishchandra, Nalini et al. 2000). Most of these patients (80) had opportunistic infections (eg, cryptococcal meningitis). Four patients “had features of cortical dementia.” However, two of these patients had cryptococcal and tuberculous meningitis, although it was felt that these opportunistic infections did not account for their dementia. In this study it seems more likely that ascertainment bias (patients were referred for neurological evaluation) would actually have resulted in a higher estimated prevalence of dementia. At any rate, the study by Satishchandra et al. does not represent a true overall prevalence of HAND (or really HAD) in South India PLHIV, because the referrals were strictly for neurological reasons and cannot take into account the entire number of PLHIV in that area. Wadia et al. studied 1527 consecutive PLHIV in Pune, India (Wadia, Pujari et al. 2001) . HAD was evaluated with standard neurological examination and minimental exam along with CT scans and CSF analysis as needed. There were 21 patients with HAD (1.4%), although another group of 26 (1.7%) was only described as “cognitive loss with impaired consciousness.” This study would seem less likely to suffer from ascertainment bias, but still represents a single site referral source for PLHIV within India. So neither of these earlier studies in India represent a broad epidemiological spectrum within India and current standards for HAND were, of course, not used. Nevertheless they suggest rough earlier estimates of HAD occurrence within India where clade C is prevalent. Taken together, these studies suggest that while a significant proportion of PLHIV in India have HAND, only a very small percentage have HAD. This percentage of HAD is far less than was seen in the US (clade B) during the pre-cART era (McArthur, Steiner et al. 2010). To summarize, the data from India suggest that mild forms of HAND are comparable between clade C and clade B individuals, but that HAD is more prevalent in clade B HIV-1 infection.
The clinical studies described above indicate that overall, HAND is prevalent in Africa and India; and recent studies suggest that HAND is prevalent in China and other countries in the Asian Pacific region as well (Zhang, Qiao et al. 2012). In general, there is a high prevalence of clade C in these areas, although clades A and D are present to the extent that it is conceivable that clade effects on the development of HAND could be estimated. The hypothesis has been that HAND severity conforms, more or less, to D>B>C>A. In Europe and the United States, in general, clade status differs from the countries highlighted above as does access to ART. What can be concluded at this point is that all recent clinical assessments of PLHIV indicate that HAND prevalence is high throughout the world, regardless of whether patients are receiving ART. The issue as to whether clade status affects the prevalence of HAND is less clear. Some studies suggest clade status is important while others do not appear to support this possibility. One might expect that if the effect were significant then there should at least be strong and consistent trends in favor of the hypothesis stated above (D>B>C>A). However, the problems discussed above with the various studies should not be taken lightly and could have resulted in the discrepancies between some of these studies. In addition and importantly, these studies were not designed to examine the rate of progression of HAND. The question remains completely open as to whether clade status determines how soon one develops HAND after infection and the rate of progression from ACI to MND to HAD. In fact, many of the studies outlined above do not even make a distinction between these subclasses of HAND. It is entirely possible that while all clades result in a prevalence of roughly 50% HAND in PLHIV, the specific prevalence of HAD could be quite different from clade to clade and could favor the D>B>C>A scenario. Basic science investigations into the affects of clades on the development of neurodegenerative findings are much more suggestive of such a picture.
Basic Science Studies
Because of reports that HAD may be less prevalent in India (Satishchandra, Nalini et al. 2000, Wadia, Pujari et al. 2001), where clade C predominates, Ranga et al. investigated the monocyte chemoattractant capability of naturally occurring clade C Tat compared to isogenic variants (Ranga, Shankarappa et al. 2004). The hypothesis included the possibility that if clade C Tat has a defective ability to act as a chemokine for monocytes, one explanation for the decreased prevalence of HAD in India could be that clade C induces less monocyte invasion of the CNS. Therefore, less HIV-infected monocytes would gain access to the CNS and since mononuclear phagocytes are believed to be the main cell type involved in HAND pathogenesis, there would be less HAD with Clade C infection. Analyses were undertaken to identify conserved Tat sequences through published sequences for various HIV clades. C31S was selected because of its highly conserved dicysteine motif and known effects on biological functions of Tat. The CS motif of Tat at positions 30 and 31 was then studied by generating mutants which encoded CC and SC variants (ie, isotypic variants). CS and CC Tats maintained their transactivating properties, while the SC variant did not. An in vitro chemotaxis system was used to determine the ability of these Tat variants to influence monocyte migration. The CC Tat (typical clade B motif) elicited migration of monocytes; however, the variants (CS or SC) did not. These results suggested that clade C Tat is deficient in its capacity to stimulate monocyte chemotaxis.
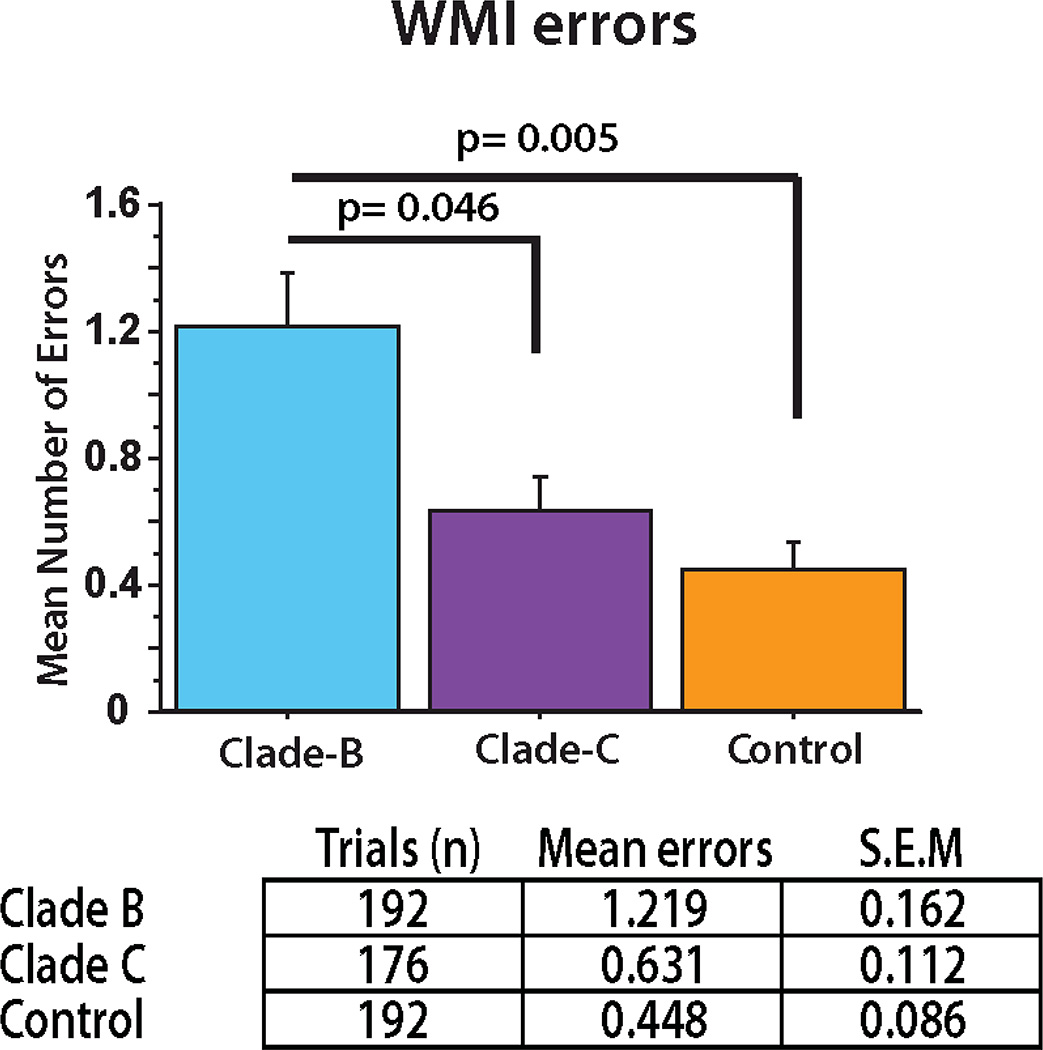
More extensive studies were undertaken to investigate the potential relationship between clade type and HAND (Rao, Sas et al. 2008). A mouse model of HAND was used to test whether there were differences between clade B (HIV-ADA) and clade C (HIV-1-IndieC) HIV. Using water radial arm maze (WRAM) testing, mice injected intracranially with human monocytes infected with clade C HIV made significantly fewer memory errors than mice injected with human monocytes infected with clade B HIV (Figure 2). In addition, the clade B mouse brain had greater astrogliosis than clade C brains and the clade B mice also had more neuronal damage, as measured by microtubule associated protein (MAP) 2 immunostaining of neurons in brain tissue sections. These findings corroborated the behavioral abnormalities and suggested that astrogliosis may play a role in addition to clade type. In vitro studies, using a monocyte migration assay, showed that clade C-infected macrophages recruited monocytes less well than clade B-infected macrophages. This recruitment was Tat and CCL2 dependent, again suggesting like Ranga et al. that clade related differences in Tat influence monocyte migration into the brain and affect the severity of HAND (Ranga, Shankarappa et al. 2004).
Figure 2. Cognitive performance by HAND mice differs based on HIV clade infection.
HAND mice infected with clade B (HIV-ADA) made significantly more WMI errors (a form of long-term memory) than HAND mice infected with clade C (HIV-IndieC) in a water radial arm maze test (p=0.046). (Rao, Sas et al. 2008). Cognitive performance was based on a series of trials (1–4) in the maze across days (9–12).
Simultaneously, Li et al. showed that recombinant clade B Tat was more toxic to primary rat hippocampal neurons than recombinant clade C Tat (Li, Huang et al. 2008); however, there was no difference in the ability of these proteins to activate HIV-LTR. This effect was dose dependent. They further demonstrated that Tat binds to the NMDA receptor to cause neurotoxicity. HEK293 NMDA receptor transfected cells showed equal toxicity to NMDA or clade B Tat. The binding of Tat to the NMDA receptor was not affected by the cys-cys motif at positions 30 and 31. They determined that binding of Tat to the NMDA receptor involves a conserved arg rich region of Tat. Two patients with HIV Tat expressing the CC motif (clade B) were compared by neurotoxicity assay to a patient with a CS Tat motif (clade C). As expected the clade B Tat demonstrated significantly greater neurotoxicty. Therefore, while binding of clade B Tat to the NMDA receptor is not affected by the above described changes at positions 30 and 31, neurotoxicity is affected by these changes through cys-cys interactions between the NR-1 subunit (cys 744) and cys at position 31 on Tat. These cys-cys linkages between Tat and NR1 subunit help activate the NMDA receptor. These differential neurotoxic effects of clade B Tat versus clade C were corroborated by Campbell et al. (Campbell, Watkins et al. 2011). They also showed that clade B Tat showed greater neurotoxcity than clade C Tat in rat hippocampal neurons. Moreover, this was NMDA receptor mediated. The findings of these studies showing greater direct neurotoxicity of clade B Tat compared to clade C Tat parallel the Ranga study outlined above (Ranga, Shankarappa et al. 2004) that showed the dependence of clade B Tat on the CC motif to modulate chemokine effects on mononuclear phagocytes. Taken together, these studies suggest a dual clade specific Tat effect on HAND related pathogenesis, influencing both migration of mononuclear phagocytes into the brain and direct neurotoxicity. Our more recent studies described below add to the evidence that position 31 of Tat is critical in conveying clade specific Tat effects in the pathogenesis of HAND (Rao, Neogi et al. 2013).
Several other in vitro studies have demonstrated clade specific differences. Clade B and C Tat were investigated using human fetal CNS progenitor cells comprised of astrocytes and neurons (Mishra, Vetrivel et al. 2008). Neuronal cell death, chemokine secretion and oxidative stress were measured after treatment of the human neurons and astrocytes with either clade C Tat (CS – see above), clade B Tat (CC) or isogenic mutants of Tat (CC and SC). Consistent with Rao et al. in vitro exposure of cells to clade B Tat resulted in greater CCL2 production, a monocyte chemokine, than clade C Tat exposure (Mishra, Vetrivel et al. 2008, Rao, Sas et al. 2008). These results suggest that clade B Tat compared to clade C induces chemotaxis of monocytes by direct and indirect mechanisms. Clade B Tat exposure also produced significantly more neuronal apoptosis and less cell viability (MTT assay) than clade C Tat. Levels of reactive oxygen species were increased in clade B Tat exposed neurons and astrocytes compared to clade C. These data, when extrapolated to the Rao et al. study, suggest that the behavioral abnormalities and decreased neuronal staining seen in clade B infected mice compared to clade C might be related to increased neuronal death that is at least in part explained by the increased presence of reactive oxygen species (Rao, Sas et al. 2008).
Other effects that could affect HAND pathogenesis and appear to be clade specific have also been reported. Primary human monocytes treated with clade B Tat [compared to clade C Tat] show significant increases of IL-6 and TNFα, cytokines that are proinflammatory and could adversely affect HAND pathogenesis (Gandhi, Saiyed et al. 2009). In addition, clade C Tat appears to upregulate IL-4 and IL-10 compared to clade B Tat. These “anti-inflammatory” cytokines might ameliorate brain inflammation during HIV infection and therefore make it less likely that HAND would develop. Gandhi et al. also showed that clade B Tat is more disruptive to membrane integrity than clade C Tat in an in vitro blood brain barrier (BBB) model (Gandhi, Saiyed et al. 2010). By disrupting the BBB clade B HIV may allow more HIV and inflammatory mediators to enter the brain, perhaps encouraging the earlier onset of HAND or more severe manifestations (ie, HAD). Samikkannu et al. demonstrated that clade B gp120 compared to clade C gp120 increases prostaglandin E2 and thromboxane A2 receptor in primary human astrocyte cultures (Samikkannu, Agudelo et al. 2011). Arachidonic acid metabolites have been shown to be increased in HAND and have been postulated to play a role in the development of cognitive dysfunction (Genis, Jett et al. 1992). However, it was also shown that clade B gp120 compared to clade C gp120 downregulates NMDA receptor (Samikkannu, Agudelo et al. 2011), which might lesson NMDA receptor mediated neurotoxic events that could lead to worsening HIVE.
Very recently, Rao et al. showed that SCID mice injected intracranially with human monocytes infected with a clade C isolate with preserved Tat dicysteine motif at positions 30 and 31 (HIV-1-1084i with clade B type Tat) display abnormalities on water radial arm maze testing that are comparable to mice injected with human monocytes infected with clade B (HIV-ADA) HIV (Rao, Neogi et al. 2013). As mentioned above, these results further support the hypothesis that the dicysteine Tat motif at positions 30 and 31 is an important determinant of the development of HAND. In vitro studies demonstrated that HIV-1-1084i, the southern Africa isolate with clade B type Tat at positions 30 and 31, induces chemotaxis similarly to clade B HIV. These in vitro findings again support the idea that some portion of clade specific effects on HAND pathogenesis are related to Tat chemokine properties and recruitment of monocytes into the brain. They may also explain why there could be discrepancies in the prevalence of HAD in India compared to South Africa. Tat mediated effects are particularly important in the era of antiretroviral therapy since currently available drugs have no effect of the production of Tat protein once the proviral DNA has been formed. Protease inhibitors act downstream of Tat production. All other antiretrovirals act in the viral lifecycle before proviral DNA is formed. Hence once viral reservoirs are established, Tat mediated effects may be the prime driver of the HIV pathophysiology.
Although other regions of HIV have not been studied in the context of clades to determine if they can influence neuropathogenesis, several studies have shown that mutations in the env, nef and LTR regions can be associated with the presence or absence of HAND. Accordingly, the nef gene acquires unique sequences in patients with HAD compared to those without (Lamers, Poon et al. 2011). Normalized nonsynonymous substitutions in the nef gene are more frequent in brain compared to lymphoid tissue (Olivieri, Agopian et al. 2010). The brain-specific nonsynonymous substitutions are in regions of functional importance resulting in efficient replication in macrophages (Olivieri, Agopian et al. 2010). Brain-derived HIV-1 V1-V2 envelope sequences from HIV demented and nondemented patients displayed significant sequence differences between clinical groups, and by phylogenetic analysis, sequences from the demented group showed clustering. Infectious recombinant viruses containing brain-derived V3 sequences from both clinical groups were macrophage-tropic, and viruses containing brain-derived V1, V2, and V3 sequences from both clinical groups spread efficiently in macrophages. In an indirect in vitro neurotoxicity assay using supernatant fluid from HIV-1-infected macrophages, recombinant viruses from demented patients induced greater neuronal death than viruses from nondemented patients. Thus, the HIV-1 envelope diversity observed in these patient groups appeared to influence the release of neurotoxic molecules from macrophages and might account in part for the variability in occurrence of dementia in AIDS patients (Power, McArthur et al. 1998).
Conclusions
Clinical studies have given conflicting results as to whether different HIV clades possess varying propensities for the development of HAND. Some studies have suggested that clade D may be most neurovirulent (Joseph et al. 2013, J NeuroVirol), followed by clades B, C and A in that order (Spira, Wainberg et al. 2003, Sacktor, Nakasujja et al. 2009). However, other studies have suggested that HAND prevalence is not affected by clade status (Maj, Satz et al. 1994, Lawler, Mosepele et al. 2010). All of these studies suffer from one or more limitations such as relatively small numbers of patients, limited area of sampling, potential referral bias, lack of genetic typing of HIV (no clade status), patient populations that may or may not receive cART or even a single agent, lack of patient characterization with detailed neuropsychological testing and/or neuroimaging, lack of a proper control population and other factors which could obfuscate findings. Importantly, many of the studies do not differentiate the less severe forms of HAND (ie, ASI and MND) from HAD or they do not attempt to identify patients with HAD. One critical possibility is that clade status affects the severity of HAND, not necessarily the total number of PLHIV with HAND. Many of the studies outlined above may have missed this important aspect of clinical epidemiology. Perhaps the most important consideration is that none of these studies were designed to detect changes over time. It is also possible that clade status determines the speed at which one progresses from cognitively normal to ASI, then to MND and finally HAD.
The basic science studies discussed above show overwhelming evidence that viral sequences can influence neuropathogenesis of HIV infection. Although clade specific studies have focused on the Tat gene, they strongly suggest that these clade differences are important in HAND pathogenesis. They indicate that differences in Tat, particularly at positions 30 and 31, are strongly associated with at least two properties that could influence the onset or severity of HAND (Rao, Neogi et al. 2013). One is the ability of Tat to act as a chemokine and attract mononuclear phagocytes. These cells are highly implicated in HAND pathogenesis (Glass, Wesselingh et al. 1993). The other Tat effect is its direct toxic effects on neurons that is mediated through the NMDA receptor (Magnuson, Knudsen et al. 1995, Nath, Psooy et al. 1996, Cheng, Nath et al. 1998, Self, Mulholland et al. 2004).
Clearly larger, longitudinal, and more detailed clinical studies are needed in order to prove or disprove these important clade implications. Until these kinds of clinical studies are published, the clade controversy will continue (Joseph, Achim et al. 2013). The potential implications of clade differences are that they may provide insight into critical elements of HAND pathogenesis. This is particularly important when one considers that the brain is affected by HIV early (Ragin, Du et al. 2012) and HAND occurs in approximately 50% of the roughly 34 million PLHIV despite cART. Clearly better therapies are needed that address important pathogenic mechanisms in HAND.
Footnotes
Conflict of Interest
The authors, Dr. William R. Tyor, Cari Fritz-French, and Dr. Avindra Nath, declare that they have no conflict of interest.
References
- Boivin MJ, Ruel TD, Boal HE, Bangirana P, Cao H, Eller LA, Charlebois E, Havlir DV, Kamya MR, Achan J, Akello C, Wong JK. HIV-subtype A is associated with poorer neuropsychological performance compared with subtype D in antiretroviral therapy-naive Ugandan children. AIDS. 2010;24(8):1163–1170. doi: 10.1097/qad.0b013e3283389dcc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell GR, Watkins JD, Loret EP, Spector SA. Differential induction of rat neuronal excitotoxic cell death by human immunodeficiency virus type 1 clade B and C tat proteins. AIDS Res Hum Retroviruses. 2011;27(6):647–654. doi: 10.1089/aid.2010.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Nath A, Knudsen B, Hochman S, Geiger JD, Ma M, Magnuson DS. Neuronal excitatory properties of human immunodeficiency virus type 1 Tat protein. Neuroscience. 1998;82(1):97–106. doi: 10.1016/s0306-4522(97)00174-7. [DOI] [PubMed] [Google Scholar]
- Choi Y, Townend J, Vincent T, Zaidi I, Sarge-Njie R, Jaye A, Clifford DB. Neurologic manifestations of human immunodeficiency virus-2: dementia, myelopathy, and neuropathy in West Africa. J Neurovirol. 2011;17(2):166–175. doi: 10.1007/s13365-011-0022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford DB, Mitike MT, Mekonnen Y, Zhang J, Zenebe G, Melaku Z, Zewde A, Gessesse N, Wolday D, Messele T, Teshome M, Evans S. Neurological evaluation of untreated human immunodeficiency virus infected adults in Ethiopia. J Neurovirol. 2007;13(1):67–72. doi: 10.1080/13550280601169837. [DOI] [PubMed] [Google Scholar]
- Gandhi N, Saiyed Z, Thangavel S, Rodriguez J, Rao KV, Nair MP. Differential effects of HIV type 1 clade B and clade C Tat protein on expression of proinflammatory and antiinflammatory cytokines by primary monocytes. AIDS Res Hum Retroviruses. 2009;25(7):691–699. doi: 10.1089/aid.2008.0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi N, Saiyed ZM, Napuri J, Samikkannu T, Reddy PV, Agudelo M, Khatavkar P, Saxena SK, Nair MP. Interactive role of human immunodeficiency virus type 1 (HIV-1) clade-specific Tat protein and cocaine in blood-brain barrier dysfunction: implications for HIV-1-associated neurocognitive disorder. J Neurovirol. 2010;16(4):294–305. doi: 10.3109/13550284.2010.499891. [DOI] [PubMed] [Google Scholar]
- Genis P, Jett M, Bernton EW, Boyle T, Gelbard HA, Dzenko K, Keane RW, Resnick L, Mizrachi Y, Volsky DJ, et al. Cytokines and arachidonic metabolites produced during human immunodeficiency virus (HIV)-infected macrophage-astroglia interactions: implications for the neuropathogenesis of HIV disease. J Exp Med. 1992;176(6):1703–1718. doi: 10.1084/jem.176.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass JD, Wesselingh SL, Selnes OA, McArthur JC. Clinical-neuropathologic correlation in HIV-associated dementia. Neurology. 1993;43(11):2230–2237. doi: 10.1212/wnl.43.11.2230. [DOI] [PubMed] [Google Scholar]
- Gupta JD, Satishchandra P, Gopukumar K, Wilkie F, Waldrop-Valverde D, Ellis R, Ownby R, Subbakrishna DK, Desai A, Kamat A, Ravi V, Rao BS, Satish KS, Kumar M. Neuropsychological deficits in human immunodeficiency virus type 1 clade C-seropositive adults from South India. J Neurovirol. 2007;13(3):195–202. doi: 10.1080/13550280701258407. [DOI] [PubMed] [Google Scholar]
- Heaps JM, Joska J, Hoare J, Ortega M, Agrawal A, Seedat S, Ances BM, Stein DJ, Paul R. Neuroimaging markers of human immunodeficiency virus infection in South Africa. J Neurovirol. 2012;18(3):151–156. doi: 10.1007/s13365-012-0090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemelaar J, Gouws E, Ghys PD, Osmanov S. Global and regional distribution of HIV-1 genetic subtypes and recombinants in 2004. AIDS. 2006;20(16):W13–W23. doi: 10.1097/01.aids.0000247564.73009.bc. [DOI] [PubMed] [Google Scholar]
- Holguin A, Banda M, Willen EJ, Malama C, Chiyenu KO, Mudenda VC, Wood C. HIV-1 effects on neuropsychological performance in a resource-limited country, Zambia. AIDS Behav. 2011;15(8):1895–1901. doi: 10.1007/s10461-011-9988-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph J, Achim CL, Boivin MJ, Brew BJ, Clifford DB, Colosi DA, Ellis RJ, Heaton RK, Gallo-Diop A, Grant I, Kanmogne GD, Kumar M, Letendre S, Marcotte TD, Nath A, Pardo CA, Paul RH, Pulliam L, Robertson K, Royal W, 3rd, Sacktor N, Sithinamsuwan P, Smith DM, Valcour V, Wigdahl B, Wood C. Global NeuroAIDS Roundtable. J Neurovirol. 2013;19(1):1–9. doi: 10.1007/s13365-013-0211-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joska JA, Fincham DS, Stein DJ, Paul RH, Seedat S. Clinical correlates of HIV-associated neurocognitive disorders in South Africa. AIDS Behav. 2010;14(2):371–378. doi: 10.1007/s10461-009-9538-x. [DOI] [PubMed] [Google Scholar]
- Joska JA, Westgarth-Taylor J, Hoare J, Thomas KG, Paul R, Myer L, Stein DJ. Validity of the International HIV Dementia Scale in South Africa. AIDS Patient Care STDS. 2011;25(2):95–101. doi: 10.1089/apc.2010.0292. [DOI] [PubMed] [Google Scholar]
- Joska JA, Westgarth-Taylor J, Myer L, Hoare J, Thomas KG, Combrinck M, Paul RH, Stein DJ, Flisher AJ. Characterization of HIV-Associated Neurocognitive Disorders among individuals starting antiretroviral therapy in South Africa. AIDS Behav. 2011;15(6):1197–1203. doi: 10.1007/s10461-010-9744-6. [DOI] [PubMed] [Google Scholar]
- Lamers SL, Poon AF, McGrath MS. HIV-1 nef protein structures associated with brain infection and dementia pathogenesis. PLoS One. 2011;6(2):e16659. doi: 10.1371/journal.pone.0016659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler K, Mosepele M, Ratcliffe S, Seloilwe E, Steele K, Nthobatsang R, Steenhoff A. Neurocognitive impairment among HIV-positive individuals in Botswana: a pilot study. J Int AIDS Soc. 2010;13:15. doi: 10.1186/1758-2652-13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Huang Y, Reid R, Steiner J, Malpica-Llanos T, Darden TA, Shankar SK, Mahadevan A, Satishchandra P, Nath A. NMDA receptor activation by HIV-Tat protein is clade dependent. J Neurosci. 2008;28(47):12190–12198. doi: 10.1523/JNEUROSCI.3019-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liner KJ, 2nd, Ro MJ, Robertson KR. HIV, antiretroviral therapies, and the brain. Curr HIV/AIDS Rep. 2010;7(2):85–91. doi: 10.1007/s11904-010-0042-8. [DOI] [PubMed] [Google Scholar]
- Magnuson DS, Knudsen BE, Geiger JD, Brownstone RM, Nath A. Human immunodeficiency virus type 1 tat activates non-N-methyl-D-aspartate excitatory amino acid receptors and causes neurotoxicity. Ann Neurol. 1995;37(3):373–380. doi: 10.1002/ana.410370314. [DOI] [PubMed] [Google Scholar]
- Maj M, Satz P, Janssen R, Zaudig M, Starace F, D'Elia L, Sughondhabirom B, Mussa M, Naber D, Ndetei D, et al. WHO Neuropsychiatric AIDS study, cross-sectional phase II. Neuropsychological and neurological findings. Arch Gen Psychiatry. 1994;51(1):51–61. doi: 10.1001/archpsyc.1994.03950010051007. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Hoover DR, Bacellar H, Miller EN, Cohen BA, Becker JT, Graham NM, McArthur JH, Selnes OA, Jacobson LP, et al. Dementia in AIDS patients: incidence and risk factors. Multicenter AIDS Cohort Study. Neurology. 1993;43(11):2245–2252. doi: 10.1212/wnl.43.11.2245. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Ann Neurol. 2010;67(6):699–714. doi: 10.1002/ana.22053. [DOI] [PubMed] [Google Scholar]
- Mishra M, Vetrivel S, Siddappa NB, Ranga U, Seth P. Clade-specific differences in neurotoxicity of human immunodeficiency virus-1 B and C Tat of human neurons: significance of dicysteine C30C31 motif. Ann Neurol. 2008;63(3):366–376. doi: 10.1002/ana.21292. [DOI] [PubMed] [Google Scholar]
- Nath A, Psooy K, Martin C, Knudsen B, Magnuson DS, Haughey N, Geiger JD. Identification of a human immunodeficiency virus type 1 Tat epitope that is neuroexcitatory and neurotoxic. J Virol. 1996;70(3):1475–1480. doi: 10.1128/jvi.70.3.1475-1480.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivieri KC, Agopian KA, Mukerji J, Gabuzda D. Evidence for adaptive evolution at the divergence between lymphoid and brain HIV-1 nef genes. AIDS Res Hum Retroviruses. 2010;26(4):495–500. doi: 10.1089/aid.2009.0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power C, McArthur JC, Nath A, Wehrly K, Mayne M, Nishio J, Langelier T, Johnson RT, Chesebro B. Neuronal death induced by brain-derived human immunodeficiency virus type 1 envelope genes differs between demented and nondemented AIDS patients. J Virol. 1998;72(11):9045–9053. doi: 10.1128/jvi.72.11.9045-9053.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power C, Selnes OA, Grim JA, McArthur JC. HIV Dementia Scale: a rapid screening test. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8(3):273–278. doi: 10.1097/00042560-199503010-00008. [DOI] [PubMed] [Google Scholar]
- Ragin AB, Du H, Ochs R, Wu Y, Sammet CL, Shoukry A, Epstein LG. Structural brain alterations can be detected early in HIV infection. Neurology. 2012;79(24):2328–2334. doi: 10.1212/WNL.0b013e318278b5b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranga U, Shankarappa R, Siddappa NB, Ramakrishna L, Nagendran R, Mahalingam M, Mahadevan A, Jayasuryan N, Satishchandra P, Shankar SK, Prasad VR. Tat protein of human immunodeficiency virus type 1 subtype C strains is a defective chemokine. J Virol. 2004;78(5):2586–2590. doi: 10.1128/JVI.78.5.2586-2590.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VR, Neogi U, Talboom JS, Padilla L, Rahman M, Fritz-French C, Gonzalez-Ramirez S, Verma A, Wood C, Ruprecht RM, Ranga U, Azim T, Joska J, Eugenin E, Shet A, Bimonte-Nelson H, Tyor WR, Prasad VR. Clade C HIV-1 isolates circulating in Southern Africa exhibit a greater frequency of dicysteine motif-containing Tat variants than those in Southeast Asia and cause increased neurovirulence. Retrovirology. 2013;10:61. doi: 10.1186/1742-4690-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VR, Sas AR, Eugenin EA, Siddappa NB, Bimonte-Nelson H, Berman JW, Ranga U, Tyor WR, Prasad VR. HIV-1 clade-specific differences in the induction of neuropathogenesis. J Neurosci. 2008;28(40):10010–10016. doi: 10.1523/JNEUROSCI.2955-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson K, Liner J, Hakim J, Sankale JL, Grant I, Letendre S, Clifford D, Diop AG, Jaye A, Kanmogne G, Njamnshi A, Langford TD, Weyessa TG, Wood C, Banda M, Hosseinipour M, Sacktor N, Nakasuja N, Bangirana P, Paul R, Joska J, Wong J, Boivin M, Holding P, Kammerer B, Van Rie A, Ive P, Nath A, Lawler K, Adebamowo C, Royal W, 3rd, Joseph J. NeuroAIDS in Africa. J Neurovirol. 2010;16(3):189–202. doi: 10.3109/13550284.2010.489597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal W, 3rd, Cherner M, Carr J, Habib AG, Akomolafe A, Abimiku A, Charurat M, Farley J, Oluyemisi A, Mamadu I, Johnson J, Ellis R, McCutchan JA, Grant I, Blattner WA. Clinical features and preliminary studies of virological correlates of neurocognitive impairment among HIV-infected individuals in Nigeria. J Neurovirol. 2012;18(3):191–199. doi: 10.1007/s13365-012-0097-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor N, Nakasujja N, Robertson K, Clifford DB. HIV-associated cognitive impairment in sub-Saharan Africa--the potential effect of clade diversity. Nat Clin Pract Neurol. 2007;3(8):436–443. doi: 10.1038/ncpneuro0559. [DOI] [PubMed] [Google Scholar]
- Sacktor N, Nakasujja N, Skolasky RL, Rezapour M, Robertson K, Musisi S, Katabira E, Ronald A, Clifford DB, Laeyendecker O, Quinn TC. HIV subtype D is associated with dementia, compared with subtype A, in immunosuppressed individuals at risk of cognitive impairment in Kampala, Uganda. Clin Infect Dis. 2009;49(5):780–786. doi: 10.1086/605284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor N, Skolasky R, Selnes OA, Watters M, Poff P, Shiramizu B, Shikuma C, Valcour V. Neuropsychological test profile differences between young and old human immunodeficiency virus-positive individuals. J Neurovirol. 2007;13(3):203–209. doi: 10.1080/13550280701258423. [DOI] [PubMed] [Google Scholar]
- Sacktor NC, Wong M, Nakasujja N, Skolasky RL, Selnes OA, Musisi S, Robertson K, McArthur JC, Ronald A, Katabira E. The International HIV Dementia Scale: a new rapid screening test for HIV dementia. AIDS. 2005;19(13):1367–1374. [PubMed] [Google Scholar]
- Samikkannu T, Agudelo M, Gandhi N, Reddy PV, Saiyed ZM, Nwankwo D, Nair MP. Human immunodeficiency virus type 1 clade B and C gp120 differentially induce neurotoxin arachidonic acid in human astrocytes: implications for neuroAIDS. J Neurovirol. 2011;17(3):230–238. doi: 10.1007/s13365-011-0026-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satishchandra P, Nalini A, Gourie-Devi M, Khanna N, Santosh V, Ravi V, Desai A, Chandramuki A, Jayakumar PN, Shankar SK. Profile of neurologic disorders associated with HIV/AIDS from Bangalore, south India (1989–96) Indian J Med Res. 2000;111:14–23. [PubMed] [Google Scholar]
- Self RL, Mulholland PJ, Nath A, Harris BR, Prendergast MA. The human immunodeficiency virus type-1 transcription factor Tat produces elevations in intracellular Ca2+ that require function of an N-methyl-D-aspartate receptor polyamine-sensitive site. Brain Res. 2004;995(1):39–45. doi: 10.1016/j.brainres.2003.09.052. [DOI] [PubMed] [Google Scholar]
- Spira S, Wainberg MA, Loemba H, Turner D, Brenner BG. Impact of clade diversity on HIV-1 virulence, antiretroviral drug sensitivity and drug resistance. J Antimicrob Chemother. 2003;51(2):229–240. doi: 10.1093/jac/dkg079. [DOI] [PubMed] [Google Scholar]
- Tyor WR. Handbook of Neurochemistry and Molecular Neurobiology. Berlin Heidelberg: N. Banik, Spinger-Verlag; 2009. Pathogenesis and treatment of HIV-associated dementia: Recent studies in a SCID mouse model; pp. 472–489. [Google Scholar]
- Wadia RS, Pujari SN, Kothari S, Udhar M, Kulkarni S, Bhagat S, Nanivadekar A. Neurological manifestations of HIV disease. J Assoc Physicians India. 2001;49:343–348. [PubMed] [Google Scholar]
- Wong MH, Robertson K, Nakasujja N, Skolasky R, Musisi S, Katabira E, McArthur JC, Ronald A, Sacktor N. Frequency of and risk factors for HIV dementia in an HIV clinic in sub- Saharan Africa. Neurology. 2007;68(5):350–355. doi: 10.1212/01.wnl.0000252811.48891.6d. [DOI] [PubMed] [Google Scholar]
- Yepthomi T, Paul R, Vallabhaneni S, Kumarasamy N, Tate DF, Solomon S, Flanigan T. Neurocognitive consequences of HIV in southern India: a preliminary study of clade C virus. J Int Neuropsychol Soc. 2006;12(3):424–430. doi: 10.1017/s1355617706060516. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Qiao L, Ding W, Wei F, Zhao Q, Wang X, Shi Y, Li N, Smith D, Chen D. An initial screening for HIV-associated neurocognitive disorders of HIV-1 infected patients in China. J Neurovirol. 2012;18(2):120–126. doi: 10.1007/s13365-012-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]