Abstract
The nuclear lamina is an extensive protein network that underlies the inner nuclear envelope. This network includes the LAP2-emerin-MAN1-domain (LEM-D) protein family, proteins that share an association with the chromatin binding protein Barrier-to-autointegration factor (BAF). Loss of individual LEM-D proteins causes progressive, tissue-restricted diseases, known as laminopathies. Mechanisms associated with laminopathies are not yet understood. Here we present our studies of one of the Drosophila nuclear lamina LEM-D proteins, Otefin (Ote), a homologue of emerin. Previous studies have shown that Ote is autonomously required for the survival of female germline stem cells (GSCs). We demonstrate that Ote is also required for survival of somatic cells in the ovarian niche, with loss of Ote causing a decrease in cap cell number and altered signal transduction. We show germ cell-restricted expression of Ote rescues these defects, revealing a non-autonomous function for Ote in niche maintenance and emphasizing that GSCs contribute to the maintenance of their own niches. Further, we investigate the requirement of Ote in the male fertility. We show that ote−/− males become prematurely sterile as they age. Parallel to observations in females, this sterility is associated with GSC loss and changes in somatic cells of the niche, phenotypes that are largely rescued by germ cell-restricted Ote expression. Taken together, our studies demonstrate that Ote is required autonomously for survival of two stem cell populations, as well as non-autonomously for maintenance of two somatic niches. Finally, our data add to growing evidence that LEM-D proteins have critical roles in stem cell survival and tissue homeostasis.
Keywords: nuclear lamina, LEM-domain proteins, spermatogenesis, oogenesis, germline stem cells, Drosophila
Introduction
The nuclear lamina is a filamentous protein network that lies underneath the inner membrane of the nuclear envelope. The major constituents of the nuclear lamina are the A- and B-type lamins that provide a scaffold for interactions between hundreds of inner nuclear membrane proteins and other lamina-associated polypeptides (Korfali et al., 2012; Schirmer and Gerace, 2005). This extensive protein network has multiple functions, serving as a mechanical support for the nucleus and regulating transcription, DNA replication and genome stability (Davidson and Lammerding, 2014; Gerace and Huber, 2012; Shimi et al., 2010).
One nuclear lamina protein family is the LEM-domain (LEM-D) family named for the three founding human proteins, LAP2, emerin and MAN1 (Lin et al., 2000; Mansharamani and Wilson, 2005; Wagner and Krohne, 2007). The hallmark feature of proteins in this family is an ~40 amino acid domain, the LEM-D, that interacts with Barrier-to-Autointegration Factor (BAF or BANF), a conserved metazoan histone and sequence-independent DNA binding protein (Cai et al., 2001; Zheng et al., 2000). As a result of BAF association, LEM-D proteins contribute to nuclear organization, serving as bridges that tether chromatin to the lamina at the nuclear periphery. Notably, nuclear envelope proteins that carry domains related to the LEM-D are found in organisms that lack BAF and lamins, such as yeast (Gonzalez et al., 2012; Grund et al., 2008; Mekhail et al., 2008). These observations suggest that LEM-D proteins may confer ancient regulatory functions of the nuclear lamina.
LEM-D proteins differ in sequence outside of the LEM-D (Barton et al., 2015; Brachner et al., 2012; Lee and Wilson, 2004). Unique, non-LEM-D regions associate with proteins involved in signal transduction, cellular architecture, transcriptional regulation, and chromatin tethering (Berk et al., 2013; Wagner and Krohne, 2007). In many cases, LEM-D proteins interact with the same regulatory proteins, possibly due to conformational plasticity imparted by the presence of unstructured regions within these proteins (Berk et al., 2014). Such sharing of protein partners provides a framework for understanding why individual loss of these globally expressed LEM-D proteins causes limited developmental defects (Barton et al., 2014; Huber et al., 2009; Liu et al., 2003; Reil and Dabauvalle, 2013). Indeed, loss of single LEM-D proteins causes tissue-restricted human diseases, including bone density disorders, cardiomyopathies and muscular dystrophies (Worman et al., 2010). Emerging evidence suggests that these progressive diseases result from altered maintenance of progenitor cell populations that support tissue homeostasis (Barkan et al., 2012; Barton et al., 2013; Liu et al., 2003; Melcon et al., 2006; Ozawa et al., 2006). Mechanisms underlying how LEM-D proteins support such stem cell function are not yet understood.
Drosophila melanogaster serves as an excellent model to study how LEM-D proteins contribute to tissue homeostasis. The Drosophila LEM-D family includes four genes (Pinto et al., 2008; Wagner and Krohne, 2007), of which three encode proteins that localize to the nuclear lamina. These include Otefin (Ote) and Bocksbeutel (Bocks), two fly homologues of emerin, and dMAN1, the fly homologue of MAN1. Mutations in genes encoding all of these Drosophila nuclear lamina LEM-D proteins have been identified, revealing that loss of individual proteins causes distinct developmental defects (Barton et al., 2013; Barton et al., 2014; Jiang et al., 2008; Pinto et al., 2008; Wagner et al., 2010). Even so, these Drosophila LEM-D proteins share functions. While loss of individual LEM-D proteins does not affect viability, complete loss of any two of nuclear lamina LEM-D proteins causes death during development (Barton et al., 2014). Although a reasonable explanation for such overlapping requirements is the additive loss of interactions with the shared partner BAF, phenotypes of baf and lem-d double mutants differ (Barton et al., 2014; Furukawa et al., 2003). These observations imply that the common functions of the Drosophila nuclear lamina LEM-D proteins extend beyond BAF recruitment.
Studies of the emerin homologue Ote have provided insights into developmental functions of the LEM-D proteins. Loss of Ote causes a complex, age-dependent phenotype in the ovary (Barton et al., 2013; Jiang et al., 2008). Drosophila ovaries are divided into sixteen to twenty ovarioles, each containing a germline stem cell (GSC) niche housed within a germarium (Fig. 1A). Within each niche, somatic cap cells directly anchor two to three GSCs and produce the Bone morphogenetic protein (BMP) ligands Decapentaplegic (Dpp) and Glass bottom boat (Gbb) to promote GSC self-renewal (Xie, 2013). BMP signaling in GSCs represses the key differentiation gene bag-of-marbles (bam). Upon asymmetric division of GSCs, one daughter remains associated with cap cells, maintains repression of bam and stem cell identity. The second daughter is displaced from the niche, experiences reduced BMP signaling, resulting in activation of bam and entrance into the differentiation program. In newly emerged ote−/− females, the majority of GSC niches carry expanded numbers of GSC-like cells, with a minority devoid of germ cells (Barton et al., 2013). As ote−/− females age, the numbers of GSC-like cells per niche increases only to undergo premature loss within two weeks. This loss occurs independently of bam activation (Barton et al., 2013), indicating that ote−/− GSCs die rather than differentiate. Although Ote is present throughout the ovary, maintenance of ote−/− GSCs requires production of Ote only in germ cells (Jiang et al., 2008). Together, these studies indicate that Ote is autonomously required for the survival of adult ovarian GSCs.
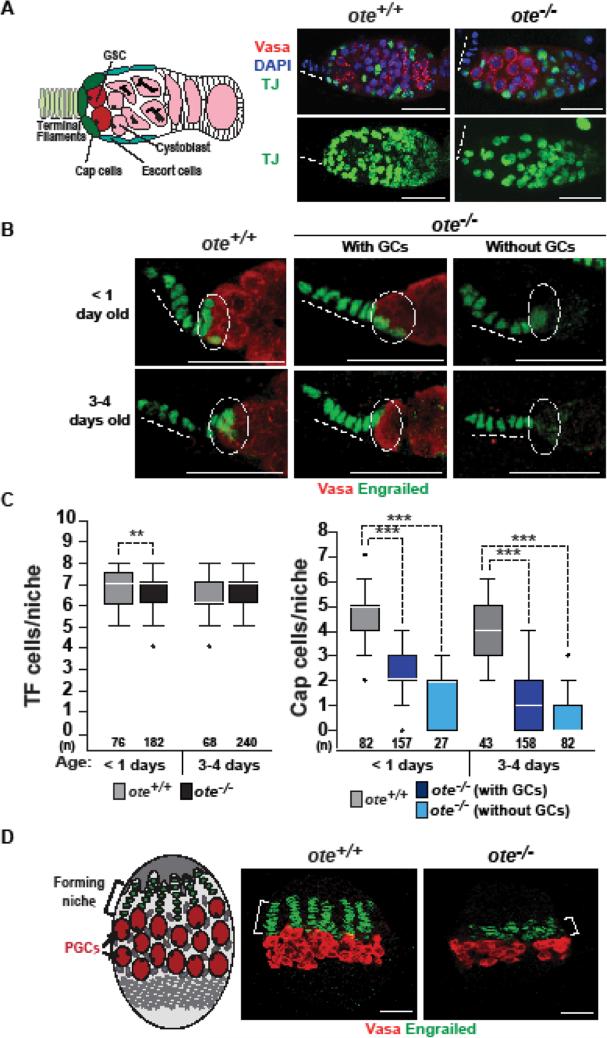
Figure 1. Loss of Ote disrupts somatic cells in the germarium.
A. Left: Schematic of the ovarian stem cell niche, showing somatic cells that include terminal filament (TF) cells (light green), cap cells (dark green), escort cells (blue), and germ cells that include germline stem cells (GSCs; red), cytoblasts and differentiating germ cells (pink). Right: Confocal images of ote+/+ and ote−/− germaria stained with antibodies against TJ (detects cap and escort cells, green), Vasa (detects germ cells, red), and DNA stained with DAPI (blue). Panels on the top are single confocal slices of a Z-stack through an entire germarium. Images on the bottom are maximum projections of each slice in the Z-stack, showing all TJ-positive cells in the germarium. Dashed lines indicate position of the TF. Scale bars, 25 μm. B. Confocal images of one- and three-to-four-day-old ote+/+ and ote−/− germaria stained with antibodies against Vasa and Engrailed (detects TF and cap cells). Dotted circles indicate the position of cap cells. C. Quantification of the number of TF cells and cap cells present within ote+/+ (gray) and ote−/− germaria with (black-C or dark blue-D) or without GSCs (light blue). The number of niches analyzed is shown below each bar. For each box, the box represents the 25th to 75th percentile interval, the line represents the median, and the whiskers represent the 5th to 95th percentile interval and non-outlier range. The Mann-Whitney-Wilcoxon Test was used to determine statistics (*p<0.05; **p<0.01; and ***p<0.001). D. Left: Schematic of the developing female larval gonad, showing TF cells (green), primordial germline stem cells (PGCs, red), and Intermingled cells (blue). The bracket highlights the region of the forming niche. Right: Confocal images ote+/+ and ote−/− stem cell niche in third instar larvae, using antibodies against Engrailed (green) and Vasa (red). Scale bars, 25 μm.
Here we investigate age-dependent phenotypes associate with loss of the LEM-D protein Ote. We find that defects in the ovary are not restricted to germ cells. Indeed, in the absence of Ote, the ovarian somatic niche breaks down, evidenced by accelerated loss of cap cells and altered signaling associated with GSC self-renewal. These defects are rescued by germ cell-restricted Ote expression, uncovering a non-autonomous requirement for Ote maintenance of the stem cell niche. Identification of age-associated defects in the ovarian GSC niche prompted investigations into whether similar defects occurred in the male GSC niche, where Ote is also globally expressed. We tested ote−/− male fertility, using a sperm exhaustion assay and found that Ote is required for sustained fertility in aging males. Premature sterility of ote−/− males was accompanied by GSC loss, indicating that Ote is required for survival of a second adult GSC population. Similar to the ovary, GSC loss in ote−/− males was associated with other niche defects that have been associated with aging (Boyle et al., 2007; Wallenfang et al., 2006), including decreased expression of cell adhesion proteins in the niche. Reminiscent of our findings in the ovary, these testes mutant phenotypes are largely rescued by germ cell-restricted Ote expression, suggesting that Ote has a non-autonomous requirement in the male GSC niche too. Notably, these data emphasize that GSCs have a role in maintenance of their own niches. Together, our studies build evidence that LEM-D proteins are required for tissue homeostasis and maintenance of progenitor cell populations.
Materials and Methods
Drosophila stocks and culture conditions
Drosophila stocks were raised at 25°C on standard cornmeal/agar medium, with p-hydroxybenzoic acid methyl ester as a mold inhibitor. Crosses were carried out in vials at 25°C at 70% humidity. In these studies, the ote+/+ reference strain was y1w67c23, while the ote−/− strain was oteB279G/halPk. The oteB279-G allele carries a piggyBac transposon insertion at +764 and the otehalPK allele carries a premature stop codon at +381 [amino acid R127; (Supplemental Fig.1A); (Barton et al., 2013)]. Dad-lacZ flies were kindly provided by Allan Spradling.
Immunohistochemical analyses
Gonad staining was done using previously described protocols (Barton et al., 2013; Baxley et al., 2011). To ensure an unbiased sampling of germarial phenotypes, we analyzed at least ten gonads per experiment, and completed a minimum of two independent experiments. Primary antibodies include: Armadillo/β-catenin [Developmental Studies Hybridoma Bank, (DSHB, N2 7A1)] at 1:50, mouse α-Engrailed (DSHB, 4D9) at 1:50, goat α-Ote at 1:500 (Barton et al., 2014), mouse α-Spectrin (DSHB, 3A9) at 1:50, guinea pig Traffic Jam at 1:10,000 (D. Godt), rabbit α-Vasa (Santa Cruz, sc-30210) at 1:500 or 1:1000, goat α-Vasa (Santa Cruz, sc-6817) at 1:500, mouse β-galactosidase (DHSB, 40-1a) at 1:200 and mouse Fascilin (Fas) III (DSHB, 7G10) at 1:50. Images were collected on a Bio-Rad Radiance 2100 Multiphoton/Confocal Microscope or a Zeiss 710 Confocal Microscope and processed using ImageJ software.
Cell-type restricted expression of Ote
Two methods were used to test the cell-specific requirements for Ote. First, we generated the P[nosP:ote, w+] transposon to restrict Ote production to germ cells (Supplemental Fig. 1A). This transposon carries a nanos (nos)-ote fusion gene and the mini-white gene for a transformation marker. The nosP-ote fusion gene contains 445 bp of the nos promoter and 5’ untranslated region (nosP, −184 to +261) fused to the ote gene (+98 to +1,477) that was PCR amplified from y1w67c23 genomic DNA. The nosP-ote fusion gene was cloned into the pCaSpeR3 transposon that directs random integration into the genome. Once transgenic lines were obtained, the P[nosP:ote, w+] transposon was crossed into an ote−/− mutant background to generate P[nosP:ote, w+], ote−/− flies. Second, we used the two-component GAL4/UAS expression system to restrict Ote production to somatic cells in the ovarian niche (Brand and Perrimon, 1993). For these studies, we generated y1w67c23, P[UASP-ote, w+] transgenic lines, carrying the ote coding region under the control of the P transposase promoter, designed for optimal germ cell expression (Rorth, 1998). Several GAL4 driver lines were used, including engrailed (en)-gal4 and bric-a-brac (bab)-gal4 that direct expression only in TF and cap cells, and c587-gal4 that directs expression in cap, escort and follicle cells. All expression patterns were confirmed by crossing these driver lines to UASp-GFP and staining using antibodies against GFP (data not shown).
qPCR analysis of gene expression
For each biological replicate, 25 ovary pairs per genotype were dissected from less than two-hour-old females in PBS and stored at −80°C. RNA was isolated with TRIzol (Invitrogen) extraction and treated with DNase I using DNA-free (Ambion). RNA was reverse-transcribed using the High Capacity cDNA kit with random hexamer primers (Applied Biosystems) and diluted five fold prior to setting up qPCR reactions. Cycle threshold levels were normalized to the housekeeping gene, RpL32, or the germline-restricted gene vasa. Fold-enrichment was calculated using the ΔΔCt method (Livak and Schmittgen, 2001). Statistical significance was obtained by comparing fold change of ote−/− samples to those of ote+/− samples.
Sperm exhaustion assay
Less than one-day-old ote−/− males were collected and individually mated in vials with three virgin ote+/+ females. As a control, less than one-day-old ote+/+ males were individually mated in vials with three virgin ote+/+ females. After three days, males were isolated and transferred to a new vial with three fresh virgin ote+/+ females. This procedure was repeated four times, until males were fifteen-days-old. All vials were scored for the presence of progeny. If five or more progeny were produced in a vial, then the male was scored as fertile at the tested age. For each experiment, at least eight to ten crosses were scored per genotype. Each experiment was repeated at least three times.
Results
Loss of Ote disrupts ovarian niche composition and function
Ovaries from young ote−/− females contain expanded numbers of GSC-like cells at the expense of differentiated daughter cells (Barton et al., 2013). Previous studies have shown that such increases in GSC numbers can be caused by altered signaling in the somatic niche (Zhu and Xie, 2003). As such, we wondered whether the ote−/− stem cell niche was compromised. Each stem cell niche contains three types of somatic cells, including terminal filament (TF) cells, cap cells and escort cells [Fig. 1; (Chen et al., 2011; Harris and Ashe, 2011; Losick et al., 2011; Xie, 2013)]. TF cells are disc shaped cells that do not contact GSCs. Cap cells are small, round cells that directly anchor two to three GSCs in each germarium. These cells produce BMP ligands critical for GSC self-renewal (Xie, 2013). Escort cells are cells that are intermingled with GSCs and differentiating germ cells. To examine the structure of the niche, we co-stained ote+/+ and ote−/− ovaries with antibodies against the germline specific DEAD-box helicase Vasa (Lasko and Ashburner, 1988) in combination with antibodies against markers that identify different somatic cell types. First, cap cells and escort cells were identified using antibodies against the transcription factor Traffic Jam (TJ) (Li et al., 2003). In comparison to ote+/+ germaria, fewer TJ positive cells were present in ote−/− germaria (Fig. 1A). Second, TF and cap cells were identified using antibodies against the transcription factor Engrailed (Xie, 2013). We found that newly eclosed ote+/+ and ote−/− germaria carry similar numbers of TF cells (Fig. 1B, C) but dramatically differ in numbers of cap cells (Fig. 1B, D). In less than one-day-old females, ote+/+ germaria had a median number of four cap cells, whereas age matched ote−/− germaria had a median number of one to two. Similar results were obtained when ovaries were stained with Lamin C antibodies, a nuclear lamina component that is enriched in TF and cap cells (data not shown). To determine whether ote−/− cap cells were retained as females aged, we quantified numbers of these cells in the germaria in three- to four-day-old ote+/+ and ote−/− ovaries. We found that cap cells numbers declined even further in niches from older ote−/− females, even though the median number of cap cells was unchanged in ote+/+ niches (Fig. 1B, D). Although an age-dependent decline occurred in all ote−/− germaria, cap cell loss was more dramatic in those lacking germ cells (Fig. 1D). These findings suggest that germ cell absence and cap cell loss are linked. Together, these analyses show that Ote loss causes declines in two of the three types of somatic cells in the ovarian niche.
We wondered whether changes in the ote−/− adult stem cell niche resulted from compromised development. Ovarian niches begin to form during larval stages, when TF cells are specified and intercalate to form sixteen to twenty stacks of cells (Godt and Laski, 1995). Subsequently, cap cells are specified in a process that involves Notch signaling (Hsu and Drummond-Barbosa, 2011; Hsu et al., 2008; Song et al., 2007). To examine niche development, we stained larval gonads with Vasa and Engrailed antibodies, finding that formation of ote−/− TFs was compromised. Notably, fewer Engrailed-positive cells were present in ote−/− gonads and TF stacks were only beginning to form in late third instar larvae, a time when ote+/+ TF formation was completed (Fig. 1D). Even so, the number of TF cells in adult ote−/− germaria was comparable to that found in ote+/+ germaria (Fig. 1C), implying that although TF formation is delayed in ote mutants, development is completed. Recent studies have shown that niche formation involves a balance of TF cell proliferation and differentiation (Lengil et al., 2015). As such, the slowed rate of TF formation may be linked to decreased rates of proliferation. We postulate that the delay in TF development might interfere with cap cell specification, leading to the lowered numbers observed in newly eclosed ote−/− females.
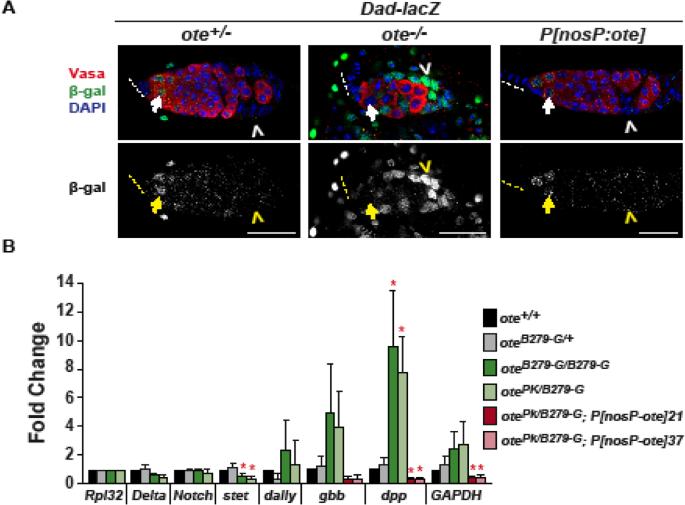
Cap cells are the major source of BMP ligands needed for GSC self-renewal (Song et al., 2004; Xie and Spradling, 1998). As such, cap cell loss is predicted to result in activation of bam transcription and inappropriate germ cell differentiation. Yet, we do not find differentiating cells in ote−/− germaria (Figs. 1,2). Further, our previous studies showed that bam remains repressed in ote−/− GSCs (Barton et al., 2013). These data suggest that active BMP signaling remains in ote−/− germ cells, even with the lower number of cap cells. To examine BMP signaling in more detail, we used the Dad-lacZ reporter line, in which the Dpp target gene Daughters-against-Dpp (Dad) carries a P element insertion that includes the lacZ gene (Tsuneizumi et al., 1997). Within wild type germaria, Dad-lacZ is expressed in GSCs and their immediate daughter cystoblasts [Fig. 2A, (Casanueva and Ferguson, 2004; Song et al., 2007; Song et al., 2004)]. In addition, Dad-lacZ is stochastically expressed in some somatic cells in the germarium, but at lower levels than in GSCs (Casanueva and Ferguson, 2004). We found that 80-95% of ote−/−, Dad-lacZ germaria had β-galactosidase staining in germ cells (Fig. 2A), providing evidence that these cells are receiving BMP signals. Strikingly, we also observed very high levels of Dad-LacZ expression in somatic cells. In our analysis of over 300 ote−/−, Dad-lacZ germaria, all had high levels of lacZ expression in somatic cells (Fig. 2A, data not shown). These data suggest that BMP signaling might be increased in ote−/− germaria. To test this possibility, we isolated RNA from newly eclosed ote+/+ and ote−/− ovaries and measured levels of dpp and gbb RNAs using quantitative real time PCR. Consistent with high levels of Dad-LacZ expression, ote−/− ovaries had statistically increased levels of dpp, with levels of gbb RNAs trending higher. Level of Delta, Notch and dally RNAs were unchanged (Fig. 2B). Taken together, our findings suggest that loss of Ote disrupts somatic cells in the ovarian GSC niche, accompanied by delayed TF formation, cap cell loss and widespread BMP signaling.
Figure 2. Analysis of BMP signaling in the ote−/− germaria.
A. Analysis of BMP signaling in ote+/+ and ote−/− germaria using the Dad-LacZ reporter gene. This reporter carries a P element insertion of lacZ in the Daughters-against-Dpp gene (Tsuneizumi et al., 1997). Top panels show confocal images from germaria obtained from one-day-old ote+/+ and ote−/− Dad-lacZ ovaries stained for Vasa (red), β-galactosidase (green) and the DNA dye DAPI (blue). Bottom panels show confocal images of β-galactosidase staining shown in white. Arrows indicate germ cells and chevrons indicate somatic cells. Dashed lines indicate the position of the TF. Scale bars, 25 μm. B. Quantitative reverse transcription PCR of RNAs isolated from ote+/+ and ote−/− ovaries obtained from less than two-hour-old females. RNAs were obtained from females representing two ote+/+ (black, gray bars), two ote−/− (green bars) genotypes and two ote−/−, P[nosP:ote, w+] lines (red bars). RNA levels were normalized to Rpl32 and fold change was set relative to the ote+/+ genotype. Error bars indicate standard deviation from three biological replicates (* = p<0.05, Student's t-test).
Germline restricted expression of Ote restores the somatic niche
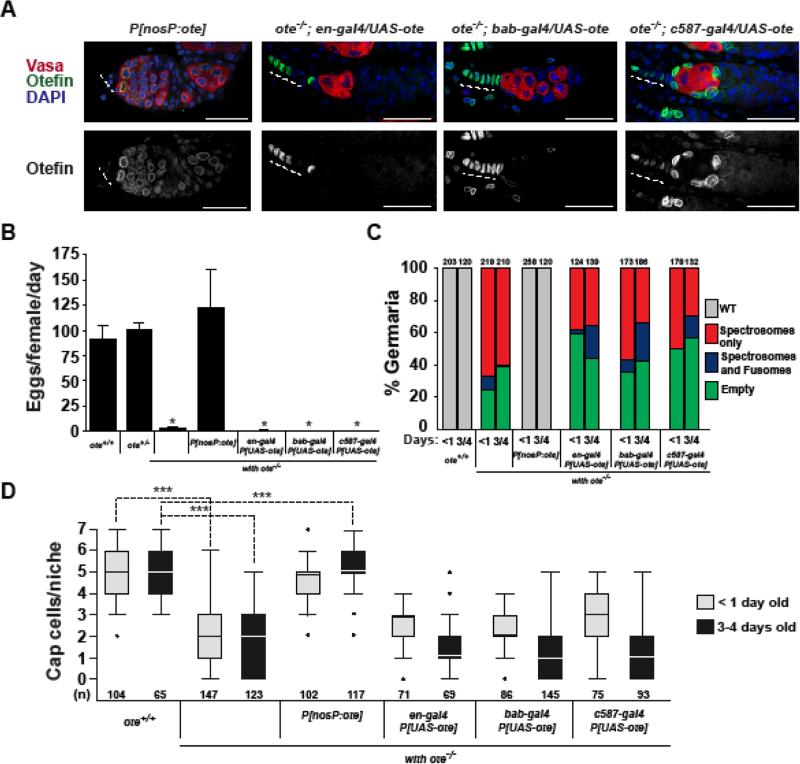
The germline defects observed in ote−/− ovaries reflect an autonomous requirement for Ote in the germline (Jiang et al., 2008). As our new studies demonstrate that loss of Ote also has profound effects on somatic cells in the GSC niche (Figs. 1, 2), we questioned whether somatic defects were due to a second, autonomous requirement of Ote in these cells. To this end, we determined the extent of rescue of somatic defects following soma-restricted expression of Ote using the GAL4-UAS system. Three GAL4 drivers were tested. Two expressed Ote in TF and cap cells [en-gal4 and bab-gal4] and one expressed Ote in all somatic cells ([c587-gal4]; Fig. 3A). As expected, soma-restricted niche Ote expression did not rescue germ cell defects, as GSCs were lost and germ cell differentiation was absent (Fig. 3A-C). To determine whether niche defects were rescued, we used stained ote−/−; P[UAST-ote], GAL4-driver ovaries with Engrailed antibodies to quantify cap cell numbers. Surprisingly, we found that somatic expression of Ote did not rescue cap cell loss (Fig. 3D). These findings indicate that Ote might be non-autonomously required to maintain somatic cells in the niche. To test this postulate, we generated ote−/−; P[nosP:ote] females (Supplemental Fig. 1A), wherein Ote is expressed only in germ cells (Fig. 3A). As expected, ote−/−; P[nosP:ote] females are fertile (Fig. 3B), carrying a normal distribution of germ cells in all germaria (Fig. 3A, C). In addition, we found that somatic niche defects were rescued ote−/−; P[nosP:ote] germaria (Fig. 3A-D), including the increased expression of the Dad-lacZ reporter gene (Fig. 2A). Indeed, quantification of cap cell numbers in ote−/−; P[nosP:ote] ovaries using staining with Engrailed antibodies revealed that cap cell loss was prevented (Fig. 3D). Our data show that germ cell expression of Ote is sufficient to rescue somatic niche defects in ote−/− germaria, demonstrating that these defects are a secondary consequence of a compromised germline.
Figure 3. Germ cell-restricted Ote expression rescues somatic niche defects in ovaries.
A. Confocal images of three-day-old germaria in ovaries isolated from ote−/−; P[nosP:ote, w+] females and several one-day-old ote−/− females carrying three different cell-type restricted GAL4 drivers and the P[UASt-ote] responder. Ovaries were stained with Vasa (red), Ote (green), and DAPI (blue) in the top panels and Ote only (white) in the bottom panels. Scale bars represent 25 μm. B. Quantification of fecundity of three-day-old females of the following genotypes: ote+/+, ote+/−, ote−/−, P[nosP:ote, w+] and the indicated GAL4-P[UASt-ote] responders. Bars indicate the variation from three independent experiments. C. Quantification of germarial class prevalence in the genetic backgrounds listed in B. The total number of germaria analyzed is shown above each bar. D. Quantification of numbers of cap cells in less than one-day-old (gray) and in three-to-four-day-old (black) females of the indicated genotypes. The number of niches analyzed is shown below each bar. The limits of box plots and analyses are as described in Fig. 1.
Loss of Ote causes age-dependent male sterility
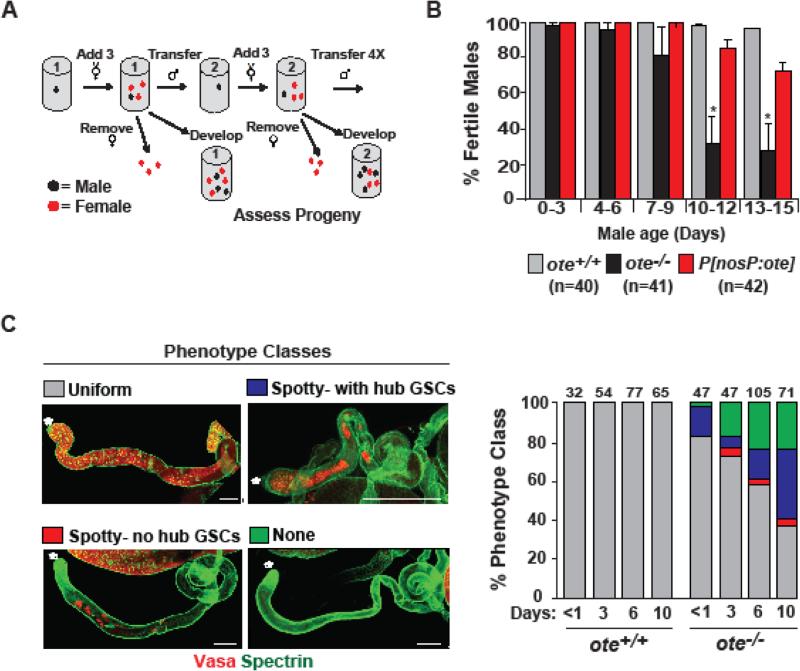
GSC maintenance in ovaries and testes shares many features (Fuller and Spradling, 2007; Spradling et al., 2011). Even so, ote−/− males appear fertile. We reasoned that sex-specific differences in oogenesis and spermatogenesis might influence the onset of fertility defects in ote−/− males. For example, female germ cells take on characteristics of adult GSCs during late larval development (Gilboa and Lehmann, 2004; Song et al., 2007), whereas male germ cells take on characteristics of adult GSCs by the end of embryogenesis (Le Bras and Van Doren, 2006; Sheng et al., 2009). As such, it is possible that maternally deposited Ote (Padan et al., 1990) permits early ote−/− GSC function, with defects only apparent as males age. To test whether male fertility is sustained in the absence of Ote, we used a sperm exhaustion assay (Fig. 4A). In this assay, ote+/+ and ote−/− males were individually mated to virgin females that were freshly supplied to each male every three days over a two-week period (Fig. 4A). The fertility of each male was determined by examining progeny output. We found that the fertility of ote+/+ males was largely unchanged over the 15-day assay period, as the vast majority of vials contained progeny. In contrast, fertility of ote−/− males decreased significantly, with only ~30% of the vials from 13- to 15-day-old ote−/− males containing progeny (Fig. 4B). These experiments reveal that Ote is also needed for male fertility requirements, implying that its contributions are not restricted to the ovary.
Figure 4. Loss of Ote causes age-dependent male sterility.
A. Schematic of sperm exhaustion assay to test fertility of aging ote−/− males. Single males (black dot) were mated to three to four females (red dots). After three days, each male was transferred to a new vial and mated to new virgin females; a process continued four times. B. Quantification of the fertility of ote+/+, ote−/−, and ote−/−, P[nosP:ote, w+] males. The number of males of each genotype is shown. Bars indicate the variation from six biological replicates (P>0.05; Student's t-test). C. Shown are confocal images of the four representative phenotypic testes classes identified using staining with Vasa and Spectrin antibodies. Testis images are oriented with anterior to the left. Asterisks mark the location of the hub. Scale bars, 200 μm. Graphs indicate the quantification of the prevalence of each phenotypic class in testes obtained from ote+/+ and ote−/− males of the indicated age.
Adult testes were examined to define phenotypes associated with the age-dependent sterility of ote−/− males. To this end, testes were isolated from different ages of ote+/+ and ote−/− males and stained with Vasa and Spectrin (Lin et al., 1994). Each testis has a single stem cell niche, called a hub, which is surrounded by a rosette of GSCs. Upon GSC division, differentiating germ cells move posterior, such that advancing stages of spermatogenesis occur along the testes length. Indeed, wild type testes carry maturing Vasa-positive cells along their entire length, even in males ten-days of age. In contrast, ote−/− testes showed complex phenotypes, including one wild type and three broad mutant phenotypic classes. These mutant classes included 1) testes with few germ cells distal to the hub but with GSCs [spotty with hub GSCs], 2) testes with few germ cells distal to the hub and no GSCs [spotty-no hub GSCs], and 3) testes with no germ cells [none] (Fig. 4C). We found that the distribution of the ote−/− mutant phenotypes changed as males aged. The first mutant phenotype observed corresponded to testes with the spotty distribution of early differentiating germ cells with GSCs remaining at the hub. These findings suggest that compromised differentiation is an early event (Fig. 4C). As ote−/− males aged, germ cell loss became pervasive, such that by ten days 60% of niches lacked GSCs and 25% of testes were devoid of germ cells. Reminiscent of events in ote−/− ovaries (Barton et al., 2013), germ cell loss was not associated with canonical apoptosis. Using antibodies against activated caspase-3, we showed that in ote−/− testes only surviving cysts undergoing individualization were stained (data not shown). Taken together, these data imply that the age-dependent sterility of ote−/− males is associated with defects in germ cell differentiation that ultimately lead to germ cell loss.
The structure of the stem cell niche is altered in ote−/− testes
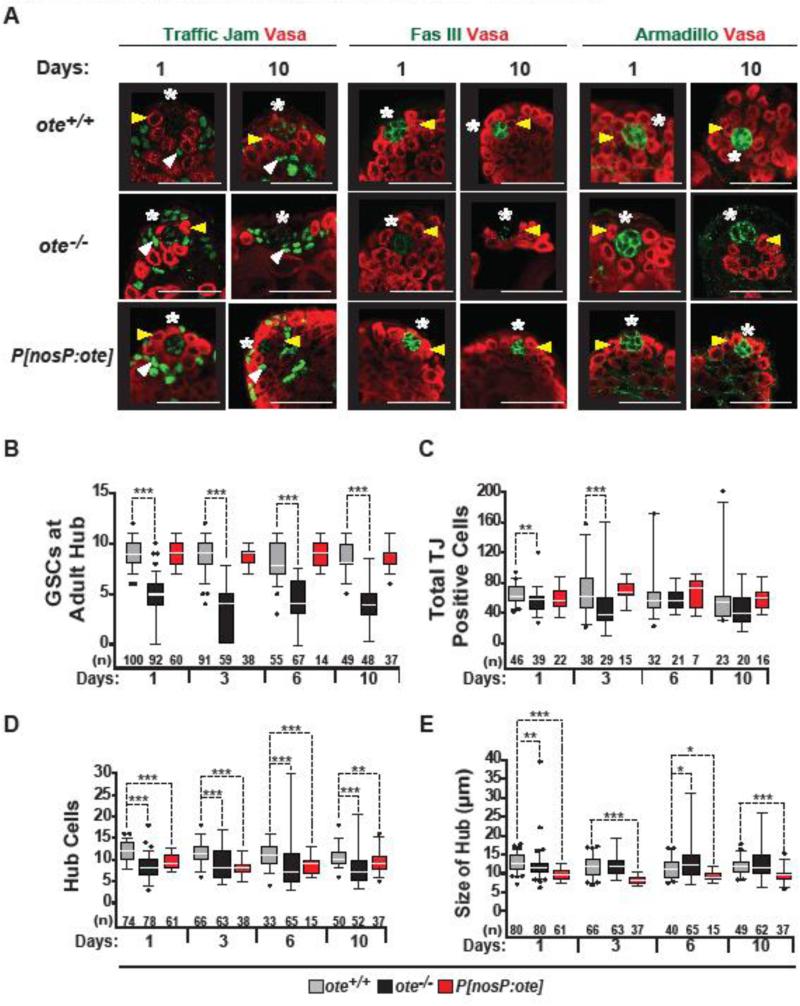
To understand the origin of the age-related defects, we analyzed the structure of the stem cell niche in the ote−/− testes. The hub is comprised of a cluster of post-mitotic somatic cells that maintain two stem cell populations, the GSCs and somatic cyst stem cells [CySCs, (de Cuevas and Matunis, 2011; Gonen and Toledano, 2014)]. GSCs make broad contacts with the hub, while CySCs make narrow contacts, causing CySC nuclei to be positioned underneath GSCs. Hub cells provide JAK-STAT signaling that promotes adhesion and is critical for self-renewal of both GSCs and CySCs (Kiger et al., 2001; Tulina and Matunis, 2001). CySCs provide BMP signaling needed for GSC self-renewal (Leatherman and Dinardo, 2010). The organization and number of GSCs associated with the hub was examined in aging ote+/+ and ote−/− testes using antibodies against Vasa. We found that one-day-old ote−/− testes had a broad distribution of numbers of GSCs, with median numbers lower than that found in wild type testes (ote−/− median of 5 versus ote+/+ median of 9; Fig. 5A, B). The observed median number of GSCs in ten-day-old ote−/− testes is lower than that reported for thirty-day-old wild type testes [4 versus an average of 6; (Boyle et al., 2007; Wallenfang et al., 2006)], implying premature GSC loss. To examine numbers of CySCs, we used antibodies against TJ. In testes, TJ accumulates at high levels in CySCs and early cyst cell nuclei, and low levels in hub cell nuclei (Li et al., 2003). Unlike observation in ote+/+ hubs (Fig. 5A), TJ positive ote−/− CySC nuclei were positioned closer to the hub, correlating with the level of GSC loss. While the total number of TJ positive cells in ote−/− niches decreased in three-day-old males, these numbers were restored to control levels in six-day-old ote−/− males (Fig. 5C). These observations suggest that Ote is not required for maintenance of CySCs. Similar observations were made using antibodies against Zinc finger homeodomain 1 (Zfh-1), another CySC marker [data not shown; (Leatherman and Dinardo, 2008)]. Together, these analyses demonstrate that Ote is required for maintaining male GSCs and not CySCs.
Figure 5. Age-dependent changes in the adult ote−/− testis niche.
A. Confocal images of one- and ten-day-old ote+/+, ote −/−, and ote−/−, P[nosP:ote, w+] testes stained with Vasa (red) and one of the following in green: TJ, Fascilin (Fas) III or Armadillo. Yellow arrowheads mark positions of GSCs; white arrowheads mark positions of CySCs; asterisks mark positions of the hubs. Scale bars, 20 μm. B-E. Quantification of the number of GSCs (B), the total number of TJ-positive cells (C), the total number of hub cells (D) and hub size (E) in aged testes obtained from ote+/+ (gray), ote−/− (black), and ote−/−, P[nosP:ote, w+] (red) males. The number of testes analyzed is shown below each bar. The limits of box plots and analyses are as described in Fig. 1.
To determine the timing of events in testes, we examined the structure of the GSC niche in third instar larvae. At this developmental stage, the stem cell niche is mature and gonads contain all premeiotic stages of spermatogenesis (Papagiannouli and Lohmann, 2012). Staining of ote+/+ and ote−/− gonads with antibodies against Vasa and TJ revealed several differences. First, we found that ote−/− gonads were smaller than ote+/+ gonads (Fig 6A, B). These size differences suggest the presence of fewer cysts, indicating that Ote may be required for proliferation of germ cells. Second, we observed that mutant niches in larval gonads had reduced GSC numbers compared to wild type niches, with a median number of eight ote−/− GSCs in the hub relative to eleven in ote+/+ controls (Fig. 6A, C). Notably, the median number of GSCs in ote−/− larval gonads was higher than the median number found in one-day-old testes of adult ote−/− males (5; Fig. 5B), suggesting that GSC maintenance is compromised during development of ote−/− testes.
Figure 6. Larval ote−/− testes show defects.
A. Confocal images of ote+/+ and ote−/− third instar larval gonads. Gonads were stained with antibodies against Vasa (red), TJ (green) and costained with DAPI (blue). Yellow arrowheads mark positions of GSCs; white arrowheads mark positions of CySCs; asterisks mark positions of the hub. Scale bars, 50 μm. B-C. Quantification of the length (B) and number of GSCs (C) in ote+/+ (gray) and ote−/− (black) third instar larval gonads. The number of gonads analyzed is shown below each bar. The limits of box plots and analyses are as described in Fig. 1.
Loss of ote−/− GSCs might reflect changes in GSC adhesion to hub cells. Within the niche, the eight to sixteen densely packed hub cells express high levels of cell adhesion molecules, such as Fascilin III (Fas III) and Armadillo/β-catenin (Davies and Fuller, 2008; Gonczy et al., 1992; Gonen and Toledano, 2014; Leatherman and Dinardo, 2010; Yamashita et al., 2003). To access adhesion in the niche, we stained ote−/− testes with Fas III. We found that in ten-day-old ote−/− testes, the majority had altered Fas III expression, with either low levels of diffuse staining (48%) or no staining (13%, Fig. 5A). In comparison, all ote+/+ testes stained with Fas III, with only ~15% showing staining at low levels. Notably, previous studies also demonstrated age-dependent declines in the expression of adhesion proteins, which were coupled with increases in hub size (Boyle et al., 2007; Gonen and Toledano, 2014). Even so, changes in those wild type males were less dramatic that what we observe in ote−/− males, as only ~2% of wild type 50-day-old testes had a complete loss of staining of Fas III staining (Boyle et al., 2007). To understand whether altered Fas III expression was associated with a loss of hub cells, we stained ote−/− testes with Armadillo/β-catenin antibodies. In this case, hubs consistently stained (Fig. 5A), which allowed us to quantify the number of hub cells in these niches. Our studies found that the median number of hub cells was stably lower than the number found in ote−/− relative to ote+/+ testes. In addition, mutant nicheshad broader distribution of hub cell numbers. In some cases, ote−/− mutant niches had double the number of Armadillo/β-catenin-positive cells (Fig. 5D). As ote−/− males aged, the size distribution of hubs expanded, although the median size was largely unchanged (Fig. 5E). Collectively, these observations reveal that loss of Ote affects somatic cells in the testis niche, with ote−/− niches showing defects that have been previously observed during normal aging.
Germline restricted expression of Ote restores male fertility
To define cell-type requirements of Ote in spermatogenesis, we first determined the distribution of Ote in the testis. Similar to findings in the ovary, antibody staining revealed that Ote localizes to the nuclear envelope in all somatic and germ cells (Supplemental Fig. 1B). Next, we analyzed spermatogenesis in P[nosP:ote, w+], ote−/− males. In the male germline, the nanos promoter directs expression of Ote only in GSCs, gonialblasts and mitotically active gonialblasts (Supplemental Fig. 1). Despite this restricted expression pattern, the sperm exhaustion assay uncovered that P[nosP:ote, w+], ote−/− males had sustained fertility at levels reaching those of wild type males (Fig 4B). Staining of P[nosP:ote, w+], ote−/− testes for Vasa and Spectrin showed that fertility was accompanied by rescued GSC maintenance (Fig. 5B), establishing an autonomous function for Ote in male germ cells. To understand whether Ote requirements in the male niche match those in the female niche, we examined hub phenotypes in P[nosP:ote, w+], ote−/− males. These analyses showed that germ cell-restricted Ote expression restored the number of TJ positive cells (Fig. 5C) and stabilized Fas III expression in all hubs (Fig. 5A). Armadillo/β-catenin staining showed that the broad distributions of hub cell numbers and sizes in ote mutants were rescued, although P[nosP:ote, w+], ote−/− hubs were smaller than wild type controls (5D, E). Together, these data imply that Ote contributions in the testes are parallel to those in ovaries, consisting of an autonomous requirement in GSCs and a non-autonomous requirement in maintenance of the somatic niche.
DISCUSSION
LEM-D proteins are a prominent family of nuclear lamina proteins that organize chromatin within the nucleus. Metazoan genomes encode multiple LEM-D proteins that possess overlapping developmental functions, thereby restricting the consequences of mutations in individual LEM-D genes. The Drosophila LEM-D family shares this feature. Indeed, Ote loss causes restricted developmental defects, with ovarian phenotypes resulting from limiting amounts of Bocks, a second Drosophila LEM-D protein that shares structural features with human emerin (Barton et al., 2014). Further, the requirement for Ote in the development of non-essential tissues provides an excellent system in which to understand how individual LEM-D proteins contribute to processes critical for tissue homeostasis. Using this model, we have defined primary and secondary consequences of LEM-D loss on a natural stem cell niche.
Ote has a non-autonomous requirement for ovarian niche maintenance
The ote mutant ovarian phenotype is complex (Barton et al., 2013). Most germaria in these mutant ovaries contain undifferentiated germ cells, with fewer completely lacking germ cells. In ote−/− germaria that contain germ cells, the number of cells increases for a few days, but ultimately declines within one week of adulthood (Barton et al., 2013). Here, we show that this altered germ cell development in ote mutants is associated with changes in the composition of somatic cells (Fig. 1). Whereas TF cell numbers are unchanged, numbers of escort cells and cap cells are reduced. Initially, these observations were surprising, as previous studies have correlated numbers of cap cells with numbers of GSCs, because cap cells produce the BMP ligands that are needed for GSC maintenance (Song et al., 2004; Xie and Spradling, 1998). However, we show that even though cap cells are lost, BMP ligands are produced. Indeed, BMP signaling becomes widespread in the ote−/− germaria (Fig. 2), extending to somatic cells far from the niche. These observations provide an explanation why ote−/− germ cells do not differentiate. Collectively, our studies indicate that both GSC niche structure and function of somatic cells are altered in the absence of Ote. Germ cell restricted-expression of Ote rescues all defects in ote−/− ovarian niches (Fig. 3). These observations imply that GSCs contribute to the maintenance of their own niche. The mechanism by which Ote expression in germ cells prevents rapid somatic cell loss is unclear. Previous studies have noted that loss of GSCs causes declines in escort cell numbers (Kai and Spradling, 2003; Margolis and Spradling, 1995; Xie and Spradling, 2000), suggesting that loss of ote−/− escort cells is linked to GSC loss. Yet, GSC loss does not affect cap cell numbers, which remain constant for weeks in the absence of GSCs (Bonfini et al., 2015; Kai and Spradling, 2003). One possibility might be that ote−/− cap cells are lost due to reduced Notch signaling, as the Notch/Delta signaling pathway is critical in the formation and maintenance of cap cells (Hsu and Drummond-Barbosa, 2011; Hsu et al., 2008; Song et al., 2007). Further, the mouse homologue of Ote, emerin, is required for expression of Notch signaling components (Koch and Holaska, 2012). However, our findings that cap cell survival depends on a non-autonomous function argues that loss of Ote changes Notch signaling components in germ cells. Although GSCs have been reported to produce Notch ligands (Ward et al., 2006), the role of these ligands remains unclear (Hsu and Drummond-Barbosa, 2011). Alternatively, it is possible that ote−/− cap cell loss is caused by the release of damaging signals emanating from unhealthy or dying GSCs. This prediction is consistent with data that uncouples cap cell and germ cell survival (Bonfini et al., 2015; Kai and Spradling, 2003). Further studies will be needed to distinguish between these alternatives.
Ote is required for maintenance of GSCs in the testis niche
Drosophila males also carry adult GSCs that are supported by a niche. The role of Ote in the maintenance of these stem cells had not previously been explored because ote−/− males appear fertile. Here, we show that fertility rapidly declines as ote−/− males age, with sterility linked to GSC loss (Fig. 4). The reason for the early fertility in ote−/− males is unknown. One possibility is maternally provided Ote protein might temporarily protect newly specified male GSCs from zygotic loss of Ote. Alternatively, Ote loss might result in accumulated defects in GSCs that ultimately lead to death and sterility. Regardless of mechanism, our observations imply that other proteins thought to only function in oogenesis may in fact have requirements in spermatogenesis, but these related functions have gone unnoticed.
Many defects were uncovered prior to the onset of ote−/− male sterility. As early as third instar larval development, the ote−/− gonads were abnormal, with a smaller size and fewer GSCs than ote+/+ gonads (Fig. 6). In one-day-old ote−/− males, defects became more severe, even though these males were fertile (Fig. 5). In these young males, the lower GSC number was coupled with a loss of differentiating germ cells. By ten-days of adulthood, ote−/− testes had significantly reduced GSC and hub cell numbers, though CySC numbers appeared stable (Fig. 5). Further, expression of hub adhesion proteins declined. These ote mutant phenotypes are similar to mutant phenotypes found in testes during natural aging and have been linked to a decline in the niche function (Boyle et al., 2007; Gonczy and DiNardo, 1996; Gonen and Toledano, 2014; Wallenfang et al., 2006), although ote mutant phenotypes are accelerated relative to wild type males. Analysis of phenotypes of ote−/−; P[nosP:ote, w+] testes showed that germ cell restricted expression rescued GSC loss, restored expression of cell adhesion markers and stabilized hub structure (Fig. 5). However, not all phenotypes were rescued, evidenced by the smaller size of P[nosP:ote, w+], ote−/− hubs (5D, E). The reason for incomplete rescue of somatic defects is unclear. The limited distribution of Ote resulting from expression using the nanos promoter may have contributed to a partial rescue, as the levels and distribution of Ote protein are not entirely restored to wild type. Even so, these data demonstrate that Ote has both autonomous and non-autonomous functions in the male GSC niche, and provide additional evidence that the germline contributes to the health of somatic cells in a stem cell niche. Our studies emphasize the importance of cell-cell communication within niches. Our studies emphasize the importance of cell-cell communication within niches.
LEM-D proteins contribute to the maintenance and differentiation of progenitor cells
Our studies reveal that Ote is required for maintenance of two adult stem cell populations, male and female GSCs. These data add to growing evidence that LEM-D proteins play critical roles in tissue differentiation and maintenance of progenitor cell populations (Barton et al., 2015; Gesson et al., 2014). Such roles for LEM-D proteins are consistent with the age-dependent, tissue-specific phenotypes of the associated human diseases. Indeed, the atypical Néstor-Guillermo progeria syndrome was linked to mutations in BANF1, the gene that encodes BAF (Puente et al., 2011). In patients carrying BANF1 mutation, profound nuclear abnormalities were observed that were associated with reduced levels of emerin within the nuclear envelope (Puente et al., 2011). It will be interesting to determine whether defects in ote−/− ovaries and testes are linked to altered BAF function. Our new data highlight the important role of the nuclear lamina in stem cell maintenance and aging.
Supplementary Material
Highlights.
- Death of ote−/− ovarian GSCs coincides with cap cell loss and altered BMP signaling.
- Loss of Ote causes premature male sterility.
- Sterility of ote−/− males is associated with GSC loss and somatic niche defects.
- Ote has autonomous and non-autonomous functions in germ cell niches.
- Defects in the nuclear lamina alter survival of two adult stem cell populations.
Acknowledgements
We thank Dorothea Godt for generously supplying antibodies (Li et al., 2003). We thank Allan Spradling, Ting Xie and Acaimo Gonzalez-Reyes for graciously providing Drosophila lines. We are grateful for technical assistance provided by James Bullard, Daniel Cook, Melinda Martin and members of the University of Iowa Central Microscopy Facility. We thank members of the Geyer laboratory for comments on the manuscript. A NIH R01 (GM087341) to P.K.G. supported this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barkan R, Zahand AJ, Sharabi K, Lamm AT, Feinstein N, Haithcock E, Wilson KL, Liu J, Gruenbaum Y. Ce-emerin and LEM-2: essential roles in Caenorhabditis elegans development, muscle function, and mitosis. Mol Biol Cell. 2012;23:543–552. doi: 10.1091/mbc.E11-06-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton LJ, Pinto BS, Wallrath LL, Geyer PK. The Drosophila nuclear lamina protein otefin is required for germline stem cell survival. Dev Cell. 2013;25:645–654. doi: 10.1016/j.devcel.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton LJ, Soshnev AA, Geyer PK. Networking in the nucleus: a spotlight on LEM-domain proteins. Curr Opin Cell Biol. 2015;34:1–8. doi: 10.1016/j.ceb.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton LJ, Wilmington SR, Martin MJ, Skopec HM, Lovander KE, Pinto BS, Geyer PK. Unique and Shared Functions of Nuclear Lamina LEM Domain Proteins in Drosophila. Genetics. 2014;197:653–665. doi: 10.1534/genetics.114.162941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxley RM, Soshnev AA, Koryakov DE, Zhimulev IF, Geyer PK. The role of the Suppressor of Hairy-wing insulator protein in Drosophila oogenesis. Dev Biol. 2011;356:398–410. doi: 10.1016/j.ydbio.2011.05.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk JM, Simon DN, Jenkins-Houk CR, Westerbeck JW, Gronning-Wang LM, Carlson CR, Wilson KL. The molecular basis of emerin-emerin and emerin-BAF interactions. J Cell Sci. 2014;127:3956–3969. doi: 10.1242/jcs.148247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk JM, Tifft KE, Wilson KL. The nuclear envelope LEM-domain protein emerin. Nucleus. 2013;4:298–314. doi: 10.4161/nucl.25751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfini A, Wilkin MB, Baron M. Reversible regulation of stem cell niche size associated with dietary control of Notch signalling. BMC developmental biology. 2015;15:8. doi: 10.1186/s12861-015-0059-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle M, Wong C, Rocha M, Jones DL. Decline in self-renewal factors contributes to aging of the stem cell niche in the Drosophila testis. Cell Stem Cell. 2007;1:470–478. doi: 10.1016/j.stem.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Brachner A, Braun J, Ghodgaonkar M, Castor D, Zlopasa L, Ehrlich V, Jiricny J, Gotzmann J, Knasmuller S, Foisner R. The endonuclease Ankle1 requires its LEM and GIY-YIG motifs for DNA cleavage in vivo. J Cell Sci. 2012;125:1048–1057. doi: 10.1242/jcs.098392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Cai M, Huang Y, Ghirlando R, Wilson KL, Craigie R, Clore GM. Solution structure of the constant region of nuclear envelope protein LAP2 reveals two LEM-domain structures: one binds BAF and the other binds DNA. Embo J. 2001;20:4399–4407. doi: 10.1093/emboj/20.16.4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanueva MO, Ferguson EL. Germline stem cell number in the Drosophila ovary is regulated by redundant mechanisms that control Dpp signaling. Development. 2004;131:1881–1890. doi: 10.1242/dev.01076. [DOI] [PubMed] [Google Scholar]
- Chen S, Wang S, Xie T. Restricting self-renewal signals within the stem cell niche: multiple levels of control. Curr Opin Genet Dev. 2011;21:684–689. doi: 10.1016/j.gde.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Davidson PM, Lammerding J. Broken nuclei--lamins, nuclear mechanics, and disease. Trends in cell biology. 2014;24:247–256. doi: 10.1016/j.tcb.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies EL, Fuller MT. Regulation of self-renewal and differentiation in adult stem cell lineages: lessons from the Drosophila male germ line. Cold Spring Harb Symp Quant Biol. 2008;73:137–145. doi: 10.1101/sqb.2008.73.063. [DOI] [PubMed] [Google Scholar]
- de Cuevas M, Matunis EL. The stem cell niche: lessons from the Drosophila testis. Development. 2011;138:2861–2869. doi: 10.1242/dev.056242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller MT, Spradling AC. Male and female Drosophila germline stem cells: two versions of immortality. Science. 2007;316:402–404. doi: 10.1126/science.1140861. [DOI] [PubMed] [Google Scholar]
- Furukawa K, Sugiyama S, Osouda S, Goto H, Inagaki M, Horigome T, Omata S, McConnell M, Fisher PA, Nishida Y. Barrier-to-autointegration factor plays crucial roles in cell cycle progression and nuclear organization in Drosophila. J Cell Sci. 2003;116:3811–3823. doi: 10.1242/jcs.00682. [DOI] [PubMed] [Google Scholar]
- Gerace L, Huber MD. Nuclear lamina at the crossroads of the cytoplasm and nucleus. J Struct Biol. 2012;177:24–31. doi: 10.1016/j.jsb.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesson K, Vidak S, Foisner R. Lamina-associated polypeptide (LAP)2alpha and nucleoplasmic lamins in adult stem cell regulation and disease. Semin Cell Dev Biol. 2014;29:116–124. doi: 10.1016/j.semcdb.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa L, Lehmann R. Repression of primordial germ cell differentiation parallels germ line stem cell maintenance. Curr Biol. 2004;14:981–986. doi: 10.1016/j.cub.2004.05.049. [DOI] [PubMed] [Google Scholar]
- Godt D, Laski FA. Mechanisms of cell rearrangement and cell recruitment in Drosophila ovary morphogenesis and the requirement of bric a brac. Development. 1995;121:173–187. doi: 10.1242/dev.121.1.173. [DOI] [PubMed] [Google Scholar]
- Gonczy P, DiNardo S. The germ line regulates somatic cyst cell proliferation and fate during Drosophila spermatogenesis. Development. 1996;122:2437–2447. doi: 10.1242/dev.122.8.2437. [DOI] [PubMed] [Google Scholar]
- Gonczy P, Viswanathan S, DiNardo S. Probing spermatogenesis in Drosophila with P-element enhancer detectors. Development. 1992;114:89–98. doi: 10.1242/dev.114.1.89. [DOI] [PubMed] [Google Scholar]
- Gonen O, Toledano H. Why adult stem cell functionality declines with age? Studies from the fruit fly Drosophila melanogaster model organism. Curr Genomics. 2014;15:231–236. doi: 10.2174/1389202915666140421213243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez Y, Saito A, Sazer S. Fission yeast Lem2 and Man1 perform fundamental functions of the animal cell nuclear lamina. Nucleus. 2012;3:60–76. doi: 10.4161/nucl.18824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grund SE, Fischer T, Cabal GG, Antunez O, Perez-Ortin JE, Hurt E. The inner nuclear membrane protein Src1 associates with subtelomeric genes and alters their regulated gene expression. J Cell Biol. 2008;182:897–910. doi: 10.1083/jcb.200803098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RE, Ashe HL. Cease and desist: modulating short-range Dpp signalling in the stem-cell niche. EMBO Rep. 2011;12:519–526. doi: 10.1038/embor.2011.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu HJ, Drummond-Barbosa D. Insulin signals control the competence of the Drosophila female germline stem cell niche to respond to Notch ligands. Dev Biol. 2011;350:290–300. doi: 10.1016/j.ydbio.2010.11.032. [DOI] [PubMed] [Google Scholar]
- Hsu HJ, LaFever L, Drummond-Barbosa D. Diet controls normal and tumorous germline stem cells via insulin-dependent and -independent mechanisms in Drosophila. Dev Biol. 2008;313:700–712. doi: 10.1016/j.ydbio.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber MD, Guan T, Gerace L. Overlapping functions of nuclear envelope proteins NET25 (Lem2) and emerin in regulation of extracellular signal-regulated kinase signaling in myoblast differentiation. Mol Cell Biol. 2009;29:5718–5728. doi: 10.1128/MCB.00270-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Xia L, Chen D, Yang Y, Huang H, Yang L, Zhao Q, Shen L, Wang J, Chen D. Otefin, a nuclear membrane protein, determines the fate of germline stem cells in Drosophila via interaction with Smad complexes. Dev Cell. 2008;14:494–506. doi: 10.1016/j.devcel.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Kai T, Spradling A. An empty Drosophila stem cell niche reactivates the proliferation of ectopic cells. Proc Natl Acad Sci U S A. 2003;100:4633–4638. doi: 10.1073/pnas.0830856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiger AA, Jones DL, Schulz C, Rogers MB, Fuller MT. Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science. 2001;294:2542–2545. doi: 10.1126/science.1066707. [DOI] [PubMed] [Google Scholar]
- Koch AJ, Holaska JM. Loss of emerin alters myogenic signaling and miRNA expression in mouse myogenic progenitors. PLoS One. 2012;7:e37262. doi: 10.1371/journal.pone.0037262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korfali N, Wilkie GS, Swanson SK, Srsen V, de Las Heras J, Batrakou DG, Malik P, Zuleger N, Kerr AR, Florens L, Schirmer EC. The nuclear envelope proteome differs notably between tissues. Nucleus. 2012;3:552–564. doi: 10.4161/nucl.22257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasko PF, Ashburner M. The product of the Drosophila gene vasa is very similar to eukaryotic initiation factor-4A. Nature. 1988;335:611–617. doi: 10.1038/335611a0. [DOI] [PubMed] [Google Scholar]
- Le Bras S, Van Doren M. Development of the male germline stem cell niche in Drosophila. Dev Biol. 2006;294:92–103. doi: 10.1016/j.ydbio.2006.02.030. [DOI] [PubMed] [Google Scholar]
- Leatherman JL, Dinardo S. Zfh-1 controls somatic stem cell self-renewal in the Drosophila testis and nonautonomously influences germline stem cell self-renewal. Cell Stem Cell. 2008;3:44–54. doi: 10.1016/j.stem.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leatherman JL, Dinardo S. Germline self-renewal requires cyst stem cells and stat regulates niche adhesion in Drosophila testes. Nat Cell Biol. 2010;12:806–811. doi: 10.1038/ncb2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KK, Wilson KL. All in the family: evidence for four new LEM-domain proteins Lem2 (NET-25), Lem3, Lem4 and Lem5 in the human genome. Symp Soc Exp Biol. 2004:329–339. [PubMed] [Google Scholar]
- Lengil T, Gancz D, Gilboa L. Activin signaling balances proliferation and differentiation of ovarian niche precursors and enables adjustment of niche numbers. Development. 2015;142:883–892. doi: 10.1242/dev.113902. [DOI] [PubMed] [Google Scholar]
- Li MA, Alls JD, Avancini RM, Koo K, Godt D. The large Maf factor Traffic Jam controls gonad morphogenesis in Drosophila. Nat Cell Biol. 2003;5:994–1000. doi: 10.1038/ncb1058. [DOI] [PubMed] [Google Scholar]
- Lin F, Blake DL, Callebaut I, Skerjanc IS, Holmer L, McBurney MW, Paulin-Levasseur M, Worman HJ. MAN1, an inner nuclear membrane protein that shares the LEM domain with lamina-associated polypeptide 2 and emerin. J Biol Chem. 2000;275:4840–4847. doi: 10.1074/jbc.275.7.4840. [DOI] [PubMed] [Google Scholar]
- Lin H, Yue L, Spradling AC. The Drosophila fusome, a germline-specific organelle, contains membrane skeletal proteins and functions in cyst formation. Development. 1994;120:947–956. doi: 10.1242/dev.120.4.947. [DOI] [PubMed] [Google Scholar]
- Liu J, Lee KK, Segura-Totten M, Neufeld E, Wilson KL, Gruenbaum Y. MAN1 and emerin have overlapping function(s) essential for chromosome segregation and cell division in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2003;100:4598–4603. doi: 10.1073/pnas.0730821100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Losick VP, Morris LX, Fox DT, Spradling A. Drosophila stem cell niches: a decade of discovery suggests a unified view of stem cell regulation. Dev Cell. 2011;21:159–171. doi: 10.1016/j.devcel.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansharamani M, Wilson KL. Direct binding of nuclear membrane protein MAN1 to emerin in vitro and two modes of binding to barrier-to-autointegration factor. J Biol Chem. 2005;280:13863–13870. doi: 10.1074/jbc.M413020200. [DOI] [PubMed] [Google Scholar]
- Margolis J, Spradling A. Identification and behavior of epithelial stem cells in the Drosophila ovary. Development. 1995;121:3797–3807. doi: 10.1242/dev.121.11.3797. [DOI] [PubMed] [Google Scholar]
- Mekhail K, Seebacher J, Gygi SP, Moazed D. Role for perinuclear chromosome tethering in maintenance of genome stability. Nature. 2008;456:667–670. doi: 10.1038/nature07460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcon G, Kozlov S, Cutler DA, Sullivan T, Hernandez L, Zhao P, Mitchell S, Nader G, Bakay M, Rottman JN, Hoffman EP, Stewart CL. Loss of emerin at the nuclear envelope disrupts the Rb1/E2F and MyoD pathways during muscle regeneration. Hum Mol Genet. 2006;15:637–651. doi: 10.1093/hmg/ddi479. [DOI] [PubMed] [Google Scholar]
- Ozawa R, Hayashi YK, Ogawa M, Kurokawa R, Matsumoto H, Noguchi S, Nonaka I, Nishino I. Emerin-lacking mice show minimal motor and cardiac dysfunctions with nuclear-associated vacuoles. The American journal of pathology. 2006;168:907–917. doi: 10.2353/ajpath.2006.050564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padan R, Nainudel-Epszteyn S, Goitein R, Fainsod A, Gruenbaum Y. Isolation and characterization of the Drosophila nuclear envelope otefin cDNA. J Biol Chem. 1990;265:7808–7813. [PubMed] [Google Scholar]
- Papagiannouli F, Lohmann I. Shaping the niche: lessons from the Drosophila testis and other model systems. Biotechnology journal. 2012;7:723–736. doi: 10.1002/biot.201100352. [DOI] [PubMed] [Google Scholar]
- Pinto BS, Wilmington SR, Hornick EE, Wallrath LL, Geyer PK. Tissue-specific defects are caused by loss of the drosophila MAN1 LEM domain protein. Genetics. 2008;180:133–145. doi: 10.1534/genetics.108.091371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puente XS, Quesada V, Osorio FG, Cabanillas R, Cadinanos J, Fraile JM, Ordonez GR, Puente DA, Gutierrez-Fernandez A, Fanjul-Fernandez M, Levy N, Freije JM, Lopez-Otin C. Exome sequencing and functional analysis identifies BANF1 mutation as the cause of a hereditary progeroid syndrome. Am J Hum Genet. 2011;88:650–656. doi: 10.1016/j.ajhg.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reil M, Dabauvalle MC. Essential roles of LEM-domain protein MAN1 during organogenesis in Xenopus laevis and overlapping functions of emerin. Eur J Cell Biol. 2013;92:280–294. doi: 10.1016/j.ejcb.2013.10.008. [DOI] [PubMed] [Google Scholar]
- Rorth P. Gal4 in the Drosophila female germline. Mech Dev. 1998;78:113–118. doi: 10.1016/s0925-4773(98)00157-9. [DOI] [PubMed] [Google Scholar]
- Schirmer EC, Gerace L. The nuclear membrane proteome: extending the envelope. Trends Biochem Sci. 2005;30:551–558. doi: 10.1016/j.tibs.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Sheng XR, Posenau T, Gumulak-Smith JJ, Matunis E, Van Doren M, Wawersik M. Jak-STAT regulation of male germline stem cell establishment during Drosophila embryogenesis. Dev Biol. 2009;334:335–344. doi: 10.1016/j.ydbio.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimi T, Butin-Israeli V, Adam SA, Goldman RD. Nuclear lamins in cell regulation and disease. Cold Spring Harb Symp Quant Biol. 2010;75:525–531. doi: 10.1101/sqb.2010.75.045. [DOI] [PubMed] [Google Scholar]
- Song X, Call GB, Kirilly D, Xie T. Notch signaling controls germline stem cell niche formation in the Drosophila ovary. Development. 2007;134:1071–1080. doi: 10.1242/dev.003392. [DOI] [PubMed] [Google Scholar]
- Song X, Wong MD, Kawase E, Xi R, Ding BC, McCarthy JJ, Xie T. Bmp signals from niche cells directly repress transcription of a differentiation-promoting gene, bag of marbles, in germline stem cells in the Drosophila ovary. Development. 2004;131:1353–1364. doi: 10.1242/dev.01026. [DOI] [PubMed] [Google Scholar]
- Spradling A, Fuller MT, Braun RE, Yoshida S. Germline stem cells. Cold Spring Harb Perspect Biol. 2011;3:a002642. doi: 10.1101/cshperspect.a002642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuneizumi K, Nakayama T, Kamoshida Y, Kornberg TB, Christian JL, Tabata T. Daughters against dpp modulates dpp organizing activity in Drosophila wing development. Nature. 1997;389:627–631. doi: 10.1038/39362. [DOI] [PubMed] [Google Scholar]
- Tulina N, Matunis E. Control of stem cell self-renewal in Drosophila spermatogenesis by JAK-STAT signaling. Science. 2001;294:2546–2549. doi: 10.1126/science.1066700. [DOI] [PubMed] [Google Scholar]
- Wagner N, Krohne G. LEM-Domain proteins: new insights into lamin-interacting proteins. Int Rev Cytol. 2007;261:1–46. doi: 10.1016/S0074-7696(07)61001-8. [DOI] [PubMed] [Google Scholar]
- Wagner N, Weyhersmuller A, Blauth A, Schuhmann T, Heckmann M, Krohne G, Samakovlis C. The Drosophila LEM-domain protein MAN1 antagonizes BMP signaling at the neuromuscular junction and the wing crossveins. Dev Biol. 2010;339:1–13. doi: 10.1016/j.ydbio.2009.11.036. [DOI] [PubMed] [Google Scholar]
- Wallenfang MR, Nayak R, DiNardo S. Dynamics of the male germline stem cell population during aging of Drosophila melanogaster. Aging Cell. 2006;5:297–304. doi: 10.1111/j.1474-9726.2006.00221.x. [DOI] [PubMed] [Google Scholar]
- Ward EJ, Shcherbata HR, Reynolds SH, Fischer KA, Hatfield SD, Ruohola-Baker H. Stem cells signal to the niche through the Notch pathway in the Drosophila ovary. Curr Biol. 2006;16:2352–2358. doi: 10.1016/j.cub.2006.10.022. [DOI] [PubMed] [Google Scholar]
- Worman HJ, Ostlund C, Wang Y. Diseases of the nuclear envelope. Cold Spring Harb Perspect Biol. 2010;2:a000760. doi: 10.1101/cshperspect.a000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie T. Control of germline stem cell self-renewal and differentiation in the Drosophila ovary: concerted actions of niche signals and intrinsic factors. Wiley interdisciplinary reviews. Developmental biology. 2013;2:261–273. doi: 10.1002/wdev.60. [DOI] [PubMed] [Google Scholar]
- Xie T, Spradling AC. decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell. 1998;94:251–260. doi: 10.1016/s0092-8674(00)81424-5. [DOI] [PubMed] [Google Scholar]
- Xie T, Spradling AC. A niche maintaining germ line stem cells in the Drosophila ovary. Science. 2000;290:328–330. doi: 10.1126/science.290.5490.328. [DOI] [PubMed] [Google Scholar]
- Yamashita YM, Jones DL, Fuller MT. Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science. 2003;301:1547–1550. doi: 10.1126/science.1087795. [DOI] [PubMed] [Google Scholar]
- Zheng R, Ghirlando R, Lee MS, Mizuuchi K, Krause M, Craigie R. Barrier-to-autointegration factor (BAF) bridges DNA in a discrete, higher-order nucleoprotein complex. Proc Natl Acad Sci U S A. 2000;97:8997–9002. doi: 10.1073/pnas.150240197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu CH, Xie T. Clonal expansion of ovarian germline stem cells during niche formation in Drosophila. Development. 2003;130:2579–2588. doi: 10.1242/dev.00499. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.