Abstract
Background and Objectives
To examine the effectiveness of behavioral interventions for melanoma prevention targeted to individuals at elevated risk due to personal and/or family history.
Methods
Through literature searches in 5 search databases (through July 2014), 20 articles describing 14 unique interventions focused on melanoma prevention among individuals at elevated risk for the disease were identified. Interventions targeting only patients undergoing active treatment for melanoma were excluded.
Results
The average study quality was moderate. The majority of interventions (6 out of 9, 66% of studies) led to improvements in one or more photoprotective behaviors, particularly for improvements in use of protective clothing (3 out of 5, 60% of studies), and frequency and/or thoroughness of skin self-examinations (9 out of 12, 75%). Fewer interventions (5 out of 14, 36%) targeted uptake of total body skin examinations (60% led to improvements). Also, fewer interventions targeted all three preventive behaviors (5 out of 14, 36%).
Conclusions
Findings suggest future interventions should aim to improve adherence across multiple preventive behaviors, over a longer time period (past 8 months post-intervention), and target high-risk children. Studies should include adequate sample sizes to investigate moderators and mediators of intervention effectiveness. Interventions may be strengthened by new techniques, such as incorporating family members (e.g., to improve thoroughness of skin self-examinations) and eHealth technology.
Keywords: Melanoma, prevention, intervention, review, high-risk
Introduction
Melanoma is the 6th most common form of cancer in the United States and is associated with significant morbidity and mortality (Guy, Machlin, Ekwueme, & Yabroff, 2014; Surveillance Epidemiology and End Results Program, 2013, 2015). Prevention of melanoma is therefore a major public health priority (U.S. Department of Health and Human Services, 2014). Thus far, the majority of interventions designed to prevent melanoma have consisted of primary prevention strategies for the general population (Buller & Borland, 1999; Lin, Eder, & Weinmann, 2011; Poochareon, Federman, & Kirsner, 2004). Interventions typically target individuals’ use of photoprotection, including regular use of sunscreen and physical barriers such as protective clothing and shade structures, and reducing engagement in risk behaviors such as use of tanning booths. While primary prevention of melanoma is essential, recent literature has emphasized the importance of targeting cancer prevention approaches to those at elevated risk due to biological or behavioral risk factors (Diao & Lee, 2013; Manne et al., 2004; McLoone, Menzies, Meiser, Mann, & Kasparian, 2013; Miller et al., 2015; Niendorf & Tsao, 2006; Pharoah et al., 2002). Focusing on individuals at elevated risk for melanoma is particularly relevant because skin cancer prevention guidelines emphasize the importance of screening higher risk populations, as opposed to the general population, to facilitate early detection (U.S. Preventive Services Task Force, 2009), and screening may improve melanoma-related mortality (Katalinic et al., 2012; Schneider, Moore, & Mendelsohn, 2008).
For melanoma, individuals who have a personal or family history of the disease are at particularly elevated risk for developing the disease (Burden et al., 1994; Ferrone et al., 2005; Leachman et al., 2009; Siskind, Aitken, Green, & Martin, 2002). Risk for melanoma is 2-fold for individuals with a first-degree relative with melanoma (Cho, Rosner, Feskanich, & Colditz, 2005; Ford et al., 1995). In contrast, risk for developing melanoma is up to 70-fold (Bishop et al., 2002) for individuals who carry the CDKN2A/p16 genetic mutation. In addition to photoprotection to reduce risk for developing melanoma (Balk, 2011; Green, Wallingford, & McBride, 2011), early detection is vital and effective for high-risk groups (Yagerman & Marghoob, 2013). Early detection includes obtaining annual total body skin exams (TBSEs) from health providers, typically thought of as the screening gold standard, and implementing regular skin self-examinations (SSEs), particularly important since melanoma can develop and spread rapidly.
To date, there is no comprehensive, systematic review of behavioral interventions to prevent melanoma in individuals (both pediatric and adult populations) at elevated risk due to personal and/or family history. Pediatric populations are important to include in melanoma prevention efforts because modifiable environmental risk factors for melanoma, such as ultraviolet radiation (UVR) exposure and severe sunburns, date back to childhood (Dennis et al., 2008; Oliveria, Saraiya, Geller, Heneghan, & Jorgensen, 2006; Pustisek, Sikanic-Dugic, Hirsl-Hecej, & Domljan, 2010; Wu, Han, Laden, & Qureshi, 2014). Prior reviews focused on efforts to improve the psychological and health behavior functioning of individuals with a personal history of melanoma (McLoone et al., 2013) or on melanoma prevention in the general pediatric or adult population (Buller & Borland, 1999; Poochareon et al., 2004). The goals of the current systematic review were to summarize the empirical literature on melanoma preventive interventions targeting children and adults at elevated risk and to use the review results to provide recommendations for future development of interventions for at-risk populations.
Materials and Methods
Search Strategy
We conducted searches in PubMed, SCOPUS, PsycINFO, Ebscohost (including CINAHL), and GoogleScholar for all literature indexed in these search engines through July 2014. Librarian-designed search strategies that incorporated the following terms were used: melanoma, prevention, screening, intervention, sun protection, photoprotection, risk, behavior, behaviour, teenage, child, infant, pediatric. As an example, the PubMed search strategy is provided in the Appendix. The wildcard (*) symbol was used whenever possible. Reference lists of articles collected were also hand-searched to identify additional articles that could be included in the current review.
Inclusion and exclusion criteria
Articles were eligible for inclusion in the current review if they: 1) included participants at elevated risk for melanoma, defined as a higher level of risk compared to the general population due to personal or family history for melanoma; 2) tested an intervention expected to promote adherence to at least one melanoma preventive behavior (i.e., photoprotection, SSE, TBSE); 3) included ≥1 outcome assessing implementation of the preventive behavior(s) outside of the intervention session; 4) were published in a peer-reviewed journal; and 5) were written in English. Articles were excluded if they focused exclusively on individuals currently undergoing active treatment for melanoma because treatment, not prevention, is typically the main priority.
Data extraction
Each article was coded for characteristics describing the sample, study design, the intervention, and outcomes. Study sample characteristics coded included participant age and type of elevated melanoma risk. Study design characteristics included the sample size at baseline, participant recruitment strategies, number of assessment timepoints, and length of follow-up. Intervention characteristics and outcomes included intervention participants (i.e., individual alone, individual + partner or other family members), content, theoretical model underlying the intervention, intervention format, number of sessions, interventionist (e.g., nurse), preventive behavior(s) targeted (determined based on which preventive behaviors the study author reported they were intending to change and the presence of outcome measures for those behavior), outcome measures and length of follow-up, and changes in preventive behavior(s). We also coded for moderators and mediators of intervention effectiveness. Moderator findings that differentiate participant characteristics associated with better or worse intervention outcomes could inform methods for tailoring interventions to maximize intervention effectiveness. Mediator findings explain the mechanisms by which interventions work and augment understanding of the processes through which interventions impact preventive behaviors. Study quality was assessed using the GRADE method (Higgins & Green, 2008). Interrater reliability coding on a subset (25%) of articles was adequate (85% agreement). Disagreements about coding were resolved via discussion.
Results
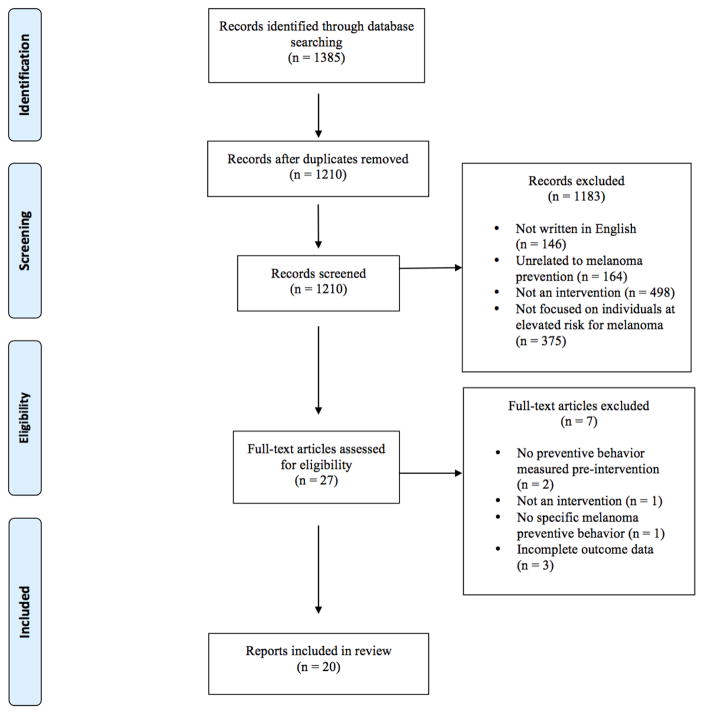
The search strategies identified 1,385 articles (Figure 1). Of those, 20 met the inclusion criteria and are summarized in the current review (see Tables 1 and 2). The 20 published reports describe findings from 14 studies.
Figure 1.
Selection of reports included in systematic review
Table 1.
Summary of study characteristics, design and theoretical basis across all studies included in the review
| Sample Characteristics | n | SD | Range |
|---|---|---|---|
| Mean Sample Size | 194 | 208 | 11–724 |
|
| |||
| Median Sample Size | 100 | - | - |
|
| |||
| Mean Age of Participants | 43.0 | * | 7–60a |
|
| |||
| Elevated Risk Sample Targeted | # of unique studies | % | |
|
| |||
| Family history of melanoma | 4 | 29 | |
| Multiple risk factorsb | 4 | 29 | |
| CDKN2A/p16 mutation | 3 | 21 | |
| Personal history of melanoma | 2 | 14 | |
| Presence of dysplastic nevi | 1 | 7 | |
|
| |||
| Study Design and Recruitment | |||
|
| |||
| Study Design | # of unique studies | % | |
|
| |||
| Randomized controlled trial | 10 | 71 | |
| Single group, pre-post design | 4 | 29 | |
|
| |||
| Recruitment Strategy | # of unique studies | % | |
|
| |||
| Clinic | 7 | 50 | |
| Registry/medical records | 5 | 36 | |
| Family members of clinic patient | 1 | 7 | |
| Newspaper advertisement | 1 | 7 | |
|
| |||
| Intervention Content and Theoretical Basis | |||
|
| |||
| Content | # of unique studies | % | |
|
| |||
| General melanoma education | 7 | 50 | |
| Sunscreen education, limit UVR exposure | 6 | 43 | |
| Photoprotection strategies for children | 1 | 7 | |
| SSE importance and/or instruction | 10 | 71 | |
| Mole mapping | 1 | 7 | |
| TBSE education | 5 | 36 | |
| Melanoma genetic testing & education | 4 | 29 | |
| Behavior change strategies | 2 | 14 | |
| Assessment of risk factors | 4 | 29 | |
| Dangers of tanning | 1 | 7 | |
|
| |||
| Theoretical Model Underlying Intervention | # of reports | % | |
| Intervention grounded in ≥1 Model | 9 | 45 | |
| Social Cognitive Theory | 5 | 25 | |
| Health Belief Model | 5 | 25 | |
| Theory of Planned Behavior | 2 | 10 | |
|
| |||
| Preventive Behaviors Targeted by Interventions | # of reports | % | |
|
| |||
| Photoprotection | 10 | 71 | |
| SSE | 11 | 79 | |
| TBSE | 5 | 36 | |
|
| |||
| Adherence Outcome Assessment | # of reports | % | |
|
| |||
| Investigator designed self-reported questionnaire | 15 | 75 | |
| Validated self-reported questionnaire | 5 | 25 | |
| Diary assessment | 3 | 15 | |
| Telephone interview | 2 | 10 | |
| Skin exam by healthcare provider | 1 | 5 | |
Notes. SSE = Skin Self-Examination, TBSE = Total Body Skin Exam, UVR = Ultraviolet Radiation
Could not be calculated based on data provided in reports.
Range of mean ages of participants across studies.
Risk factors included phenotypic factors (e.g., red hair, freckling, sun-sensitive skin), personal/family history of melanoma, number of nevi, presence of sunburn in childhood.
Table 2.
Study and intervention features for each report included in the review
| Authors (Year) | Sample Size, Gender, Study Groups | Participant Age (Mean/Mode, Total Sample and By Group) | Type of Melanoma Risk | Intervention Format | Intervention Content | Post-Intervention Outcomes |
|---|---|---|---|---|---|---|
| Aspinwall, Leaf, Dola, Kohlmann, Leachman (2008) | 64a (48.4% female) Carrier/no history (n=14); Carrier/with history (n=18); Noncarrier/no history (n=27); Noncarrier/with history (n=3) |
M=46.0, SD=16.1 Unaffected carriers: M=38.0, SD=14.6 Affected carriers: M=49.6, SD=14.1 Unaffected noncarriers: M=44.6, SD=14.4 Affected noncarriers: M=76.3, SD=14.8 |
CDKN2A/p16/ family history of melanoma as confirmed by the Utah Cancer Registry and the Utah Population Database | Individual session | Genetic test reporting + melanoma genetics education and risk communication1 | 1 month post-intervention: SSE
|
| Aspinwall, Leaf, Kohlmann, Dola, Leachman (2009) | See Aspinwall et al. (2008)a | See Aspinwall et al. (2008) | See Aspinwall et al. (2008) | Individual session | Genetic test reporting + melanoma genetics education and risk communication1 | 1 month post-intervention: Photoprotection
|
| Aspinwall, Taber, Leaf, Kohlmann, Leachman (2013) | 37a (45.9% female) | M=45.2, SD=14.7 | See Aspinwall et al. (2008) | Individual session | Genetic test reporting + melanoma genetics education and risk communication1 |
24 months post-intervention: SSE
|
| Aspinwall, Taber, Kohlmann, Leaf, Leachman (2014) | See Aspinwall et al. (2013)a | See Aspinwall et al. (2013) | See Aspinwall et al. (2008) | Individual session | Genetic test reporting + melanoma genetics education and risk communication1 |
24 months post-intervention: Photoprotection
|
| Bergenmar, Hansson, Brandberg (2009) | 11 (45% female) Carriers n=4; non-carriers n=5; inconclusive result n=2 |
M=36, SD=8.1 carriers: M=40.3, SD=9.4 noncarriers: M=36.6, SD=4.8 inconclusive result: M=25.5, SD=2.1 |
Potential CDKN2A mutation-carriers identified through genetic testing offered to their family members who were patients at a pigmented lesion clinic | Individual session | Genetic test reporting + recommendations for skin exams and to be careful in the sun for those with CDKN2A+ reports and with dysplastic nevi regardless of genetic risk1 | 6 month post-intervention: Risk behaviors
|
| Berwick, Oliveria, Luo, Headley, Bolognia (2000) | 75 (55% female) high-risk n=60; low- risk n=15 |
M=44.0 low-risk (no history of melanoma, participating in population study): M=55.0 high-risk (history of melanoma or multiple atypical nevi attending a pigmented lesion clinic): M=33.0 |
Family history of melanoma; high-risk phenotypic risk factors; personal histories of CM or atypical nevi | Individual session + educational material | Educational session on clinical characteristics of CM, risk factors for melanoma, and methods for conducting SSE1 | 6–18 months post-intervention: SSE
|
| Boone, Stapleton, Turrisi, Ortiz, Robinson, Mallett (2009) | 130 pairsb (56.9% female) Included in analysis: Dyadic training group n=40; Solo training group n=16 |
Age ranges from 18–29 to ≥70 Dyadic: mode age groups=30–39, 40–49; Solo: mode age group=60–692 |
Diagnosed with melanoma | Dyadic or solo intervention + enabling kit (see next column) |
Treatment Group: Education and skills training session with demonstration of ABCDE rule and a 15-minute skills training with partner, and enabling kit with laminated card with color examples of ABCDE rule, five drawings illustrating checking body sites using a hand-held mirror, a lighted hand magnifying glass, a millimeter ruler, and diagrams of the body positions needed to perform a complete SSE. Control Group: Same intervention without partner present. |
4 months post-intervention SSE
|
| Geller, Emmons, Brooks, Powers, Zhang, Koh, Heeren, Sober, Li, Gilchrest (2006) | 494 (237 intervention, 51.9% female; 297 usual care, 54.9% female) | Intervention: 55.7% 18–50 years old; 44.3% older than 51 years Usual care: 60.6% 18–50 years old, 39.4% older than 51 years |
Biological siblings of patients with melanoma | Telephone consult + tailored information packets mailed to home vs. usual care |
Treatment Group: Motivational and goal-setting telephone intervention session + computer-generated tailored print materials targeting level of participation in SSE, TBSE, and sun protection. Control Group (Usual care): Physicians recommended that patients diagnosed with melanoma inform family about their diagnosis and encourage family members to receive screening. |
12 months post-intervention (includes data 6 months post-intervention): Photoprotection
|
| Glanz, Schoenfeld, Steffen (2010) | 724 (77.5% female) Intervention Completers n=307 (79.8% female); Control Completers n=289 (79.9% female) |
M=41.7, SD=11.0 Intervention Completers M=42.4, SD=10.6; Control Completers M=41.3, SD=11.0 |
Moderate or high risk for skin cancer as measured by the Brief Skin Cancer Risk Assessment Tool (BRAT) | Tailored print material mailed to home |
Treatment Group: Tailored risk feedback and recommendations + UVR self-monitoring aids, SSE instructions and practice tools, and skin cancer prevention and detection information Control Group: Standard sun safety booklet, a tip sheet on sunscreen use, and a bookmark encouraging SSE |
3 months post-intervention: Photoprotection
|
| Glanz, Volpicelli, Kanetsky, Ming, Schuchter, Jepson, Domchek, Armstrong (2013) | 73 (68.5% female) Treatment group n=35, 80% female; Control group n=38, 57.9% female |
M=59.5, SD=15.5 Treatment group M=62.4 SD=14.6; Control group M=56.9, SD=16.1 |
Family history of melanoma (defined as 3 or more verified cases of melanoma in first-degree relatives) | In-person genetic consultation + information brochure |
Treatment Group: Background on genotyping and genetic testing for melanoma, including a description of CDKN2A and MC1R; For those who tested positive (n=5), a genetic counselor reviewed the risks associated with these genes + one- page skin cancer prevention brochure. Control Group: Standard practice – No offer of genetic counseling or disclosure of genotyping results. Participants received a one-page generic skin cancer prevention brochure via mail. |
4 months post-intervention: Photoprotection
|
| Glazebrook, Garrud, Avery, Coupland, Williams (2006) | 589 (80.3% female) Intervention group n=259, 82.6% female; Control group n=330, 78.5% female |
Intervention: M=38.2, SD=14.3; Control: M=38.4, SD=15.2 | Screened to have at least one high-risk characteristic: red hair, multiple moles, history of sunburn as a child, freckling, family history of melanoma, fair sun- sensitive skin | Individual computer session |
Treatment Group (Skinsafe): Computer program comprising 8 sections (10–15 minutes total) on dangers of excessive sun exposure; how to protect skin from the sun; features of skin at risk; early signs of melanoma; how to reduce risk from melanoma; how to check skin for suspicious lesions. The final section prompted consideration of personal risk factors and provided tailored feedback regarding relative risk. Control Group: No details provided. |
6 months post-intervention: Photoprotection & SSE
|
| Gritz, Tripp, Peterson, Prokhorov, Shete, Urbauer, Fellman, Lee, Gershenwald (2013) | 340 child- parent pairs (61.8% female) Survivor/Intervention group n=170, 61.2% female; Survivor/Control group n=170, 62.4% female Child/Intervention group: n=170, 44.7% female; Child/Control group n=170, 53.5% female |
Survivor/Intervention: M=40.4, SD=6.4 Survivor/Control: M=40.5, SD=6.5 Child/Intervention: M=7.3, SD=3.9 Child/Control: M=7.3, SD=3.8 |
Children of melanoma survivors | Mailings to home (DVD, print booklet, children’s activity book, magnet) |
Treatment Group: Targeted sun- protection information for children, including testimonials/videos about survivors protecting their children and how, with an emphasis on overcoming barriers. Also included were children’s activity booklet and magnet. Control Group: Three health-related brochures available to the public on sun protection, physical activity, and nutrition. |
1 month post-intervention: Photoprotection
Photoprotection
|
| Hay, Oliveria, Dusza, Phelan, Ostroff, Halpern (2006) | 100c (63% female) Treatment group n=49, 59% female; Control group n=51, 67% female* |
M=40.0, SD=11.7 | Patients with 5 or more clinically dysplastic or atypical nevi | 2-hour individual session + personalized photobook/p amphlet and mole diary |
Treatment Group: Dermatologist presentation on SSE, types of skin cancer, and sun-protection advice + whole body photograph + 3-minute video on SSE + nurse-guided visualization exercise (i.e., visualizing “being at home in a comfortable, well-lit room”) + personalized photobook for SSE. Control Group: Written pamphlet on how to perform SSE and how to record moles in a diary format (no photobook). |
4 months post-intervention: SSE
|
| Kasparian, Meiser, Butow, Simpson, Mann (2009) | 119 completed baseline (52% female) Treatment group (offered genetic testing) n=29, 62% female; Control group (declined testing) n=76, 45% female |
Mode age= >60 (age ranges from 18–29 to >60) | Strong family history of melanoma (i.e., families with at least three relatives with a confirmed melanoma diagnosis) and a known family- specific CDKN2A mutation | Individual sessions |
Treatment Group: Received genetic testing. Non-experimental Control Group: Declined and did not receive genetic testing. |
12 months post-intervention: Photoprotection
|
| Manne, Jacobsen, Ming, Winkel, Dessureault, Lessin (2010) | 443 (63% female) Treatment group n=225, 60% female; Control group n=218, 66% female |
M=47.6, SD=13.2 Intervention: M=47.1, SD=13.9; Control: M=48.1, SD=12.6 |
First-degree relatives of patients recruited from the cutaneous oncology practices at 3 medical centers | 1 tailored telephone session + tailored intervention material mailed to home on a monthly basis (3 mailings total) for both groups |
Treatment Group: Printed mailing tailored based on participants’ responses to baseline questionnaires + tailored phone consultation on risks for CM, benefits of TBSE/SSE, barriers to SSE, ways to self-motivate and conduct SSEs, and ways to increase sun-protection habits. Control Group: Generic telephone counseling and print based on “best available public health materials.” |
6 months post-intervention: Photoprotection
Photoprotection
|
| Oliveria, Dusza, Phelan, Ostroff, Berwick, Halpern (2004) | See Hay et al. (2006)c | Mode age=30–39 (age ranges from <20 to ≥60) Intervention: Mode=30–39; Control: Mode=30–39 | 5 or more clinical dysplastic/atypical nevi | Individual session + personalized photobook/pamphlet and mole diary |
Treatment Group: Teaching intervention (physician and nurse education modules) with personalized photo book including digital whole-body photography. Control Group: Pamphlet and diary, “Skin Cancer: If You Can Spot It” (no photobook). |
4 month post-intervention: SSE
|
| Rat, Quereux, Riviere, Clouet, Senand, Volteau, Dreno, Nguyen (2014) | 173 (76% female) Intervention group n=97, 76% female; Control group n=76, 76% female |
Intervention: M=43.6; SD=17.1; Control: M=42.8; SD=14.6 | Elevated melanoma risk as assessed by the Self-Assessment Melanoma Risk Score (SAMScore) | Individual session tailored by SAMScore risk calculator results vs. individual session (not tailored) + public health posters in office |
Treatment Group: If determined to be at high risk with SAMScore calculator, practitioners tailored session with TBSE, counseling, and handout on primary and secondary prevention behaviors (i.e., sunbathing, tanning bed use, taking protective action during sun exposure, performing SSE, using mirror or having another person assist with SSE, taking a photograph for use with SSE, “feeling able to detect a change in a mole”, and experiencing a sunburn). Control Group: Public health campaign poster in the waiting room; no use of risk calculator; TBSE performed at practitioner’s discretion. All patients with suspicious lesions referred to dermatologist. |
5 months post-intervention: Photoprotection
|
| Robinson, Turrisi, Stapleton (2007) | 130 pairsb (50% female) Intervention group n=65, 51% female; Control group n=65, 49% female |
Age ranges from 18–29 to ≥70 Dyadic: mode age group=30–39; Solo: mode age group=60–692 |
Personal history of CM treated at least 6 months ago, family history of CM, large number of nevi (50), or 2 or more pathologically confirmed atypical nevi | Solo vs. dyadic (with spouse or co-habiting partner) session |
Treatment Group: Visual presentation on SSE and ABCD rule for mole screening and in-vivo skills demonstration with partner present. Control Group: Same intervention in a solo learning condition (i.e., without partner present). All participants received enabling kit with ABCD card, lighted handheld magnifying glass, and set of body maps to use as a diary to record areas of concern found during the monthly SSE. |
4 months post-intervention: SSE
|
| Robinson, Turrisi, Mallett, Stapleton, Pion (2010) | 40 (52.5% female) Intervention group n=19, 53% female; Control group n=21, 52% female |
Age ranges from 18–29 to ≥70 In-Person: mode age group=40–49; Workbook: mode age groups=18- 29, 50–59 |
History of stage I or IIA melanoma | In-person training vs. workbook intervention session |
Treatment Group: Participants and their partners received a 39- page illustrated workbook about melanoma, enabling kit use, and 12 confidence-building/skills training exercises developed based on the authors’ clinical experiences. Dyads were instructed to read the workbook together and write questions/comments in the workbook. Control Group: Participants and their partners jointly completed a skills quiz on assessing visual qualities of melanoma and received additional instruction on ABCDE criteria with an enabling kit that contained a ruler, a magnifying lens, a laminated card with color examples of the ABCDE rule, and body maps. |
4 month post-intervention (includes 1 month data): SSE
|
| Taber, Aspinwall, Leaf, Kohlmann, Leachman (2013) | 61a (45.9% female) | No age information provided | CDKN2A/p16 risk | Individual session + follow-up letter | Genetic test reporting and counseling + monthly SSE recommendations reiterated in a mailed follow-up letter.1 |
24 months post-intervention: SSE
|
Notes: UVR = Ultraviolet Radiation, CM = Cutaneous Melanoma, SSE = Skin Self-Examination, TBSE = Total Body Skin Exam, ABCD/ABCDE = acronym for detecting potentially problematic moles: asymmetry, border, color, diameter, and evolving. Bolded assessment titles in the post-intervention column are follow-up assessments (occurring after the first reported assessment post-intervention).
Study design had only an intervention group and no identified control group.
Age of participants was reported in ranges (i.e., 18–29 years, 30–39, 40–49, 50–59, 60–69, ≥70). The most frequently endorsed ranges (mode groups) are reported in this table.
These study samples included the same participants (i.e., all publications marked with an ‘a’ included participants from the same study).
No information about the gender composition of the sample was provided.
Sample characteristics and study design
A summary of the average number of participants, definitions of elevated risk, study design, and recruitment strategies is contained in Table 1. Across the studies included, participants’ mean ages ranged from 7 to 60 years (mean=43.0 years). All but one study focused on adults (Gritz et al., 2013). Of the studies that examined photoprotection and/or UVR exposure as an outcome (n=9), 33% (n=3) controlled for season. Most of the 14 studies measured preventive behavior outcomes at a single timepoint after the intervention (n=9, 64%), and the remainder reported 2 assessments. The average length of follow-up post-intervention across the 14 studies was 8 months (SD=6), with the maximum follow-up period being 24 months. Across the 14 studies, the first reported assessment of preventive behavior outcomes occurred on average at 5 months (SD=3, range: 1–12). Second assessments occurred on average at 8 months (SD=5, range: 4–24).
Intervention content, theoretical basis, and format
The content of interventions is presented in Tables 1 and 2. The most common content included education on the importance of and/or how to conduct SSEs (71%) and education on melanoma and skin cancer and their prevention (50%). Less common content included behavior change strategies (14%), and mole mapping and digital photography to assist in performing TBSEs and SSEs (7%). Almost half of the reports stated that the intervention tested was grounded in one or more theoretical models (Table 1). Interventions varied in their format. The most common format consisted of in-person intervention sessions with a healthcare provider or trained study staff member (n=9, 60%). Of the studies that tested in-person interventions, other formats used included tools to assist with SSEs (e.g., magnifying glass; n=3), mole diaries (n=2), and written materials on melanoma prevention and/or screening (n=10). Other formats used less frequently included print/multimedia materials mailed to the home (n=2) (Glanz, Schoenfeld, & Steffen, 2010; Gritz et al., 2013), telephone session plus written materials (n=2) (Geller et al., 2006; Manne et al., 2010), and computer-delivered material (n=1) (Glazebrook, Garrud, Avery, Coupland, & Williams, 2006).
Intervention outcome(s)
The primary preventive behavior outcome for the vast majority of interventions was the occurrence, frequency, and/or thoroughness of SSEs (n=11, 79%). A similar proportion of interventions targeted photoprotection and UVR exposure (n=10, 71%). Fewer intervened on TBSEs, which are typically recommended on an annual or semi-annual basis for high-risk populations without a history of melanoma (n=5, 36%). Approximately one-third of interventions (n=5, 36%) targeted all three preventive behaviors. Approximately one-quarter each of interventions targeted photoprotection only (n=3, 21%), and SSE only (n=4, 29%), and the remainder targeted both photoprotection and SSE (n=2, 14%).
Intervention delivery
The vast majority of interventions included only the individual at elevated risk for melanoma (n=11, 79%). Some interventions included both the individual at risk for melanoma and a family member, such as a partner/spouse or parent (n=3, 21%) (Boone et al., 2009; Gritz et al., 2013; Robinson, Turrisi, & Stapleton, 2007). Interventions were delivered by genetic counselors (n=3, 21%), physician and/or nurse (n=4, 29%), trained research assistant (n=2, 14%), health educator (n=2, 14%), via postal mailings (n=2, 14%), and via a computerized program (n=1, 7%). On average, sessions took place during 2 contacts with participants (SD=1.8, range: 1–7, mode=1).
Intervention effectiveness
Photoprotection
6 out of the 9 interventions targeting photoprotective behaviors (66%) produced statistically significant improvements in at least one self-reported photoprotective behaviors at one or more post-intervention assessments (Aspinwall, Leaf, Kohlmann, Dola, & Leachman, 2009; Aspinwall, Taber, Kohlmann, Leaf, & Leachman, 2014; Geller et al., 2006; Glanz et al., 2010; K. Glanz et al., 2013; Glazebrook et al., 2006; Gritz et al., 2013; Kasparian, Meiser, Butow, Simpson, & Mann, 2009; Manne et al., 2010; Rat et al., 2014). Photoprotective methods assessed included use of protective clothing, sunscreen, and decreasing UVR exposure. At the first assessment post-intervention, the majority of interventions targeting protective clothing (3 out of 5, 60%) reported significant improvements in these behaviors. Fewer interventions targeting sunscreen use (2 out of 6, 33%), decreased UVR exposure (1 out of 5, 20%) and composite measures of photoprotective behaviors (1 out of 3, 33%) reported significant improvements. Three interventions had follow-up assessments where the photoprotective outcomes were measured a second time post-intervention. Both interventions targeting protective clothing (2 out of 2, 100%), the lone intervention targeting composite photoprotective behaviors (1 out of 1, 100%), and 1 intervention targeting UVR exposure (1 out of 2, 50%) reported significant improvements in behaviors at follow-up compared with baseline. The interventions targeting sunscreen use did not report significant improvements in this behavior at follow-up as compared with baseline (0 out of 2, 0%). Photoprotection was most often assessed using self-report measures, and some studies also included interviews. Specific intervention findings are described below and in Table 2.
Three interventions employed genetic test reporting and counseling (Aspinwall et al., 2009; Aspinwall et al., 2014; Glanz et al., 2013; Kasparian et al., 2009). Significant improvements in the use of protective clothing was the most consistently reported preventive behavior (Aspinwall et al., 2009; Aspinwall et al., 2014; Glanz et al., 2013). There were no significant improvements in sunscreen use reported in any of the genetic testing interventions.
Three interventions targeted photoprotective behaviors among first-degree relatives of individuals with melanoma (Geller et al., 2006; Gritz et al., 2013; Manne et al., 2010). One intervention led to significant improvements in a composite photoprotection measure 1 year post-intervention but not at 6 months (Manne et al., 2010). The second intervention that included tailored educational materials, and telephone counseling sessions reported no significant improvements across photoprotective behaviors (Geller et al., 2006). Among children at risk for melanoma, an intervention that provided print, multimedia, and interactive materials on melanoma prevention produced significant improvements in sunscreen reapplication 1 month post-intervention and improvements in use of wide-brimmed hats 4 months post-intervention (Gritz et al., 2013).
Two interventions focused on individuals at risk for melanoma due to a range of risk factors (e.g., phenotypic factors, family history) (Glanz et al., 2010; Glazebrook et al., 2006). Glazebrook et al. tested a computer-based intervention, with results of increased photoprotective behaviors measured by a composite self-report measure (Glazebrook et al., 2006). Another intervention provided information on participant’s individualized risk for melanoma, associated recommendations for preventive behaviors, tools for monitoring UVR exposure, and SSE instructions and tools (Glanz et al., 2010). Use of hat and sunglasses improved significantly. There were significant improvements in sunscreen application as assessed via a diary, but not when assessed via the Sun Habits Survey.
Self skin-examination
Most interventions targeting SSE behaviors (9 out of 12 interventions, 75%) led to statistically significant improvements in the frequency and/or thoroughness of SSEs at least one follow-up assessment post-intervention (Aspinwall, Leaf, Dola, Kohlmann, & Leachman, 2008; Aspinwall, Taber, Leaf, Kohlmann, & Leachman, 2013; Berwick, Oliveria, Luo, Headley, & Bolognia, 2000; Boone et al., 2009; Geller et al., 2006; K. Glanz et al., 2010; Glanz et al., 2013; Glazebrook et al., 2006; Hay et al., 2006; Kasparian et al., 2009; Manne et al., 2010; Oliveria et al., 2004; Rat et al., 2014; Robinson, Turrisi, Mallett, Stapleton, & Pion, 2010; Robinson et al., 2007; Taber, Aspinwall, Leaf, Kohlmann, & Leachman, 2013). At the first assessment post-intervention, the majority of interventions targeting SSE reported significant improvements in SSE frequency (8 out of 12, 67%), but fewer targeting SSE thoroughness (1 out of 4, 25%) or both frequency and thoroughness (1 out of 3, 33%) reported improvements. Three interventions had follow-up assessments and reported significant improvements in SSE frequency (2 out of 3, 67%), SSE thoroughness (2 out of 2, 100%), and both SSE frequency and thoroughness (2 out of 2, 100%), relative to baseline levels. The specific intervention findings are described below and in Table 2.
Interventions ranged widely in how SSE recommendations were delivered. Some provided recommendations to perform SSEs in the context of delivering melanoma genetic test results (Aspinwall et al., 2008; Glanz et al., 2013), with improvements observed in SSE frequency (Aspinwall et al., 2008; Aspinwall et al., 2013; Glanz et al., 2013; Taber et al., 2013) and thoroughness (Aspinwall et al., 2008; Aspinwall et al., 2013; Taber et al., 2013). Other interventions provided more detailed instruction and tools for SSEs through in-person sessions (Berwick et al., 2000; Hay et al., 2006; Oliveria et al., 2004; Rat et al., 2014). These interventions led to significant improvements in SSE frequency but not thoroughness. Another intervention involved individuals’ partners in the intervention to facilitate partner-assisted skin exams (Boone et al., 2009; Robinson et al., 2007). Boone et al. was the only study to use an objective measure of SSE thoroughness (i.e., dermatologist-identified lesions) and demonstrated that participants missed concerning skin lesions in certain body areas, even if partners were involved in assisting with the skin exams (Boone et al., 2009).
Total body skin examination
Fewer interventions targeted TBSE adherence. Three out of the 5 interventions (60%) targeting TBSE adherence demonstrated statistically significant improvements. Two genetic testing interventions reported improved adherence to TBSE recommendations (Aspinwall et al., 2013; Kasparian et al., 2009) while another genetic testing intervention reported no improvements 4 months post-testing (Glanz et al., 2013). Two other interventions for relatives of melanoma patients had mixed findings for TBSE adherence at 1 year (Geller et al., 2006; Manne et al., 2010).
Other outcomes: Risk behaviors
Half of the studies (n=7, 50%) assessed risk behavior outcomes at post-intervention, including intentional sunbathing or being tan (n=4), tanning bed use (n=2), and sunburn occurrence (n=5). Studies reported decreased frequency of sunbathing or participant reports of being tan (Aspinwall et al., 2014; Bergenmar, Hansson, & Brandberg, 2009; Geller et al., 2006; Rat et al., 2014). There was limited evidence for changes in tanning bed use, which was generally low, with one study reporting no changes (Rat et al., 2014) and another study reporting mixed findings on changes in tanning bed use among the 2 participants who reported any tanning bed use (Aspinwall et al., 2014). Only one study demonstrated significant decreases in sunburn occurrence (Aspinwall et al., 2014) among participants (K. Glanz et al., 2010; K. Glanz et al., 2013; Gritz et al., 2013; Rat et al., 2014).
Moderators of intervention effectiveness
More than half of the reports (n=13, 65%, mean sample size=207, SD=223) examined potential moderators of intervention effectiveness. These included analyses of participant demographic characteristics such as gender and age (Boone et al., 2009; Glanz et al., 2010; Gritz et al., 2013), personal or family diagnosis of melanoma during the study (Aspinwall et al., 2014), knowledge of preventive behaviors (Robinson et al., 2010), seasonality associated with the intervention (Aspinwall et al., 2009), SSE self-efficacy (Geller et al., 2006; Robinson et al., 2010), and perceived barriers and benefits (Glanz et al., 2010; Taber et al., 2013). For example, demographic factors associated with improved preventive behavior adherence among children were parent participants who had younger children (Gritz et al., 2013), males having partner-assisted SSEs conducted by females (Boone et al., 2009), lack of melanoma family history (Gritz et al., 2013), socioeconomic factors (Aspinwall et al., 2013), and study site (Glanz et al., 2010). Studies with adults indicated that older participants had significantly greater improvements in adherence to melanoma preventive behaviors (Boone et al., 2009; Glanz et al., 2010). Psychosocial factors that were not found to be significant moderators of intervention outcomes included SSE efficacy, SSE attitude, and SSE knowledge (Robinson et al., 2010).
Some studies examined the extent to which illness- or risk-specific characteristics moderated intervention effectiveness (Aspinwall et al., 2009; Berwick et al., 2000; Gritz et al., 2013; Manne et al., 2010). Increases in SSE frequency were associated with younger age of diagnosis among the participant’s first-degree relative (Manne et al., 2010). Individuals at higher risk for melanoma generally had greater improvements in melanoma preventive behaviors compared with those at lower or unknown levels of risk (Aspinwall et al., 2008; Aspinwall et al., 2013; Glanz et al., 2010; Kasparian et al., 2009). One study noted, however, that although CDKN2A/p16 carriers were more likely to obtain TBSEs, there were no differences in sunscreen use or SSE behavior compared with individuals who had declined testing (Kasparian et al., 2009). Another study presented contrary findings that there was no difference in SSE occurrence post-intervention between those at high vs. low risk due to a personal history of melanoma or the presence of atypical nevi (Berwick et al., 2000).
Mediators of intervention effectiveness
Only a few reports (n=3, 15%, mean sample size=422, SD=313) examined potential mediators of intervention effectiveness, which are often constructs of interest in health behavior theories such as the Health Belief Model (Janz & Becker, 1984). Mediators examined included self-efficacy, perceived barriers and benefits, risk perceptions, social norms, intentions, and worry/anxiety related to preventive behaviors. Findings for self-efficacy varied: in one study, higher levels of SSE self-efficacy accounted for improvements in SSE outcomes (Hay et al., 2006), but in another study, sunscreen self-efficacy was not a significant mediator of photoprotection outcomes (Manne et al., 2010). Perceived barriers and benefits to melanoma preventive behaviors were generally not significantly related to preventive behavior implementation (Glanz et al., 2010; Manne et al., 2010). Perceived benefits of TBSEs and of photoprotection were not mediators of TBSE and photoprotection adherence, respectively (Manne et al., 2010).
There was mixed evidence for the role of intentions as mediators between intervention group and melanoma preventive behavior outcomes among two RCTs of interventions tailored to participants’ melanoma risk level (n=724, n=443) (Glanz et al., 2010; Manne et al., 2010). Other psychosocial factors that were not found to be significant mediators of photoprotection and SSE outcomes included melanoma worry, SSE anxiety, skin cancer knowledge, skin awareness, risk perception, and social norms (K. Glanz et al., 2010; Hay et al., 2006).
Risk of bias and study quality
Using the GRADE method recommended by the Cochrane Collaboration (Higgins & Green, 2008), we evaluated study quality and the potential risk for bias based on information in the reports. Study quality ranged from very low to high with average study quality rated as moderate. For reports of RCTs (n=12), we assessed for adequate sequence generation (33%, n=4), adequate allocation concealment (17%, n=2), and adequate blinding procedures (50%, n=6). For all reports, we assessed for adequate explanation of incomplete outcome data (32%, n=6), reports free of selective outcome reporting (47%, n=9), and reports free of other problems indicating the study was at high risk for bias (37%, n=7). In general, there was limited information in reports on the risk of bias dimensions.
Conclusions
The occurrence of melanoma and associated mortality are thought to be at least partially preventable through photoprotective strategies, and screening may facilitate early detection (Green, Williams, Logan, & Strutton, 2011; U.S. Department of Health and Human Services, 2014; Yagerman & Marghoob, 2013). As a result, behavioral interventions that promote individuals’ adherence to melanoma preventive behaviors, particularly among high-risk populations, are of utmost importance. The present review aims to inform future research by providing a systematic summary of the current state of the literature on behavioral interventions aimed at preventing melanoma among individuals at elevated risk due to personal or family history.
The majority of interventions demonstrated statistically significant improvements in the preventive behaviors targeted. Specifically, 66% of interventions targeting photoprotection, 75% of interventions targeting SSE, and 60% of interventions targeting TBSE led to significant improvements. These findings also underscore that individuals at elevated risk for melanoma were not, on average, optimally adherent to melanoma preventive behaviors prior to intervention. A larger proportion of interventions yielded improved use of protective clothing and SSE frequency than improvements in sunscreen use, decreased UVR exposure and SSE thoroughness. There were mixed findings on improvements in obtaining TBSEs. Our findings indicate that interventions are needed to improve high-risk individuals’ engagement in all photoprotective behaviors and to target not only SSE frequency but also thoroughness.
Follow-up assessments of outcomes were limited in duration. There were mixed findings on improvements in or sustaining of photoprotection outcomes. However, SSE improvements were consistently observed or sustained at follow-up. Follow-ups were unlikely to extend into and take place during the same season in which baseline assessments occurred, which could affect photoprotection outcomes due to seasonal differences. Future interventions could include follow-ups that allow assessments of adherence to preventive behaviors over a sufficiently long period (e.g., at least one year for TBSE) and seasonally-timed assessments for photoprotective behaviors.
The moderator findings indicated that personal history of melanoma, more recent personal diagnosis of melanoma, and younger age of family member’s diagnosis were associated with better intervention outcomes (Aspinwall et al., 2009; Berwick et al., 2000; Manne et al., 2010). These findings underscore the importance of targeting interventions that promote preventive behaviors to high-risk groups. Initial evidence from mediator findings was mixed for the role of self-efficacy and intentions as mechanisms underlying preventive behavior change. Future work is needed to elucidate intervention mechanisms accounting for improvements in preventive behavior adherence.
The results of the current review should be considered within the context of a few limitations. While the average study quality was moderate, quality of studies varied. The studies included in the current review defined elevated risk for melanoma in several ways. As the evidence base for behavioral interventions to improve adherence to melanoma preventive behaviors among high-risk individuals grows, it will be useful to summarize intervention outcomes by type of elevated risk.
Future interventions for high-risk populations could target the range of recommended preventive behaviors, including photoprotection, SSE, and TBSE. When targeting SSEs, interventions should ideally focus on both SSE frequency and thoroughness, which was done in only one-third of interventions. Thorough SSEs allow detection of problematic moles that could appear anywhere on the body, including areas less immediately visible (e.g., bottoms of feet, scalp).
Based on moderator findings, future interventions may benefit from tailoring content to the type of melanoma risk that individuals have (Glanz et al., 2010; Glazebrook et al., 2006; Manne et al., 2010). For instance, individuals at more moderate risk for melanoma or who do not have a personal history of the disease may require different interventions that increase the salience of risk. In addition, interventions are needed to address preventive behavior adherence among minor children at elevated risk for melanoma who have an opportunity to implement preventive behaviors from a young age (Dennis et al., 2008; Oliveria et al., 2006; Pustisek et al., 2010).
With the exception of one study that included individuals’ partners (Boone et al., 2009; Robinson et al., 2007), the interventions included in this review targeted high-risk individuals alone. However, it may be particularly useful to involve other family members in interventions so that participants learn to collaborate on or receive support for preventive behavior implementation (e.g., reminders to use photoprotection, assistance with SSEs) (Taber et al., 2013). To maximize sustainability of interventions, which can be intensive and expensive, future interventions could integrate technology platforms (e.g., web-based resources, virtual sessions) to increase convenience and exposure to intervention material (Cushing & Steele, 2010; Nilsen et al., 2012). In addition, sustainability could be augmented by incorporating intervention components delivered by health educators or trained bachelor’s or master’s level individuals. The interventions in the current review relied primarily on in-person intervention formats delivered by healthcare providers.
The results of this review hold several implications for the design of future studies. Half of the studies reviewed included moderator analyses and a smaller proportion (15%) included mediation analyses. Ideally, future interventions will continue to be grounded in theoretical frameworks relevant to health behaviors and utilize behavior change strategies with known effectiveness (Davis, Campbell, Hildon, Hobbs, & Michie, 2014; Glanz & Bishop, 2010; Hillhouse, Turrisi, & Kastner, 2000; Jackson & Aiken, 2000; Michie, van Stralen, & West, 2011). Studies that incorporate larger samples will be better powered to conduct moderator and mediator analyses that can guide intervention design and understanding of the mechanisms underlying intervention effectiveness.
Additional work to improve assessments of participants’ engagement in preventive behaviors is needed, particularly to validate self-reported measures with objective measures (e.g., UVR dosimetry or reflectance spectroscopy, skin exam implemented by healthcare provider, medical records documenting skin exam obtained from healthcare provider) (Glanz & Mayer, 2005). All of the studies reviewed used self-report measures of adherence to preventive behaviors, and relatively few used validated self-reported measures. It would also be useful to devise strategies to assess functionally overlapping photoprotective behaviors (e.g., use of protective clothing negates the need for sunscreen on covered skin).
Children and adults at high risk for melanoma are a vulnerable population that could benefit from targeted melanoma preventive interventions. Existing interventions for these at-risk individuals show promise for improving implementation of preventive behaviors. Future efforts are needed to build on these existing interventions and other interventions to promote cancer prevention health behaviors (Sabatino et al., 2012) by increasing efficacy and effectiveness across multiple melanoma preventive behaviors through incorporation of behavior change strategies, to target different at-risk populations, to identify mechanisms underlying effective interventions, and to continue innovations and progress in the formats and content of interventions. Melanoma preventive interventions that are tailored for high-risk populations are a unique opportunity to provide personalized cancer prevention leading to improved health outcomes.
Highlights.
Preventive interventions for those at-risk can improve photoprotection & screening.
Relatively few interventions targeted uptake of total body skin examinations.
Future interventions could promote adherence across multiple preventive behaviors.
Melanoma preventive interventions for high-risk children are needed.
Acknowledgments
This work was supported, in part, by the National Cancer Institute (NCI) of the National Institutes of Health (NIH) K07CA196985 and the Huntsman Cancer Foundation (Y.P.W.); and NCI R01 CA158322 (L.G.A., T.S., S.A.L.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Appendix
PubMed literature search strategy:
((Melanoma OR melanomas) AND (prevention OR screening OR intervention OR interventions OR protection OR protections)) AND (risk OR risks) AND (behavior OR behaviors OR behavior* OR behaviour*) AND (adolescent[tw] OR adolescents[tw] OR adolescence[tw] OR teenage*[tw] OR child[tw] OR children[tw] OR infant[tw] OR infants[tw] OR pediatric[tw] OR pediatrics[tw])
Footnotes
Conflict of Interest Statement: Dr. Leachman serves on a Medical and Scientific Advisory Board for Myriad Genetics and Castle Biosciences, for which she has received an honorarium. She collaborated with Myriad to validate an assay that is unrelated to research reported here. The other authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aspinwall LG, Leaf SL, Dola ER, Kohlmann W, Leachman SA. CDKN2A/p16 genetic test reporting improves early detection intentions and practices in high-risk melanoma families. Cancer Epidemiology Biomarkers and Prevention. 2008;17(6):1510–1519. doi: 10.1158/1055-9965.epi-08-0010. [DOI] [PubMed] [Google Scholar]
- Aspinwall LG, Leaf SL, Kohlmann W, Dola ER, Leachman SA. Patterns of photoprotection following CDKN2A/p16 genetic test reporting and counseling. Journal of the American Academy of Dermatology. 2009;60(5):745–757. doi: 10.1016/j.jaad.2008.12.034. [DOI] [PubMed] [Google Scholar]
- Aspinwall LG, Taber JM, Kohlmann W, Leaf SL, Leachman SA. Unaffected family members report improvements in daily routine sun protection 2 years following melanoma genetic testing. Genetics in Medicine. 2014;16:846–853. doi: 10.1038/gim.2014.37. 2014/04/26 ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspinwall LG, Taber JM, Leaf SL, Kohlmann W, Leachman SA. Melanoma genetic counseling and test reporting improve screening adherence among unaffected carriers 2 years later. Cancer Epidemiology Biomarkers and Prevention. 2013;22(10):1687–1697. doi: 10.1158/1055-9965.epi-13-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balk SJ. Ultraviolet radiation: A hazard to children and adolescents. Pediatrics. 2011;127(3):588–597. doi: 10.1542/peds.2010-3501. [DOI] [PubMed] [Google Scholar]
- Bergenmar M, Hansson J, Brandberg Y. Family members’ perceptions of genetic testing for malignant melanoma--a prospective interview study. European Journal of Oncology Nursing. 2009;13(2):74–80. doi: 10.1016/j.ejon.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Berwick M, Oliveria S, Luo ST, Headley A, Bolognia JL. A pilot study using nurse education as an intervention to increase skin self-examination for melanoma. Journal of Cancer Education. 2000;15(1):38–40. doi: 10.1080/08858190009528651. [DOI] [PubMed] [Google Scholar]
- Bishop DT, Demenais F, Goldstein AM, Bergman W, Bishop JN, Bressac-de Paillerets B, Hansson J. Geographical variation in the penetrance of CDKN2A mutations for melanoma. Journal of the National Cancer Institute. 2002;94:894–903. doi: 10.1093/jnci/94.12.894. [DOI] [PubMed] [Google Scholar]
- Boone SL, Stapleton J, Turrisi R, Ortiz S, Robinson JK, Mallett KA. Thoroughness of skin examination by melanoma patients: Influence of age, sex and partner. Australasian Journal of Dermatology. 2009;50:176–180. doi: 10.1111/j.1440-0960.2009.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller DB, Borland R. Skin cancer prevention for children: a critical review. Health Education and Behavior. 1999;26:317–343. doi: 10.1177/109019819902600304. [DOI] [PubMed] [Google Scholar]
- Burden A, Vestey J, Sirel J, Aitchison T, Hunter J, MacKie R. Multiple primary melanoma: risk factors and prognostic implications. BMJ. 1994;309(6951):376. doi: 10.1136/bmj.309.6951.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho E, Rosner BA, Feskanich D, Colditz GA. Risk factors and individual probabilities of melanoma for whites. Journal of Clinical Oncology. 2005;23:2669–2675. doi: 10.1200/JCO.2005.11.108. [DOI] [PubMed] [Google Scholar]
- Cushing CC, Steele RG. A meta-analytic review of eHealth interventions for pediatric health promoting and maintaining behaviors. Journal of Pediatric Psychology. 2010 doi: 10.1093/jpepsy/jsq023. [DOI] [PubMed] [Google Scholar]
- Davis R, Campbell R, Hildon Z, Hobbs L, Michie S. Theories of behaviour and behaviour change across the social and behavioural sciences: a scoping review. Health Psychology Review. 2014 doi: 10.1080/17437199.2014.941722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis LK, Vanbeek MJ, Beane Freeman LE, Smith BJ, Dawson DV, Coughlin JA. Sunburns and risk of cutaneous melanoma: Does age matter? A comprehensive meta-analysis. Annals of Epidemiology. 2008;18(8):614–627. doi: 10.1016/j.annepidem.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao DY, Lee TK. Sun-protective behaviors in populations at high risk for skin cancer. Psychology Research and Behavior Management. 2013;7:9–18. doi: 10.2147/prbm.s40457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrone CR, Porat LB, Panageas KS, Berwick M, Halpern AC, Patel A, Coit DG. Clinicopathological features of and risk factors for multiple primary melanomas. JAMA. 2005;294:1647–1654. doi: 10.1001/jama.294.13.1647. [DOI] [PubMed] [Google Scholar]
- Ford D, Bliss JM, Swerdlow AJ, Armstrong BK, Franceschi S, Green A, Østerlind A. Risk of cutaneous melanoma associated with a family history of the disease. International Journal of Cancer. 1995;62:377–381. doi: 10.1002/ijc.2910620403. [DOI] [PubMed] [Google Scholar]
- Geller AC, Emmons KM, Brooks DR, Powers C, Zhang Z, Koh HK, … Gilchrest BA. A randomized trial to improve early detection and prevention practices among siblings of melanoma patients. Cancer. 2006;107(4):806–814. doi: 10.1002/cncr.22050. [DOI] [PubMed] [Google Scholar]
- Glanz K, Bishop DB. The role of behavioral science theory in development and implementation of public health interventions. Annual Review of Public Health. 2010;31:399–418. doi: 10.1146/annurev.publhealth.012809.103604. [DOI] [PubMed] [Google Scholar]
- Glanz K, Mayer JA. Reducing ultraviolet radiation exposure to prevent skin cancer methodology and measurement. American Journal of Preventive Medicine. 2005;29(2):131–142. doi: 10.1016/j.amepre.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Glanz K, Schoenfeld ER, Steffen A. A randomized trial of tailored skin cancer prevention messages for adults: Project SCAPE. American Journal of Public Health. 2010;100:735. doi: 10.2105/AJPH.2008.155705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanz K, Volpicelli K, Kanetsky PA, Ming ME, Schuchter LM, Jepson C, … Armstrong K. Melanoma genetic testing, counseling, and adherence to skin cancer prevention and detection behaviors. Cancer Epidemiology Biomarkers and Prevention. 2013;22(4):8. doi: 10.1158/1055-9965.EPI-12-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook C, Garrud P, Avery A, Coupland C, Williams H. Impact of a multimedia intervention “Skinsafe” on patients’ knowledge and protective behaviors. Preventive Medicine. 2006;42(6):449–454. doi: 10.1016/j.ypmed.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Green AC, Wallingford SC, McBride P. Childhood exposure to ultraviolet radiation and harmful skin effects: Epidemiological evidence. Progress in Biophysics and Molecular Biology. 2011;107:349–355. doi: 10.1016/j.pbiomolbio.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AC, Williams GM, Logan V, Strutton GM. Reduced melanoma after regular sunscreen use: randomized trial follow-up. Journal of Clinical Oncology. 2011;29(3):257–263. doi: 10.1200/jco.2010.28.7078. [DOI] [PubMed] [Google Scholar]
- Gritz E, Tripp M, Peterson S, Prokhorov A, Shete S, Urbauer D, Gershenwald J. Randomized controlled trial of a sun protection intervention for children of melanoma survivors. Cancer Epidemiology Biomarkers and Prevention. 2013;22:1813–1824. doi: 10.1158/1055-9965.EPI-13-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy GP, Machlin SR, Ekwueme DU, Yabroff KR. Prevalence and Costs of Skin Cancer Treatment in the US, 2002–2006 and 2007–2011. American Journal of Preventive Medicine. 2014 doi: 10.1016/j.amepre.2014.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay JL, Oliveria SA, Dusza SW, Phelan DL, Ostroff JS, Halpern AC. Psychosocial mediators of a nurse intervention to increase skin self-examination in patients at high risk for melanoma. Cancer Epidemiology Biomarkers and Prevention. 2006;15(6):1212–1216. doi: 10.1158/1055-9965.epi-04-0822. [DOI] [PubMed] [Google Scholar]
- Higgins J, Green S. Cochrane Book Series. West Sussex: John Wiley & Sons; 2008. Cochrane handbook for systematic reviews of interventions. [Google Scholar]
- Hillhouse JJ, Turrisi R, Kastner M. Modeling tanning salon behavioral tendencies using appearance motivation, self-monitoring and the theory of planned behavior. Health Education Research. 2000;15(4):405–414. doi: 10.1093/her/15.4.405. [DOI] [PubMed] [Google Scholar]
- Jackson KM, Aiken LS. A psychosocial model of sun protection and sunbathing in young women: the impact of health beliefs, attitudes, norms, and self-efficacy for sun protection. Health Psychology. 2000;19(5):469. doi: 10.1037/0278-6133.19.5.469. [DOI] [PubMed] [Google Scholar]
- Janz NK, Becker MH. The Health Belief Model: a decade later. Health Education Quarterly. 1984 Spring;11:1–47. doi: 10.1177/109019818401100101. [DOI] [PubMed] [Google Scholar]
- Kasparian NA, Meiser B, Butow PN, Simpson JM, Mann GJ. Genetic testing for melanoma risk: a prospective cohort study of uptake and outcomes among Australian families. Genetics in Medicine. 2009;11(4):265–278. doi: 10.1097/GIM.0b013e3181993175. [DOI] [PubMed] [Google Scholar]
- Katalinic A, Waldmann A, Weinstock MA, Geller AC, Eisemann N, Greinert R, … Breitbart E. Does skin cancer screening save lives?: an observational study comparing trends in melanoma mortality in regions with and without screening. Cancer. 2012;118(21):5395–5402. doi: 10.1002/cncr.27566. [DOI] [PubMed] [Google Scholar]
- Leachman SA, Carucci J, Kohlmann W, Banks KC, Asgari MM, Bergman W, … Tsao H. Selection criteria for genetic assessment of patients with familial melanoma. Journal of the American Academy of Dermatology. 2009;61(4):677.e671–614. doi: 10.1016/j.jaad.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JS, Eder M, Weinmann S. Behavioral counseling to prevent skin cancer: a systematic review for the U.S. Preventive Services Task Force. Annals of Internal Medicine. 2011;154(3):190–201. doi: 10.7326/0003-4819-154-3-201102010-00009. [DOI] [PubMed] [Google Scholar]
- Manne S, Fasanella N, Connors J, Floyd B, Wang H, Lessin S. Sun protection and skin surveillance practices among relatives of patients with malignant melanoma: prevalence and predictors. Preventive Medicine. 2004;39(1):36–47. doi: 10.1016/j.ypmed.2004.02.028. [DOI] [PubMed] [Google Scholar]
- Manne S, Jacobsen PB, Ming ME, Winkel G, Dessureault S, Lessin SR. Tailored versus generic interventions for skin cancer risk reduction for family members of melanoma patients. Health Psychology. 2010;29(6):10. doi: 10.1037/a0021387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoone J, Menzies S, Meiser B, Mann GJ, Kasparian NA. Psycho-educational interventions for melanoma survivors: a systematic review. Psycho-Oncology. 2013;22(7):1444–1456. doi: 10.1002/pon.3165. [DOI] [PubMed] [Google Scholar]
- Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Science. 2011;6:42. doi: 10.1186/1748-5908-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KA, Huh J, Unger JB, Richardson JL, Allen MW, Peng DH, Cockburn MG. Patterns of sun protective behaviors among Hispanic children in a skin cancer prevention intervention. Preventive Medicine. 2015;81:303–308. doi: 10.1016/j.ypmed.2015.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niendorf KB, Tsao H. Cutaneous Melanoma: Family screening and genetic testing. Dermatologic Therapy. 2006;19:8. doi: 10.1111/j.1529-8019.2005.00050.x. [DOI] [PubMed] [Google Scholar]
- Nilsen W, Kumar S, Shar A, Varoquiers C, Wiley T, Riley WT, … Atienza AA. Advancing the science of mHealth. J Health Commun. 2012;17(sup1):5–10. doi: 10.1080/10810730.2012.677394. [DOI] [PubMed] [Google Scholar]
- Oliveria SA, Dusza SW, Phelan DL, Ostroff JS, Berwick M, Halpern AC. Patient adherence to skin self-examination. effect of nurse intervention with photographs. American Journal of Preventive Medicine. 2004;26:152–155. doi: 10.1016/j.amepre.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Oliveria SA, Saraiya M, Geller AC, Heneghan MK, Jorgensen C. Sun exposure and risk of melanoma. Archives of Disease in Childhood. 2006;91(2):131–138. doi: 10.1136/adc.2005.086918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pharoah PD, Antoniou A, Bobrow M, Zimmern RL, Easton DF, Ponder BA. Polygenic susceptibility to breast cancer and implications for prevention. Nature Genetics. 2002;31:33–36. doi: 10.1038/ng853. [DOI] [PubMed] [Google Scholar]
- Poochareon VN, Federman DG, Kirsner RS. Primary prevention efforts for melanoma. J Drugs Dermatol. 2004;3(5):506–519. [PubMed] [Google Scholar]
- Pustisek N, Sikanic-Dugic N, Hirsl-Hecej V, Domljan ML. Acute skin sun damage in children and its consequences in adults. Collegium Antropologicum. 2010;34(2):233–237. [PubMed] [Google Scholar]
- Rat C, Quereux G, Riviere C, Clouet S, Senand R, Volteau C, Nguyen J-M. Targeted melanoma prevention intervention: A cluster randomized controlled trial. Annals of Family Medicine. 2014;12:21–28. doi: 10.1370/afm.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JK, Turrisi R, Mallett K, Stapleton J, Pion M. Comparing the efficacy of an in-person intervention with a skin self-examination workbook. Archives of Dermatology. 2010;146:91–94. doi: 10.1001/archdermatol.2009.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JK, Turrisi R, Stapleton J. Efficacy of a partner assistance intervention designed to increase skin self-examination performance. Archives of Dermatology. 2007;143:37–41. doi: 10.1001/archderm.143.1.37. [DOI] [PubMed] [Google Scholar]
- Sabatino SA, Lawrence B, Elder R, Mercer SL, Wilson KM, DeVinney B, … Glanz K. Effectiveness of interventions to increase screening for breast, cervical, and colorectal cancers: nine updated systematic reviews for the guide to community preventive services. American Journal of Preventive Medicine. 2012;43(1):97–118. doi: 10.1016/j.amepre.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Moore DH, Mendelsohn ML. Screening program reduced melanoma mortality at the Lawrence Livermore National Laboratory, 1984 to 1996. Journal of the American Academy of Dermatology. 2008;58(5):741–749. doi: 10.1016/j.jaad.2007.10.648. [DOI] [PubMed] [Google Scholar]
- Siskind V, Aitken J, Green A, Martin N. Sun exposure and interaction with family history in risk of melanoma, Queensland, Australia. International Journal of Cancer. 2002;97:90–95. doi: 10.1002/ijc.1563. [DOI] [PubMed] [Google Scholar]
- Surveillance Epidemiology and End Results Program. SEER Cancer Statistics Review. 2013 Retrieved from http://seer.cancer.gov/statfacts/html/melan.html.
- Surveillance Epidemiology and End Results Program. SEER Stat Fact Sheets: Melanoma of the Skin. 2015 Retrieved from http://seer.cancer.gov/statfacts/html/melan.html.
- Taber JM, Aspinwall LG, Leaf SL, Kohlmann W, Leachman SA. Partner involvement in conduct of skin self-examinations remains low following CDKN2A/p16 genetic test reporting and counseling. Journal of the American Academy of Dermatology. 2013;69(5):842–844. doi: 10.1016/j.jaad.2013.06.048. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. The Surgeon General’s Call to Action to Prevent Skin Cancer. Washington, DC: 2014. [Google Scholar]
- U.S. Preventive Services Task Force. Screening for skin cancer: U.S. Preventive Services Task Force recommendation statement. Annals of Internal Medicine. 2009;150:188–193. doi: 10.7326/0003-4819-150-3-200902030-00008. [DOI] [PubMed] [Google Scholar]
- Wu S, Han J, Laden F, Qureshi AA. Long-term ultraviolet flux, other potential risk factors, and skin cancer risk: A cohort study. Cancer Epidemiology Biomarkers and Prevention. 2014;23:1080–1089. doi: 10.1158/1055-9965.EPI-13-0821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagerman S, Marghoob A. Melanoma patient self-detection: A review of efficacy of the skin self-examination and patient-directed educational efforts. Expert Review of Anticancer Therapy. 2013;13(12):1423–1431. doi: 10.1586/14737140.2013.856272. [DOI] [PubMed] [Google Scholar]