SUMMARY
Non-invasive respiratory support is increasingly used in lieu of intubated ventilator support for the management of neonatal respiratory failure, particularly in very low birth weight infants at risk for bronchopulmonary dysplasia. The optimal approach and mode for non-invasive support remains uncertain. This article reviews the application of high-frequency ventilation for non-invasive respiratory support in neonates, including basic science studies on mechanics of gas exchange, animal model investigations, and a review of current clinical use in human neonates.
Keywords: Neonatal, Respiratory, Non-invasive, High frequency
1. Introduction
Great strides have been made in the respiratory support of neonatal lung disease since the initial respirator efforts by Donald and Lord in 1953 [1]. Advances have included the introduction of continuous positive airway pressure (CPAP) (endotracheal, then nasal) [2], incorporation of CPAP (to provide positive end expiratory pressure, PEEP) as an integral component of the ventilator [3], development of extremely low tidal volume support via high-frequency ventilation (HFV) [4], surfactant replacement therapy [5], and sophisticated computer-based technical improvements in conventional mechanical ventilators [6]. Perhaps the most important of these was the introduction of CPAP, as first reported by Gregory et al. in 1971 [2].
Despite advances in neonatal respiratory care over the past 50 years, bronchopulmonary dysplasia (BPD) remains the most prevalent chronic morbidity affecting surviving preterm infants, with rates of >40% for infants <29 weeks of gestation and/or <1000 g at birth [7–10]. The importance of this problem in preterm infants is underscored by more than 5500 publications related to BPD since the first description by Northway in 1967. Pathologically, current BPD is most usually defined as interrupted or simplified alveolization and represents the immature lung’s response to a variety of inflammatory insults including perinatal infection, oxidative injury, nutritional deficiencies, and ventilator-mediated stretch [11–14].
It is indeed ironic that more than 40 years after the introduction of CPAP as the first effective therapy for neonatal respiratory distress syndrome (RDS) (survival rates improved by >50%), and despite the marked technological advances made during that time span, there has been a renewed emphasis on the application of non-invasive (NIV) respiratory approaches to support neonatal lung disease and reduce the frequency and severity of BPD [15]. The optimal NIV approach for neonatal respiratory disease, and especially for the prevention of BPD, remains unclear. In this article we describe what is known about the development and application of high-frequency ventilation as one potential approach to NIV in the neonate. One could describe this NIV mode in various ways; here we term it “high-frequency nasal ventilation” or HFNV, as an inclusive term for any NIV approach with high-frequency breath rates via any device designed to provide neonatal high-frequency ventilation.
2. High-frequency nasal ventilation: gas exchange
2.1. Ventilator features and interface factors
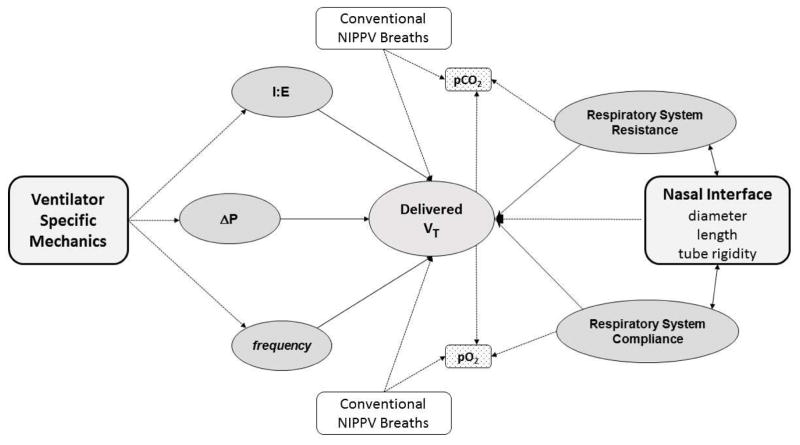
As shown in Fig. 1, various factors may contribute to the effectiveness of gas exchange during HFNV, including: (i) the specific mechanical features of the ventilator used to deliver HFNV, (ii) inspiratory time, (iii) the set pressure or amplitude, (iv) the high frequency rate, (v) the underlying lung pathophysiology, and (vi) characteristics of the nasal interface. Many of these factors are interactive. One additional variable involved in the efficacy of gas exchange during HFNV is the potential interposition of conventional breaths (i.e., combining nasal intermittent positive pressure ventilation, or NIPPV, with HFNV). This option is possible with most available high-frequency ventilators, though the 3110A (CareFusion, San Diego, CA, USA) is a notable exception (Table 1). The dynamics of gas exchange during HFNV remain less clear than during invasive HFV [16]. Laboratory evidence demonstrates substantial loss of pressure and volume across the non-invasive interface during conventional nasal ventilation [17,18]. Similar data have also been reported during HFNV in simulated studies on test lungs [19–21] and in clinical studies in preterm lambs [22].
Fig. 1.
Interactive factors contributing to delivered tidal volume (VT) and effectiveness of gas exchange during non-invasive high-frequency nasal ventilation. I:E, inspiratory to expiratory ratio; ΔP, pressure amplitude. NIPPV, nasal intermittent positive pressure ventilation.
Table 1.
Potential ventilator driver, nasal interface options and support features for initiation of neonatal high frequency nasal ventilation.
| Variable | Comments |
|---|---|
| Ventilator | A variety of “drivers” are possible – see Table 7 Not all have been used clinically or studied in the lab |
| Frequency | Dependent on device & active v passive expiratory phase Optimum f unclear Recommendation: Start oscillators at 6 – 8 Hz; others at 4 – 6 Hz |
| Inspiratory time | May be expressed as I:E, I-time or “On-time” VT ≫ at 50% I:E compared to 33% Recommendation: Set I:E at 50%; for Jet use longer “on-time” than 20 msec |
| Amplitude/ΔP | Device dependent Increased ΔP → larger VT ; appears to plateau ~ 70% Recommendations: Start ~ 50% max ΔP for device Adjust as possible to achieve visible chest wall vibration |
| Nasal interface | Single naso-pharyngeal tube Standard binasal CPAP prongs or nasal CPAP mask ? other nasal cannula interfaces Recommendations: Maximize internal diameter → Larger VT Minimize dead space |
| Conventional breaths | Not all devices can provide this additional support Studied in animal studies, but not described in neonatal reports |
2.2. Factors affecting tidal volume delivery
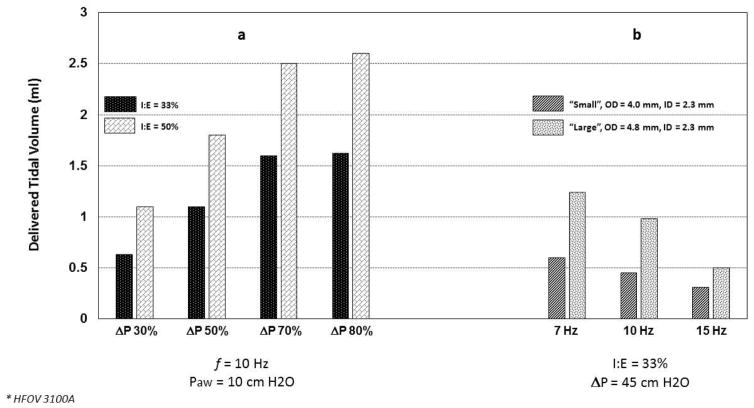
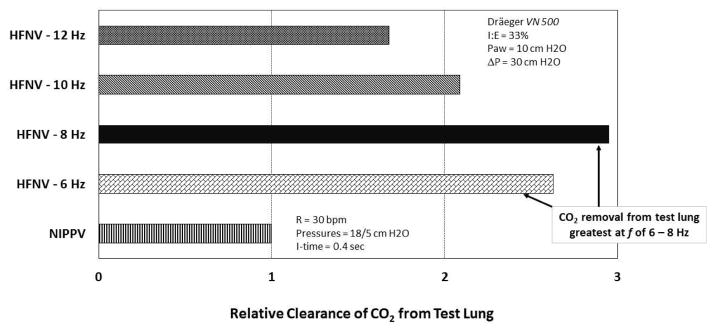
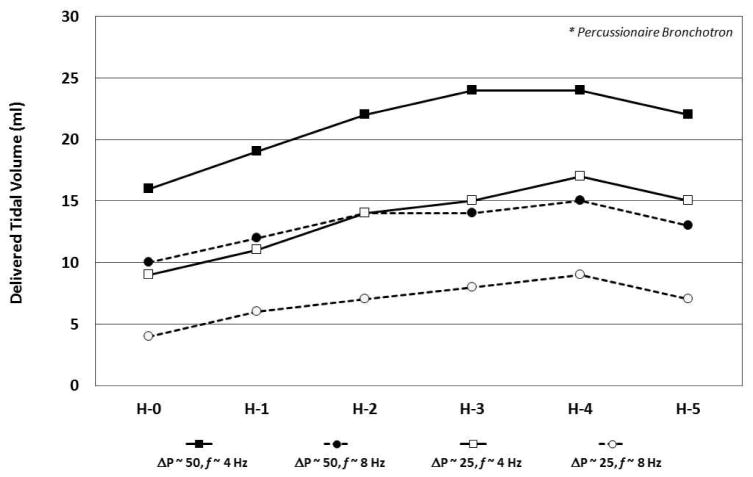
In test lung studies using the 3100A, De Luca et al. demonstrated that tidal volume (VT) delivery is affected by the set amplitude, inspiratory time, and frequency; variables also known to impact VT during invasive HFV [19,20] (Fig. 2a, b). As expected, VT increased as pressure amplitude increased, though a plateau effect was noted at around 70% maximum amplitude for the 3100A. VT also increased as inspiratory time increased, either by changing the I:E ratio from 33% to 50% or by decreasing frequency (with f at 10 Hz this change is accomplished by increasing the inspiratory time from 33 to 50 ms). De Luca et al. also found increased VT using larger nasal prongs interface [19] (Fig. 2b). Using the Dräger VN 500 in high-frequency mode, Mukerji et al. performed bench testing to assess the effect of high frequency rate on CO2 removal via a non-invasive nasal prong interface [21]. With mean pressure, amplitude and I:E fixed, they found marked increase in CO2 removal from a test lung at all HFNV rates compared to conventional NIPPV (Fig. 3). The optimal frequency for CO2 removal was 8 Hz in their test lung model (Fig. 3). In unpublished data from our laboratory, using the Bronchotron (Percussionaire, Sand Point, ID, USA) interfaced via nasal CPAP prongs to a test lung, we found loss of pressure and volume with smaller nasal prongs, with lower pressure amplitude, and at higher frequency rates (Fig. 4). Similar to the application of oscillatory HFV through an endotracheal tube, the largest delivered VT is found at lower frequency (longer I-time), higher amplitude, and across lower resistance interfaces [16].
Fig. 2.
Variable effect of pressure amplitude (ΔP), frequency (f), and nasal prong size on tidal volume (VT) delivery during test-lung simulated high-frequency nasal ventilation. (a) At a fixed frequency (10 Hz) and mean airway pressure (10 cmH2O), increasing ΔP is accompanied by increasing VT with an apparent plateau effect around 70% maximum amplitude. There is a significant increase in VT when the I:E ratio is increased from 33% to 50%. (Adapted with permission from De Luca et al. [20].) (b) With a set I:E ratio and ΔP, increasing high frequency breath rate is accompanied by decreasing VT. The effect on VT is affected by the caliber of the nasal prong interface. High frequency via 3100A (CareFusion, San Diego, CA, USA). (Adapted with permission from De Luca et al. [19].)
Fig. 3.
Comparison of relative effectiveness of gas exchange by non-invasive mode (nasal intermittent positive pressure ventilation (NIPPV) vs high-frequency nasal ventilation (HFNV)). High frequency rate has a significant impact on the effectiveness of gas exchange in a test-lung model, with apparent optimal rate for this model at 8 Hz. High frequency via VN500 (Dräger Medical, Lübeck, Germany). (Adapted from Ref. [21].)
Fig. 4.
Effect of nasal prong size, amplitude (ΔP) and frequency (f) on delivered tidal volume in lung simulated high-frequency nasal ventilation via a Bronchotron high-frequency ventilator (Percussionaire, Sand Point, ID, USA). Delivered tidal volume is significantly decreased at lower ΔP and at higher f. Nasal interface via variously sized Hudson (H) nasal CPAP prongs (Hudson-RCI, Temecula, CA, USA). (Unpublished data.)
3. Clinical studies: animal
Though bench studies are important to assist us in trying to understand the variables that may have an effect on gas exchange during HFNV, bench models can never exactly mirror all the features of in-vivo gas exchange. Unfortunately, relevant data are more difficult to obtain in the clinical setting, particularly in extremely preterm human neonates. A series of studies in our premature lamb laboratory model of BPD have allowed us to investigate further the potential efficacy of HFNV in the pre-clinical setting. Lamb-based studies by other investigators have assessed the effects of various forms of NIV, including HFNV, on laryngeal muscle activity and respiratory function.
3.1. Preterm lamb model of BPD
Over the past two decades, our laboratory has developed a sustainable preterm lamb model of BPD that has allowed us to study and compare the effects of prolonged mechanical ventilation (MV) to early, sustained NIV on lung injury and development [22–26]. Our NIV approach in the preterm lamb includes supra-physiological respiratory frequency coupled with low-rate conventional background breaths through a single nasopharyngeal tube. This approach is providing new insights on variables that have an effect on sustained respiratory gas exchange during HFNV. It also provides us with a “positive control” for physiologic, morphologic, and molecular outcomes for comparison to the lungs of chronically ventilated premature lambs.
3.1.1. Approach to HFNV in preterm lamb BPD model
As noted above, our HFNV approach uses a combination of high-frequency percussive breaths coupled with low-frequency conventional breaths. The primary reason we adopted this approach was practical: we were unable to support premature lambs with significant respiratory distress beyond more than 3–4 h using only bubble CPAP through nasal prongs or nasopharyngeal tube due to progressive deterioration of respiratory gas exchange [24]. Despite antenatal exposure to corticosteroids, postnatal treatment with surfactant and caffeine, and prone positioning via a nylon sling, preterm lambs managed on bubble CPAP developed progressive, unsustainable pathologic rises in arterial carbon dioxide (PaCO2) with declining arterial oxygenation (PaO2) and pH. We do not know the specific reasons for this progressive deterioration in these CPAP-managed preterm lambs, but we suspect that species-specific anatomic dead space may be a major factor.
Our model prospectively targets PaO2 in a range between 60 and 90 mmHg and PaCO2 in a range between 45 and 60 mmHg. Examples of achieved respiratory gas exchange values are shown for day-of-life 6 among three ventilated groups of premature lambs (Fig. 5). Results are compared among preterm lambs supported via pressure-limited, volume-targeted MV and HFNV respiratory support with either VDR4 (Percussionaire, Sand Point, ID, USA) ventilator or VN500 (Dräger Medical, Lübeck, Germany) ventilator in high-frequency mode. Initial settings for MV were rate 60 breaths/min, inspiratory time 0.3 s, PEEP 7 cmH2O, and targeted expiratory tidal volumes of 5–7 mL/kg. The VDR4 ventilator’s high-frequency initial settings were rate at 10 Hz, I:E ratio at 1:1 and oscillatory PEEP of 7 cmH2O; with background convective breaths at 10 breaths per min, peak inspiratory pressure (PIP) of 25 cmH2O, inspiratory time of 0.5 s and PEEP of 7 cmH2O. The VN500 ventilator’s initial settings in the high-frequency mode were high-frequency rate at 8 Hz and amplitude at 25 mbar, with background convective breaths at 10 breaths per min, PIP of 25 cmH2O, and I:E ratio of 1:1. Mean airway pressure (Paw) was set 2 cmH2O higher than the Paw during MV support. At day-of-life 6, HFNV respiratory support resulted in sustained, physiologically targeted PaO2 while using ~10% lower FiO2 compared to MV support. Pressure setting for PIP at the ventilators was comparable among MV and both HFNV modes of respiratory support. However, PIP at the ventilator is not the same as mean intra-tracheal pressure during HFNV [24,25].
Fig. 5.
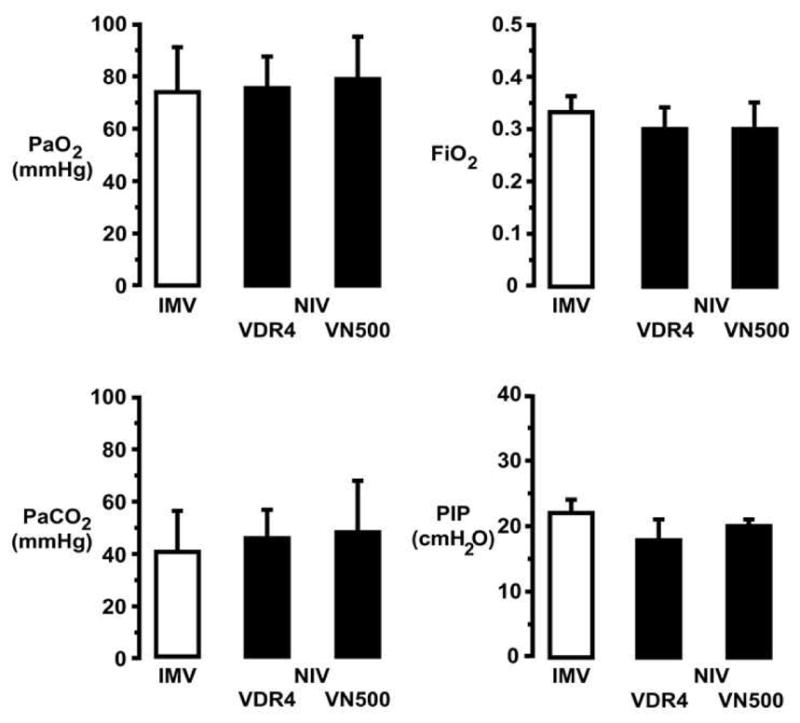
Physiologic targets for arterial blood gases in premature lambs supported for 21 days by invasive mechanical ventilation (IMV; VIP Bird, CareFusion, San Diego, CA, USA) or two approaches to non-invasive high-frequency nasal ventilation (NIV): VDR4 (Percussionaire, Sand Point, ID, USA) or VN500 (Dräger Medical, Lübeck, Germany). Our model prospectively targets arterial oxygenation (PaO2) range between 60 and 90 mmHg and ventilation (PaCO2) range between 45 and 60 mmHg. At day-of-life 6, either mode of NIV respiratory support required ~10% lower FiO2 to maintain the targeted PaO2 range compared to IMV respiratory support. Pressure setting for peak inspiratory pressure (PIP) at the ventilators was comparable among IMV and both NIV modes of respiratory support, but PIP at the ventilator is not the same as mean intra-tracheal pressure during NIV. (See Fig. 7; adapted with permission from Null et al. [25].)
3.1.2. Airway pressure measurements during HFNV in preterm lambs
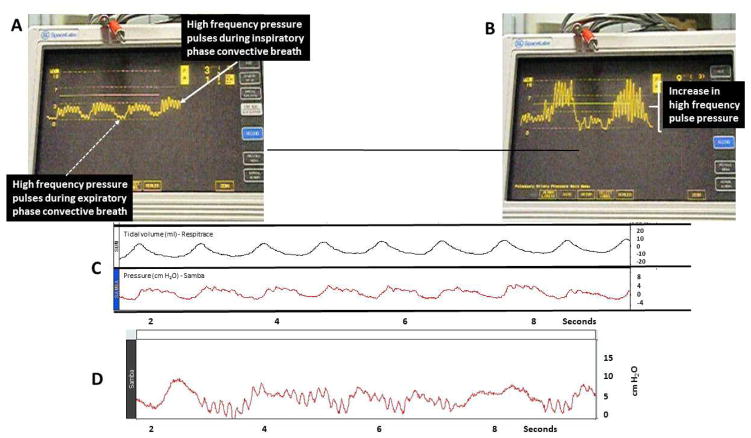
We measured intra-tracheal pressure in all three groups of premature lambs. Example traces shown in Fig. 6A and B are for a premature lamb supported by HFNV, using the VDR4 ventilator. High-frequency pressure pulses are evident during the expiratory phase and inspiratory phase of the convective breath. The intra-tracheal PIP and PEEP traces shown in Fig. 2C reveal that tidal volume measured simultaneously follows the intra-tracheal pressure trace, as should be the case. Figure 6D shows the intra-tracheal PIP and PEEP trace for a different premature lamb supported by HFNV, using the VN500 in its HF mode. During HFNV support with the VN500, high-frequency pressure pulses are evident only during the expiratory phase of the convective breath. (Unlike the VDR4, the VN500 does not provide HF pulses during the inspiratory phase of a conventional breath.)
Fig. 6.
Graphic representation of in-vivo data from preterm lambs supported by high-frequency nasal ventilation via VDR4 high-frequency ventilator (Percussionaire, Sand Point, ID, USA). (A) High-frequency pressure pulsations are seen throughout both the inspiratory (solid white arrow) and expiratory (dashed white arrow) phases of background convective breaths. (B) Change in peak delivered pressure and convective tidal volume following increased high-frequency pressure amplitude. (C) Measured tidal volume by respiratory inductive plethysmography (Respitrace, CareFusion, San Diego, CA, USA) during convective breaths with simultaneous pressure recordings via Samba 3200 fiberoptic micropressure transducer (Harvard Apparatus Canada, Saint-Laurent, QC, Canada) integrated with a BIOPAC MP150 system (BIOPAC Systems, Goleta, CA, USA) and inserted into posterior nasopharyngeal space.
Figure 7 summarizes measurements made in premature lambs at two peak pressure settings (high PIP of ~27 cmH2O or moderate PIP of ~14 cmH2O) at the ventilators [25]. The following results stand out. First, the PIP setting at the ventilator was attenuated by ~70% across the nasopharyngeal tube to the distal nasopharynx. Second, within the nasopharynx and trachea, peak and mean airway pressures were similar, and minimal oscillatory pressure changes were observed. Third, HFNV maintained a positive mean intra-tracheal pressure of a few cmH2O, depending on PIP setting at the ventilator. Fourth, peak and mean intra-tracheal pressure were very low during HFNV, ~30% of the pressures during MV. Thus, HFNV maintains positive, albeit low, end expiratory pressure in spontaneously breathing neonatal lambs.
Fig. 7.
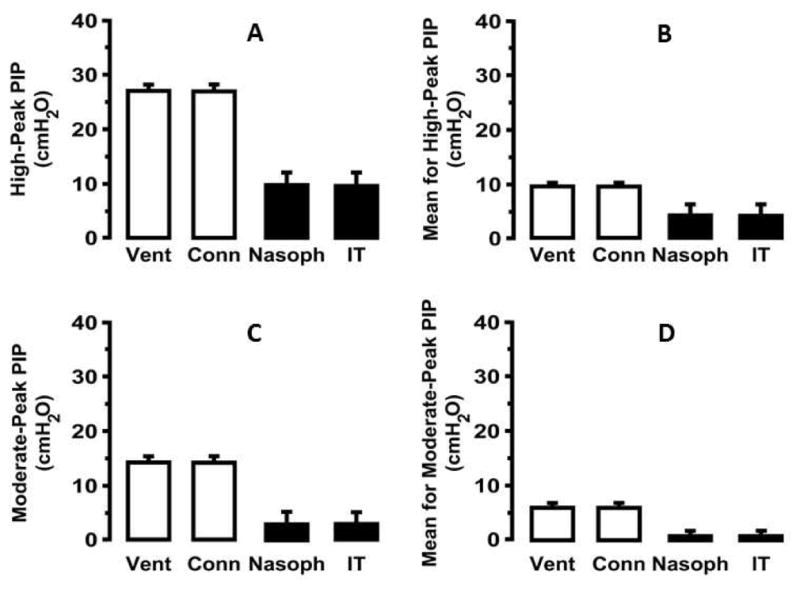
Pressure measurements in premature lambs supported by high-frequency nasal ventilation (HFNV) via the VDR4 (Percussionaire, Sand Point, ID, USA) across the spectrum from ventilator output (Vent) to the connection of the ventilator circuit to the nasopharyngeal tube interface (Conn), then beyond the tube within the nasopharyngeal space or within the trachea. (Adapted with permission from Null et al. [25].) Measurements were made at two different set peak inspiratory pressure (PIP) settings (high ~27 cmH2O, or moderate PIP ~14 cmH2O). At both high (A) and medium (C) PIP there was ~70% pressure attenuation across the tube interface, but there was essentially no pressure loss from the nasopharynx to mid trachea. Similar pressure decrements are seen for mean airway pressures (B and D) with persistent positive, low positive end expiratory pressure maintained during HFNV in spontaneously breathing neonatal lambs.
One reality of any form of NIV is leak. Our HFNV interface is a shortened, uncuffed endotracheal tube (3.0 Fr), with the tip positioned ~5 cm along the ~10 cm length of the premature lamb nose. The tube is narrower than the naris, the other naris is open, and the posterior end of the nose is open to the nasopharynx. Thus, leak may occur along three paths in our model. The major consequence of leak is pressure reduction in the nasopharynx, trachea and beyond [17,18]. In comparison, airway pressures during invasive MV reflect the pressure settings at the ventilator (data not shown) [27].
3.1.3. Physiologic consequences of long-term HFNV support
We extended the physiological comparison between invasive MV and non-invasive HFNV respiratory support by ventilating other premature lambs for 21 days [25]. To date, long-term HFNV respiratory support studies have only been completed using the VDR ventilator. The same physiologic targets for oxygenation and ventilation were used as for the shorter study. Results, at weeks-of-life 1, 2, and 3, are shown in Table 2. Targeted oxygenation range was maintained using ~30% lower FiO2 at weeks of life 2 and 3 for the HFNV group compared to the matched MV group at the corresponding weeks of life. The targeted ventilation range was maintained using ~35% and ~75% lower PIP at weeks-of-life 2 and 3, respectively, for the HFNV group compared to the matched MV group at the corresponding weeks-of-life. We did not measure intra-tracheal pressure in these groups because that would have required repeated intubation of the HFNV group, which could have affected outcomes.
Table 2.
Respiratory gas exchange parameters in preterm lambs managed by invasive or non-invasive respiratory support (mean ± SD, except as noted). (Reprinted with permission, Ref 25)
| Parameter | Preterm 3d (n=5 each) | Preterm 21d (n=4 each) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Day of life | Invasive MV | HFNV | Week of life | Intubation and IMV | HFNV | |
| PaO2 (mmHg) | 1 | 73 ± 17 | 73 ± 11 | 1 | 76 ± 13 | 75 ± 12 |
| 2 | 85 ± 9 | 81 ± 10 | 2 | 76 ± 3 | 73 ± 14 | |
| 3 | 73 ± 8 | 77 ± 16 | 3 | 74 ± 6 | 68 ± 3 | |
|
|
||||||
| FiO2 | 1 | 0.33 ± 0.03 | 0.32 ± 0.03 | 1 | 0.42 ± 0.11 | 0.30 ± 0.04 |
| 2 | 0.35 ± 0.04 | 0.31 ± 0.03 | 2 | 0.36 ± 0.04 | 0.26 ± 0.03* | |
| 3 | 0.38 ± 0.04 | 0.27 ± 0.03* | 3 | 0.33 ± 0.05 | 0.23 ± 0.02* | |
|
|
||||||
| PaCO2 (mmHg) | 1 | 49 ± 7 | 50 ± 16 | 1 | 46 ± 7 | 52 ± 4 |
| 2 | 44 ± 5 | 55 ± 8 | 2 | 49 ± 4 | 45 ± 5 | |
| 3 | 53 ± 10 | 64 ± 11 | 3 | 47 ± 8 | 42 ± 6 | |
|
|
||||||
| PIP (cmH2O)a | 1 | 22 ± 2 | 13 ± 4* | 1 | 14 ± 1 | 18 ± 3 |
| 2 | 20 ± 2 | 13 ± 4* | 2 | 15 ± 2 | 10 ± 4 | |
| 3 | 25 ± 2 | 13 ± 2* | 3 | 19 ± 5 | 4 ± 1* | |
|
|
||||||
| ITP (cmH2O) | 1 | 22 ± 2 | 2 ± 2* | 1 | na | na |
| 2 | 20 ± 2 | 2 ± 3* | 2 | na | na | |
| 3 | 25 ± 2 | 2 ± 2* | 3 | na | na | |
|
|
||||||
| Paw (cmH2O)a | 1 | 11 ± 1 | 6 ± 1* | 1 | 10 ± 1 | 9 ± 3 |
| 2 | 12 ± 2 | 6 ± 1* | 2 | 10 ± 1 | 5 ± 1* | |
| 3 | 12 ± 1 | 7 ± 1* | 3 | 10 ± 1 | 4 ± 1* | |
|
|
||||||
| PEEP (cmH2O)a | 1 | 6 ± 1 | 5 ± 1 | 1 | 7 ± 2 | 7 ± 3 |
| 2 | 7 ± 1 | 6 ± 2 | 2 | 7 ± 1 | 4 ± 1* | |
| 3 | 7 ± 1 | 6 ± 3 | 3 | 7 ± 1 | 4 ± 1* | |
|
|
||||||
| pH | 1 | 7.34 ± 0.05 | 7.35 ± 0.09 | 1 | 7.34 ± 0.10 | 7.33 ± 0.06 |
| 2 | 7.43 ± 0.07 | 7.37 ± 0.03 | 2 | 7.30 ± 0.04 | 7.43 ± 0.06* | |
| 3 | 7.37 ± 0.14 | 7.34 ± 0.08 | 3 | 7.35 ± 0.07 | 7.45 ± 0.04 | |
|
|
||||||
| Oxygenation Index [Median (IQR)] | 1 | 5.7 (0.8) | 2.3 (0.5)† | 1 | 5.0 (2.7) | 1.9 (0.9)† |
| 2 | 4.6 (1.8) | 2.2 (0.2)† | 2 | 4.8 (0.9) | 4.0 (1.6)† | |
| 3 | 5.6 (1.7) | 2.5 (1.3)† | 3 | 3.2 (0.8) | 1.4 (0.1)† | |
|
|
||||||
| A-a gradient (mmHg) [Median (IQR)] | 1 | 65 (14) | 47 (15) | 1 | 83 (124) | 33 (40) |
| 2 | 55 (24) | 20 (26)† | 2 | 70 ( 48) | 53 (20)† | |
| 3 | 90 (47) | 9 (11)† | 3 | 40 ( 53) | 18 (10)† | |
|
|
||||||
| PaO2/FiO2 ratio [Median (IQR)] | 1 | 209 (24) | 243 (59) | 1 | 212 (102) | 261 (133) |
| 2 | 243 (58) | 284 (53) | 2 | 220 ( 49) | 233 ( 50) | |
| 3 | 184 (62) | 262 (88)† | 3 | 256 (121) | 294 ( 23) | |
P < 0.05 versus IMV group, unpaired t-test;
P < 0.05 versus IMV group by Mann-Whitney U-test
Pressure at the ventilator
Abbreviations: IMV, intermittent mandatory ventilation; HFNV, high-frequency nasal ventilation; PaO2, arterial partial pressure of oxygen; FiO2, fractional inspired oxygen concentration; PaCO2, arterial partial pressure of carbon dioxide; PIP, peak inspiratory pressure; ITP, intra-tracheal pressure; na, measurement not made
We also calculated indices of respiratory gas exchange during the 21-day study including oxygenation index (OI), alveolar–arterial (A–a) gradient, and PaO2/FiO2 (P/F) ratio at weeks-of-life 1, 2, and 3 (Table 2). To calculate OI, in lieu of mean intra-tracheal pressure, we used mean airway pressure detected at the ventilators. Mean airway pressure for the MV group was 10 ± 1 cmH2O at weeks-of-life 1, 2, and 3; for the HFNV group, mean airway pressure was 9 ± 3, 5 ± 1, and 4 ± 1 cmH2O at weeks-of-life 1, 2, and 3, respectively. Median OI and A–a gradient were significantly lower for the HFNV group compared to the MV group. Median P:F ratio was larger for the HFNV group at each week-of-life; however, the time-matched differences were not statistically significant.
3.1.4. Radiologic and histologic effect of HFNV compared to MV in preterm lambs
Our studies also provide function–structure insights. The better physiologic outcomes for respiratory gas exchange for the HFNV groups at 3 and 21 days are accompanied by improved radiographic and histologic appearances of the lungs. Radiographically, HFNV groups have more translucent lung fields, less obvious air bronchograms, and larger lung volumes compared to matched MV groups. Histologically, alveoli formed in the lung of both HFNV groups whereas alveolar simplification typified the histologic appearance of both MV groups (Fig. 8). Together, these results show that non-invasive ventilation with HFNV leads to better respiratory gas exchange and alveolar formation than MV.
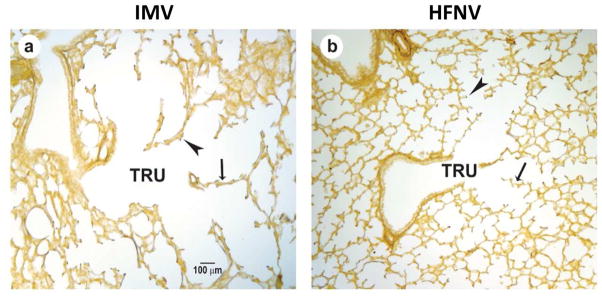
Fig. 8.
Histopathology at 21 days of preterm lambs (131 days) supported by (a) invasive mechanical ventilation (IMV) or (b) non-invasive high-frequency nasal ventilation (HFNV). The terminal respiratory unit (TRU) is dilated and non-uniform in MV animals compared to HFNV lambs. Blunted secondary crest formation is seen in the lungs of MV lambs (large arrowhead, a) but numerous well-developed secondary crests are noted in the HFNV lambs (large arrowhead, b). The arrows point to thickened saccular/alveolar walls in the lambs managed by IMV contrasted to thin distal airspace walls in the HFNV lambs.
3.1.5. Potential molecular effects of HFNV
On a molecular level, the mechanisms that impart better functional and structural outcomes with the use of early HFNV remain under investigation. Some studies in our preterm lamb model show that NIV support with HFNV results in a distinctly different shift in the signaling pathways related to lung maturation and development when compared to invasive MV. One cellular mechanism that has been studied is the balance between apoptosis and proliferation of mesenchymal cells, which contribute to the transitioning cellularity of the walls of immature airspace during the canalicular and saccular stages of lung development. For efficient respiratory gas exchange to occur, the cellular, and therefore thick, immature walls of the airspace have to thin. Thinning occurs, in part, through apoptosis of mesenchymal cells. Our results show that 3 days of HFNV is associated with more apoptosis, and less proliferation, among mesenchymal cells in the walls of the distal airspaces compared to 3 days of MV [24] (Table 3).
Table 3.
Effect of invasive mechanical ventilation (MV) compared to high frequency nasal ventilation (HFNV) on the lung and brain tissues of preterm lambs with respiratory distress syndrome.
| LUNG | MV | HFNV |
|---|---|---|
|
| ||
| Alveolization/Septal formation | Decreased | Increased |
| Mesenchymal apoptosis | Decreased | Increased |
| PTHrP-PPAR-γ pathway | No change | Enhanced |
| Angiogenic pathways | Decreased | Increased |
| IGF-1 | Increased | Decreased |
| TLR-4 | Increased | Decreased |
| VASP | Increased | Decreased |
| Histone acetylation | Decreased | Increased |
|
| ||
| BRAIN | ||
|
| ||
| Reactive gliosis/astrocytosis | Increased | Decreased |
| Glial/neuronal apoptosis | Increased | Decreased |
| Angiogenic pathways | Decreased | Increased |
| IGF-1 | Decreased | Increased |
| TLR-4 | Increased | Decreased |
| BDNF | Decreased | Increased |
| Histone acetylation | Decreased | Increased |
PTHrP-PPAR-γ = Parathyroid Hormone-related Protein-Peroxisome Proliferator-Activated Receptor γ; IGF = insulin-like growth factor; TLR = toll-like receptor; VASP = vasodilator-activated phosphoprotein (stretch mediated regulator of epithelial actin-cytoskeletal remodeling); BDNF = brain derived neurotrophic factor
Early, sustained HFNV support also promotes up-regulation of key markers involved in normal development processes related to alveolar epithelial–mesenchymal interaction, including markers of alveolar parathyroid hormone-related protein–peroxisome proliferator-activated receptor-γ (PTHrP–PPAR-γ) signaling and surfactant proteins B and C [26] (Table 3). In contrast, invasive MV either reduced or did not change the expression of these markers. Additionally, functional markers promoting persistent mesenchymal cellularity and immature lung development were either down-regulated or unchanged by HFNV in comparison to invasive MV [26]. Unpublished studies also indicate important differences between HFNV and invasive MV in the expression and activity of certain membrane-based mechano-transducer molecules, such as vasodilator-activated phosphoprotein (VASP) (Table 3).
3.1.6. Comparative model for alternate/additional BPD interventions
At the beginning of this section, we stated that we use HFNV as a form of NIV for two main reasons. First, because we could not sustain these preterm lambs only using nCPAP. The second reason is that HFNV positively affects physiologic, morphologic, and molecular outcomes in the lungs of chronically ventilated premature lambs. Prior to using HFNV, we relied on reference fetal and term lambs for comparisons. The value of these reference groups is that they provide context for the stage of lung development at the time that fetal lambs are delivered (matched for postconceptional age) and when the chronically ventilated premature lamb studies end (also matched for postconceptional age). However, these reference groups do not approximate the effects related to the clinical setting of preterm birth, respiratory failure, and IMV. Long-term NIV support with HFNV allows a closer approximation of the current NIV respiratory approach to human preterm infants, with a model outcome that shows near-normal respiratory function and alveolarization [24,25].
Finally, another value of the HFNV mode of respiratory support for chronically ventilated premature lambs is unraveling the contribution of other aspects of care employed in the management of preterm infants, such as inadequate nutrition and sedation, not only on the lung but also on other major organ systems (Table 3). Enteral feeding intolerance is a challenge with chronically ventilated premature lambs, as in premature human infants, related in part to immaturity of the gastrointestinal tract blood flow, motility, and absorption [28,29]. Enteral feeding volumes were compared between HFNV and MV groups because of the potential for differences in nutrition to influence lung development and injury. Enteral nutrition was targeted for 60–80 kcal/kg/day during the first week of life and 120–150 kcal/kg/day for the remaining two weeks of life. Enteral feeding was started with ewe’s colostrum for the first 2 days of life, followed by ewe’s milk thereafter. Enteral feeding volume was gradually increased as tolerated. Although historically we gave total parenteral nutrition solution in combination with enteral feedings of ewe’s colostrum and milk [22,23], this approach did not promote weight gain or growth. In more contemporary 21-day studies, the weekly volume of milk increased for the HFNV group (week 1: 107 ± 10; week 2: 149 ± 59; week 3: 188 ± 70 mL/kg/day), whereas the corresponding weekly milk volume decreased for the MV group (106 ± 10, 81 ± 55, and 53 ± 23 mL/kg/day, respectively), associated with residual milk in the stomach [25]. Over the course of 21 days, the HFNV group gained ~1 kg of body weight from their delivery weight whereas the MV group lost ~0.5 kg during the same period. This degree of extrauterine growth restriction for the MV group of premature lambs is consistent with the degree of extrauterine growth restriction that occurs in premature infants and may contribute to adverse pulmonary and neurodevelopmental outcomes in preterm humans [30–32]. Current studies in our laboratory are underway to further dissect the roles of micro- and macronutrients in preterm lung outcomes.
3.2. Laryngeal effects of non-invasive respiratory support
One seemingly minimal but potentially very important advantage for HFNV compared to the more conventional approach to NIPPV is the different response of the laryngo-pharyngeal musculature during each of these modes. During normal spontaneous breathing, laryngeal muscles act in synchrony to open the upper airway during inspiration, decreasing resistance and easing the flow of gas through the glottis [33]. Recent studies have demonstrated that normal muscle activity during inspiration is markedly altered with the application of pressure-supported NIPPV (Table 4) [33–35]. NIPPV induces an increase in inspiratory constrictor (thryoarytenoid) muscle activity while suppressing normal inspiratory dilator (cricothyroid) muscle activity, with these effects augmented as NIPPV support is increased [33–35]. Contrary to the effect of NIPPV, the application of HFNV was shown not to increase inspiratory constrictor muscle activity while maintaining normal dilator muscle activity [35] (Table 4). Furthermore, in support of improving VT delivery at lower high-frequency rates, HFNV at 4 Hz (compared to 8 Hz) was accompanied by lower rates of spontaneous breathing, often apneic in nature, while maintaining normal pCO2 values [35].
Table 4.
Effect of various non-invasive respiratory support modes on laryngeal muscle activity.
| Thyroarytenoid muscle | Cricothyroid muscle | Comment | |
|---|---|---|---|
| Normal function | Laryngeal constriction early expiratory phase | Laryngeal relaxation during inspiration | Synchronized support of inspiratory dilation/expiratory constriction |
| PS – NIPPV | Increased inspiratory activity | Decreased inspiratory activity | Increased inspiratory resistance, decreased VT |
| NAVA – NIPPV | No increase | Normal activity | Improved VT v PS |
| High frequency nasal oscillatory ventilation | No increase | Normal activity | Spontaneous RR decreased at lower f |
PS-NIPPV: pressure supported nasal intermittent positive pressure ventilation; NAVA: neutrally adjusted ventilator assist;
4. Clinical studies: human
4.1. Review of published reports
The first report on the use of high-frequency ventilation as a non-invasive technique in neonates was almost 20 years ago by van der Hoeven et al. [36]. As shown in Table 5, there are now four published studies reporting the application of HFNV in 107 preterm neonates [36–39]. There is considerable variability across these four studies in terms of the median gestational age, age at initiation, and duration of HFNV. There are also differences between these studies with respect to the high frequency ventilator used and the initial settings applied. Three of these trials used a shortened endotracheal tube inserted through the nares into the nasopharyngeal space; the most recent report from Mukerji et al. applied HFNV via standard nasal CPAP prongs or mask. Each of the studies suggested that HFNV could successfully support many (but not all) infants who were otherwise failing more conventional nasal CPAP or nasal ventilation. None of the studies reported significant adverse events associated with HFNV use, although in many cases HFNV was only used for relatively short periods of time. Nonetheless, all four studies were retrospective analyses and a definitive randomized trial is needed. In addition to the reports identified in Table 5, there is an additional single case report of HFNV rescue [40] and one randomized trial of 46 infants comparing HFNV to CPAP in the management of transient tachypnea of the newborn in late-preterm/term infants [41]. In this latter study, though the overall duration of support was significantly less for infants managed by HFNV, the transient nature of the disease process makes it difficult to assess the cost-effectiveness of such an approach.
Table 5.
High frequency nasal ventilation (HFNV): Demographics, nasal interface, ventilator driver and settings, and effects for published studies of at least 10 infants.
| Van der Hoeven | Colaizy | Czernik | Mukerji | |
|---|---|---|---|---|
| # infants | 21 | 14 | 20 | 52 (79 uses) |
| Gestation, wk | 29 (27–32) | 27 (25–30) | 25 (23–27) | 25 (23–35) |
| Birth Wt, g | 1010 (750–2170) | 995 (438–1371) | 635 (382–1020) | 740 (500–2860) |
| Study age, d | 1 (0.2–20) | 30 (18–147) | 31 (10–183) | 20 (2–147) |
| Duration, hr | 36 (12–108) | 2 | 136 (7–456) | 57 (1–415) |
| Nasal interface | NP tube | NP Tube | NP Tube | Nasal prongs/mask |
| Ventilator | InfantStar | InfantStar | Leoni Plus | Drager |
| Settings |
Initial Rate: 10 Hz Paw: 6–8 cm H2O Amp: 24–48 cm H2O |
Initial Rate: 10 Hz Paw: 4–7 cm H2O Amp: 29–60 cm H2O |
Initial Rate: 10 Hz Paw: 8 cm H2O Amp: 20 cm H2O |
Varied over time Rate: 6–14 Hz Paw: 6–8 cm H2O Amp: 24–48 cm H2O |
| Comments | Success 16 (76%) Improved ventilation No adverse effects |
Success 13 (93%) Improved ventilation Short-term use only |
Success 14 (70%) 91% if 1st attempt No adverse effects |
Success 46 (58%) Improved ventilation No adverse effects |
There is limited evidence to support the optimal device or approach for HFNV. Recently, Fischer et al. reported on the use of HFNV in Europe [42]. Among 172 responding neonatal intensive care units (NICU s) across five European countries, 30 (17% overall; with 25 of the units in Germany) reported the use of HFNV clinically in the management of neonates. HFNV was most often used in preterm infants and the most frequent indication was failure of standard nasal CPAP (90%). These investigators noted significant variation in the devices used to support HFNV and in the range of initial support parameters, but most infants were managed via standard short binasal prongs or via a single nasopharyngeal tube.
4.2. Combining NIV approaches: HFNV plus NIPPV
None of the studies published to date on the use of HFNV in human neonates has incorporated conventional background breaths during HFNV support. This approach combines conventional NIPPV with HFNV. Such breaths have been part of the approach we have used for investigation in our premature lamb model of BPD, as described elsewhere in this review, but we have also incorporated these convective breaths during clinical management of neonates via HFNV (see Section 4.3). Intermittent provision of a conventional NIPPV breath may potentially augment VT delivery, improve functional resting lung volume, and improve gas exchange. We are not aware of any clinical or laboratory studies that have compared the combined approach to only HFNV (or only NIPPV) support.
4.3. Current application of HFNV at University of Utah
We have used HFNV clinically in two different ways: (i) as rescue for infants failing standard nasal ventilation and (ii) as a mode of non-invasive respiratory support during neonatal transport. Clinically we have managed HFNV via the Percussionaire VDR-4 or Bronchotron devices in the NICU, with the Bronchotron ventilator exclusively used during neonatal transport. These devices typically provide high-frequency pressure pulses on top of a baseline CPAP pressure with variable frequency of convective background rate and tidal volume (Fig. 9). All variables are modifiable, including CPAP pressure (typically 5–12 cmH2O), pulsatile pressure, I:E ratio and high frequency rate (typical range 5–10 Hz), as well as the inspiratory time (typically 0.5–1.0 s), pressure, and frequency (typically 8–20) of convective breaths. Some care is required to insure that excess volume delivery does not occur with the convective breaths. A turbohub is used to facilitate adequate humidification, placement of the phasitron, and adequate pressure pulsations during non-invasive support (Fig. 10). Amplitudes are adjusted to achieve some minimal visible chest wall vibrations and then adjusted as needed for desired pCO2 values. The choice of mean distending pressure is related to Paw prior to extubation or CPAP prior to switching to HFNV, FiO2 needed to achieve desired O2 saturations, and radiographic assessment of lung inflation. Specific caveats we have observed during our clinical experiences include:
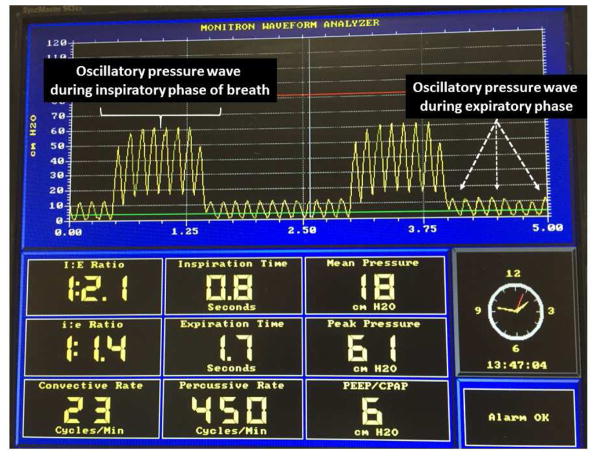
Fig. 9.
Monitor view showing waveforms and variable settings interfaced with VDR4 (Percussionaire, Sand Point, ID, USA) for HFNV support. The oscillatory pressure pulses are delivered continuously throughout both the inspiratory phase of convective breaths as well as during the expiratory or CPAP phase. The pressure measurements are obtained proximally at the ventilator, not at or beyond the nasal prong interface.
Fig. 10.
Configuration of the “phasitron” to the “turbohub” for non-invasive respiratory support with the VDR4 or Bronchotron ventilators (Percussionaire, Sand Point, ID, UT, USA).
-
Lower high-frequency rates (4–8 Hz) may be indicated for:
inadequate ventilation despite increasing amplitudes
infants with pathophysiology associated with air trapping
infants with non-homogeneous lung disease
-
To improve oxygenation if FiO2 >40%, consider:
-
optimize mean/resting lung volume
increase CPAP if there is evidence for sub-optimal inflation
reduce CPAP for evidence of air trapping and lung overinflation
for non-homogeneous lung disease, use lower frequencies and higher pressure amplitudes
-
-
To improve ventilation consider:
increase pulse pressure amplitude and decrease CPAP if lung is overinflated
adjust high-frequency rate as noted in “A” above
adjust convective breath rate, inspiratory time, and/or pressure.
Using these devices and approaches over the past few years, we have been able to effectively manage more than 100 neonates in the NICU and during neonatal transport. In almost all instances we have used HFNV in the NICU as a “rescue” therapy for infants failing other NIV modes, in an attempt to prevent reintubation. Of the more than 60 infants transported on HFNV, none has required in-transport intubation. The characteristics of transport infants are shown in Table 6.
Table 6.
Patient characteristics of over 60 neonates managed during transport on high frequency nasal ventilation.
| Gestation at birth | 23 – 42+ weeks |
| Birth weight | 470 – 4800 g |
| Weight at transport | 680 – >5000 g |
| Age at transport | 1 – 60+ days |
| FiO2 (Median) | 0.37 pre v 0.33 post |
| CPAP (cm H2O) | 5 – 10 (80% in 5–7 range) |
| Transport time | 80% > 2 hours |
| Primary diagnosis | RDS or BPD |
| Airway related complications | None |
4.4. Device-specific comments and recommendations
In Table 7 we present device-specific recommendations and comments related to initial settings. It is important to understand that each high-frequency device may have an “optimal” approach for HFNV. Current evidence suggests that ventilation via HFNV may be optimized with the use of lower frequencies (6–8 Hz for high-frequency ventilators with “active” exhalation; 4–6 Hz for high-frequency ventilators with “passive” exhalation). Additionally, studies suggest improved VT delivery with larger nasal prong/cannula interface, with a longer inspiratory time, and with a higher pressure amplitude. However, no clinical studies (animal or human) have compared the effects of these potential modifiers on in-vivo gas exchange. It is also important to remember that there is marked loss of pressure, and thus volume, across the interface, as well as within the respiratory passages; thus the differences noted in bench studies may not necessarily translate to clinical care, where respiratory system mechanics and the spontaneous breathing activity of the patient also come into play [17–22].
Table 7.
Available high frequency ventilators and potential ventilator settings for initiation of neonatal high frequency nasal ventilation.
| Ventilator | Suggested Initial Settings High Frequency Support |
Suggested Initial Setting Conventional Breaths * |
|---|---|---|
| 3100A | Frequency: 8–10 Hz I:E Ratio: 1:2 or 1:1 Paw: Similar to MV or CPAP Amp/ΔP: 2X Paw; adjust as needed |
Cannot provide conventional breaths; Limited by stiff tubing interface Relative high pressure amplitude |
| VDR4 or Bronchotron | Frequency: 5–8 Hz I:E ratio: 1:1 Paw: Similar to MV or CPAP Amp/ΔP: 2X Paw; adjust as needed |
OPTIONAL NIPPV breaths Frequency: 6–12 bpm PIP: as needed to move chest; be careful to not provide excessive VT |
| Drager VN500 or Babylog 8000 @ | Frequency: 6–10 Hz I:E Ratio: 1:1 Paw: Similar to MV or CPAP Amp/ΔP: 2X Paw; adjust as needed ΔP may be less than above devices, particularly with Babylog 8000 |
OPTIONAL NIPPV breaths Frequency: 6–12 bpm PIP: 15–20 above Paw; adjust PRN to obtain minimal chest rise |
| Other devices: Infant Star 950 Leoni Plus @ Stephanie/Sophie @ Life Pulse Jet |
Comments: Fixed I-time at 18 msec, “synchronized” via Graseby capsule Variable I:E ratio Variable I:E; supports up to 5 kg “On-time” usually set at 20 msec |
Optional NIPPV breaths in all Limited availability & ΔP Probably similar function to Drager HFOV possible in insp & exp phase No clinical or lab studies to date; probably needs longer “on-time” |
We routinely employ convective breaths during HFNV with Percussionaire devices;
Devices are not currently approved for HFV in the US
Device manufacturers: 3100A (Carefusion, San Diego, CA, USA); VDR4/Bronchotron (Percussionaire, Sand Point, ID, USA); Drager VN500/Babylog 8000 (Draeger Medical, Lubeck, Germany); Infant Star 950 (Mallinckrodt, St Louis, MO, USA); Leoni Plus (Heinen+Lowenstein, Bad Ems, Germany); Stephanie/Sophie (Fritz Stephan-GmbH, Gackenbach, Germany); Life Pulse Jet (Bunnell Inc, Salt Lake City, UT, USA)
5. Future directions
Many issues need to be clarified in relation to HFNV. First, not every high-frequency ventilator functions the same or has the same capabilities (Tables 1 and 7). Issues to consider include active versus passive expiratory phase, ability to adjust inspiratory time, variability of maximum delivered high-frequency VT, flexibility of ventilator circuits, ability to provide conventional breaths during high-frequency support, and ease of user interface. Second, there are no data from well-designed randomized trials documenting clinical safety and efficacy of HFNV compared to other NIV approaches (Table 5). Such trials are urgently needed. Additionally, it is unclear what the optimal approach to HFNV should be, and whether the approach is the same or different between available devices and nasal interfaces. Whether the coupling of NIPPV to some forms of HFNV, compared to HFNV only, provides additional benefit for successful NIV support also remains to be determined. Finally, the main reason underlying the promotion of any mode of NIV in lieu of invasive mechanical ventilation, including HFNV, is the potential to minimize injury to the immature lung and to restore or improve lung growth and development, thereby preventing the long-term sequelae associated with both preterm birth and BPD. Such studies will require a long-term investment in time, money, and effort.
Practice points.
There is increasing use of high-frequency ventilation for non-invasive support in neonates.
During non-invasive high-frequency ventilation frequency, inspiratory time and pressure amplitude affect delivered tidal volume.
Effective gas exchange is also dependent on the device and the nasal interface.
Research directions.
Need studies to identify optimal approach(es) for using high-frequency nasal ventilation.
Need adequately designed randomized trials comparing non-invasive high-frequency ventilation to other modes of non-invasive support.
Need additional mechanistic studies in animal models to assess systemic effects as well as the effects on lung injury and developmental lung biology.
Acknowledgments
The authors wish to acknowledge the outstanding support from Kevin Crezee, RRT, Director of Neonatal Respiratory Care, Primary Children’s Hospital, Salt Lake City, UT, USA, for his technical assistance in laboratory studies and clinical application of HFNV devices.
Funding sources
K. Albertine: NIH R01 HL110002 and T32 HL-07744.
Footnotes
Conflict of interest statement
Dr Yoder discloses that he has received grant and equipment support for clinical research from Dräger Medical (Lübeck, Germany). Dr Albertine discloses that he has received research support in the form of equipment from Percussionaire (Sand Point, ID, USA) and Dräger Medical. Dr Null discloses that he has received grant and equipment support from Dräger Medical and equipment support from Percussionaire. He has also received financial support from the above companies as well as from Bunnell, Inc. (Salt Lake City, UT, USA) and CareFusion (San Diego, CA, USA) via sponsorship of an annual conference on high-frequency ventilation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Donald I, Lord J. Augmented respiration; studies in atelectasis neonatorum. Lancet. 1953;1:9–17. doi: 10.1016/s0140-6736(53)92511-2. [DOI] [PubMed] [Google Scholar]
- 2.Gregory GA, Kitterman JA, Phibbs RH, Tooley WH, Hamilton WK. Treatment of the idiopathic respiratory-distress syndrome with continuous positive airway pressure. N Engl J Med. 1971;284:1333–40. doi: 10.1056/NEJM197106172842401. [DOI] [PubMed] [Google Scholar]
- 3.DeLemos RA, McLaughlin GW, Robison EJ, Schulz J, Kirby RR. Continuous positive airway pressure as an adjunct to mechanical ventilation in the newborn with respiratory distress syndrome. Anesth Analg. 1973;52:328–32. [PubMed] [Google Scholar]
- 4.Marchak BE, Thompson WK, Duffty P, et al. Treatment of RDS by high-frequency oscillatory ventilation: a preliminary report. J Pediatr. 1981;99:287–92. doi: 10.1016/s0022-3476(81)80480-5. [DOI] [PubMed] [Google Scholar]
- 5.Polin RA, Carlo WA Committee on Fetus and Newborn, American Academy of Pediatrics. Surfactant replacement therapy for preterm and term neonates with respiratory distress. Pediatrics. 2014;133:156–63. doi: 10.1542/peds.2013-3443. [DOI] [PubMed] [Google Scholar]
- 6.Hummler H, Schulze A. New and alternative modes of mechanical ventilation in neonates. Semin Fetal Neonatal Med. 2009;14:42–8. doi: 10.1016/j.siny.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Yoder BA, Harrison M, Clark RH. Time-related changes in steroid use and bronchopulmonary dysplasia in preterm infants. Pediatrics. 2009;124:673–9. doi: 10.1542/peds.2008-2793. [DOI] [PubMed] [Google Scholar]
- 8.Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–56. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farstad T, Bratlid D, Medbø S, Markestad T for the Norwegian Extreme Prematurity Study Group. Bronchopulmonary dysplasia – prevalence, severity and predictive factors in a national cohort of extremely premature infants. Acta Paediatr. 2011;100:53–8. doi: 10.1111/j.1651-2227.2010.01959.x. [DOI] [PubMed] [Google Scholar]
- 10.Maitre NL, Ballard RA, Ellenberg JH, et al. Prematurity and Respiratory Outcomes Program. Respiratory consequences of prematurity: evolution of a diagnosis and development of a comprehensive approach. J Perinatol. 2015;35:313–21. doi: 10.1038/jp.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Husain AN, Siddiqui NH, Stocker JT. Pathology of arrested acinar development in post surfactant bronchopulmonary dysplasia. Hum Pathol. 1998;29:710–17. doi: 10.1016/s0046-8177(98)90280-5. [DOI] [PubMed] [Google Scholar]
- 12.Coalson JJ, Winter VT, Siler-Khodr T, Yoder BA. Neonatal chronic lung disease in extremely immature baboons. Am J Respir Crit Care Med. 1999;160:1333–46. doi: 10.1164/ajrccm.160.4.9810071. [DOI] [PubMed] [Google Scholar]
- 13.Jobe AH. The new BPD: an arrest of lung development. Pediatr Res. 1999;46:641–3. doi: 10.1203/00006450-199912000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Jobe AH. Animal models, learning lessons to prevent and treat neonatal chronic lung disease. Front Med (Lausanne) 2015;2:49. doi: 10.3389/fmed.2015.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmölzer GM, Kumar M, Pichler G, Aziz K, O’Reilly M, Cheung PY. Non-invasive versus invasive respiratory support in preterm infants at birth: systematic review and meta-analysis. BMJ. 2013;347:f5980. doi: 10.1136/bmj.f5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pillow JJ. High-frequency oscillatory ventilation: mechanisms of gas exchange and lung mechanics. Crit Care Med. 2005;33:S135–S141. doi: 10.1097/01.ccm.0000155789.52984.b7. [DOI] [PubMed] [Google Scholar]
- 17.Mukerji A, Belik J. Neonatal nasal intermittent positive pressure ventilation efficacy and lung pressure transmission. J Perinatol. 2015;35:716–19. doi: 10.1038/jp.2015.61. [DOI] [PubMed] [Google Scholar]
- 18.Owen LS, Morley CJ, Davis PG. Pressure variation during ventilator generated nasal intermittent positive pressure ventilation in preterm infants. Archs Dis Childh Fetal Neonatal Ed. 2010;95:F359–F364. doi: 10.1136/adc.2009.172957. [DOI] [PubMed] [Google Scholar]
- 19.De Luca D, Carnielli VP, Conti G, Piastra M. Noninvasive high frequency oscillatory ventilation through nasal prongs: bench evaluation of efficacy and mechanics. Int Care Med. 2010;36:2094–2100. doi: 10.1007/s00134-010-2054-7. [DOI] [PubMed] [Google Scholar]
- 20.De Luca D, Piastra M, Pietrini D, Conti G. Effect of amplitude and inspiratory time in a bench model of non-invasive HFOV through nasal prongs. Pediatr Pulmonol. 2012;47:1012–18. doi: 10.1002/ppul.22511. [DOI] [PubMed] [Google Scholar]
- 21.Mukerji A, Finelli M, Belik J. Nasal high-frequency oscillation for lung carbon dioxide clearance in the newborn. Neonatology. 2013;103:161–5. doi: 10.1159/000345613. [DOI] [PubMed] [Google Scholar]
- 22.Pierce RA, Albertine KH, Starcher BC, Bohnsack JF, Carlton DP, Bland RD. Chronic lung injury in preterm lambs: disordered pulmonary elastin deposition. Am J Physiol. 1997;272:L452–L460. doi: 10.1152/ajplung.1997.272.3.L452. [DOI] [PubMed] [Google Scholar]
- 23.Albertine KH, Jones GP, Starcher BC, et al. Chronic lung injury in preterm lambs. Disordered respiratory tract development. Am J Respir Crit Care Med. 1999;159:945–58. doi: 10.1164/ajrccm.159.3.9804027. [DOI] [PubMed] [Google Scholar]
- 24.Reyburn B, Li M, Metcalfe DB, et al. Nasal ventilation alters mesenchymal cell turnover and improves alveolarization in preterm lambs. Am J Respir Crit Care Med. 2008;178:407–18. doi: 10.1164/rccm.200802-359OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Null DM, Alvord J, Leavitt W, et al. High-frequency nasal ventilation for 21 d maintains gas exchange with lower respiratory pressures and promotes alveolarization in preterm lambs. Pediatr Res. 2014;75:507–16. doi: 10.1038/pr.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rehan VK, Fong J, Lee R, et al. Mechanism of reduced lung injury by high-frequency nasal ventilation in a preterm lamb model of neonatal chronic lung disease. Pediatr Res. 2011;70:462–6. doi: 10.1203/PDR.0b013e31822f58a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sondergaard S, Karason S, Hanson A, et al. Direct measurement of intratracheal pressure in pediatric respiratory monitoring. Pediatr Res. 2002;51:339–45. doi: 10.1203/00006450-200203000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Neu J. Gastrointestinal maturation and implications for infant feeding. Early Hum Dev. 2007;83:767–75. doi: 10.1016/j.earlhumdev.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Di Mauro A, Neu J, Riezzo G, et al. Gastrointestinal function development and microbiota. Ital J Pediatr. 2013;39:15. doi: 10.1186/1824-7288-39-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biniwale MA, Ehrenkranz RA. The role of nutrition in the prevention and management of bronchopulmonary dysplasia. Semin Perinatol. 2006;30:200–8. doi: 10.1053/j.semperi.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Stephens BE, Walden RV, Gargus RA, et al. First week protein and energy intakes are associated with 18-month developmental outcomes in extremely low birth weights infants. Pediatrics. 2009;123:1337–43. doi: 10.1542/peds.2008-0211. [DOI] [PubMed] [Google Scholar]
- 32.Ehrenkranz RA, Das A, Wrage LA, et al. Early nutrition mediates the influence of severity of illness on extremely LBW infants. Pediatr Res. 2011;69:522–9. doi: 10.1203/PDR.0b013e318217f4f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moreau-Bussière F, Samson N, St-Hilaire M, et al. Laryngeal response to nasal ventilation in non-sedated newborn lambs. J Appl Physiol. 2007;102:2149–57. doi: 10.1152/japplphysiol.00891.2006. [DOI] [PubMed] [Google Scholar]
- 34.Hadj-Ahmed MA, Samson N, Bussières M, Beck J, Praud JP. Absence of inspiratory laryngeal constrictor muscle activity during nasal neurally adjusted ventilatory assist in newborn lambs. J Appl Physiol (1985) 2012;113:63–70. doi: 10.1152/japplphysiol.01496.2011. [DOI] [PubMed] [Google Scholar]
- 35.Hadj-Ahmed MA, Samson N, Nadeau C, Boudaa N, Praud JP. Laryngeal muscle activity during nasal high-frequency oscillatory ventilation in non-sedated newborn lambs. Neonatology. 2015;107:199–205. doi: 10.1159/000369120. [DOI] [PubMed] [Google Scholar]
- 36.van der Hoeven M, Brouwer E, Blanco CE. Nasal high frequency ventilation in neonates with moderate respiratory insufficiency. Archs Dis Childh Fetal Neonatal Ed. 1998;79:F61–F63. doi: 10.1136/fn.79.1.f61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colaizy TT, Younis UM, Bell EF, Klein JM. Nasal high-frequency ventilation for premature infants. Acta Paediatr. 2008;97:1518–22. doi: 10.1111/j.1651-2227.2008.00900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Czernik C, Schmalisch G, Bührer C, Proquitté H. Weaning of neonates from mechanical ventilation by use of nasopharyngeal high-frequency oscillatory ventilation: a preliminary study. J Matern Fetal Neonatal Med. 2012;25:374–8. doi: 10.3109/14767058.2011.580401. [DOI] [PubMed] [Google Scholar]
- 39.Mukerji A, Singh B, Helou SE, et al. Use of noninvasive high-frequency ventilation in the neonatal intensive care unit: a retrospective review. Am J Perinatol. 2015;30:171–6. doi: 10.1055/s-0034-1381317. [DOI] [PubMed] [Google Scholar]
- 40.Hoehn T, Krause MF. Effective elimination of carbon dioxide by nasopharyngeal high-frequency ventilation. Respir Med. 2000;94:1132–4. doi: 10.1053/rmed.2000.0889. [DOI] [PubMed] [Google Scholar]
- 41.Dumas De La Roque E, Bertrand C, et al. Nasal high frequency percussive ventilation versus nasal continuous positive airway pressure in transient tachypnea of the newborn: a pilot randomized controlled trial ( NCT00556738) Pediatr Pulmonol. 2011;46:218–23. doi: 10.1002/ppul.21354. [DOI] [PubMed] [Google Scholar]
- 42.Fischer HS, Bohlin K, Bührer C, et al. Nasal high-frequency oscillation ventilation in neonates: a survey in five European countries. Eur J Pediatr. 2015;174:465–71. doi: 10.1007/s00431-014-2419-y. [DOI] [PubMed] [Google Scholar]