Abstract
Background
Among cardiac patients, positive psychological factors are consistently linked with superior clinical outcomes and improvement in key markers of inflammation and hypothalamic-pituitary-adrenal axis functioning. Further, positive psychology interventions (PPI) have effectively increased psychological well-being in a wide variety of populations. However, there has been minimal study of PPIs in cardiac patients, and no prior study has evaluated their effect on key prognostic biomarkers of cardiac outcome. Accordingly, we investigated the effect of three distinct PPIs on risk biomarkers in cardiac patients.
Methods
In an exploratory trial, 69 patients with recent coronary artery bypass graft surgery or percutaneous intervention were randomized to a) one of three 6-week in-person PPIs (based on the work of Seligman, Lyubomirsky, or Fordyce) or b) a wait-list control group. Risk biomarkers were assessed at baseline, post-intervention (7 weeks), and at 15 week follow-up. Between-group differences in change from baseline biomarker levels were examined via random effects models.
Results
Compared to the control group, participants randomized to the Seligman (B= −2.06; p= .02) and Fordyce PPI (B= −1.54; p= .04) had significantly lower high-sensitivity C-reactive protein (hs-CRP) levels at 7 weeks. Further, the Lyubomirsky PPI (B= −245.86; p= .04) was associated with a significantly lower cortisol awakening response (CARg) at 7 weeks compared to control participants. There were no other significant between-group differences.
Conclusion
Despite being an exploratory pilot study with multiple between-group comparisons, this initial trial offers the first suggestion that PPIs might be effective in reducing risk biomarkers in high-risk cardiac patients.
Keywords: Positive psychology, intervention, coronary artery disease, inflammation, HPA-axis functioning, randomized controlled trial
Coronary artery disease (CAD) is the world’s leading cause of death.1 Psychological factors play an important role in the morbidity and mortality associated with CAD. For example, depression in CAD patients is associated with approximately double the risk of mortality and other adverse cardiac events, and these relationships are independent of traditional cardiac risk factors. 2–4 Anxiety is also prospectively associated with a higher risk of mortality and major cardiac events in CAD patients. 5,6 However, interventions targeting these negative psychological syndromes in patients with heart disease patients have shown limited effects on cardiac morbidity and mortality. 7,8
In contrast, positive psychological constructs are associated with superior cardiovascular outcomes. 9,10 Positive affect has been associated with beneficial cardiovascular health and possibly superior immune functioning.11 Likewise, other positive constructs, including optimism. 12,13 vitality, 14,15 positive affect,16,17 well-being,18,19 and sense of control 20 have also been associated with reduced cardiac morbidity and mortality, along with improved status of physiological markers of cardiac prognosis, in many cases independent of the adverse effects of negative psychological states such as depression. 21
The relationship between positive psychological factors and cardiac outcomes is often considered to be mediated via two potential pathways: health behavior and physiologic mechanisms. Positive psychological factors, measured at baseline, are associated with greater participation in key health behaviors, including following a healthy diet, smoking cessation, and increased physical exercise, 9,10 all of which are strongly related to cardiac outcome. 22 Though physiologic effects have been less well-studied, positive psychological factors have also been linked to adaptive hypothalamic-pituitary-adrenal (HPA) axis functioning and reduced inflammation. 23–25 Such findings may be important given that these markers are associated with adverse cardiac events and that HPA axis dysfunction and inflammation may be mediational pathways by which depression leads to higher rates of mortality in CAD patients. 26
Positive psychology interventions (PPI) utilize systematic exercises to cultivate positive affect, optimism, and other positive psychological factors. PPIs have consistently reduced distress and improved well-being in healthy populations and patients with mental health symptoms. 27,28 However, despite the clear relationships between positive psychological constructs and cardiac outcomes, very few studies so far have specifically investigated the application of PPI in cardiac populations. In a small three-arm pilot trial, an 8-week, telephone-based PPI lead to greater (though nonsignificant) improvements in mood, anxiety, and quality of life compared to both an active control group (Relaxation Response) or an attention-matched control group (a control condition that matches the amount of attention patients receive in the intervention condition, but lacks specific therapeutic content) among patients hospitalized for an acute coronary syndrome or heart failure. 29 Among patients who had just received a percutaneous coronary intervention, Peterson and colleagues 30 compared a positive affect/self affirmation (PA) intervention to patient education alone. Patients in the PA group had a significant increase in physical activity and a decrease in depressive symptoms, though there were no differences in rates of cardiac events and cardiac biomarkers were not assessed.
The question of whether PPIs can lead to improvements in physiological biomarkers associated with CAD progression and outcomes has remained unanswered. Accordingly, in this secondary analysis of a randomized controlled trial of 6-week PPIs in CAD patients (in which PPIs led to significant improvements in psychological outcomes 31), we examined the effects of PPIs on inflammatory makers and HPA axis function. We hypothesized that the PPIs would be associated with greater reductions of inflammatory markers and CARg post-intervention, compared to participants in the wait-list control condition.
Methods
Participants and Recruitment
Permission to conduct the study was obtained from the Ethics Committee at The University of Isfahan. The trial was registered at the Iranian Registry of Clinical Trials (IRCT2014010516081N1). Patients who had received coronary artery bypass surgery (CABG) or percutaneous coronary intervention at Sina Cardiovascular Hospital or Izamin Cardiac Rehabilitation Center within the preceding 5 months and were living in the immediate Isfahan area were identified as eligible participants. Patients meeting these criteria were approached based on random selection from printed patients lists of patients undergoing CABG or percutaneous intervention. Willing patients were invited to attend an introductory session explaining the role of psychological factors in heart disease and describing the rationale, intervention procedures and assessment appointments of the study. All study visits took place at Izamin Cardiac Rehabilitation Center.
Patients were excluded if they had a medical condition limiting their ability to participate in the trial, if they were unwilling to participate in all stages of the study, or if they were undergoing current treatment with psychiatric medication (e.g., antidepressants, antipsychotics, benzodiazepines), steroids, or psychotherapy. Patients receiving nonsteroidal anti-inflammatory medications were not excluded because the vast majority of patients were receiving aspirin, which has a highly similar mechanism and effect on acute and chronic inflammation. Those willing to participate and meeting inclusion/exclusion criteria completed written informed consent and were provided with self-report questionnaires to be completed prior to the first study meeting.
Procedures
Enrolled subjects were randomly assigned to one of three PPIs (Seligman, Lyubomirsky, Fordyce) or a wait-list control (i.e., participants had a 3 in 4 chance of receiving a PPI). Randomization was achieved using concealed cards with group assignment listed. Participants and study staff were unaware of group assignment until the card was revealed following pre-randomization baseline measures. Subjects assigned to one of the PPIs received a 6-week intervention, described below. Subjects in the control group were placed on a waiting list and received treatment as usual for the duration of the study. When their study assessments were completed, participants in the control group were offered participation in one of the six-week PPIs. Participants in all conditions were assessed at baseline, 7 weeks (i.e., a week after the 6 week intervention for those in PPI conditions), and 15 weeks (i.e., follow-up assessment 8 weeks after the post-treatment assessment).
Interventions
The PPIs were developed based on the empirical work of three seminal positive psychology researchers (Seligman, 32,33 Lyubomirsky, 34,35 Fordyce, 36,37). Participants attended six weekly 90-minute in-person group sessions. PPI exercises were designed in conjunction with a cardiologist to ensure that participants would be able to complete them despite any physical limitations. Each PPI intervention was strictly manualized and the PPI trainer used a content checklist for each session to ensure treatment was delivered in accordance with the manual. All interventions were delivered by an experienced PPI trainer (Gh. N) with specific training in each of the studies PPI manuals.
During each session, the study trainer introduced up to three strategies to boost psychological well-being. Specific exercises to implement these strategies were explained by the trainer and subsequently practiced by the participants in the group setting with supervision from the trainer. Finally, participants were encouraged to perform at least one exercise using each of that week’s strategies prior to the next group session. They were also asked to continue to practice exercises from previous weeks and to incorporate them into their daily lives.
At the beginning of the subsequent session, strategies introduced in the previous week and participants’ experiences with those exercises were reviewed. After the final session, participants received a chart to plan exercise continuation for future weeks.
Seligman group intervention (intervention content for all PPI groups are listed in Table 1)
Table 1.
Intervention content and according exercises of the three different PPIs.
| Seligman PPI | Lyubomirsky PPI | Fordyce PPI | |
|---|---|---|---|
| Week1 |
Increasing satisfaction about the past
|
Experiencing positive emotions
|
Increasing activity and social relationships
|
| Week 2 |
Enhancing happiness in the present
|
Enhancing social relationships and physical activity
|
Increasing productivity and organization
|
| Week 3 |
Optimism about the future
|
Cultivating optimism andgratitude
|
Reducing worry and setting realistic goals
|
| Week 4 |
Renewing strength and virtue
|
Developing positive coping skills
|
Cultivating optimism and focusing on the present
|
| Week 5 |
Valuing and using strengths and virtues
|
Forgiveness and spirituality
|
Focusing on positive personality traits
|
| Week 6 |
Enhancing meaning in life
|
Commitment to goals, flow, and mindfulness
|
Prioritizing positive thoughts and feelings
|
Seligman posits these three distinct pathways to achieve the full life, with a special emphasis on engagement and a ‘meaningful’ life. Accordingly, the Seligman exercises 32,33 focused on enhancing positive feelings (“Pleasant life”), identifying and using personal strengths (“Engagement life”), and finding meaning in one’s life (“Meaningful life”).
Lyubomirsky group
The Lyubomirsky program 34,35 content had moderate overlap with the Seligman group regarding specific exercises targeting optimism and gratitude. However, it uniquely included exercises focusing on religion and spirituality, physical activity, and developing strategies for coping.
Fordyce group
The Fordyce program 36,37 had some common elements with the two previous programs in that some happiness activities focused on optimism, becoming present-oriented, and eliminating negative cognitions and feelings. In contrast to the other interventions, the Fordyce intervention focused on increasing organizational skills, setting realistic goals, and focusing on positive personality traits.
Data collection
Baseline sociodemographic and medical characteristics were gathered via participant report. To measure changes in depression over time, the Beck Depression Inventory II (BDI) 38 was administered at baseline, post-intervention (Week 7) and follow-up (Week 15). The BDI contains 21 items measuring depressive symptoms over the preceding two weeks. The range of scores is between 0 and 63. The Persian translation of the BDI has shown high internal consistency (α=.87) and acceptable test-retest reliability (r =.74) in an Iranian sample. 39
Regarding biomarkers, inflammatory markers were collected at baseline, post- intervention (Week 7), and follow-up (Week 15) and markers of HPA-axis activity (i.e.,CARg) were assessed at baseline and post-intervention. Blood samples were drawn at each assessment point between 8 and 10 am. Participants were instructed to fast and to avoid caffeine 12 hours prior to sampling. The samples, collected in tubes with no additives, were stored at room temperature for 40 minutes and then were refrigerated until they were centrifuged within three hours of collection to isolate serum. Serum aliquots were frozen at −70°C until the time of assay. Specimens were coded so the operator was blinded to interventions and other identifying characteristics. hs-CRP was measured with nephelometric assay for quantitative determination of low levels of CRP. IL-1 and IL-6 were assayed with the enzyme-linked immunosorbent assay (ELISA) kits.
Regarding HPA-axis activity, saliva sampling for CARg took place at home. Participants were instructed to start sampling immediately at awakening (0) and then 15 and 45 min after awakening in the morning. Saliva samples were stored in participants’ freezers before being transferred to the laboratory where they were stored at −20° C until the time of assay. Cortisol was assayed with ELISA kits. Area under the curve (AUC) with respect to ground (CARg) [see 40 for formula] was calculated.
Statistical analysis
The primary study outcome for this analysis was group differences in high-sensitivity C-reactive protein (hs-CRP). This marker was selected because, among all inflammatory markers, it has been most consistently linked to cardiac outcomes; it is also the inflammatory marker most used in clinical care. 41,42 Secondary outcome measures were interleukin-1 (IL-1), interleukin-6 (IL-6), and cortisol awakening response (CARg), a marker of HPA axis activity.
Baseline data were compared between study groups using chi-square analysis for categorical outcome and one-way ANOVA for continuous variables. To compare biomarker levels across groups, linear random effects models, with a random intercept for each patient, were used to assess biomarkers at each time point. Use of random effects models allows inclusion of all participants, even those with missing data at specific time points, in an intent-to-treat analysis, providing advantages over including only participants with complete data or using other methods for missing data (e.g., last observation carried forward).43
Each PPI was compared to the control group. Between group differences in improvement of the outcome measures from baseline at 7 weeks and 15 weeks were compared between each PPI and the control condition. Age, sex, time since medical intervention, and change in BDI were evaluated as covariates for each model and included in the model, if they significantly increased model fit (-2 log likelihood). In analysis on the CARg, patients awakening time was included as a covariate as well. All analyses were completed using SPSS version 22. All statistical tests were two-tailed. Given the exploratory, hypothesis-generating nature of this pilot study, significance was set at p=.05.
Results
Enrollment and Retention
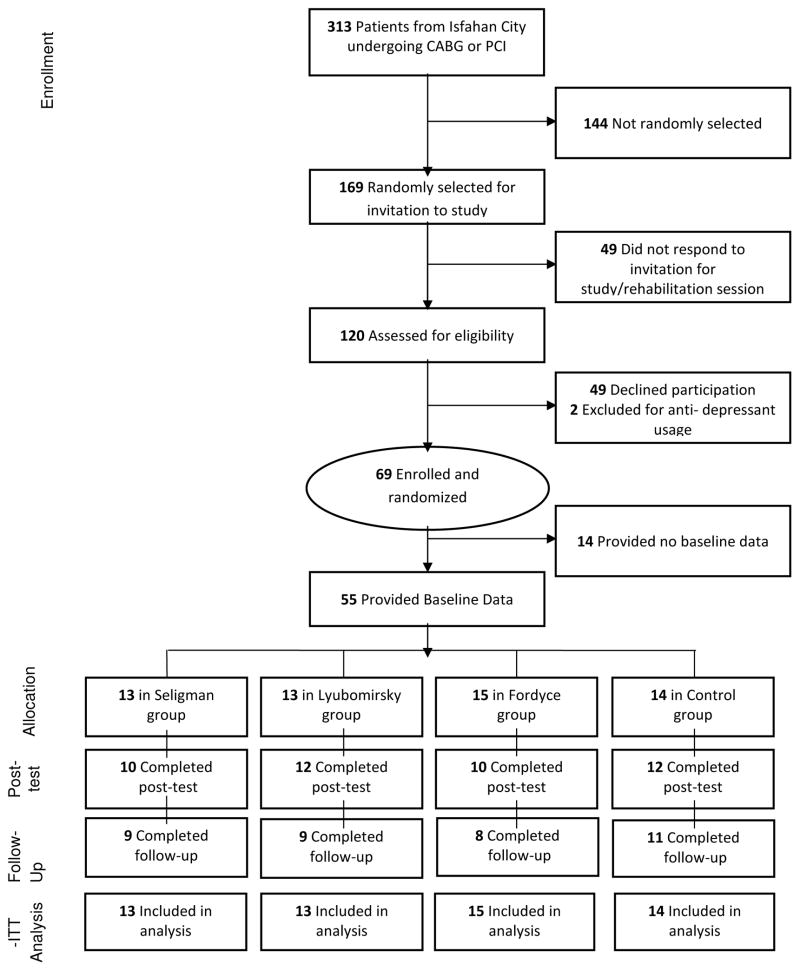
Patient flow throughout the study can be seen in Figure 1. Fifty-eight percent of eligible patients were ultimately enrolled and randomized. There were no significant differences in baseline characteristics between participants who provided follow-up data and those who dropped out, though there was a trend toward higher rates of CABG in those who dropped out (57.1% dropout vs. 30.9% retained; χ2 = 3.32 ; p = .07).
Figure 1.
CONSORT Diagram of study recruitment and throughput.
Baseline characteristics
Participants’ baseline characteristics and scores are listed in Table 2. There were no statistically significant differences in baseline medical or sociodemographic groups at baseline, and no statistically significant group differences in risk biomarkers at baseline. Table 2 also lists BDI scores and biomarker results over time. Though there were no significant group differences in BDI scores, the scores did appear to differ across groups and change in BDI score was therefore included as a covariate in the main analyses.
Table 2.
Sample Characteristics of Sociodemographic, Medical, and Risk Biomarker variables.
| Seligman Group (N=13) | Lyubomirsky Group (N=13) | Fordyce Group (N=15) | Control Group (N=14) | p | |
|---|---|---|---|---|---|
| Sociodemographic Characteristics | |||||
| Male sex f (%) | 9 (69.2) | 11 (84.6) | 13 (86.7) | 9 (64.3) | .41 |
| Age M (SD) | 55.8 (5.3) | 59.2 (11.5) | 54.7 (10.1) | 56.9 (6.7) | .58 |
| High school education or less f (%) | 9 (69.2) | 9 (69.2) | 10 (66.7) | 9 (64.3) | .99 |
| Married f (%) | 11 (84.6) | 12 (92.3) | 14 (93.3) | 12 (85.7) | .84 |
| Medical Characteristics | |||||
| Recent CABG f (%) | 7 (53.8) | 4 (30.8) | 3 (20) | 3 (21.4) | .20 |
| Days since medical procedure M (SD; MD=4) | 339.25 (67.54) | 119.18 (127.25) | 120.33 (149.78) | 98.76 (67.54) | .14 |
| Diabetes f (%) | 5 (38.5) | 2 (15.4) | 4 (26.7) | 3 (21.4) | .58 |
| Hypertension f (%) | 1 (7.7) | 1 (7.7) | 5 (33.3) | 2 (15.4) | .20 |
| Current smoking f (%) | 2 (16.6) | 2 (15.4) | 1 (7.1) | 2 (15.4) | .88 |
| Risk Biomarkers | |||||
| Baseline M (SD) | |||||
| hs-CRP (mg/dl) | 2.01 (1.48) | 1.74 (0.72) | 1.91 (0.83) | 1.69 (0.49) | .73 |
| IL-1 (pg/ml) | 2.48 (0.69) | 2.56 (0.66) | 2.75 (0.41) | 2.67 (0.96) | .77 |
| IL-6 (pg/ml) | 1.19 (0.64) | 1.26 (0.59) | 1.22 (0.63) | 1.57 (0.64) | .37 |
| CARga | 229.05 (55.30) | 283.65 (203.22) | 139.50 (155.26) | 106.33 (66.14) | .19 |
| Post-intervention M (SD) | |||||
| hs-CRP (mg/dl) | 1.08 (0.31) | 3.01 (1.77) | 1.39 (0.57) | 2.57 (2.86) | |
| IL-1 (pg/ml) | 1.96 (1.18) | 1.96 (1.21) | 1.94 (1.32) | 1.28 (0.73) | |
| IL-6 (pg/ml) | 2.82 (0.98) | 2.55 (1.01) | 2.32 (1.35) | 3.04 (0.47) | |
| CARgA | 254.24 (70.52) | 263.04 (134.21)) | 340.33 (168.03) | 286.83 (182.94) | |
| Follow-up M (SD) | |||||
| hs-CRP (mg/dl) | 1.72 (1.38) | 1.72 (0.38) | 2.50 (1.25) | 1.92 (1.18) | |
| IL-1 (pg/ml) | 2.01 (1.97) | 1.72 (1.53) | 3.60 (3.31) | 3.58 (3.74) | |
| IL-6 (pg/ml) | 3.28 (1.07) | 2.90 (0.99) | 3.80 (2.46) | 3.96 (2.12) | |
| Depressiveness (BDI) | |||||
| Baseline M (SD) | 10.13 (10.83) | 16.49 (6.29) | 16.27 (10.12) | 15.23 (7.76) | .27 |
| Post-intervention M (SD) | 9.00 (7.45) | 18.95 (9.60) | 11.72 (9.96) | 14.30 (6.96) | .06 |
| Follow-up M (SD) | 8.46 (9.31 | 12.98 (4.40) | 9.33 (7.17) | 15.94 (8.79) | .13 |
Notes:
Cortisol awakening response CARg has been computed as area under the curve of participants’ cortisol levels 0, 15 and 45 minutes after awakening.
Primary study outcome: hs-CRP (Table 3)
Table 3.
Between-group comparisons on study risk biomarkers at post-intervention and follow-up.
| Outcome | Coefficient | 95% CI | p | Coefficient | 95% CI | p | |
|---|---|---|---|---|---|---|---|
| Post-intervention (7 weeks) | Follow-up (15 weeks) | ||||||
| Seligman vs. control | |||||||
| hs-CRP | −2.01 | −3.93; −0.08 | 0.04 | −0.49 | −2.32; 1.32 | 0.59 | |
| IL-1 A | −1.23 | −0.98, 3.44 | 0.27 | −1.53 | −3.69; 0.62 | 0.16 | |
| IL-6 A | −0.01 | −1.23; 1.22 | 0.99 | −0.23 | −1.50; 1.03 | 0.72 | |
| CARg B | −129.54 | −334.63; 75.56 | 0.21 | ||||
| Lyubomirsky vs. control | |||||||
| hs-CRP | 0.30 | −1.56, 2.16 | 0.75 | 0.19 | −2.15, 1.75 | 0.84 | |
| IL-1 | 0.62 | −1.45; 2.42 | 0.55 | −2.06 | −4.26; 0.13 | 0.07 | |
| IL-6 A | 0.01 | −1.23; 1.25 | 0.99 | −1.12 | −2.59; 0.35 | 0.13 | |
| CARg B | −263.17 | −492.63; −33.68 | 0.03 | ||||
| Fordyce vs. control | |||||||
| hs-CRP | −1.63 | −3.23; −0.04 | 0.04 | 0.65 | −0.95; 1.45 | 0.38 | |
| IL-1 | 0.01 | −2.10; 2.13 | 0.99 | −0.34 | −2.71; 2.04 | 0.78 | |
| IL-6 A | −0.28 | −1.78; 1.22 | 0.71 | 0.25 | −1.39; 1.92 | 0.75 | |
| CARg B | 3.01 | −203.74; 197.72 | 0.98 | ||||
Notes: All analyses have been controlled for the time between medical intervention and first biomarker sampling and for change in depressiveness (BDI).
Additionally controlled for age and sex.
Cortisol measures have been additionally controlled for awakening time.
Participants randomized to the Seligman and Fordyce PPIs had significantly greater reductions in hs-CRP levels post-intervention (7 weeks) compared to the control group; there were no between-group differences between the Lyubomirsky and control groups at 7 weeks. There were no differences in hs-CRP change from baseline between the control group and any of the PPIs at 15-week follow-up.
Secondary outcome: IL-1, IL-6, CARg (Table 3)
There was no significant difference in IL-1 or IL-6 change from baseline between any of the PPIs and the control group, though there was a statistical trend showing greater improvement in IL-1 levels at 15-week follow-up for the Lyubomirsky PPI compared to the control group. Comparing change in CARg from baseline between the PPIs and the control group rendered a significantly lower level of CARg for the Lyubomirsky PPI compared to the control group.
Discussion
This is, to our knowledge, the first randomized controlled trial that investigated the effects of PPI on inflammatory markers and regulation of HPA axis activity in cardiac patients. Though our analyses were highly exploratory, we found that PPIs may be associated with greater reductions in hs-CRP, and perhaps other biomarkers, in CAD patients. Specifically, both the Seligman PPI and Fordyce PPI had greater improvements of hs-CRP (our primary outcome variable) post-intervention. In contrast, the Lyubomirsky PPI, was associated with reduced CARg at post-intervention and possibly reduced IL-1 levels at follow-up, but had no effects on hs-CRP.
Across all risk biomarkers, hs-CRP appeared to depict the clearest pattern of intervention effects. Given that hs-CRP may be most consistent measure to link inflammatory processes with cardiac outcome,41,42 and that it is the most commonly used marker in clinical care, hs-CRP is the most promising candidate to further evaluate the effects of PPIs on biomarkers in cardiac patients.
The differential effects across the three PPIs may have been related to the different intervention content in the different PPIs. Eudaimonic well-being (personal growth, purposeful engagement, meaning and direction in life) has been linked to lower levels of pro-inflammatory cytokines, daily salivary cortisol, and cardiovascular risk, while hedonic well-being (which is more related to momentary joy, happiness, and pleasure) has shown comparatively less linkage to biomarkers.44 Differences in the interventions’ focus on eudiamonic well-being vs. hedonic well-being might have led to differences in the effects on biomarkers.
While plasma biomarkers may not always directly correlate with clinical outcomes, in cardiac patients, biomarkers have been consistently linked with prognosis.41,45 For example, large-scale prospective studies have found that hs-CRP strongly and independently predicts adverse cardiovascular events, including myocardial infarction, ischemic stroke, and sudden cardiac death in CAD patients.46 In addition, there is increasing focus on the use of biomarkers in clinical management, such as the work of Januzzi and colleagues 47 finding that use of N- terminal pro-B-type natriuretic peptide (NT-proBNP) levels to guide treatment of heart failure patients lead to lower event rates than standard care. This suggests that examining the effects of interventions on biomarkers of cardiac risk may have clinical application.
This exploratory study had several important limitations. The small sample size and four-arm design limited statistical power to detect significant relationships. Given the hypothesis-generating nature of this study, significance (including secondary outcome variables) was set at p=.05, and all significant findings, especially on secondary outcomes, must be interpreted with caution. Although there were no significant differences on baseline characteristics, small differences in medical characteristics like hypertension or recent CABG may impact the effects of interventions on biomarkers, possibly skewing group differences in this sample, and larger studies are needed to identify potential moderators of PPI effects in cardiac patients. Other limitations include recruitment from a single city, delivery of PPIs from a single trainer, and lack of an attention-matched control condition. It is possible that effects may have been derived from the effects specific to the trainer or to attention alone. Finally, this pilot study also did not longitudinally assess rates of cardiac events or mediating variables such as health behaviors.
In conclusion, in an exploratory study, two PPIs were associated with greater improvements in hs-CRP compared to a wait-list control condition. This study provides the first suggestion that PPIs could have effects on important physiological processes in patients with heart disease. These findings should be confirmed in larger, well-controlled studies, and, if possible, correlated with cardiac events. Further research is also needed to identify the most effective PPI content and delivery modality.
Acknowledgments
Funding: This study was supported by Center of Excellence for Psychology of Spirituality and Happiness, The University of Isfahan, Isfahan, Iran. Editing/analysis time for Jeff Huffman was supported by NIH grant R01HL113272. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Johannes A. C. Laferton was supported by a fellowship within the Postdoc-Program of the German Academic Exchange Service (DAAD).
Footnotes
Conflict of interest
All authors declare that they have no conflict of interest in relation to this study.
Ethical standards
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.GBD Mortality and Causes of Death Collaborators. Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;385(9963):117–71. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med. 2004;66(6):802–13. doi: 10.1097/01.psy.0000146332.53619.b2. [DOI] [PubMed] [Google Scholar]
- 3.van Melle JP, de Jonge P, Spijkerman TA, et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med. 2004;66(6):814–22. doi: 10.1097/01.psy.0000146294.82810.9c. [DOI] [PubMed] [Google Scholar]
- 4.Celano CM, Huffman JC. Depression and cardiac disease: a review. Cardiol Rev. 2011;19(3):130–42. doi: 10.1097/CRD.0b013e31820e8106. [DOI] [PubMed] [Google Scholar]
- 5.Watkins LL, Koch GG, Sherwood A, et al. Association of anxiety and depression with all-cause mortality in individuals with coronary heart disease. J Am Heart Assoc. 2013;2(2):e000068. doi: 10.1161/JAHA.112.000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martens EJ, de Jonge P, Na B, Cohen BE, Lett H, Whooley Ma. Scared to death? Generalized anxiety disorder and cardiovascular events in patients with stable coronary heart disease:The Heart and Soul Study. Arch Gen Psychiatry. 2010;67(7):750–8. doi: 10.1001/archgenpsychiatry.2010.74. [DOI] [PubMed] [Google Scholar]
- 7.Baumeister H, Hutter N, Bengel J. Psychological and pharmacological interventions for depression in patients with coronary artery disease. Cochrane Database Syst Rev. 2011;(9):CD008012. doi: 10.1002/14651858.CD008012.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whalley B, Thompson DR, Taylor RS. Psychological Interventions for Coronary Heart Disease: Cochrane Systematic Review and Meta-analysis. Int J Behav Med. 2012 doi: 10.1007/s12529-012-9282-x. [DOI] [PubMed] [Google Scholar]
- 9.Dubois CM, Beach SR, Kashdan TB, et al. Positive psychological attributes and cardiac outcomes: associations, mechanisms, and interventions. Psychosomatics. 2012;53(4):303–18. doi: 10.1016/j.psym.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Boehm JK, Kubzansky LD. The heart’s content: the association between positive psychological well-being and cardiovascular health. Psychol Bull. 2012;138(4):655–91. doi: 10.1037/a0027448. [DOI] [PubMed] [Google Scholar]
- 11.Steptoe A, Wardle J, Marmot M. Positive affect and health-related neuroendocrine, cardiovascular, and inflammatory processes. Proc Natl Acad Sci U S A. 2005;102(18):6508–12. doi: 10.1073/pnas.0409174102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giltay EJ, Kamphuis MH, Kalmijn S, Zitman FG, Kromhout D. Dispositional optimism and the risk of cardiovascular death: the Zutphen Elderly Study. Arch Intern Med. 2006;166(4):431–6. doi: 10.1001/.431. [DOI] [PubMed] [Google Scholar]
- 13.Tindle HA, Chang Y-F, Kuller LH, et al. Optimism, cynical hostility, and incident coronary heart disease and mortality in the Women’s Health Initiative. Circulation. 2009;120(8):656–62. doi: 10.1161/CIRCULATIONAHA.108.827642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boehm JK, Peterson C, Kivimaki M, Kubzansky L. A prospective study of positive psychological well-being and coronary heart disease. Health Psychol. 2011;30(3):259–67. doi: 10.1037/a0023124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kubzansky LD, Thurston RC. Emotional vitality and incident coronary heart disease: benefits of healthy psychological functioning. Arch Gen Psychiatry. 2007;64(12):1393–401. doi: 10.1001/archpsyc.64.12.1393. [DOI] [PubMed] [Google Scholar]
- 16.Denollet J, Pedersen SS, Daemen J, de Jaegere P, Serruys PW, van Domburg RT. Reduced positive affect (anhedonia) predicts major clinical events following implantation of coronary-artery stents. J Intern Med. 2008;263(2):203–11. doi: 10.1111/j.1365-2796.2007.01870.x. [DOI] [PubMed] [Google Scholar]
- 17.Brummett BH, Boyle SH, Siegler IC, Williams RB, Mark DB, Barefoot JC. Ratings of positive and depressive emotion as predictors of mortality in coronary patients. Int J Cardiol. 2005;100(2):213–6. doi: 10.1016/j.ijcard.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 18.Barefoot JC, Brummett BH, Helms MJ, Mark DB, Siegler IC, Williams RB. Depressive symptoms and survival of patients with coronary artery disease. Psychosom Med. 2000;62(6):790–5. doi: 10.1097/00006842-200011000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Boehm JK, Peterson C, Kivimaki M, Kubzansky LD. Heart health when life is satisfying: evidence from the Whitehall II cohort study. Eur Heart J. 2011;32(21):2672–7. doi: 10.1093/eurheartj/ehr203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Surtees PG, Wainwright NWJ, Luben R, Khaw K-T, Day NE. Mastery, sense of coherence, and mortality: evidence of independent associations from the EPIC-Norfolk Prospective Cohort Study. Health Psychol. 2006;25(1):102–10. doi: 10.1037/0278-6133.25.1.102. [DOI] [PubMed] [Google Scholar]
- 21.DuBois CM, Lopez OV, Beale EE, Healy BC, Boehm JK, Huffman JC. Relationships between positive psychological constructs and health outcomes in patients with cardiovascular disease: A systematic review. Int J Cardiol. 2015;195:265–80. doi: 10.1016/j.ijcard.2015.05.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case- control study. Lancet. 2004;364(9438):937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 23.Steptoe A, Dockray S, Wardle J. Positive affect and psychobiological processes relevant to health. J Pers. 2009;77(6):1747–76. doi: 10.1111/j.1467-6494.2009.00599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dockray S, Steptoe A. Positive affect and psychobiological processes. Neurosci Biobehav Rev. 2010;35(1):69–75. doi: 10.1016/j.neubiorev.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Low CA, Bower JE, Moskowitz JT, Epel ES. Positive Psychological State and Biological Processes. In: Sheldon KM, Kashdan TB, Steger MF, editors. Designing Positive Psychology: Taking Stock and Moving Forward. New York: Oxford University Press; 2011. pp. 41–50. [Google Scholar]
- 26.Goldston K, Baillie AJ. Depression and coronary heart disease: a review of the epidemiological evidence, explanatory mechanisms and management approaches. Clin Psychol Rev. 2008;28(2):288–306. doi: 10.1016/j.cpr.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Bolier L, Haverman M, Westerhof GJ, Riper H, Smit F, Bohlmeijer E. Positive psychology interventions: a meta-analysis of randomized controlled studies. BMC Public Health. 2013;13:119. doi: 10.1186/1471-2458-13-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sin NL, Lyubomirsky S. Enhancing well-being and alleviating depressive symptoms with positive psychology interventions: a practice-friendly meta-analysis. J Clin Psychol. 2009;65(5):467–87. doi: 10.1002/jclp.20593. [DOI] [PubMed] [Google Scholar]
- 29.Huffman JC, Mastromauro CA, Boehm JK, et al. Development of a positive psychology intervention for patients with acute cardiovascular disease. Heart Int. 2011;6(2):e14. doi: 10.4081/hi.2011.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peterson JC, Charlson ME, Hoffman Z, et al. A randomized controlled trial of positive-affect induction to promote physical activity after percutaneous coronary intervention. Arch Intern Med. 2012;172(4):329–36. doi: 10.1001/archinternmed.2011.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nikrahan Gh, Laferton JAC, Asgari K, et al. Positive Psychologie Interventionen für Patienten mit koronarer Herzerkrankung: Ergebnisse einer randomisiert kontrollierten Pilotstudie [Positive Psychology Interventions for Coronary Heart Disease Patients: Results of a Randomized Controlled Pilot Study] Mainz, Germany: 2016. 15th Bi-anual Meeting of the German Society of Behavioral Medicine. [Google Scholar]
- 32.Seligman MEP, Steen TA, Park N, Peterson C. Positive psychology progress: empirical validation of interventions. Am Psychol. 2005;60(5):410–21. doi: 10.1037/0003-066X.60.5.410. [DOI] [PubMed] [Google Scholar]
- 33.Seligman MEP, Rashid T, Parks AC. Positive psychotherapy. Am Psychol. 2006;61(8):774–88. doi: 10.1037/0003-066X.61.8.774. [DOI] [PubMed] [Google Scholar]
- 34.Lyubomirsky S, Dickerhoof R, Boehm JK, Sheldon KM. Becoming happier takes both a will and a proper way: an experimental longitudinal intervention to boost well-being. Emotion. 2011;11(2):391–402. doi: 10.1037/a0022575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheldon KM, Lyubomirsky S. How to increase and sustain positive emotion: The effects of expressing gratitude and visualizing best possible selves. J Posit Psychol. 2006;1:73–82. [Google Scholar]
- 36.Fordyce MW. Development of a program to increase personal happiness. J Couns Psychol. 1977;26:511–21. [Google Scholar]
- 37.Fordyce MW. A program to increase happiness: Further studies. J Couns Psychol. 1983;30:480–98. [Google Scholar]
- 38.Beck A, Steer R, Brown G. Manual for Beck Depression Inventory-II (BDI-II) San Antonio, TX: Psychology Corporation; 1996. [Google Scholar]
- 39.Ghassemzadeh H, Mojtabai R, Karamghadiri N, Ebrahimkhani N. Psychometric properties of a Persian-language version of the Beck Depression Inventory--Second edition: BDI-II-PERSIAN. Depress Anxiety. 2005;21(4):185–92. doi: 10.1002/da.20070. [DOI] [PubMed] [Google Scholar]
- 40.Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28(7):916–31. doi: 10.1016/S0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- 41.Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation. 2004;109(21 Suppl 1):II2–10. doi: 10.1161/01.CIR.0000129535.04194.38. [DOI] [PubMed] [Google Scholar]
- 42.Ridker P, Hennekens C. C-Reactive Protein and other Markers of Inflammation in the Prediction of Cardiovascular Disease in Women. N Engl J Med. 2000 doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 43.Gibbons RD, Hedeker D, DuToit S. Advances in Analysis of Longitudinal Data. Annu Rev Clin Psychol. 2010;6:79–107. doi: 10.1146/annurev.clinpsy.032408.153550.Advances. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ryff CD. Psychological Well-Being Revisited: Advances in the Science and Practice of Eudaimonia. Psychother Psychosom. 2014;83(1):10–28. doi: 10.1159/000353263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ridker P, Buring J, Shih J. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98:1998–2001. doi: 10.1161/01.cir.98.8.731. [DOI] [PubMed] [Google Scholar]
- 46.Kaptoge S, Di Angelantonio E, Lowe G, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375(9709):132–40. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Januzzi JL, Rehman SU, Mohammed AA, et al. Use of amino-terminal pro-B-type natriuretic peptide to guide outpatient therapy of patients with chronic left ventricular systolic dysfunction. J Am Coll Cardiol. 2011;58(18):1881–9. doi: 10.1016/j.jacc.2011.03.072. [DOI] [PubMed] [Google Scholar]