Abstract
Purpose
Little is known about how the number of HPV vaccine doses affect adherence to screening guidelines. This study compared adherence to cervical cancer screening by the number of HPV vaccine doses received by young women and assessed whether the specialty of vaccinating providers affected behavior.
Methods
This retrospective cohort study using administrative insurance claims records included 24,964 19–26 year old women who received at least 1 injection of the HPV vaccine between January 2006 and November 2009. Vaccinated young women continuously enrolled in a nationally-representative private insurance plan for 6 months prior to and 37 months after HPV vaccine administration were included. Logistic regression was used to compare the odds of Papanicolaou (Pap) testing 3 years after vaccine initiation by number of vaccine doses and provider type.
Results
In this sample, 79.3% had a Pap test 3 years following vaccine initiation. Receiving 1 (aOR: 0.60, 95% CI 0.55–0.65) or 2 (aOR: 0.80, 95% CI 0.74–0.87) doses was associated with decreased odds of Pap testing compared to 3 doses. Many young women in our sample (16.5%) were diagnosed with cervical dysplasia prior to HPV vaccination. Patients vaccinated by non-obstetrician/gynecologists were less likely to get a Pap test following vaccination.
Conclusions
Women who received 1 or 2 doses of the HPV vaccine were less likely that those who received 3 doses to be screened for cervical cancer 3 years following vaccine initiation. Pediatricians and primary care physicians should convey the importance of initiating and continuing screening to HPV vaccinated patients.
Keywords: Human papillomavirus, papillomavirus vaccines, cervical intraepithelial neoplasia, Papanicolaou test, papanicolaou smear, health care providers
INTRODUCTION
Human papillomavirus (HPV) vaccination is an important strategy to reduce HPV-related diseases and cancers in the US. The most commonly used vaccine in the US protects against 4 types of HPV that cause genital warts (types 6 and 11) and a high proportion of cervical cancers (types 16 and 18). In a randomized clinical trial, HPV vaccination among 18–25 year old women reduced abnormal Pap tests, referral for colposcopy, and treatment related to abnormal cervical cytology [1]. However, since the vaccine does not provide protection against all HPV types that cause cervical cancer, and does not clear infections that are present before vaccination, vaccinated women still need to get regular Papanicolaou (Pap) tests every 3 years, starting at age 21 [2–4].
More than 98% of vaccinated women report they intend to be screened after vaccination,[5] but intention does not always lead to actual participation [6,7]. Recent literature has indicated that young women who initiated HPV vaccination were more likely than unvaccinated women to report having a Pap test in the previous 3 years [8]. Although the effect of vaccination on compliance with Pap testing guidelines has been examined, there is little information about how the number of vaccine doses affects subsequent cervical cancer screening behavior. Pap testing behavior among young women who received the vaccine after 16 is particularly important, as they may have already been exposed to HPV, and may still be at risk, as the vaccine is not effective at clearing established infections [9]. In addition, although 2 doses of the vaccine appear to produce an adequate immune response in girls <16 years of age, women ≥16 years of age have a lower immunological response to HPV vaccination compared to younger adolescents [10], and it is unknown what level of immunogenicity is needed to protect against HPV infection long-term. For these reasons, young women need appropriate screening after vaccination, particularly those who received fewer than 3 doses.
The purpose of this study was to examine the association between the number of HPV vaccine doses that young women (19–26 years old at vaccination) received and receipt of a Pap test during the 3 year interval after vaccine initiation. We also assessed whether the specialty of the vaccinating physician and receiving the vaccine at appropriate intervals was associated with guideline-consistent screening behaviors.
METHODS
Participants and Procedure
We conducted a retrospective cohort study of health insurance claims from Clinformatics™ DataMart, a product of OptumInsight Life Sciences, Inc. (Eden Prairie, MN). Clinformatics™ DataMart consists of all claims paid for enrollees of one nationally available insurance group, including vaccinations, medical services, and prescriptions. Although they may have enrolled in different types of plans, all enrollees had the same eligibility for HPV vaccination, but reimbursement may have varied depending on a particular plan’s premiums, co-insurance, and deductibles. Enrollees include over 53 million Americans, with more than 4.8 million female 19–26 year olds. Enrollees are largely representative of working adults in the U.S., with slightly higher representation in Northern states. We included females who received at least 1 HPV vaccination (either bivalent or quadrivalent) any time between January 2006 and November 2009, with 37 months of follow-up. Data were available through December 31st, 2012. Although 19–20 year olds were no longer recommended to receive screening after 2009 according to guidelines from the American College of Obstetricians & Gynecologists (ACOG) [11,12,4], we included these women because the youngest would have reached age 21 by the third year of follow-up.
Records for 24,964 subjects were obtained that 1) contained a Current Procedural Terminology (CPT) code for HPV quadrivalent (90649) or bivalent (90650) vaccine, 2) were enrolled continuously 6 months before and 37 months after vaccine series initiation, and 3) were between 19 and 26 years at first vaccination (Figure 1). We included the enrollment criteria to ensure that we had the most accurate information on the women in our study. We chose 6 months of enrollment before vaccination to avoid missing observations among those with shorter enrollment, while optimizing sample size. The final 37 months of enrollment allowed us to assess the first Pap test that occurred at least 30 days after vaccine series initiation (not during vaccine initiation), and then within 3 years following the first 30 days after initiation.
Figure 1.
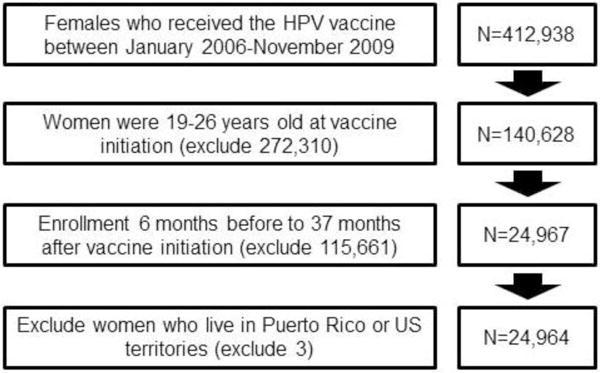
Cohort selection of vaccinated 19–26 year old females from administrative health insurance records
Analyses were based on current guidelines to ensure our findings were relevant across the study period and to reduce sample bias. We excluded vaccine initiators ≥ 27+ years of age because they received the vaccine off-label, and screening recommendations would have changed during follow-up when they reached 30 years of age. Informed consent was not required as this was an analysis of de-identified secondary data. This study was exempted from review by the institutional review board of the University of Texas Medical Branch, Galveston, TX.
Measures
The number of HPV vaccinations received by an individual separated by at least 10 days that occurred during the study period (37 months after earliest recorded HPV vaccine) was examined. A 10-day period was used to ensure that vaccines were not repeat entries or given too soon after an initial dose to be effective. A 6-month look-back period was included to ensure that the earliest record was the first HPV vaccine received. The sequence of the dose for each vaccine given was not recorded. Therefore, we used a 6-month time period before vaccination to make sure that the first vaccine recorded in the dataset was the first actual vaccine received by the enrollee, as those enrolled for a shorter period of time prior to the first vaccine dose may have received other doses that were not recorded in this dataset. HPV vaccination was categorized into those who had received 1, 2, or 3+ doses anytime during the period of time examined for each enrollee. Young women who received 4+ doses were rare (n=167). Therefore, we combined those who received 4+ doses with those who received 3 doses. Excessive immunization (receipt of more doses of a vaccine than recommended) has been found to be common among young children in the U.S.,[13] although the problem has not been studied among young adults. It is likely that excessive immunization also occurs in this group, especially since immunization registries in the U.S. were mostly developed for children, and may not be as reliable for ascertaining vaccination history for young adults. Further, providers may not have been aware of the flexibility of the vaccination schedule for the HPV vaccine series soon after the vaccine was introduced, and may have given extra doses to those who were not vaccinated at the recommended time intervals.
Interval appropriateness was also measured. Intervals between 6 and 8 months for the 1st and 3rd doses were considered appropriate, similar to the recommended interval [14]. We used this measure, rather than the standard 1-year measure because we wanted to determine whether young women who followed 1 guideline more appropriately were likely to follow the other guideline. One-year completion for women in this dataset is reported elsewhere [15]. Those with shorter or longer intervals were considered inappropriate, and we included those who did not complete in a separate category. Enrollees were not included in the data set more than once.
Pap testing was included as a dichotomized variable. We counted the first Pap test using CPT codes, Healthcare Common Procedure Coding System (HCPCS), and International Classification of Diseases, 9th Edition (ICD-9) codes established by the Healthcare Effectiveness Data and Information Sets (HEDIS)[16,17] which occurred between 30 days and 37 months after the initial vaccine dose.
Enrollees were categorized into two groups based on their age at first vaccination: 19–21 years and 22–26 years. The 22–26 year olds were already eligible to receive Pap testing, whereas 19–21 year olds were either not yet eligible, or newly eligible according to cancer screening guidelines [4,3,2]. Since cervical cancer screening guidelines changed during the study, it was determined that these age cutoffs would be best. ACOG guidelines first recommended annual Pap testing according to sexual debut among women less than 21 years of age [11]. In 2009, these guidelines were changed, with a recommendation for Pap testing every 3 years starting at 21 years of age regardless of sexual activity, until 30 years of age [12]. Due to the way age was calculated (birth year subtracted from year of vaccine initiation), it was determined that including 21 year olds in the younger age category would be most appropriate.
The type of providers who administered the first HPV vaccination were grouped into 4 categories. These included: “obstetrics/gynecology,” which included obstetricians or gynecologists, “primary care providers,” which included family practitioners, general practitioners, or internal medicine physicians, “adolescent/pediatric providers” which included pediatricians and adolescent medicine specialists, and “other” providers not included in the previous categories. Obstetricians/gynecologists were used as the referent group because they initiated vaccination in the highest proportion of the sample. We also evaluated the proportion of the sample with at least 2 vaccines that changed provider type between the 1st and 2nd dose, as well as the proportion of those that received 3 doses that changed provider type between the 1st and 3rd dose. The US was divided into 4 regions reflecting the Census regions, including: Northeast, South, Midwest, and West. South was the comparison category because it represented the largest proportion of enrollees.
The frequency of other exams and procedures which may have been related to the outcomes included: well woman exams, colposcopies, and loop electrosurgical excision procedures (LEEP). Any tests/exams which occurred between 30 days and 37 months after initial HPV vaccination were examined in bivariate analyses. ICD-9 codes for cervical dysplasia included: abnormal Pap smears, atypical squamous cells of undetermined significance (ASCUS), malignant neoplasms of the cervix, carcinoma in situ of cervix uteri, and colposcopies or LEEPs. These codes were combined and used to indicate recent cervical dysplasia history in order to evaluate its effect on observed associations. A dichotomous variable for recent cervical dysplasia history that was observed 6 months prior and 1 month after 1st vaccination for each was used.
Statistical Analyses
Frequencies and proportions for each variable were examined in bivariate analysis. Multivariable logistic regression was used to examine the association of the number of doses of the HPV vaccine with Pap testing after controlling for all variables, including age, region of residence, provider type, and year at first dose in Model 1. In addition, Model 2 included an adjustment for young women with a recent history of cervical dysplasia to evaluate whether including this variable in the model would change any of the previously observed associations. Logistic regression was also used to examine whether appropriateness of vaccine timing (6–8 months between 1st and 3rd vaccine doses) was associated with Pap testing in the next 3 years. In the first model, we excluded a recent history of cervical dysplasia, and in the second model, we included recent history of cervical dysplasia to examine its effect on the observed associations. Sensitivity analyses, which included 13,805 women who were vaccinated between 21 and 26 years old, were conducted to determine whether the associations observed among 19–26 year old women were the same among women eligible for Pap testing at the time of vaccination. Finally, among 19–26 year olds, we tested the interaction between time and number of HPV vaccine doses to examine whether there was a change in the association between the number of doses received and subsequent Pap testing using an adjusted logistic regression model. Data analysis for this study was done using SAS ® 9.3 (SAS Institute Inc., Cary, NC, USA.)
RESULTS
More than half of young women who initiated vaccination were between 19 and 21 years of age (Table 1). Almost half received their 1st HPV vaccine dose from an obstetrician/gynecologist, and more than half received all 3 doses. Out of all the vaccinations administered, more than 40% occurred during 2007. More than a third of the sample received their 1st and 3rd shot between 6 and 8 months apart. Among those that received 3 vaccines, 92% (n=12,556) completed the series within 12 months. Almost 80% of the sample had a Pap test in the 3 years following HPV vaccine initiation.
Table 1.
Cohort characteristics of vaccinated 19–26 year old females from administrative health insurance records with HPV vaccine initiation between 2006–2009 (N=24,964)
| N | % | |
|---|---|---|
| Overall | 24,964 | 100 |
| Age at the 1st dose | ||
| 19–21 | 14,111 | 56.5 |
| 22–26 | 10,853 | 43.5 |
| Region | ||
| Midwest | 7,113 | 28.5 |
| Northeast | 3,361 | 13.5 |
| South | 10,841 | 43.4 |
| West | 3,649 | 14.6 |
| Provider giving the 1st dose | ||
| Obstetrics/gynecology | 12,180 | 48.8 |
| Primary care provider | 8,725 | 35.0 |
| Adolescent/pediatric provider | 3,295 | 13.2 |
| Other | 764 | 3.1 |
| Year of the 1st dose | ||
| 2006 | 1,369 | 5.5 |
| 2007 | 10,398 | 41.7 |
| 2008 | 7,929 | 31.8 |
| 2009 | 5,268 | 21.1 |
| Number of doses received | ||
| 1 | 5,369 | 21.5 |
| 2 | 5,951 | 23.8 |
| 3 | 13,477 | 54.0 |
| 4+ | 167 | 0.7 |
| Appropriate interval between doses 1 and 3 | ||
| Appropriatea | 8,719 | 34.9 |
| Inappropriate | 4,925 | 19.7 |
| < 3 doses | 11,320 | 45.3 |
| History of cervical dysplasiab | ||
| No | 20,847 | 83.5 |
| Yes | 4,117 | 16.5 |
| Number of women who received tests between months 1 and 37 after the 1st dosec | ||
| Pap test | 19,797 | 79.3 |
| Median (Q1–Q3) | ||
| Days between doses 1 and 2 | 66 (61–94) | |
| Days between doses 1 and 3 | 194 (183–231) | |
defined as 6–8 months
defined as any woman having a diagnosis for cervical dysplasia (622.10–622.12), abnormal Pap smear (795.00–795.04), cervical malignant neoplasm (180.1, 180.8), carcinoma in situ of cervix uteri (233.1), or having any colposcopy or LEEP between 6 months before and 1 month following the 1st dose.
Well woman exams (ICD-9 code V72.31), Pap tests (CPT codes 88141-88143, 88147, 88148, 88150, 88152-88155, 88164-88167, 88174, 88175; HCPCS codes G0123, G0124, G0141, G0143G0145, G0147, G0148, P3000, P3001, Q0091; and ICD-9 code 91.46), HPV test (CPT codes 87620-87622; CPT code V73.81), colposcopies (CPT codes 56820, 56821, 57420, 57421, 57452, 47454, 57455, 57456, 57460, and 57461), and loop electrosurgical excision procedures (LEEP; CPT codes 57460, 57522, and 57461).
In the adjusted analyses shown in Model 1, age, region, provider type, year of vaccine administration, and number of doses received were associated with Pap testing following HPV vaccine initiation (Table 2). Young women vaccinated by primary care providers, adolescent/pediatric providers, or other types of providers had lower odds of receiving a Pap test compared to those vaccinated by obstetricians/gynecologists. Patients rarely changed provider types between vaccine doses. Among those that received 2 doses, 5.9% (n=1,149) switched to another provider type between doses 1 and 2. Among those that received 3 doses, 6.9% (n=936) switched to another provider type between doses 1 and 3. Vaccine initiation before 2009 was associated with increased odds of Pap testing compared to young women vaccinated in 2009. Fewer doses were associated with lower odds of Pap testing. In Model 2, after adjusting for recent dysplasia history, there were no changes in the associations observed in Model 1. However, a recent cervical dysplasia history was associated with significantly increased odds of having a Pap test. Sensitivity analyses revealed similar findings, although earlier year of vaccination was no longer associated with increased likelihood of subsequent Pap testing among 21–26 year olds (supplemental Table 1).
Table 2.
Adjusted multivariable logistic regression evaluating characteristics associated with Papanicolaou (Pap) testing in 3 years following HPV vaccine series initiation (N=24,964)
| Pap test | |||
|---|---|---|---|
| % received Pap test |
Model 1 aOR (95% CI) |
Model 2 aOR (95% CI) |
|
| Age | |||
| 19–21 | 73.6 | 1.00 | 1.00 |
| 22–26 | 86.8 | 1.97 (1.84, 2.12) | 1.90 (1.77, 2.05) |
| Region | |||
| Midwest | 77.9 | 0.74 (0.69, 0.80) | 0.75 (0.69, 0.81) |
| Northeast | 71.3 | 0.48 (0.43, 0.52) | 0.48 (0.44, 0.53) |
| South | 83.1 | 1.00 | 1.00 |
| West | 78.0 | 0.75 (0.68, 0.83) | 0.75 (0.68, 0.82) |
| Provider giving the 1st dose | |||
| Obstetrics/gynecology | 86.2 | 1.00 | 1.00 |
| Primary care provider | 76.2 | 0.59 (0.55, 0.64) | 0.66 (0.61, 0.71) |
| Adolescent/pediatric provider | 62.8 | 0.38 (0.35, 0.42) | 0.44 (0.40, 0.49) |
| Other | 76.7 | 0.65 (0.54, 0.77) | 0.69 (0.58, 0.83) |
| Year of the 1st dose | |||
| 2006 | 84.4 | 1.51 (1.27, 1.78) | 1.46 (1.23, 1.73) |
| 2007 | 80.3 | 1.24 (1.14, 1.36) | 1.25 (1.14, 1.36) |
| 2008 | 79.5 | 1.14 (1.05, 1.25) | 1.16 (1.06, 1.26) |
| 2009 | 75.7 | 1.00 | 1.00 |
| Number of doses received | |||
| 1 | 72.2 | 0.60 (0.56, 0.66) | 0.60 (0.55, 0.65) |
| 2 | 78.4 | 0.81 (0.75, 0.88) | 0.80 (0.74, 0.87) |
| 3+ | 82.5 | 1.00 | 1.00 |
| History of cervical dysplasia | |||
| No | 76.7 | – | 1.00 |
| Yes | 92.3 | – | 2.73 (2.42, 3.09) |
Interval appropriateness between doses 1 and 3 of the HPV vaccine series was not associated with Pap testing (Table 3). Having a recent history of cervical dysplasia was associated with more than a 2 times greater odds of having a Pap test in the next 3 years. However, including recent history of dysplasia in Model 2 did not change the associations observed in the Model 1 analyses. Sensitivity analyses revealed similar findings, with the exception that earlier years of vaccination were no longer associated with increased likelihood of Pap testing among 21–26 year olds (supplemental Table 2).
Table 3.
Percent of women receiving Pap tests within 3 years of vaccination by appropriateness of timing (6–8 month gap) between the 1st and 3rd doses (N=24,964)
| Pap test | |||
|---|---|---|---|
| % received Pap test |
Model 1 OR (95% CI) |
Model 2 OR (95% CI) |
|
| Age | |||
| 19–21 | 73.6 | 1.00 | 1.00 |
| 22–26 | 86.8 | 1.97 (1.83, 2.12) | 1.90 (1.77, 2.04) |
| Region | |||
| Midwest | 77.9 | 0.75 (0.69, 0.81) | 0.75 (0.70, 0.82) |
| Northeast | 71.3 | 0.48 (0.44, 0.53) | 0.48 (0.44, 0.53) |
| South | 83.1 | 1.00 | 1.00 |
| West | 78.0 | 0.75 (0.68, 0.83) | 0.75 (0.68, 0.83) |
| Provider giving the 1st dose | |||
| Obstetrics/gynecology | 86.2 | 1.00 | 1.00 |
| Primary care physician | 76.2 | 0.59 (0.55, 0.63) | 0.65 (0.61, 0.71) |
| Adolescent/pediatric primary care physician | 62.8 | 0.38 (0.34, 0.41) | 0.43 (0.39, 0.48) |
| Other | 76.7 | 0.64 (0.53, 0.76) | 0.68 (0.57, 0.82) |
| Year of the 1st dose | |||
| 2006 | 84.4 | 1.55 (1.31, 1.83) | 1.50 (1.27, 1.77) |
| 2007 | 80.3 | 1.27 (1.17, 1.39) | 1.28 (1.17, 1.40) |
| 2008 | 79.5 | 1.15 (1.06, 1.26) | 1.17 (1.07, 1.28) |
| 2009 | 75.7 | 1.00 | 1.00 |
| Appropriate interval between doses 1 and 3 | |||
| Appropriate | 82.3 | 1.00 | 1.00 |
| Inappropriate | 82.7 | 1.10 (1.00, 1.21) | 1.10 (1.00, 1.21) |
| < 3 doses | 75.5 | 0.73 (0.68, 0.79) | 0.72 (0.67, 0.78) |
| History of cervical dysplasia | |||
| No | 76.7 | – | 1.00 |
| Yes | 92.3 | – | 2.73 (2.42, 3.09) |
The interaction between year of vaccine initiation and number of HPV vaccine doses was significant (Table 4). The proportion of those who received a Pap test dropped in all 3 dose groups, but the decrease was most apparent among women who received 3 doses of the vaccine. The odds of a Pap test were lower among young women who received the first of 2 doses in 2006 and 2007 compared to those who received the first of 3 in 2006 and 2007, but women who had received 2 doses starting in 2008 or 2009 were no longer less likely to have had a Pap test compared to those who received 3 doses. Although the difference in Pap testing among the 3 dose groups became smaller across time, the odds of having a Pap test for those who received 1 dose remained significantly lower in 2009 compared to those who completed 3 doses.
Table 4.
Multivariable logistic regression evaluating the association between the interaction of time and number of HPV vaccine doses on Pap testinga (N=24,964)
| N | % received Pap test | aOR (95% CI) | |
|---|---|---|---|
| 2006 | |||
| 1 | 143 | 74.1 | 0.47 (0.30, 0.73) |
| 2 | 255 | 82.4 | 0.85 (0.58, 1.25) |
| 3+ | 971 | 86.4 | 1.00 |
| 2007 | |||
| 1 | 1292 | 73.0 | 0.53 (0.46, 0.61) |
| 2 | 2064 | 76.6 | 0.68 (0.60, 0.77) |
| 3+ | 7042 | 82.7 | 1.00 |
| 2008 | |||
| 1 | 1758 | 71.7 | 0.60 (0.53, 0.69) |
| 2 | 1724 | 80.6 | 0.92 (0.80, 1.07) |
| 3+ | 4447 | 82.2 | 1.00 |
| 2009 | |||
| 1 | 2176 | 72.1 | 0.79 (0.67, 0.95) |
| 2 | 1908 | 78.0 | 1.03 (0.85, 1.23) |
| 3+ | 1184 | 78.6 | 1.00 |
The logistic regression model was adjusted for age at the 1st dose, region, provider type that gave first dose, and history of cervical dysplasia.
CONCLUSION
Our main finding was that young women who received the HPV vaccine were less likely to have a Pap test within the next 3 years if they received only 1 or 2 doses compared to 3+ doses. An earlier study asserted that young women understand the need to continue to be screened regularly for cervical cancer after HPV vaccination [5]. It is possible that young women who complete the HPV vaccine series have a higher general knowledge about healthy behaviors than those who did not receive the recommended number of doses. Our results indicate that those who practice healthy behaviors are more likely to engage in other health behaviors [18]. Increased exposure to health providers also offers more opportunities to discuss screening. Thus, patients who receive fewer doses of the HPV vaccine may have less of an opportunity to be screened or be educated about screening. Our results indicate the importance of discussing cervical cancer screening with young women when they initiate vaccination, as vaccine series completion may be decreasing among young women across time [15], and it is impossible to know if a patient will return to receive their remaining doses.
Overall, 79% of our sample received a Pap test 3 years after initiating vaccination. This rate is lower than the national level of 84% among 21–30 year olds who self-reported Pap tests within the past 3 years [19]. The study samples are comparable by age, as the NHIS sample were asked about screening behavior in the past 3 years, meaning that they would have been 18–27 years old 3 years previous, similar to the starting age of our cohort. However, differences in screening rates between the studies may be due to variations in methodology, recall bias in the National Health Interview Survey (NHIS), changes in guidelines, or due to lack of knowledge about the need for cervical cancer screening in our sample [4,3,20].
We found that young women who received their first vaccine from an adolescent or pediatric care provider were less likely to get a Pap test in the next 3 years compared to those who received their 1st vaccine from an obstetrician/gynecologist. Health care practices have been shown to differ by provider type in other studies as well. In a national study that examined provider type and cancer screening behaviors, a higher proportion of women who visited both a primary care physician in addition to either a registered nurse or physician assistant in the past year reported having had a Pap test in the past 3 years compared to those who had only seen a primary care physician, another type of healthcare provider, or no provider in the past year [21]. According to a review of electronic medical records, gynecologists were more compliant with HPV co-testing guidelines among 30 year old women compared to other physician types at one medical center [22]. Provider type may play an important role through the providers’ experiences and exposure to guidelines. For example, pediatricians see more children and young adolescents who are not recommended to have a Pap test, whereas gynecologists not only screen young women, but more often treat patients with abnormal Pap smears. Therefore, gynecologists may more routinely inform their patients of the necessity of following the cervical cancer prevention guidelines. However, we only examined provider type for the initial vaccine, and therefore, cannot account for the variations in beliefs and actual knowledge of different types of providers.
We also found that appropriate spacing between the 1st and 3rd HPV vaccine doses was not associated with the odds of future Pap testing. Although the association between vaccine dose spacing and Pap testing has not previously been examined, it has been found that longer spacing between doses of the HVP vaccine can occur due to a number of reasons. For example, a chart review that examined young women who initiated HPV vaccination found that those who became pregnant were less likely to complete on time [23]. Therefore, longer intervals between the first and third doses of the HPV vaccine may be due to other factors which may not affect adherence to screening guidelines.
The 2010 NHIS data revealed that young women who were informed that they had HPV in the past were more likely to have received at least 1 vaccine dose compared to those who did not have a history, a finding consistent with our results [24]. A high proportion of young women in our sample had diagnostic codes indicating a history of cervical dysplasia. Current evidence indicates that high-grade cervical intraepithelial neoplasia and adenocarcinoma in situ has generally been decreasing among young women between 18 and 29 years of age [25]. Although rates of these conditions appear to be decreasing in the general population, it is possible that young women in this age group, upon finding out about cervical abnormalities, are motivated to get vaccinated afterward. Young women with a history of cervical dysplasia before vaccination were more than 2 and a half times more likely to have a Pap test within 3 years of HPV vaccine initiation compared to those without a history, which is important, as dysplasia may recur in these women. Finally, we found that a recent history of a cervical dysplasia diagnosis did not change the association between the number of HPV vaccine doses and subsequent Pap testing.
Although young women who were vaccinated soon after the introduction of the vaccine with 1 or 2 doses had lower odds of guideline-adherent cervical cancer screening compared to those who received 3 doses, this study provides evidence that these associations are changing across time. Our sensitivity analysis of 21–26 year olds showed that the change in Pap testing across time was largely due to younger women. This is likely due to guidelines adopted in 2009 that recommended young women ≤21 years old should not be screened – a guideline that has been shown to be associated with a decrease in Pap testing among women ≤21 years old. [26] Future research should examine at what age young women are initiating cervical cancer screening, as it appears that trends in age at adoption of cervical cancer screening may be changing.
The foremost strength of this study is that it was conducted using a large national dataset of insurance enrollees. Also, we were able to examine screening practices after HPV vaccination and confirm the number of vaccine doses received. Further, we examined the effect of cervical dysplasia on subsequent cervical cancer screening. This study also has some limitations. The data did not contain information on race/ethnicity, socioeconomic status, and other demographic factors that may have influenced Pap testing compliance in this group. We were also unable to determine whether women in the sample had been partially or fully vaccinated at a younger age due to changes in insurance enrollment. Moreover, the results in this study may not be applicable to uninsured populations, or younger females vaccinated before they are eligible for Pap testing. The high proportion of young women in our sample who had dysplasia in the 6 months preceding vaccination indicates that those vaccinated in this age group may not be representative of the general population. Increasingly, young women are being vaccinated at the recommended age. [27] Future studies are needed to examine Pap testing behaviors among those vaccinated at the recommended age after they turn 21 years old.
In conclusion, despite current efforts to educate young women, HPV vaccine recipients may not be getting the message about guideline consistent Pap testing after vaccination, unless they have already been diagnosed with cervical dysplasia before vaccination. It is critical that young women are aware of the need for screening at the first vaccine visit, particularly those vaccinated by pediatric and primary care providers. Educational materials focusing on the need for beginning and continuing cervical cancer screening could be distributed through adolescent/pediatric and primary care providers to young women who are vaccinated after they are 18 years of age.
Supplementary Material
Highlights.
Number of HPV vaccines associated with later cervical cancer screening.
Time between vaccines does not affect cervical cancer screening.
Women with cervical dysplasia before vaccination are being screened at high rates.
Cervical cancer screening adherence may vary by provider specialty.
Acknowledgments
This work was supported by the Institute for Translational Sciences (ITS) at the University of Texas Medical Branch, which is partially funded by a Clinical and Translational Science Award (UL1TR000071) from the National Center for Advancing Translational Sciences, National Institutes of Health. Dr. Hirth is a Scholar supported by a research career development award (K12HD052023: Building Interdisciplinary Research Careers in Women’s Health Program –BIRCWH; Principal Investigator: Berenson) from the Office of Research on Women’s Health (ORWH), the Office of the Director (OD), the National Institute of Allergy and Infectious Diseases (NIAID), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) at the National Institutes of Health. The sponsors had no role in the design or conduct of this study. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Contributor Information
Jacqueline M. Hirth, Email: jmhirth@utmb.edu, Department of Obstetrics and Gynecology and Center for Interdisciplinary Research in Women’s Health, University of Texas Medical Branch, 301 University Blvd Rte 0587, Galveston, TX 77555, Phone: (409) 772-1021, Fax: (409) 747-5129.
Yu-Li Lin, Office of Biostatistics, Department of Preventive Medicine and Community Health, University of Texas Medical Branch, 301 University Blvd Rte 0587, Galveston, TX 77555.
Yong-Fang Kuo, Office of Biostatistics, Department of Preventive Medicine and Community Health, University of Texas Medical Branch, 301 University Blvd Rte 0587, Galveston, TX 77555.
Abbey B. Berenson, Department of Obstetrics and Gynecology and Center for Interdisciplinary Research in Women’s Health, University of Texas Medical Branch, 301 University Blvd Rte 0587, Galveston, TX 77555.
References
- 1.Rodríguez AC, Solomon D, Herrero R, Hildesheim A, González P, Wacholder S, Porras C, Jiménez S, Schiffman M. Impact of human papillomavirus vaccination on cervical cytology screening, colposcopy, and treatment. American Journal of Epidemiology. 2013;178(5):752–760. doi: 10.1093/aje/kwt047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, Garcia FAR, Moriarty AT, Waxman AG, Wilbur DC, Wentzensen N, Downs LS, Spitzer M, Moscicki A-B, Franco EL, Stoler MH, Schiffman M, Castle PE, Myers ER. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA: A Cancer Journal for Clinicians. 2012;62:147–172. doi: 10.3322/caac.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.U.S. Preventive Services Task Force. Final recommendation statement: cervical cancer: screening. 2014 Jan 15; 2015. [Google Scholar]
- 4.ACOG Committee on Practice Bulletins-Gynecology. Screening for cervical cancer. Obstetrics & Gynecology. 2012;120(5):1222–1238. doi: 10.1097/aog.0b013e318277c92a. [DOI] [PubMed] [Google Scholar]
- 5.Price RA, Koshiol J, Kobrin S, Tiro JA. Knowledge and intention to participate in cervical cancer screening after the human papillomavirus vaccine. Vaccine. 2011;29:4238–4243. doi: 10.1016/j.vaccine.2011.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armitage CJ, Conner M. Efficacy of the Theory of Planned Behavior: a meta-analytic review. British Journal of Social Psychology. 2001;40:471–499. doi: 10.1348/014466601164939. [DOI] [PubMed] [Google Scholar]
- 7.Hofman R, van Empelen P, Richardus JH, de Kok IMCM, de Koning HJ, van Ballegooijen M, Korfage IJ. Predictors of HPV vaccination uptake: a longitudinal study among parents. Health Education Research. 2014;29(1):83–96. doi: 10.1093/her/cyt092. [DOI] [PubMed] [Google Scholar]
- 8.Sauer AG, Jemal A, Simard EP, Fedewa SA. Differential uptake of recent Papanicolaou testing by HPV vaccination status among young women in the United States, 2008–2013. Cancer Epidemiology. 2015 doi: 10.1016/j.canep.2015.05.002. In press. [DOI] [PubMed] [Google Scholar]
- 9.Hildesheim A, Herrero R, Wacholder S, Rodríguez AC, Solomon D, Bratti MC, Schiller JT, González P, Dubin G, Porras C, Jiménez S, Lowy DR. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. JAMA. 2007;298(7):743–753. doi: 10.1001/jama.298.7.743. [DOI] [PubMed] [Google Scholar]
- 10.Block SL, Nolan T, Sattler C, Barr E, Giacoletti KED, Marchant CD, Castellsagué X, Rusche SA, Lukac S, Bryan JT, Cavanaugh PF, Reisinger KS. Comparison of the immunogenicity and reactogenicity of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in male and female adolescents and young adult women. Pediatrics. 2006;118(5):2135–2145. doi: 10.1542/peds.2006-0461. [DOI] [PubMed] [Google Scholar]
- 11.ACOG Committee on Practice. ACOG practice bulletin clinical management guidelines for obstetrician-gynecologists. Obstetrics & Gynecology. 2003;102(2):417–427. doi: 10.1016/s0029-7844(03)00745-2. [DOI] [PubMed] [Google Scholar]
- 12.ACOG Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin no109: cervical cytology screening. Obstetrics & Gynecology. 2009;114(6):417–427. doi: 10.1097/AOG.0b013e3181c6f8a4. [DOI] [PubMed] [Google Scholar]
- 13.Feikema SM, Klevens M, Washington ML, Barker L. Extraimmunization among US children. JAMA. 2000;283(10):1311–1317. doi: 10.1001/jama.283.10.1311. [DOI] [PubMed] [Google Scholar]
- 14.Advisory Committee on Immunization Practices (ACIP) FDA licensure of bivalant human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP) MMWR. 2010;59(20):626–629. [PubMed] [Google Scholar]
- 15.Hirth JM, Tan A, Wilkinson GS, Berenson AB. Completion of the human papillomavirus vaccine series among insured females between 2006 and 2009. Cancer. 2012;118:5623–5629. doi: 10.1002/cncr.27598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.HEDIS. HEDIS 2013 technical specification. V. Washington DC; 2013. [Google Scholar]
- 17.HEDIS. HEDIS 2012 technical specification. IV. Washington DC; 2012. [Google Scholar]
- 18.Meissner HI, Yabroff KR, Dodd KW, Leader AE, Ballard-Barbash R, Berrigan D. Are patterns of health behavior associated with cancer screening? American Journal of Health Promotion. 2009;23(3):168–175. doi: 10.4278/ajhp.07082085. [DOI] [PubMed] [Google Scholar]
- 19.Klabunde CN, Brown M-B, Rachel, White MC, Thompson T, Plescia M, King SC. Cancer screening - United States, 2010. MMWR. 2012;61(3):41–45. [PubMed] [Google Scholar]
- 20.Bulletins-Gynecology. ACoP. ACOG practice bulletin no109: cervical cytology screening. Obstetrics and Gynecology. 2009;114(6):1409–1420. doi: 10.1097/AOG.0b013e3181c6f8a4. [DOI] [PubMed] [Google Scholar]
- 21.Kepka D, Smith A, Zeruto C, Yabroff KR. Is provider type associated with cancer screening and prevention: advanced practice registered nurses, physician assistants, and physicians. BMC Cancer. 2014;14(233) doi: 10.1186/1471-2407-14-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langsjoen J, Goodell C, Castro E, Thomas J, Kuehl TJ, Wehbe-Janek H, Hinsky M. Improving compliance with cervical cancer screening guidelines. Proceedings (Baylor University Medical Center) 2015;28(4):450–453. doi: 10.1080/08998280.2015.11929305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perry R, Rankin K, Yu MC, Harwood B. Factors associated with human papillomavirus vaccination completion on a catch-up schedule. Obstetrics & Gynecology. 2014;124(1):76–81. doi: 10.1097/AOG.0000000000000319. [DOI] [PubMed] [Google Scholar]
- 24.Williams WW, Lu P-J, Saraiya M, Yankey D, Dorell C, Rodriguez JL, Kepka D, Markowitz LE. Factors associated with human papillomavirus vaccination among young adult women in the United States. Vaccine. 2013;31(28):2937–2946. doi: 10.1016/j.vaccine.2013.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hariri S, Johnson ML, Bennett NM, Bauer HM, Park IU, Schafer S, Niccolai LM, Unger ER, Markowitz LE, HPV-IMPACT Working Group Population-based trends in high-grade cervical lesions in the early human papillomavirus vaccine era in the United States. Cancer. 2015;121(16):2775–2781. doi: 10.1002/cncr.29266. [DOI] [PubMed] [Google Scholar]
- 26.Hirth JM, Tan A, Wilkinson GS, Berenson AB. Compliance with cervical cancer screening and human papillomavirus testing guidelines among insured young women. American Journal of Obstetrics and Gynecology. 2013;209(3):200.e201–200.e207. doi: 10.1016/j.ajog.2013.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rahman M, McGrath CJ, Hirth JM, Berenson AB. Age at HPV vaccine initiation and completion among US adolescent girls: trend from 2008 to 2012. Vaccine. 2015;33(5):585–587. doi: 10.1016/j.vaccine.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


