Abstract
Aim
The American Heart Association (AHA) recommends monitoring invasive arterial diastolic blood pressure (DBP) and end-tidal carbon dioxide (ETCO2) during cardiopulmonary resuscitation (CPR) when available. In intensive care unit patients, both may be available to the rescuer. The objective of this study was to compare DBP versus ETCO2 during CPR as predictors of cardiac arrest survival.
Methods
In two models of cardiac arrest (primary ventricular fibrillation [VF] and asphyxia-associated VF), 3-month old swine received either standard AHA guideline-based CPR or patient-centric, BP-guided CPR. Mean values of DBP and ETCO2 in the final two minutes before the first defibrillation attempt were compared using receiver operating characteristic curves (area under curve [AUC] analysis). The optimal DBP cut point to predict survival was derived and subsequently validated in two independent, randomly generated cohorts.
Results
Of 60 animals, 37 (61.7%) survived to 45 minutes. DBP was higher in survivors than in non-survivors (40.6±1.8mmHg vs. 25.9±2.4mmHg; p<0.001), while ETCO2 was not different (30.0±1.5mmHg vs. 32.5±1.8mmHg; p=0.30). By AUC analysis, DBP was superior to ETCO2 (0.82 vs. 0.60; p=0.025) in discriminating survivors from non-survivors. The optimal DBP cut point in the derivation cohort was 34.1mmHg. In the validation cohort, this cut point demonstrated a sensitivity of 0.78, specificity of 0.81, positive predictive value of 0.64, and negative predictive value of 0.89 for survival.
Conclusions
In both primary and asphyxia-associated VF porcine models of cardiac arrest, DBP discriminates survivors from non-survivors better than ETCO2. Failure to attain a DBP >34mmHg during CPR is highly predictive of non-survival.
INTRODUCTION
In the United States, cardiac arrest occurs greater than 300,000 times annually in the pre-hospital setting and greater than 200,000 times annually in the hospital.1,2 Although an increasingly substantial proportion of these in-hospital cardiac arrests (IHCAs) occur in intensive care units (ICUs),3–5 current cardiopulmonary resuscitation (CPR) guidelines and training do not differ significantly between that provided by laypersons in the pre-hospital setting or by experienced practitioners in a highly monitored environment.6,7 ICU providers have the knowledge and tools necessary to titrate resuscitation efforts to the actual physiologic response of the patient.3,4 Utilization of these tools can be expected to save lives.8
To that end, a 2013 American Heart Association (AHA) consensus statement recommended physiologic monitoring during CPR. Specifically, it endorsed targeting coronary perfusion pressure (CoPP), the difference between the aortic pressure and the right atrial pressure during the relaxation phase of chest compressions, invasively measured arterial diastolic blood pressure (DBP), or end-tidal carbon dioxide (ETCO2).8 These clinical measures are established indicators of CPR quality and correlate with survival.9–20 Moreover, pre-clinical CPR models that target intra-arrest hemodynamics or ETCO2 improve survival outcomes21–23 and CPR quality,24 respectively. Newly published 2015 AHA Advanced Cardiac Life Support (ACLS) guidelines endorse physiologic monitoring during cardiac arrest but do not distinguish between different modalities or offer specific physiologic goals.25 Indeed, while tailoring resuscitation efforts in a patient-centric fashion seems logical, the ideal manner in which to do this remains unclear. In-hospital rescuers are frequently confronted with multiple intra-arrest indicators of CPR quality, but no previous study has directly compared DBP to ETCO2 in their respective abilities to gauge CPR quality or predict outcome.
Therefore, the primary objective of this study was to determine whether DBP or ETCO2 was more likely to discriminate survivors from non-survivors during CPR in a realistic large animal model of cardiac arrest and, if possible, to determine the values of each that could predict the return of spontaneous circulation (ROSC) and short-term survival. DBP was selected as a primary comparator rather than CoPP because it is a more commonly clinically available measurement. We hypothesized that mean aortic DBP would perform better than ETCO2 as a discriminator between survivors and non-survivors. We addressed this hypothesis through a retrospective analysis of data collected during experiments in porcine models of ventricular fibrillation (VF) cardiac arrest.
MATERIALS AND METHODS
Overview
This study entailed an analysis of data from all laboratory experiments performed in three-month old female domestic swine over a three-year period. These experiments consisted of two different injury groups (primary VF cardiac arrest and asphyxia-associated VF cardiac arrest) and two different resuscitation methods (AHA guideline-based CPR and patient-centric blood pressure-targeted CPR), which are described below. Comprehensive laboratory records were reviewed and hemodynamic recordings from all animals were examined for completeness. Any experiments without complete hemodynamic recordings available from the resuscitation period were excluded.
Animal Preparation and Data Acquisition and Measurement
The Children’s Hospital of Philadelphia Institutional Animal Care and Use Committee approved all experimental protocols. A full description of animal preparation and instrumentation methodology is available in our previous publications.21–23,26,27 Briefly, swine were anesthetized, mechanically ventilated, and high-fidelity, solid-state, micromanometer-tipped vascular catheters were placed in the right atrium, pulmonary artery, and aorta for continuous hemodynamic measurements. A bipolar pacing wire was advanced through a vascular introducer sheath into the right ventricle for VF induction. Prior to and during the experimental protocol, the electrocardiograph, aortic blood pressure, right atrial pressure, pulse oximetry, and ETCO2 waveforms were displayed and recorded. CoPP was calculated and displayed in real time by subtracting the mid-diastolic right atrial pressure from the mid-diastolic aortic pressure.
Experimental Protocol
Injury Period
Animals were randomized to one of two different injury groups:
Untreated VF cardiac arrest: VF was induced and left untreated for 7 minutes, after which the resuscitation period commenced.
Asphyxia-associated VF cardiac arrest: Asphyxia was induced and left untreated for 7 minutes, after which VF was induced and the resuscitation period commenced. Further descriptions of these protocols are available in previous publications.21–23
Resuscitation Period
In all animals, after completion of the 7-minute injury period, chest compressions (100 per minute), ventilations (6 per minute with 100% inhaled oxygen), and vasopressor therapy were initiated. Animals from each injury group were randomized to one of two treatment groups:
AHA guideline-based care (“Guideline Care”): Chest compression depth was targeted to the AHA-recommended depth of 51mm with standard ACLS epinephrine dosing.28
Patient-centric blood pressure-targeted CPR (“BP Care”): Chest compression depth was actively titrated to maintain a systolic blood pressure of 100mmHg and vasopressors (epinephrine and vasopressin) were titrated to maintain a CoPP of at least 20mmHg.
Full descriptions of both treatment groups are available in our previous publications.21–23 In both groups, after 10 minutes of CPR, an initial defibrillation attempt was provided. Waiting 10 minutes for the initial defibrillation attempt allowed for a substantial period of time in which to study CPR prior to achieving ROSC. Resuscitation according to treatment group continued with defibrillation attempts up to every two minutes until sustained ROSC was achieved or until an additional 10 minutes of resuscitation efforts after the initial defibrillation attempt failed to result in ROSC. The primary survival outcome was 45-minute ICU survival.
Outcomes
Continuous waveform data was recorded in PowerLab (ADInstruments, Colorado Springs, CO) and converted to numerical data that was then summarized into 15-second data epochs utilizing a custom code (Python, Enthought Canopy; Austin, TX). The mean aortic DBP and mean ETCO2 from the two minutes (eight 15-second epochs) immediately preceding the initial defibrillation attempt were chosen a priori for primary analysis. This time period was chosen in order to minimize the differences in ETCO2 between the animals with and without pre-VF asphyxia and because the last two minutes of CPR may be most reflective of myocardial perfusion and readiness at the time of defibrillation. The final two epochs were eliminated due to artifact from the movement of pressure transducers immediately prior to the countershock. The mean values for each variable of the remaining six epochs for each animal (minutes 15–16.5 of the experimental protocol) constitute the data contributing to the final analysis (Table 1).
Table 1.
Resuscitation Variables in Survivors Versus Non-Survivors
| Variable | Survivors (n=37) | Non-survivors (n=23) | p-value |
|---|---|---|---|
| ETCO2 | 30.02 (9.03) | 32.48 (8.37) | 0.20 |
| CoPP | 25.29 (10.30) | 12.28 (10.33) | <0.001 |
| MAP | 56.60 (9.91) | 43.42 (10.54) | <0.001 |
| SBP | 107.45 (22.62) | 100.88 (42.14) | 0.08 |
| DBP | 40.60 (10.82) | 25.90 (11.68) | <0.001 |
| RAP | 27.12 (11.09) | 30.40 (15.85) | 0.97 |
| RAS | 72.68 (50.41) | 94.20 (63.51) | 0.03 |
| RAD | 13.36 (11.35) | 7.67 (9.15) | 0.003 |
| CC Depth | 42.37 (6.49) | 49.13 (4.69) | <0.001 |
| CC Rate | 100.85 (2.14) | 100.43 (0.97) | 0.96 |
| ICP | 20.40 (15.20) | 22.92 (45.04) | 0.76 |
Mean measurements from minutes 15–16.5 of the experimental protocol, representing time during CPR preceding the initial defibrillation attempt. Displayed values are means (standard deviations). Units are millimeters for CC depth, min−1 for CC rate, and mmHg for all other variables. ETCO2 = end-tidal carbon dioxide; CoPP = coronary perfusion pressure; MAP = mean aortic blood pressure; SBP = systolic aortic blood pressure; DBP = diastolic aortic blood pressure; RAP = mean right atrial pressure; RAS = systolic right atrial pressure; RAD = diastolic right atrial pressure; CC = chest compression; ICP = intracranial pressure.
Statistical Methods
Wilcoxon rank-sum tests were used to compare the distribution of resuscitation variables between survivors and non-survivors. Receiver operating characteristic (ROC) curves with area under the curve (AUC) analyses were used to compare the ability of mean aortic DBP and mean ETCO2 to discriminate between survivors versus non-survivors. The optimal cut point in mean aortic DBP for discriminating survival versus non-survival, as determined by the Youden index, was generated using 10-fold cross validation. In each of 10 iterations, 15 animals from the primary VF cardiac arrest group and 15 animals from the asphyxia-associated VF cardiac arrest group were randomly sampled. The optimal cut point was then applied to the remaining animals. Summary statistics related to the ability of this cut point to predict survival were reported. Statistical analyses were performed with Stata 14 (StataCorp, College Station, TX) or R 3.0 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
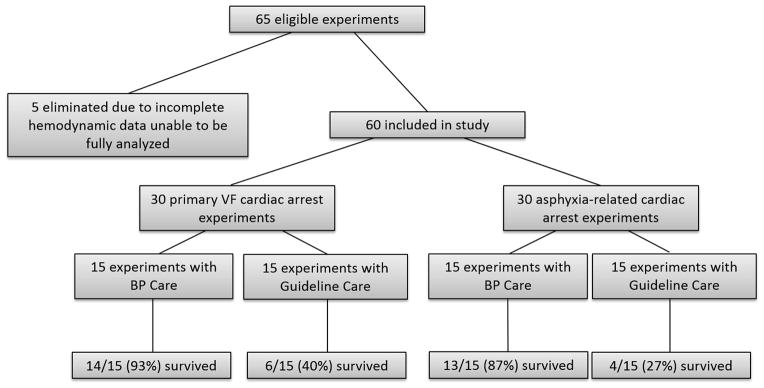
Of the 65 eligible experiments performed on three-month old swine in our laboratory, five were eliminated from analysis due to incomplete hemodynamic data recordings, leaving 60 individual animal experiments for inclusion in this study (Figure 1). There were 30 experiments in each of the primary VF cardiac arrest and asphyxia-associated cardiac arrest groups. Half of the experiments in each of these groups were conducted with Guideline Care and the other half were conducted with BP Care. As previously reported, 45-minute survival was more likely in animals treated with BP Care than in those treated with Guideline Care (27/30 vs. 10/30; p<0.001).21–23
Figure 1. Study Design.
65 experiments were evaluated for adherence to protocol and completeness of intra-arrest hemodynamic data. Five were eliminated due to incomplete hemodynamic data, leaving 60 for inclusion in this study. 30 of these were asphyxia-related cardiac arrest experiments and 30 were primary VF cardiac arrest experiments. Within each injury group, half of animals were treated with BP Care and half were treated with Guideline Care. VF = ventricular fibrillation; BP = blood pressure.
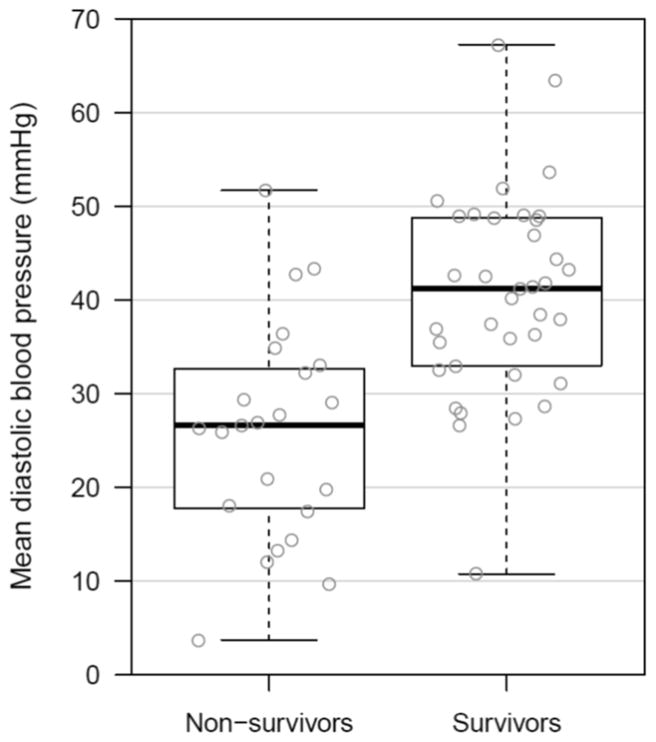
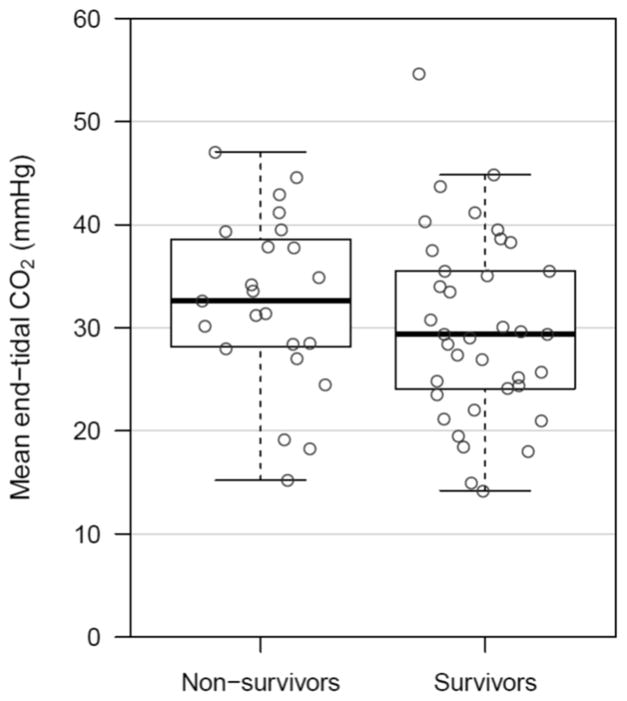
Mean aortic DBP was significantly higher in survivors than in non-survivors (40.6±1.8mmHg vs. 25.9±2.4mmHg; p<0.001) (Figure 2a), whereas ETCO2 did not differ between survivors and non-survivors (30.0±1.5mmHg vs. 32.5±1.8mmHg; p=0.20) (Figure 2b). Other resuscitation variables are summarized in Table 1. Chest compression depth and right atrial systolic pressure were significantly higher in non-survivors, while mean aortic pressure, right atrial diastolic pressure, and CoPP were higher in survivors. In the subgroup analysis of animals with primary VF cardiac arrest (n=30), mean aortic DBP was significantly higher in survivors than in non-survivors (39.9±2.6mmHg vs. 24.3±4.1mmHg; p=0.005), whereas ETCO2 did not differ between survivors and non-survivors (28.7±2.0mmHg vs. 30.0±2.9mmHg; p=0.43). In animals with asphyxia-associated cardiac arrest (n=30), mean aortic DBP was significantly higher in survivors than in non-survivors (41.4±2.5mmHg vs. 27.1±3.1mmHg; p=0.001), whereas ETCO2 did not differ between survivors and non-survivors (31.6±2.3mmHg vs. 34.4±2.1mmHg; p=0.46).
Figure 2. Mean Aortic Diastolic Blood Pressure (2a) and Mean End-tidal Carbon Dioxide (2b) in Survivors Versus Non-Survivors.
Box plots comparing survivors and non-survivors for each of the two primary comparators. Depicted values are means of aortic diastolic blood pressure and end-tidal carbon dioxide for each animal during minutes 15–16.5 of the experimental protocol.
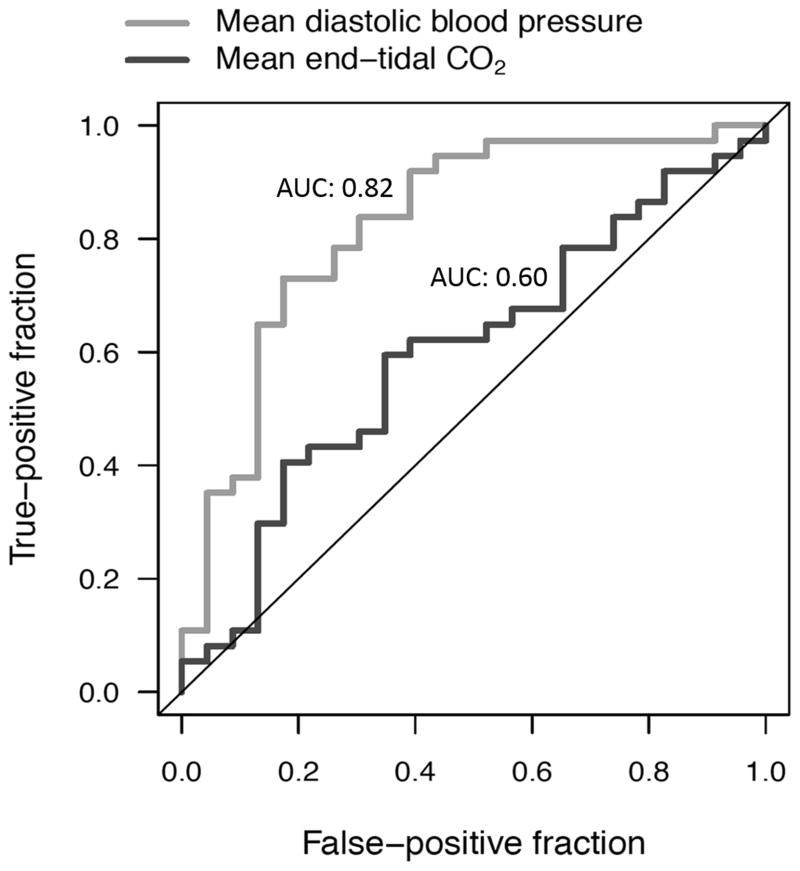
The AUC for mean aortic DBP was 0.82 vs. 0.60 for ETCO2 (p=0.025; Figure 3). The optimal cut point from the derivation cohort (n=30 in each of 10 iterations) in mean aortic DBP for discriminating survivors from non-survivors was 34.1mmHg. When applied to the validation cohort (n=30 in each of 10 iterations), this cut point demonstrated a sensitivity of 0.78 (95% CI: 0.40–0.97), a specificity of 0.81 (95% CI: 0.58–0.95), a positive predictive value of 0.64 (95% CI: 0.31–0.89) and a negative predictive value of 0.89 (95% CI: 0.67–0.99). The positive likelihood ratio was 4.08 (95%CI: 1.58–10.5) and the negative likelihood ratio was 0.27 (95% CI: 0.08–0.95). In a post hoc supplemental analysis of the final five minutes of CPR, mean aortic DBP was significantly higher in survivors than in non-survivors (39.6±1.29mmHg vs. 26.8±2.00mmHg; p<0.001), whereas ETCO2 did not differ between survivors and non-survivors (31.9±1.69mmHg vs. 31.6±1.31mmHg; p=0.87). The AUC for mean aortic DBP in the final five minutes was 0.83 (95%CI: 0.72–0.94) versus 0.50 (95%CI: 0.35–0.65) for ETCO2 (p<0.001).
Figure 3. Receiver Operating Characteristic Curves Evaluating the Ability of Diastolic Blood Pressure and End-tidal Carbon Dioxide to Discriminate between Survivors and Non-survivors.
Area under the curve (AUC) of DBP: 0.82, 95% CI: (0.70, 0.93). AUC of ETCO2: 0.60, 95% CI: (0.45, 0.74).
DISCUSSION
Our data establish that mean arterial DBP is a superior discriminator of survival than mean ETCO2 in porcine models of IHCA. Whereas DBP values during the final two minutes of CPR preceding the first defibrillation attempt were substantially higher among survivors compared with non-survivors, the concomitant ETCO2 measurements did not differ between survivors and non-survivors. The optimal DBP cut point of 34.1mmHg from the derivation dataset had a negative predictive value of 89% in the validation dataset, providing evidence that values lower than this during CPR are unlikely to be survivable. These data suggest that maintaining DBP >34mmHg enhances the likelihood of a successful resuscitation.
These findings demonstrating the predictive value of DBP during CPR are relevant because IHCAs occur greater than 200,000 times annually in the United States and frequently occur in settings where both invasive arterial blood pressure monitoring and ETCO2 measurement are feasible. Greater than half of adult IHCAs occur in the ICU and many have invasive arterial catheters at the time of cardiac arrest.29 More than 95% of pediatric IHCAs occur in the ICU, and 40% of children have invasive arterial blood pressure monitoring in place during CPR.3,30 Therefore, titration of chest compression depth and vasopressor dosing to DBP is feasible.
It is important to note that these experiments were conducted in a controlled environment in young, healthy swine with presumably normal coronary vasculature. We acknowledge that greater variability exists among human cardiac arrest victims. However, our findings were robust across cohorts from two different injury groups (primary VF and asphyxia-associated cardiac arrest) and two different treatment groups (Guideline Care and BP Care). Further, in subgroup analyses of DBP and ETCO2 within each injury group, DBP remained an effective discriminator between survivors and non-survivors, while ETCO2 once again was not. Therefore, we believe that the underlying benefit of titrating CPR to patient physiology should apply to all patients in whom it is possible (i.e., invasive monitoring in place at the time of the arrest).
Notably, these findings are biologically plausible and consistent with the well-established importance of CoPP in the physiology of cardiac arrest. The driving pressure for myocardial blood flow during CPR is CoPP;16,31,32 therefore, higher values of CoPP during CPR are associated with survival.15–17,32 Recent work in the swine cardiac arrest models reported here has demonstrated that a patient-centric method of CPR, relying upon CoPP as an intra-arrest target, results in improved rates of ROSC22,23 and 24-hour survival21 compared with AHA guideline-based care. However, the use of CoPP during cardiac arrest is dependent upon the ability to monitor both invasive arterial blood pressure and central venous pressure and promptly calculate the difference between them during the relaxation phase of chest compressions.3 We therefore chose to evaluate DBP rather than CoPP during CPR as a predictor of outcome because DBP is the major driver of CoPP and is more readily available during CPR. Importantly, both DBP and CoPP during CPR discriminated between survivors and non-survivors in this study, while ETCO2 did not.
A number of factors influence ETCO2, including minute ventilation, pulmonary pathology, and the ventilation/perfusion defects that occur with vasopressor administration.19,33–36 During CPR, ETCO2 primarily reflects pulmonary blood flow, and therefore cardiac output rather than myocardial blood flow.9,11,37 Animal studies9,38 and investigations in adults with out-of-hospital cardiac arrest10,12,13,18,19 demonstrated that ETCO2 values <10mmHg are incompatible with ROSC. Presumably, animals and patients with ETCO2 <10mmHg have very low pulmonary blood flow, cardiac output, and systemic blood pressures, precluding successful resuscitation. ETCO2 was not useful as a predictor of outcome in this swine study because the mean ETCO2 levels during CPR among both survivors and non-survivors were >30mmHg, reflecting reasonably adequate pulmonary blood flow and cardiac output. Cardiac output does not necessarily correlate with adequacy of DBP and its downstream effects on myocardial blood flow. That the survivors had mean DBP >40mmHg and non-survivors had mean DBP <26mmHg suggests that systemic vascular resistance was a key determinant of myocardial blood flow and survival.
Interestingly, chest compression depth was inversely associated with survival in this study. Since most of the survivors were in the BP Care group, in which chest compression depth was actively titrated to systolic blood pressure goals, we hypothesize that the depth necessary to generate these pressures is not uniform between individuals. Though in concordance with CPR guidelines, the standardized chest compression depths provided to animals in the Guideline Care group may not have been physiologically necessary. Recent evidence points to potential harm with chest compressions that are too deep,39 leading to the inclusion of an upper depth limit of 6cm in the 2015 AHA guidelines.40 Arguably, the optimal scenario in an ICU setting would be to provide the smallest depth necessary to achieve a targeted physiologic goal.
This study examined short-term, 45-minute survival from cardiac arrest as the primary outcome. With improvement in IHCA survival rates in recent years,5 increased focus has been on long-term survival and functional status after cardiac arrest. Notably, in an investigation by our group of longer-term survival within these models of CPR, 9 of 10 (90%) animals treated with BP Care survived to 45-minutes as compared to 0/10 animals treated with Guideline Care. Of those 9 survivors, 8 (89%) survived to 24 hours and 7 (78%) had favorable neurologic status at 24 hours as evidenced by a swine cerebral performance category of 1 or 2.21 Other previous work has established that both cerebral perfusion pressure and brain tissue oxygen tension were strongly associated with higher CoPPs with the same resuscitation methods that are described in this report.26 Thus, within these pre-clinical models, targeting intra-arrest hemodynamics not only results in the improved short-term survival reported here, but also longer-term survival with favorable neurologic outcomes.21,26
This study has important limitations. First, this was a retrospective analytic study of previously performed experiments. This exposes the results to potential confounding by treatment group membership and vasopressor administration, as the majority of survivors were in the BP Care group, in which animals have on average received more vasopressors in our previous publications.21–23 Therefore, the increased DBP seen in survivors was likely driven, at least in part, by vasopressor administration. As further evidence of this, right atrial diastolic blood pressures were also higher in survivors, likely due to vasopressor-induced increases in pulmonary vascular resistance.35 Clearly, a prospective comparison of DBP-guided versus ETCO2-guided resuscitation strategies would limit the inherent biases of this study design but does not detract from the findings of better discrimination of survival by DBP versus ETCO2. Second, ETCO2 in an asphyxiated animal is elevated upon commencing ventilation regardless of pulmonary blood flow or cardiac output; therefore, this presumably could have lowered the ability of ETCO2 to discriminate between survivors and non-survivors in half of our cohort. However, to minimize this bias, our predictor variables (ETCO2 and hemodynamics) were acquired 8 minutes into CPR, long after the time period when these values would equilibrate between injury groups.21,41 Third, while the AUC for DBP (0.82) was significantly superior to that of ETCO2 (0.60), the reader should note that it is an imperfect predictor of survival. Caution should be applied in using DBP as a standalone predictor of survival or as an indication to cease resuscitation efforts.
CONCLUSIONS
In these porcine models of primary VF cardiac arrest and asphyxia-related cardiac arrest, diastolic blood pressure was an effective predictor of short-term survival, while end-tidal carbon dioxide was not. Specifically, inability to obtain an adequate diastolic blood pressure was highly predictive of death. These biologically plausible findings support the contention that patient-centric titration of CPR to hemodynamics may improve outcomes from in-hospital cardiac arrest.
Acknowledgments
We would like to acknowledge Drs. Thomas Conlon, Joshua Lampe, and Lance Becker who have supported the cardiopulmonary resuscitation laboratory at the Children’s Hospital of Philadelphia.
Footnotes
CONFLICTS OF INTEREST:
Financial support was provided through Russell Raphaely Endowed Chair funds, awarded to Robert A. Berg, at The Children’s Hospital of Philadelphia. Robert M. Sutton is supported through a National Institutes of Health career development award (Eunice Kennedy Shriver National Institute of Child Health & Human Development – K23HD072629). No other authors have relevant conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Merchant RM, Yang L, Becker LB, et al. Incidence of treated cardiac arrest in hospitalized patients in the United States. Crit Care Med. 2011;39(11):2401–2406. doi: 10.1097/CCM.0b013e3182257459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berg RA, Sutton RM, Holubkov R, et al. Ratio of PICU versus ward cardiopulmonary resuscitation events is increasing. Crit Care Med. 2013;41(10):2292–2297. doi: 10.1097/CCM.0b013e31828cf0c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brady WJ, Gurka KK, Mehring B, Peberdy MA, O’Connor RE American Heart Association’s Get with the Guidelines I. In-hospital cardiac arrest: impact of monitoring and witnessed event on patient survival and neurologic status at hospital discharge. Resuscitation. 2011;82(7):845–852. doi: 10.1016/j.resuscitation.2011.02.028. [DOI] [PubMed] [Google Scholar]
- 5.Girotra S, Nallamothu BK, Spertus JA, et al. Trends in survival after in-hospital cardiac arrest. N Engl J Med. 2012;367(20):1912–1920. doi: 10.1056/NEJMoa1109148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berg MD, Schexnayder SM, Chameides L, et al. Part 13: pediatric basic life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 Suppl 3):S862–875. doi: 10.1161/CIRCULATIONAHA.110.971085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berg RA, Hemphill R, Abella BS, et al. Part 5: adult basic life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 Suppl 3):S685–705. doi: 10.1161/CIRCULATIONAHA.110.970939. [DOI] [PubMed] [Google Scholar]
- 8.Meaney PA, Bobrow BJ, Mancini ME, et al. Cardiopulmonary resuscitation quality: [corrected] improving cardiac resuscitation outcomes both inside and outside the hospital: a consensus statement from the American Heart Association. Circulation. 2013;128(4):417–435. doi: 10.1161/CIR.0b013e31829d8654. [DOI] [PubMed] [Google Scholar]
- 9.Gudipati CV, Weil MH, Bisera J, Deshmukh HG, Rackow EC. Expired carbon dioxide: a noninvasive monitor of cardiopulmonary resuscitation. Circulation. 1988;77(1):234–239. doi: 10.1161/01.cir.77.1.234. [DOI] [PubMed] [Google Scholar]
- 10.Levine RL, Wayne MA, Miller CC. End-tidal carbon dioxide and outcome of out-of-hospital cardiac arrest. N Engl J Med. 1997;337(5):301–306. doi: 10.1056/NEJM199707313370503. [DOI] [PubMed] [Google Scholar]
- 11.Weil MH, Bisera J, Trevino RP, Rackow EC. Cardiac output and end-tidal carbon dioxide. Crit Care Med. 1985;13(11):907–909. doi: 10.1097/00003246-198511000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Kalenda Z. The capnogram as a guide to the efficacy of cardiac massage. Resuscitation. 1978;6(4):259–263. doi: 10.1016/0300-9572(78)90006-0. [DOI] [PubMed] [Google Scholar]
- 13.Sanders AB, Kern KB, Otto CW, Milander MM, Ewy GA. End-tidal carbon dioxide monitoring during cardiopulmonary resuscitation. A prognostic indicator for survival. JAMA. 1989;262(10):1347–1351. [PubMed] [Google Scholar]
- 14.Sheak KR, Wiebe DJ, Leary M, et al. Quantitative relationship between end-tidal carbon dioxide and CPR quality during both in-hospital and out-of-hospital cardiac arrest. Resuscitation. 2015;89:149–154. doi: 10.1016/j.resuscitation.2015.01.026. [DOI] [PubMed] [Google Scholar]
- 15.Sanders AB, Ewy GA, Taft TV. Prognostic and therapeutic importance of the aortic diastolic pressure in resuscitation from cardiac arrest. Crit Care Med. 1984;12(10):871–873. doi: 10.1097/00003246-198410000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Kern KB, Ewy GA, Voorhees WD, Babbs CF, Tacker WA. Myocardial perfusion pressure: a predictor of 24-hour survival during prolonged cardiac arrest in dogs. Resuscitation. 1988;16(4):241–250. doi: 10.1016/0300-9572(88)90111-6. [DOI] [PubMed] [Google Scholar]
- 17.Paradis NA, Martin GB, Rivers EP, et al. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA. 1990;263(8):1106–1113. [PubMed] [Google Scholar]
- 18.Kolar M, Krizmaric M, Klemen P, Grmec S. Partial pressure of end-tidal carbon dioxide successful predicts cardiopulmonary resuscitation in the field: a prospective observational study. Crit Care. 2008;12(5):R115. doi: 10.1186/cc7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eckstein M, Hatch L, Malleck J, McClung C, Henderson SO. End-tidal CO2 as a predictor of survival in out-of-hospital cardiac arrest. Prehosp Disaster Med. 2011;26(3):148–150. doi: 10.1017/S1049023X11006376. [DOI] [PubMed] [Google Scholar]
- 20.Ahrens T, Schallom L, Bettorf K, et al. End-tidal carbon dioxide measurements as a prognostic indicator of outcome in cardiac arrest. Am J Crit Care. 2001;10(6):391–398. [PubMed] [Google Scholar]
- 21.Sutton RM, Friess SH, Naim MY, et al. Patient-centric blood pressure-targeted cardiopulmonary resuscitation improves survival from cardiac arrest. Am J Respir Crit Care Med. 2014;190(11):1255–1262. doi: 10.1164/rccm.201407-1343OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friess SH, Sutton RM, Bhalala U, et al. Hemodynamic directed cardiopulmonary resuscitation improves short-term survival from ventricular fibrillation cardiac arrest. Crit Care Med. 2013;41(12):2698–2704. doi: 10.1097/CCM.0b013e318298ad6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sutton RM, Friess SH, Bhalala U, et al. Hemodynamic directed CPR improves short-term survival from asphyxia-associated cardiac arrest. Resuscitation. 2013;84(5):696–701. doi: 10.1016/j.resuscitation.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamrick JL, Hamrick JT, Lee JK, Lee BH, Koehler RC, Shaffner DH. Efficacy of chest compressions directed by end-tidal CO2 feedback in a pediatric resuscitation model of basic life support. J Am Heart Assoc. 2014;3(2):e000450. doi: 10.1161/JAHA.113.000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Link MS, Berkow LC, Kudenchuk PJ, et al. Part 7: Adult Advanced Cardiovascular Life Support: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132(18 Suppl 2):S444–464. doi: 10.1161/CIR.0000000000000261. [DOI] [PubMed] [Google Scholar]
- 26.Friess SH, Sutton RM, French B, et al. Hemodynamic directed CPR improves cerebral perfusion pressure and brain tissue oxygenation. Resuscitation. 2014;85(9):1298–1303. doi: 10.1016/j.resuscitation.2014.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kilbaugh TJ, Sutton RM, Karlsson M, et al. Persistently Altered Brain Mitochondrial Bioenergetics After Apparently Successful Resuscitation From Cardiac Arrest. J Am Heart Assoc. 2015;4(9) doi: 10.1161/JAHA.115.002232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neumar RW, Otto CW, Link MS, et al. Part 8: adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 Suppl 3):S729–767. doi: 10.1161/CIRCULATIONAHA.110.970988. [DOI] [PubMed] [Google Scholar]
- 29.Goldberger ZD, Chan PS, Berg RA, et al. Duration of resuscitation efforts and survival after in-hospital cardiac arrest: an observational study. Lancet. 2012;380(9852):1473–1481. doi: 10.1016/S0140-6736(12)60862-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meaney PA, Nadkarni VM, Cook EF, et al. Higher survival rates among younger patients after pediatric intensive care unit cardiac arrests. Pediatrics. 2006;118(6):2424–2433. doi: 10.1542/peds.2006-1724. [DOI] [PubMed] [Google Scholar]
- 31.Raessler KL, Kern KB, Sanders AB, Tacker WA, Jr, Ewy GA. Aortic and right atrial systolic pressures during cardiopulmonary resuscitation: a potential indicator of the mechanism of blood flow. Am Heart J. 1988;115(5):1021–1029. doi: 10.1016/0002-8703(88)90071-3. [DOI] [PubMed] [Google Scholar]
- 32.Berg RA, Sanders AB, Kern KB, et al. Adverse hemodynamic effects of interrupting chest compressions for rescue breathing during cardiopulmonary resuscitation for ventricular fibrillation cardiac arrest. Circulation. 2001;104(20):2465–2470. doi: 10.1161/hc4501.098926. [DOI] [PubMed] [Google Scholar]
- 33.Touma O, Davies M. The prognostic value of end tidal carbon dioxide during cardiac arrest: a systematic review. Resuscitation. 2013;84(11):1470–1479. doi: 10.1016/j.resuscitation.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 34.Tang W, Weil MH, Gazmuri RJ, Sun S, Duggal C, Bisera J. Pulmonary ventilation/perfusion defects induced by epinephrine during cardiopulmonary resuscitation. Circulation. 1991;84(5):2101–2107. doi: 10.1161/01.cir.84.5.2101. [DOI] [PubMed] [Google Scholar]
- 35.Lindberg L, Liao Q, Steen S. The effects of epinephrine/norepinephrine on end-tidal carbon dioxide concentration, coronary perfusion pressure and pulmonary arterial blood flow during cardiopulmonary resuscitation. Resuscitation. 2000;43(2):129–140. doi: 10.1016/s0300-9572(99)00129-x. [DOI] [PubMed] [Google Scholar]
- 36.Martin GB, Gentile NT, Paradis NA, Moeggenberg J, Appleton TJ, Nowak RM. Effect of epinephrine on end-tidal carbon dioxide monitoring during CPR. Ann Emerg Med. 1990;19(4):396–398. doi: 10.1016/s0196-0644(05)82345-5. [DOI] [PubMed] [Google Scholar]
- 37.Idris AH, Staples ED, O’Brien DJ, et al. End-tidal carbon dioxide during extremely low cardiac output. Ann Emerg Med. 1994;23(3):568–572. doi: 10.1016/s0196-0644(94)70080-x. [DOI] [PubMed] [Google Scholar]
- 38.Trevino RP, Bisera J, Weil MH, Rackow EC, Grundler WG. End-tidal CO2 as a guide to successful cardiopulmonary resuscitation: a preliminary report. Crit Care Med. 1985;13(11):910–911. doi: 10.1097/00003246-198511000-00012. [DOI] [PubMed] [Google Scholar]
- 39.Stiell IG, Brown SP, Nichol G, et al. What is the optimal chest compression depth during out-of-hospital cardiac arrest resuscitation of adult patients? Circulation. 2014;130(22):1962–1970. doi: 10.1161/CIRCULATIONAHA.114.008671. [DOI] [PubMed] [Google Scholar]
- 40.Kleinman ME, Brennan EE, Goldberger ZD, et al. Part 5: Adult Basic Life Support and Cardiopulmonary Resuscitation Quality: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132(18 Suppl 2):S414–435. doi: 10.1161/CIR.0000000000000259. [DOI] [PubMed] [Google Scholar]
- 41.Grmec S, Lah K, Tusek-Bunc K. Difference in end-tidal CO2 between asphyxia cardiac arrest and ventricular fibrillation/pulseless ventricular tachycardia cardiac arrest in the prehospital setting. Crit Care. 2003;7(6):R139–144. doi: 10.1186/cc2369. [DOI] [PMC free article] [PubMed] [Google Scholar]