Abstract
The neurodevelopmental fetal alcohol spectrum disorder (FASD) is characterized by cognitive and behavioral deficits in the offspring. Conferring the deficits to the next generation would increase overall FASD disease burden and prevention of this transmission could be highly significant. Prior studies showed the reversal of these behavioral deficits by low dose thyroxine (T4) supplementation to the ethanol-consuming mothers. Here we aim to identify whether prenatal ethanol (PE) exposure impairs hippocampus-dependent learning and memory in the second-generation (F2) progeny, and whether T4 administration to the ethanol-consuming dam can prevent it. Sprague-Dawley (S) dams received control diets (ad libitum and nutritional control) or ethanol containing liquid diet with and without simultaneous T4 (0.3mg/l diet) administration. Their offspring (SS F1) were mated with naïve Brown Norway (B) males and females generating the SB F2 and BS F2 progeny. Hippocampus-dependent contextual fear memory and hippocampal expression of the thyroid hormone-regulated type 3 deiodinase, (Dio3) and neurogranin (Nrgn) were assessed. SS F1 PE-exposed females and their SB F2 progeny exhibited fear memory deficits. T4 administration to the mothers of F1 females reversed these deficits. Although SS F1 PE-exposed males also experienced fear memory deficit, this was neither transmitted to their BS F2 offspring nor reversed by prenatal T4 treatment. Hippocampal Dio3 and Nrgn expression showed similar pattern of changes. Grandmaternal ethanol consumption during pregnancy affects fear memory of the matrilineal second-generation progeny. Low dose T4 supplementation prevents this process likely via altering allele-specific and total expression of Dio3 in the hippocampus.
Keywords: prenatal ethanol, fear conditioning, thyroxine administration, Dio3, neurogranin, free T3
Introduction
The consumption of alcohol during pregnancy has been linked to a compendium of disabilities collectively referred to as fetal alcohol spectrum disorder (FASD) (Manning and Eugene Hoyme, 2007). These disabilities range from dismorphology and mental retardation as seen in Fetal Alcohol Syndrome, to cognitive and behavioral deficits that characterize Alcohol-Related Neurodevelopmental Disorder (ARND). FASD affects approximately 2-5% of young children in the United States (May et al., 2014) and puts a significant strain on the health care system, while the prevalence of ARND is not really known, as it does not yet have a defined diagnostic category. Despite the high prevalence, we have limited knowledge to the mechanisms through which ethanol produces these effects. Even less is known about whether and how ethanol might affect future generations.
The deficits and severity of symptoms that arise as a result of prenatal ethanol (PE) exposure vary greatly from one individual to another, even when controlling for the time, and level of ethanol exposure (Guerri et al., 2009). Some of the potential causes of this variability are explored by looking at the genetic vulnerability of the ethanol-consuming mother or their offspring (Tunc-Ozcan et al., 2014). Additionally, vulnerability can be caused by intergenerational effects of ancestral ethanol exposure as environmental factors such as diet, stress, or exposure to teratogens (i.e. ethanol) cause a wide range of physiological and behavioral changes across multiple generations (Brasset and Chambeyron, 2013; Gluckman and Hanson, 2004; Govorko et al., 2012; Harper et al., 2014b; Miller et al., 2014). This form of intergenerational effect could result in behavioral or cognitive deficits in individuals not directly exposed to the teratogen. The mechanism of the intergenerational effect is not known, but is likely to be epigenetic in its nature, including changes in the allele-specific expression of imprinted genes across generations (Downing et al., 2011; Haycock, 2009; Mead and Sarkar, 2014; Ramsay, 2010; Sittig et al., 2011b; Tunc-Ozcan et al., 2014).
Hippocampal development is impaired in human FASD (Willoughby et al., 2008) that is paralleled by animal models (Gil-Mohapel et al., 2010; Gil-Mohapel et al., 2011). Consequently, some of the most debilitating effects of ARND are on hippocampus-based learning and memory (Dudek et al., 2014). Animal models employed by most laboratories model ARND, and PE-exposure in rats results in impaired hippocampus-dependent spatial learning and memory as measured by the Morris Water Maze (Sittig et al., 2011b; Wilcoxon et al., 2005) or a contextual fear conditioning paradigm (Weeber et al., 2001) that uses the entire experimental environment as the conditioned stimulus and requires the hippocampal formation (Kim et al., 1992; Maren et al., 1998; Phillips and LeDoux, 1992).
The cause of the hippocampus-specific vulnerability in FASD is not known. One of the possible mechanisms is related to PE-induced abnormal thyroid hormone levels during development (Scott et al., 1998; Wilcoxon and Redei, 2004), and the abundance of thyroid hormone receptors (TR) and thyroid hormone regulated genes in the hippocampus (Bastian et al., 2012; Bernal, 2007; Desouza et al., 2005). It is well known that thyroid hormone is essential for normal brain development (Heindel and Zoeller, 2003). Clinical or subclinical hypothyroidism of the mother negatively affects the neuropsychological development of the child (Haddow et al., 1999; Zoeller and Rovet, 2004), and experimental hypothyroidism in developing rats results in impaired learning (Taylor et al., 2014). Decreased serum TSH and thyroxine (T4) has been found in alcohol-consuming pregnant women (Herbstman et al., 2008), and in newborns exposed to alcohol in utero (Hernandez et al., 1992). Similar findings in animal models are reported with decreased peripheral free T4, fT3, and TSH in ethanol-consuming pregnant dams (Wilcoxon and Redei, 2004).
We have shown previously that supplementation with T4 during pregnancy can alleviate behavioral and cognitive deficits caused by PE exposure (Gottesfeld and Silverman, 1990; Tunc-Ozcan et al., 2013; Wilcoxon et al., 2005; Wilcoxon and Redei, 2004). One of the mechanisms by which abnormal thyroid homeostasis of the developing brain could result in long term cognitive deficit is alteration in the hippocampal expression of deiodinase 3 (Dio3) that metabolizes the biologically active thyroid hormone triiodothyronine (T3) into an inactive metabolite (Gereben et al., 2008). Dio3 is a preferentially paternally imprinted gene that show allele-specific expression in the adult rat brain as well (Sittig et al., 2011a). PE exposure affects the allele-specific expression of Dio3 in the hippocampus together with total expression changes (Sittig et al., 2011a; Sittig et al., 2011b). Another thyroid hormone-mediated gene is neurogranin (Nrgn), which encodes a neuron-specific postsynaptic protein and plays an important role in synaptic plasticity, learning, and memory (Miyakawa et al., 2001; Wilcoxon et al., 2007).
The work presented here continues the examination of the effects of PE on context-dependent fear memory, activity and anxiety in the first generation offspring directly exposed to alcohol (SS F1), as well as in the second-generation matrilinear (SB F2) and patrilinear (BS F2) progeny. We chose to generate these reciprocal crosses to measure allele-specific expression of the imprinted Dio3 as we have done previously (Sittig et al., 2011b), but now in the second generation. The main goal of this study was to investigate the intergenerational consequences of PE and attempt to prevent them by simultaneous T4 administration with ethanol during in utero development of the SS F1 offspring.
Materials and Methods
Animals
The Institutional Animal Care and Use Committee of Northwestern University have approved all animal procedures. Sprague-Dawley (S, Harlan - Indianapolis, IN) and Brown Norway (B, Charles River – Wilmington, MA) rats were housed in a climate-controlled environment with a 14:10 hour light/dark cycle (lights on at 6am) with water provided ad libitum throughout the duration of the study. We chose these strains with the knowledge that B is the most phylogenetically divergent inbred rat strain from all others, and S is the most commonly utilized outbred strain (Swerdlow et al., 2008) that has also been employed in all our previous studies (Sittig and Redei, 2010; Wilcoxon et al., 2005). The B and S genomes have been sequenced by the Rat Genome Project and Celera respectively, and therefore, allele-specific expression in imprinted genes can be explored in these crosses as we have done previously (Sittig et al., 2011b).
Female S rats were mated with male S rats and the day of finding sperm in vaginal smears was considered gestational day 1 (GD1). The dams were divided into 4 separate feeding groups: control (C), pair-fed (PF), ethanol (E), and ethanol + thyroxine (E+T4). Control dams received laboratory rat chow diet ad libitum throughout gestation while the remaining 3 groups received a liquid diet (Lieber-DeCarli '82; Bio-Serv. Frenchtown, NJ) beginning on GD4 and assigned diet was started at GD8. For E dams, the ethanol percentage in the diet was increased until a final concentration of 5% (w/v) from GD 8-10 and kept constant until G21. The E+T4 group received 0.3 mg/l thyroxine (Sigma-Aldrich Co, St Louis, MO, USA) in the E-containing liquid diet, which, based on the daily diet consumption, is equivalent to approximately 8ug/100gBW/day of T4 (Tunc-Ozcan et al., 2013). Each PF dam received liquid diet without E, the volume was matched to the amount of E diet consumed by an E dam. Since we found in our previous study that the low levels of T4 only reversed the PE-induced changes and normalized the thyroid homeostasis, no control groups with added T4 were employed (Tunc-Ozcan et al., 2013).
After GD21 all rats received standard laboratory chow ad libitum for the remainder of the study. Around postnatal day (PND) 70, separate cohorts of one-two male and female rats from each litter were used for open field test (OFT), fear conditioning (FC), or brain sample collection to avoid potential litter effects. The morphological parameters of the different groups have been described previously (Harper et al., 2014a).
Upon reaching adulthood (∼PND 70), experimentally naïve SS F1 male and female offspring of all treatment groups were mated with naïve B female and male rats, respectively, thereby generating BS F2 and SB F2 progeny (maternal strain first). All F1 mating pairs received standard laboratory chow and water, ad libitum, throughout the experiment. All offspring were weaned at PND 24. Beginning at PND 70, F2 progeny were used either for behavioral testing or for hippocampal expression analyses as was done in the F1 generation.
Experimentally naive adult rat male and female offspring from all generations and treatment groups were sacrificed by decapitation between 10:00 and 12:00 h. Trunk blood was collected into EDTA-coated tubes on ice, and plasma was obtained by centrifugation. Whole hippocampus were immediately dissected and collected directly into RNAlater reagent (Ambion, Austin, TX) and stored at -80°C.
Behavioral tests
Context dependent fear conditioning (FC) studies were performed on adult animals (PND 70-90), using an automated fear conditioning apparatus (TSE, Bad Homburg, Germany). On the first day of the test, rats were placed in the fear-conditioning chamber for 3 minutes to habituate to the novel environment. This period was followed by a series of three mild shocks (0.8 mA, 1 sec each, 60 sec between each shock) administered through an electrified floor grid. Twenty-four hours later the rats were placed in the same chamber for 3 minutes, and examined for contextual fear memory as measured by freezing duration, velocity, distance traveled and total locomotion through the use of an infrared beam system (detection rate 100Hz). Any rats that did not respond to the initial shock were excluded from the study.
To test activity and anxiety, the open field test (OFT) was carried out on a separate cohort of adult rats (PND 70-90). The rats were placed in a circular arena (diameter 82 cm) surrounded by a 30 cm high wall and lit to a brightness of approximately 60 lux by indirect overhead lighting. The arena contained an inner concentric circle with a diameter of 50 cm designated as the inner zone. Rats were placed in the center of the arena and allowed to move freely for 10 minutes with the activity being recorded and tracked by TSE Videomot software (version 5.75, Bad Homburg, Germany). The software recorded and analyzed total distance traveled and time spent in the inner and outer areas of the arena. Between each test, the arena was cleaned with a 1.25% acetic acid solution to eliminate cues caused by odor.
Radioimmunoassay
Experimentally naïve rats from different prenatal diet groups were sacrificed by decapitation for tissue and blood collection. Plasma was collected from trunk blood was used for measurements of free T3 (fT3). fT3 levels were measured by RIA as described previously (Sittig and Redei, 2010). Assay was manufactured by MP Biomedicals, LLC (Irvine, CA, USA) and the assay sensitivity and coefficient of variation were 0.6 pg/ml and 8%, respectively.
RNA isolation and Quantitative RT-PCR
Total RNA extraction, reverse transcription and quantitative PCR (qPCR) were performed as described previously (Tunc-Ozcan et al., 2013). Briefly, RNA was isolated using Trizol reagent according to the manufacturer's instructions (Life Technologies, Grand Island, NY, USA). Reverse transcription of 1ug total RNA was performed by using the TaqMan Reverse Transcription kit (Applied Biosystems, Branchburg, NJ, USA). Real-time PCR was conducted with the ABI 7900 system using SYBR Green Master Mix (Applied Biosystems, Foster City, CA, USA). Reactions were performed in triplicate and reached threshold amplification within 32 cycles. Levels of transcripts were determined relative to GAPDH and a general calibrator using the 2−ΔΔCt method. Primers were designed using the ABI Primer Express 3.0 program (given sequences are 5′ to 3′) and were constructed to span an exon junction: Dio3 (F:TGGCTCGAACTGGCAACTTT; R:ATTGCGCTTTGCTGAAATAGC), Nrgn: (F:CCTGAACTACCACCCAGCAT; R:ATCTTCTTCCTCGCCATGTG) and Gapdh: (F:CAACTCCCTCAAGATTGTCAGCAA; R:GGCATGGACTGTGGTCATGA).
Pyrosequencing
To facilitate the identification of allelic Dio3 expression in SB F2 and BS F2 hybrid offspring, we used the identified SNP in the Dio3 exon between S and B strains (Sittig et al., 2011a). To measure the allelic ratio, PCR was conducted using forward primer and a biotinylated reverse primer flanking the SNP: F:ATCTGCGTATCCGACGACAAC, R:biotin-TCATGGGCCTGCTTGAAGAA. Purification of biotinylated PCR products and pyrosequencing were performed by EpigenDx, Inc. (Worcester, MA, USA) using a sequencing forward primer.
Statistical analyses
Data are presented as means +/- SEM. The plasma fT3, total Dio3 and Nrgn expression data was normalized to the C offspring value. Data were analyzed within generation first by 2-way ANOVA (diet, sex), and subsequently subjected to hypothesis testing analyses using one-way ANOVA, or, in some cases, Student's t-test. When there was no significant effect of sex, or sex by diet interaction, male and female data were combined and analyzed by one-way ANOVA (diet). When there was either a sex effect or sex by diet interaction, male and female data were additionally analyzed, separately, by one-way ANOVA (diet). Bonferroni adjusted post hoc tests were used to identify differences between C, PF, E and E+T4 groups, with significance set at P<0.05, as shown in the figures. Cohen's d effect sizes were calculated by using the online effect size calculator at http://www.campbellcollaboration.org/escalc/html/EffectSizeCalculator-SMD30.php for ANOVA as well as pair-wise comparisons.
Results
Behavior
Contextual fear memory of the SS F1 offspring exposed to PE was impaired as measured by decreased freeze duration upon re-exposure to the context on the second day of the FC test (diet: F(3,81)=4.00, p<0.05, Cohen's d=1.50). Although there were no significant sex differences in this effect, we also analyzed male and female SS F1 offspring separately, to illustrate the intergenerational transmission by lineage. PE-exposed SS F1 male offspring showed contextual fear memory deficit with a significantly decreased freeze duration in the second day of the FC (F(3,49)=3.39, p<0.05, d=-0.60) (Figure 1A). However, administration of T4 to the ethanol-consuming dam did not reverse this impairment in their SS F1 male offspring. These findings were mirrored by the distance traveled measure; PE-exposure resulted in a significant increase in distance traveled compared to controls, with no recovery as a result of T4 supplementation (F(3,49)=10.73, p<0.01, d=0.73) (Figure 1A). Examining FC behavior of the SS F1 males' BS F2 progeny revealed no sex differences; therefore, male and female data were combined. There were no significant differences in freeze duration or distance traveled in the FC test between the BS F2 groups whose fathers were exposed to the different prenatal diets in utero (Figure 1B).
Figure 1. Maternal ethanol consumption leads to deficit in fear memory in male offspring that is not transmitted to their BS F2 progeny. No effect of prenatal T4 treatment in either generation.
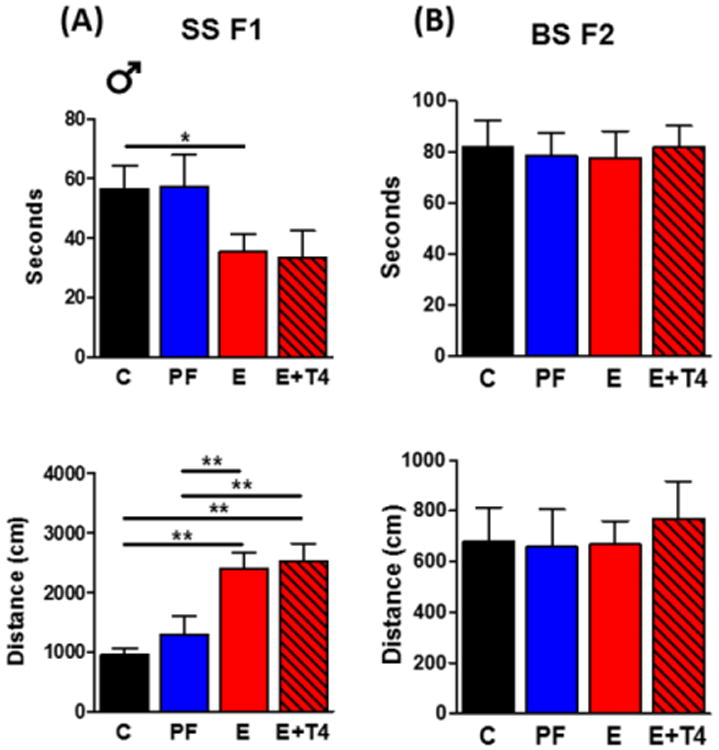
Time (in seconds) spent freezing and distance (in centimeters) travelled in the second day of contextual fear conditioning paradigm by A) SS F1 males, and B) their BS F2 offspring (male and female data combined). Data represented as mean +/- SEM. N=16-24/group. *p<0.05, **p<0.01 by one-way ANOVA followed by Bonferroni post-hoc test.
PE-exposed SS F1 females showed impaired contextual fear memory with a significantly decreased freeze duration (F(3,35)=7.42, p<0.01, d=-1.15; PF vs E t(15)=2.29, p<0.05, d=1.11) and increased distance travelled (F(3,35=6.02, p<0.01, d=1.00; PF vs E t(15)=2.18, p<0.05, d=1.06), which were both reversed by T4 supplementation to the E-consuming mothers (Figure 2A). SB F2 progeny of the PE-exposed SS F1 females also exhibited significant decreases in freeze duration (F(3,58)=4.19, p<0.01, d=-0.53) and increases in distance travelled (F(3,58)=6.07, p<0.01, d=0.51) compared to controls; the latter effect remained to be reversed by T4 supplementation to their E-consuming grandmothers (Figure 2B). There were no sex differences within groups, therefore, male and female SB F2 data were combined.
Figure 2. Maternal ethanol consumption leads to deficit in fear memory in female offspring that is transmitted to their SB F2 progeny. Prenatal T4 treatment of the ethanol consuming mother eliminated the deficit in the SS F1 females and in their SB F2 offspring.

Time (in seconds) spent freezing and distance (in centimeters) travelled in the second day of contextual fear conditioning paradigm by A) SS F1 females, and B) their SB F2 offspring (male and female data combined). Data presented as mean +/- SEM. N=12-21/group. *p<0.05, **p<0.01 by oneway ANOVA followed by Bonferroni post-hoc test; ˆp<0.05 by Student's t-test.
Anxiety and activity levels were measured in the OFT to investigate whether they contributed to the fear memory deficit observed in the SSF1 and SB F2 offspring. Neither activity, nor anxiety level findings were commensurate with the fear-memory deficit. Total activity levels were not affected by prenatal diets (Supplemental Figures 1 and 2). PE increased anxiety levels of the SS F1 male offspring, but decreased it in the females, as measured by distance travelled in the inner area of the OFT (diet: F(3,82)=4.15, p<0.05; sex: F(1,82)=7.17, p<0.01; interaction: F(3,82)=6.21, p<0.01, d=-0.46). T4 supplementation reversed the PE effects in the SS F1 offspring. Since total activity was not altered by PE or T4 supplementation, the increased activity, and decreased freezing observed in the FC (Figures 1 and 2) represented a specific memory deficit induced by PE and reversed by T4 in the SS F1 offspring.
Thyroid hormone status and expression of thyroid hormone-regulated Dio3 and Nrgn
There were no sex differences in the plasma free T3 (fT3) levels, in total expression of Dio3 and Nrgn and allele-specific expressions of Dio3 for both BS F2 and SB F2 progeny, therefore, male and female data were combined in the following analysis.
Plasma fT3 levels were significantly higher in PE-exposed SS F1 males and females compared to control offspring (diet: F(3,48)=8.03, p<0.01, d=0.78) (Figure 3A). This PE-induced increase was normalized by simultaneous administration of T4 to the E consuming mothers in both SS F1 males and females. BS F2 progeny of the SS F1 males did not show any difference in fT3 levels by diet (Figure 3B), while the SB F2 progeny of the SS F1 females showed elevated levels of fT3 in the E group that was reversed in E+T4 group (F(3,80)=13.0, p<0.01, d=0.51) (Figure 3C).
Figure 3. Prenatal ethanol exposure causes elevated plasma free T3 levels in both male and female SS F1 rats and the latter's SB F2 progeny, which is normalized by administration of T4 in the ethanol containing liquid diet.
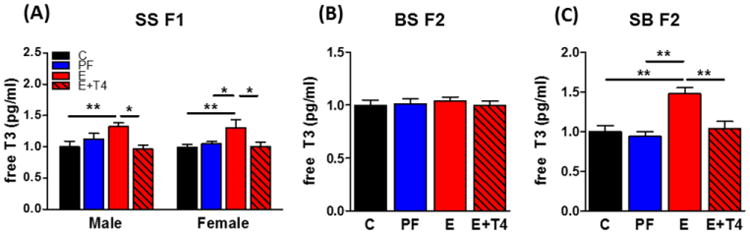
Plasma free T3 levels of A) SS F1 males and females, B) their BS F2 and C) SB F2 progeny (there were no sex differences, so male and female data were combined for the BS and SB F2 progeny). Free T3 was measured by radioimmunoassay. Data presented as mean +/- SEM. N=12-25/group. *p<0.05, **p<0.01 by one-way ANOVA followed by Bonferroni post-hoc test.
Both male and female SS F1 offspring of E consuming dams displayed significantly decreased transcript levels of Nrgn in the hippocampus compared to both C and PF offspring, which was increased by prenatal T4 supplementation (diet: F(3,33)=8.52, p<0.01, d=2.84) (Figure 4A). Similar to fT3, BS F2 progeny showed no prenatal diet-induced differences in Nrgn transcript levels (Figure 4B), while SB F2 progeny mimicked the SS F1 pattern with a significantly decreased Nrgn expression in E group that was reversed in the E+T4 group (F(3,38)= 18.31, p<0.01, d=-1.11) (Figure 4C).
Figure 4. The decrease in hippocampal neurogranin (Nrgn) transcript levels of prenatal ethanol-exposed SS F1 rats and their SB F2 progeny is normalized by administration of T4 in the ethanol containing liquid diet to the mother.
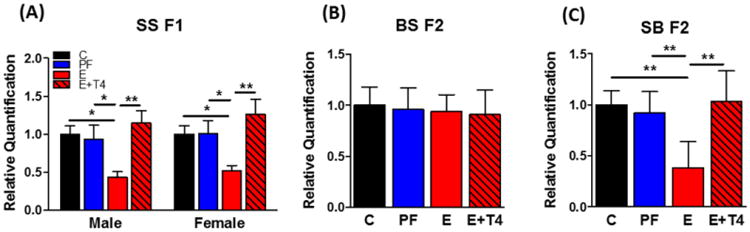
Nrgn transcript levels of A) SS F1 males and females, B) their BS F2 and C) SB F2 progeny (there were no sex differences, so male and female data were combined for BS and SB F2 progeny). Transcript levels were measured by quantitative real-time RT-PCR and normalized to Gapdh and a general calibrator using the 2−ΔΔCt method. Data presented as mean +/- SEM. N=6-14/group. *p<0.05, **p<0.01 by one-way ANOVA followed by Bonferroni post-hoc test.
Hippocampal transcript levels of Dio3 were significantly higher in PE-exposed SS F1 females compared to either controls, while there was only a trend of increased Dio3 expression in PE-exposed SS F1 males (diet: F(3,18)=6.23, p<0.01, sex: F(1,28)=5.02, p<0.05, d=2.14) (Figure 5A). Maternal T4 administration reversed the PE-induced increase in the SS F1 females. BS F2 progeny showed no differences among diet groups in their hippocampal Dio3 transcript levels (Figure 5B), while SB F2 progeny had increased total expression of hippocampal Dio3 in the E group compared to both controls that was normalized in the E+T4 group (F(3,40)=5.25, p<0.05, d=0.58) (Figure 5C).
Figure 5. The increase in Dio3 transcript levels in the hippocampus of prenatal ethanol-exposed SS F1 female rats and their SB F2 progeny is normalized by administration of T4 in the ethanol containing liquid diet.
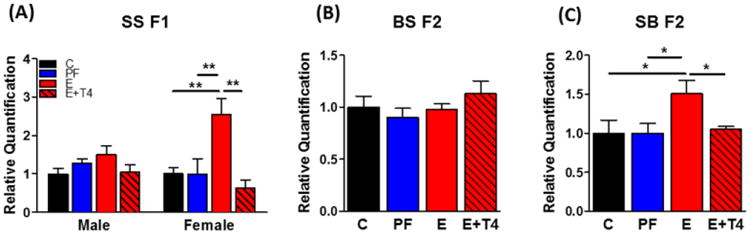
Dio3 transcript levels of A) SS F1 males and females, B) their BS F2 and C) SB F2 progeny (there were no sex differences, so male and female data were combined for the BS F2 and SB F2 progeny). Transcript levels were measured by quantitative real-time RT-PCR and normalized to Gapdh and a general calibrator using the 2−ΔΔCt method. Data presented as mean +/- SEM. N=6-13/group. *p<0.05, **p<0.01 by one-way ANOVA followed by Bonferroni post-hoc test.
The Dio3 gene is a partially imprinted gene. Allele-specific expression analyses by pyrosequencing revealed preferentially paternal Dio3 expression in the hippocampus of the BS F2 progeny with no significant effect of grandmaternal diets (Figure 6A). Hippocampus of the SB F2 offspring of SS F1 females also exhibited preferential paternal expression in all groups except the E group, which had an inverted imprinting pattern such that the maternal Dio3 allele predominated (F(3,35)=3.54, p<0.05, d=-0.34) (Figure 6B). Grandmaternal T4 administration during pregnancy concomitantly with E reversed this imprinting pattern.
Figure 6. Maternal prenatal ethanol exposure increases maternal expression of hippocampal Dio3 in the SB F2 progeny that is reversed by the presence of concomitant T4.
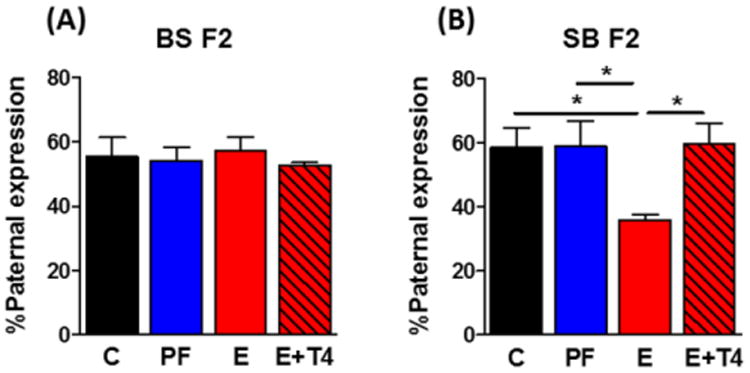
Allele-specific expression of Dio3 in the hippocampus of A) BS F2 and B) SB F2 progeny (there were no sex differences, so male and female data were combined). Data presented as mean +/- SEM. N=5-12/group. *p<0.05 by one-way ANOVA followed by Bonferroni post-hoc test.
Discussion
This study demonstrated for the first time that hippocampus-associated phenotypes that are affected by prenatal ethanol exposure are transferred to the next generation via matrilineal transmission. Furthermore, as T4 supplementation to the ethanol-consuming dam consistently reversed the affected phenotypes in the SS F1 E females, their offspring showed this normalization as well.
We focused on hippocampus-related phenotypes because this brain region is affected in individuals with FASD (Astley et al., 2009; Berman and Hannigan, 2000; Kodituwakku, 2007; Willoughby et al., 2008). Animals exposed to ethanol in utero exhibit specific alterations in hippocampal-dependent spatial learning and memory tests (Sutherland et al., 1997; Wilcoxon et al., 2005). Contextual fear conditioning is also hippocampus-dependent (Maren et al., 2013; Saxe et al., 2006), and it is known to be affected by PE (Weeber et al., 2001). In agreement with these findings, both male and female offspring of ethanol consuming dams showed deficit in fear memory as measured by decreased freezing and inversely increased activity in the fear-provoking context. Simultaneous administration of a low dose T4 to the ethanol-consuming dam did not reverse this deficit in the male SS F1 offspring, but restored fear memory in the PE-exposed SS F1 female to the level of controls. The sex difference in the T4 effect is not in agreement with the reversal of the elevated fT3 levels by maternal T4 administration in both males and females exposed to PE. The cause of this difference in T4 efficacy is not known, but similar sex difference has been observed in T4 efficacy previously (Rumbaugh et al., 1978).
The elevated fT3 levels in the SS F1 offspring of E dams were concomitant with decreased expression of hippocampal Nrgn and both were normalized by maternal T4 administration. Neurogranin (or RC3) is a neuron-specific protein that affects cognitive function and is thought to be directly regulated by T3 (Chaalal et al., 2014; Guadano-Ferraz et al., 1997; Huang et al., 2006). However, the decreased expression of hippocampal Nrgn in the light of increased plasma fT3 in response to PE suggests that the local thyroid hormone-milieu may differ from the peripheral one and/or it is affected by PE (Shukla et al., 2010; Sittig et al., 2011b). The local thyroid hormone availability is regulated by hippocampal Dio3, which metabolizes the biologically active T3 to the inactive T2. The PE-induced increase in the total expression of Dio3 likely results in local hypothyroidism, as T3 and Dio3 levels are closely and inversely related in the hippocampus (Sittig et al., 2011b). Should that be the case, local T3 levels would be independent of plasma fT3, similarly as we have shown it previously (Shukla et al., 2010). The increased hippocampal Dio3 expression, therefore, could result in decreased Nrgn levels in the same brain region. The only caveat in this explanation is that SS F1 E females showed a dramatically greater increase in their hippocampal Dio3 levels compared to their male counterparts, while their suppressed Nrgn expression was comparable. As Dio3 expression is known to be induced by estrogen, the female-specific exaggerated increase of Dio3 expression in the PE hippocampus is likely a synergistic effect between estrogen and the PE-induced elevated fT3 (Hernandez, 2005). Future studies could address this phenomenon.
The explanation for the decreased paternal allelic expression, but increased total Dio3 expression in the SB F2 hippocampus is a complex one. Dio3 expression is known to be positively regulated by thyroid hormones (Tu et al., 1999). However, the elevated total levels of hippocampal Dio3 in the SB F2 offspring of PE-exposed mothers and the reversal of this elevation by grandmaternal T4 administration is the opposite of the expected effect if it is due to thyroid hormone levels. One potential mechanism by which total Dio3 expression could increase while paternal contribution to Dio3 is decreased is increased methylation of the intergenic differentially methylated region (IGDMR) on the maternal allele. This increased methylation could produce results similar to that of maternal deletion of the IGDMR, namely increased Dio3 total expression (Lin et al., 2003). Alternatively, because total expression of Dio3 was significantly higher, while the paternal contribution was significantly lower in the SB F2 E hippocampus, the total Dio3 increase is very likely due to increased maternal expression. Since, in the control offspring, hippocampal expression of Dio3 is about 60% paternal, maternal expression is mostly silenced. Thus, the increased maternal expression in the SB F2 E hippocampus might be the result of a lack of silencing of maternal Dio3. We could only speculate that this lack of silencing of the maternal allele is an unorthodox imprinting mechanism. One mechanism by which this could occur is the reduced expression of non-coding RNAs, such as Rian and Mirg, in the maternal allele of the SB F2 E progeny (see Figure 7). A somewhat similar phenomenon has been described, where maternal transmission of the Gtl2LacZ insertion “paternalized” the maternal allele leading to an increase in the maternal expression of Dio3 (Charalambous et al., 2012).
Figure 7. Schematic representation of the ∼1.01 Mb imprinted Dlk1-Dio3 region on the rat chromosome 6:134-135 Mb.

Genes, thought to be expressed from the paternally allele (Dlk1, Rtl1 and Dio3), are marked by dark blue boxes and those expressed from the maternally allele (Rian and Mirg: C/D snoRNA and miRNA genes) by red boxes, respectively. Silent alleles are marked with light grey boxes. Differentially methylated regions are indicated by filled and open lollipops (methylated and unmethylated, respectively). The bigger lollipops indicate the location of intergenic differentially methylated region (IGDMR).
Most described phenotypes showed a transmission to the next generation via the matrilineal lineage. The lineage specificity of this transmission could be assigned to maternal genetic- or behavior-specific effects, or to a differential influence of ethanol on female and male germ cells of the SS F1 fetus. Genetic differences between the S and the B mothers could result in the behavioral and hippocampal gene expression differences between the SB F2 and the BS F2 crosses. We found clear behavioral differences between the SB and BS F1 offspring of SS and BB mothers, respectively (Sittig et al., 2011a). These differences could be the results of strain differences in maternal behavior independent of PE effects. Although we and others found no differences in the maternal behavior of the ethanol consuming dams compared to controls (Marino et al., 2002; Matta and Elberger, 2007; McMurray et al., 2008), the increased anxiety and depression-like behaviors (Hellemans et al., 2010; Wilcoxon et al., 2005) of the female PE-exposed offspring could affect their maternal behavior towards their F2 progeny (Meaney, 2001; O'Connor et al., 2002).
When the dam is exposed to ethanol (or other toxicant), both the F1 embryo and the F2 generation germ cells are also directly exposed. Therefore, disease phenotypes in the F1 and F2 generations might still be due to the toxicology of direct exposure to the environmental factor, in this case ethanol. The sex difference in transmission, the observed matrilineal transmission, can occur via a direct effect of E on the SS F1 oocyte leading to a deficit in fear memory in their offspring. This possibility is supported by human studies investigating whether the growth of the fetus of a non-smoking mother Influenced by the smoking of either grandmother while pregnant (Miller et al., 2014). They found, together with three other studies (Hypponen et al., 2003; Misra et al., 2005), that maternal, but not paternal, prenatal exposure to cigarette affects their child's birth characteristics.
The matrilineal transmission could also be the result of differential vulnerability of the male and female germline to E, the sex differences in the establishment of imprinting genes contributing to the phenotypes, or the sex differences in the schedule of reprogramming the germ cells. The first two possibilities need further exploration. Assessing the vulnerability of the F3 generation to ancestral maternal alcohol consumption could in part clarify the validity of these possibilities. However, regarding the sex differences in the schedule of reprogramming the germ cells during embryonic development and gonadal sex determination, genome-wide demethylation does not have a significant time difference between male and female gametes, but remethylation follows sex specific timelines (Reik et al., 2001). Additional information would help to determine if these schedule differences interact with ethanol exposure to lead to the matrilineal transmission.
Many adverse prenatal conditions lead to intergenerational transmission of specific deficits. This has been shown for humans (Chamorro-Garcia and Blumberg, 2014; Veenendaal et al., 2013) and animals (Constantinof et al., 2015; Iqbal et al., 2012). Whether maternal alcohol consumption is specific in its effect on the next generation cannot be ascertained at this date. The matrilineal nature of the intergenerational transmission we described here suggests some level of specificity for alcohol. The fact that the effect sizes for many significant measures decreased from the SS F1 E offspring to the SB F2 E progeny imply the attenuation of these deficits to the second generation. Should this be the case in humans, without further alcohol consumption by the daughters with FASD, these deficits would eventually wash out throughout the generations. However, the increased rate of alcoholism in FASD (Nizhnikov et al., 2016) makes the probability high of this intergenerational transmission to persist into subsequent generations.
In conclusion, the present study signifies the effect of maternal prenatal exposure to ethanol on the cognitive performance of their offspring. If the alterations induced by the maternal prenatal exposure are immediately corrected, such that seems to be the case by the concomitant administration of T4 to the ethanol consuming dam, the offspring of these PE-exposed mothers are cognitively rehabilitated. Should these intergenerational consequences of PE exposure be present in human FASD, such as was shown for nicotine exposure, they would have major socioeconomic and health consequences for the future generations.
Supplementary Material
Highlights.
Prenatal ethanol exposure affects fear memory of males and females
Deficits from maternal prenatal ethanol exposure are transmitted to progeny
Maternal T4 with ethanol lessens deficit in female offspring and their progeny
Acknowledgments
We thank Tim Ullman and Brian Andrus for their contributions to this study.
Funding for this research provided by NIH AA017978
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Astley SJ, Aylward EH, Olson HC, Kerns K, Brooks A, Coggins TE, Davies J, Dorn S, Gendler B, Jirikowic T, Kraegel P, Maravilla K, Richards T. Magnetic resonance imaging outcomes from a comprehensive magnetic resonance study of children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2009;33:1671–1689. doi: 10.1111/j.1530-0277.2009.01004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian TW, Anderson JA, Fretham SJ, Prohaska JR, Georgieff MK, Anderson GW. Fetal and neonatal iron deficiency reduces thyroid hormone-responsive gene mRNA levels in the neonatal rat hippocampus and cerebral cortex. Endocrinology. 2012;153:5668–5680. doi: 10.1210/en.2012-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RF, Hannigan JH. Effects of prenatal alcohol exposure on the hippocampus: spatial behavior, electrophysiology, and neuroanatomy. Hippocampus. 2000;10:94–110. doi: 10.1002/(SICI)1098-1063(2000)10:1<94::AID-HIPO11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Bernal J. Thyroid hormone receptors in brain development and function. Nat Clin Pract Endocrinol Metab. 2007;3:249–259. doi: 10.1038/ncpendmet0424. [DOI] [PubMed] [Google Scholar]
- Brasset E, Chambeyron S. Epigenetics and transgenerational inheritance. Genome Biol. 2013;14:306. doi: 10.1186/gb-2013-14-5-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaalal A, Poirier R, Blum D, Gillet B, Le Blanc P, Basquin M, Buee L, Laroche S, Enderlin V. PTU-induced hypothyroidism in rats leads to several early neuropathological signs of Alzheimer's disease in the hippocampus and spatial memory impairments. Hippocampus. 2014;24:1381–1393. doi: 10.1002/hipo.22319. [DOI] [PubMed] [Google Scholar]
- Chamorro-Garcia R, Blumberg B. Transgenerational effects of obesogens and the obesity epidemic. Curr Opin Pharmacol. 2014;19:153–158. doi: 10.1016/j.coph.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charalambous M, Ferron SR, da Rocha ST, Murray AJ, Rowland T, Ito M, Schuster-Gossler K, Hernandez A, Ferguson-Smith AC. Imprinted gene dosage is critical for the transition to independent life. Cell Metab. 2012;15:209–221. doi: 10.1016/j.cmet.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinof A, Moisiadis VG, Matthews SG. Programming of stress pathways: A transgenerational perspective. J Steroid Biochem Mol Biol. 2015 doi: 10.1016/j.jsbmb.2015.10.008. [DOI] [PubMed] [Google Scholar]
- Desouza LA, Ladiwala U, Daniel SM, Agashe S, Vaidya RA, Vaidya VA. Thyroid hormone regulates hippocampal neurogenesis in the adult rat brain. Mol Cell Neurosci. 2005;29:414–426. doi: 10.1016/j.mcn.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Downing C, Johnson TE, Larson C, Leakey TI, Siegfried RN, Rafferty TM, Cooney CA. Subtle decreases in DNA methylation and gene expression at the mouse Igf2 locus following prenatal alcohol exposure: effects of a methyl-supplemented diet. Alcohol. 2011;45:65–71. doi: 10.1016/j.alcohol.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek J, Skocic J, Sheard E, Rovet J. Hippocampal abnormalities in youth with alcohol-related neurodevelopmental disorder. J Int Neuropsychol Soc. 2014;20:181–191. doi: 10.1017/S1355617713001343. [DOI] [PubMed] [Google Scholar]
- Gereben B, Zeold A, Dentice M, Salvatore D, Bianco AC. Activation and inactivation of thyroid hormone by deiodinases: local action with general consequences. Cell Mol Life Sci. 2008;65:570–590. doi: 10.1007/s00018-007-7396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Mohapel J, Boehme F, Kainer L, Christie BR. Hippocampal cell loss and neurogenesis after fetal alcohol exposure: insights from different rodent models. Brain Res Rev. 2010;64:283–303. doi: 10.1016/j.brainresrev.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Gil-Mohapel J, Boehme F, Patten A, Cox A, Kainer L, Giles E, Brocardo PS, Christie BR. Altered adult hippocampal neuronal maturation in a rat model of fetal alcohol syndrome. Brain Res. 2011;1384:29–41. doi: 10.1016/j.brainres.2011.01.116. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. Developmental origins of disease paradigm: a mechanistic and evolutionary perspective. Pediatr Res. 2004;56:311–317. doi: 10.1203/01.PDR.0000135998.08025.FB. [DOI] [PubMed] [Google Scholar]
- Gottesfeld Z, Silverman PB. Developmental delays associated with prenatal alcohol exposure are reversed by thyroid hormone treatment. Neurosci Lett. 1990;109:42–47. doi: 10.1016/0304-3940(90)90535-h. [DOI] [PubMed] [Google Scholar]
- Govorko D, Bekdash RA, Zhang C, Sarkar DK. Male germline transmits fetal alcohol adverse effect on hypothalamic proopiomelanocortin gene across generations. Biol Psychiatry. 2012;72:378–388. doi: 10.1016/j.biopsych.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadano-Ferraz A, Escamez MJ, Morte B, Vargiu P, Bernal J. Transcriptional induction of RC3/neurogranin by thyroid hormone: differential neuronal sensitivity is not correlated with thyroid hormone receptor distribution in the brain. Brain Res Mol Brain Res. 1997;49:37–44. doi: 10.1016/s0169-328x(97)00119-8. [DOI] [PubMed] [Google Scholar]
- Guerri C, Bazinet A, Riley EP. Foetal Alcohol Spectrum Disorders and alterations in brain and behaviour. Alcohol Alcohol. 2009;44:108–114. doi: 10.1093/alcalc/agn105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, O'Heir CE, Mitchell ML, Hermos RJ, Waisbren SE, Faix JD, Klein RZ. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341:549–555. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- Harper KM, Tunc-Ozcan E, Graf EN, Herzing LB, Redei EE. Intergenerational and parent of origin effects of maternal calorie restriction on Igf2 expression in the adult rat hippocampus. Psychoneuroendocrinology. 2014a;45:187–191. doi: 10.1016/j.psyneuen.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper KM, Tunc-Ozcan E, Graf EN, Redei EE. Intergenerational effects of prenatal ethanol on glucose tolerance and insulin response. Physiol Genomics. 2014b;46:159–168. doi: 10.1152/physiolgenomics.00181.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycock PC. Fetal alcohol spectrum disorders: the epigenetic perspective. Biol Reprod. 2009;81:607–617. doi: 10.1095/biolreprod.108.074690. [DOI] [PubMed] [Google Scholar]
- Heindel JJ, Zoeller RT. Thyroid hormone and brain development: translating molecular mechanisms to population risk. Thyroid. 2003;13:1001–1004. doi: 10.1089/105072503770867165. [DOI] [PubMed] [Google Scholar]
- Hellemans KG, Sliwowska JH, Verma P, Weinberg J. Prenatal alcohol exposure: fetal programming and later life vulnerability to stress, depression and anxiety disorders. Neurosci Biobehav Rev. 2010;34:791–807. doi: 10.1016/j.neubiorev.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbstman J, Apelberg BJ, Witter FR, Panny S, Goldman LR. Maternal, infant, and delivery factors associated with neonatal thyroid hormone status. Thyroid. 2008;18:67–76. doi: 10.1089/thy.2007.0180. [DOI] [PubMed] [Google Scholar]
- Hernandez A. Structure and function of the type 3 deiodinase gene. Thyroid. 2005;15:865–874. doi: 10.1089/thy.2005.15.865. [DOI] [PubMed] [Google Scholar]
- Hernandez JT, Hoffman L, Weavil S, Cvejin S, Prange AJ., Jr The effect of drug exposure on thyroid hormone levels of newborns. Biochem Med Metab Biol. 1992;48:255–262. doi: 10.1016/0885-4505(92)90072-7. [DOI] [PubMed] [Google Scholar]
- Huang FL, Huang KP, Wu J, Boucheron C. Environmental enrichment enhances neurogranin expression and hippocampal learning and memory but fails to rescue the impairments of neurogranin null mutant mice. J Neurosci. 2006;26:6230–6237. doi: 10.1523/JNEUROSCI.1182-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hypponen E, Smith GD, Power C. Effects of grandmothers' smoking in pregnancy on birth weight: intergenerational cohort study. BMJ. 2003;327:898. doi: 10.1136/bmj.327.7420.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal M, Moisiadis VG, Kostaki A, Matthews SG. Transgenerational effects of prenatal synthetic glucocorticoids on hypothalamic-pituitary-adrenal function. Endocrinology. 2012;153:3295–3307. doi: 10.1210/en.2012-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS, DeCola JP, Landeira-Fernandez J. Selective impairment of long-term but not short-term conditional fear by the N-methyl-D-aspartate antagonist APV. Behav Neurosci. 1992;106:591–596. doi: 10.1037//0735-7044.106.4.591. [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW. Defining the behavioral phenotype in children with fetal alcohol spectrum disorders: a review. Neurosci Biobehav Rev. 2007;31:192–201. doi: 10.1016/j.neubiorev.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Lin SP, Youngson N, Takada S, Seitz H, Reik W, Paulsen M, Cavaille J, Ferguson-Smith AC. Asymmetric regulation of imprinting on the maternal and paternal chromosomes at the Dlk1-Gtl2 imprinted cluster on mouse chromosome 12. Nat Genet. 2003;35:97–102. doi: 10.1038/ng1233. [DOI] [PubMed] [Google Scholar]
- Manning MA, Eugene Hoyme H. Fetal alcohol spectrum disorders: a practical clinical approach to diagnosis. Neurosci Biobehav Rev. 2007;31:230–238. doi: 10.1016/j.neubiorev.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Maren S, Anagnostaras SG, Fanselow MS. The startled seahorse: is the hippocampus necessary for contextual fear conditioning? Trends Cogn Sci. 1998;2:39–42. doi: 10.1016/s1364-6613(98)01123-1. [DOI] [PubMed] [Google Scholar]
- Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci. 2013;14:417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino MD, Cronise K, Lugo JN, Jr, Kelly SJ. Ultrasonic vocalizations and maternal-infant interactions in a rat model of fetal alcohol syndrome. Developmental psychobiology. 2002;41:341–351. doi: 10.1002/dev.10077. [DOI] [PubMed] [Google Scholar]
- Matta SG, Elberger AJ. Combined exposure to nicotine and ethanol throughout full gestation results in enhanced acquisition of nicotine self-administration in young adult rat offspring. Psychopharmacology (Berl) 2007;193:199–213. doi: 10.1007/s00213-007-0767-2. [DOI] [PubMed] [Google Scholar]
- May PA, Baete A, Russo J, Elliott AJ, Blankenship J, Kalberg WO, Buckley D, Brooks M, Hasken J, Abdul-Rahman O, Adam MP, Robinson LK, Manning M, Hoyme HE. Prevalence and characteristics of fetal alcohol spectrum disorders. Pediatrics. 2014;134:855–866. doi: 10.1542/peds.2013-3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray MS, Williams SK, Jarrett TM, Cox ET, Fay EE, Overstreet DH, Walker CH, Johns JM. Gestational ethanol and nicotine exposure: effects on maternal behavior, oxytocin, and offspring ethanol intake in the rat. Neurotoxicol Teratol. 2008;30:475–486. doi: 10.1016/j.ntt.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead E, Sarkar D. Fetal Alcohol Spectrum Disorders and their Transmission through Genetic and Epigenetic Mechanisms. Front Genet. 2014;5:154. doi: 10.3389/fgene.2014.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Miller LL, Pembrey M, Davey Smith G, Northstone K, Golding J. Is the growth of the fetus of a non-smoking mother influenced by the smoking of either grandmother while pregnant? PLoS One. 2014;9:e86781. doi: 10.1371/journal.pone.0086781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra DP, Astone N, Lynch CD. Maternal smoking and birth weight: interaction with parity and mother's own in utero exposure to smoking. Epidemiology. 2005;16:288–293. doi: 10.1097/01.ede.0000158198.59544.cf. [DOI] [PubMed] [Google Scholar]
- Miyakawa T, Yared E, Pak JH, Huang FL, Huang KP, Crawley JN. Neurogranin null mutant mice display performance deficits on spatial learning tasks with anxiety related components. Hippocampus. 2001;11:763–775. doi: 10.1002/hipo.1092. [DOI] [PubMed] [Google Scholar]
- Nizhnikov ME, Popoola DO, Cameron NM. Transgenerational Transmission of the Effect of Gestational Ethanol Exposure on Ethanol Use-Related Behavior. Alcohol Clin Exp Res. 2016;40:497–506. doi: 10.1111/acer.12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor TG, Heron J, Golding J, Beveridge M, Glover V. Maternal antenatal anxiety and children's behavioural/emotional problems at 4 years. Report from the Avon Longitudinal Study of Parents and Children. Br J Psychiatry. 2002;180:502–508. doi: 10.1192/bjp.180.6.502. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Ramsay M. Genetic and epigenetic insights into fetal alcohol spectrum disorders. Genome Med. 2010;2:27. doi: 10.1186/gm148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science. 2001;293:1089–1093. doi: 10.1126/science.1063443. [DOI] [PubMed] [Google Scholar]
- Rumbaugh RC, Kramer RE, Colby HD. Dose-dependent actions of thyroxine on hepatic drug metabolism in male and female rats. Biochem Pharmacol. 1978;27:2027–2031. doi: 10.1016/0006-2952(78)90062-x. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott HC, Sun GY, Zoeller RT. Prenatal ethanol exposure selectively reduces the mRNA encoding alpha-1 thyroid hormone receptor in fetal rat brain. Alcohol Clin Exp Res. 1998;22:2111–2117. [PubMed] [Google Scholar]
- Shukla PK, Sittig LJ, Andrus BM, Schaffer DJ, Batra KK, Redei EE. Prenatal thyroxine treatment disparately affects peripheral and amygdala thyroid hormone levels. Psychoneuroendocrinology. 2010;35:791–797. doi: 10.1016/j.psyneuen.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittig LJ, Herzing LB, Shukla PK, Redei EE. Parent-of-origin allelic contributions to deiodinase-3 expression elicit localized hyperthyroid milieu in the hippocampus. Mol Psychiatry. 2011a;16:786–787. doi: 10.1038/mp.2011.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittig LJ, Redei EE. Paternal genetic contribution influences fetal vulnerability to maternal alcohol consumption in a rat model of fetal alcohol spectrum disorder. PLoS One. 2010;5:e10058. doi: 10.1371/journal.pone.0010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittig LJ, Shukla PK, Herzing LB, Redei EE. Strain-specific vulnerability to alcohol exposure in utero via hippocampal parent-of-origin expression of deiodinase-III. FASEB J. 2011b;25:2313–2324. doi: 10.1096/fj.10-179234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland RJ, McDonald RJ, Savage DD. Prenatal exposure to moderate levels of ethanol can have long-lasting effects on hippocampal synaptic plasticity in adult offspring. Hippocampus. 1997;7:232–238. doi: 10.1002/(SICI)1098-1063(1997)7:2<232::AID-HIPO9>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Breier M, Mora AB, Ko D, Shoemaker JM. A novel rat strain with enhanced sensitivity to the effects of dopamine agonists on startle gating. Pharmacol Biochem Behav. 2008;88:280–290. doi: 10.1016/j.pbb.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PN, Okosieme OE, Murphy R, Hales C, Chiusano E, Maina A, Joomun M, Bestwick JP, Smyth P, Paradice R, Channon S, Braverman LE, Dayan CM, Lazarus JH, Pearce EN. Maternal perchlorate levels in women with borderline thyroid function during pregnancy and the cognitive development of their offspring: data from the Controlled Antenatal Thyroid Study. J Clin Endocrinol Metab. 2014;99:4291–4298. doi: 10.1210/jc.2014-1901. [DOI] [PubMed] [Google Scholar]
- Tu HM, Legradi G, Bartha T, Salvatore D, Lechan RM, Larsen PR. Regional expression of the type 3 iodothyronine deiodinase messenger ribonucleic acid in the rat central nervous system and its regulation by thyroid hormone. Endocrinology. 1999;140:784–790. doi: 10.1210/endo.140.2.6486. [DOI] [PubMed] [Google Scholar]
- Tunc-Ozcan E, Sittig LJ, Harper KM, Graf EN, Redei EE. Hypothesis: genetic and epigenetic risk factors interact to modulate vulnerability and resilience to FASD. Front Genet. 2014;5:261. doi: 10.3389/fgene.2014.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunc-Ozcan E, Ullmann TM, Shukla PK, Redei EE. Low-dose thyroxine attenuates autism-associated adverse effects of fetal alcohol in male offspring's social behavior and hippocampal gene expression. Alcohol Clin Exp Res. 2013;37:1986–1995. doi: 10.1111/acer.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenendaal MV, Painter RC, de Rooij SR, Bossuyt PM, van der Post JA, Gluckman PD, Hanson MA, Roseboom TJ. Transgenerational effects of prenatal exposure to the 1944-45 Dutch famine. BJOG. 2013;120:548–553. doi: 10.1111/1471-0528.12136. [DOI] [PubMed] [Google Scholar]
- Weeber EJ, Savage DD, Sutherland RJ, Caldwell KK. Fear conditioning-induced alterations of phospholipase C-beta1a protein level and enzyme activity in rat hippocampal formation and medial frontal cortex. Neurobiol Learn Mem. 2001;76:151–182. doi: 10.1006/nlme.2000.3994. [DOI] [PubMed] [Google Scholar]
- Wilcoxon JS, Kuo AG, Disterhoft JF, Redei EE. Behavioral deficits associated with fetal alcohol exposure are reversed by prenatal thyroid hormone treatment: a role for maternal thyroid hormone deficiency in FAE. Mol Psychiatry. 2005;10:961–971. doi: 10.1038/sj.mp.4001694. [DOI] [PubMed] [Google Scholar]
- Wilcoxon JS, Nadolski GJ, Samarut J, Chassande O, Redei EE. Behavioral inhibition and impaired spatial learning and memory in hypothyroid mice lacking thyroid hormone receptor alpha. Behav Brain Res. 2007;177:109–116. doi: 10.1016/j.bbr.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcoxon JS, Redei EE. Prenatal programming of adult thyroid function by alcohol and thyroid hormones. Am J Physiol Endocrinol Metab. 2004;287:E318–326. doi: 10.1152/ajpendo.00022.2004. [DOI] [PubMed] [Google Scholar]
- Willoughby KA, Sheard ED, Nash K, Rovet J. Effects of prenatal alcohol exposure on hippocampal volume, verbal learning, and verbal and spatial recall in late childhood. J Int Neuropsychol Soc. 2008;14:1022–1033. doi: 10.1017/S1355617708081368. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Rovet J. Timing of thyroid hormone action in the developing brain: clinical observations and experimental findings. J Neuroendocrinol. 2004;16:809–818. doi: 10.1111/j.1365-2826.2004.01243.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


