Abstract
Background
The Johns Hopkins Hospital Emergency Department (JHHED) has served as an observational window on the HIV epidemic in a socioeconomically depressed, urban population. We previously reported that HIV incidence among JHHED patients is decreasing and that prevalence has declined from 11.4% in 2003 to 5.6% in 2013.
Objectives
This study sought to observe temporal trends in hepatitis C virus (HCV) and herpes simplex virus type 2 (HSV-2) seroprevalence, which are surrogate markers for parenteral and sexual risk behavior, respectively.
Study Design
Identity unlinked-serosurveys were conducted over 6–8 weeks in the adult JHHED in 2003, 2007, and 2013. Excess sera from 10,274 patients, previously tested for HIV, were assayed for HSV-2 and HCV antibodies.
Results
Overall HCV seroprevalence declined steadily from 22.0% in 2003 to 13.8% in 2013 (Ptrend<0.01), and was significant by all gender and race strata. Overall HSV-2 prevalence declined from 55.3% in 2003 to 50.0% in 2013 (Ptrend<0.01), but was non-significant after adjustment for demographics. Among HIV+ individuals <45 years of age, there was a significant decrease in the proportion of individuals with HCV co-infection [without HSV-2] (Ptrend=0.02) from 2003 to 2013, however, there was an increase in individuals with HSV-2 co-infection [without HCV] (Ptrend<0.01).
Discussion
Little change in age-specific HSV-2 prevalence suggests the decrease in HIV prevalence was likely not associated with changes in sexual risk behavior. In addition to clinical interventions, strategies to address sexual health disparities and continued parenteral harm-reduction efforts are needed to further drive the decline in HIV.
Keywords: HIV, hepatitis C, HCV, herpes, HSV-2, emergency department
BACKGROUND
Urban emergency departments (EDs) serve as primary health care facilities for millions of underserved populations [1], providing a window into the state of public health within the local community. Pioneered by the Johns Hopkins Hospital Emergency Department (JHHED) in Baltimore City, ED-based identity-unlinked serosurveys have provided sentinel observations regarding HIV epidemiology since the late 1980s [2–10]. Most recently, the JHHED noted a two-fold reduction in HIV prevalence from 11.4% in 2003 to 5.6% in 2013 [9]. Although increases in HIV viral suppression during this period correlated with observed declines in HIV incidence estimates [9], changes in sexual or parenteral risk behavior may have also contributed to this waning epidemic.
Herpes simplex virus type 2 (HSV-2) infection is a surrogate marker for risky lifetime sexual behavior and has a biologic role in sexual HIV transmission [11–14]. Urban settings have higher levels of HSV-2 seroprevalence compared to the national prevalence (15.7%) [15–18]. HIV infection is also spread by injection drug use, and an excellent marker of parenteral risk behavior is hepatitis C virus (HCV) infection [19]. There is interest in expanding ED-based HIV screening and linkage-to-care (LTC) programs to now include HCV screening, for which curative therapy now exists [20–27].
OBJECTIVES
This study assessed trends in HIV, HCV and HSV-2 seroepidemiology among patients in the JHHED to: (1) characterize the local burden of disease, and (2) explore potential changes in parenteral and sexual risk behavior during a waning HIV epidemic.
STUDY DESIGN
Study Population
An identity-unlinked serosurvey was conducted during a 6 to 8-week period in 2003, 2007 and 2013 at the adult JHHED in Baltimore, Maryland. The protocol has been described elsewhere [2–4, 8, 9]. Briefly, eligible patients were ≥ 18 years of age, required blood drawn for a medical reason, and had matched chart review data recorded in real-time. Excess sera were collected and assigned a unique study ID—individuals with repeat ED visits were excluded. All patient identifiers were irretrievably stripped from the study database prior to initiating laboratory testing. This study was approved by the Johns Hopkins School of Medicine Institutional Review Board (IRB).
Laboratory Testing
HIV and HSV-2 testing was performed as previously described [8, 9, 17]. HCV serostatus was determined by the GENEDIA HCV 3.0 ELISA (Green Cross Medical) in 2003 & 2013, and the Ortho HCV 3.0 ELISA (Ortho Clinical Diagnostics) in 2007 [29].
Statistical Analyses
Temporal trends by study year were examined using the Cochran-Armitage test. Prevalence ratios (PR) for seropositivity were determined by Poisson regression with robust variance estimation. Adjusted prevalence ratios (aPRs) were calculated from multivariate models including age, gender, race, and co-infection status.
RESULTS
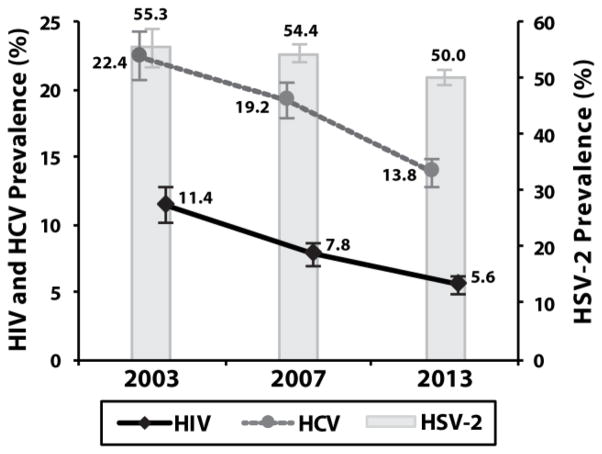
The study included 10,274 individuals (Table 1). There were unadjusted declines in total HIV, HCV, and HSV-2 prevalence by study year (Ptrend<0.05; Figure 1). After stratification by race and gender, the temporal age-adjusted decline in HIV prevalence was only significant among black males and black females (Ptrend<0.01; Table S1), while age-adjusted temporal declines in HCV prevalence were observed for all race-gender strata (Ptrend<0.05; Table S1). There were no age-adjusted temporal declines in HSV-2 prevalence for any race-gender strata (Table S1).
Table 1.
Study demographics.
| Characteristic | Study Year, %
|
||
|---|---|---|---|
| 2003 | 2007 | 2013 | |
| Gender | |||
| Female | 54.5 | 54.0 | 55.0 |
| Male | 45.5 | 46.0 | 45.0 |
| Race | |||
| White | 26.4 | 26.9 | 29.4 |
| Black | 69.0 | 67.3 | 63.1 |
| Other | 4.6 | 5.9 | 7.6 |
| Age | |||
| 18–24 | 9.8 | 10.5 | 11.3 |
| 25–34 | 14.0 | 16.7 | 19.4 |
| 35–44 | 22.2 | 20.1 | 15.3 |
| 45–54 | 22.3 | 23.2 | 21.6 |
| ≥55 | 31.7 | 28.8 | 32.3 |
|
| |||
| Total, no. | 2,144 | 3,417 | 4,713 |
Note: 5 individuals had missing age data and 4 had missing gender data.
Figure 1. HIV, HCV and HSV-2 seroprevalence in the overall study population.
Error bars represent 95% confidence intervals.
Note: 20 individuals had missing HCV data and 23 had missing HSV-2 data.
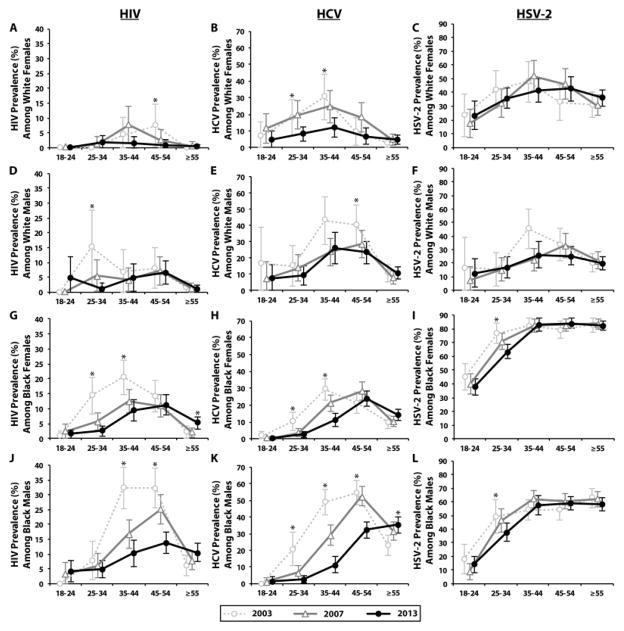
There were differential reductions in prevalence estimates between 2003 and 2013 among various age-groups (Figure 2). For white females, HIV and HSV-2 prevalence did not significantly decline among any age group, however, a 58% reduction in HCV prevalence was observed among the 25–44 age group (PR=0.42; 95%CI=0.26–0.69; Figure 2A,B). For white males, there was a 93% reduction in HIV prevalence among the 25–34 age-group (PR=0.07; 95%CI=0.01–0.58; Figure 2D), a 41% reduction in HCV prevalence among the 35–54 age group (PR=0.59; 95%CI=0.43–0.81; Figure 2E), and a 42% reduction in HSV-2 prevalence among the 35–44 age group (PR=0.58; 95%CI=0.35–0.94; Figure 2F).
Figure 2. Temporal trends in age-specific HIV, HCV and HSV-2 seroprevalence by race and gender.
Error bars represent 95% confidence intervals (excluded if prevalence was 0%).
* Denotes a significant linear trend from 2003 to 2013 (P<0.05).
For black females among the 25–44 age group, there was a 69% and 71% decline in HIV prevalence (PR=0.31; 95%CI=0.21–0.46; Figure 2G) and HCV prevalence (PR=0.29; 95%CI=0.20–0.42; Figure 2H), respectively. There was also a 17% decrease in HSV-2 prevalence among the 25–34 age group of black females (PR=0.83; 95%CI=0.73–0.94; Figure 2I). For black males among the 35–54 age group, there was a 61% and 52% decline in HIV prevalence (PR=0.39; 95%CI=0.30–0.51; Figure 2E) and HCV prevalence (PR=0.48; 95%CI=0.41–0.58; Figure 2K), respectively. There was also a 39% decline in HCV prevalence among the 25–34 age group of black males (PR=0.61; 95%CI=0.53–0.70; Figure 2K).
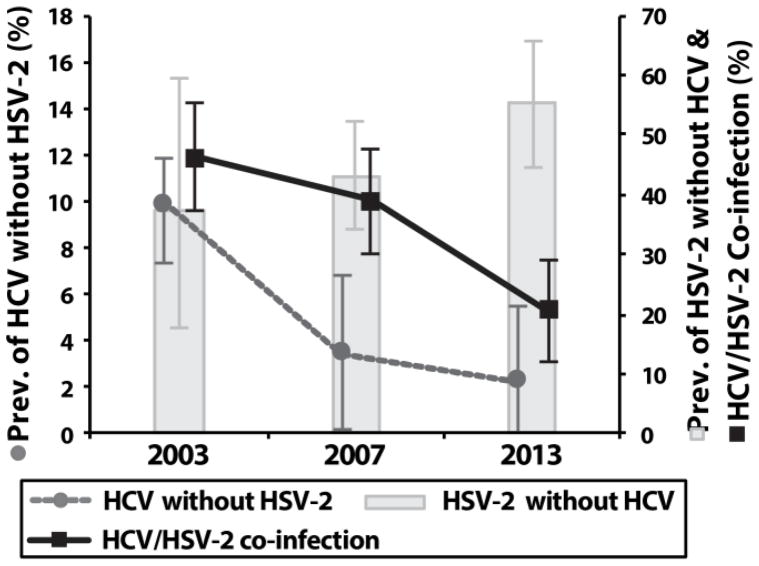
Between 2003 and 2013, there was a 21% reduction in HCV prevalence (aPR=0.79; 95%CI=0.67–0.93) and no change in HSV-2 prevalence among HIV positive individuals (Table 3). Among HIV positive individuals <45 years of age, there were decreasing temporal trends in HCV/HSV-2 co-infection and HCV infection [without HSV-2] (Ptrend<0.05), however, there was an increase in HSV-2 infection [without HCV] (Ptrend<0.05; Figure 3). These temporal trends remained significant after adjustment for demographics. In 2013, HCV infection [without HSV-2] was associated with HIV infection among individuals >45 years of age (aPR=8.49; 95%CI=4.12–17.56), but not among individuals <45 years of age (aPR=1.99; 95%CI=0.48–8.21; Table S2).
Figure 3. Temporal trends in HCV infection, HSV-2 infection, and HCV/HSV-2 co-infection among HIV-positive individuals <45 years of age.
Error bars represent 95% confidence intervals
Figure S1 illustrates a higher and earlier peak prevalence of HSV-2 among HIV positive individuals compared to HIV negative individuals.
DISCUSSION
This study characterizes HIV, HCV and HSV-2 seroepidemiology during the evolution of a waning HIV epidemic among an inner-city population. Reductions in HCV prevalence were observed in this population, and were most pronounced among black men who were most at-risk for HIV infection. The reduction in HIV/HCV co-infection [without HSV-2] among adults <45 years of age suggests a decrease in parenteral risk behavior, and this hypothesis is supported by local studies of people who inject drugs and the scale-up of harm-reduction efforts [29–33]. However, an aging-cohort effect was also observed for HIV and HCV infection, with notable declines among older black populations, suggestive of an increase in mortality [34, 35]. Nonetheless, the continued high prevalence of HIV and HCV among patients attending the JHHED supports the utility of ED-based screening and LTC programming.
Additionally, these results highlight the sexual health disparities of this population. The black population and HIV-infected persons were disproportionately infected with HSV-2, and minimal temporal declines were observed. Most notable was the high prevalence of HSV-2 among black women, which consistently reached >80% by age 35. The rise in HIV/HSV-2 co-infection [without HCV infection] among individuals <45 years and early age of HSV-2 acquisition in this study is concerning. These data emphasize the need for early and effective control programs for sexually transmitted diseases among inner-city minorities and young adults.
There were limitations to this study. Changes in ED attendance by key populations may have affected our results. Patients were not systematically interviewed for risk behavior, and temporal inferences were solely based on biomarker data that require additional confirmation. Furthermore, the study was limited to a single-site, and results may not be generalizable to other populations. However, the temporal trends observed in this report are compatible with previous serological studies [13, 16, 18, 36–38].
In addition to observed increases in patients on antiretroviral therapy in this ED population [9], reductions in parenteral risk may have contributed to the waning HIV epidemic and prevalence in HCV in this population. Given the biologic synergism of HIV/HSV-2 co-infection and minimal changes in age-specific HSV-2 prevalence, the observed decline in HIV incidence was likely not associated with changes in sexual risk behavior. In order to further drive the decline of HIV, strategies to reduce sexual risk behavior and continued parenteral risk-reduction efforts are needed to supplement therapeutic interventions.
Supplementary Material
Table 2.
Reduction in HCV prevalence but not HSV-2 prevalence among HIV positive individuals.
| Study Year | HCV Co-infection
|
HSV-2 Coinfection
|
||
|---|---|---|---|---|
| N (% Pos.) | aPR (95% CI) | N (% Pos.) | aPR (95% CI) | |
| 2003 | 228 (59.6) | (ref) | 228 (79.8) | (ref) |
| 2007 | 265 (53.6) | 0.89 (0.77–1.04) | 265 (79.6) | 1.02 (0.94–1.11) |
| 2013 | 262 (48.1) | 0.79 (0.67–0.93) | 258 (78.3) | 1.01 (0.92–1.10) |
Adjusted prevalence ratios (aPR) were calculated from a multivariate model that included HCV/HSV-2 co-infection, age group, gender, and race. Prevalence in 2003 was the reference group, and aPRs with a P<0.05 are shown in bold. N represents the number of individuals analyzed.
Highlights.
Emergency departments can serve as key venues for HIV, HCV, & HSV-2 surveillance.
There were minimal declines in age-specific HSV-2 prevalence, unlike for HIV & HCV.
Prevalence of HIV/HCV co-infection [without HSV-2] decreased in persons <45 years.
Prevalence of HIV/HSV-2 co-infection [without HCV] increased in persons <45 years.
Gender and racial disparities were persistent throughout the study period.
Acknowledgments
Funding:
This work was supported by the Division of Intramural Research and extramural support [K01AI100681] from the National Institute of Allergy and Infectious Diseases.
Footnotes
Presentation:
This work was presented in-part at the 2015 World STI & HIV Congress of the International Society for Sexually Transmitted Diseases in Brisbane, Australia.
CONFLICT OF INTEREST
Competing Interests:
The authors declare no conflicts of interest.
Ethical Approval:
The study was approved by the Johns Hopkins School of Medicine IRB.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.CDC/National Center for Health Statistics. [Accessed 24 Feb 2016];National Hospital Ambulatory Medical Care Survey: 2011 Emergency Department Summary Tables. Available at: http://www.cdc.gov/nchs/fastats/emergency-department.htm.
- 2.Kelen GD, Fritz S, Qaqish B, et al. Unrecognized human immunodeficiency virus infection in emergency department patients. The New England journal of medicine. 1988;318:1645–50. doi: 10.1056/NEJM198806233182503. [DOI] [PubMed] [Google Scholar]
- 3.Kelen GD, DiGiovanna T, Bisson L, Kalainov D, Sivertson KT, Quinn TC. Human immunodeficiency virus infection in emergency department patients. Epidemiology, clinical presentations, and risk to health care workers: the Johns Hopkins experience JAMA : the journal of the American Medical Association. 1989;262:516–22. doi: 10.1001/jama.262.4.516. [DOI] [PubMed] [Google Scholar]
- 4.Kelen GD, Hexter DA, Hansen KN, Tang N, Pretorius S, Quinn TC. Trends in human immunodeficiency virus (HIV) infection among a patient population of an inner-city emergency department: implications for emergency department-based screening programs for HIV infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 1995;21:867–75. doi: 10.1093/clinids/21.4.867. [DOI] [PubMed] [Google Scholar]
- 5.Goggin MA, Davidson AJ, Cantril SV, O’Keefe LK, Douglas JM. The extent of undiagnosed HIV infection among emergency department patients: results of a blinded seroprevalence survey and a pilot HIV testing program. The Journal of emergency medicine. 2000;19:13–9. doi: 10.1016/s0736-4679(00)00175-x. [DOI] [PubMed] [Google Scholar]
- 6.Hall MR, Ray D, Payne JA. Prevalence of hepatitis C, hepatitis B, and human immunodeficiency virus in a Grand Rapids, Michigan emergency department. The Journal of emergency medicine. 2010;38:401–5. doi: 10.1016/j.jemermed.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 7.Mohammed DY, Martin E, Sadashige C, Jaker M, Paul S. An anonymous unlinked sero-prevalence survey of HIV/HCV in an urban Emergency Department. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2013;58(Suppl 1):e19–23. doi: 10.1016/j.jcv.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 8.Hsieh Y-H, Kelen GD, Laeyendecker O, Kraus CK, Quinn TC, Rothman RE. HIV care continuum for HIV-infected emergency department patients in an inner-city academic emergency department. Annals of emergency medicine. 2015;66:69–78. doi: 10.1016/j.annemergmed.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelen GD, Hsieh YH, Rothman RE, et al. Improvements in the continuum of HIV care in an inner-city emergency department. AIDS (London, England) 2016;30:113–20. doi: 10.1097/QAD.0000000000000896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oster AM. The evolving contribution of emergency department testing studies: from risk to care. AIDS (London, England) 2016;30:151–2. doi: 10.1097/QAD.0000000000000883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tobian AA, Quinn TC. Herpes simplex virus type 2 and syphilis infections with HIV: an evolving synergy in transmission and prevention. Current opinion in HIV and AIDS. 2009;4:294–9. doi: 10.1097/COH.0b013e32832c1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Behling J, Chan AK, Zeh C, Nekesa C, Heinzerling L. Evaluating HIV Prevention Programs: Herpes Simplex Virus Type 2 Antibodies as Biomarker for Sexual Risk Behavior in Young Adults in Resource-Poor Countries. PloS one. 2015;10:e0128370. doi: 10.1371/journal.pone.0128370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Des Jarlais DC, Arasteh K, McKnight C, et al. HSV–2 co-infection as a driver of HIV transmission among heterosexual non-injecting drug users in New York City. PloS one. 2014;9:e87993. doi: 10.1371/journal.pone.0087993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith JS, Robinson NJ. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. The Journal of infectious diseases. 2002;186(Suppl 1):S3–28. doi: 10.1086/343739. [DOI] [PubMed] [Google Scholar]
- 15.Bradley H, Markowitz LE, Gibson T, McQuillan GM. Seroprevalence of herpes simplex virus types 1 and 2--United States, 1999–2010. The Journal of infectious diseases. 2014;209:325–33. doi: 10.1093/infdis/jit458. [DOI] [PubMed] [Google Scholar]
- 16.Oster AM, Sternberg M, Nebenzahl S, et al. Prevalence of HIV, sexually transmitted infections, and viral hepatitis by Urbanicity, among men who have sex with men, injection drug users, and heterosexuals in the United States. Sexually transmitted diseases. 2014;41:272–9. doi: 10.1097/OLQ.0000000000000110. [DOI] [PubMed] [Google Scholar]
- 17.Patel EU, Frank MA, Hsieh YH, et al. Prevalence and factors associated with herpes simplex virus type 2 infection in patients attending a Baltimore City emergency department. PloS one. 2014;9:e102422. doi: 10.1371/journal.pone.0102422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schillinger JA, McKinney CM, Garg R, et al. Seroprevalence of herpes simplex virus type 2 and characteristics associated with undiagnosed infection: New York City, 2004. Sexually transmitted diseases. 2008;35:599–606. doi: 10.1097/OLQ.0b013e3181666fb1. [DOI] [PubMed] [Google Scholar]
- 19.Vickerman P, Hickman M, May M, Kretzschmar M, Wiessing L. Can hepatitis C virus prevalence be used as a measure of injection-related human immunodeficiency virus risk in populations of injecting drug users? An ecological analysis. Addiction. 2010;105:311–8. doi: 10.1111/j.1360-0443.2009.02759.x. [DOI] [PubMed] [Google Scholar]
- 20.Allison WE, Chiang W, Rubin A, et al. Hepatitis C virus infection in the 1945–1965 birth cohort (baby boomers) in a large urban ED. The American Journal of Emergency Medicine. 2015 doi: 10.1016/j.ajem.2015.12.072. [DOI] [PubMed] [Google Scholar]
- 21.Donnelly JP, Franco RA, Wang HE, Galbraith JW. Emergency Department Screening for Hepatitis C Virus: Geographic Reach and Spatial Clustering in Central Alabama. Clinical Infectious Diseases. 2015;civ984 doi: 10.1093/cid/civ984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galbraith JW, Franco RA, Donnelly JP, et al. Unrecognized chronic hepatitis C virus infection among baby boomers in the emergency department. Hepatology (Baltimore, Md) 2015;61:776–82. doi: 10.1002/hep.27410. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh Y-H, Rothman RE, Laeyendecker OB, et al. Evaluation of The CDC Recommendations for HCV Testing in an Urban Emergency Department. Clinical Infectious Diseases. 2016 doi: 10.1093/cid/ciw074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyons MS, Kunnathur VA, Rouster SD, et al. Prevalence of Diagnosed and Undiagnosed Hepatitis C in a Midwestern Urban Emergency Department. Clinical Infectious Diseases. 2016 doi: 10.1093/cid/ciw073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White DA, Anderson ES, Pfeil SK, Trivedi TK, Alter HJ. Results of a rapid hepatitis C virus screening and diagnostic testing program in an urban emergency department. Annals of Emergency Medicine. 2016;67:119–28. doi: 10.1016/j.annemergmed.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 26.Galbraith JW. Hepatitis C Virus Screening: An Important Public Health Opportunity for United States Emergency Departments. Annals of emergency medicine. 2016;67:129–30. doi: 10.1016/j.annemergmed.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Allison WE, Chiang W, Rubin A, Oshva L, Carmody E. Knowledge about Hepatitis C Virus Infection and Acceptability of Testing in the 1945–1965 Birth Cohort (Baby Boomers) Presenting to a Large Urban Emergency Department: A Pilot Study. The Journal of emergency medicine. 2016 doi: 10.1016/j.jemermed.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Lim YA, Kwak YS, Cho YS, et al. An Evaluation of Commercial Reagent Kits for Detecting HCV Antibodies. Korean Journal of Clnical Pathology. 1998;18:220–7. [Google Scholar]
- 29.Mehta SH, Astemborski J, Kirk GD, et al. Changes in blood-borne infection risk among injection drug users. The Journal of infectious diseases. 2011;203:587–94. doi: 10.1093/infdis/jiq112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bailey SL, Ouellet LJ, Mackesy-Amiti ME, et al. Perceived risk, peer influences, and injection partner type predict receptive syringe sharing among young adult injection drug users in five U.S. cities. Drug and alcohol dependence. 2007;91(Suppl 1):S18–29. doi: 10.1016/j.drugalcdep.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 31.Hagan H, Pouget ER, Des Jarlais DC, Lelutiu-Weinberger C. Meta-regression of hepatitis C virus infection in relation to time since onset of illicit drug injection: the influence of time and place. Am J Epidemiol. 2008;168:1099–109. doi: 10.1093/aje/kwn237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore E, Han J, Serio-Chapman C, Mobley C, Watson C, Terplan M. Contraception and clean needles: feasibility of combining mobile reproductive health and needle exchange services for female exotic dancers. American journal of public health. 2012;102:1833–6. doi: 10.2105/AJPH.2012.300842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allen ST, Ruiz MS, Jones J. Assessing Syringe Exchange Program Access among Persons Who Inject Drugs (PWID) in the District of Columbia. J Urban Health. 2016 doi: 10.1007/s11524-015-0018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Annals of internal medicine. 2012;156:271–8. doi: 10.7326/0003-4819-156-4-201202210-00004. [DOI] [PubMed] [Google Scholar]
- 35.Ly KN, Hughes EM, Jiles RB, Holmberg SD. Rising Mortality Associated with Hepatitis C Virus in the United States, 2003–2013. Clinical Infectious Diseases. 2016 doi: 10.1093/cid/ciw111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Des Jarlais DC, Arasteh K, McKnight C, Perlman DC, Cooper HL, Hagan H. HSV-2 Infection as a Cause of Female/Male and Racial/Ethnic Disparities in HIV Infection. PloS one. 2013;8:e66874. doi: 10.1371/journal.pone.0066874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Operario D, Gamarel KE, Grin BM, et al. Sexual Minority Health Disparities in Adult Men and Women in the United States: National Health and Nutrition Examination Survey, 2001–2010. American journal of public health. 2015;105:e27–34. doi: 10.2105/AJPH.2015.302762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Operario D, Lee JH, Kuo C, Zaller N. Racial and Ethnic Disparities in HIV and STIs in the United States-National Health and Nutrition Examination Survey 1999–2012. AIDS patient care and STDs. 2015;29:635–8. doi: 10.1089/apc.2015.0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.