Abstract
Drosophila eye development is a complex process that involves many transcription factors (TFs) and interactions with their cofactors and targets. The TF Sine oculis (So) and its cofactor Eyes absent (Eya) are highly conserved and are both necessary and sufficient for eye development. Despite their many important roles during development, the direct targets of So are still largely unknown. Therefore the So-dependent regulatory network governing eye determination and differentiation is poorly understood. In this study, we intersected gene expression profiles of so or eya mutant eye tissue prepared from three different developmental stages and identified 1731 differentially expressed genes across the Drosophila genome. A combination of co-expression analyses and motif discovery identified a set of twelve putative direct So targets, including three known and nine novel targets. We also used our previous So ChIP-seq data to assess motif predictions for So and identified a canonical So binding motif. Finally, we performed in vivo enhancer reporter assays to test predicted enhancers from six candidate target genes and find that at least one enhancer from each gene is expressed in the developing eye disc and that their expression patterns overlap with that of So. In summary, we expand the set of putative So targets and show for the first time that the combined use of expression profiling of so with its cofactor eya is an effective method to identify novel So targets. Moreover, since So is highly conserved throughout the metazoa, our results provide the basis for future functional studies in a wide variety of organisms.
Keywords: Drosophila, Sine oculis, Eyes absent, Transcription factor, RNA-Seq, Eye development
Introduction
Transcriptional regulation involves a variety of complex biological processes that are dependent on the interactions among transcription factors (TFs) and their target genes. TFs activate and/or repress downstream target genes through binding to their regulatory motifs. This regulation often involves feed-forward and feedback loops. Despite their vital regulatory roles during development, much remains to be learned about the underlying mechanisms, especially the direct targets of TFs. There are two main approaches utilized for gene regulatory network (GRN) mapping. First, ChIP-Seq, which combines chromatin immunoprecipitation (ChIP) with massively parallel DNA sequencing (Seq), can identify the binding regions of TFs genome-wide. The second is gene expression profiling using RNA sequencing (RNA-Seq) or microarrays, which rely on genetic perturbations of TF function (Walhout, 2011). However, both approaches have disadvantages. For example, ChIP-Seq can be technically challenging in the absence of high-specificity antibodies. On other hand, gene expression profiling does not provide cis-regulatory information. However, a combination of gene expression profiling and motif discovery is an effective way to discover direct targets of TFs. In Drosophila, this approach has been successfully used to identify targets for Atonal and Glass, two key TFs that regulate retinal differentiation (Aerts et al., 2010; Potier et al., 2014).
One of the pivotal transcription factors controlling retinal cell fate specification in Drosophila is Sine oculis (So). So recruits Eyes absent (Eya), a transcriptional coactivator and a protein phosphatase, to target DNA where they synergistically direct many developmental processes, including cell fate determination, differentiation, proliferation, and survival in organisms ranging from flies to humans (Abdelhak et al., 1997; Bonini et al., 1993; Cheyette et al., 1994; Dozier et al., 2001; Hoshiyama et al., 2007; Kawakami et al., 2000; Sahly et al., 1999; Seo et al., 1999; Serikaku and O'Tousa, 1994; Xu et al., 1997; Zimmerman et al., 1997). The role of the So/Eya complex during development was first revealed in Drosophila. Specifically, coexpression of eya and so greatly increases ectopic eye formation, both in size and frequency, compared with expression of either eya or so alone (Pignoni et al., 1997). Cell culture assays have shown that cotransfection of eya and so appreciably activate transcriptional reporters while either gene alone does not (Silver et al., 2003). More importantly, this synergy between eya and so is conserved in vertebrates. Loss- and gain-of-function studies with Eya1 and Six1 mutants in fish, frogs and chick demonstrate that they play critical roles in sensory placode development (Tadjuidje and Hegde, 2012; Xu, 2013). A clear synergistic activation of targets is observed with combinations of mouse Six and Eya in cell culture studies (Ohto et al., 1999). Moreover, Six1 and Eya1 synergistically regulate organogenesis in mice (Li et al., 2003). Additionally, recent findings have demonstrated that Sox2 physically interacts with Eya1 and Six1 to synergistically activate transcription of Eya1/Six1 targets during sensory cell development (Ahmed et al., 2012a) and neurogenesis (Ahmed et al., 2012b) in the inner ear. In addition to Eya, there are two other proteins, Groucho (Gro) and Fl(2)d, that physically interact with So during eye development (Anderson et al., 2014; Silver et al., 2003).
so and eya are two key members of the Retinal Determination (RD) gene network, and are necessary and sufficient for eye formation (Pappu and Mardon, 2004). In Drosophila, loss of eya or so function causes massive apoptosis and the complete failure of eye development (Bonini et al., 1993; Cheyette et al., 1994; Zimmerman et al., 2000). This cell death phenotype resembles those seen in the ear and kidney primordia of Eya1 and Six1 mutant mouse embryos (Li et al., 2003; Xu et al., 1999). In addition, eya and so are required posterior to the morphogenetic furrow (MF) for normal retinal development (Atkins et al., 2013; Karandikar et al., 2014; Pignoni et al., 1997). In contrast to these loss-of-function phenotypes, ectopic overexpression of eya or so in other imaginal discs is sufficient to induce the formation of ectopic eyes (Bonini et al., 1997; Weasner et al., 2007). Although much has been learned from the genetic dissection of eya and so function during eye development, the direct targets of the So homeodomain transcription factor are largely unknown. To date, only a few direct So targets have been identified in Drosophila, including the RD network genes eyeless (ey) and dachshund (dac), as well as genes that regulate retinal differentiation and MF progression such as atonal (ato), lozenge (lz), prospero (pros), and hedgehog (hh) (Hayashi et al., 2008; Pappu et al., 2003; Pauli et al., 2005; Yan et al., 2003; Zhang et al., 2006). To increase our understanding of the So-dependent regulatory network during eye development, we first generated expression profiles from eye tissue mutant for so or eya at several stages of eye development. Co-expression analysis and motif discovery were then applied to screen candidate So targets. We also used our previous So ChIP-Seq data (Jusiak et al., 2014) to assess So motif predictions. Finally, to validate predicted So targets, we performed in vivo enhancer reporter assays and tested the expression levels of predicted direct So targets in so or eya conditional knockout eye discs using antibody staining. Together, these data expand the known regulatory landscape directly downstream of So and Eya.
Materials and Methods
Fly work
Fly stocks used for RNA-Seq experiments and the Janelia FlyLight enhancer-Gal4 lines used for enhancer reporter assays are listed in Table S7. All flies were maintained with standard corn meal and yeast extract medium at 25°C.
RNA isolation and RNA-Seq
30 eye discs were homogenized in 500 μl of TRIzol (Ambion) using a pestle. RNA was purified using the PureLink RNA Mini Kit (Ambion). We used the Illumina TruSeq protocol to build libraries after RNA extraction and sequenced them on the Illumina HiSeq 2000.
Immunohistochemistry
For immunohistochemistry, imaginal discs of wandering third instar larvae were dissected and processed as described in (Jin et al., 2013). Primary antibodies used were guinea pig anti-So (1:2000, gift from Dr. Ilaria Rebay), chick anti-GFP (1:1000, Abcam), rabbit anti-Pnt (1:500, gift from Dr. Jim Skeath), guinea pig anti-Rau (1:100, gift from Dr. Christian Klämbt), mouse anti-E(spl) (1:1, gift from Dr. Sarah Bray, E(spl) antibody recognize multiple E(spl)-complex members, including HLHmdelta (Cooper et al., 2000), and rabbit anti-E2f (1:100, gift from Dr. Nicholas Dyson). All secondary antibodies were made in goat and used at a final dilution of 1:500; Cy3, Cy5 and Alexa Fluor 488 secondary antibodies were obtained from Jackson ImmunoResearch and Life Technologies, respectively.
RNA-Seq preprocessing
RNA-Seq raw reads and splice junction sites were mapped to Drosophila melanogaster genome Dm3 with Bowtie (v0.12.7) and TopHat (v2.0.0), respectively. The reference Drosophila genome annotation file was downloaded from http://genome.ucsc.edu/. Read counts mapped to each gene were calculated by HTseq (http://www-huber.embl.de/users/anders/HTSeq/doc/overview.html). Normalization and differential expression analysis was performed through DESeq (Anders and Huber, 2010) (v1.20.0, R/Bioconductor package).
Identification of potential targets of eya and so
In order to select potential targets of eya and so, we performed differential expression contrasts of samples with altered “eya”, “so” and “eya and so” function versus “wild-type” at each of the different developmental stages, and another contrast with the three stages at once (DESeq using nbinomTest as the DE method). For each contrast, the standard DESeq workflow was followed, filtering out the 25% of genes with the lowest interquartile range (IQR). The samples included on the contrasts are shown in the Summary tab of Table S2. For “eya and so” vs. “wild-type”, a total of 6 samples (3 so samples and 3 eya samples) were put into the same group (since they function as a complex) for comparison to the corresponding 3 wild type samples from the same stage. Genes differentially expressed in any of the contrasts (p-value<0.05 and log2 (Fold Change)>1), were selected as input for Gene Ontology enrichment analysis with GOrilla (http://cbl-gorilla.cs.technion.ac.il/) (Eden et al., 2009) and for GENIE3 (Huynh-Thu et al., 2010). As possible regulators (also required as input for running GENIE3), we used the 120 TFs (out of the 753 TFs list available in FlyTF (Adryan and Teichmann, 2006)) that were differentially expressed, plus eya. We selected the 112 genes that were targets of both eya and so within the top quartile of GENIE3 output (importance measure (IM) > 0.009482). We then used HTC clustering (Eisen et al., 1998) in TM4 MultiExperiment Viewer (MeV) (Saeed et al., 2003) to discard genes with inverse expression patterns to eya and so, and kept only the positively correlated genes (marked with the pink vertical bar in Figure 3). These genes were analyzed by gene ontology enrichment analysis with PANTHER (http://pantherdb.org/) (Mi et al., 2013). This provided a list of genes which were potential targets of eya and so based on co-expression. However, these co-expressed genes probably include many indirect downstream genes. To identify the potential direct targets, we looked for enriched TF binding motifs within the co-expressed genes using i-cisTarget (Imrichova et al., 2015). The source of the Six motif identified by i-cisTarget is the Homer Motif Database, which obtained the motif from a dataset from Six1 ChIP-on-Chip from myoblasts (GSE20150). Figure 3B and the interaction network were created in Cytoscape (Shannon et al., 2003) and the online software STRING (Szklarczyk et al., 2015), respectively.
Figure 3.
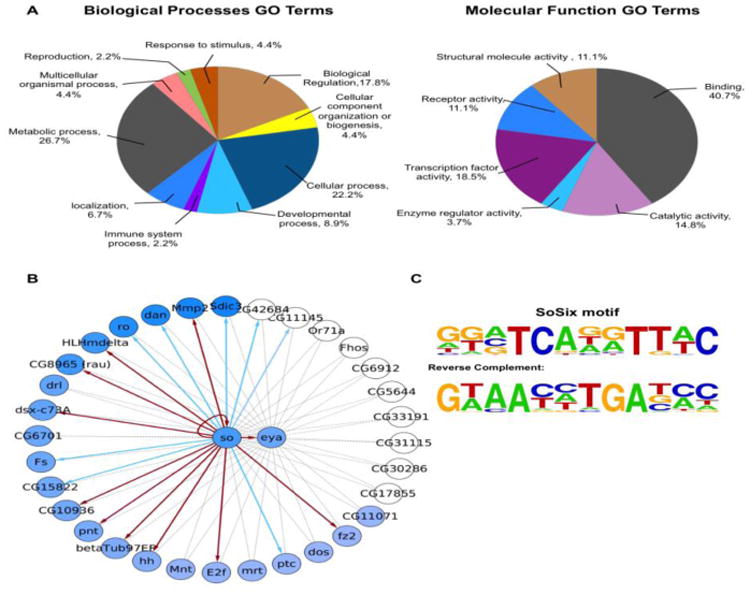
GO and regulatory network analyses of so/eya positively correlated genes. (A) Gene Ontology analysis, left: Biological processes, right: Molecular Function. The percentage of each section is proportional to the number of differentially expressed genes in GO categories. (B) Gene regulatory network showing predicted regulatory interactions of so/eya positively correlated genes obtained from hierarchical clustering analysis on the top 25% differentially expressed genes. The dashed links represent associations found by GENIE3, links that are confirmed by motif enrichment are indicated by solid lines. If the motif enrichment is significant (ranking higher than 775), arrows are red; otherwise they are blue. One exception is CG11145, which is ranked higher than 775 and therefore should be marked in red. However, its locus overlaps with so, which confounds interpretation. Therefore, we have marked CG11145 in blue. The node color intensity represents the fold change (FC) in differential expression. White nodes: not significant or lower FC than threshold in this contrast (“eya and so” vs. “wild-type”); however, these nodes may be significant in the individual contrasts “eya” vs. “wild-type” or “so” vs. “wild-type” (C) Logo of the SoSix1 motif.
Results
Transcriptome profiling of eya and so mutant eye tissue
So is known to function as a transcription factor with its coactivator Eya and the genetic and physical synergy between so and eya has been widely observed in multiple developmental processes from Drosophila to humans (Abdelhak et al., 1997; Bonini et al., 1993; Cheyette et al., 1994; Dozier et al., 2001; Hoshiyama et al., 2007; Kawakami et al., 2000; Sahly et al., 1999; Seo et al., 1999; Serikaku and O'Tousa, 1994; Xu et al., 1997; Zimmerman et al., 1997). To identify downstream target genes of So, we determined the gene expression profiles of wild-type and so, eya, and ato loss-of-function mutant or RNAi-knockdown eye discs at three distinct developmental stages. The first time point assayed was 68-70 hrs after egg laying (AEL), during which time eye discs are undergoing the late second and early third larval instar stage transition. At this time, so1 and eya2 eye discs are about to begin or have just started differentiation but massive apoptosis has not yet begun. Moreover, no So or Eya protein expression is observed in so1 and eya2 eye discs, respectively, and the size of eye discs is only slightly smaller compared to wild-type (Halder et al., 1998; Jin and Mardon, 2016). We also included expression profiles of ato1 from the AEL stage to identify target genes expressed posterior to the MF. Since so1 or eya2 eye discs die by apoptosis after the early third instar larval stage, this genotype would not be appropriate for RNA-Seq posterior to the morphogenetic furrow (MF). Therefore, we used RNA interference (RNAi) to knock down eya and so posterior to the MF. Specifically, we dissected GMR>soRNAi and GMR>eyaRNAi eye discs at the white pre-pupal (WP) stage and 24 hrs after puparium formation (APF) for late-stage RNA-Seq. Genotypes of all samples are shown in Table S1. All samples were dissected and collected in three biological replicates and expression profiles of all replicates of a given genotype are closely correlated (Figure S1). To analyze relationships among samples at different developmental stages, principal component analysis (PCA) was applied. As expected, the expression profiles of 68-70 hrs AEL, WP, and 24 hrs APF samples are clearly separated (Figure 1), consistent with the idea that distinct developmental processes occur at these three time points.
Figure 1.
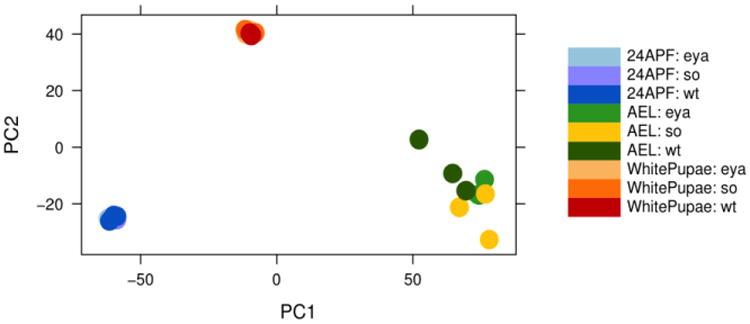
Principal component analysis (PCA). PCA of RNA-Seq expression data from three distinct developmental stages: 68-70 hrs AEL, WP and 24 hrs APF.
Identification of candidate so and eya-regulated genes by co-expression analysis
The main approach used for identification of candidate So targets by co-expression analysis with so and eya was to use GENIE3, a co-expression network inference algorithm (Huynh-Thu et al., 2010). As input to GENIE3, we used all differentially expressed genes between any two relevant conditions, being “eya”, “so” or “eya and so” vs. “wild-type” from each of the three developmental stages separately, and one analysis using all stages at once. This resulted in 1731 genes that are differentially expressed in any of these contrasts (Table S2). We performed Gene Ontology (GO) analysis on these 1731 genes with GOrilla (Eden et al., 2009) and found that they are enriched in Biological Processes GO terms related to eye, photoreceptor, and neuronal development and are also very strongly enriched for TF activity (Table S3 and Figure S2). Notably, the top 10 terms include “sensory organ development” (GO:0007423; P-value 6.11*10-14), “eye development” (GO:0001654; P-value 7.38*10-14), “compound eye development” (GO:0048749, P-value 2.46*10-12), and “regulation of transcription” (GO:0006355, P-value is 3.05E-11).
We used the top 25% of the co-expression scores based on GENIE3 to select so and eya targets for further analysis. The intersection between the targets for so and eya, resulted in a set of 112 genes which are potential downstream targets for both so and eya (Table S4). Hierarchical clustering was performed on these 112 genes. We also intersected expression profiles of corresponding genes in 68-70 hrs AEL ato1 mutant eye discs to identify genes that are specifically involved in differentiation (Zhang et al., 2006) and improve the accuracy of our predictions (Potier et al., 2014). As a result, we generated two distinct groups based on clustering, one positively correlated gene set (which follows the same overall expression pattern as eya and so, marked with a pink vertical bar in the heatmap, Figure 2) and one negatively correlated gene set consisting of all remaining genes. Since Eya acts as a coactivator of So and, together, they synergistically regulate downstream genes, the positively correlated genes in so and eya are likely to act downstream of So/Eya and play specific roles during Drosophila retinal development. This set has 34 genes (Table S4, marked in red), containing the positive control hh, so itself as an auto-regulatory target gene and eya, and is enriched in relevant developmental processes, such as neuron differentiation (P-value 2.36*10-6), eye development (P-value 1.45*10-5), sensory organ development (P-value 1.53*10-5), and Bolwig's organ morphogenesis and development (P-value 2*10-5) (Figure 3A and Table S5). Consistent with previous findings that most of the known So targets are transcription factors, positively correlated genes are over-represented in the Molecular Function GO terms “Transcription factor activity” (18.5%) and “Binding” (40.7%) (Figure 3A). The interconnectivity of So/Eya positively correlated candidates is shown in Figure 3B.
Figure 2.
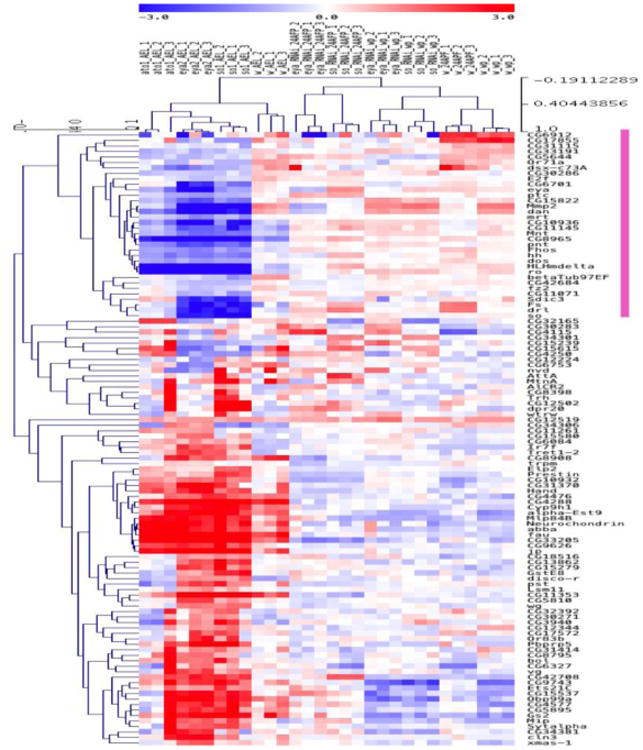
Hierarchical clustering of the top 25% differentially expressed genes. The heatmap shows the expression profiles of 112 genes identified as potential targets of so and eya by GENIE3. The color scale illustrates relative expression levels across all samples: blue represents down-regulated genes and red represents up-regulated genes. The dendrograms on the left and top of the heatmap show the hierarchical clustering of the transcripts for gene names and for all different samples, respectively. Clusters of genes which positively correlate in so and eya mutant tissue are indicated by the pink vertical bar.
Motif discovery using known direct So targets
Although so plays an important role in Drosophila eye development as a core retinal determination gene, only six genes are currently known as direct targets. To check whether any So motif or Six family DNA binding motif is enriched among the known So target genes, we applied i-cisTarget, an integrative genomics tool to predict transcriptional regulatory modules, to this set of genes (Imrichova et al., 2015). This analysis reveals that a predicted Six1 motif is indeed present in the known So targets (Figure 3C), and has a normalized enriched score (NES) of 3.74. This motif, which we call SoSix1, closely matches the reported consensus motif for So, GTAANYNGANAYC/G (Y=C or T, N=A or C or G or T) (Pauli et al., 2005). This analysis of known direct So targets indicates that if a critical number of true direct So target genes are present among a set of candidate genes, it is possible to retrieve associated binding motifs.
Direct So target prediction by motif enrichment analysis
To screen for direct target genes of So, we performed motif enrichment analysis on the So/Eya positively correlated candidate targets, similarly as performed above on the set of previously known So targets. The SoSix1 motif again appears as significantly enriched at rank 6 (out of nearly 10,000 motifs), with a normalized enrichment score (NES) of 5.32. The optimal cut-off at position 775, defined by i-cisTarget, yields a subset of 21 So binding region predictions among the 34 putative So target genes (Table 1). As a result, we found 12 predicted direct target genes with this motif that are significantly differentially expressed and positively correlated with eya and so. We name this gene set as “confident hits” and are HLHmdelta, pointed (pnt), E2F transcription factor 1 (E2f), hh, so, eya, Matrix metalloproteinase 2 (Mmp2), CG10936, frizzled 2 (fz2), rau, doublesex cognate 73A (dsx-c73A), and betaTub97EF (Table 1). Nine of these are novel putative direct So targets.
Table 1.
List of confident hits. This table shows the rank, associated genes, SoSix1 motif locations predicted by i-cisTarget and ChIP-Seq data validation, as well as reporter assay results and corresponding human homologues. Gray highlights indicate motif predictions for which no corresponding Gal4 lines were available.
| Rank | Associated genes | i-cis Target predicted So binding region | Overlap with So ChIP peak? | Reporter assay Positive? | Human homologue |
|---|---|---|---|---|---|
| 14 | HLHmdelta | chr3R: 21822368-21823453 | YES | YES | N/A |
| 17 | Mmp2 | chr2R: 5547784-5548876 | YES | N/A | MMP |
| 26 | hh | chr3R: 18962365-18964702 | YES | YES | SHH |
| 48 | so | chr2R: 3316058-3317633 | NO | NO | SIX1-6 |
| 63 | betaTub97EF | chr3R: 23801615-23803245 | YES | N/A | TUBB1 |
| 85 | so | chr2R: 3314710-3315493 | YES | YES | SIX1-6 |
| 143 | so | chr2R: 3304563-3305585 | NO | NO | SIX1-6 |
| 144 | eya | chr2L: 6549825-6550357 | NO | YES (ocelli) | EYA1-4 |
| 180 | eya | chr2L: 6544459-6546448 | NO | NO | EYA1-4 |
| 228 | CG10936 | chr2R: 13515427-13516089 | YES | N/A | N/A |
| 266 | fz2 | chr3L: 19161223-19162160 | YES | N/A | FZD1-10 |
| 278 | rau | chr2L: 5869522-5870420 | NO | N/A | N/A |
| 279 | eya | chr2L: 6541716-6543559 | YES | NO | EYA1-4 |
| 307 | Mmp2 | chr2R: 5541749-5544247 | YES | N/A | MMP |
| 377 | fz2 | chr3L: 19176425-19177998 | YES | N/A | FZD1-10 |
| 503 | E2f | chr3R: 17471133-17472022 | YES | YES | E2F1 |
| 529 | dsx-c73A | chr3L: 16428779-16430005 | NO | N/A | N/A |
| 645 | fz2 | chr3L: 19200054-19200895 | NO | N/A | FZD1-10 |
| 676 | E2f | chr3R: 17475343-17476006 | YES | YES | E2F1 |
| 732 | pnt | chr3R: 19145518-19146845 | YES | YES | ETS1-2 |
| 775 | fz2 | chr3L: 19140486-19141324 | NO | N/A | FZD1-10 |
Predicted interaction network for So, Eya, and their direct target genes
To investigate predicted interactions for the 12 confident hits, and to assess if they are linked with each other and known So targets, we used the STRING database, which provides co-localization, as well as direct (physical) and indirect (functional) associations (Szklarczyk et al., 2015). Associations among proteins predicted by STRING are shown in Figure 4A. The interaction network shows So associations with most of the confident hits, either directly or indirectly, except for candidate genes dsx-c73A and CG10936. The network also shows a high degree of regulatory cross talk, with many genes regulated by more than one gene. Importantly, the pnt gene, which encodes a transcription factor, associates with so, eya, and all known So target genes. In addition, the STRING output confirms the previously reported physical and genetic interactions of Eya-So and Eya-Dac (Chen et al., 1997; Pignoni et al., 1997) and the genetic interactions of Ey with Eya, So, Dac and Hh; Pnt with E2f and Rau; and Ato with Hh (Bonini et al., 1997; Chen et al., 1999; Halder et al., 1998; Kango-Singh et al., 2003; Pappu et al., 2003; Sieglitz et al., 2013; Staehling-Hampton et al., 1999; White and Jarman, 2000).
Figure 4.
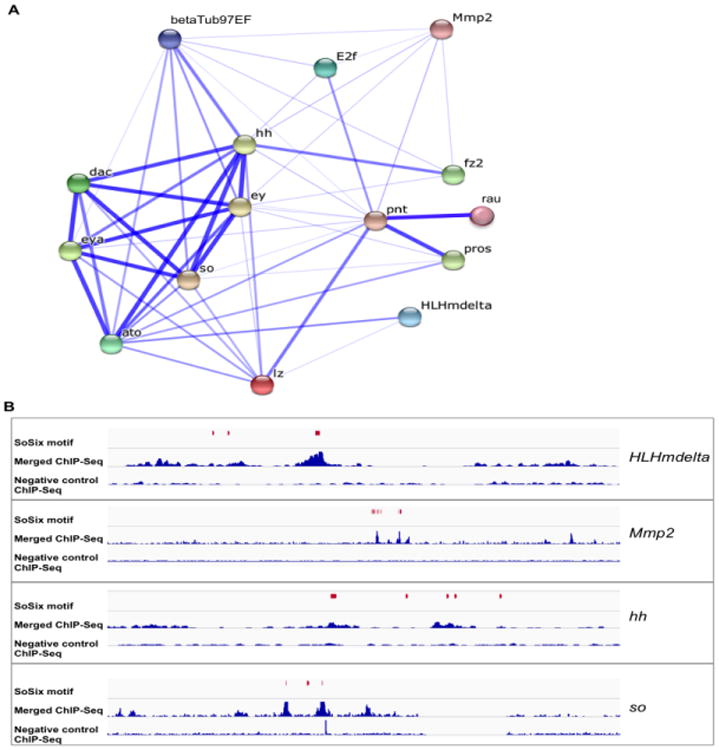
Predicted interaction network and validation using ChIP-Seq data. (A) The associations shown were generated based on STRING analysis; stronger associations are represented by thicker lines. This network includes previously known So targets and predicted targets (confident hits) identified in this study. (B) Examples showing the overlap between SoSix1 motif predictions and ChIP-Seq peaks for the predicted target genes HLHmdelta, Mmp2, hh and so. Each panel includes ChIP-Seq data (2 replicates merged, middle line and negative control, bottom line) and the positions of each SoSix1 motif (in red).
Validation of motif predictions with ChIP-Seq data
To validate So motif predictions, we used our previous ChIP-Seq data for assessment (Jusiak et al., 2014). We first applied i-cisTarget on the top 10% of significantly enriched ChIP-Seq peaks (n=757) and found the most strongly enriched motif in these peaks is SoSix1, with a high significance score (rank=1, NES = 11.88) (Table S6). This finding strongly supports the proposal that SoSix1 is a bona fide So motif. By comparing locations of the SoSix1motif predictions with the ChIP-Seq peaks of confident hits, we found that 62% of motif predictions overlap with a So ChIP-Seq peak (13 out of 21) (Table 1). Examples in Figure 4B show the overlap between SoSix1 motif predictions and ChIP-Seq peaks for HLHmdelta, Mmp2, hh and so, the top four predicted target genes in Table 1.
Validation of predicted direct So targets in vivo
Each of the 12 predicted So target genes harbor a cluster of one or more So binding regions (Table 1). To test if any of these motif predictions overlap with retinal enhancer activity, we performed in vivo enhancer reporter assays using the Janelia collection of Gal4 lines (Pfeiffer et al., 2008). We found eleven Gal4 lines that cover a predicted motif, representing six target genes: HLHmdelta, hh, pnt (one Gal4 line each), E2f (two different Gal4 lines each), so, eya (three different Gal4 lines each). These lines were crossed to UAS-GFP for detection of enhancer activity. We also visualized So expression in the same eye discs. Remarkably, we found GFP expression in eye discs from at least one Gal4 for each of the six predicted target genes we tested (HLHmdelta, E2f, pnt, hh, so and eya), of which five are active in photoreceptor cells and overlap with So staining (Figure 5). One Gal4 line from the eya gene showed enhancer activity in the ocelli, which also colocalizes with So expression (Cheyette et al., 1994). Moreover, the SoSix motifs associated with each predicted So target genes are conserved (Figure S3). We furthermore confirmed that the predicted direct So targets Pnt, E2f, HLHmdelta and Rau, for which antibodies are available, are reduced in an so or eya post-mitotic knockout background (soGMRcKO and eyaGMRcKO, respectively, unpublished data), which allows removing so or eya function in differentiating cells posterior to the MF (Figure 6). Overall, by intersecting genome-wide data from so and eya, we achieved a high success rate of So direct target gene prediction.
Figure 5.
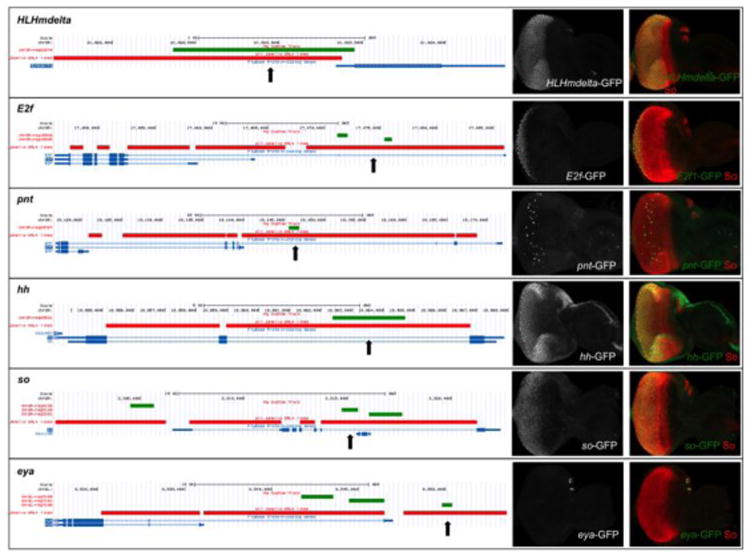
Validation of predicted So target enhancers by in vivo enhancer-reporter assays. Predicted So binding motifs were tested for 3 predicted target genes (HLHmdelta, E2f and pnt) and 3 known target genes (hh, so and eya). (Left) UCSC Genome Browser screenshots showing the locations of So binding regions predicted by i-cisTarget (green horizontal bars) and the enhancer-GAL4 lines from the Janelia FlyLight collection (red horizontal bars, the enhancer-Gal4 lines tested in vivo and are positive in reporter assays are indicated by vertical black arrows). (Right) SoSix1 motif predictions were tested by crossing the Janelia FlyLight GAL4 lines indicated in red in the panels to the left with UAS-GFP. Third instar larvae from these crosses were stained using anti-GFP (green) and anti-So (red).
Figure 6.
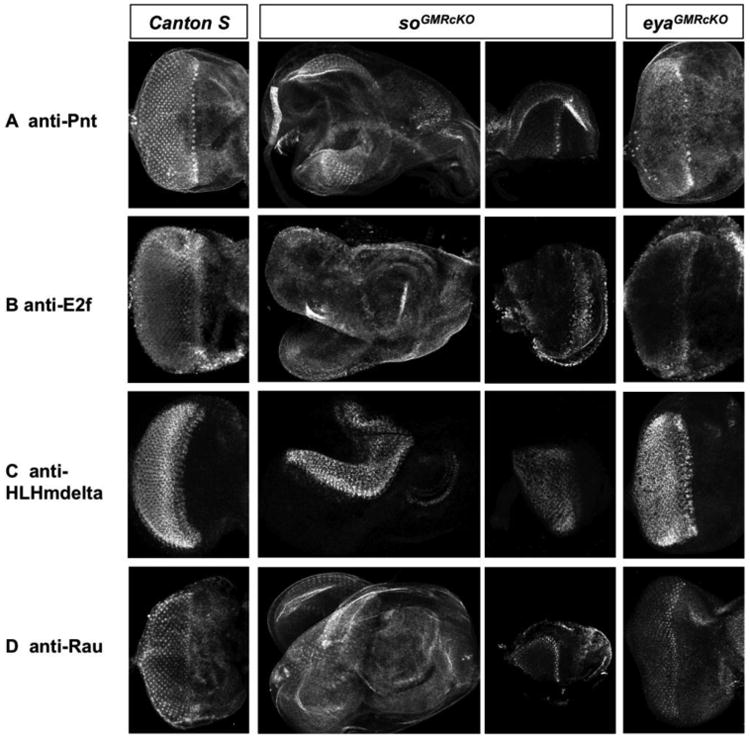
so and eya are required for normal expression of putative direct So targets posterior to the MF. Antibody stainings for a set of predicted direct So targets in wild-type and post-mitotic knockout of so (soGMRcKO) or eya (eyaGMRcKO) imaginal discs. So and Eya expression are eliminated posterior to the MF in soGMRcKO and eyaGMRcKO third instar larval eye discs, respectively. Since third instar larval eye discs of soGMRcKO flies are misshapen (Figure S4), disc fragments are shown to improve visualization of putative direct So targets expression (third column). Expression of Pnt (A) and E2f (B) are strongly reduced, and that of HLHmdelta (C) and Rau (D) are moderately reduced in both soGMRcKO and eyaGMRcKO eye discs.
Discussion
The homeodomain transcription factor So regulates multiple aspects of eye development for a wide range of higher eukaryotes. However, the mechanisms underlying the So-dependent regulatory network during eye development largely remain unknown, in part since only a few direct So targets are currently identified. To end this, we used a combinatorial approach of expression profiling and motif discovery to identify 12 putative So direct targets, including 9 novel targets. Through in vivo enhancer reporter assays, we found at least one predicted enhancers from all six target genes tested is able to drive reporter expression in the developing eye disc. Our results expand the list of putative So direct targets in the eye, providing the basis for further functional studies.
Intersecting expression profiles of so and eya mutant eye tissue
Transcription factors usually regulate their downstream targets by binding cooperatively with their cofactors or other TFs (Hayashi et al., 2008; Zhang et al., 2006). To identify direct targets of So and infer a regulatory network, we performed co-expression analyses by intersecting expression profiles of so and its cofactor gene eya at three different developmental stages. The “AEL” samples are a mixture of eye discs that are immediately before, during and after differentiation. The RNAs from AEL eye discs were prepared in parallel (same time point of collection, same temperature and same manipulation) to minimize variation within a single genotype and make direct comparison of different genotypes meaningful. Moreover, we included AEL data from ato1 mutant eye discs with that of so1 and eya2 to identify genes that are specifically involved in differentiation posterior to the MF. In addition to expression profiles at an early developmental stage, we included RNAi knockdown data of eya and so at two developmental stages after differentiation to gain a larger number of perturbations and minimize nonspecific candidate genes. In particular, numerous studies have suggested that co-expression analysis requires many related perturbations to achieve good prediction power (Hsu et al., 2015; Potier et al., 2014; Torkamani et al., 2010).
As expected, the 1731 genes that are differentially expressed in response to loss of eya or so function are enriched in GO categories that are relevant to eye development. For further analysis, 34 genes that positively correlate in so and eya mutant tissue were identified. Applying GO enrichment on this gene set, we found they are not only enriched in GO terms pertaining to the eye, but also rank high in the GO term “regulation of fibroblast growth factor receptor (FGFR) signaling pathway” (P-value 1.93*10-3). FGFR signaling regulates the onset of retinal wrapping glial cell differentiation (Franzdottir et al., 2009), suggesting that So may regulate glial differentiation during eye development. By using in vivo enhancer reporter assays and validation of putative target expression in the absence of so or eya function posterior to the MF, we achieved a high rate of success for So direct target prediction. Our findings provide an example where intersection of expression profiling of a TF with its cofactor can increase specificity in subsequent analyses of RNA-Seq data, which should be generally applicable to target prediction for other TFs.
Integrative analysis of gene expression profiling and cis-regulatory analysis
Sequencing-based expression profiling, such as RNA-Seq, is widely used to obtain genome-wide quantitative gene expression levels in many organisms (Hong et al., 2011; McManus et al., 2010). RNA-Seq based on genetic perturbations provides a powerful approach to gain a deeper understanding of TF-regulated networks. However, this approach does not provide cis-regulatory information. To solve this problem, we applied regulatory motif discovery to putative target genes derived from RNA-Seq data and used our previous ChIP-Seq data to validate the locations of motif predictions. First, we identified the “SoSix1” motif as a putative So binding motif by using all known direct So targets as input. We then observed that this motif is over-represented across our predicted targets. The targetome of the SoSix1 motif comprises 12 out of the 34 putative So target genes, including the known So direct target hh and so itself. Finally, significantly enriched So ChIP-Seq peaks were used to assess motif predictions. We found that more than 62% of predicted motifs overlap with highly enriched ChIP-Seq peaks in the 12 genes studied. A similar strategy has been successfully employed to identify target genes for eye-specific TFs expressed within and posterior to the MF, including Atonal, Glass and other TFs in Drosophila (Aerts et al., 2010; Menoret et al., 2013; Oliva et al., 2015; Potier et al., 2014).
In this paper, we first applied this approach to So and identified a canonical So binding motif, which was extracted from a mouse Six1 ChIP-Seq dataset (GSE20150). This is not surprising since Six1/2 are the mammalian homologues of so (Kawakami and Kobayashi, 1998) and so, Six1/2, and Six4/5 share very similar DNA-binding sequences (Pauli et al., 2005). Moreover, previous protein-DNA interaction studies of Six and So found that a stretch of 13 nucleotides, GTAANYNGANAYC/G, is necessary for Six and So binding to DNA. Nucleotides G, A, A at positions 1, 4, 9, respectively, are most important for this interaction (Hazbun et al., 1997; Pauli et al., 2005; Rogers et al., 2005; Silver et al., 2003; Zhang et al., 2006). Our predicted SoSix1 motif closely matches this known consensus sequence. More importantly, the three nucleotides at positions 1, 4, and 9 are present and conserved within the SoSix1 motif.
Novel direct So targets in eye development
Through our analysis, we identified 12 predicted direct So target genes, including nine novel targets: HLHmdelta, pnt, E2f, Mmp2, CG10936, fz2, rau, dsx-c73A and betaTub97EF. Gene regulatory network analysis predicts a high degree of regulatory cross talk between novel and known So targets (including So and Eya). Interestingly, Mmp2, rau and pnt are involved in the EGFR and FGFR signaling pathways (Franzdottir et al., 2009). Specifically, Rau and its transcriptional activator Pnt act in a positive feedback loop downstream of FGFR- and EGFR-mediated signaling to regulate R7 photoreceptor development, while Mmp2 is implicated in cancer (Bossuyt et al., 2009; Sieglitz et al., 2013). These findings suggested that So may mediate FGFR and EGFR signaling pathways and cancer cell proliferation via its direct downstream targets. Additionally, Pnt and So directly regulate hh (Pauli et al., 2005; Rogers et al., 2005), providing an intricate model of cross-talk among the retinal determination genes and the EGFR, FGFR, and Hh signaling pathways.
HLHmdelta, pnt, E2f are three predicted targets which have FlyLight enhancer-Gal4 lines, all of which can drive reporter activation during eye development. HLHmdelta and E2f are involved in Notch signaling and are required for R3/R4 photoreceptor cell induction and cell proliferation in eye discs, respectively (Baonza and Freeman, 2005; Cooper and Bray, 1999). During Drosophila eye development, Notch signaling plays important roles in the growth of eye discs, dorsal/ventral boundary establishment, and MF progression (Bhattacharya and Baker, 2009; Dominguez and de Celis, 1998). Our work suggests that So regulates these processes by acting upstream of HLHmdelta and E2f in Notch signaling, consistent with the known roles of So during Drosophila eye development, including cell survival, MF initiation, and photoreceptor differentiation (Cheyette et al., 1994). Moreover, the putative So target Fz2 can bind Wingless (Wg) and act as a receptor for Wg in Drosophila (Chen and Struhl, 1999). Along with previous studies suggesting that the Wg signaling pathway regulates eya during ocelli development (Blanco et al., 2009) and our finding that a predicted enhancer of eya is able to drive reporter expression in ocelli, we hypothesize that So regulates this process through eya, fz2, and wg. In contrast, little is currently known about the function of CG10936, dsx-c73A and betaTub97EF, although they may play a role in sensory perception, cuticle development, and centrosome duplication, respectively. A detailed characterization of the regulatory interactions between these novel putative targets and So awaits further studies.
Conclusions
In conclusion, by integrating gene expression profiles of so and eya, we have obtained a high-quality set of so/eya target genes which show a strong GO term enrichment in eye development and TF regulation. Applying motif discovery to this gene set, we identified twelve predicted direct So target genes. Six of these were tested by in vivo enhancer reporter assays and at least one predicted enhancer from each gene is able to activate reporter expression in eye discs, suggesting that this approach for target prediction is robust. Since many eye-specific genes are highly conserved, our studies may yield new insights into the regulatory mechanisms underlying So-dependent eye development throughout the metazoa.
Supplementary Material
Highlights.
Determining the gene expression profiles of so or eya mutant eye tissues.
Applying co-expression analysis and motif discovery to differentially expressed genes.
A canonical So binding motif are identified from motif discovery and ChIP-seq data.
Enhancer reporter assays confirm predicted So binding motifs in vivo.
Acknowledgments
We thank Dr, Ilaria Rebay, Dr. Christian Klämbt, Dr. Jim Skeath, Dr. Sarah Bray, and Dr. Nicholas Dyson for antibodies. We are grateful to Dr. Ming Fa and Dr. Baojun Wu for critical reading of manuscript, Xuan Zhu for technical help, and the Bloomington Stock Center for Drosophila stocks.
Funding: This work was funded by the National Eye Institute grant R01 EY011232 (GM).
Footnotes
Competing interests: The authors declare that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Meng Jin, Email: mjin@bcm.edu.
Sara Aibar, Email: sara.aibar@kuleuven.be.
Zhongqi Ge, Email: Zhongqi.Ge@bcm.edu.
Rui Chen, Email: ruichen@bcm.edu.
Stein Aerts, Email: stein.aerts@med.kuleuven.be.
References
- Abdelhak S, Kalatzis V, Heilig R, Compain S, Samson D, Vincent C, Weil D, Cruaud C, Sahly I, Leibovici M, Bitner-Glindzicz M, Francis M, Lacombe D, Vigneron J, Charachon R, Boven K, Bedbeder P, Van Regemorter N, Weissenbach J, Petit C. A human homologue of the Drosophila eyes absent gene underlies branchio-oto-renal (BOR) syndrome and identifies a novel gene family. Nat Genet. 1997;15:157–164. doi: 10.1038/ng0297-157. [DOI] [PubMed] [Google Scholar]
- Adryan B, Teichmann SA. FlyTF: a systematic review of site-specific transcription factors in the fruit fly Drosophila melanogaster. Bioinformatics. 2006;22:1532–1533. doi: 10.1093/bioinformatics/btl143. [DOI] [PubMed] [Google Scholar]
- Aerts S, Quan XJ, Claeys A, Naval Sanchez M, Tate P, Yan J, Hassan BA. Robust target gene discovery through transcriptome perturbations and genome-wide enhancer predictions in Drosophila uncovers a regulatory basis for sensory specification. PLoS biology. 2010;8:e1000435. doi: 10.1371/journal.pbio.1000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed M, Wong EY, Sun J, Xu J, Wang F, Xu PX. Eya1-Six1 interaction is sufficient to induce hair cell fate in the cochlea by activating Atoh1 expression in cooperation with Sox2. Developmental cell. 2012a;22:377–390. doi: 10.1016/j.devcel.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed M, Xu J, Xu PX. EYA1 and SIX1 drive the neuronal developmental program in cooperation with the SWI/SNF chromatin-remodeling complex and SOX2 in the mammalian inner ear. Development. 2012b;139:1965–1977. doi: 10.1242/dev.071670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral AJ, Brito FF, Chobanyan T, Yoshikawa S, Yokokura T, Van Vactor D, Gama-Carvalho M. Quality assessment and control of tissue specific RNA-seq libraries of Drosophila transgenic RNAi models. Frontiers in genetics. 2014;5:43. doi: 10.3389/fgene.2014.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S, Huber W. Differential expression analysis for sequence count data. Genome biology. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AM, Weasner BP, Weasner BM, Kumar JP. The Drosophila Wilms Tumor 1-Associating Protein (WTAP) homolog is required for eye development. Developmental biology. 2014;390:170–180. doi: 10.1016/j.ydbio.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins M, Jiang Y, Sansores-Garcia L, Jusiak B, Halder G, Mardon G. Dynamic rewiring of the Drosophila retinal determination network switches its function from selector to differentiation. PLoS genetics. 2013;9:e1003731. doi: 10.1371/journal.pgen.1003731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baonza A, Freeman M. Control of cell proliferation in the Drosophila eye by Notch signaling. Developmental cell. 2005;8:529–539. doi: 10.1016/j.devcel.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A, Baker NE. The HLH protein Extramacrochaetae is required for R7 cell and cone cell fates in the Drosophila eye. Developmental biology. 2009;327:288–300. doi: 10.1016/j.ydbio.2008.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco J, Seimiya M, Pauli T, Reichert H, Gehring WJ. Wingless and Hedgehog signaling pathways regulate orthodenticle and eyes absent during ocelli development in Drosophila. Developmental biology. 2009;329:104–115. doi: 10.1016/j.ydbio.2009.02.027. [DOI] [PubMed] [Google Scholar]
- Bonini NM, Bui QT, Gray-Board GL, Warrick JM. The Drosophila eyes absent gene directs ectopic eye formation in a pathway conserved between flies and vertebrates. Development. 1997;124:4819–4826. doi: 10.1242/dev.124.23.4819. [DOI] [PubMed] [Google Scholar]
- Bonini NM, Leiserson WM, Benzer S. The eyes absent gene: genetic control of cell survival and differentiation in the developing Drosophila eye. Cell. 1993;72:379–395. doi: 10.1016/0092-8674(93)90115-7. [DOI] [PubMed] [Google Scholar]
- Bossuyt W, De Geest N, Aerts S, Leenaerts I, Marynen P, Hassan BA. The atonal proneural transcription factor links differentiation and tumor formation in Drosophila. PLoS biology. 2009;7:e40. doi: 10.1371/journal.pbio.1000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CM, Struhl G. Wingless transduction by the Frizzled and Frizzled2 proteins of Drosophila. Development. 1999;126:5441–5452. doi: 10.1242/dev.126.23.5441. [DOI] [PubMed] [Google Scholar]
- Chen R, Amoui M, Zhang Z, Mardon G. Dachshund and eyes absent proteins form a complex and function synergistically to induce ectopic eye development in Drosophila. Cell. 1997;91:893–903. doi: 10.1016/s0092-8674(00)80481-x. [DOI] [PubMed] [Google Scholar]
- Chen R, Halder G, Zhang Z, Mardon G. Signaling by the TGF-beta homolog decapentaplegic functions reiteratively within the network of genes controlling retinal cell fate determination in Drosophila. Development. 1999;126:935–943. doi: 10.1242/dev.126.5.935. [DOI] [PubMed] [Google Scholar]
- Cheyette BN, Green PJ, Martin K, Garren H, Hartenstein V, Zipursky SL. The Drosophila sine oculis locus encodes a homeodomain-containing protein required for the development of the entire visual system. Neuron. 1994;12:977–996. doi: 10.1016/0896-6273(94)90308-5. [DOI] [PubMed] [Google Scholar]
- Cooper MT, Bray SJ. Frizzled regulation of Notch signalling polarizes cell fate in the Drosophila eye. Nature. 1999;397:526–530. doi: 10.1038/17395. [DOI] [PubMed] [Google Scholar]
- Cooper MT, Tyler DM, Furriols M, Chalkiadaki A, Delidakis C, Bray S. Spatially restricted factors cooperate with notch in the regulation of Enhancer of split genes. Developmental biology. 2000;221:390–403. doi: 10.1006/dbio.2000.9691. [DOI] [PubMed] [Google Scholar]
- Dominguez M, de Celis JF. A dorsal/ventral boundary established by Notch controls growth and polarity in the Drosophila eye. Nature. 1998;396:276–278. doi: 10.1038/24402. [DOI] [PubMed] [Google Scholar]
- Dozier C, Kagoshima H, Niklaus G, Cassata G, Burglin TR. The Caenorhabditis elegans Six/sine oculis class homeobox gene ceh-32 is required for head morphogenesis. Developmental biology. 2001;236:289–303. doi: 10.1006/dbio.2001.0325. [DOI] [PubMed] [Google Scholar]
- Eden E, Navon R, Steinfeld I, Lipson D, Yakhini Z. GOrilla: a tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC bioinformatics. 2009;10:48. doi: 10.1186/1471-2105-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzdottir SR, Engelen D, Yuva-Aydemir Y, Schmidt I, Aho A, Klambt C. Switch in FGF signalling initiates glial differentiation in the Drosophila eye. Nature. 2009;460:758–761. doi: 10.1038/nature08167. [DOI] [PubMed] [Google Scholar]
- Halder G, Callaerts P, Flister S, Walldorf U, Kloter U, Gehring WJ. Eyeless initiates the expression of both sine oculis and eyes absent during Drosophila compound eye development. Development. 1998;125:2181–2191. doi: 10.1242/dev.125.12.2181. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Xu C, Carthew RW. Cell-type-specific transcription of prospero is controlled by combinatorial signaling in the Drosophila eye. Development. 2008;135:2787–2796. doi: 10.1242/dev.006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazbun TR, Stahura FL, Mossing MC. Site-specific recognition by an isolated DNA-binding domain of the sine oculis protein. Biochemistry. 1997;36:3680–3686. doi: 10.1021/bi9625206. [DOI] [PubMed] [Google Scholar]
- Hong LZ, Li J, Schmidt-Kuntzel A, Warren WC, Barsh GS. Digital gene expression for non-model organisms. Genome Res. 2011;21:1905–1915. doi: 10.1101/gr.122135.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshiyama D, Iwabe N, Miyata T. Evolution of the gene families forming the Pax/Six regulatory network: isolation of genes from primitive animals and molecular phylogenetic analyses. FEBS letters. 2007;581:1639–1643. doi: 10.1016/j.febslet.2007.03.027. [DOI] [PubMed] [Google Scholar]
- Hsu CL, Juan HF, Huang HC. Functional Analysis and Characterization of Differential Coexpression Networks. Scientific reports. 2015;5:13295. doi: 10.1038/srep13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh-Thu VA, Irrthum A, Wehenkel L, Geurts P. Inferring regulatory networks from expression data using tree-based methods. PloS one. 2010;5 doi: 10.1371/journal.pone.0012776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imrichova H, Hulselmans G, Atak ZK, Potier D, Aerts S. i-cisTarget 2015 update: generalized cis-regulatory enrichment analysis in human, mouse and fly. Nucleic acids research. 2015;43:W57–64. doi: 10.1093/nar/gkv395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, Jusiak B, Bai Z, Mardon G. Eyes absent tyrosine phosphatase activity is not required for Drosophila development or survival. PloS one. 2013;8:e58818. doi: 10.1371/journal.pone.0058818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, Mardon G. Distinct Biochemical Activities of Eyes absent During Drosophila Eye Development. Scientific reports. 2016;6:23228. doi: 10.1038/srep23228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jusiak B, Karandikar UC, Kwak SJ, Wang F, Wang H, Chen R, Mardon G. Regulation of Drosophila eye development by the transcription factor Sine oculis. PloS one. 2014;9:e89695. doi: 10.1371/journal.pone.0089695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kango-Singh M, Singh A, Henry Sun Y. Eyeless collaborates with Hedgehog and Decapentaplegic signaling in Drosophila eye induction. Developmental biology. 2003;256:49–60. doi: 10.1016/s0012-1606(02)00123-9. [DOI] [PubMed] [Google Scholar]
- Karandikar UC, Jin M, Jusiak B, Kwak S, Chen R, Mardon G. Drosophila eyes absent is required for normal cone and pigment cell development. PloS one. 2014;9:e102143. doi: 10.1371/journal.pone.0102143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami K, Kobayashi M. Structure and function of novel homeobox gene family six and implications in development and differentiation. Tanpakushitsu kakusan koso Protein, nucleic acid, enzyme. 1998;43:2120–2125. [PubMed] [Google Scholar]
- Kawakami K, Sato S, Ozaki H, Ikeda K. Six family genes--structure and function as transcription factors and their roles in development. BioEssays : news and reviews in molecular, cellular and developmental biology. 2000;22:616–626. doi: 10.1002/1521-1878(200007)22:7<616::AID-BIES4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Li X, Oghi KA, Zhang J, Krones A, Bush KT, Glass CK, Nigam SK, Aggarwal AK, Maas R, Rose DW, Rosenfeld MG. Eya protein phosphatase activity regulates Six1-Dach-Eya transcriptional effects in mammalian organogenesis. Nature. 2003;426:247–254. doi: 10.1038/nature02083. [DOI] [PubMed] [Google Scholar]
- Lizardi PM, Forloni M, Wajapeyee N. Genome-wide approaches for cancer gene discovery. Trends in biotechnology. 2011;29:558–568. doi: 10.1016/j.tibtech.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus CJ, Coolon JD, Duff MO, Eipper-Mains J, Graveley BR, Wittkopp PJ. Regulatory divergence in Drosophila revealed by mRNA-seq. Genome research. 2010;20:816–825. doi: 10.1101/gr.102491.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menoret D, Santolini M, Fernandes I, Spokony R, Zanet J, Gonzalez I, Latapie Y, Ferrer P, Rouault H, White KP, Besse P, Hakim V, Aerts S, Payre F, Plaza S. Genome-wide analyses of Shavenbaby target genes reveals distinct features of enhancer organization. Genome biology. 2013;14:R86. doi: 10.1186/gb-2013-14-8-r86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi H, Muruganujan A, Casagrande JT, Thomas PD. Large-scale gene function analysis with the PANTHER classification system. Nature protocols. 2013;8:1551–1566. doi: 10.1038/nprot.2013.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohto H, Kamada S, Tago K, Tominaga SI, Ozaki H, Sato S, Kawakami K. Cooperation of six and eya in activation of their target genes through nuclear translocation of Eya. Mol Cell Biol. 1999;19:6815–6824. doi: 10.1128/mcb.19.10.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva C, Molina-Fernandez C, Maureira M, Candia N, Lopez E, Hassan B, Aerts S, Canovas J, Olguin P, Sierralta J. Hindsight regulates photoreceptor axon targeting through transcriptional control of jitterbug/Filamin and multiple genes involved in axon guidance in Drosophila. Developmental neurobiology. 2015;75:1018–1032. doi: 10.1002/dneu.22271. [DOI] [PubMed] [Google Scholar]
- Pappu KS, Chen R, Middlebrooks BW, Woo C, Heberlein U, Mardon G. Mechanism of hedgehog signaling during Drosophila eye development. Development. 2003;130:3053–3062. doi: 10.1242/dev.00534. [DOI] [PubMed] [Google Scholar]
- Pappu KS, Mardon G. Genetic control of retinal specification and determination in Drosophila. The International journal of developmental biology. 2004;48:913–924. doi: 10.1387/ijdb.041875kp. [DOI] [PubMed] [Google Scholar]
- Pauli T, Seimiya M, Blanco J, Gehring WJ. Identification of functional sine oculis motifs in the autoregulatory element of its own gene, in the eyeless enhancer and in the signalling gene hedgehog. Development. 2005;132:2771–2782. doi: 10.1242/dev.01841. [DOI] [PubMed] [Google Scholar]
- Pfeiffer BD, Jenett A, Hammonds AS, Ngo TT, Misra S, Murphy C, Scully A, Carlson JW, Wan KH, Laverty TR, Mungall C, Svirskas R, Kadonaga JT, Doe CQ, Eisen MB, Celniker SE, Rubin GM. Tools for neuroanatomy and neurogenetics in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:9715–9720. doi: 10.1073/pnas.0803697105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignoni F, Hu B, Zavitz KH, Xiao J, Garrity PA, Zipursky SL. The eye-specification proteins So and Eya form a complex and regulate multiple steps in Drosophila eye development. Cell. 1997;91:881–891. doi: 10.1016/s0092-8674(00)80480-8. [DOI] [PubMed] [Google Scholar]
- Potier D, Davie K, Hulselmans G, Naval Sanchez M, Haagen L, Huynh-Thu VA, Koldere D, Celik A, Geurts P, Christiaens V, Aerts S. Mapping gene regulatory networks in Drosophila eye development by large-scale transcriptome perturbations and motif inference. Cell reports. 2014;9:2290–2303. doi: 10.1016/j.celrep.2014.11.038. [DOI] [PubMed] [Google Scholar]
- Rogers EM, Brennan CA, Mortimer NT, Cook S, Morris AR, Moses K. Pointed regulates an eye-specific transcriptional enhancer in the Drosophila hedgehog gene, which is required for the movement of the morphogenetic furrow. Development. 2005;132:4833–4843. doi: 10.1242/dev.02061. [DOI] [PubMed] [Google Scholar]
- Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, Sturn A, Snuffin M, Rezantsev A, Popov D, Ryltsov A, Kostukovich E, Borisovsky I, Liu Z, Vinsavich A, Trush V, Quackenbush J. TM4: a free, open-source system for microarray data management and analysis. BioTechniques. 2003;34:374–378. doi: 10.2144/03342mt01. [DOI] [PubMed] [Google Scholar]
- Sahly I, Andermann P, Petit C. The zebrafish eya1 gene and its expression pattern during embryogenesis. Development genes and evolution. 1999;209:399–410. doi: 10.1007/s004270050270. [DOI] [PubMed] [Google Scholar]
- Seo HC, Curtiss J, Mlodzik M, Fjose A. Six class homeobox genes in drosophila belong to three distinct families and are involved in head development. Mechanisms of development. 1999;83:127–139. doi: 10.1016/s0925-4773(99)00045-3. [DOI] [PubMed] [Google Scholar]
- Serikaku MA, O'Tousa JE. sine oculis is a homeobox gene required for Drosophila visual system development. Genetics. 1994;138:1137–1150. doi: 10.1093/genetics/138.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome research. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieglitz F, Matzat T, Yuva-Aydemir Y, Neuert H, Altenhein B, Klambt C. Antagonistic feedback loops involving Rau and Sprouty in the Drosophila eye control neuronal and glial differentiation. Science signaling. 2013;6:ra96. doi: 10.1126/scisignal.2004651. [DOI] [PubMed] [Google Scholar]
- Silver SJ, Davies EL, Doyon L, Rebay I. Functional dissection of eyes absent reveals new modes of regulation within the retinal determination gene network. Mol Cell Biol. 2003;23:5989–5999. doi: 10.1128/MCB.23.17.5989-5999.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehling-Hampton K, Ciampa PJ, Brook A, Dyson N. A genetic screen for modifiers of E2F in Drosophila melanogaster. Genetics. 1999;153:275–287. doi: 10.1093/genetics/153.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, Kuhn M, Bork P, Jensen LJ, von Mering C. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic acids research. 2015;43:D447–452. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadjuidje E, Hegde RS. The Eyes Absent proteins in development and disease. Cell Mol Life Sci. 2012 doi: 10.1007/s00018-012-1144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torkamani A, Dean B, Schork NJ, Thomas EA. Coexpression network analysis of neural tissue reveals perturbations in developmental processes in schizophrenia. Genome research. 2010;20:403–412. doi: 10.1101/gr.101956.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhout AJ. Gene-centered regulatory network mapping. Methods in cell biology. 2011;106:271–288. doi: 10.1016/B978-0-12-544172-8.00010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weasner B, Salzer C, Kumar JP. Sine oculis, a member of the SIX family of transcription factors, directs eye formation. Developmental biology. 2007;303:756–771. doi: 10.1016/j.ydbio.2006.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NM, Jarman AP. Drosophila atonal controls photoreceptor R8-specific properties and modulates both receptor tyrosine kinase and Hedgehog signalling. Development. 2000;127:1681–1689. doi: 10.1242/dev.127.8.1681. [DOI] [PubMed] [Google Scholar]
- Xu PX. The EYA-SO/SIX complex in development and disease. Pediatric nephrology. 2013;28:843–854. doi: 10.1007/s00467-012-2246-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu PX, Adams J, Peters H, Brown MC, Heaney S, Maas R. Eya1-deficient mice lack ears and kidneys and show abnormal apoptosis of organ primordia. Nat Genet. 1999;23:113–117. doi: 10.1038/12722. [DOI] [PubMed] [Google Scholar]
- Xu PX, Woo I, Her H, Beier DR, Maas RL. Mouse Eya homologues of the Drosophila eyes absent gene require Pax6 for expression in lens and nasal placode. Development. 1997;124:219–231. doi: 10.1242/dev.124.1.219. [DOI] [PubMed] [Google Scholar]
- Yan H, Canon J, Banerjee U. A transcriptional chain linking eye specification to terminal determination of cone cells in the Drosophila eye. Developmental biology. 2003;263:323–329. doi: 10.1016/j.ydbio.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Zhang T, Ranade S, Cai CQ, Clouser C, Pignoni F. Direct control of neurogenesis by selector factors in the fly eye: regulation of atonal by Ey and So. Development. 2006;133:4881–4889. doi: 10.1242/dev.02669. [DOI] [PubMed] [Google Scholar]
- Zimmerman JE, Bui QT, Liu H, Bonini NM. Molecular genetic analysis of Drosophila eyes absent mutants reveals an eye enhancer element. Genetics. 2000;154:237–246. doi: 10.1093/genetics/154.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman JE, Bui QT, Steingrimsson E, Nagle DL, Fu W, Genin A, Spinner NB, Copeland NG, Jenkins NA, Bucan M, Bonini NM. Cloning and characterization of two vertebrate homologs of the Drosophila eyes absent gene. Genome research. 1997;7:128–141. doi: 10.1101/gr.7.2.128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


