Abstract
Objectives. To determine the effect of sodium (Na) reduction on occurrence of headaches.
Methods. In the Trial of Nonpharmacologic Interventions in the Elderly, 975 men and woman (aged 60–80 years) with hypertension were randomized to a Na-reduction intervention or control group and were followed for up to 36 months. The study was conducted between 1992 and 1995 at 4 clinical centers (Johns Hopkins University, Wake Forest University School of Medicine, Robert Wood Johnson Medical School, and the University of Tennessee).
Results. Mean difference in Na excretion between the Na-reduction intervention and control group was significant at each follow-up visit (P < .001) with an average difference of 38.8 millimoles per 24 hours. The occurrence of headaches was significantly lower in the Na-reduction intervention group (10.5%) compared with control (14.3%) with a hazard ratio of 0.59 (95% confidence interval = 0.40, 0.88; P = .009). The risk of headaches was significantly associated with average level of Na excretion during follow-up, independent of most recent blood pressure. The relationship appeared to be nonlinear with a spline relationship and a knot at 150 millimoles per 24 hours.
Conclusions. Reduced sodium intake, currently recommended for blood pressure control, may also reduce the occurrence of headaches in older persons with hypertension.
Headache is a common health problem in adults, resulting in approximately 18 million physician visits in the United States each year.1 Globally, the estimated lifetime prevalence of headaches in adults is 66%.2 The most common types of headaches are nonvascular and are commonly termed “tension headaches.”1 Tension headaches have been attributed to muscle spasm in the head, neck, and shoulders in response to stress, fatigue or environmental factors like noise or bright lights.3 However, the pathophysiology of tension headaches is uncertain.
Headaches have been associated with elevated blood pressure (BP), including sustained severe hypertension, malignant hypertension, and paroxysmal hypertension.4 In the Hypertension Optimal Treatment trial, in which participants were randomized to 1 of 3 diastolic BP goals, headaches were reduced in all treatment groups, independent of BP goal and type of antihypertensive drug.5
High sodium intake is associated with elevated BP, and clinical trials have demonstrated that sodium-reduction (NaD) lowers BP in individuals with or without hypertension.6–13 A high intake of sodium potentially leads to headaches through a direct effect on BP or indirectly through a BP-independent mechanism. However, few studies have investigated the relationship of sodium intake to the occurrence of headaches. In preliminary observations from the Trial of Nonpharmacologic Interventions in the Elderly (TONE), we reported that individuals who were assigned to the NaD intervention had a lower incidence of headaches.13 A more recent analysis using data collected from the Dietary Approaches to Stop Hypertension (DASH)-sodium trial reported that NaD was associated with a lower risk of headaches,14 replicating the earlier observation from the TONE study. We aimed to expand on the original observation from TONE by examining the relationship between sodium intake and headaches, with a particular focus on assessing dose–response relationship.
METHODS
TONE was a multicenter, randomized controlled trial designed to test the efficacy of nonpharmacologic interventions as a means to control hypertension in the elderly. A detailed description of the design and methods of this trial has been published elsewhere.15 Eligible individuals were aged 60 to 80 years, were community dwelling, and had hypertension controlled on single antihypertensive medication. Major exclusion criteria included history of a heart attack or stroke within the preceding 6 months, current angina pectoris, congestive heart failure, insulin-dependent diabetes mellitus, serious mental or physical illness, unexplained or involuntary weight loss of 4.5 kilograms or greater during the previous year, hypercreatinemia (> 2.0 mg/dL), hyperkalemia (> 5.5 mmol/L), and anemia (hemoglobin level < 11 g/dL).
We randomly assigned overweight persons (body mass index [BMI; defined as weight in kilograms divided by the square of height in meters] > 27.3 kg/m2 in men, > 27.8 kg/m2 in women) to 1 of 4 groups in a 2 × 2 factorial design (usual care, weight loss alone, reduced sodium alone, or combined weight loss and reduced sodium). We assigned nonoverweight persons to usual care or reduced sodium intake. The NaD goal for the reduced sodium groups, both NaD alone and NaD combined with weight loss, was to achieve and maintain a 24-hour dietary sodium intake of 80 millimoles (1800 mg) or less. Three months after the start of intervention, we employed a standardized protocol to gradually taper and withdraw antihypertensive medication in participants whose BP remained less than 150/90 millimeters of mercury.
The primary trial outcome was a composite endpoint that included recurrence of high BP, resumption of antihypertensive medication, or a clinical cardiovascular event. During follow-up, we restarted antihypertensive medication if (1) systolic BP was 190 millimeter of mercury or more or diastolic BP was 110 millimeters of mercury or more at a single visit (average of 3 BP measurements), (2) mean systolic BP was 170 millimeters of mercury or more or diastolic BP was 100 millimeters of mercury or more over 2 consecutive visits (average of 6 BP measurements), or (3) mean systolic BP was 150 millimeters of mercury or more or diastolic BP rose to 90 millimeters of mercury or more at 3 consecutive visits (average of 9 BP measurements).
We enrolled participants between August 30, 1992, and June 27, 1994. We collected TONE data at 2 screening visits to establish study eligibility, a randomization visit, drug withdrawal visits (90 days after the start of intervention), and 11 subsequent quarterly follow-up visits (beginning 6 months after randomization). Closeout visits occurred between July and December 1995. The median follow-up in TONE was 29 months (range = 1–36 months). We conducted the study at 4 clinical centers (Johns Hopkins University, Wake Forest University School of Medicine, Robert Wood Johnson Medical School, and the University of Tennessee).13 Staff members, who were blinded to the participant’s randomized treatment assignment, obtained outcome information.
Variables
We estimated dietary sodium intake by measurement of carefully collected 24-hour urinary sodium excretion. We analyzed urinary sodium levels by flame photometry.13 In TONE we ascertained headaches by participant self-report of an adverse event at any follow-up visit. At each follow-up visit, participants completed a brief self-reported medical history and adverse events questionnaire. A study nurse evaluated any participant who reported an intercurrent health-related concern. If a participant reported a medical problem that was potentially serious (e.g., angina) or a symptom that was severe (e.g., headache or chest pain) the nurse completed an adverse event form. A physician then reviewed and coded the adverse event by type (e.g., stroke, myocardial infarction, headache). We also collected and coded the date of the event. Both the nurse and physician were unaware on the participant’s treatment assignment.
Trained observers who were masked to intervention assignment measured BP. At each visit, we obtained 3 BP measurements while participants rested in the seated position. We collected detailed demographic and socioeconomic information including age, gender, race, physical activity, smoking habits, and alcohol intake at baseline.15 We also collected interval medical information, medication use, and body weight measurement at each visit.
Statistical Analyses
We expressed descriptive data for identification of baseline characteristics as well as urinary sodium excretion at each visit as means (SD) for continuous variables and counts (%) for categorical variables. We used the Student t test and χ2 test to compare continuous variables and discrete variables, respectively. For all the analyses, the primary outcome was headache, which we identified by means of adverse event reports.
First, we compared incidence of headaches between those in the NaD intervention group (i.e., the NaD alone plus combined weight loss and NaD interventions) and their counterparts in the control group (i.e., usual care plus weight loss alone interventions). We used Kaplan-Meier plots to explore temporal patterns for cumulative incidence of headaches and Cox proportional hazard regressions to estimate the hazard ratio (HR) and 95% confidence interval (CI) for comparison of first occurrence of a headache between the 2 groups. Because this comparison was on the basis of randomized assignments, we only adjusted for variables that were significantly different between the NaD intervention and control groups (i.e., time-varying drug withdraw status and BPs). Although we conducted the main analyses between the NaD and control groups, we also performed a sensitivity analysis to compare headaches among the 4 original group assignments of the factorial design (i.e., usual care, weight loss alone, NaD alone, and combined weight loss and NaD interventions).
Second, we examined whether there were a dose–response relationship and threshold effect between sodium intake and the occurrence of headaches. The exposure was 24-hour urinary sodium excretion, measured as the average level during follow-up. We used Cox proportional hazard regressions to investigate the frequency of new onset headaches, assessed as an adverse event (incident headaches postrandomization). In these models, we combined participants from all intervention groups and we included potential confounders such as age, gender, race, physical activity, smoking, alcohol intake, headaches at baseline, average weight loss during follow-up, and drug withdraw status (time-varying variable) in the adjusted analyses. To explore whether the relationship of headaches with sodium intake was independent of BP, we additionally adjusted for the most recent BP. We performed all statistical analyses using SAS version 9.4 (SAS Institute, Cary, NC). We considered a P value of less than .05 (2 sides) statistically significant.
RESULTS
Table 1 displays the baseline characteristics of all TONE study participants by assignment to the NaD intervention or control. Of the 975 individuals, 52% were men, 76% were White, and the mean (SD) age was 65.8 (4.6) years. Mean (SD) 24-hour urinary sodium excretion at baseline was 148.5 (54) millimoles per 24 hours. Overall, there was no statistically significant difference in baseline characteristics by group (NaD vs control).
TABLE 1—
Baseline Characteristics of Participants by Randomly Assigned Group: Trial of Nonpharmacologic Interventions in the Elderly, United States, 1992–1995
| Characteristic | Control (n = 488), Mean (SD) or % | Sodium Reduction (n = 487), Mean (SD) or % | Alla (n = 975), Mean (SD) or % |
| Age, y | 65.8 (4.5) | 65.8 (4.7) | 65.8 (4.6) |
| Male | 51.8 | 52.6 | 52.2 |
| White | 74.8 | 77.2 | 76.0 |
| SBP, mmHg | 128.1 (9.3) | 128.4 (9.4) | 128.2 (9.3) |
| DBP, mmHg | 71.3 (7.4) | 71.4 (7.2) | 71.4 (7.3) |
| Urine sodium, mmol/24 h | 148.6 (54.9) | 148.4 (53.4) | 148.5 (54.2) |
| Weight, lb | 180.0 (26.3) | 180.7 (28.4) | 180.3 (27.4) |
| BMI, kg/m2 | 28.9 (3.5) | 28.9 (3.6) | 28.9 (3.5) |
| Alcohol user | 34.8 | 35.5 | 35.2 |
| Currently smokes | 4.9 | 5.7 | 5.3 |
Note. BMI = body mass index; DBP = diastolic blood pressure; SBP = systolic blood pressure.
We used the Student t test and χ2 test to compare continuous variables and discrete variables, respectively. There were no statistically significant differences (all P > .05) in baseline characteristics between the control and sodium-reduction study groups.
Participant attendance rates were 90%, 86%, and 86% at the 9-, 18-, and 30-month follow-up visits. BP measurements were available for 100% of the visits. We collected 24-hour urine samples in 867 (88.9%), 804 (82.5%), and 421 (82.1%) participants at the 9-, 18-, and 30-month visits, respectively.
Urinary Sodium Excretion and Headache Occurrence
Baseline urinary sodium excretion did not differ between the NaD and control groups (Table A, available as a supplement to the online version of this article at http://www.ajph.org). We observed significantly greater reductions in urinary sodium from baseline in the NaD group compared with the control group at the 9-, 18-, and 30-month follow-up visits. The mean changes in urinary sodium excretion in the NaD group were −40.9, −43.2, and −45.3 millimoles per 24 hours at the 9-, 18-, and 30-month follow-up visits, respectively. Corresponding mean changes in the control group were 0.4, −3.1, and −4.5 millimoles per 24 hours, respectively. During follow-up, the mean between-group difference in average urinary sodium excretion was 38.8 millimoles per 24 hours.
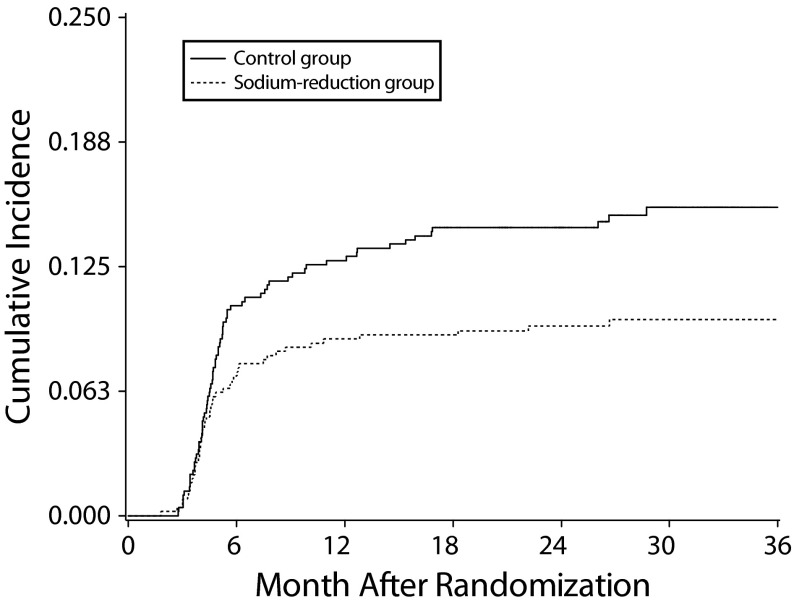
During follow-up, 126 participants reported headache as an adverse event, and 13 (10.3%) reported headache twice as an adverse event. We used the first reported headache as our study outcome. Figure 1 displays the cumulative incidence of headaches during follow-up by randomized assignment. In the control group, 14.3% reported headaches during follow-up. By contrast, only 10.5% reported headaches in the NaD group (log-rank test, P = .012).
FIGURE 1—
Cumulative Incidence of Headaches by Intervention Group (Reduced Sodium vs Control): Trial of Nonpharmacologic Interventions in the Elderly, United States, 1992–1995
Note. We used the log-rank test to compare the group difference (P = .012).
The HR for headaches in the NaD group compared with control was 0.56 (95% CI = 0.38, 0.83; P = .01) after adjustment for clinical center, time-varying drug withdrawal status, and average weight loss during follow-up (model 2). After further adjustment for most recent systolic and diastolic BP before the occurrence of headaches (model 3), the HR was essentially unchanged (HR = 0.59; 95% CI = 0.40, 0.88; P = .02). In the sensitivity analysis, the HR for headaches (model 3) was 0.83 (95% CI = 0.49, 1.40; P = .48) in the weight loos alone intervention, 0.61 (95% CI = 0.39, 0.95; P = .03) in the NaD alone intervention, and 0.47 (95% CI = 0.25, 0.92; P = .03) in the combined weight loos and NaD intervention compared with the control group.
Observational Analyses and Dose–Response Relationship
Table 2 summarizes results from the Cox proportion hazard models using average urinary sodium excretion during follow-up as the primary exposure. We identified a statistically significant direct association of headaches with average urinary sodium excretion. After adjustment for clinical center, age, smoking, race, gender, alcohol use, physical activity, average weight loss during follow-up, and drug withdrawal status (model 2), a 10 millimoles per 24 hour higher level of urinary sodium excretion was associated with a hazard of headaches that was 7.0% higher (95% CI = 4%, 11%, P ≤ .001). The association between urinary sodium excretion and risk of headaches persisted after further adjustment for most recent systolic BP and diastolic BP in model 3. Because headaches were more common in women (17.0%) than in men (8.8%), we further stratified our analyses by gender. The magnitude of the associations was similar in both gender; the HR of headaches associated with a 10 millimoles per 24 hours higher level of urinary sodium excretion was 1.09 (95% CI = 1.03, 1.15) for men and 1.07 (95% CI = 1.02, 1.12) for women.
TABLE 2—
HR (95% CI) of Headaches by Level of Urinary Sodium Excretion During Follow-Up: Trial of Nonpharmacologic Interventions in the Elderly, United States, 1992–1995
| Average Urinary Sodium as Categorical Variableb |
|||
| Model | Average Urinary Sodium Excretion as a Continuous Variable,a HR (95% CI) | 120–150 mmol/24 h, HR (95% CI) | > 150 mmol/24 h, HR (95% CI) |
| Model 1c | 1.04 (1.00, 1.07) | 1.01 (0.62, 1.66) | 1.65 (1.11, 2.46) |
| Model 2d | 1.07 (1.04, 1.11) | 1.15 (0.67, 1.95) | 2.27 (1.45, 3.55) |
| Model 3e | 1.07 (1.04, 1.11) | 1.26 (0.74, 2.16) | 2.32 (1.48, 3.65) |
Note. CI = confidence interval; HR = hazard ratio. Cox proportional hazard models showing HR of headaches associated with average urinary sodium excretion.
Higher by 10 mmol/24 h.
< 120 mmol/24 h is the reference group.
Cox proportional hazards models adjusted for clinical center.
Model 1 plus smoking, race, gender, age, alcohol use, baseline headache, average weight loss during follow-up, physical activity and time-varying drug withdrawal status.
Model 2 plus most recent systolic blood pressure and diastolic blood pressure before the occurrence of headaches.
In exploratory analyses, the relationship between headaches and absolute urinary sodium level appeared to be nonlinear, with a threshold between 120 to 150 millimoles per 24 hours. We noted the greatest evidence for an association between urinary sodium excretion and occurrence of headaches above this threshold. For this reason, we categorized the participants into 3 groups on the basis of their average urinary sodium excretion during follow-up: (1) less than 120 millimoles per 24 hours (below the threshold, lower sodium intake), (2) 120–150 millimoles per 24 hours (close to threshold, moderate sodium intake), and (3) more than 150 millimoles per 24 hours (above threshold, higher sodium intake). Compared with the category of lower sodium intake, individuals with moderate sodium intake did not exhibit a significantly higher risk of headaches (HR = 1.26; 95% CI = 0.74, 2.16; P = .39). However, individuals in the higher sodium intake category had a significantly higher risk of headaches than did those in the lower sodium intake category (HR = 2.32; 95% CI = 1.48, 3.65; P ≤ .001).
During spline interpolation, we identified knots at 120 millimoles per 24 hours and 150 millimoles per 24 hours. Above the 150 millimoles per 24 hours knot, an increase of 10 millimoles per 24 hours in urinary sodium excretion was associated with an increase of 9.7% in the hazard of headaches (95% CI = 3.4%, 16.9%; P = .002) after adjusting for age, smoking, race, gender, alcohol use, physical activity, average weight loss during follow-up, and drug withdrawal status. This positive association remained significant with further adjustment for most recent BPs in model 3 (HR = 1.09; 95% CI = 1.02, 1.18; P = .004). The risk of headaches increased dramatically and significantly as the urinary sodium excretion level was above the 150 millimoles per 24 hours (Figure A, available as a supplement to the online version of this article at http://www.ajph.org).
DISCUSSION
In this analysis of 975 persons, aged 60 to 80 years who participated in the TONE trial, we found that NaD was associated with a lower risk of headaches over the course of 36 months of follow-up. Specifically, individuals who were randomly assigned to a NaD intervention had a lower cumulative incidence of headaches than did their counterparts in the control group. In an observational analysis, urinary sodium excretion, measured as an average level during follow-up, was associated with occurrence of incident headaches, independent of other risk factors, baseline headaches, use of BP lowering medications, and most recent level of BP. We observed an apparent threshold effect, with the risk of headaches increasing progressively above a urinary sodium excretion of 150 millimoles per 24 hours. Above the sodium excretion threshold of 150 millimoles per 24 hours, risk of incident headaches was higher by 7.8% for every 10 millimoles average increase in 24-hour urinary sodium; below this threshold, there was no significant relationship.
Although numerous studies have assessed the effects of a reduced sodium intake on BP6,7,11–13 and several studies have assessed the relationship between headaches and BP,4,5,16,17 the relationship between sodium intake and the occurrence of headaches has received scant attention. The most relevant study is the DASH-Sodium trial, a randomized, controlled feeding study that tested the effects of 3 levels of sodium intake (50, 100, and 150 mmol/24 hr) in 2 different diets (the DASH diet and a typical American diet). In a recent secondary analysis of this trial, a reduced sodium intake was associated with significantly lower risk of headache (odds ratio[OR] = 0.69; P = .05, among those consuming a typical American diet, and OR = 0.69; P = .04, among those consuming the DASH diet) in adults with prehypertension and stage 1 hypertension.14
By contrast to TONE, study participants in the DASH-Sodium trial tended to be younger, with a mean (SD) age of 48 years.10 Also, the 2 trial designs and duration of follow-up were different (a cross-over trial with approximately 30 days of follow-up for each period of intervention in the DASH-Sodium trial and a parallel arm trial with a median follow-up of 29 months in the TONE trial), the extent of NaD was almost twofold greater in the DASH-Sodium trial than in the TONE trial, and the methods used to ascertain headaches were different in the 2 studies (symptom check list in the DASH-Sodium trial and adverse event reporting in the TONE trial). Overall, the findings from our analysis and the corresponding DASH-Sodium report provide consistent evidence regarding the apparent effect of sodium intake on the occurrence of headaches. Because of the use of adverse events reporting as the method of ascertainment for headaches in the TONE trial, it seems likely that the results from this trial may have highlighted the relationship between NaD and more severe headaches.
The mechanisms underlying an association between sodium intake and headaches are uncertain. Sodium intake may be related to headaches through a direct effect on BP or indirectly through other BP-independent mechanisms. Excessive intake of sodium is a well-established risk factor for high BP, and NaD can lower BP in hypertensive and nonhypertensive individuals.6–13 Elevated BP has been associated with headaches independent of weight status and antihypertensive drug therapy.4,5 Therefore, it is possible that a high intake of sodium may lead to headaches through a direct effect on BP. However, controlling for recent BP in the Cox regression models did not attenuate the association between sodium and headaches in our analyses, suggesting that the effect of sodium on headaches might be independent of BP.
One proposed BP-independent mechanism relates to changes in vascular smooth muscle tone. Sodium intake is an important determinant of smooth muscle cell reactivity, which is in part mediated by changes in extracellular volume and perhaps by changes in intracellular calcium. A high sodium intake increases the reactivity of arterioles and the BP response to stress or sympathetic simulation.18 In a study of patients with asthma, a moderate reduction in sodium intake led to an improvement in asthma symptoms, potentially related to changes in bronchial smooth muscle tone.19 Such findings suggest that nonvascular headaches might be part of a continuum with vascular headaches at 1 extreme.20 Still, we cannot totally rule out an effect of BP in our study because we measured BP at prespecified follow-up visits, not at the time of the headaches.
Strengths and Limitations
Our study has several strengths. First, the sample size of TONE trial was relatively large (n = 975), and the duration of follow-up was relatively long (up to 3 years). Second, we collected information on potential adverse events at each study visit; a study clinician who was blinded to the participant’s treatment allocation verified these. Third, we estimated sodium intake by means of carefully collected 24-hour urine samples, with averaging of several samples for the observational analyses: twice before randomization and once at the 9-month, 18-month, and 30-month follow-up visits. Of the available methods, mean 24-hour urinary excretion of sodium collected from multiple occasions provides the most accurate estimate of dietary sodium intake for observational analyses.21 Study limitations include a lack of specific information regarding participant headaches severity and duration and prior history of headaches.
Nevertheless, headaches reported as an adverse event in TONE were associated with baseline headache experience,22 and we adjusted for baseline headaches in our observational analyses. Again, because we did not measured BP at the time of the event, inferences about the relationship between BP and headaches are uncertain. However, there were no differences when we compared BP measured at the study visit closest to the adverse event and the average BP during the follow-ups for study participants. The average time between most recent BP measurement and occurrence of adverse event headache was 12.3 days for those who had adverse event headache, which is relative short. Although the assortment of headaches in this study is probably not subject to reporting bias or directly related to the intervention assignments, we were unable to rule out the possibility that the observed improvement in occurrence of headaches in the NaD group could be a surrogate of improving general well-being.
Conclusions
Our findings suggest that reducing sodium intake lowers the risk of headaches in older persons with hypertension. Our findings are consistent with results from the DASH-Sodium trial, which enrolled a middle-aged study population and used a different approach to ascertainment. Whether a reduced sodium intake lowers the risk of headaches in other populations (e.g., persons with migraine headaches) is unknown. From a policy perspective, these data provide an additional rationale in support of recommendations by the American Heart Association, US federal government, Institute of Medicine, World Health Organization, and others to reduce dietary sodium intake.23
ACKNOWLEDGMENTS
The Trial of Nonpharmacologic Interventions in the Elderly (TONE) trial was supported by the National Institutes of Health (grants R01 AG-09799, R01 H-48642, R01 AG-09771, R01 AG-09773, P60 AG-10484, and K08 HLO2642).
We thank the TONE participants and staff for their contributions to the study.
HUMAN PARTICIPANT PROTECTION
The institutional review boards at Johns Hopkins University, Wake Forest University School of Medicine, Robert Wood Johnson Medical School, and the University of Tennessee approved the trial protocol. Oversight of the trial was also provided by an external data and safety monitoring board appointed by staff at the National Institute on Aging and the National Heart, Long, and Blood Institute. All potential study participants provided written informed consent. Clinical Trial Registration: clinicaltrials.gov identifier NCT00000535.
REFERENCES
- 1.DuBose CD, Cutlip AC, Cutlip WD., 2nd Migraines and other headaches: an approach to diagnosis and classification. Am Fam Physician. 1995;51(6):1498–1504. 1507–1509. [PubMed] [Google Scholar]
- 2.Stovner L, Hagen K, Jensen R et al. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia. 2007;27(3):193–210. doi: 10.1111/j.1468-2982.2007.01288.x. [DOI] [PubMed] [Google Scholar]
- 3.Rasmussen BK, Olesen J. Epidemiology of migraine and tension-type headache. Curr Opin Neurol. 1994;7(3):264–271. doi: 10.1097/00019052-199406000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Cirillo M, Stellato D, Lombardi C, De Santo NG, Covelli V. Headache and cardiovascular risk factors: positive association with hypertension. Headache. 1999;39(6):409–416. doi: 10.1046/j.1526-4610.1999.3906409.x. [DOI] [PubMed] [Google Scholar]
- 5.Wiklund I, Halling K, Ryden-Bergsten T. [What is the effect of lowering the arterial blood pressure on the quality of life? An auxiliary study to the HOT (Hypertension Optimal Treatment) trial] Arch Mal Coeur Vaiss. 1999;92(8):1079–1082. [PubMed] [Google Scholar]
- 6.The effects of nonpharmacologic interventions on blood pressure of persons with high normal levels. Results of the Trials of Hypertension Prevention, phase I. JAMA. 1992;267(9):1213–1220. doi: 10.1001/jama.1992.03480090061028. [Erratum JAMA 1992;267(17):2330] [DOI] [PubMed] [Google Scholar]
- 7.Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high-normal blood pressure. The Trials of Hypertension Prevention, phase II. The Trials of Hypertension Prevention Collaborative Research Group. Arch Intern Med. 1997;157(6):657–667. [PubMed] [Google Scholar]
- 8.Aburto NJ, Ziolkovska A, Hooper L, Elliott P, Cappuccio FP, Meerpohl JJ. Effect of lower sodium intake on health: systematic review and meta-analyses. BMJ. 2013;346:f1326. doi: 10.1136/bmj.f1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Appel LJ American Society of Hypertension Writing Group. ASH position paper: dietary approaches to lower blood pressure. J Clin Hypertens. 2009;11(7):358–368. doi: 10.1111/j.1751-7176.2009.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mozaffarian D, Fahimi S, Singh GM et al. Global sodium consumption and death from cardiovascular causes. N Engl J Med. 2014;371(7):624–634. doi: 10.1056/NEJMoa1304127. [DOI] [PubMed] [Google Scholar]
- 11.Sacks FM, Svetkey LP, Vollmer WM et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344(1):3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 12.Stamler R, Stamler J, Grimm R et al. Nutritional therapy for high blood pressure. Final report of a four-year randomized controlled trial—the Hypertension Control Program. JAMA. 1987;257(11):1484–1491. doi: 10.1001/jama.257.11.1484. [DOI] [PubMed] [Google Scholar]
- 13.Whelton PK, Appel LJ, Espeland MA et al. Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of nonpharmacologic interventions in the elderly (TONE). TONE Collaborative Research Group. JAMA. 1998;279(11):839–846. doi: 10.1001/jama.279.11.839. [DOI] [PubMed] [Google Scholar]
- 14.Amer M, Woodward M, Appel LJ. Effects of dietary sodium and the DASH diet on the occurrence of headaches: results from randomised multicentre DASH-sodium clinical trial. BMJ Open. 2014;4(12):e006671. doi: 10.1136/bmjopen-2014-006671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Appel LJ, Espeland M, Whelton PK et al. Trial of Nonpharmacologic Intervention in the Elderly (TONE). Design and rationale of a blood pressure control trial. Ann Epidemiol. 1995;5(2):119–129. doi: 10.1016/1047-2797(94)00056-y. [DOI] [PubMed] [Google Scholar]
- 16.Cooper WD, Glover DR, Hormbrey JM, Kimber GR. Headache and blood pressure: evidence of a close relationship. J Hum Hypertens. 1989;3(1):41–44. [PubMed] [Google Scholar]
- 17.Mathew NT. Migraine and hypertension. Cephalalgia. 1999;19(suppl 25):17–19. doi: 10.1177/0333102499019s2504. [DOI] [PubMed] [Google Scholar]
- 18.Burney P. A diet rich in sodium may potentiate asthma. Epidemiologic evidence for a new hypothesis. Chest. 1987;91(6 suppl):143S–148S. doi: 10.1378/chest.91.6_supplement.143s. [DOI] [PubMed] [Google Scholar]
- 19.Carey OJ, Locke C, Cookson JB. Effect of alterations of dietary sodium on the severity of asthma in men. Thorax. 1993;48(7):714–718. doi: 10.1136/thx.48.7.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merikangas KR, Fenton BT, Cheng SH, Stolar MJ, Risch N. Association between migraine and stroke in a large-scale epidemiological study of the United States. Arch Neurol. 1997;54(4):362–368. doi: 10.1001/archneur.1997.00550160012009. [DOI] [PubMed] [Google Scholar]
- 21.Whelton PK, Appel LJ, Sacco RL et al. Sodium, blood pressure, and cardiovascular disease: further evidence supporting the American Heart Association sodium reduction recommendations. Circulation. 2012;126(24):2880–2889. doi: 10.1161/CIR.0b013e318279acbf. [DOI] [PubMed] [Google Scholar]
- 22.Lipton RB, Pfeffer D, Newman LC, Solomon S. Headaches in the elderly. J Pain Symptom Manage. 1993;8(2):87–97. doi: 10.1016/0885-3924(93)90106-6. [DOI] [PubMed] [Google Scholar]
- 23.Whelton PK. Sodium, potassium, blood pressure, and cardiovascular disease in humans. Curr Hypertens Rep. 2014;16(8):465. doi: 10.1007/s11906-014-0465-5. [DOI] [PubMed] [Google Scholar]