Abstract
Despite a century of research into the factors that generate and maintain biodiversity, we know remarkably little about the drivers of parasite diversity. To identify the mechanisms governing parasite diversity, we combined surveys of 8,100 amphibian hosts with an outdoor experiment that tested theory developed for free-living species. Our analyses revealed that parasite diversity increased consistently with host diversity due to habitat (i.e., host) heterogeneity, with secondary contributions from parasite colonization and host abundance. Results of the experiment, in which host diversity was manipulated while parasite colonization and host abundance were fixed, further reinforced this conclusion. Finally, the coefficient of host diversity on parasite diversity increased with spatial grain, which was driven by differences in their species-area curves: while host richness quickly saturated, parasite richness continued to increase with neighborhood size. These results offer mechanistic insights into drivers of parasite diversity and provide a hierarchical framework for multi-scale disease research.
Keywords: biodiversity, disease ecology, dilution effect, macroecology, biogeography, emerging disease
Introduction
Why biodiversity is concentrated in some areas and not others remains one of the most central questions in community ecology, for which three explanations are often advanced (Connell 1978; Rosenzweig 1995; Allouche et al. 2012). First, species richness may increase with resource availability, as suggested by positive correlations between productivity and species richness (Waide et al. 1999; Mittelbach et al. 2001; Jetz et al. 2009; but see Adler et al. 2011). Increased energy availability is hypothesized to increase opportunities for “species packing” while decreasing the risk of local extinction by increasing population size (MacArthur 1970). Second, some habitats may support a large number of species because they offer more colonization opportunities, due to large patch size or close proximity to a propagule source (i.e., island biogeography theory, (MacArthur & Wilson 1967) and neutral theory, (Hubbell 2001). Finally, resource heterogeneity can promote coexistence and species richness by offering a greater diversity of niches, thereby limiting interspecific competition (habitat heterogeneity hypothesis, (Hutchinson 1959; MacArthur & MacArthur 1961; Rosenzweig 1995). For instance, variation in both structural habitat complexity and the diversity of prey resources have been strongly linked to consumer diversity (Tews et al. 2004). Collectively, these three factors have been shown to shape patterns of species richness across a wide range of taxa (Tews et al. 2004; Jetz et al. 2009), although consensus on their relative importance and potential interactions has remained elusive (Venail et al. 2008; Allouche et al. 2012; Chase & Knight 2013).
Despite nearly a century of empirical and theoretical research devoted to the study of free-living biodiversity, we know considerably less about parasite species richness and the factors underlying its variation. As written by Dunn et al. (2010), “The fact that warbler species distributions are better understood than the distribution of human pathogens is a gap that clearly deserves research attention.” This knowledge gap is even greater for parasites of wildlife, and has recently taken on added urgency in light of increased disease emergence across a range of non-human hosts (Fisher et al. 2012; Smith et al. 2014). One of the most frequently cited correlates of parasite diversity is the richness of host species (i.e., the “host-diversity-begets-parasite-diversity hypothesis”, (Hechinger & Lafferty 2005; Thieltges et al. 2011; Poulin 2014). Given the intimate association between hosts and parasites as well as the expectation that many parasites are specialized to infect a small number of host species (Poulin 2014), an increase in host species richness would be expected to increase parasite diversity. For example, in a recent meta-analysis of 21 published studies, Kamiya et al. (2014) reported a consistent, positive correlation between host and parasite species richness, independent of parasite type or host taxon. Similarly, Dunn et al. (2010) reported that bird and mammal richness were strong, positive predictors of the diversity of zoonotic parasites at the country level. Nonetheless, the ecological mechanisms that drive this pattern have rarely been explored – what are the processes that generate and maintain the correlation between host diversity and parasite diversity? While it is often assumed to stem from niche diversification alone, this explanation overlooks alternative mechanisms, such as changes in resource availability (e.g., host abundance) and colonization opportunities, both of which could correlate cryptically with host diversity in nature.
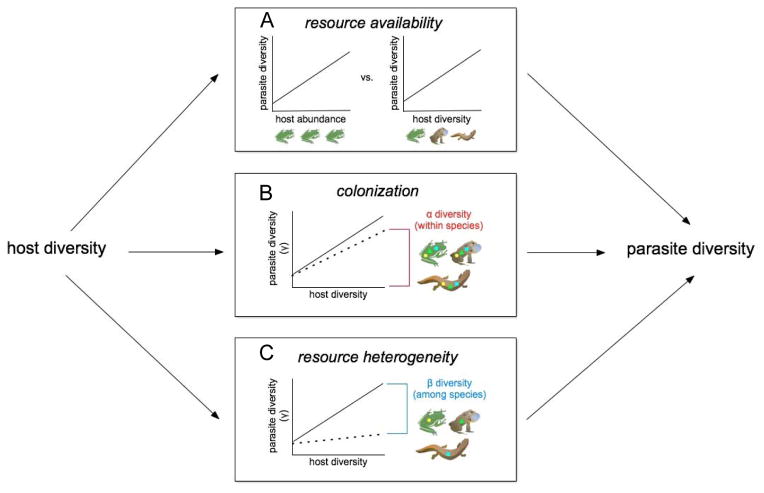
Building from community ecology theory, we can develop testable predictions to assess the contributions of hypothesized drivers of parasite diversity, such as colonization opportunities, resource availability, and resource heterogeneity. For instance, if parasite diversity is driven primarily by changes in resource availability (i.e., the diversity–productivity hypothesis, (Poulin 2014) – analogous with instances in which free-living diversity and productivity covary (Waide et al. 1999; Jetz et al. 2009) – host biomass or abundance should be a better predictor of parasite richness than host diversity, assuming host abundance and diversity are not overly collinear (Stevens & Carson 2002) (Fig. 1a). Decomposing community diversity (or γ diversity) into the sum of average richness per patch (α diversity) and among-patch variation (β diversity) can offer further insights into the structuring processes (Lande 1996; Gering and Crist 2002). If increased host diversity provides more opportunities for parasite colonization (i.e., the propagule-pressure hypothesis,(Levine 2000), as might occur when newly arriving hosts bring generalist parasites that spread to established hosts, then host diversity should correlate most strongly with average parasite richness per host, or parasite α diversity. Thus, increases in host diversity drive an overall increase in parasite richness in all host species, rather than by adding specialized parasites infectious to only one or a few host species (Fig. 1b). Finally, if the key determinant of parasite diversity is variation in niche opportunities (i.e., the habitat-heterogeneity hypothesis), host diversity should correlate most strongly with parasite β diversity, or the among-host diversity in parasite species. In this scenario, the overall richness of parasites within the community will increase owing to greater variation in parasite species composition among different host species, even while the average richness of parasites per host species remains constant (Fig. 1c).
Figure 1.
Host diversity can influence parasite diversity through at least three possible mechanisms. (A) First, the relationship may be driven by changes in resource availability (the diversity-productivity relationship), which might be expected if increased host richness correlates with an increase in total host species abundance. In this scenario, the total abundance or biomass of hosts should be a better predictor of parasite richness than is host species richness. (B) Second, variation in parasite colonization (the propagule-pressure hypothesis) may underlie the correlation between host and parasite diversity. If newly arriving host species are accompanied by generalist parasites that spread to already established hosts, we would predict that increases in host diversity should correlate with an increase in the average number of parasite species per host (α diversity), regardless of the identity of that host species. (C) Alternatively, the diversity covariance between hosts and parasites may emerge from changes in resource heterogeneity (the habitat-heterogeneity hypothesis). Here, new host species represent novel habitats for parasites that otherwise could not establish, which would lead to a stronger relationship between host diversity and the among-host diversity in parasite species composition (β diversity). Thus, parasite diversity is increasing only at the level of the host community, rather than within individual hosts or host populations.
Changes in the form or magnitude of the host-diversity-begets-parasite-diversity relationship across scales can offer further insights into the underlying drivers. Previous research in community ecology has highlighted the importance of both biological and spatial scale in determining the influence of putative biodiversity drivers (e.g., Levin 1992; Scheiner et al. 2000; Cadotte & Fukami 2005; Chase & Knight 2013). For instance, while free-living species richness often increases linearly with productivity at regional scales (i.e., for γ diversity), this relationship becomes unimodal at local scales (i.e., for α diversity) due to increased heterogeneity in species composition among sites (Chase & Leibold 2002). How the link between host and parasite diversity varies across biological levels (e.g., individual, population, community) remains uncertain, but there are many reasons to expect the factors influencing parasite richness at the individual host level differ from those affecting richness at the host community level. Similarly, the strength or slope of the host–parasite diversity correlation could vary with spatial grain for several reasons. First, if parasite species are more spatially aggregated than their hosts, increases in spatial grain will likely yield greater numbers of parasite species per host species, increasing the slope between host and parasite richness. Alternatively, the slope may decrease with spatial grain if the local-scale interactions (i.e., host–parasite and parasite–parasite interactions) driving the relationship are swamped by processes operating at larger scales (e.g., climatic gradients, biogeographical history) (Fridley et al. 2007). To date, however, the challenge of quantifying host and parasite diversity across multiple scales has limited opportunities to test the mechanistic links underlying this relationship.
Here, we use a hierarchically-nested dataset involving 8,100 individual amphibian hosts representing 696 host populations and 424 host communities across a 758,100-ha region to systematically evaluate the mechanisms and scale-dependence of the diversity-begets-diversity relationship. Specifically, we asked the following questions: (i) What drives the positive covariance between host and parasite diversity? And (ii) how does this relationship change with scale, including the biological level of organization (hosts, populations, and communities) and spatial grain (ponds, parks, and metacommunities)? For consistency with previous work, here we use parasite richness as our proxy for diversity, although we acknowledge the importance of additional metrics such as evenness, genetic diversity, and functional diversity (e.g., Ostfeld and Keesing 2012). Adopting analytical approaches developed for free-living community ecology, we contrasted the individual and joint contributions of colonization (propagule hypothesis), resource availability (diversity–productivity hypothesis), and resource heterogeneity (habitat-heterogeneity hypothesis) (Fig. 1). We complemented our observational data with a mesocosm experiment that tested how variation in host diversity affected parasite α, β, and γ diversity while controlling host abundance and parasite colonization. The current work also helps form a bridge between scale-dependent approaches in parasitology (e.g., Bush et al. 1997) and diversity-partitioning methods applied to free-living communities (e.g., Gering et al. 2003).
Materials and Methods
Field sampling
To quantify the richness of host and parasites species across natural ecosystems, we sampled pond communities in the East Bay region of central California between 2009 and 2014 (see Fig. S1 in Supporting Information). We defined the community as the assemblage of larval amphibian species within a pond. Each pond was sampled two times per year using visual encounter surveys, standardized dip-net sweeps, and seine hauls to ensure adequate detection of all amphibian host species (Johnson et al. 2013c; Richgels et al. 2013). We focused on larval amphibians because this developmental stage is the most reliable indicator of breeding activity and the period when many water-borne infections are acquired. Sampling amphibians as they reached metamorphosis – rather than as more mobile adults – also helped to ensure that infections reflected conditions of the system in which the animal was collected. To measure parasite richness, we collected a subsample (10 to 15) of each host species (excluding federally-protected species) as they approached metamorphosis, and performed a systematic examination of all major tissues and organs for parasites (Hartson et al. 2011). These included 21 taxa of larval and adult digeneans, nematodes, cestodes, acanthocephalans, and protists (Table S1). Based on previous rarefaction analyses, these techniques were effective in quantifying both amphibian and parasite species richness at the saturation of their richness–sampling effort curves (Johnson et al. 2013a; Johnson et al. 2013b). This approach allowed us to evaluate how the richness of hosts affected parasite richness at multiple, nested levels, including within individual hosts, across host populations, and among amphibian communities (i.e., ponds). These scales parallel the oft-used levels from parasitology: infracommunity richness, component community richness, and compound community richness (Bush et al. 1997).
Mesocosm experiment
To better understand the mechanisms linking host and parasite diversity, we performed a mesocosm experiment in which we manipulated the diversity of amphibian hosts (1 versus 4 species) while maintaining the same total number of host individuals (40) and the same propagule input of larval trematodes (4 species). These richness values are broadly representative of the study system; among 424 host communities, the average ± 95% CI for host and parasite richness were 2.80 ± 0.095 (range: 1 to 6) and 4.10 ± 0.199 (range: 0 to 12), respectively. Our goal was to examine how shifts in host species richness affected the average parasite richness per host (α diversity), the among-host variation in parasite richness (β diversity), and the cumulative parasite richness per mesocosm (γ diversity). Mesocosms (378 L) were filled with well water and seeded with sediment, zooplankton, and algae using standard methods (Johnson et al. 2013b). In the low host diversity treatment, we added 40 larvae of Pseudacris regilla, the most commonly encountered amphibian species in the region; in the high host diversity treatment, we added 10 individual larvae of Pseudacris regilla, Taricha torosa, Anaxyrus americanus, and Lithobates catesbeianus, which collectively comprise the four most common, non-endangered amphibian species in the study region. To obtain infectious parasites, we collected snails (Helisoma trivolvis) infected with different larval trematodes (Ribeiroia ondatrae, Echinostoma spp., Alaria spp., and Cephalogonimus spp.) and added between 694 and 11,141 parasite cercariae of each species over a 12-day period (see (Johnson et al. 2013b). Each of these parasites is a generalist capable of infecting a wide range of different amphibians, although their infection success is expected to vary by host (Johnson et al. 2013b). Two weeks after initiating the experiment, we measured each host and quantified parasite infection.
Analysis
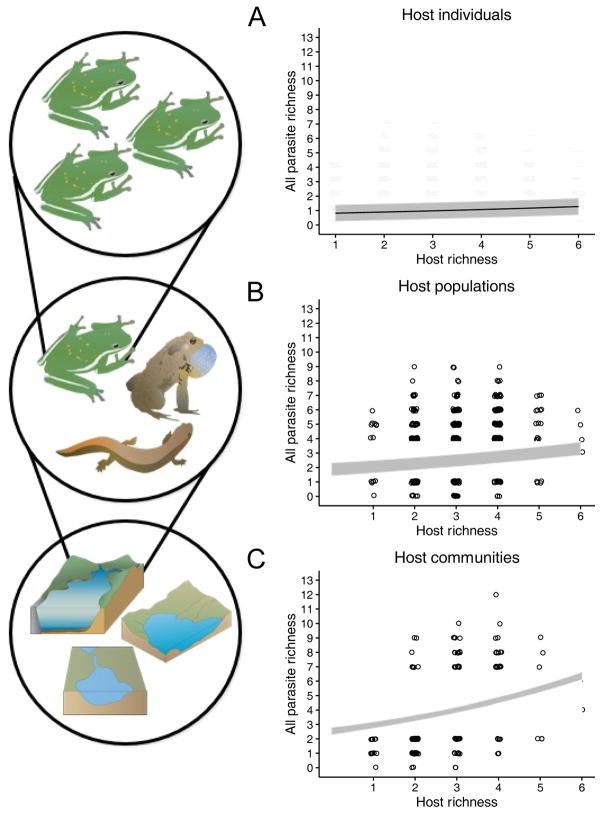
We analyzed the relationship between amphibian and parasite diversity in nature at three biological levels of organization: the host individual, for which we used parasite richness within individual hosts as the response; the host population, for which we used the total number of parasite species within all individuals of a given host species in a given pond; and the host community, for which we used the total richness of parasites across all host species in the same pond (Fig. 2). For each, we used generalized linear mixed models (GLMMs) with parasite richness as a Poisson-distributed response, host species richness in the pond as a fixed effect, and wetland, sample year, and host species as random effects (depending on the level of the analysis). To subsequently evaluate the ecological drivers of detected relationships between host and parasite diversity, we ran the same GLMM described above but added a level-dependent measure of host resource availability as an additional fixed effect. At the individual host level, we used host body size (snout–vent length in mm) as an indicator of resource availability; at the population level, we multiplied the average number of hosts of a given species per dip-net sweep by the pond perimeter as a proxy for population abundance; at the community level, we multiplied the total number of hosts per sweep by pond perimeter as a proxy for community abundance. The population and community measures of host availability were log10-transformed to help normalize their distributions. We used perimeter rather than pond area because larval amphibians tend to occur in the littoral rather than pelagic zone, although results are comparable with either variable. After verifying that host diversity and host resource availability were not collinear, we compared their coefficient estimates at each scale and evaluated their statistical significance.
Figure 2.
Effects of host diversity on parasite richness within individual hosts, host populations, and host communities. For each biological scale, the richness of amphibian host species detected at the pond level was positively related to the taxonomic richness of parasites. (A) Host scale: coefficient=0.088, conditional R2=0.47, p<0.0001, n=8100; (B) Population scale: coefficient=0.095, conditional R2=0.43, p=0.0002, n=696; (C) Community scale: coefficient=0.151, conditional R2=0.31, p<0.00001, n=423.
To evaluate the roles of parasite colonization and niche heterogeneity in driving covariation between host and parasite diversity, we applied diversity-decomposition methods by examining how host diversity affected the subcomponents of parasite diversity: the within-patch or α diversity, the among-patch or β diversity, and their sum (or product), which is γ diversity (Whittaker 1960; Lande 1996; Gering & Crist 2002; Gering et al. 2003). If host diversity affects parasite diversity predominantly through changes in colonization opportunities (propagule-pressure hypothesis), the strongest effects should manifest on parasite α diversity. If, instead, the mechanism involves changes in niche heterogeneity, such that new hosts represent novel habitats for parasites, the effect of host diversity should be mediated through β diversity, or among-host species diversity.
We applied these additive partitioning methods at two biological levels: from individual hosts to populations (i.e., γpopulation = αhost + βhost), and from host populations to host communities (i.e., γcommunity = αpopulation + βpopulation). Thus, αhost is the average parasite richness per host of species i, while αpopulation is the average parasite richness per host species population within pond j. Following Lande (1996), we used the additive approach to estimate β (i.e., βi = γi − αi) and complemented this by also estimating Jaccard’s dissimilarity index. While Jaccard’s index offers a measure of β diversity that is independent of γ, it requires exclusions of any hosts or populations with no parasites, forcing the loss of biologically meaningful data. Because γpopulation = αpopulation, we can combine these equations together to assess the cross-scale contributions of host diversity: γcommunity = αhost + βhost + βpopulation (Gering & Crist 2002; Gering et al. 2003). To evaluate how host diversity affected parasite α and β diversity at the host and population levels (averaged values), we used linear mixed models with a fixed effect of host richness; at the host level, we included random effects for host species, pond, and year, and at the population level we included random effects of site and year.
Finally, to evaluate the influence of spatial grain, we used a neighborhood-based approach that combined communities together into progressively larger neighborhoods of connected sites and assessed resultant changes in the relationship between host and parasite species richness. We considered 100 evenly-spaced buffer distances ranging from 0 km, in which each pond was an independent community, to 15 km, when all wetlands fell into one of two neighborhoods – the minimum number necessary to compare host and parasite richness. For each buffer distance, we recalculated parasite and host richness aggregated at the neighborhood scale and fit a Poisson generalized linear model for the effect of host richness on parasite richness. We then compared how host and parasite richness changed with buffer distance (i.e., the species–area curve of each), as well as how the coefficient of the host–parasite diversity relationship varied with buffer distance.
Results
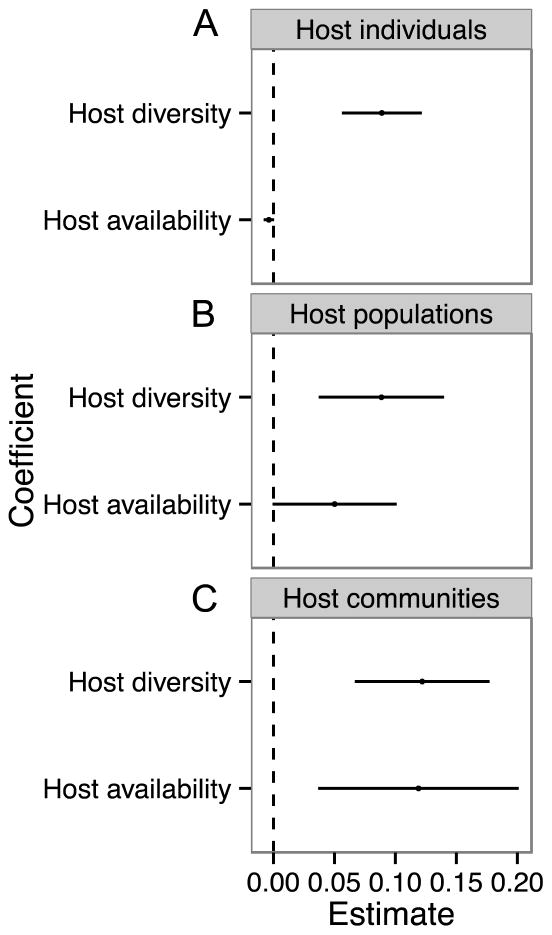
Over five years, we sampled 198 unique ponds, many of which were sampled across multiple years (424 site-by-year combinations). We encountered six amphibian species (Ambystoma californiense, Anaxyrus boreas, Lithobates catesbeianus, Pseudacris regilla, Rana draytonii, Taricha torosa) and 21 parasite taxa, including larval trematodes (n=8), adult trematodes (n=4), nematodes (n=5), acanthocephalans (n=1), protists (n=2), and cestodes (n=1). Among sites, host richness varied from 1 to 6 (average ± 1 SE=2.79 ± 0.048), while parasite richness ranged from 0 to 12 (average ± 1 SE=4.08 ± 0.101). Host species richness was a consistent, positive predictor of parasite richness; however, the magnitude of this relationship varied with biological level of organization, increasing progressively from individual hosts to host populations to host communities (Fig. 2). The coefficient of host diversity on parasite diversity at the community scale was ~2× that observed for individual hosts (Fig. 2). Host resource availability received marginal support as a predictor of parasite diversity at the host and population levels (p=0.089 and p=0.053, respectively), but became a more important co-contributor at the community scale (p=0.004) (Fig. 3).
Figure 3.
Assessing the roles of resource availability versus resource heterogeneity on parasite diversity at the (A) host, (B) population and (C) community scales. Depicted are the coefficient estimates ± 95% of host availability and host richness when included together in regression models. Host availability was defined as host body size (snout–vent length) at the individual scale, as the log10-transformed abundance of hosts of a given species at the population level, and the log10-transformed total abundance of all host species at the community level. While host richness was a positive predictor of parasite richness across all three scales, host availability was a co-contributor only at the community scale.
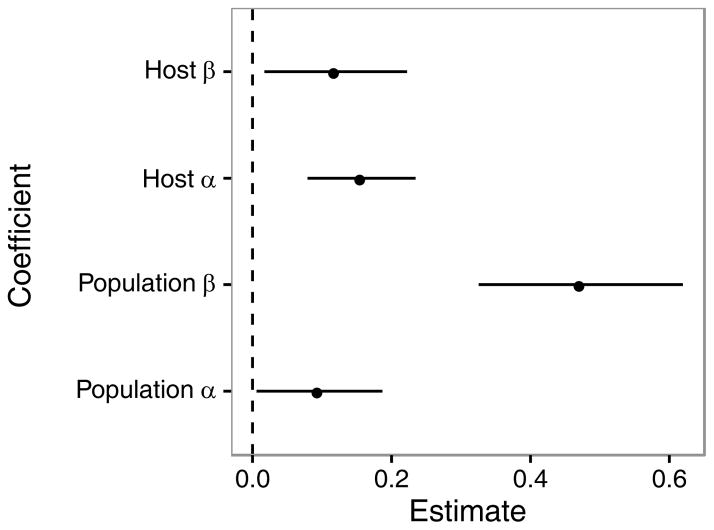
Decomposition of parasite γ diversity into its component contributions from α and β illustrated the importance of resource heterogeneity over parasite colonization in driving the host-diversity-begets-parasite-diversity relationship. Although host diversity had positive effects on parasite α and β diversity at both the host and population scales (Fig. 4), the largest coefficient was for parasite βpopulation, which was ~4× the coefficient of host diversity on parasite αhost, βhost, or αpopulation. Thus, while the increases in α diversity within individual hosts and populations suggested that changes in parasite colonization helped explain the effects of host diversity on parasite richness, our overall results indicated that the strongest influence of host diversity was on parasite diversity at the among-host species level, consistent with predictions of the habitat-heterogeneity hypothesis. Comparable effects were observed when using Jaccard’s dissimilarity index as the response variable. By recalculating γcommunity = αhost + βhost + βpopulation, we found that the proportional contribution of βpopulation to γcommunity increased monotonically with host diversity, from an average of 23.4% at low host diversity (1 to 3 species) to ~47% at high host diversity (4 or more species).
Figure 4.
Diversity decomposition analysis of the host-diversity-begets-parasite diversity relationship. Host diversity had positive but variable effects on the average richness of parasites per host (αhost; coefficient=0.157, p=0.0008), the among-host individual variation in parasite composition (βhost; coefficient=0.119, p=0.019), the average richness of parasites per host population (αpopulation; coefficient=0.096, p=0.035), and especially the among-host population variation in parasite composition (βpopulation; coefficient=0.423, p<0.0001). Depicted is the coefficient estimate ± 95% confidence interval of host diversity on parasite diversity as determined through linear mixed effects models.
Results of the mesocosm study indicated that increases in host richness from one to four species had no effect on parasite richness at the community level (γ diversity); indeed, in all but one mesocosm, each administered parasite species established successfully, regardless of the host community (t=1.00, p=0.331; n=40), consistent with the generalist nature of these parasites. Similarly, there was no effect of host richness on parasite richness per host individual after accounting for host species as a random effect (Poisson GLMM; coefficient ± 1 SE=0.117 ± 0.083, z=1.418, p=0.156). However, αhost decreased from an average of 2.446, 95% CI: (2.346, 2.545) at low-host diversity to 2.123, CI: (2.013, 2.233) at high-host diversity. Similarly, βhost decreased from 1.454, CI: (1.244, 1.664) to 0.923, CI: (0.793, 1.053) between the low- and high-host diversity treatments, respectively. Because total parasite richness was unaffected by host diversity (i.e., γcommunity was constant), these decreases led to a concurrent increase in βpopulation, which was fixed at 0 in the low diversity treatment (i.e., there was only 1 host population) but increased to 0.95, CI: (0.79, 1.11) when host richness was high (~24% of parasite γcommunity). These values were similar in magnitude to field observations, for which βpopulation increased from zero in single-host species communities to ~21% of gamma diversity when four host species were present. Results were comparable if we used Jaccard’s dissimilarity index, which is unconstrained by gamma diversity, for which compositional dissimilarity increased from 0.22 to 0.44 between the treatments (GLM; coefficient ± 1 SE=0.217 ± 0.023, t=9.65, p<0.0001), which compared with field observations of 0.245 and 0.433 at low (1–3) and high (4–6) host species. Within P. regilla specifically, which was the only host species shared between the low and high host diversity treatments, there was no effect of host diversity on either parasite richness per host individual or Jaccard’s dissimilarity index (p=0.17), further emphasizing the role of among-species variation in driving the observed results. Host survival among mesocosms averaged ~97% and was unrelated to treatment.
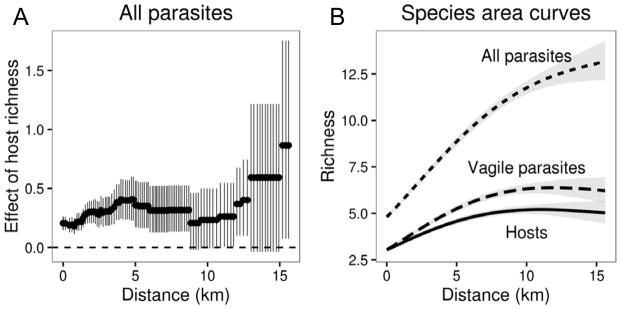
With respect to spatial scale, increasing the size of the spatial neighborhoods in our field survey led to nonlinear increases in the estimated coefficient between host and parasite diversity (Fig. 5). Initial increases in buffer distance (e.g., 0 to 5 km) tended to connect ponds within the same park and led to an increase in estimated coefficient from 0.206, CI: (0.148, 0.263) to 0.351, CI: (0.166, 0.555). At distances between 5 and 10 km, there was little change in the number of neighborhoods or the coefficient estimate, in large part because the minimum distance between parks averaged ~7 km and this increase in buffer led to few changes in connectivity. Further increases in buffer distance (>10 km) connected parks together (i.e., into metacommunities) and led to additional increases in the coefficient (Fig. 5a), although the 95% confidence intervals also included zero due to the decrease in sample size as neighborhoods became larger (i.e., each pond was assigned to only one neighborhood without replacement). Importantly, the species–area curves for hosts and parasites revealed that host richness increased much more slowly than parasite richness and reached a plateau at a lower buffer distance, such that progressive increases in spatial grain disproportionately added new parasites rather than new hosts (Fig. 5b). Correspondingly, a Mantel test indicated that parasite community dissimilarity increased with geographic distance (r=0.06, p=0.006), whereas no such relationship was detected for hosts (r=0.011, p=0.331) or when selecting only parasites with highly mobile definitive hosts (r=−0.015, p=0.73), such as birds and mammals.
Figure 5.
Influence of spatial grain and neighborhood size on the relationship between host and parasite diversity. (A) Increases in neighborhood size tended to non-linearly increase the coefficient of the relationship between host and parasite richness; error bars represent the 95% CI of the estimate, for which sampling was done without replacement (i.e., sample size decreases as spatial grain is increased). (B) Species–area curves for hosts, highly vagile parasites (e.g., larval trematodes that have avian and mammalian definitive hosts, Table S1), and all parasites as a nested function of buffer distance.
Discussion
By applying theory derived for free-living communities to a hierarchically-nested dataset of host and parasites, our analyses revealed that host diversity is a consistent, positive predictor of parasite diversity, but that the strength of this relationship and the underlying mechanisms varied with both the biological level of organization and the spatial scale. The magnitude of the coefficient between host and parasite diversity increased three-fold as the level of biological organization moved from individual hosts to host communities. Similarly, the coefficient increased as we connected communities into progressively larger metacommunities and recalculated their cumulative richness, emphasizing both the generality of the diversity-begets-diversity relationship, as well as its scale dependence. The patterns observed are consistent with the idea that niche heterogeneity causes covariance between host and parasite diversity (i.e., our results support the habitat-heterogeneity hypothesis), as indicated by the strong relationship between host diversity and parasite β diversity at the population-scale. However, both colonization opportunities and resource availability in the form of total host abundance also contributed to the effects of host diversity on parasite diversity, demonstrating that multiple mechanisms explain the diversity covariance between hosts and parasites.
Although host diversity has often been identified as a significant correlate of parasite diversity (e.g., Kamiya et al. 2014), the ecological mechanisms that drive this pattern are not readily apparent. The positive correlation between host and parasite diversity is frequently assumed to emerge from the fact that communities with higher host diversity offer additional niches in the form of more host species, especially for parasites that are highly host-specific or require multiple hosts to complete their life cycles (Hechinger & Lafferty 2005; Poulin 2014). However, ecological theory developed for free-living species has shown that, alongside resource heterogeneity, species diversity is also influenced by both propagule pressure and total resource availability (Rosenzweig 1995; Levine 2000; Jetz et al. 2009; Stevens et al. 2012; Haegeman & Loreau 2014). Our analyses revealed that each of these factors contributed to the observed changes in parasite diversity, but to different degrees and at different biological scales. Comparisons of host availability and host richness as predictors suggested that host richness was the primary driver of parasite richness, particularly at the host- and population levels. Host availability, which can influence parasite richness both through the amount of habitat available for parasite colonization and by sustaining larger parasite populations, became a significant co-contributor only at the community scale. Use of the diversity-decomposition approach allowed us to further assess whether the effects of host diversity on parasite richness were mediated through changes in parasite colonization or niche heterogeneity. The positive and consistent relationship between host diversity and parasite α diversity is consistent with an influential role of colonization with progressive increases in host richness, which might stem from colonization by generalist parasites as new host species are added or from increased visitation by transport hosts such as bird or mammals at high-diversity sites (e.g., Hechinger & Lafferty 2005). However, the strongest effect of host diversity was on parasite βpopulation diversity (i.e., among species), for which the coefficient of host diversity was 3–4× greater than those for other responses. This suggests that niche variation in the form of added host species was the dominant influence on the link between host and parasite richness, consistent with the habitat-heterogeneity hypothesis and an overall emphasis in community ecology on heterogeneity as a driver of biodiversity. Tews et al. (2004), for instance, reported that 85% of studies published between 1960 and 2003 found significant and positive effects of heterogeneity on the richness of taxa ranging from microbes to mammals.
Results of the mesocosm experiment provided additional support for the role of host heterogeneity in driving parasite diversity. By holding constant both host resource availability (total host abundance) and parasite colonization (propagule pressure), we found that the only effects of increasing host richness were on parasite β diversity, or the among-host-species differences in parasite community composition. Increases in host richness from one to four species had no effect on either the average parasite richness per host individual (α diversity) or the total number of parasite species within each mesocosm (γ diversity). The levels of β diversity observed at high host richness, whether measured as the difference between γ and α or as Jaccard’s dissimilarity index, were comparable to those observed in natural systems at similar diversity levels. Given the complexities inherent to manipulating host and parasite communities simultaneously, here we used a relatively small yet realistic number of generalist parasites and maintained a constant total number of hosts, but we highlight the importance of subsequent efforts to concurrently vary host evenness, host abundance, and include a mixture of specialist and generalist parasites. Our results are also consistent with those of the only two previous experimental studies of the diversity-begets-diversity relationship for parasites. As part of the Jena Biodiversity Experiment, experimental increases in plant species richness and functional diversity enhanced both the richness and prevalence of colonizing pathogens (Scherber et al. 2010; Rottstock et al. 2014), although whether these effects stemmed from changes in host heterogeneity alone or a combination of mechanisms was not assessed.
The strength of the relationship between host and parasite diversity also tended to increase with spatial aggregation. Thus, as ponds were connected together into parks and metacommunities, the coefficient of host diversity on parasite diversity increased in magnitude. This increase was non-linear with respect to buffer distance, largely reflecting the uneven distributions of accessible ponds across the landscape. Based on the species–area curves for hosts and parasites, this amplification of the coefficient emerged from the fact that parasite richness increased more rapidly with increasing buffer distance and saturated more gradually than did host richness. This likely stemmed from several factors. First, parasite species richness is often greater than host species richness, as observed here, and parasites tend to be more spatially aggregated than hosts, leading to stronger increases in parasite richness as larger areas are sampled. Moreover, because dispersal limitation is more likely to manifest at local scales (i.e., parasites cannot instantaneously colonize all suitable host habitats), the transition from local to landscape scales is expected to narrow the gap between the potential and realized niches of parasites. Depending on the mode of parasite dispersal, for instance, there will probably be temporal lags between stochastic local extinctions of parasites and their successful recolonization, particularly at fine spatial scales. Indeed, for highly vagile parasites, such as larval trematodes with avian or mammalian definitive hosts (Table S1), parasite richness saturated much more quickly with buffer distance than for than for all parasites combined, highlighting the influence of dispersal limitation (Fig. 5b and Fig. S2). This result contrasts with previous findings suggesting that the slopes of species–area curves are steeper for large-bodied organisms (Drakare et al. 2006), which could stem from the tendency of parasites to decouple the association between body size and trophic level observed among free-living species (Lafferty & Kuris 2002).
A major priority in disease ecology is to understand the diversity and distribution of parasites, including how these patterns change with scale. Our results offer insight into the mechanisms linking host and parasite species richness, as well as how this relationship changes across spatial scales from local communities to metacommunities and biological levels from individual hosts to host communities. This complements macroecological studies aimed at characterizing parasite diversity at regional to biogeographic scales (Guernier et al. 2004; Dunn et al. 2010; Smith et al. 2014), for which the influence of evolutionary history and diversification will also have a more prominent signal. Such findings also have immediate relevance for the ongoing debate about whether increasing free-living biodiversity reduces the transmission of infectious disease (e.g., Ostfeld & Keesing 2012; Randolph & Dobson 2012; Wood & Lafferty 2013). Resolution of this debate requires careful attention to both the scale involved (e.g., local to regional) and the epidemiological process (e.g., parasite colonization vs. transmission) (Johnson et al. 2015). For instance, the richness of parasite species in an area is not equivalent to the risk of disease, which is often more closely aligned with the prevalence or load of an especially pathogenic infection. Our results indicate that increases in host species and the niches they provide allow for increases in parasite richness, particularly at higher biological scales and coarser spatial grains. Previous work has shown how changes in host richness can depress local transmission of parasites and reduce host pathology (e.g., Ostfeld & Keesing 2012; Johnson et al. 2013c). Thus, quantifying the concurrent and scale-dependent effects of host diversity on parasite colonization as well as transmission represents an important area of future research for understanding the net effects of diversity on disease.
Supplementary Material
Acknowledgments
For assistance in collecting data, we thank D. Calhoun, J. Gregory, and T. McDevitt-Galles. For their wisdom in providing funding to support this research, we gratefully acknowledge the National Science Foundation (DEB-1149308), the National Institutes of Health (R01GM109501) and the David and Lucile Packard Foundation. We further thank members of the Macroecology of Infectious Disease Research Coordination Network for fruitful discussions (funded by NSF/NIH/USDA DEB 131223) and two anonymous reviewers for helpful feedback on the manuscript.
Footnotes
Author contributions: PTJJ designed the study, coordinated data collection, and drafted the manuscript; CLW contributed to the project design and manuscript development; MBJ assisted with data analysis and analytical framework; DLP coordinated the mesocosm experiment; SEH and YPS contributed to the project design and analytical framework. All authors contributed to editing of the manuscript.
References
- 1.Adler PB, Seabloom EW, Borer ET, Hillebrand H, Hautier Y, Hector A, et al. Productivity is a poor predictor of plant species richness. Science. 2011;333:1750–1753. doi: 10.1126/science.1204498. [DOI] [PubMed] [Google Scholar]
- 2.Allouche O, Kalyuzhny M, Moreno-Rueda G, Pizarro M, Kadmon R. Area-heterogeneity tradeoff and the diversity of ecological communities. Proc Natl Acad Sci U S A. 2012;109:17495–17500. doi: 10.1073/pnas.1208652109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bush AO, Lafferty KD, Lotz JM, Shostak AW. Parasitology meets ecology on its own terms: Margolis et al revisited. J Parasitol. 1997;83:575–583. [PubMed] [Google Scholar]
- 4.Cadotte MW, Fukami T. Dispersal, spatial scale, and species diversity in a hierarchically structured experimental landscape. Ecol Lett. 2005;8:548–557. doi: 10.1111/j.1461-0248.2005.00750.x. [DOI] [PubMed] [Google Scholar]
- 5.Chase JM, Knight TM. Scale-dependent effect sizes of ecological drivers on biodiversity: why standardised sampling is not enough. Ecol Lett. 2013;16:17–26. doi: 10.1111/ele.12112. [DOI] [PubMed] [Google Scholar]
- 6.Chase JM, Leibold MA. Spatial scale dictates the productivity-biodiversity relationship. Nature. 2002;416:427–430. doi: 10.1038/416427a. [DOI] [PubMed] [Google Scholar]
- 7.Connell JH. Diversity in tropical rain forests and coral feefs: high diversity of trees and corals is maintained only in a non-equilibrium state. Science. 1978;199:1302–1310. doi: 10.1126/science.199.4335.1302. [DOI] [PubMed] [Google Scholar]
- 8.Drakare S, Lennon JJ, Hillebrand H. The imprint of the geographical, evolutionary and ecological context on species-area relationships. Ecol Lett. 2006;9:215–227. doi: 10.1111/j.1461-0248.2005.00848.x. [DOI] [PubMed] [Google Scholar]
- 9.Dunn RR, Davies TJ, Harris NC, Gavin MC. Global drivers of human pathogen richness and prevalence. Proc R Soc Lond B. 2010;277:2587–2595. doi: 10.1098/rspb.2010.0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, McCraw SL, et al. Emerging fungal threats to animal, plant and ecosystem health. Nature. 2012;484:186–194. doi: 10.1038/nature10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fridley JD, Stachowicz JJ, Naeem S, Sax DF, Seabloom EW, Smith MD, et al. The invasion paradox: Reconciling pattern and process in species invasions. Ecology. 2007;88:3–17. doi: 10.1890/0012-9658(2007)88[3:tiprpa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 12.Gering JC, Crist TO. The alpha-beta-regional relationship: providing new insights into local-regional patterns of species richness and scale dependence of diversity components. Ecol Lett. 2002;5:433–444. [Google Scholar]
- 13.Gering JC, Crist TO, Veech JA. Additive partitioning of species diversity across multiple spatial scales: Implications for regional conservation of biodiversity. Conserv Biol. 2003;17:488–499. [Google Scholar]
- 14.Guernier V, Hochberg ME, Guegan JF. Ecology drives the worldwide distribution of human diseases. PLoS Biol. 2004;2:740–746. doi: 10.1371/journal.pbio.0020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haegeman B, Loreau M. General relationships between consumer dispersal, resource dispersal and metacommunity diversity. Ecol Lett. 2014;17:175–184. doi: 10.1111/ele.12214. [DOI] [PubMed] [Google Scholar]
- 16.Hartson RB, Orlofske SA, Melin VE, Dillon RT, Johnson PTJ. Land use and wetland spatial position jointly determine amphibian parasite communities. EcoHealth. 2011;8:485–500. doi: 10.1007/s10393-011-0715-9. [DOI] [PubMed] [Google Scholar]
- 17.Hechinger RF, Lafferty KD. Host diversity begets parasite diversity: bird final hosts and trematodes in snail intermediate hosts. Proc R Soc Lond B. 2005;272:1059–1066. doi: 10.1098/rspb.2005.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hubbell SP. The unified neutral theory of biodiversity and biogeography. Princeton University Press; Princeton: 2001. [DOI] [PubMed] [Google Scholar]
- 19.Hutchinson GE. Homage to Santa-Rosalia or why are there so many kinds of animals. Am Nat. 1959;93:145–159. [Google Scholar]
- 20.Jetz W, Kreft H, Ceballos G, Mutke J. Global associations between terrestrial producer and vertebrate consumer diversity. Proc R Soc Lond B. 2009;276:269–278. doi: 10.1098/rspb.2008.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson PTJ, Hoverman JT, McKenzie VJ, Blaustein AR, Richgels KLD. Urbanization and wetland communities: applying metacommunity theory to understand the local and landscape effects. J Appl Ecol. 2013a;50:34–42. [Google Scholar]
- 22.Johnson PTJ, Ostfeld RS, Keesing F. Frontiers in research on biodiversity and disease. Ecol Lett. 2015;18:1119–1133. doi: 10.1111/ele.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson PTJ, Preston DL, Hoverman JT, LaFonte BE. Host and parasite diversity jointly control disease risk in complex communities. Proc Natl Acad Sci U S A. 2013b;110:16916–16921. doi: 10.1073/pnas.1310557110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson PTJ, Preston DL, Hoverman JT, Richgels KLD. Biodiversity decreases disease through predictable changes in host community competence. Nature. 2013c;494:230–233. doi: 10.1038/nature11883. [DOI] [PubMed] [Google Scholar]
- 25.Kamiya T, O’Dwyer K, Nakagawa S, Poulin R. Host diversity drives parasite diversity: meta-analytical insights into patterns and causal mechanisms. Ecography. 2014:689–697. [Google Scholar]
- 26.Lafferty KD, Kuris AM. Trophic strategies, animal diversity and body size. Trends Ecol Evol. 2002;17:507–513. [Google Scholar]
- 27.Lande R. Statistics and partitioning of species diversity, and similarity among multiple communities. Oikos. 1996;76:5–13. [Google Scholar]
- 28.Levin SA. The problem of pattern and scale in ecology. Ecology. 1992;73:1943–1967. [Google Scholar]
- 29.Levine JM. Species diversity and biological invasions: Relating local process to community pattern. Science. 2000;288:852–854. doi: 10.1126/science.288.5467.852. [DOI] [PubMed] [Google Scholar]
- 30.MacArthur R. Species packing and competitive equilibrium for many species. Theor Popul Biol. 1970;1:1–11. doi: 10.1016/0040-5809(70)90039-0. [DOI] [PubMed] [Google Scholar]
- 31.MacArthur R, MacArthur JW. On bird species-diversity. Ecology. 1961;42:594. [Google Scholar]
- 32.MacArthur RH, Wilson EO. The theory of island biogeography. Princeton University Press; Princeton, N.J: 1967. [Google Scholar]
- 33.Mittelbach GG, Steiner CF, Scheiner SM, Gross KL, Reynolds HL, Waide RB, et al. What is the observed relationship between species richness and productivity? Ecology. 2001;82:2381–2396. [Google Scholar]
- 34.Ostfeld RS, Keesing F. Effects of host diversity on infectious disease. Annu Rev Ecol Evol Syst. 2012;43:157–182. [Google Scholar]
- 35.Poulin R. Parasite biodiversity revisited: frontiers and constraints. Int J Parasitol. 2014;44:581–589. doi: 10.1016/j.ijpara.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Randolph SE, Dobson ADM. Pangloss revisited: a critique of the dilution effect and the biodiversity-buffers-disease paradigm. Parasitology. 2012;139:847–863. doi: 10.1017/S0031182012000200. [DOI] [PubMed] [Google Scholar]
- 37.Richgels KLD, Hoverman JT, Johnson PTJ. Evaluating the role of regional and local processes in structuring a larval trematode metacommunity of Helisoma trivolvis. Ecography. 2013;36:854–863. [Google Scholar]
- 38.Rosenzweig ML. Species diversity in space and time. Cambridge University Press; Cambridge; New York: 1995. [Google Scholar]
- 39.Rottstock T, Joshi J, Kummer V, Fischer M. Higher plant diversity promotes higher diversity of fungal pathogens, while it decreases pathogen infection per plant. Ecology. 2014;95:1907–1917. doi: 10.1890/13-2317.1. [DOI] [PubMed] [Google Scholar]
- 40.Scheiner SM, Cox SB, Willig M, Mittelbach GG, Osenberg C, Kaspari M. Species richness, species-area curves and Simpson’s paradox. Evol Ecol Res. 2000;2:791–802. [Google Scholar]
- 41.Scherber C, Eisenhauer N, Weisser WW, Schmid B, Voigt W, Fischer M, et al. Bottom-up effects of plant diversity on multitrophic interactions in a biodiversity experiment. Nature. 2010;468:553–556. doi: 10.1038/nature09492. [DOI] [PubMed] [Google Scholar]
- 42.Smith KF, Goldberg M, Rosenthal S, Carlson L, Chen J, Chen C, et al. Global rise in human infectious disease outbreaks. J R Soc Interface. 2014;11:20140950. doi: 10.1098/rsif.2014.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stevens MHH, Carson WP. Resource quantity, not resource heterogeneity, maintains plant diversity. Ecol Lett. 2002;5:420–426. [Google Scholar]
- 44.Stevens RD, Gavilanez MM, Tello JS, Ray DA. Phylogenetic structure illuminates the mechanistic role of environmental heterogeneity in community organization. J Anim Ecol. 2012;81:455–462. doi: 10.1111/j.1365-2656.2011.01900.x. [DOI] [PubMed] [Google Scholar]
- 45.Tews J, Brose U, Grimm V, Tielborger K, Wichmann MC, Schwager M, et al. Animal species diversity driven by habitat heterogeneity/diversity: the importance of keystone structures. J Biogeogr. 2004;31:79–92. [Google Scholar]
- 46.Thieltges DW, Hof C, Dehling DM, Brandle M, Brandl R, Poulin R. Host diversity and latitude drive trematode diversity patterns in the European freshwater fauna. Global Ecol Biogeogr. 2011;20:675–682. [Google Scholar]
- 47.Venail PA, MacLean RC, Bouvier T, Brockhurst MA, Hochberg ME, Mouquet N. Diversity and productivity peak at intermediate dispersal rate in evolving metacommunities. Nature. 2008;452:210–U257. doi: 10.1038/nature06554. [DOI] [PubMed] [Google Scholar]
- 48.Waide RB, Willig MR, Steiner CF, Mittelbach G, Gough L, Dodson SI, et al. The relationship between productivity and species richness. Annu Rev Ecol Syst. 1999;30:257–300. [Google Scholar]
- 49.Whittaker RH. Vegetation of the Siskiyou Mountains, Oregon and California. Ecol Monogr. 1960;30:280–338. [Google Scholar]
- 50.Wood CL, Lafferty KD. Biodiversity and disease: a synthesis of ecological perspectives on Lyme disease transmission. Trends Ecol Evol. 2013;28:239–247. doi: 10.1016/j.tree.2012.10.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.