Abstract
Seasonal influenza virus vaccines have to be re-formulated and re-administered on an annual basis due to antigenic drift of the influenza virus surface glycoproteins. In addition, seasonal vaccines show limited efficacy against novel pandemic influenza virus strains, and producing tailored vaccines for these strains in a timely manner is challenging. Several novel broadly protective vaccine candidates targeting the conserved stalk domain of the viral hemagglutinin have been developed. Here we review these novel constructs and discuss several important findings and considerations regarding the protective efficacy of stalk-based vaccines.
Introduction
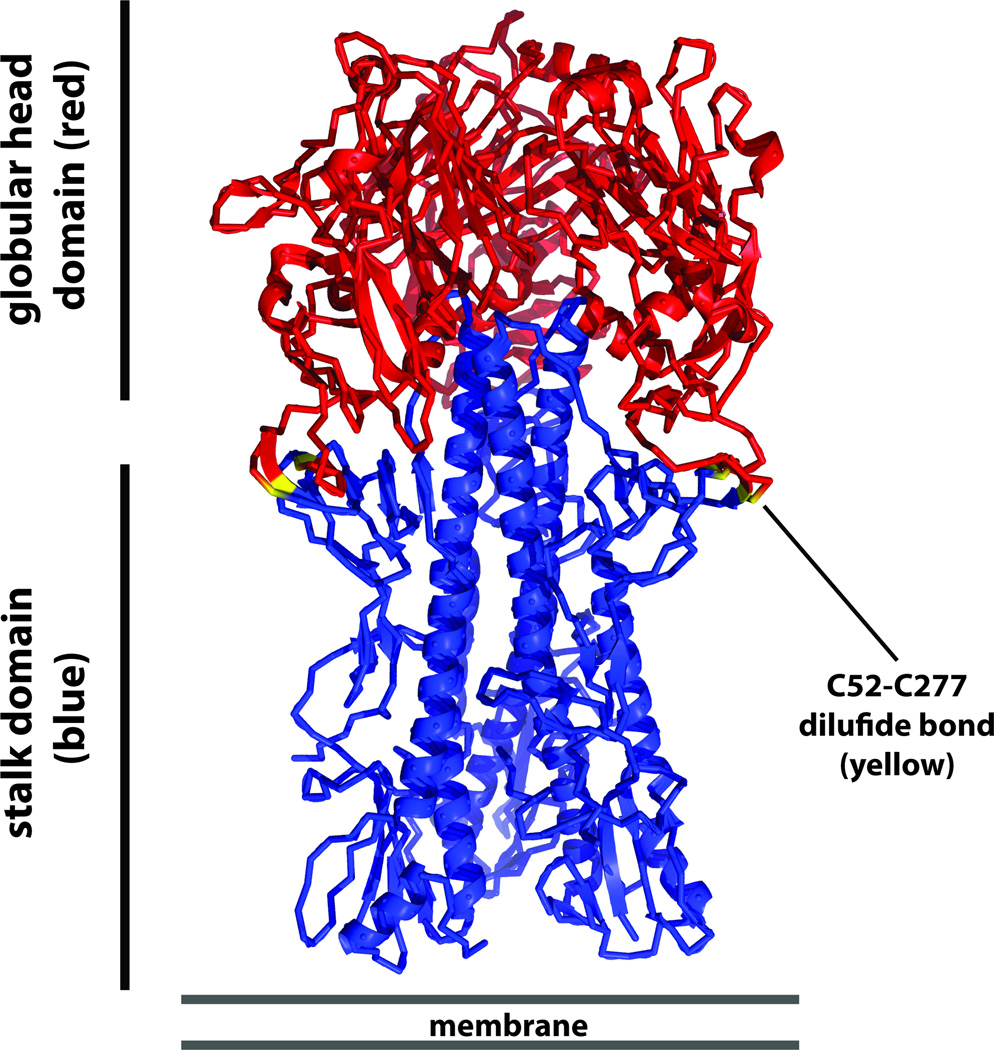
Influenza virus infections cause significant morbidity and mortality worldwide [1]. Current influenza virus vaccines provide good protection against disease but have to be re-formulated and re-administered on an annual bases due to antigenic drift of the virus [2]. This antigenic drift is caused by human herd immunity, which is mostly directed against the globular head domain of the viral hemagglutinin (HA, Figure 1). Antibodies against this immunodominant, membrane distal HA head domain are potent neutralizers of the virus. However, the high plasticity of the head domain [3] makes it easy for the virus to escape immune pressure. The membrane proximal stalk domain of the HA (Figure 1) is more conserved and antibodies that target this domain have been shown to broadly neutralize influenza viruses across several subtypes [4–12]. Unfortunately, the stalk domain is immunosubdominant compared to the head domain and is usually not targeted by the immune system following exposure to influenza virus vaccines. In the past it has been difficult to design vaccines that target the stalk domain due to the immunosubdominant and fragile nature of the conformational epitopes to which most neutralizing anti-stalk antibodies bind.
Figure 1. Schematic of the trimeric influenza virus HA.
The membrane distal globular head domain is shown in red and the membrane proximal stalk domain is shown in blue. Cysteines 52 and 277 - which form a disulfide bond that demarcates head and stalk domain - are shown in yellow. The schematic is based on the H1 HA of A/PR/8/34 (PDB 1RU7 as described in [92]).
Stalk-based vaccine approaches
Two major strategies to induce stalk-based immunity have been developed so far. The first focuses on removal of the entire immunosubdominant head domain to construct headless HAs [13]. (Table 1) Graves and colleagues recognized in the 1980s that the HA2 subunit (which forms the majority of the stalk domain) is more conserved than the HA1 subunit (which includes the globular head domain) [14]. In order to unmask the HA2 on the viral surface they treated virus preparations with acid (to induce a post-fusion conformation) and then removed the HA1 using a reducing agent [15]. Unfortunately, this treatment most likely destroyed the conformational epitopes which can induce neutralizing anti-stalk antibodies. In the 1990s, the first anti-stalk antibody, mAb C179 was isolated [8], and cells expressing a construct including the HA2 domain were used as immunogens in mice providing partial protection against heterosubtypic H1N1 challenge [16]. Steel and colleagues expressed their headless HA construct on virus-like particles and achieved homologous protection [17]. A construct based on the same design but expressed as soluble protein in insect cells showed full homologous and partial heterosubtypic protection following challenge of vaccinated mice [18]. Several other constructs were developed and provided protection against viral challenge in the mouse model [19–21]. However, the structural integrity of these constructs with respect to complex, conformational stalk epitopes was most likely suboptimal. Lu and colleagues improved on these constructs using an iterative design process and a cell free expression platform [22]. They were the first to show binding of broadly-neutralizing stalk mAbs to their immunogen (in an ELISA). However, animal studies with this headless HA construct have not been published. Recently, Yassine et al. and Impagliazzo et al. independently reported stable, correctly folded headless immunogens [23,24]. Interestingly both groups used a similar strategy to stabilize their respective stalk structures. Removal of the globular head domain exposes an area at the membrane distal part of the stalk at the very end of the HA2 long alpha helix (LAH) that is usually covered by the head domain. In both studies, this membrane distal part of the stalk was stabilized by a trimerization domain. While Yassine et al. used an HIV gp41 trimerization domain that was later removed, Impagliazzo et al. replaced the upper part of the LAH with a helical leucine zipper trimerization domain (which is present in the final construct). In addition, Yassine et al. fused their construct to a bacterial ferritin, which forms nanoparticles. This strategy was chosen to further stabilize the stalk and to make the construct more immunogenic. Structures based on X-ray crystallography and electron microscopy show binding of stalk mAbs to both constructs suggesting that their structure closely resembles the native HA stalk with respect to conformational stalk epitopes. Both constructs induced stalk-reactive antibodies in animal models and protected from challenge with highly pathogenic H5N1 viruses. Interestingly, despite robust protection, neutralizing antibody titers against homologous viruses were low and titers against heterosubtypic viruses were almost undetectable, a finding that will be further discussed below.
Table 1.
Overview of HA stalk-based influenza vaccine approaches
| Candidate | Development stage | Key points | References |
|---|---|---|---|
| Headless HA | pre-clinical | removal of globular head domain allows the immune system to focus on the stalk domain; must be expressed recombinantly and cannot be produced using traditional influenza vaccine production platforms | [15–24], reviewed in [13] |
| Chimeric HA (cHA) | pre-clinical, clinical phase in preparation | sequential presentation of the same stalk domain in combination with exotic head domains breaks the immunodominance of the head domain and refocuses the immune response to the stalk; can be produced using traditional influenza virus vaccine production platforms | [27,29–33,35,36] |
| Glycan shielding | pre-clinical | hyperglycosylation of the globular head domain shields it from the immune system | [58,59] |
| Prime-boost strategies | clinical | have been developed to increase the efficacy of seasonal, H5 and H7 influenza virus vaccines but have also been shown to broaden the immune response | [45,60,61,63,64] |
| Peptides | pre-clinical | allow the immune response to focus on the epitope of choice without distraction by the globular head domain; might not capture the right conformation of complex structural epitopes | [56,57] |
| VLP-based approaches | pre-clinical | present key epitopes on the surface of immunogenic VLPs, might not capture the right conformation of complex structural epitopes | [54,55] |
The second major strategy seeks to break the immunodominance of the head domain by sequential exposure of the immune system to chimeric HAs (cHAs) [14,25,26] (Table 1). cHAs consist of stalk domains from H1 (group 1), H3 (group 2) or influenza B viruses in combination with head domains of exotic – mostly avian – influenza virus subtypes [27–29]. By sequential vaccination with cHAs that have different head domains but the same stalk domain, it is possible to refocus the immune response towards the (usually) subdominant stalk domain (Figure 2A). This concept has proven successful in mouse and ferret models, using constructs from both group 1 and group 2 HAs [30–35]. Importantly, this strategy provided complete protection against a heterosubtypic challenge using H5N1, H6N1 or H7N9 viruses [30,31]. cHA vaccination also reduced lung titers in mice challenged with H3N8, H10N7, and non-lethal H3N2 variant viruses [32] and reduced transmission of pandemic H1N1 virus in the ferret model [36]. Typically, three sequential vaccinations with cHAs that have the same stalk domain but different head domains are necessary in naive animals to induce protective immunity. However, humans already have low levels of B-cells and antibodies with specificities for stalk epitopes and are therefore already primed [12,37–40]. This pre-existing immunity is most likely induced by natural infection with influenza viruses and/or influenza vaccinations [41–44]. Therefore, it is likely that the administration of one or two cHA-based vaccines induces high titers of stalk-reactive antibodies. It remains to be tested if these titers will be sufficiently high to confer protection. Clinical studies with pre-pandemic avian influenza virus vaccines provide evidence for this hypothesis. When subtype H5 or H7 vaccines are administered in clinical studies these vaccines significantly boost anti-stalk titers [38,39,45,46]. Importantly, these subtypes have completely different head domains in combination with stalk domains that express conserved group 1 (H5) or group 2 (H7) stalk epitopes. To some extent, this phenomenon of an enhanced stalk response also occurred during the H1N1 pandemic of 2009 [28,40,47–50]. While the 2009 pandemic H1N1 and the pre-pandemic seasonal H1N1 strains both share a highly conserved stalk domain, the HA head domains of the two strains are largely antigenically distinct. In contrast, vaccination with seasonal influenza virus vaccines or repeated administration of the pandemic H1N1 strain results in more narrow and head-specific responses [38,42,51–53].
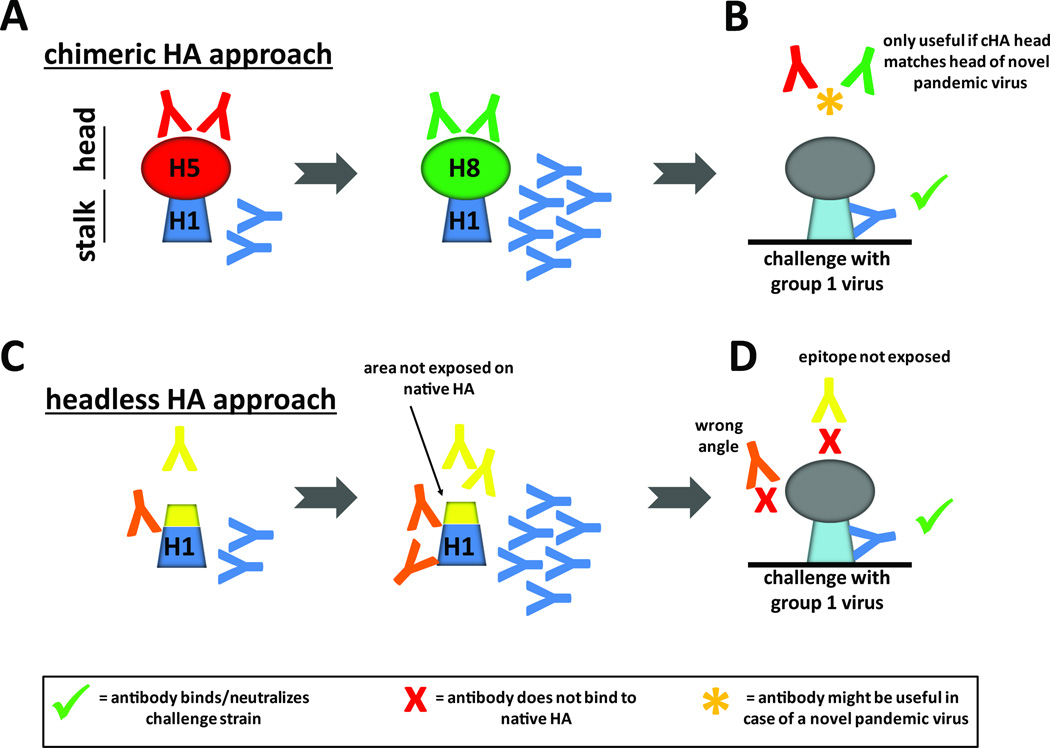
Figure 2. Vaccination with cHA and headless HA immunogens may result in different antibody profiles.
A cHA vaccination induces stalk-reactive antibodies by sequential exposure of the immune system to constructs with a conserved stalk domain but divergent head domains (cH5/1 and cH8/1 HAs which share the H1 stalk domain have very different H5 and H8 head domains). B Anti-stalk antibodies (blue) generated in response to cHA vaccination bind and neutralize incoming viruses. Since these antibodies were induced by full length HAs they should bind efficiently to stalk epitopes on wild type full length HAs. Low levels of antibodies induced against the different head domains are most likely irrelevant to protection if the head domain of the incoming virus is not matched (red and green). If the head domains of the challenge virus and the cHA constructs match, these head antibodies might be beneficial for protection as well. C and D Antibodies induced by headless HA constructs may belong to three different categories: First, antibodies that bind stalk epitopes on headless HA vaccine constructs bind and neutralize wild type HA (blue). Second, antibodies that bind to surfaces on headless HAs are not exposed on wild type HA (yellow). These antibodies might not bind to wild type HA and might not be able to neutralize incoming virus. Third, antibodies that bind to stalk epitopes at angles may be unable to bind to stalk epitopes (due to steric hindrance) when a head domain is present (orange). These antibodies are unable to bind and neutralize incoming virus.
In addition to headless HA and cHA immunogens, a number of other antigen designs and strategies have been proposed (Table 1). These include the presentation of stalk epitopes on virus-like particles [54,55], peptide based approaches [56,57], strategies that shield the HA head domain from recognition by the immune system [58,59] and heterologous prime-boost strategies [60–64] that consist of a prime with DNA, a live virus-vector or live-attenuated virus followed by a protein or inactivated vaccine boost.
Vaccination with cHA and headless HA immunogens may result in different antibody profiles
The type of antibody response induced by headless and chimeric HAs on a monoclonal level is currently unknown. cHAs are full length HAs that have H1, H3 or influenza B stalk domains combined with 'exotic' globular head domains and can be produced using traditional influenza vaccine production platforms as inactivated or live-attenuated vaccines. Stalk antibodies induced by cHAs will likely bind and affect wild type HAs on virions and infected cells (Figure 2A and B). Antibodies induced against the 'exotic' globular head domains will be irrelevant against currently circulating strains (but may be useful in protecting against pandemic viruses of the matched HA subtype). Headless HA constructs, however, may induce antibodies that bind to the immunogen but not to wild type HA on virions (Figure 2C and D). This scenario is likely since headless HAs expose areas on the top of the stalk domain that are not accessible on wild type HAs because they are covered by the head domain. Furthermore, the absence of the head domain might allow for angles of antibody binding which are not possible in the presence of a head domain on infectious virus. An immune response against headless HAs might therefore result in the induction of antibodies that do not bind to wild type HAs and therefore cannot contribute to protection.
Stalk neutralization titers are typically low in mice
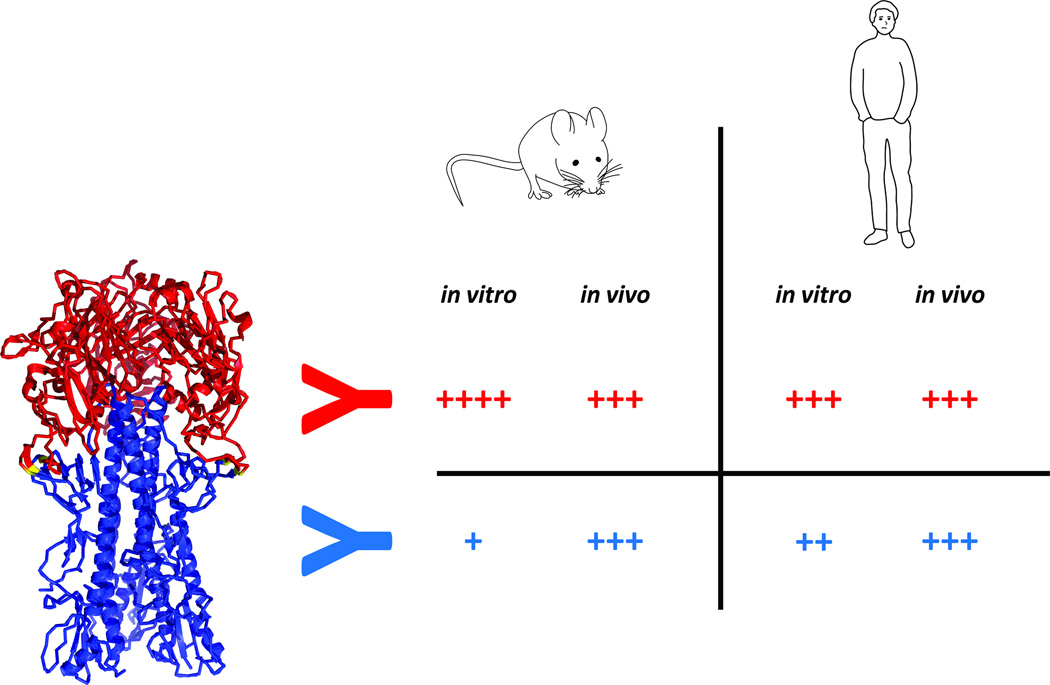
It has been noted that neutralization titers induced by stalk-based vaccines are low in animal models specifically mice. Although this observation is complicated by the different types of assays used in the studies, low or undetectable neutralization titers appear to be common [18,23,24,30,31,42]. This is in stark contrast to studies in humans, in which stalk-based neutralization titers can be readily measured when individuals have experienced pandemic H1N1 infections or H5N1 vaccinations (both scenarios have shown to stimulate anti-stalk antibodies) [38,39,41,65]. Several reasons may explain these findings. First, the in vitro neutralizing potency of stalk reactive antibodies is generally lower than that of head-reactive hemagglutination inhibition (HI) antibodies. As an example, He and colleagues [66] reported a potency difference between murine head and stalk antibodies of 4–6 logs, a finding confirmed by Dilillo and colleagues [67]. This divergence between the potency of murine head and stalk antibodies is not observed for human head and stalk antibodies (Figure 3). The latter are generally more similar with respect to their potency when unbiased data from plasmablast responses are considered [47,68]. For example Li and colleagues report 23 HI active, head reactive and 3 stalk-reactive mAbs recovered from plasmablasts after pandemic H1N1 vaccination. While only 8 (34.8%) of their recovered anti-head mAbs had neutralization IC50 values of below 1 ug/ml (with the remaining 15 HI active mAbs having IC50 values between 32 and 1 ug/ml) all three recovered anti-stalk antibodies showed IC50 values below 1 ug/ml [47]. Second, the serum IgG concentration of human blood is approximately 10 fold higher than that of standard laboratory mouse strains. IgG serum concentrations in human adults range from 4–22 mg/ml serum with averages between 11–12 mg/ml depending on age, ethnicity and gender [69–71]. The level of serum IgG in mice usually used in vaccine studies (6–20 weeks) has been shown to be between 1–2 mg/ml [72,73]. Third, inherent differences in CDR composition and length between humans and mice might influence neutralization potency as well [74]. Based on these considerations, anti-stalk neutralization titers should be readily measured in adult human sera while very high levels of anti-stalk antibody levels would be needed in the mouse model to reach the limit of detection in common microneutralization assays.
Figure 3. In vitro neutralizing potency and in vivo protective efficacy for murine and human anti-head and anti-stalk antibodies.
Murine anti-head antibodies usually exhibit high potency in vitro as well as good protective efficacy in vivo. Their in vitro IC50 is 2–6 logs lower (better) than those of murine anti-stalk antibodies. However, the in vivo protective efficacy of murine head and stalk mAbs is similar. On the other hand human head and stalk antibodies behave similar in in vitro potency assays with anti-head mAbs performing slightly better. The in vivo protective efficacy of both types of human mAbs is similar as well. Anti-stalk antibodies gain in vivo potency through Fc-mediated immune mechanisms.
Stalk antibodies show enhanced protective potency in vivo as compared to in vitro
While anti-head antibodies show higher potency than anti-stalk antibodies in vitro, it has been demonstrated that the protective effect of both types of antibodies is almost equal in vivo in murine challenge models with mouse-adapted and non-mouse-adapted viruses (Figure 3) [67,75,76]. As discussed above differences between head- and stalk-stalk antibodies in in vitro potency can be more than thousand fold [66,67,76]. Monoclonal antibody CR9114 shows an even stronger difference and displays no neutralizing activity against influenza B viruses in vitro, while affording robust protection against challenge with divergent influenza B strains in the mouse model [75]. This enhancement of potency of anti-stalk antibodies in vivo is most likely caused by Fc-mediated mechanisms like antibody dependent cell-mediated cytotoxicity (ADCC) and complement dependent cytotoxicity (CDC) which are not readily measured in (classical) in vitro neutralization assays. Recent studies have clearly demonstrated the efficacy-enhancing effect of ADCC on cross-reactive antibodies both on a monoclonal [67] as well as a polyclonal level [77–80]. A possible effect of CDC was also reported in vitro [81] but the significance of mediating complement activity for anti-stalk antibodies in vivo has not yet been elucidated. In addition to ADCC and CDC, more complex mechanisms like interactions between alveolar macrophages, CD8+ T-cells and antibodies may play a role as well [82]. Finally, it cannot be ruled out that the neutralizing activity of anti-stalk antibodies is enhanced in vivo by yet to be described interactions with host defense proteins, specific cell types or by the micro-environment of the lung architecture.
How broad is 'broad protection'?
An important feature of stalk-reactive antibodies is their breadth of binding and neutralization. Typical stalk-reactive antibodies bind to HAs within the phylogenetic group 1 (H1, H2, H5, H6, H8, H9, H11, H12, H13, H16, H17 and H18) or group 2 (H3, H4, H7, H10, H14, H15) [7–9,83–85]. Exceptions are mAbs 12D1 [11] and 6F12 [10] which are restricted to one subtype but bind broadly within this subtype and several antibodies that are capable of cross-group binding [6,12,86–88] with one mAb even recognizing influenza B HAs [75]. Polyclonal antibodies induced by stalk-immunogens in mice [23,24,30] or vaccination with pandemic influenza virus vaccines [38,39,45,46] in humans usually follow the same trend with good group 1 cross-reactivity induced by H1 stalks and good group 2 cross-reactivity induced by H3 stalks. Cross-group reactivity at a very low level can be detected but does not provide full protection from challenge [30]. It is important to note that reactivity against different viruses within the same group varies. While there is still considerable cross-reactivity and cross-protection towards members within the same HA group, cross-reactivity in both animals [18,23,24,30,32,33] and humans [38,39] is highest towards the stalks that were used to induce the antibodies. For, example, Impagliazzo and colleagues reported approximately 100–1000 ELISA units (EU)/ml reactivity against various H1 strains, 10 EU/ml against H5 and 0.1 EU against H9 for sera from mice vaccinated with their H1-based stalk construct [24]. Reactivity to group 2 HAs (H3, H7) was close to or below the limit of detection. This makes sense, since stalk epitopes change only slightly from subtype to subtype and affinity maturation will likely result in antibodies that bind most efficiently to HAs that the immune system actually encountered. Nevertheless, lower titers against heterosubtypic HAs can still confer full protection as demonstrated by several studies [18,23,24,30–32]. These data suggests that a vaccine formulation comprised of a group 1 stalk, a group 2 stalk and an influenza B HA stalk component will make an effective universal influenza virus vaccine for humans.
Longevity of broadly protective vaccines
The primary goal for the development of universal influenza virus vaccines is to induce a broadly protective immune response. However, it is crucial that this immune response is long-lived. One of the most significant caveats of current inactivated influenza virus vaccines is that the immune response they elicit is relatively short lived and may wane over the course of one influenza season [89]. A universal influenza virus vaccine that would induce short-lived immunity could be an appealing tool in case of a new pandemic but would be less useful in protecting against seasonal influenza viruses since it would have to be given annually like current vaccines. It is not well understood why current inactivated influenza virus vaccines induce relatively short-lived immune responses. Natural influenza virus infection can certainly induce lifelong immunity against a specific strain (which is not necessarily an advantage due to antigenic drift) [90]. A better understanding of the difference - in quality and quantity - of the immune responses to vaccination versus natural infection will be very important in order to design a novel generation of vaccines that induce long-lasting immunity. Several strategies to enhance the longevity of the immune response to influenza have been tested and might be suitable for stalk-based vaccines as well [34,91].
Conclusions
The discovery of stalk-reactive antibodies has spurred the development of universal influenza virus vaccine candidates. Several new designs for stalk-based immunogens and vaccination strategies have been proposed and successfully tested in pre-clinical studies. Future clinical trials will show if these vaccine candidates perform well in humans in terms of safety, immunogenicity and efficacy. Along the way we will learn important lessons that will help us in better understanding the mechanisms of immune responses to conserved influenza virus epitopes in humans.
Highlights.
Two different types of stalk-based universal influenza virus vaccine candidates - chimeric hemagglutinins and headless hemagglutinins - are currently in development
The two types of vaccines might induce a different antibody profile
Efficacy of anti-stalk antibodies in vivo is high despite low in vitro neutralization potential
Long lasting immunity is important for universal influenza virus vaccines
Acknowledgments
I would like to thank Raffael Nachbagauer (Mount Sinai) for his help with preparing the figures. Work in the Krammer laboratory is supported by NIH grants R56 AI117287 and U19 AI109946 and the Centers of Influenza Virus Research and Surveillance (CEIRS) contract HHSN272201400008C. The Krammer laboratory also receives funding from GlaxoSmithKline and the Biomedical Advanced Research and Development Authority (BARDA, HHSO100201500010C).
Conflict of interest
The Icahn School of Medicine at Mount Sinai has filed several patents regarding influenza virus vaccines.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jayasundara K, Soobiah C, Thommes E, Tricco AC, Chit A. Natural attack rate of influenza in unvaccinated children and adults: a meta-regression analysis. BMC Infect Dis. 2014;14:670. doi: 10.1186/s12879-014-0670-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerdil C. The annual production cycle for influenza vaccine. Vaccine. 2003;21:1776–1779. doi: 10.1016/s0264-410x(03)00071-9. [DOI] [PubMed] [Google Scholar]
- 3.Heaton NS, Sachs D, Chen CJ, Hai R, Palese P. Genome-wide mutagenesis of influenza virus reveals unique plasticity of the hemagglutinin and NS1 proteins. Proc Natl Acad Sci U S A. 2013;110:20248–20253. doi: 10.1073/pnas.1320524110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ekiert DC, Bhabha G, Elsliger MA, Friesen RH, Jongeneelen M, Throsby M, Goudsmit J, Wilson IA. Antibody recognition of a highly conserved influenza virus epitope. Science. 2009;324:246–251. doi: 10.1126/science.1171491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ekiert DC, Friesen RH, Bhabha G, Kwaks T, Jongeneelen M, Yu W, Ophorst C, Cox F, Korse HJ, Brandenburg B, et al. A highly conserved neutralizing epitope on group 2 influenza A viruses. Science. 2011;333:843–850. doi: 10.1126/science.1204839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corti D, Voss J, Gamblin SJ, Codoni G, Macagno A, Jarrossay D, Vachieri SG, Pinna D, Minola A, Vanzetta F, et al. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science. 2011;333:850–856. doi: 10.1126/science.1205669. [DOI] [PubMed] [Google Scholar]
- 7.Sui J, Hwang WC, Perez S, Wei G, Aird D, Chen LM, Santelli E, Stec B, Cadwell G, Ali M, et al. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat Struct Mol Biol. 2009;16:265–273. doi: 10.1038/nsmb.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okuno Y, Isegawa Y, Sasao F, Ueda S. A common neutralizing epitope conserved between the hemagglutinins of influenza A virus H1 and H2 strains. J Virol. 1993;67:2552–2558. doi: 10.1128/jvi.67.5.2552-2558.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan GS, Lee PS, Hoffman RM, Mazel-Sanchez B, Krammer F, Leon PE, Ward AB, Wilson IA, Palese P. Characterization of a broadly neutralizing monoclonal antibody that targets the fusion domain of group 2 influenza a virus hemagglutinin. J Virol. 2014;88:13580–13592. doi: 10.1128/JVI.02289-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan GS, Krammer F, Eggink D, Kongchanagul A, Moran TM, Palese P. A pan-h1 anti-hemagglutinin monoclonal antibody with potent broad-spectrum efficacy in vivo. J Virol. 2012;86:6179–6188. doi: 10.1128/JVI.00469-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang TT, Tan GS, Hai R, Pica N, Petersen E, Moran TM, Palese P. Broadly protective monoclonal antibodies against H3 influenza viruses following sequential immunization with different hemagglutinins. PLoS Pathog. 2010;6:e1000796. doi: 10.1371/journal.ppat.1000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henry Dunand CJ, Leon PE, Kaur K, Tan GS, Zheng NY, Andrews S, Huang M, Qu X, Huang Y, Salgado-Ferrer M, et al. Preexisting human antibodies neutralize recently emerged H7N9 influenza strains. J Clin Invest. 2015;125:1255–1268. doi: 10.1172/JCI74374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krammer F. The Quest for a Universal Flu Vaccine: Headless HA 2.0. Cell Host Microbe. 2015;18:395–397. doi: 10.1016/j.chom.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Krammer F, Palese P. Influenza virus hemagglutinin stalk-based antibodies and vaccines. Curr Opin Virol. 2013;3:521–530. doi: 10.1016/j.coviro.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graves PN, Schulman JL, Young JF, Palese P. Preparation of influenza virus subviral particles lacking the HA1 subunit of hemagglutinin: unmasking of cross-reactive HA2 determinants. Virology. 1983;126:106–116. doi: 10.1016/0042-6822(83)90465-8. [DOI] [PubMed] [Google Scholar]
- 16.Sagawa H, Ohshima A, Kato I, Okuno Y, Isegawa Y. The immunological activity of a deletion mutant of influenza virus haemagglutinin lacking the globular region. J Gen Virol. 1996;77(Pt 7):1483–1487. doi: 10.1099/0022-1317-77-7-1483. [DOI] [PubMed] [Google Scholar]
- 17.Steel J, Lowen AC, T TW, Yondola M, Gao Q, Haye K, Garcia-Sastre A, Palese P. Influenza virus vaccine based on the conserved hemagglutinin stalk domain. MBio. 2010;1:1–9. doi: 10.1128/mBio.00018-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wohlbold TJ, Nachbagauer R, Margine I, Tan GS, Hirsh A, Krammer F. Vaccination with soluble headless hemagglutinin protects mice from challenge with divergent influenza viruses. Vaccine. 2015;33:3314–3321. doi: 10.1016/j.vaccine.2015.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bommakanti G, Citron MP, Hepler RW, Callahan C, Heidecker GJ, Najar TA, Lu X, Joyce JG, Shiver JW, Casimiro DR, et al. Design of an HA2-based Escherichia coli expressed influenza immunogen that protects mice from pathogenic challenge. Proc Natl Acad Sci U S A. 2010;107:13701–13706. doi: 10.1073/pnas.1007465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bommakanti G, Lu X, Citron MP, Najar TA, Heidecker GJ, ter Meulen J, Varadarajan R, Liang X. Design of Escherichia coli-expressed stalk domain immunogens of H1N1 hemagglutinin that protect mice from lethal challenge. J Virol. 2012;86:13434–13444. doi: 10.1128/JVI.01429-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mallajosyula VV, Citron M, Ferrara F, Lu X, Callahan C, Heidecker GJ, Sarma SP, Flynn JA, Temperton NJ, Liang X, et al. Influenza hemagglutinin stem-fragment immunogen elicits broadly neutralizing antibodies and confers heterologous protection. Proc Natl Acad Sci U S A. 2014;111:E2514–E2523. doi: 10.1073/pnas.1402766111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu Y, Welsh JP, Swartz JR. Production and stabilization of the trimeric influenza hemagglutinin stem domain for potentially broadly protective influenza vaccines. Proc Natl Acad Sci U S A. 2014;111:125–130. doi: 10.1073/pnas.1308701110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yassine HM, Boyington JC, McTamney PM, Wei CJ, Kanekiyo M, Kong WP, Gallagher JR, Wang L, Zhang Y, Joyce MG, et al. Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection. Nat Med. 2015;21:1065–1070. doi: 10.1038/nm.3927. [DOI] [PubMed] [Google Scholar]
- 24.Impagliazzo A, Milder F, Kuipers H, Wagner M, Zhu X, Hoffman RM, van Meersbergen R, Huizingh J, Wanningen P, Verspuij J, et al. A stable trimeric influenza hemagglutinin stem as a broadly protective immunogen. Science. 2015 doi: 10.1126/science.aac7263. [DOI] [PubMed] [Google Scholar]
- 25.Krammer F, Palese P. Universal influenza virus vaccines: need for clinical trials. Nat Immunol. 2014;15:3–5. doi: 10.1038/ni.2761. [DOI] [PubMed] [Google Scholar]
- 26.Krammer F, Palese P, Steel J. Advances in universal influenza virus vaccine design and antibody mediated therapies based on conserved regions of the hemagglutinin. Curr Top Microbiol Immunol. 2015;386:301–321. doi: 10.1007/82_2014_408. [DOI] [PubMed] [Google Scholar]
- 27.Hai R, Krammer F, Tan GS, Pica N, Eggink D, Maamary J, Margine I, Albrecht RA, Palese P. Influenza viruses expressing chimeric hemagglutinins: globular head and stalk domains derived from different subtypes. J Virol. 2012;86:5774–5781. doi: 10.1128/JVI.00137-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pica N, Hai R, Krammer F, Wang TT, Maamary J, Eggink D, Tan GS, Krause JC, Moran T, Stein CR, et al. Hemagglutinin stalk antibodies elicited by the 2009 pandemic influenza virus as a mechanism for the extinction of seasonal H1N1 viruses. Proc Natl Acad Sci U S A. 2012;109:2573–2578. doi: 10.1073/pnas.1200039109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen CJ, Ermler ME, Tan GS, Krammer F, Palese P, Hai R. Influenza A viruses expressing intra- or inter-group chimeric hemagglutinins. J Virol. 2016 doi: 10.1128/JVI.03060-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krammer F, Pica N, Hai R, Margine I, Palese P. Chimeric hemagglutinin influenza virus vaccine constructs elicit broadly protective stalk-specific antibodies. J Virol. 2013;87:6542–6550. doi: 10.1128/JVI.00641-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krammer F, Margine I, Hai R, Flood A, Hirsh A, Tsvetnitsky V, Chen D, Palese P. H3 Stalk-Based Chimeric Hemagglutinin Influenza Virus Constructs Protect Mice from H7N9 Challenge. J Virol. 2014;88:2340–2343. doi: 10.1128/JVI.03183-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Margine I, Krammer F, Hai R, Heaton NS, Tan GS, Andrews SA, Runstadler JA, Wilson PC, Albrecht RA, García-Sastre A, et al. Hemagglutinin Stalk-Based Universal Vaccine Constructs Protect against Group 2 Influenza A Viruses. J Virol. 2013;87:10435–10446. doi: 10.1128/JVI.01715-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krammer F, Hai R, Yondola M, Tan GS, Leyva-Grado VH, Ryder AB, Miller MS, Rose JK, Palese P, García-Sastre A, et al. Assessment of influenza virus hemagglutinin stalk-based immunity in ferrets. J Virol. 2014;88:3432–3442. doi: 10.1128/JVI.03004-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goff PH, Eggink D, Seibert CW, Hai R, Martínez-Gil L, Krammer F, Palese P. Adjuvants and immunization strategies to induce influenza virus hemagglutinin stalk antibodies. PLoS One. 2013;8:e79194. doi: 10.1371/journal.pone.0079194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryder AB, Nachbagauer R, Buonocore L, Palese P, Krammer F, Rose JK. Vaccination with VSV-vectored chimeric hemagglutinins protects mice against divergent influenza virus challenge strains. J Virol. 2015 doi: 10.1128/JVI.02598-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nachbagauer R, Miller MS, Hai R, Ryder AB, Rose JK, Palese P, García-Sastre A, Krammer F, Albrecht RA. Hemagglutinin stalk immunity reduces influenza virus replication and transmission in ferrets. J Virol. 2015 doi: 10.1128/JVI.02481-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nachbagauer R, Choi A, Izikson R, Cox MM, Palese P, Krammer F. Age Dependence and Isotype Specificity of Influenza Virus Hemagglutinin Stalk-Reactive Antibodies in Humans. MBio. 2016;7 doi: 10.1128/mBio.01996-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ellebedy AH, Krammer F, Li GM, Miller MS, Chiu C, Wrammert J, Chang CY, Davis CW, McCausland M, Elbein R, et al. Induction of broadly cross-reactive antibody responses to the influenza HA stem region following H5N1 vaccination in humans. Proc Natl Acad Sci U S A. 2014;111:13133–13138. doi: 10.1073/pnas.1414070111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nachbagauer R, Wohlbold TJ, Hirsh A, Hai R, Sjursen H, Palese P, Cox RJ, Krammer F. Induction of broadly reactive anti-hemagglutinin stalk antibodies by an H5N1 vaccine in humans. J Virol. 2014;88:13260–13268. doi: 10.1128/JVI.02133-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sangster MY, Baer J, Santiago FW, Fitzgerald T, Ilyushina NA, Sundararajan A, Henn AD, Krammer F, Yang H, Luke CJ, et al. B cell response and hemagglutinin stalk-reactive antibody production in different age cohorts following 2009 H1N1 influenza virus vaccination. Clin Vaccine Immunol. 2013;20:867–876. doi: 10.1128/CVI.00735-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pica N, Hai R, Krammer F, Wang TT, Maamary J, Eggink D, Tan GS, Krause JC, Moran T, Stein CR, et al. Hemagglutinin stalk antibodies elicited by the 2009 pandemic influenza virus as a mechanism for the extinction of seasonal H1N1 viruses. Proc Natl Acad Sci U S A. 2012;109:2573–2578. doi: 10.1073/pnas.1200039109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Margine I, Hai R, Albrecht RA, Obermoser G, Harrod AC, Banchereau J, Palucka K, García-Sastre A, Palese P, Treanor JJ, et al. H3N2 influenza virus infection induces broadly reactive hemagglutinin stalk antibodies in humans and mice. J Virol. 2013;87:4728–4737. doi: 10.1128/JVI.03509-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krammer F, Pica N, Hai R, Tan GS, Palese P. Hemagglutinin Stalk-Reactive Antibodies Are Boosted following Sequential Infection with Seasonal and Pandemic H1N1 Influenza Virus in Mice. J Virol. 2012;86:10302–10307. doi: 10.1128/JVI.01336-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller MS, Gardner TJ, Krammer F, Aguado LC, Tortorella D, Basler CF, Palese P. Neutralizing Antibodies Against Previously Encountered Influenza Virus Strains Increase over Time: A Longitudinal Analysis. Sci Transl Med. 2013;5:198ra107. doi: 10.1126/scitranslmed.3006637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Halliley JL, Khurana S, Krammer F, Fitzgerald T, Coyle EM, Chung KY, Baker SF, Yang H, Martínez-Sobrido L, Treanor JJ, et al. High-Affinity H7 Head and Stalk Domain-Specific Antibody Responses to an Inactivated Influenza H7N7 Vaccine After Priming With Live Attenuated Influenza Vaccine. J Infect Dis. 2015;212:1270–1278. doi: 10.1093/infdis/jiv210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krammer F, Jul-Larsen A, Margine I, Hirsh A, Sjursen H, Zambon M, Cox RJ. An H7N1 Influenza Virus Vaccine Induces Broadly Reactive Antibody Responses against H7N9 in Humans. Clin Vaccine Immunol. 2014;21:1153–1163. doi: 10.1128/CVI.00272-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li GM, Chiu C, Wrammert J, McCausland M, Andrews SF, Zheng NY, Lee JH, Huang M, Qu X, Edupuganti S, et al. Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc Natl Acad Sci U S A. 2012;109:9047–9052. doi: 10.1073/pnas.1118979109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wrammert J, Koutsonanos D, Li GM, Edupuganti S, Sui J, Morrissey M, McCausland M, Skountzou I, Hornig M, Lipkin WI, et al. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J Exp Med. 2011 doi: 10.1084/jem.20101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller MS, Tsibane T, Krammer F, Hai R, Rahmat S, Basler CF, Palese P. 1976 and 2009 H1N1 Influenza Virus Vaccines Boost Anti-Hemagglutinin Stalk Antibodies in Humans. J Infect Dis. 2012 doi: 10.1093/infdis/jis652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomson CA, Wang Y, Jackson LM, Olson M, Wang W, Liavonchanka A, Keleta L, Silva V, Diederich S, Jones RB, et al. Pandemic H1N1 Influenza Infection and Vaccination in Humans Induces Cross-Protective Antibodies that Target the Hemagglutinin Stem. Front Immunol. 2012;3:87. doi: 10.3389/fimmu.2012.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andrews SF, Huang Y, Kaur K, Popova LI, Ho IY, Pauli NT, Henry Dunand CJ, Taylor WM, Lim S, Huang M, et al. Immune history profoundly affects broadly protective B cell responses to influenza. Sci Transl Med. 2015;7:316ra192. doi: 10.1126/scitranslmed.aad0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moody MA, Zhang R, Walter EB, Woods CW, Ginsburg GS, McClain MT, Denny TN, Chen X, Munshaw S, Marshall DJ, et al. H3N2 influenza infection elicits more cross-reactive and less clonally expanded anti-hemagglutinin antibodies than influenza vaccination. PLoS One. 2011;6:e25797. doi: 10.1371/journal.pone.0025797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, Zheng NY, Mays I, Garman L, Helms C, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667–671. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schneemann A, Speir JA, Tan GS, Khayat R, Ekiert DC, Matsuoka Y, Wilson IA. A virus-like particle that elicits cross-reactive antibodies to the conserved stem of influenza virus hemagglutinin. J Virol. 2012;86:11686–11697. doi: 10.1128/JVI.01694-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen S, Zheng D, Li C, Zhang W, Xu W, Liu X, Fang F, Chen Z. Protection against multiple subtypes of influenza viruses by virus-like particle vaccines based on a hemagglutinin conserved epitope. Biomed Res Int. 2015;2015:901817. doi: 10.1155/2015/901817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang TT, Tan GS, Hai R, Pica N, Ngai L, Ekiert DC, Wilson IA, Garcia-Sastre A, Moran TM, Palese P. Vaccination with a synthetic peptide from the influenza virus hemagglutinin provides protection against distinct viral subtypes. Proc Natl Acad Sci U S A. 2010;107:18979–18984. doi: 10.1073/pnas.1013387107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Staneková Z, Király J, Stropkovská A, Mikušková T, Mucha V, Kostolanský F, Varečková E. Heterosubtypic protective immunity against influenza A virus induced by fusion peptide of the hemagglutinin in comparison to ectodomain of M2 protein. Acta Virol. 2011;55:61–67. doi: 10.4149/av_2011_01_61. [DOI] [PubMed] [Google Scholar]
- 58.Eggink D, Goff PH, Palese P. Guiding the immune response against influenza virus hemagglutinin toward the conserved stalk domain by hyperglycosylation of the globular head domain. J Virol. 2014;88:699–704. doi: 10.1128/JVI.02608-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin SC, Liu WC, Jan JT, Wu SC. Glycan masking of hemagglutinin for adenovirus vector and recombinant protein immunizations elicits broadly neutralizing antibodies against H5N1 avian influenza viruses. PLoS One. 2014;9:e92822. doi: 10.1371/journal.pone.0092822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wei CJ, Boyington JC, McTamney PM, Kong WP, Pearce MB, Xu L, Andersen H, Rao S, Tumpey TM, Yang ZY, et al. Induction of broadly neutralizing H1N1 influenza antibodies by vaccination. Science. 2010;329:1060–1064. doi: 10.1126/science.1192517. [DOI] [PubMed] [Google Scholar]
- 61.Babu TM, Levine M, Fitzgerald T, Luke C, Sangster MY, Jin H, Topham D, Katz J, Treanor J, Subbarao K. Live attenuated H7N7 influenza vaccine primes for a vigorous antibody response to inactivated H7N7 influenza vaccine. Vaccine. 2014;32:6798–6804. doi: 10.1016/j.vaccine.2014.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Halliley JL, Khurana S, Krammer F, Fitzgerald T, Coyle EM, Chung KY, Baker SF, Yang H, Martínez-Sobrido L, Treanor JJ, et al. High-Affinity H7 Head and Stalk Domain-Specific Antibody Responses to an Inactivated Influenza H7N7 Vaccine After Priming With Live Attenuated Influenza Vaccine. J Infect Dis. 2015 doi: 10.1093/infdis/jiv210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luke CJ, Subbarao K. Improving pandemic H5N1 influenza vaccines by combining different vaccine platforms. Expert Rev Vaccines. 2014;13:873–883. doi: 10.1586/14760584.2014.922416. [DOI] [PubMed] [Google Scholar]
- 64.Talaat KR, Luke CJ, Khurana S, Manischewitz J, King LR, McMahon BA, Karron RA, Lewis KD, Qin J, Follmann DA, et al. A live attenuated influenza A(H5N1) vaccine induces long-term immunity in the absence of a primary antibody response. J Infect Dis. 2014;209:1860–1869. doi: 10.1093/infdis/jiu123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miller MS, Tsibane T, Krammer F, Hai R, Rahmat S, Basler CF, Palese P. 1976 and 2009 H1N1 influenza virus vaccines boost anti-hemagglutinin stalk antibodies in humans. J Infect Dis. 2013;207:98–105. doi: 10.1093/infdis/jis652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.He W, Mullarkey CE, Duty JA, Moran TM, Palese P, Miller MS. Broadly neutralizing anti-influenza virus antibodies: enhancement of neutralizing potency in polyclonal mixtures and IgA backbones. J Virol. 2015;89:3610–3618. doi: 10.1128/JVI.03099-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dilillo DJ, Tan GS, Palese P, Ravetch JV. Broadly neutralizing hemagglutinin stalk-specific antibodies require FcγR interactions for protection against influenza virus in vivo. Nat Med. 2014;20:143–151. doi: 10.1038/nm.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wrammert J, Koutsonanos D, Li GM, Edupuganti S, Sui J, Morrissey M, McCausland M, Skountzou I, Hornig M, Lipkin WI, et al. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J Exp Med. 2011;208:181–193. doi: 10.1084/jem.20101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maddison SE, Stewart CC, Farshy CE, Reimer CB. The relationship of race, sex, age to concentrations of serum immunoglobulins expressed in international units in healthy adults in the USA. Bull World Health Organ. 1975;52:179–185. [PMC free article] [PubMed] [Google Scholar]
- 70.Gonzalez-Quintela A, Alende R, Gude F, Campos J, Rey J, Meijide LM, Fernandez-Merino C, Vidal C. Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities. Clin Exp Immunol. 2008;151:42–50. doi: 10.1111/j.1365-2249.2007.03545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stoop JW, Zegers BJ, Sander PC, Ballieux RE. Serum immunoglobulin levels in healthy children and adults. Clin Exp Immunol. 1969;4:101–112. [PMC free article] [PubMed] [Google Scholar]
- 72.Bos NA, Kimura H, Meeuwsen CG, De Visser H, Hazenberg MP, Wostmann BS, Pleasants JR, Benner R, Marcus DM. Serum immunoglobulin levels and naturally occurring antibodies against carbohydrate antigens in germ-free BALB/c mice fed chemically defined ultrafiltered diet. Eur J Immunol. 1989;19:2335–2339. doi: 10.1002/eji.1830191223. [DOI] [PubMed] [Google Scholar]
- 73.Natsuume-Sakai S, Motonishi K, Migita S. Quantitative estimations of five classes of immunoglobulin in inbred mouse strains. Immunology. 1977;32:861–866. [PMC free article] [PubMed] [Google Scholar]
- 74.Shi B, Ma L, He X, Wang X, Wang P, Zhou L, Yao X. Comparative analysis of human and mouse immunoglobulin variable heavy regions from IMGT/LIGM-DB with IMGT/HighV-QUEST. Theor Biol Med Model. 2014;11:30. doi: 10.1186/1742-4682-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dreyfus C, Laursen NS, Kwaks T, Zuijdgeest D, Khayat R, Ekiert DC, Lee JH, Metlagel Z, Bujny MV, Jongeneelen M, et al. Highly conserved protective epitopes on influenza B viruses. Science. 2012;337:1343–1348. doi: 10.1126/science.1222908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wohlbold TJ, Chromikova V, Tan GS, Meade P, Amanat F, Comella P, Hirsh A, Krammer F. Hemagglutinin Stalk- and Neuraminidase-Specific Monoclonal Antibodies Protect against Lethal H10N8 Influenza Virus Infection in Mice. J Virol. 2015;90:851–861. doi: 10.1128/JVI.02275-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Florek NW, Weinfurter JT, Jegaskanda S, Brewoo JN, Powell TD, Young GR, Das SC, Hatta M, Broman KW, Hungnes O, et al. Modified vaccinia Ankara encoding influenza virus hemagglutinin induces heterosubtypic immunity in macaques. J Virol. 2014 doi: 10.1128/JVI.01219-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jegaskanda S, Weinfurter JT, Friedrich TC, Kent SJ. Antibody-dependent cellular cytotoxicity is associated with control of pandemic H1N1 influenza virus infection of macaques. J Virol. 2013;87:5512–5522. doi: 10.1128/JVI.03030-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jegaskanda S, Job ER, Kramski M, Laurie K, Isitman G, de Rose R, Winnall WR, Stratov I, Brooks AG, Reading PC, et al. Cross-reactive influenza-specific antibody-dependent cellular cytotoxicity antibodies in the absence of neutralizing antibodies. J Immunol. 2013;190:1837–1848. doi: 10.4049/jimmunol.1201574. [DOI] [PubMed] [Google Scholar]
- 80.Jegaskanda S, Reading PC, Kent SJ. Influenza-specific antibody-dependent cellular cytotoxicity: toward a universal influenza vaccine. J Immunol. 2014;193:469–475. doi: 10.4049/jimmunol.1400432. [DOI] [PubMed] [Google Scholar]
- 81.Terajima M, Cruz J, Co MD, Lee JH, Kaur K, Wrammert J, Wilson PC, Ennis FA. Complement-dependent lysis of influenza a virus-infected cells by broadly cross-reactive human monoclonal antibodies. J Virol. 2011;85:13463–13467. doi: 10.1128/JVI.05193-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Laidlaw BJ, Decman V, Ali MA, Abt MC, Wolf AI, Monticelli LA, Mozdzanowska K, Angelosanto JM, Artis D, Erikson J, et al. Cooperativity between CD8+ T cells, non-neutralizing antibodies, and alveolar macrophages is important for heterosubtypic influenza virus immunity. PLoS Pathog. 2013;9:e1003207. doi: 10.1371/journal.ppat.1003207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Throsby M, van den Brink E, Jongeneelen M, Poon LL, Alard P, Cornelissen L, Bakker A, Cox F, van Deventer E, Guan Y, et al. Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells. PLoS ONE. 2008;3:e3942. doi: 10.1371/journal.pone.0003942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ekiert DC, Bhabha G, Elsliger MA, Friesen RH, Jongeneelen M, Throsby M, Goudsmit J, Wilson IA. Antibody recognition of a highly conserved influenza virus epitope. Science. 2009;324:246–251. doi: 10.1126/science.1171491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Friesen RH, Lee PS, Stoop EJ, Hoffman RM, Ekiert DC, Bhabha G, Yu W, Juraszek J, Koudstaal W, Jongeneelen M, et al. A common solution to group 2 influenza virus neutralization. Proc Natl Acad Sci U S A. 2014;111:445–450. doi: 10.1073/pnas.1319058110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nakamura G, Chai N, Park S, Chiang N, Lin Z, Chiu H, Fong R, Yan D, Kim J, Zhang J, et al. An in vivo human-plasmablast enrichment technique allows rapid identification of therapeutic influenza A antibodies. Cell Host Microbe. 2013;14:93–103. doi: 10.1016/j.chom.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 87.Wu Y, Cho M, Shore D, Song M, Choi J, Jiang T, Deng YQ, Bourgeois M, Almli L, Yang H, et al. A potent broad-spectrum protective human monoclonal antibody crosslinking two haemagglutinin monomers of influenza A virus. Nat Commun. 2015;6:7708. doi: 10.1038/ncomms8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tharakaraman K, Subramanian V, Viswanathan K, Sloan S, Yen HL, Barnard DL, Leung YH, Szretter KJ, Koch TJ, Delaney JC, et al. A broadly neutralizing human monoclonal antibody is effective against H7N9. Proc Natl Acad Sci U S A. 2015;112:10890–10895. doi: 10.1073/pnas.1502374112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Belongia EA, Sundaram ME, McClure DL, Meece JK, Ferdinands J, VanWormer JJ. Waning vaccine protection against influenza A (H3N2) illness in children and older adults during a single season. Vaccine. 2015;33:246–251. doi: 10.1016/j.vaccine.2014.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yu X, Tsibane T, McGraw PA, House FS, Keefer CJ, Hicar MD, Tumpey TM, Pappas C, Perrone LA, Martinez O, et al. Neutralizing antibodies derived from the B cells of 1918 influenza pandemic survivors. Nature. 2008;455:532–536. doi: 10.1038/nature07231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Goff PH, Hayashi T, Martínez-Gil L, Corr M, Crain B, Yao S, Cottam HB, Chan M, Ramos I, Eggink D, et al. Synthetic Toll-like receptor 4 (TLR4) and TLR7 ligands as influenza virus vaccine adjuvants induce rapid, sustained, and broadly protective responses. J Virol. 2015;89:3221–3235. doi: 10.1128/JVI.03337-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gamblin S, Haire L, Russell R, Stevens D, Xiao B, Ha Y, Vasisht N, Steinhauer D, Daniels R, Elliot A, et al. The structure and receptor binding properties of the 1918 influenza hemagglutinin. Science. 2004;303:1838–1842. doi: 10.1126/science.1093155. [DOI] [PubMed] [Google Scholar]