Abstract
On July 10, 1976, an explosion at a chemical plant in Seveso, Italy, released up to 30 kg of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)—the most potent dioxin congener. Twenty years later, the Seveso Women’s Health Study (SWHS) initiated a follow-up assessment of a cohort of female Seveso residents. Researchers collected serial blood, measured for TCDD levels, and recorded information about the women’s medical history after the explosion. The study’s aims were to: 1) modify the human PBPK model for TCDD (Emond et al. 2004; Emond et al. 2005; NCEA-USEPA 2010) to include repetitive gestation and lactation; 2) simulate TCDD blood concentrations during different life stages including pregnancy and lactation, under different exposure scenarios; and 3) use this PBPK model to compare the influence of gestation and lactation on elimination of TCDD. After optimization of the model, it was assessed using data from the SWHS cohort. The 23 women in Subcohort A, were 4–39 years old and in Subcohort B, the 18 women were 3–17 years old when the explosion occurred. The model accurately predicted the blood concentrations during the 20 years post-exposure, including periods of pregnancy and lactation. The model was also used to analyze the contribution of gestation and lactation to the mother’s elimination of TCDD. The results suggest that gestation and lactation do not significantly impact TCDD blood elimination. Future efforts will focus on using additional data to evaluate the PBPK model and improving the mathematical descriptions of lactation and multiple gestations.
Keywords: PBPK, pharmacokinetics, dioxin, TCDD, developmental
INTRODUCTION
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is the most potent congener of the dioxin and dioxin-like chemicals. TCDD and related chemicals are associated with many biochemical, physiological, and toxicological responses, including the induction of Cytochrome P4501A (CYP1A) and Cytochrome P4501B (CYP1B) isoforms, modulation of growth factors and their receptors, immunotoxicity, developmental toxicities and cancer (Devito and Birnbaum 1994; Emond et al. 2004; Emond et al. 2005).
The pharmacokinetics of TCDD are relatively well understood in adult humans (Kerger et al. 2006; Michalek and Tripathi 1999; Milbrath et al. 2009). TCDD induces its own elimination at high exposures and its pharmacokinetics are highly influenced by the percentage of body fat at low exposures (Emond et al. 2005). However, the impact of pregnancy and lactation on the elimination of TCDD and other dioxins is not clear.
A wide variety of factors could impact the elimination of chemicals during pregnancy and lactation, including exposure level, length of lactation, age, parity, and diet as well as the individual chemical pharmacokinetics. Several studies suggest that human milk concentrations of persistent organic pollutants (POPs) decrease during the course of lactation (Hooper et al. 1998; Schecter et al. 1998; Wittsiepe et al. 2007) while other studies have reported no change or a slight increase over time (LaKind et al. 2009; Miyata et al. 2002). The pharmacokinetics of TCDD is dose dependent, but we have not found evidence for dose dependency for other POPs such as PCB 153 (LaKind et al 2009). Although pregnancy and lactation may contribute to TCDD elimination (LaKind et al. 2009; Rogan and Ragan 1994; Weiss et al. 2003), this elimination may not be linear because the pharmacokinetics of TCDD is dose dependent and dependent upon body fat composition (Emond et al. 2005; Emond et al. 2006).
On July 10, 1976, an explosion at the Industrie Chimiche Meda Società Azionaria (ICMESA) chemical factory in Seveso, Italy released an aerosol cloud of up to 30 kg of TCDD over 18.1 km2 of a residential area (di Domenica 1980). The area surrounding the factory was subdivided into exposure zones based on surface soil levels of TCDD (Mocarelli and Pocchiari 1988). In 1996, 20 years after the explosion, the Seveso Women’s Health Study (SWHS) was initiated to follow up the women who were newborn to 40 years old at the time of the accident in 1976 and resided in the most heavily contaminated areas (Zones A or B) (Eskenazi et al. 2000). The TCDD concentrations measured in 1976 serum varied widely in this population, ranging from 2.5 to 56,000 ppt, lipid adjusted, and were highest in those who were youngest (Eskenazi et al. 2004; Warner et al. 2014)
The Seveso Women’s Health Study, with detailed information on pregnancy and lactation history and serial blood sampling starting shortly after exposure (Eskenazi et al. 2000; Eskenazi et al. 2001), provides a unique opportunity to study the pharmacokinetics of TCDD in women from an exposed population. In the present study, we use these data to address three objectives: 1) to modify the human PBPK model for TCDD (Emond et al. 2004; Emond et al. 2005; NCEA-USEPA 2010) to include gestation and lactation; 2) to simulate TCDD blood concentrations during different life stages including pregnancy and lactation, under different exposure scenarios; and 3) to use this PBPK model to compare the influence of gestation and lactation on elimination of TCDD.
METHODS
PBPK Model Development and Calibration
Model Development
The PBPK model described here is based on the human TCDD model (Emond et al. 2005), which was later improved during the TCDD reassessment process to include gestational exposures (NCEA-USEPA 2010). During development of the model, it was evaluated using several human datasets (i.e., U.S. Air Force veteran cohort of Operation Ranch Hand, Viennese women poisoning cases (Emond et al. 2005)). Because gestation and lactation may be considered as important elimination routes for lipophilic environmental contaminants as well as important periods of exposure (Schecter et al. 1996; Takekuma et al. 2011), we expanded the TCDD PBPK model to account for lifetime exposure incorporating lactation.
Model Representation
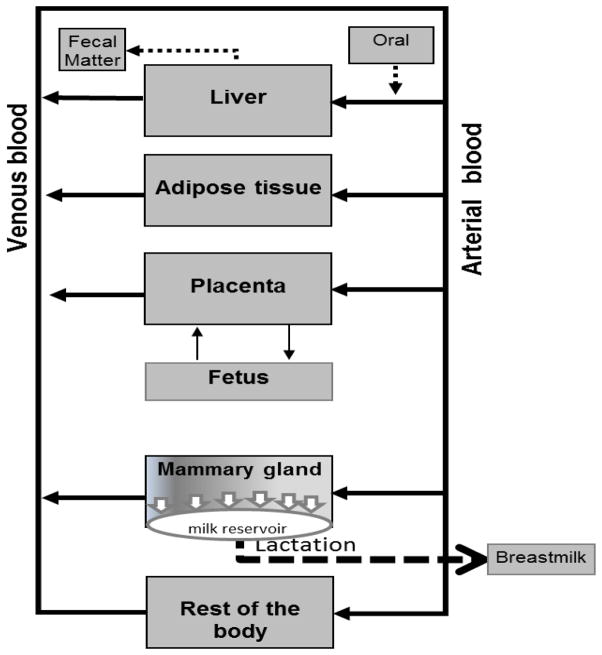
The original model contained 5 compartments: liver, adipose tissue, rest-of-the-body (maternal), plus two additional compartments during pregnancy, placenta [maternal/fetal] and whole fetus. This new PBPK model incorporates the mammary gland and a description of lactation (Fig. 1). Thus, the model includes those organs or tissues that play essential roles in the pharmacokinetics of TCDD, are required for mass balance, or are used for biological sampling of TCDD exposure. Liver and adipose tissue were included in the PBPK model because they are involved in the metabolism and storage of TCDD, and they account for almost 80% of the body burden of TCDD (Carrier et al. 1995). Depending on the body burden, TCDD may be highly sequestered in the liver due to its binding to CYP1A2, which becomes more important as the TCDD concentrations increase due to the aryl hydrocarbon receptor (AhR) –mediated induction of CYP1A2 (Diliberto et al. 1997; Emond et al. 2005). A blood compartment was also included because it describes the systemic circulation and this biological fluid is commonly used to measure TCDD exposures. The rest-of-the-body compartment (all other tissues not considered individually) was included to complete mass balance. Each maternal compartment was interconnected by the systemic circulation (blood) (Fig. 1). Liver, adipose tissue, rest-of-the-body (maternal), and the placenta were described as diffusion limited. The mammary gland was described as flow limited.
Fig. 1.
A conceptual representation of the developmental human PBPK model.
The fetus is an independent subcompartment, exchanging TCDD with the mother through cord blood across the placenta, and is considered well stirred and flow limited. Although the fetal subcompartment is considered in mass balance calculations, it is not considered as part of the maternal compartment when estimating maternal body burdens.
The PBPK model includes several physiological changes in the maternal and fetal subcompartments during pregnancy (Emond et al. 2003; Emond et al. 2004; NCEA-USEPA 2012). The blood TCDD exchange between the placenta and the fetus was described by a mathematical clearance expression, (L/day), which was activated at Week 8 of pregnancy. For the non-gestational condition, the model apportions TCDD only among the blood, adipose tissue, liver, and rest-of-the-body compartments; the placental compartment and the fetal subcompartments are deactivated (turned off). The lactational subcompartment activates at the end of gestation, if the mother breastfed the baby; otherwise it is not activated.
Absorption from the gastrointestinal (GI) tract was modeled using a first-order rate constant, (Ka) (day−1) that was fitted to an optimization dataset (Diliberto et al. 1997; Emond et al. 2005). Elimination via the feces was described by a first-order rate, (Kst) (day−1). The metabolism and elimination processes were grouped into a parameter, (KBILELI), which varied with exposure and was described in the liver compartment.
This model allowed for multiple gestations (Fig. 1) and used the pregnancy and lactation histories reported by each Seveso Women’s Health Study participant. According to the literature, the ratio of whole blood TCDD concentration (lipid adjusted) to the milk TCDD concentration (lipid adjusted) ranges from 1 to 1.5 (Schecter et al. 1995; Schecter et al. 1996). The model includes milk production beginning at the end of gestation and is continuing until the end of lactation. The model was formatted to four lactations of 30 minutes per day, with the infant receiving 150 mL of milk per kilogram of newborn per lactation (Kent et al. 1999; Kent et al. 2006).
The equations and the description of the symbol used for the milk production and lactation frequency are presented in the Supplemental Materials Section A and B. While different versions of the mathematical description of lactation are described in the literature (Byczkowski et al. 1994; Clewell et al. 2003; Clewell and Gearhart 2002; Fisher et al. 1997; You et al. 1999), we adapted the equations 1 to 3 for this model:
| (Eq. 1) |
| (Eq. 2) |
| (Eq. 3) |
Where;
| Qmlik | TCDD Clearance from the mammary gland into the milk (L/day) |
| CVMAT | Tissue blood sub-compartment TCDD concentration in the mammary gland (nmol/L) |
| RASUCKLING | Rate of change of TCDD during suckling from the milk reservoir during breast-feed (nmol/day) |
| Amilk | Amount of TCDD in milk (nmole) |
| Wmilk | Volume of milk in the milk reservoir (L) |
| Ksuck | First order constant (1/day) |
| Cmilk | Milk concentration (nmoL/L) |
Model Parameterization
Parameters of the PBPK model such as the volumes of the placenta, body weight, and adipose tissue compartments during pregnancy varied based on the physiological changes occurring during pregnancy and these parameters were obtained from the literature (Table 1) or previously optimized (Emond et al. 2003; Emond et al. 2004; NCEA-USEPA 2012) (Table 1). All of the mathematical equations and descriptions of the symbol are presented in Supplemental Materials Section A and B. Anatomical, physiological, and biochemical parameters (e.g., tissue volumes, cardiac output, blood flows, partition coefficients, and permeability constants) were extracted from the literature (Emond et al. 2004; Krishnan and Andersen 2008; Lawrence and Gobas 1997; Poiger and Schlatter 1986; Wang et al. 1997). Other parameters such as fetal weight or adipose tissue content during pregnancy were described as functions using data from literature.
Table 1.
Parameters of the PBPK model for TCDD in Human
| Parameter Description | Symbol | Parameter Values Human Gestational a |
|---|---|---|
| Body weight (kg) | BW | Calculated |
| Cardiac output (L/kg/day) | QCC | 368.64 b,c |
|
| ||
|
Tissue (intracellular) volumes (fraction of BW unitless)
| ||
| Liver | WLI0 | Calculated |
| Adipose tissue | WF0 | Calculated |
| Mammary tissue fraction | WMATINIT | 0.0062 |
| Milk production volume | WMILKINIT | 0.15 |
|
| ||
|
Tissue blood volumes (unitless)
| ||
| Liver (fraction of WLI0) | WLIB0 | 0.266 e |
| Adipose tissue (fraction of WF0) | WFB0 | 0.05 |
| Rest of the body (fraction of WRE0) | WREB0 | 0.03 |
| Placenta tissue fraction of tissue blood weight (unitless) | WPLAB0 | 0.4 g |
|
| ||
|
Tissue blood flow (fraction of cardiac output [unitless])
| ||
| Liver | QLIF | 0.26 c |
| Adipose tissue | QFF | 0.05 c |
| Placenta | QPLAF | Calculated |
| Mammary gland | QMAT | 0.1 |
|
| ||
|
Tissue permeability (fraction of tissue blood flow [unitless])
| ||
| Liver | PALIF | 0.35 d |
| Adipose tissue | PAFF | 0.12 e |
| Placenta diffusional permeability fraction (unitless) | PAPLAF | 0.3 g |
| Rest of the body | PAREF | 0.03 d |
|
| ||
|
Partition coefficient
| ||
| Liver | PLI | 6 d |
| Fetus/blood partition coefficient (unitless) | PFETUS | 4 f |
| Placenta/blood partition coefficient (unitless) | PPLA | 1.5 f |
| Adipose tissue | PF | 100 d |
| Rest of the body | PRE | 1.5 d |
|
| ||
| Mammary | PMAT | 12e |
|
| ||
|
Metabolism constants
| ||
| Urinary clearance elimination (L/day) | CLURI | 1.00−6 g |
| Clearance—transfer from mother to fetus (L/day) | CLPLA_FET | 0.024 d |
| Liver (biliary elimination and metabolism [day−1]) | KBILE_LI | Inducible |
| Interspecies constant (day−1) | KELV | 0.024 e |
|
| ||
|
AhR
| ||
| Affinity constant in liver (nmol/L) | KDLI | 0.1 d |
| Binding capacity in liver (nmol/L) | LIBMAX | 0.35 d |
| Placenta binding capacity (nmol/L) | PLABMAX | 0.2 f |
| Affinity constant protein (AhR) in placenta (nmol/L) | KDPLA | 0.1 f |
|
| ||
|
CYP1A2 induction parameters
| ||
| Dissociation constant CYP1A2 (nmol/L) | KDLI2 | 40 f |
| Degradation process CYP1A2 (nmol/L) | CYP1A2_1OUTZ | 1600 d |
| Dissociation constant during induction (nmol/L) | CYP1A2_1EC50 | 130 d |
| Basal concentration of CYP1A2 (nmol/L) | CYP1A2_1A2 | 1600 d |
| First-order rate of degradation (day−1) | CYP1A2_1KOUT | 2.4 d |
| Time delay before induction process (day) | CYP1A2_1TAU | 0.0104 d |
| Maximal induction of CYP1A2 (unitless) | CYP1A2_1EMAX | 9300 e |
|
| ||
|
Other constants
| ||
| Oral absorption constant (day−1) | KABS | 1.44 e |
| Gastric non-absorption constant (day−1) | KST | 0.24 e |
| Clearance TCDD in the mam. gland into the milk (L/day) | Qmilk | 3.0 e |
| Clearance parameter of suckling milk from (L/day) | CL_SUCK | 20 e |
Units for human non-gestational parameters are L and kg.
Units are L/kg/day.
Optimized.
Many of the parameters and constants used to describe lactation were obtained from the literature known (e.g., hourly milk production, mammary gland blood flow). However, in the description of lactation, the partition coefficient for mammary gland/blood [Pmat]) was set equal to 12 corresponding to the ratio of Cmammary gland/Cblood. The rate of transfer of TCDD from the tissue blood subcompartment of the mammary gland (CVMAT) to the milk reservoir (Fig. 1) is described as the clearance of milk from the mammary gland tissue to the milk subcompartment or the milk reservoir. Another parameter called Ksuck is a first order function (day−l) purging the milk outside the reservoir during lactation. In order to attain a whole blood concentration to milk concentration ratio of 1.5, based on Schecter et al (1995, 1996), the parameters CVMAT, Qmilk, and Ksuck were optimized.
Advanced Continuous Simulation Language (acslX) version 3.0.2.1 was used to write the PBPK model description. To reduce the time duration of a simulation, the independent variable time was converted from hours to days. The conversion of whole blood to serum lipid adjusted used in the model is presented in Equation 4, as follows:
| (Eq. 4) |
The model was initially evaluated by simulating virtual scenarios consisting of a single exposure to a women aged 20 years, with pregnancy occurring 1 year post-exposure and nursing for 6 months. The simulation was stopped 3 years after the initial exposure. Four different exposure levels were used to simulate a range of exposures which included the average daily intake of dioxins (0.001 ng/kg of TCDD), and a low (10 ng/kg of TCDD), medium (100 ng/kg of TCDD), and high (1,000 ng/kg of TCDD) single acute exposure.
Comparisons were performed for gestation and lactation, gestation but no lactation, and no pregnancy during the exposure period by examining the model predictions of body burden (ng/kg), mother whole blood concentrations (ng/L), and serum TCDD concentrations on a lipid adjusted basis (Table 2). These exposures were selected to cover the range of exposures in Seveso based on preliminary simulations and analyses of the data from pregnant women with or without lactation and nulliparous women in the Seveso cohort. In the initial analysis, the PBPK model indicated that the linkage between exposure and body burden was non-linear and that at higher exposures there was a greater rate of elimination consistent with previous findings from the model (Emond et al. 2005). More importantly, the model predicts that pregnancy and/or lactation will have limited impact on maternal body burdens, blood concentrations, or serum concentrations (Table 2).
Table 2.
A dose response comparison of the model predictions of a single exposure (i.e., 0.001, 10, 100, and 1,000 ng of TCDD/kg of body weight) to discriminate between the routes of elimination. The exposure scenario for each simulation assumes a woman was 20 years old at the start of the exposure, that pregnancy began 1 year after the single exposure and lasted for 280 days, and that lactation lasted for 6 months (180 days) starting just after the labor. The simulation was stopped 3 years post-exposure. The following different conditions were simulated: pregnancy + lactation (P+L), pregnancy and no lactation (P+NL), and no pregnancy and no lactation (NP+NL). The following three different parameters were retained for this comparison: the body burden (ng/kg), the whole blood TCDD concentration (ng/L), and the serum TCDD concentration (ng/kg in lipid adjusted).
| Single Exposure Dose (ng/kg of Body Weight) | Dose Metrics | P+L | P+NL | NP+NL |
|---|---|---|---|---|
| 0.001 ng/kg | Body burden (ng/kg of body weight) | 7.56×X10−4 | 8.01×10−4 | 8.01×10−4 |
| Whole blood TCDD (ng/L) | 2.58×10−5 | 2.60×10−5 | 2.60×10−5 | |
| Serum TCDD concentration (ng/kg lipid adjusted) | 8.24×10−3 | 8.30×10−3 | 8.30×10−3 | |
|
| ||||
| 10 ng/kg | Body burden (ng/kg of body weight) | 6.4215 | 6.45 | 6.45 |
| Whole blood TCDD (ng/L) | 0.16 | 0.164 | 0.16 | |
| Serum TCDD concentration (ng/kg lipid adjusted) | 51.97 | 52.16 | 52.21 | |
|
| ||||
| 100 ng/kg | Body burden (ng/kg of body weight) | 52.00 | 52.16 | 52.19 |
| Whole blood TCDD (ng/L) | 0.94 | 0.95 | 0.95 | |
| Serum TCDD concentration (ng/kg lipid adjusted) | 301.223 | 301.944 | 302.104 | |
|
| ||||
| 1,000 ng/kg | Body burden (ng/kg of body weight) | 430.19 | 430.19 | 430.90 |
| Whole blood TCDD (ng/L) | 4.766 | 4.77 | 4.77 | |
| Serum TCDD concentration (ng/kg lipid adjusted) | 1520.26 | 1520.26 | 1522.14 | |
Software, Algorithms, and Model Code
All simulations and parameter fits were conducted using acslX v.3.0.2.1 (AEgis Technologies, Huntsville, AL). The Gear algorithm was used to integrate double-precision variables. Parameter fitting was driven by using the relative-error model estimation. Maximization of the log likelihood function was the decisive factor for parameter fitting. A limitation of this approach is that although maximum likelihood estimates will be reasonable, error estimates will be incorrect due to violation of the assumption of independent data. Starting values for parameter fitting were obtained by visual fit, and then the subroutine parameter estimation from acslX was used.
Sensitivity Analysis
Sensitivity analysis shows the ways in which a model output is influenced by variation of individual parameter values (Easterling et al. 2000), and the results are expressed as a magnitude of change in the model output of interest (Krishnan and Andersen 2008). For the current analysis, each parameter in the PBPK model was tested for sensitivity by varying each parameter by ±10% of the optimized value. The sensitivity analysis was performed assuming the woman received a single exposure dose of 30 ng TCDD/kg body weight at 25 years of age and became pregnant at age 25 years, then lactated for 6 months (180 days) after delivery for a total simulation of 2 years. The sensitivity analysis recorded the influence of individual parameter-driven variations of area under the curve (AUC) in the mother’s blood and is presented in Equation 2.
| (Eq. 2) |
RESULTS
Model Fits to the SWHS Cohort
Data from the SWHS cohort was used to assess the model. The SWHS sample was divided into two subcohorts herein described as Subcohort A and Subcohort B. Subcohort A consisted of 23 SWHS participants who were 4 to 39 years of age at the time of the explosion and had multiple (>2) blood samples taken from shortly after the exposure until 1996. Six of the 23 women were nulliparous, eight had given birth to one child, and nine had given birth to two or more children in the 20-year window. Subcohort B consisted of 18 women who were 3 to 17 years of age at the time of the explosion and had only 2 blood samples, one shortly after exposure and a second collection in 1996.
For each subject, the initial exposure was not measured, although TCDD blood concentrations were determined soon after the exposure. The initial exposure was estimated using the PBPK model in an iterative manner by fitting a single oral exposure that would provide a reasonable fit to the TCDD blood concentration data. The assumption of a single oral exposure instead of inhalation and/or dermal route exposures for a short period of time is reasonable because of the long half-life of TCDD and the fact that the measurements were taken a few weeks after the absorption period. For each subject in the entire cohort, blood concentration profiles were simulated and compared to the measured blood concentration profiles; the oral exposure that best fit the blood concentration profiles was used as the initial exposure.
Model Simulation of SWHS Subcohort A
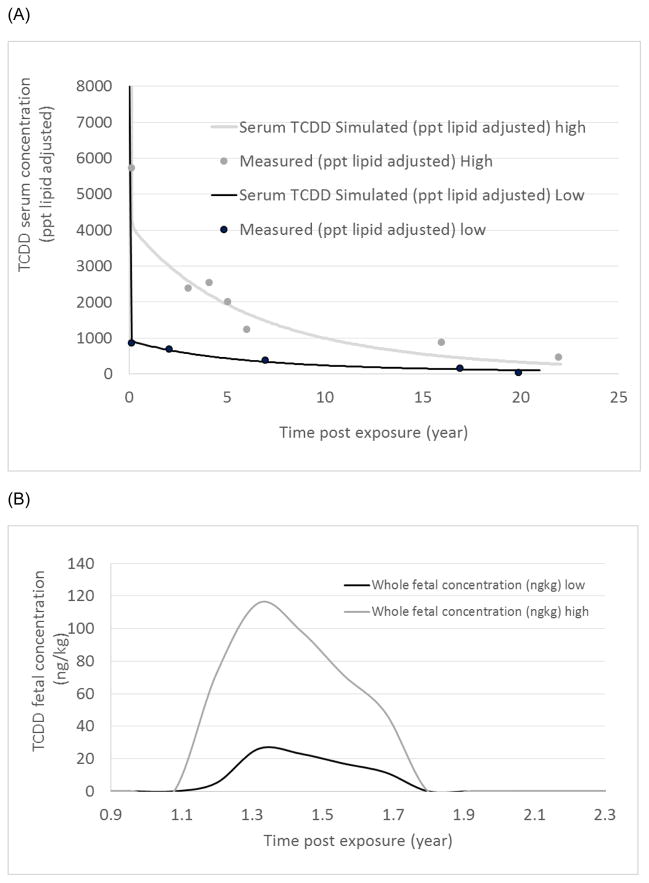
First, we evaluated the model with two women from Subcohort A, one with a high exposure level and one with a lower exposure level. The woman with the higher exposure level corresponded to an equivalent single oral dose of 2,000 ng TCDD/kg body weight. The six available data points measured were well predicted by the modeling profile (Fig. 2A). The woman with the lower exposure had an estimated exposure of 250 ng of TCDD/kg body weight, and the simulation reasonably predicted her blood TCDD concentration time course data (Fig. 2A). It is important to note that the two women became pregnant at different times during the post-exposure period; however, the data for the women during pregnancy were superimposed to compare their profiles. Fetal concentrations of TCDD were simulated and expressed as ng of TCDD/kg of body weight (Fig. 2B). A comparison between the ratio of high to low dose (high/low) regarding the exposure dose (2,000/250 ng TCDD/kg body weight), the mothers’ serum concentration ratio (5,730/864 measured or 4,274/952 predicted [ng/L lipid adjusted]), and the maximal fetal concentration ratio (116/23) corresponded to ≅8, ≅6, ≅4.5, and ≅5, respectively. This observation suggests that the fetal concentration was dependent on the maternal serum concentration and that the decreased ratio of high to low exposure in the fetus was likely because of maternal hepatic sequestration at the higher exposure.
Fig. 2.
Simulated tissue concentration time course for a woman with a single gestation who was exposed in Seveso, Italy, in 1976. The figures express (A) a TCDD serum concentration in lipid adjusted and (B) fetal concentrations at the higher exposure dose (2,000 ng of TCDD/kg of body weight) or at low exposure dose (250 ng of TCDD/kg of body weight), following a single exposure oral dose.
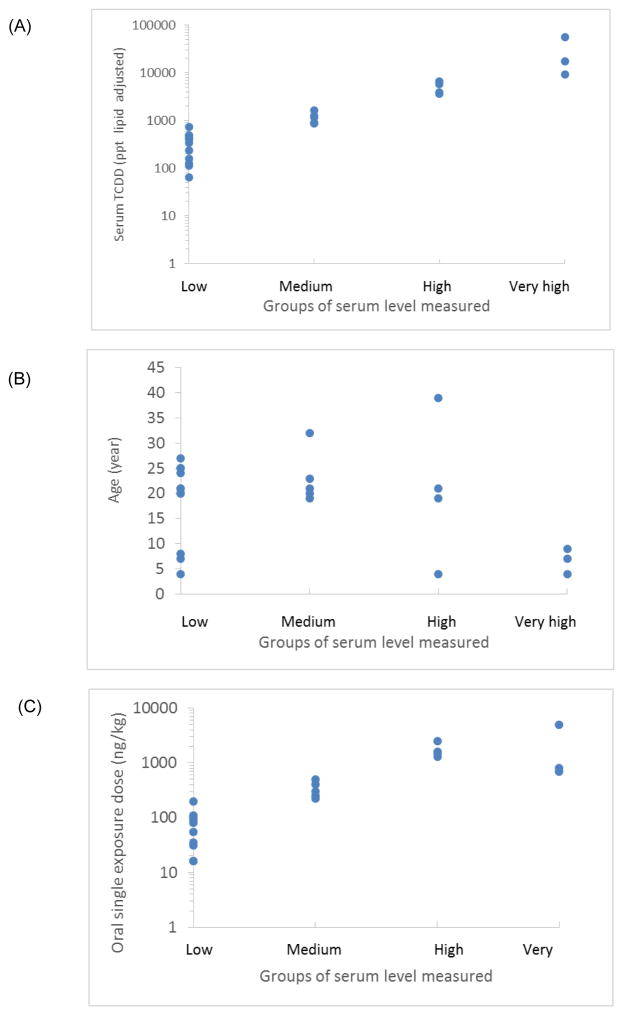
Second, we split Subcohort A into four exposure groups (i.e., low, medium, high, and very high), based on initial serum concentration expressed in ng/L lipid adjusted (Fig. 3A). The analysis indicated that the youngest women had higher exposures based on mass TCDD/kg body weight. No apparent differences with respect to age were observed within groups with low, medium, and high blood concentrations (Fig. 3B). A comparison of the oral exposure doses (ng/kg) for all four groups was conducted. As expected, the lower exposure doses were also in the lower blood concentration groups. However, some overlap was observed between the groups (Fig. 3C).
Fig. 3.
A comparison of (A) age (years), (B) serum TCDD concentration (ng/kg lipid adjusted in log scale to a subgroups of exposure, and (C) exposure dose estimated with the PBPK human model for TCDD. The parameters are compared to the subgroups of serum-level categories corresponding to low (≤200 ng/kg), medium (>200 to ≤1,000 ng/kg), high (>1,000 to <5,000 ng/kg), and very high (≤5,000 ng/kg) measured exposures.
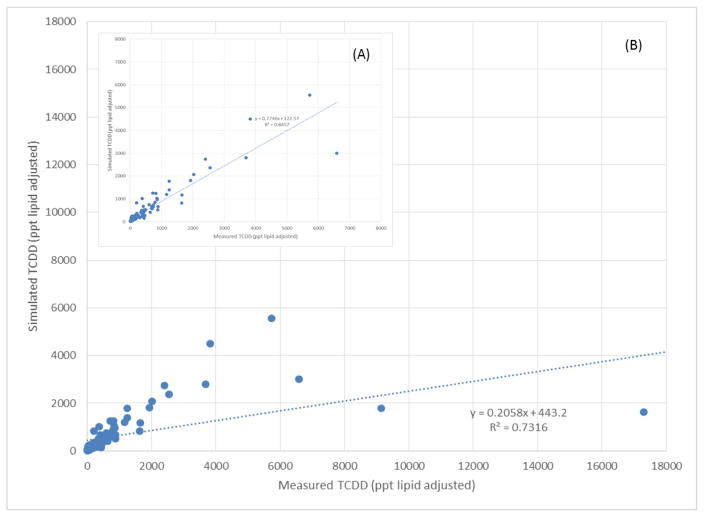
Third, a comparison was made between the TCDD concentration measured in serum collected in 1976 to the model predictions, and the profile was determined using the PBPK for all 23 women in Subcohort A (Suppl mat. Section C). A back-calculation strategy was used on the first data point measured in 1976 to determine the dose adjustment (Fig. 4A–B). Of the 23 women (96 data measurements), the model simulations showed a relatively good fit for 20 of the women; however, the model under predicted 1976 levels (ppt, lipid-adjusted) by a factor 4.8–10.6 for three women, yielding the following ratio of the 1976 measured/simulated blood concentrations expressed in ppt lipid adjusted of 9,140/1,777, 17,300/1,632, and 56,000/11,636. The model also over predicted 1996 levels (ppt, lipid-adjusted) for these three women, yielding the following ratios of the 1996 measured/simulated blood concentrations expressed in ppt lipid adjusted of ≈38/119, 103/151, and 35/73. When all of the data from 1976 were compared to the predicted blood concentrations, the R-squared (R2) determination coefficient was approximately 0.73 with a slope of 0.21, indicating that the model under predicted by approximately 5 fold (Fig. 4A). However, if the three women who were most over predicted were removed, then the correlation increased to 0.85 (Fig. 4B) with a slope of 0.77, indicating that the model predictions are approximately 1/3 less than the data.
Fig. 4.
A comparison between the measured TCDD concentrations during the follow-up study from 1976 to 1996 to the predicted concentration with the PBPK model. A total of 23 women were followed in Cohort A for a total of 96 samples. (A) Represents the correlation of all 23 women sampled and modeled individually and the expressed serum lipid adjusted for R2=0.7316. (B) Represents the correlation, if the first samples from 1976 of three highest blood concentrations of the women were removed because the concentrations were outliers compared to the cohort. The correlation is now R2=0.8457.
Model simulations of SWHS Subcohort B
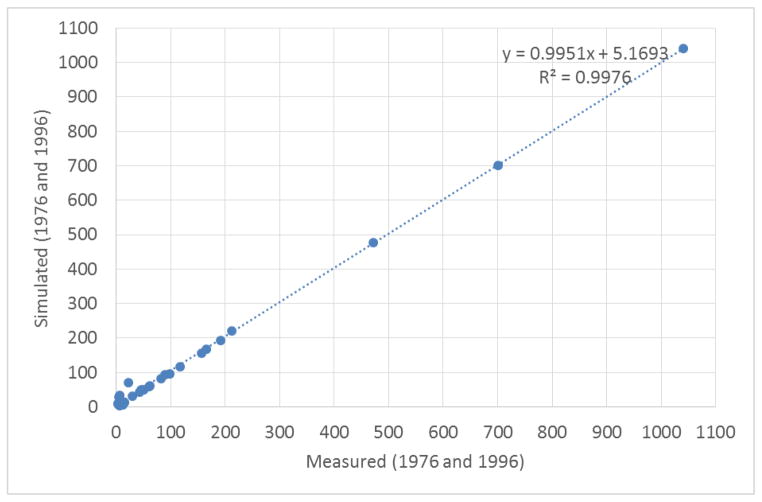
Lastly, in the second dataset, Subcohort B, containing 18 women with only two measured blood samples, one taken shortly after the exposure and the second in 1996 (Fig. 5), the model accurately fitted the data with an R2 determination coefficient of 0.9976, i.e. the model explained approximately 99% of the data measured.
Fig. 5.
A comparison of samples collected during two time points (i.e., 1976 and 1996) for each Seveso woman in Cohort B (N=18). A correlation between the measured concentration follow-up study and the predicted concentration with the PBPK model these 18 pairs of serum was R2=0.9976.
Sensitivity Analysis
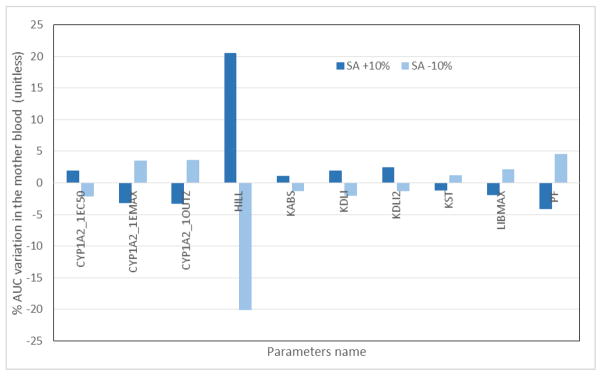
Sensitivity analysis of the PBPK model was performed for all 40 parameters and it determined that the most sensitive parameter was the Hill constant with an AUC variation of ≈ 20% for a 10% change in the parameter. Nine constants showed AUC variations higher than 1% (Fig. 6) and were considered sensitive in the model. These constants regulate the sensitivity of the induction of the CYP1A2 enzyme sequestering the TCDD in the liver and include the dissociation constant during induction (CYP1A2_1EC50) (nmoL/mL), maximal induction of CYP1A2 (CYP1A2_1EMAX) (unitless), degradation process CYP1A2 (nmoL/mL), (CYP1A2_1OUTZ) (nmoL/L), the Hill coefficient (HILL) (unitless), the affinity constant of CYP1A2 (KDLI2), the affinity constant (KDLI) (nmoL/L), the oral absorption constant (KABS) (day−1), the gastric transit for non-absorption (KST) (day−1), and the adipose tissue partition coefficient adipose tissue/blood (PF) (unitless). All other constants, including those related to gestation and lactation, had a negligible impact on the mother’s blood AUC.
Fig. 6.
Sensitivity analysis of each parameter was varied by ±10% of the optimized value. Sensitivity analysis was performed for each parameter after a single oral dose of 30 ng of TCDD/kg of body weight on women who started their pregnancies at 25 years of age at the beginning of the exposure and nursed their children for 6 months (180 days) after delivery, for a total simulation of 2 years. The AUC was calculated 2 years after the exposure, thus at the end of the simulation.
DISCUSSION
The first objective of this study was to modify a PBPK model for TCDD (Emond et al. 2004; Emond et al. 2005; NCEA-USEPA 2010) to include pregnancy and lactation. Using such a PBPK model will facilitate investigations of developmental exposure scenarios, better characterize the transfer of TCDD from the mother to the fetus, and better assess variations of body burden at different ages or windows of sensitivity. The second objective was to simulate TCDD blood concentrations during different life stages including pregnancy and lactation and to assess the influence of gestation and lactation on the elimination of TCDD. Finally we used the PBPK model to simulate the exposures and blood concentrations of a sample of women in the Seveso Woman’s Health Study.
In order to include lactation, the mammary gland was added to the model with a milk subcompartment. With the exception of TCDD partitioning into the mammary gland and milk, all other parameters in these compartments were based on physiological measurements available from the literature. The mammary gland partition coefficient (Pmat), the milk clearance (Qmilk), and the first order elimination rate (Ksuck) were optimized to obtain a whole blood:milk TCDD concentration ratio of 1.5, based on Schecter et al (1995, 1996) No other optimization was necessary for estimating these parameters (Table 1).
An initial evaluation of the model in which single exposures ranging from 0.001 – 1,000 ng/kg were simulated for a 20 year old women who gets pregnant, suggested that pregnancy and lactation had limited impact (< 1% of the body burden) on maternal body burdens. Based on the samples from the SWHS data (Subcohorts A and B), we also found that women who were exposed to TCDD and later became pregnant and lactated, had infants (including fetuses and newborns) with significant TCDD concentrations and that pregnancy and lactation minimally impacted maternal body burden or serum concentrations.
Although a number of studies suggested that pregnancy and lactation result in significant decreases in TCDD body burdens in women, these studies have limitations. The effects of pregnancy and lactation on the elimination of persistent organic pollutants are complex and uncertain. Initial studies to evaluate the impacts of pregnancy and lactation on the elimination of persistent organic pollutants were limited by their sample size (Schecter et al. 1996) and experimental designs (Weiss et al 2003). For example, Weiss et al. (2003) compared three different cohorts, each consisting of 12 women who were nursing their new child; however the samples were pooled within each cohort so no individual data were available. Hooper et al. (1999) suggested that the TCDD off-loading rates from two nursing mothers were between 4 and 5 pg/g of lipid per month of nursing (Hooper et al. 1999). Although this finding suggests that nursing is a significant route of elimination, there are only two time points with measured TCDD concentrations, with the first in blood and the second in milk. Beck et al. (1994) compared TCDD concentrations across women with different numbers of children and reported lower TCDD concentrations in milk from mothers with more children.
Lakind et al. (2009) found that depuration from milk in 10 nursing women was chemical- and individual-specific and in some cases concentrations of some POPs actually increased over time. Although the LaKind et al. study is small, it has serial samples over time, and the data are presented for individual women. The conflicting data suggest that we cannot generalize to all lipophilic persistent chemicals because the transfer from the mother’s blood to the milk is chemical specific. The advantage of the present study is that the use of a PBPK model allows for the evaluation of the impact of pregnancy and lactation on the elimination of TCDD and provides insight into the magnitude of this effect.
In this PBPK model, only the major compartments that contribute to the pharmacokinetic properties of TCDD were described. For TCDD, the major compartments include liver, adipose tissue, placenta, mammary gland, fetus, and rest-of-the-body. The rest-of-the-body compartment was a grouping of all other tissues. Individually, these tissues do not significantly influence the distribution of TCDD in particular or other highly lipophilic contaminants in general (Emond et al. 2004). The current PBPK model for adult women can predict the tissue concentrations 20 years after a major exposure and can incorporate the impact of pregnancy on the elimination of TCDD. Predictions of the maternal compartments in the pregnant women appear to accurately reflect the available data from the Seveso women as previously described. However, our work is limited because few pharmacokinetic studies on TCDD have sequential pregnancy and lactation datasets (Emond et al. 2003; LaKind et al. 2004; LaKind et al. 2009; Mocarelli and Pocchiari 1988; Schecter et al. 1996; Schecter et al. 1998; Weiss et al. 2003). In addition, although the SWHS data used here are serial measures for the same women, the collections did not necessarily coincide with reported pregnancies or lactation, and no data on TCDD levels in breastmilk, cord blood, or the infants are available.
The gestational PBPK model presented in this manuscript contains a whole fetus description, which is a relatively simple representation. Nevertheless, this approach reduces the complexity of the model and emphasizes the importance of TCDD transfer across the placenta. In 2003, our group used the same approach for TCDD (Emond et al. 2003), but at that time, data were unavailable to evaluate the predictions. Few PBPK models have been developed to study chemical transfer across the placenta to the fetus because such models require additional complexity and compartments (Abduljalil et al. 2012; Byczkowski and Lipscomb 2001; Clewell et al. 2003; Corley et al. 2003).
We determined that if a woman aged 20 years is exposed to a single oral dose of 100 ng TCDD/kg body weight during pregnancy, she will transfer less than 1% of TCDD to her fetus corresponding to an AUCfetus of 558 ng*day/kg. If that same woman also nursed her infant for 180 days, she will transfer around 1 to 2% to her infant corresponding to an AUCmilk 734 ng*day/kg of the mother body weight. Together it is an AUCfetus+milk for about 1,292 ng*day/kg compared to the mothers AUC of 39,975 ng*day/kg corresponding to about 3% of the mother.
We also observed that during gestation and lactation, there is a displacement of the free TCDD from the liver compartment to the blood, suggesting that the amount transferred to the fetus and during nursing reduces the biliary elimination accordingly. It is also important to note that simulating the data points for a woman by recreating the pregnancy and lactation scenarios or simulating without pregnancy and lactation conditions derived similar maternal blood concentration time course. This observation suggests that pregnancy and lactation have an impact on the infant’s exposure, but have a negligible impact on the mother (in fact a redistribution of the elimination was observed). However, more complete exposure studies using individual women and their offspring are required to quantify these important observations.
Seveso Cohort
Data from the SWHS cohort was divided into two subcohorts (A and B) based on whether the women had repeated (> 2) blood samplings over the 20 years of exposure (Subcohort A) or only two blood samplings, one shortly after exposure and a second in 1996 (Subcohort B). Subcohort A from Seveso was split into four different groups and classified (i.e., low, medium, high, and very high) depending on the serum concentration measured in 1976 shortly after the explosion (Figs. 3A–C). It is interesting to note that four of the seven subjects classified as high or very high were 10 years old or younger at the time of the exposure (Fig. 3B). This finding, that a large percentage of the young population was highly exposed during the explosion, is consistent with what has been reported previously in the full SWHS cohort (Eskenazi et al. 2004; Warner et al. 2014). Because the female residents were young and highly exposed, this represents a challenge for scientists to improve our understanding of the implications of short periods of high exposure to dioxins in relation to the health effect observed such as the neurological, immune system, reproductive system, endocrinological and intellectual development of such infants, including the potential for developing cancer (Birnbaum 1998; Birnbaum and Fenton 2003).
The model was also assessed by evaluating its ability to individually simulate each subject in Subcohort A from Seveso. Out of the 23 women, three had very high concentrations during the first sampling in 1976. These three women were 4, 7, and 23 years old when the explosion occurred. Unfortunately, the TCDD lipid adjusted blood concentrations from these women were under predicted by approximately 5–10 fold. While the model has difficulty predicting the highly exposed population, the model provides fairly accurate predictions of maternal exposures over 20 years in low to moderately exposed populations.
Sensitivity Analysis
Sensitivity analysis is a useful approach for understanding how the different parameters influence the model predictions. In addition, sensitivity analysis allows for a quantitative comparison of the influence of the different physiological processes described by the model. The sensitivity assessment performed here focused on the sensitivity of the model parameters to influence maternal blood concentrations and was performed using a single oral exposure of 30 ng of TCDD/kg of body weight. The sensitivity analysis in the present model was similar to the sensitivity analyses of earlier versions of the model (Emond et al. 2004; NCEA-USEPA 2010). We found the most sensitive parameters described chemical-specific phenomena. For example, parameters describing maternal CYP1A2 induction and binding of TCDD to maternal CYP1A2 had a greater influence on blood TCDD concentrations at this level. This result is consistent with the dose-dependent induction of CYP1A2 by TCDD, which influences the concentration in the mother’s blood (AUC) throughout the simulation. CYP1A2 binds and sequesters TCDD and related chemicals in hepatic tissue (Diliberto et al. 1997; Diliberto et al. 1999). At higher levels of CYP1A2 induction, the mother’s liver would sequester a greater percentage of the TCDD dose, thereby decreasing TCDD distribution to extrahepatic tissue, including systemic circulation. These results seem to be significant when extrapolating this PBPK model to interspecies, including humans, and subpopulations. Clewell et al. (2003) and Sweeney et al. (2001) also performed sensitivity analyses for their developmental models (Clewell et al. 2003; Sweeney et al. 2001) and found the most sensitive parameters described chemical-specific processes. Thus, although this model and other models describe gestational exposures, sensitivity analysis indicates that the model is not sensitive to small changes in parameters describing the physiology of gestation or lactation. Direct comparisons between these sensitivity analysis results should be viewed cautiously for many reasons. First, the molecular mechanisms of the developmental effects of perchlorate are better understood than those for TCDD. Second, Clewell et al. (2003) developed a much more complex fetal model that incorporates fetal blood flow and several other compartments, including the fetal thyroid gland, which is the target tissue for studying the effects of perchlorate. Third, TCDD and perchlorate are very different chemically. Sweeney et al. (2001) incorporated physiological changes during gestation in the maternal compartment, but did not use specific placental or fetal compartments. The rapidly perfused compartment is a surrogate for fetal concentrations of 2 methoxyethanol and 2-ethoxyethanol in the Sweeney et al. model (2001). Because of these differences, comparisons of the sensitivity analyses of these models might not provide insights regarding a broader discussion of the use and application of PBPK models during development.
Improvement of the PBPK model will require a systematic study with serial samples of maternal milk and blood across the gestational and lactational stages. We believe that gestational transfer and lactation of TCDD might contribute to maternal elimination, but more human data are required to improve confidence in the pharmacokinetic description. (Abduljalil et al. 2012).
CONCLUSION
Future efforts will focus on evaluating this model with additional Seveso data, improving the mathematical description of lactation and multiple pregnancies, and conducting a sensitivity analysis. In addition, future development might include assessing the importance of the inhalation route in this TCDD PBPK model, although, for this persistent organic chemical, oral exposure is usually considered to be the major route of exposure. The PBPK model is a good tool for risk assessment, including physiological descriptions of stages of life. By making these improvements, the model will become an even more accurate predictor of developmental exposures and will aid in understanding and estimating the risks associated with developmental exposures.
Highlights.
PBPK modeling can help to determine what influence the TCDD elimination.
The PBPK model accurately reproduces the exposure profiles of the Seveso women.
A small impact for the mother, but significant ones for the fetus and newborn.
The importance of gestation/lactation were overrated in the old literature of TCDD
The PBPK model is an important quantification tool for human risk assessments.
Acknowledgments
This work was funded in part by the intramural research programs of the NIEHS and the NCI (contract # HHSN261201500081P). This study was also supported by grant numbers R01 ES07171 and F06 TW02075-01 from the National Institutes of Health, R82471 from the U.S. Environmental Protection Agency, 2P30-ESO01896-17 from the National Institute of Environmental Health Sciences, and #2896 from Regione Lombardia and Fondazione Lombardia Ambiente, Milan, Italy. No disclaimer required.
Abbreviations
- acslX®
Advanced Continuous Simulation Language
- AhR
Aryl hydrocarbon receptor
- AUC
area under the curve
- CYP1A
Cytochrome P4501A
- CYP1A2
Cytochrome P4501A2
- CYP1B
Cytochrome P4501B
- EPA
U.S. Environmental Protection Agency
- GI
gastrointestinal
- ICMESA
Industrie Chimiche Meda Società Azionaria
- NCEA
National Center for Environmental Assessment
- NIH
National Institutes of Health
- NIEHS
National Institute of Environmental Health Sciences
- PBPK
physiologically based pharmacokinetic
- PCB
polychlorinated biphenyl
- R2
R-squared
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abduljalil K, Furness P, Johnson TN, Rostami-Hodjegan A, Soltani H. Anatomical, physiological and metabolic changes with gestational age during normal pregnancy: a database for parameters required in physiologically based pharmacokinetic modelling. Clin Pharmacokinet. 2012;51:365–396. doi: 10.2165/11597440-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Birnbaum LS. Developmental Effects of Dioxins. In: Korach KS, editor. Reproductive and Developmental Toxicology. New York: Marcel Dekker; 1998. pp. 87–112. [Google Scholar]
- Birnbaum LS, Fenton SE. Cancer and developmental exposure to endocrine disruptors. Environ Health Perspect. 2003;111:389–394. doi: 10.1289/ehp.5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byczkowski JZ, Kinkead ER, Leahy HF, Randall GM, Fisher JW. Computer Simulation of the Lactational Transfer of Tetrachloroethylene in Rats Using a Physiologically Based Model. Toxicology and Applied Pharmacology. 1994;125:228–236. doi: 10.1006/taap.1994.1068. [DOI] [PubMed] [Google Scholar]
- Byczkowski JZ, Lipscomb JC. Physiologically Based Pharmacokinetic Modeling of the Lactational Transfer of Methylmercury. Risk Analysis. 2001;21:869. doi: 10.1111/0272-4332.215158. [DOI] [PubMed] [Google Scholar]
- Carrier G, Brunet RC, Brodeur J. Modeling of the toxicokinetics of polychlorinated dibenzo-p-dioxins and dibenzofurans in mammalians, including humans 1. Nonlinear Distribution of PCDD/PCDF body burden between liver and adipose tissues. Toxicology and Applied Pharmacology. 1995;131:253–266. doi: 10.1006/taap.1995.1068. [DOI] [PubMed] [Google Scholar]
- Clewell RA, Gearhart JM. Pharmacokinetics of toxic chemicals in breast milk: use of PBPK models to predict infant exposure. Environ Health Perspect. 2002;110:A333–A337. doi: 10.1289/ehp.021100333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell RA, Merrill EA, Yu KO, Mahle DA, Sterner TR, Fisher JW, et al. Predicting neonatal perchlorate dose and inhibition of iodide uptake in the rat during lactation using physiologically-based pharmacokinetic modeling. Toxicol Sci. 2003;74:416–436. doi: 10.1093/toxsci/kfg147. [DOI] [PubMed] [Google Scholar]
- Corley RA, Mast TJ, Carney EW, Rogers JM, Daston GP. Evaluation of physiologically based models of pregnancy and lactation for their application in children’s health risk assessments. Crit Rev Toxicol. 2003;33:137–211. doi: 10.1080/713611035. [DOI] [PubMed] [Google Scholar]
- Devito MJ, Birnbaum LS. Toxicology of dioxins and related chemicals. In: Schecter A, editor. Dioxin and health. New York: Plenum Press; 1994. pp. 139–162. [Google Scholar]
- Diliberto JJ, Burgin D, Birnbaum LS. Role of CYP1A2 in hepatic sequestration of dioxin: studies using CYP1A2 knock-out mice. Biochemical and biophysical research communications. 1997;236:431–433. doi: 10.1006/bbrc.1997.6973. [DOI] [PubMed] [Google Scholar]
- Diliberto JJ, Burgin DE, Birnbaum LS. Effects of CYP1A2 on disposition of 2,3,7,8-tetrachlorodibenzo-p-dioxin, 2,3,4,7,8-pentachlorodibenzofuran, and 2,2′,4,4′,5,5′-hexachlorobiphenyl in CYP1A2 knockout and parental (C57BL/6N and 129/Sv) strains of mice. Toxicology and Applied Pharmacology. 1999;159:52–64. doi: 10.1006/taap.1999.8720. [DOI] [PubMed] [Google Scholar]
- Easterling MR, Evans MV, Kenyon EM. Comparative analysis of software for physiological based pharmacokinetic modeling: Simulation, optimization and sensibility analysis. Toxicology Mechanisms and Methods. 2000;10:203–229. [Google Scholar]
- Emond C, Birnbaum LS, DeVito M. Physiologically based pharmacokinetic model for developmental exposures to TCDD in the rat. Toxicol Sci. 2004;80:115–133. doi: 10.1093/toxsci/kfh117. [DOI] [PubMed] [Google Scholar]
- Emond C, Birnbaum LS, Devito MJ. Use of a physiologically based pharmacokinetic model for rats to study the influence of body fat mass and induction of CYP1A2 on the pharmacokinetics of TCDD. Environ Health Perspect. 2006;114:1394–1400. doi: 10.1289/ehp.8805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emond C, DeVito M, Birnbaum LS. Society of toxicology (SOT), editor . Utilization of the PBPK model to predict the distribution of 2, 3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in human during critical windows of development. Salt Lake city: SOT; 2003. [Google Scholar]
- Emond C, Michalek JE, Birnbaum LS, Devito MJ. Comparison of the use of a physiologically based pharmacokinetic model and a classical pharmacokinetic model for dioxin exposure assessments. Environ Health Perspect. 2005;113:1666–1668. doi: 10.1289/ehp.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Mocarelli P, Warner M, Needham L, Patterson DG, Jr, Samuels S, et al. Relationship of serum TCDD concentrations and age at exposure of female residents of Seveso, Italy. Environ Health Perspect. 2004;112:22–27. doi: 10.1289/ehp.6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Mocarelli P, Warner M, Samuels S, Needham L, Patterson D, et al. Seveso Women’s Health Study: does zone of residence predict individual TCDD exposure? Chemosphere. 2001;43:937–942. doi: 10.1016/s0045-6535(00)00454-9. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Mocarelli P, Warner M, Samuels S, Vercellini P, Olive D, et al. Seveso Women’s Health Study: a study of the effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on reproductive health. Chemosphere. 2000;40:1247–1253. doi: 10.1016/s0045-6535(99)00376-8. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Warner M, Sirtori M, Fuerst T, Rauch SA, Brambilla P, et al. Serum dioxin concentrations and bone density and structure in the Seveso Women’s Health Study. Environ Health Perspect. 2014;122:51–57. doi: 10.1289/ehp.1306788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J, Mahle D, Bankston L, Greene R, Gearhart J. Lactational transfer of volatile chemicals in breast milk. Am Ind Hyg Assoc J. 1997;58:425–431. doi: 10.1080/15428119791012667. [DOI] [PubMed] [Google Scholar]
- Hooper K, Chuvakova T, Kazbekova G, Hayward D, Tulenova A, Petreas MX, et al. Analysis of breast milk to assess exposure to chlorinated contaminants in Kazakhstan: sources of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) exposures in an agricultural region of southern Kazakhstan. Environ Health Perspect. 1999;107:447–457. doi: 10.1289/ehp.99107447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper K, Petreas MX, Chuvakova T, Kazbekova G, Druz N, Seminova G, et al. Analysis of breast milk to assess exposure to chlorinated contaminants in Kazakstan: high levels of 2,3,7, 8-tetrachlorodibenzo-p-dioxin (TCDD) in agricultural villages of southern Kazakstan. Environ Health Perspect. 1998;106:797–806. doi: 10.1289/ehp.98106797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent JC, Mitoulas L, Cox DB, Owens RA, Hartmann PE. Breast volume and milk production during extended lactation in women. Exp Physiol. 1999;84:435–447. [PubMed] [Google Scholar]
- Kent JC, Mitoulas LR, Cregan MD, Ramsay DT, Doherty DA, Hartmann PE. Volume and frequency of breastfeedings and fat content of breast milk throughout the day. Pediatrics. 2006;117:e387–e395. doi: 10.1542/peds.2005-1417. [DOI] [PubMed] [Google Scholar]
- Kerger BD, Leung HW, Scott P, Paustenbach DJ, Needham LL, Patterson DG, Jr, et al. Age- and concentration-dependent elimination half-life of 2,3,7,8-tetrachlorodibenzo-p-dioxin in Seveso children. Environ Health Perspect. 2006;114:1596–1602. doi: 10.1289/ehp.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan K, Andersen M. Physiologically based pharmacokinetic and toxicokinetic models. In: Hayes AW, editor. Principles and methods of toxicology. New York: CRC Press; 2008. pp. 231–291. [Google Scholar]
- LaKind JS, Amina WA, Berlin CM., Jr Environmental chemicals in human milk: a review of levels, infant exposures and health, and guidance for future research. Toxicol Appl Pharmacol. 2004;198:184–208. doi: 10.1016/j.taap.2003.08.021. [DOI] [PubMed] [Google Scholar]
- LaKind JS, Berlin CM, Jr, Sjodin A, Turner W, Wang RY, Needham LL, et al. Do human milk concentrations of persistent organic chemicals really decline during lactation? Chemical concentrations during lactation and milk/serum partitioning. Environ Health Perspect. 2009;117:1625–1631. doi: 10.1289/ehp.0900876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence GS, Gobas FA. A pharmacokinetic analysis of interspecies extrapolation in dioxin risk assessment. Chemosphere. 1997;35:427–452. doi: 10.1016/s0045-6535(97)00108-2. [DOI] [PubMed] [Google Scholar]
- Michalek JE, Tripathi RC. Pharmacokinetics of TCDD in veterans of Operation Ranch Hand: 15-year follow-up. J Toxicol Environ Health A. 1999;57:369–378. doi: 10.1080/009841099157584. [DOI] [PubMed] [Google Scholar]
- Milbrath MO, Wenger Y, Chang CW, Emond C, Garabrant D, Gillespie BW, et al. Apparent half-lives of dioxins, furans, and polychlorinated biphenyls as a function of age, body fat, smoking status, and breast-feeding. Environ Health Perspect. 2009;117:417–425. doi: 10.1289/ehp.11781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata M, Motoki K, Tamura E, Furukawa M, Gonzalez FJ, Yamazoe Y. Relative importance of maternal and embryonic microsomal epoxide hydrolase in 7,12-dimethylbenz[a]anthracene-induced developmental toxicity. Biochem Pharmacol. 2002;63:1077–1084. doi: 10.1016/s0006-2952(02)00847-x. [DOI] [PubMed] [Google Scholar]
- Mocarelli P, Pocchiari F. Preliminary report: 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure to humans--Seveso, Italy. MMWR Morb Mortal Wkly Rep. 1988;37:733–736. [PubMed] [Google Scholar]
- NCEA-USEPA. EPA’s reanalysis of key issues related to dioxin toxicity and response to NAS comments. 2010 EPA/600/R-10/038A. [Google Scholar]
- NCEA-USEPA. EPA’s reanalysis of key issues related to dioxin toxicity and response to NAS comments Volume1. 2012 EPA/600/R-10/038F. [Google Scholar]
- Poiger H, Schlatter C. Pharmacokinetics of 2,3,7,8-TCDD in man. Chemosphere. 1986;15:1489–1494. [Google Scholar]
- Rogan WJ, Ragan NB. Chemical contaminants, pharmacokinetics, and the lactating mother. Environ Health Perspect. 1994;102(Suppl 11):89–95. doi: 10.1289/ehp.94102s1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter A, Dai LC, Thuy LT, Quynh HT, Minh DQ, Cau HD, et al. Agent Orange and the Vietnamese: the persistence of elevated dioxin levels in human tissues. Am J Public Health. 1995;85:516–522. doi: 10.2105/ajph.85.4.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter A, Kassis I, Papke O. Partitioning of dioxins, dibenzofurans, and coplanar PCBS in blood, milk, adipose tissue, placenta and cord blood from five American women. Chemosphere. 1998;37:1817–1823. doi: 10.1016/s0045-6535(98)00247-1. [DOI] [PubMed] [Google Scholar]
- Schecter A, Papke O, Lis A, Ball M, Ryan JJ, Olson JR, et al. Decrease in milk and blood dioxin levels over two years in a mother nursing twins: estimates of decreased maternal and increased infant dioxin body burden from nursing. Chemosphere. 1996;32:543–549. doi: 10.1016/0045-6535(95)00248-0. [DOI] [PubMed] [Google Scholar]
- Sweeney LM, Tyler TR, Kirman CR, Corley RA, Reitz RH, Paustenbach DJ, et al. Proposed occupational exposure limits for select ethylene glycol ethers using PBPK models and Monte Carlo simulations. Toxicol Sci. 2001;62:124–139. doi: 10.1093/toxsci/62.1.124. [DOI] [PubMed] [Google Scholar]
- Takekuma M, Saito K, Falandysz J, Nakazawa H. Ratio variation of congener profiles of PCDD/Fs and dioxin-like PCBs in human milk during lactation. Sci Total Environ. 2011;409:1368–1377. doi: 10.1016/j.scitotenv.2010.12.031. [DOI] [PubMed] [Google Scholar]
- Wang X, Santostefano MJ, Evans MV, Richardson VM, Diliberto JJ, Birnbaum LS. Determination of parameters responsible for pharmacokinetic behavior of TCDD in female sprague-dawley rats. Toxicology and Applied Pharmacology. 1997;147:151–168. doi: 10.1006/taap.1997.8242. [DOI] [PubMed] [Google Scholar]
- Warner M, Mocarelli P, Brambilla P, Wesselink A, Patterson DG, Jr, Turner WE, et al. Serum TCDD and TEQ concentrations among Seveso women, 20 years after the explosion. J Expo Sci Environ Epidemiol. 2014;24:588–594. doi: 10.1038/jes.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J, Papke O, Bignert A, Jensen S, Greyerz E, Agostoni C, et al. Concentrations of dioxins and other organochlorines (PCBs, DDTs, HCHs) in human milk from Seveso, Milan and a Lombardian rural area in Italy: a study performed 25 years after the heavy dioxin exposure in Seveso. Acta Paediatr. 2003;92:467–472. doi: 10.1111/j.1651-2227.2003.tb00580.x. [DOI] [PubMed] [Google Scholar]
- Wittsiepe J, Furst P, Schrey P, Lemm F, Kraft M, Eberwein G, et al. PCDD/F and dioxin-like PCB in human blood and milk from German mothers. Chemosphere. 2007;67:S286–S294. doi: 10.1016/j.chemosphere.2006.05.118. [DOI] [PubMed] [Google Scholar]
- You L, Gazi E, Archibeque-Engle S, Casanova M, Conolly RB, Heck HA. Transplacental and lactational transfer of p,p’-DDE in Sprague-Dawley rats. Toxicol Appl Pharmacol. 1999;157:134–144. doi: 10.1006/taap.1999.8673. [DOI] [PubMed] [Google Scholar]