Abstract
Uric acid, a waste metabolite among humans, was linked to various cognitive outcomes. We describe sex and age-group specific associations of baseline SUA (SUAbase) and significant change in SUA (ΔSUA: 1 vs. 0=decrease vs. no change; 2 vs. 0=Increase vs. no change) with longitudinal annual rate of cognitive change among a large sample of urban adults. Data from the Healthy Aging in Neighborhoods of Diversity across the Life Span study, 2004–2009 (visit 1) and 2009–2013 (visit 2) were used. Of 3,720 adults selected at baseline (age range:30-64y), complete data were available for N=1,487–1,602 with a mean repeat of 1.5–1.7 visits/participant. Cognitive test domains spanned attention, processing speed, learning/memory, executive function, visuo-spatial/visuo-construction ability, language/verbal and global cognitive function. SUA was measured at both visits. Multiple mixed-effect regression analyses were conducted. In the total population, a higher SUAbase was associated with a faster annual rate of decline on a measure of visual memory/visuo-construction ability (the Benton Visual Retention Test) by γ=0.07 with a standard error of 0.02, P<0.001. Among older men, a significant increase in SUA was associated with slower decline on a test of attention/processing speed, namely Trailmaking test, Part A, measured in seconds to completion (γ=−6.91±1.73, p<0.001). In sum, a higher SUAbase was associated with faster cognitive decline over-time in a visual memory/visuo-construction ability test. ΔSUA had particular beneficial effects of an increasing ΔSUA on the domain of attention/processing speed among older men. More longitudinal studies are needed to examine cognitive domain-specific effects of over-time change in SUA within sex and age groups.
Keywords: Cognition, serum uric acid, sex differences, aging
INTRODUCTION
Uric acid, a waste metabolite among humans, triggers development of gout and kidney stones if present at elevated levels in serum, increasing risk for hypertension, cerebrovascular and renal disease.[1, 2] Although a diet low in uric acid has little influence on its serum levels, a Mediterranean dietary pattern rich in antioxidants and anti-inflammatory agents is linked to a reduced risk of hyperuricemia.[3]
Previous studies have examined the association between SUA with various cognitive outcomes among middle-aged and older adults[4–19] Some, report a potentially adverse effect of hyperuricemia on cognitive outcomes over-time,[5–13] while others suggest a beneficial effect on cognitive performance or slower rate of cognitive decline.[14–19] Prior study limitations included exposure measurement error,[14] selection bias,[14] small sample sizes (<200 subjects) and/or lack of generalizability.[6, 7, 17, 18] Moreover, many assessed only global cognitive outcomes.[9, 11, 15, 16, 18]
Recent studies point to the importance of examining sex-specific associations between hyperuricemia and cognitive performance or change.[5] First, SUA is more strongly associated with cardiovascular disease incidence and all-cause mortality among women than men, particularly among postmenopausal women [20–22]. Secondly, a study by Heo et al.[23] uncovered a dose-response relationship between SUA and brain infarction only among women. Finally, higher SUA was linked to slower rate of Parkinson’s disease progression in men, but faster progression in women.[24] Finally, in most studies reviewed as well as an earlier report, SUA was higher in older individuals and within each age group, was higher in men compared to women.[25] Most of this evidence suggests that SUA may have a beneficial or no significant cognitive effect among men, while having a potential deleterious effect among women, particularly older women. Moreover, none of the previous studies testing the effect of SUA on cognitive outcomes have examined changes in SUA over-time and its concurrent relationship with cognitive change.
Thus, our present study examines the sex- and age-specific associations of SUA at baseline (SUAbase) and change over-time (ΔSUA) with longitudinal cognitive change among a sample of urban US adults residing in Baltimore city. We hypothesize that the association SUA and cognitive outcomes is an adverse one among women, particularly older women, while being null or protective among men.
MATERIALS AND METHODS
Database and study participants
The Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) study is a prospective cohort study initiated in 2004. The study used area probability sampling to recruit a socioeconomically diverse and representative sample of African American and white urban adults (30–64 years old) residing in Baltimore, Maryland.[26] Written informed consent was obtained from all participants who were provided with a protocol booklet and a video explaining study procedures. Approval of materials was completed by MedStar Institutional Review Board. Data for the present study were derived from baseline visit 1 (2004–2009) and the first follow-up examination (visit 2; 2009–2013). Follow-up time ranged from <1y to ~8y, with a mean of 4.64±0.93y.
HANDLS initially recruited 3,720 participants (Phase I, visit 1) Given that only Phase II had in-depth data including biochemical indices and cognitive performance measures, SUAbase was available for 2,502 participants. Reliable cognitive test data were complete for N=2,088 for the California Verbal learning test-free delayed recall (CVLT-DFR) to 2,700 for the Clock Drawing Test at visit 1, and for 2,630 in the case of Mini-Mental State Examination (MMSE) at visit 1. Similarly, at the follow-up visit (visit 2), those sample sizes ranged from 1,728 (Trailmaking Test, Part B) to 1,846 (CVLT-DFR) to In the final analytic models which combined both waves, complete data on outcomes at either visit, as well as SUAbase and covariates at baseline (e.g. dietary variables and depressive symptoms) were available for N=1,487–1,602 with a mean repeat of 1.5–1.7 visits/participant and a total number of visits ranging from 2,275 to 2,753. Similar sample sizes were available when exposure was ΔSUA. Supplemental Figure 1 describes sample selection in more details.
Cognitive assessment
Cognitive performance was assessed with 7 tests yielding 11 test scores and covering 7 domains (Global, attention, learning/memory, executive function, visuo-spatial/visuo-construction ability, psychomotor speed, language/verbal): the Mini-Mental State Examination (MMSE), the California Verbal Learning Test (CVLT) immediate (List A) and Delayed Free Recall (DFR), Digit Span Forward and Backwards tests (DS-F and DS-B), the Benton Visual Retention Test (BVRT), Animal Fluency test (AF), Brief Test of Attention (BTA), Trails A and B and the Clock Drawing Test (CDT) (Appendix I). All participants were judged capable of informed consent and were probed for their understanding of the protocol. Although no formal dementia diagnosis was conducted, all participants were given mental status tests, which they completed successfully. In every case, low mental status performance was due to low literacy level without any sign of dementia.
Serum uric acid (SUA) assessment
SUA measurements are useful in the diagnosis and treatment of renal and metabolic disorders, including renal failure, gout, leukemia, psoriasis, starvation or other wasting conditions, and in patients receiving cytotoxic drugs. Using 1 ml of fasting blood serum, uric acid was measured using a standard spectrophotometry method. The reference range for adult men is 4.0–8.0 mg/dL, whereas for women, this range is cited as 2.5–7.0 mg/dL. (http://www.questdiagnostics.com/testcenter/TestDetail.action?ntc=905) Other reference ranges were also recently suggested and depend on the menopausal status of women. Those reference ranges are based on predictive value for gout outcomes among healthy individuals and do not necessarily predict other pathologies. Thus, based on recent research evidence, a “normal” SUA value is suggested to be <6.0 mg/dL for all healthy adult individuals. [27] Two main exposures were examined in the analysis: (1) SUAbase (visit 1), continuous; (2) Standardized annual rate of change in SUA between the two visits (1 and 2), categorized as significant decline (z<-1.645), non-significant change (−1.645≤z≤+1.645) and significant increase (z>+1.645); termed ΔSUA. The annual rate of change is estimated using a mixed-effects regression model that is described in further detail in Appendix II. The categorization of the annual rate of change in SUA was made due to the high level of kurtosis found in the distribution whereby the vast majority of participants had a stable SUA with only the upper and lower tails showing significant increase or decrease, respectively.
Covariates
Covariates included age, sex, race (White vs. African American), marital status, educational attainment (<High School (HS); HS, >HS), poverty income ratio (PIR<125% for “poor”), measured body mass index (BMI, kg/m2), opiate, marijuana or cocaine use (“current” vs. “never or former”), smoking status (“current” vs. “never or former”) and the Wide Range Achievement Test (WRAT) letter and word reading subtotal scores to measure literacy. (See Appendix I) To assess depressive symptoms with focus on affective, depressed mood, the 20-item CES-D was used. Baseline CES-D total score was included in the analysis as a potential confounder in the association between SUA and cognitive change or baseline performance. (See Appendix I) The Healthy Eating Index (HEI-2010), based on two 24-hr recalls administered at baseline, was used as a measure of overall dietary quality. Steps for calculating HEI-2010 are made available by the National Cancer Institute’s Applied Research (http://appliedresearch.cancer.gov/tools/hei/tools.html) and the HANDLS websites (http://handls.nih.gov/06Coll-dataDoc.htm). Total and component HEI-2010 scores were calculated for each recall day (day 1 and day 2) and then averaged to obtain the mean HEI-2010 total and component scores, thus combining both days. Only total HEI-2010 score was included in analyses.
Statistical Analysis
All analyses were conducted using Stata 13.0. First, and using survey commands that accounted for sampling weights yielded population estimates of means and proportions. Means across key binary variables were compared using svy:reg, whereas design-based F-tests were carried out to examine the relationship between categorical variables using svy:tab. Second, mixed-effects regression models with 11 continuous cognitive test score(s) as alternative outcomes were conducted. In these models the time variable was interacted with a number of covariates including the main exposure variables, namely SUAbase concentration and ΔSUA. The models assume missingness at random for the outcomes of interest, given that not all observation had 2 complete cognitive scores at the two time points (~1.5–1.7 visits/person). Moderating effect of sex and age groups was tested by adding interaction terms to separate multivariable mixed-effects regressions (3-way interactions Time×exposure×sex or Time×exposure×Age; and 4-way interaction terms: Time×exposure×sex×Age) and stratifying by sex and age to examine relationships among the following groups: (1) Younger men, (2) Older men, (3) Younger women, (4) Older women, whenever at least one 4-way interaction was deemed statistically significant. Appendix II describes the approach used in detail. Our choice of age and sex as stratifying variables were guided by the previous literature which has shown that the effect of uric acid on cognitive decline was mostly seen in older women. [5] Variable time of follow-up is accounted for in the mixed-effects regression model as annual rate of change in the outcome was of primary interest.
Moreover, selection bias may occur due to the non-random selection of participants with complete data from the target study population. Thus, in each mixed-effect regression model, a 2-stage Heckman selection process was conducted, by running a probit model to compute an inverse mills ratio at the first stage (derived from the predicted probability of being selected, conditional on the covariates in the probit model, mainly baseline age, sex, race, poverty status and education). At the second stage, this inverse mills ratio was then entered as a covariate in the final mixed-effects regression model, as was done in a previous study.[28]
The key parameter of interest was the interaction between time and the main exposures of interest (i.e. Time×SUAbase, Time×ΔSUAdecrease, Time×ΔSUAincrease). A familywise Bonferroni procedure was used to correct for multiple testing by accounting only for cognitive tests and assuming that SUA exposures related to separate substantive hypotheses.[29] Therefore, the critical p-value was reduced to 0.05/11=0.004. Due to their lower statistical power, 3-way and 4-way interaction terms between Time, exposure, age group and sex had their critical p-values set to 0.05. [30] Several sensitivity analyses were conducted: (A) Baseline use of diuretics was added into the mixed-effects regression model to examine potential attenuation of effects due to the known positive relationship between diuretics and SUA; (B.1 and B.2) For Trails A vs. change in SUA among older men two other sensitivity analyses were done whereby change in HEI-2010 and in BMI over time were added to the model. Mixed-effects regression models with the time variable were used to obtain the empirical bayes estimators of change in HEI-2010 and BMI over time, which were then entered into the main model alternatively to assess confounding effects.
RESULTS
Table 1 displays participant characteristics baseline (visit 1). This sub-set of participants had complete MMSE scores and the analysis is stratified by age group and sex. Overall, younger participants had mean difference in age of ~16y compared to older participants (41y vs. 57y). Compared to younger men, a greater proportion of women (both younger and older) were living below poverty, whereas younger women were less likely to be currently married. However, both older men and women had significantly higher proportions >HS and <HS education compared to younger men whereas lower literacy level was only found in older men when compared to younger men. The highest prevalence of current smoking was found among younger men and were lowest among older women. Similarly, younger men had the highest prevalence of illicit drug use compared to all other sex-age groups. BMI was also lowest in younger men, baseline 2010-HEI total score indicated better dietary quality among older men and women, compared to younger men. Both younger and older women had higher mean CES-D score compared to younger men. SUAbase was significantly lower in women of both age groups compared to younger men whereas the reverse was true for older men. SUAbase≥6.0 mg/dL prevalence is estimated at 33% in this sample, with older men having the higher proportion of hyperuricemia defined as such (59%) and younger women having the lowest (17%); (p<0.001, design-based F-test for difference by age group and sex). However, the distribution of proportions in each category of ΔSUA did not differ by sex and age group.
Table 1.
Selected baseline (Visit 1) study participant characteristics by age group and sex for HANDLS participants with complete and reliable baseline MMSE scores (n=2,630) a
| All | Older women (>50y) |
Older men (>50y) |
Younger women (≤50y) |
Younger men (≤50y), referent |
Pb Sex ×Age group |
|
|---|---|---|---|---|---|---|
| 21.3±1.2 | 18.4±1.1 | 33.3±0.2 | 27.0±1.6 | |||
| N=2,630 | N=686 | N=525 | N=802 | N=617 | ||
| Age at baseline, y | 47.0±0.3 | 56.7±0.3c | 56.6±0.3c | 40.6±0.4 | 40.7±0.4 | <0.001 |
| (N=2,630) | (N=686) | (N=525) | (N=802) | (N=617) | ||
| Married, % | 35.0±1.7 | 34.8±3.4 | 38.8±3.3 | 30.0±2.9c | 38.9±3.4 | 0.11 |
| (N=2,447) | (N=616) | (N=474) | (N=770) | (N=586) | ||
| Education, % | ||||||
| <HS | 4.3±0.6 | 6.6±1.5c | 7.6±1.6c | 2.5±0.6 | 2.4±0.7 | 0.010 |
| HS | 52.8±1.7 | 45.4±3.1 | 46.4±3.2 | 55.8±3.3 | 59.9±3.4 | |
| >HS | 38.5±1.7 | 43.4±3.2 | 41.9±3.4 | 38.0±3.2 | 32.9±3.2 | |
| Missing | 4.4±0.8 | 4.6±1.2 | 4.0±1.2 | 3.7±1.5 | 5.5±2.0 | |
| (N=2,630) | (N=686) | (N=525) | (N=802) | (N=617) | ||
| Literacy (WRAT score) | 43.2±0.2 | 42.7±0.4 | 42.0±0.6c | 43.7±0.4 | 43.6±0.5 | 0.05 |
| (N=2,616) | (N=682) | (N=522) | (N=798) | (N=614) | ||
| PIR <125%, % | 19.6±1.0 | 22.5±2.2c | 17.0±1.7 | 22.1±2.1c | 16.1±1.6 | 0.026 |
| (N=2,630) | (N=686) | (N=525) | (N=802) | (N=616) | ||
| Current smoking status, % | 0.005 | |||||
| Currently smoking | 43.7±1.7 | 33.0±3.1c | 43.1±3.3 | 42.4±3.2 | 54.1±3.4 | |
| Missing | 4.9±1.7 | 7.4±2.0 | 4.4±1.4 | 5.0±1.6 | 3.2±1.5 | |
| (N=2,630) | (N=686) | (N=525) | (N=802) | (N=617) | ||
| Current use of illicit drugs, % | ||||||
| Used any type | 48.4±1.7 | 30.4±3.1c | 54.2±3.2c | 43.2±3.3c | 65.1±3.2 | <0.001 |
| Missing | 7.8±0.8 | 10.0±2.1 | 8.6±1.8 | 8.0±1.6 | 5.3±1.1 | |
| (N=2,630) | (N=686) | (N=525) | (N=802) | (N=617) | ||
| Body mass index, kg.m−2 | 29.7±0.3 | 31.8±0.6c | 28.9±0.4c | 30.7±0.6c | 27.5±0.4 | <0.001 |
| (N=2,630) | (N=686) | (N=525) | (N=802) | (N=617) | ||
| HEI-2010 total score | 43.8±0.4 | 47.5±0.9c | 44.2±0.8c | 42.6±0.7 | 42.2±0.7 | <0.001 |
| (N=2,045) | (N=521) | (N=394) | (N=649) | (N=481) | ||
| Depressive symptoms | ||||||
| CES-D score | 10.5±0.3 | 11.3±0.5c | 9.7±0.4 | 11.1±0.6c | 9.5±0.5 | 0.08 |
| (N=2,079) | (N=549) | (N=409) | (N=638) | (N=483) | ||
|
Baseline serum uric acid (SUAbase) continuous, mg/dL |
5.41±0.05 | 5.28±0.09c | 6.26±0.10c | 4.71±0.08c | 5.78±0.08 | 0.97 |
| (N=2,502) | (N=659) | (N=496) | (N=760) | (N=587) | ||
|
Baseline serum uric acid (SUAbase) categorical, mg/dL |
||||||
| <6 mg/dL | 67.0±1.6 | 72.9±2.8c | 40.5±3.3c | 83.2±2.6c | 60.7±3.4 | <0.001 |
| ≥ 6 mg/dL | 33.0±1.6 | 27.1±2.8 | 59.4±3.3 | 16.8±2.6 | 39.3±3.4 | |
| (N=2,502) | (N=659) | (N=496) | (N=760) | (N=587) | ||
|
Annual rate of change in serum uric acid (***SUA), mg/dL |
||||||
| Stable [Range: 0.00; +0.11,Mean±SD: +0.05±0.02] |
92.2±0.9 | 90.3±1.7 | 91.3±1.9 | 94.2±2.0 | 92.0±1.7 | 0.61 |
| Significant decrease [Range: −0.24;0.00, Mean±SD: −0.02±0.03] |
3.3±0.7 | 2.9±1.0 | 3.5±1.4 | 3.0±1.5 | 3.8±1.3 | |
| Significant increase [Range: +0.11;+0.35, Mean±SD: +0.14±0.03] |
4.5±0.7 | 6.8±1.4 | 5.2±1.3 | 2.8±1.3 | 4.3±1.2 | |
| (N=2,585) | (N=679) | (N=515) | (N=785) | (N=606) | ||
Key: CES-D=Center for Epidemiologic Studies-Depression; MMSE=Mini-Mental State Examination; PIR=poverty income ratio; WRAT=Wide Range Achievement Test.
Values are weighted mean±SEM or percent±SEP.
P-value was based on linear regression models when row variable is continuous (svy:reg) with sex/age group coded as continuous variable (0=younger men, 1=younger women, 2=older men, 3=older women) and design-based F-test when row variable is categorical (svy:tab).
P<0.05. P-value was based on linear regression models when row variable is continuous (svy:reg) and design-based F-test when row variable is categorical (svy:tab), comparing each of the sex/age categories to the referent category of younger men.
Table 2 shows that in addition to some age group and sex differentials in cognitive performance, only 4 out of 11 cognitive tests changed markedly between visits, with verbal and visual memory scores (3 of 4) declining over time for all age-sex groups. In contrast, a possible learning effect was observed for the global cognitive measure MMSE, among study participants with available data.
Table 2.
Cognitive performance test scores at visits 1 and 2, by age group and sex for HANDLS participants with complete and reliable baseline MMSE scoresa
| All | Older women (>50y) |
Older men (>50y) |
Younger women (≤50y) |
Younger men (≤50y) |
|
|---|---|---|---|---|---|
| Mini-Mental State Exam, total score | |||||
| Visit 1 | 27.83±0.07 | 27.76±0.16 | 27.26±0.16b | 28.15±0.12 | 28.02±0.13 |
| (N=2,630) | (N=686) | (N=525) | (N=802) | (N=617) | |
| Visit 2 | 28.04±0.06 | 27.96±0.09 | 27.59±0.18b | 28.18±0.11 | 28.18±0.11 |
| (N=1,934) | (N=505) | (N=341) | (N=653) | (N=434) | |
| P (Visit2-Visit1) | 0.028 | 0.27 | 0.16 | 0.44 | 0.36 |
| California Verbal Learning Test (CVLT), List A | |||||
| Visit 1 | 25.0±0.26 | 24.95±0.39 | 22.56±0.40b | 27.07±0.49b | 24.17±0.59 |
| (N=2,172) | (N=563) | (N=426) | (N=670) | (N=513) | |
| Visit 2 | 20.08±0.26 | 19.86±0.46 | 16.46±0.50b | 21.86±0.52b | 20.21±0.48 |
| (N=1,976) | (N=509) | (N=358) | (N=650) | (N=459) | |
| P (Visit2-Visit1) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| CVLT, free delayed recall | |||||
| Visit 1 | 7.34±0.12 | 7.08±0.18 | 6.34±0.20b | 8.21±0.24b | 7.16±0.25 |
| (N=2,088) | (N=543) | (N=413) | (N=645) | (N=487) | |
| Visit 2 | 5.82±0.13 | 5.68±0.21 | 4.20±0.28b | 6.48±0.25 | 6.04±0.25 |
| (N=1,846) | (N=481) | (N=327) | (N=606) | (N=432) | |
| P (Visit2-Visit1) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Benton Visual Retention Test | |||||
| Visit 1 | 5.66±0.16 | 6.79±0.36b | 6.21±0.30b | 5.57±0.30b | 4.51±0.32 |
| (N=2,594) | (N=671) | (N=516) | (N=794) | (N=613) | |
| Visit 2 | 7.65±0.18 | 9.10±0.34b | 8.87±0.37b | 7.32±0.33b | 6.08±0.32 |
| (N=2,085) | (N=532) | (N=382) | (N=692) | (N=479) | |
| P (Visit2-Visit1) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Brief Test of Attention | |||||
| Visit 1 | 6.72±0.08 | 6.53±0.16 | 6.44±0.17 | 7.04±0.17 | 6.66±0.16 |
| (N=2,247) | (N=583) | (N=458) | (N=684) | (N=522) | |
| Visit 2 | 6.64±0.09 | 6.62±0.12 | 6.25±0.22 | 6.79±0.17 | 6.74±0.18 |
| (N=1,907) | (N=486) | (N=347) | (N=632) | (N=442) | |
| P (Visit2-Visit1) | 0.55 | 0.67 | 0.50 | 0.30 | 0.72 |
| Animal Fluency | |||||
| Visit 1 | 19.19±0.20 | 18.18±0.32b | 18.77±0.30b | 19.01±0.39b | 20.49±0.44 |
| (N=2,695) | (N=705) | (N=550) | (N=813) | (N=627) | |
| Visit 2 | 19.46±0.24 | 18.55±0.41b | 19.19±0.38b | 19.26±0.42b | 20.68±0.59 |
| (N=2,139) | (N=548) | (N=403) | (N=696) | (N=492) | |
| P (Visit2-Visit1) | 0.38 | 0.48 | 0.40 | 0.66 | 0.80 |
| Digits Span, Forward | |||||
| Visit 1 | 7.42±0.07 | 7.03±0.12 | 7.43±0.16 | 7.58±0.14b | 7.52±0.15 |
| (N=2,579) | (N=661) | (N=519) | (N=791) | (N=608) | |
| Visit 2 | 7.50±0.09 | 6.97±0.15b | 7.23±0.18b | 7.74±0.17 | 7.76±0.20 |
| (N=1,971) | (N=499) | (N=372) | (N=643) | (N=457) | |
| P (Visit2-Visit1) | 0.52 | 0.76 | 0.41 | 0.48 | 0.33 |
| Digits Span, Backward | |||||
| Visit 1 | 5.79±0.07 | 5.63±0.15 | 5.90±0.15 | 5.90±0.13 | 5.90±0.16 |
| (N=2,561) | (N=653) | (N=516) | (N=787) | (N=605) | |
| Visit 2 | 5.78±0.08 | 5.63±0.16 | 5.39±0.17b | 5.91±0.13 | 6.00±0.17 |
| (N=1,965) | (N=499) | (N=370) | (N=642) | (N=454) | |
| P (Visit2-Visit1) | 0.96 | 0.99 | 0.35 | 0.95 | 0.67 |
| Clock, command | |||||
| Visit 1 | 8.79±0.04 | 8.59±0.08 | 8.88±0.07 | 8.82±0.08 | 8.86±0.10 |
| (N=2,700) | (N=701) | (N=545) | (N=820) | (N=634) | |
| Visit 2 | 8.78±0.05 | 8.70±0.10b | 8.74±0.10 | 8.78±0.09 | 8.88±0.09 |
| (N=2,104) | (N=539) | (N=386) | (N=692) | (N=487) | |
| P (Visit2-Visit1) | 0.87 | 0.40 | 0.25 | 0.75 | 0.89 |
| Trailmaking test, Part A | |||||
| Visit 1 | 34.86±0.59 | 41.40±1.89b | 39.77±1.15b | 30.43±0.76 | 31.7±0.85 |
| (N=2,557) | (N=672) | (N=496) | (N=789) | (N=600) | |
| Visit 2 | 36.48±1.39 | 44.38±5.46 | 41.03±1.55b | 30.90±0.82 | 34.74±2.52 |
| (N=1,874) | (N=492) | (N=339) | (N=619) | (N=424) | |
| P (Visit2-Visit1) | 0.61 | 0.61 | 0.51 | 0.67 | 0.26 |
| Trailmaking test, Part B | |||||
| Visit 1 | 138.77±4.57 | 169.16±9.38b | 166.05±10.72b | 113.0±6.61 | 127.69±10.42 |
| (N=2,556) | (N=672) | (N=496) | (N=788) | (N=600) | |
| Visit 2 | 127.87±5.79 | 136.44±9.39 | 154.35±13.86b | 120.20±10.81 | 114.18±11.53 |
| (N=1,728) | (N=445) | (N=306) | (N=578) | (N=399) | |
| P (Visit2-Visit1) | 0.14 | 0.014 | 0.50 | 0.57 | 0.39 |
Most cognitive test scores were in the direction of higher score=better performance, except for BVRT (total errors), and Trailmaking Test both parts (expressed in seconds).
p<0.05 for null hypothesis of no difference in means of cognitive test scores by sex and Age group within each visit (referent category: Younger men). Wald test from svy: reg command.
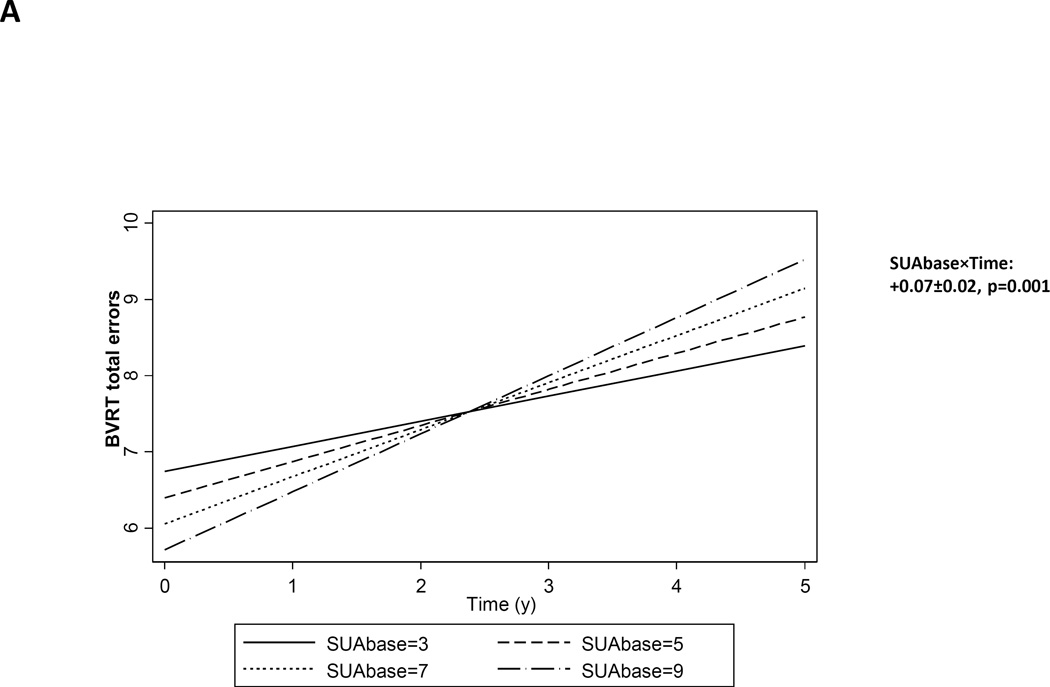
Table 3 displays associations between SUAbase and longitudinal cognitive change, based on mixed-effects regression analyses. However, a higher SUA at baseline was associated with significant increase over time in the number of errors committed on the BVRT test, indicative of faster annual rate of decline by γ=0.07 with SEE=0.02, P=0.001. When testing for interaction by sex and age groups, effects were largely homogenous across the four groups (4-way interaction terms in a separated mixed-effects regression model, p>0.05). Thus, stratum-specific findings were not presented, for simplicity. Figure 1A. depicts predictive margins from the mixed-effects regression model with BVRT test score as the outcome, given pre-set values of baseline SUA, with emphasis on differences in the predicted slopes. The observed BVRT test scores across time are also presented for three observed levels of baseline SUA, namely 3, 6 and 9, in Figure 1B, using both scatter plots and a LOWESS smoothing technique. The results confirm that the rate of increase in BVRT is faster when baseline SUA is higher.
Table 3.
Longitudinal annual rate of cognitive change by baseline serum uric acid concentration: mixed-effects linear regression models
| Intercept | Time | (SUAbase)×Time | ||||
|---|---|---|---|---|---|---|
| γ ±SEE | P | γ ±SEE | P | γ±SEE | P | |
|
Mini-Mental State Exam, total score |
+26.70±0.25 | <0.001 | +0.12±0.07 | 0.08 | −0.01±0.01 | 0.45 |
| N’=2,594 | ||||||
| N=1,583 | ||||||
| k=1.6 | ||||||
|
California Verbal Learning Test (CVLT), List A |
+24.33±0.90 | <0.001 | −1.19±0.21 | <0.001 | −0.01±0.03 | 0.64 |
| N’=2,376 | ||||||
| N=1,516 | ||||||
| k=1.6 | ||||||
| CVLT, free delayed recall | +7.37±0.43 | <0.001 | −0.36±0.10 | 0.001 | −0.01±0.01 | 0.55 |
| N’=2,275 | ||||||
| N=1,487 | ||||||
| k=1.5 | ||||||
| Benton Visual Retention Test | +9.74±0.66 | <0.001 | +0.03±0.16 | 0.84 | +0.07±0.02 | 0.001 |
| N’=2,678 | ||||||
| N=1,597 | ||||||
| k=1.7 | ||||||
| Brief Test of Attention | +6.26±0.31 | <0.001 | −0.03±0.08 | 0.69 | −0.01±0.01 | 0.27 |
| N’=2,498 | ||||||
| N=1,548 | ||||||
| k=1.6 | ||||||
| Animal Fluency | +17.06±0.71 | <0.001 | −0.06±0.15 | 0.70 | −0.01±0.02 | 0.78 |
| N’=2,753 | ||||||
| N=1,602 | ||||||
| k=1.7 | ||||||
| Digits Span, Forward | +6.69±0.29 | <0.001 | +0.07±0.06 | 0.25 | −0.01±0.01 | 0.19 |
| N’=2,628 | ||||||
| N=1,596 | ||||||
| k=1.6 | ||||||
| Digits Span, Backward | +1.31±4.59 | 0.76 | +1.13±1.13 | 0.32 | −0.01±0.02 | 0.63 |
| N’=2,612 | ||||||
| N=1,595 | ||||||
| k=1.6 | ||||||
| Clock, command | +8.93±0.17 | <0.001 | −0.09±0.05 | 0.043 | +0.00±0.01 | 0.86 |
| N’=2,749 | ||||||
| N=1,600 | ||||||
| k=1.7 | ||||||
| Trailmaking test, Part A | +39.1±4.92 | <0.001 | +1.13±1.44 | 0.43 | +0.23±0.19 | 0.22 |
| N’=2,644 | ||||||
| N=1,566 | ||||||
| k=1.7 | ||||||
| Trailmaking test, Part B | +212.80±54.21 | <0.001 | −0.09±12.68 | 0.99 | +0.51±0.55 | 0.35 |
| N’=2,550 | ||||||
| N=1,554 | ||||||
| k=1.6 | ||||||
Key: BVRT=Benton Visual Retention Test; CES-D=Center for Epidemiologic Studies-Depression; CVLT=California Verbal Learning Test; HANDLS=Healthy Aging in Neighborhoods of Diversity across the Life Span; MMSE=Mini-Mental State Examination; N=number of participants; N’=number of visits; k=mean visits per person; WRAT=Wide Range Achievement Test.
Multiple mixed-effects linear regression models adjusted for baseline age, sex, race/ethnicity, marital status, education, WRAT total score, poverty income ratio, current smoking status, current use of illicit drugs, body mass index, CES-D total score and 2010-HEI. Models are stratified and presented by sex and age group when in a separate model, the four-way interaction Time×exposure×sex×Age had at least one term that is statistically significant at the type I error level of 0.05.
Most cognitive test scores were in the direction of higher score=better performance, except for BVRT (total errors), and Trailmaking Test both parts (expressed in seconds).
FIGURE 1.
A. Baseline serum uric acid (SUAbase) and it association with longitudinal cognitive change over-time on the BVRT number of errors: mixed-effects regression model, HANDLS, 2004–2013
B. Scatterplot and LOWESS curves of observed BVRT number of errors by time at two observed level of serum uric acid (SUAbase, lowest quintile (Q1) and uppermost quintile (Q5)). HANDLS, 2004–2013
Q1: 1.6–4.1 mg/dL; Q5: 6.8–14.2 mg/dL
When examining the concurrent association between ΔSUA and longitudinal cognitive change, several findings emerged, considering a type I error of 0.05. However, after correction for multiple testing (type I error reduced to 0.004), only one key finding remained. In particular, among older men, a significant increase in SUA was associated with slower decline on a test of attention/processing speed, namely Trailmaking test, Part A, measured in seconds to completion (γ=−6.91±1.73, p<0.001). (Table 4, Figure 2). Results from the first sensitivity analysis (A) indicated that baseline use of diuretics did not have a confounding effect on our key findings (i.e. BVRT vs. SUAbase (total population) and Trails A vs. increase in SUA (older men)). It is worth noting that around 7.5% of HANDLS participants were using diuretics at baseline. Similarly, for Trails A vs. change in SUA among older men two other sensitivity analyses were done whereby change in HEI-2010 and in BMI over time were added to the model. The results were not substantially altered. (data not shown).
Table 4.
Longitudinal annual rate of cognitive change by annual rate of change in serum uric acid (0=Stable, 1=Significant decrease, 2=Significant increase): mixed-effects linear regression models
| Intercept | Time | (ΔSUA) ×Time |
||||
|---|---|---|---|---|---|---|
| γ ±SEE | P | γ ±SEE | P | γ±SEE | P | |
|
Mini-Mental State Exam, total score |
||||||
| (N’= 2,716 ; N=1,651 ; k=1.6 ) | ||||||
| 1 vs. 0 | +26.82±0.13 | <0.001 | +0.09±0.05 | 0.08 | −0.01±0.05 | 0.78 |
| 2 vs. 0 | __ | __ | −0.07±0.04 | 0.09 | ||
|
California Verbal Learning Test (CVLT), List A |
||||||
| Total population | ||||||
| (N’=2,482 ; N=1,581; k=1.6 ) | ||||||
| 1 vs. 0 | +25.57±0.68 | <0.001 | −1.25±0.16 | <0.001 | +0.09±0.17 | 0.61 |
| 2 vs. 0 | __ | __ | −0.21±0.14 | 0.12 | ||
| Older women | ||||||
| (N’=637 ; N=402 ; k=1.6 ) | ||||||
| 1 vs. 0 | +26.78±2.36 | <0.001 | −1.71±0.33 | <0.001 | +0.18±0.34 | 0.60 |
| 2 vs. 0 | __ | __ | −0.30±0.24 | 0.21 | ||
| Older men | ||||||
| (N’=466 ; N=307; k=1.5 ) | ||||||
| 1 vs. 0 | +22.06±1.33 | <0.001 | −1.71±0.33 | <0.001 | −0.46±0.38 | 0.22 |
| 2 vs. 0 | __ | __ | −0.49±0.25 | 0.05 | ||
| Younger women | ||||||
| (N’=818 N=507 ; Visits/person= ) | ||||||
| 1 vs. 0 | +23.66±2.93 | <0.001 | −0.66±0.65 | 0.31 | +0.51±0.33 | 0.13 |
| 2 vs. 0 | __ | __ | −0.80±0.35 | 0.023 | ||
| Younger men | ||||||
| (N’=561 ; N=365 ;k=1.5 ) | ||||||
| 1 vs. 0 | +24.87±1.87 | <0.001 | −0.94±0.49 | 0.06 | −0.05±0.33 | 0.89 |
| 2 vs. 0 | __ | __ | +0.51±1.38 | 0.78 | ||
| CVLT, free delayed recall | ||||||
| Total population | ||||||
| (N’=2,377 ; N=1,551; k=1.5 ) | ||||||
| 1 vs. 0 | +7.91±0.32 | <0.001 | −0.40±0.08 | <0.001 | +0.01±0.08 | 0.87 |
| 2 vs. 0 | __ | __ | −0.04±0.07 | 0.34 | ||
| Older women | ||||||
| (N’=619; N=396 ; k=1.6 ) | ||||||
| 1 vs. 0 | +7.68±0.74 | <0.001 | −0.38±0.18 | 0.030 | +0.19±0.15 | 0.19 |
| 2 vs. 0 | __ | __ | −0.21±0.10 | 0.045 | ||
| Older men | ||||||
| (N’=442 ; N=297;k=1.5 ) | ||||||
| 1 vs. 0 | +6.33±0.64 | <0.001 | −0.50±0.17 | 0.004 | −0.08±0.20 | 0.71 |
| 2 vs. 0 | __ | __ | −0.04±0.13 | 0.74 | ||
| Younger women | ||||||
| (N’=779; N=498 ; k=1.6 ) | ||||||
| 1 vs. 0 | +7.71±0.65 | <0.001 | −0.43±0.14 | 0.002 | −0.06±0.16 | 0.73 |
| 2 vs. 0 | __ | __ | −0.33±0.17 | 0.049 | ||
| Younger men | ||||||
| (N’=537; N=360 ; k=1.5) | ||||||
| 1 vs. 0 | +7.25±0.79 | <0.001 | −0.40±0.23 | 0.09 | +0.01±0.17 | 0.94 |
| 2 vs. 0 | __ | __ | +0.30±0.16 | 0.06 | ||
| Benton Visual Retention Test | ||||||
| (N’=2,803 ; N=1,665 ; k=1.7 ) | ||||||
| 1 vs. 0 | +8.80±0.51 | <0.001 | −0.38±0.13 | 0.003 | −0.01±0.13 | 0.94 |
| 2 vs. 0 | __ | __ | +0.10±0.11 | 0.37 | ||
| Brief Test of Attention | ||||||
| Total population | ||||||
| (N’=2,803 ; N=1,665;k=1.7 ) | ||||||
| 1 vs. 0 | +6.52±0.24 | <0.001 | −0.10±0.06 | 0.12 | −0.05±0.06 | 0.36 |
| 2 vs. 0 | __ | __ | +0.02±0.05 | 0.68 | ||
| Older women | ||||||
| (N’= 662; N=405; k=1.6) | ||||||
| 1 vs. 0 | +6.95±0.52 | <0.001 | −0.07±0.12 | 0.55 | +0.01±0.10 | 0.92 |
| 2 vs. 0 | __ | __ | +0.03±0.08 | 0.68 | ||
| Older men | ||||||
| (N’=500; N=317; k=1.6) | ||||||
| 1 vs. 0 | +6.09±0.51 | <0.001 | −0.10±0.14 | 0.45 | −0.24±0.16 | 0.11 |
| 2 vs. 0 | __ | __ | −0.18±0.10 | 0.08 | ||
| Younger women | ||||||
| (N’=850 ; N=514; k=1.7) | ||||||
| 1 vs. 0 | +5.98±0.47 | <0.001 | +0.07±0.10 | 0.50 | +0.01±0.10 | 0.96 |
| 2 vs. 0 | __ | __ | −0.03±0.13 | 0.82 | ||
| Younger men | ||||||
| (N’=590; N=375; k=1.6) | ||||||
| 1 vs. 0 | +6.67±0.60 | <0.001 | −0.15±0.17 | 0.39 | +0.01±0.12 | 0.94 |
| 2 vs. 0 | __ | __ | +0.24±0.10 | 0.019 | ||
| Animal Fluency | ||||||
| (N’=2,879; N=1,670; k=1.7) | ||||||
| 1 vs. 0 | +17.58±0.54 | <0.001 | −0.08±0.11 | 0.47 | −0.13±0.66 | 0.25 |
| 2 vs. 0 | __ | __ | +0.05±0.09 | 0.57 | ||
| Digits Span, Forward | ||||||
| Total population | ||||||
| (N’=2,749; N=1,664; k=1.7) | ||||||
| 1 vs. 0 | +6.85±0.22 | <0.001 | +0.01±0.05 | 0.81 | +0.05±0.05 | 0.34 |
| 2 vs. 0 | __ | __ | +0.04±0.04 | 0.30 | ||
| Older women | ||||||
| (N’=697; N=421; k=1.7) | ||||||
| 1 vs. 0 | +6.60±0.46 | <0.001 | +0.05±0.10 | 0.64 | −0.01±0.10 | 0.85 |
| 2 vs. 0 | __ | __ | +0.10±0.07 | 0.13 | ||
| Older men | ||||||
| (N’=542; N=331; k=1.6) | ||||||
| 1 vs. 0 | +6.67±0.46 | <0.001 | −0.14±0.09 | 0.12 | +0.12±0.10 | 0.24 |
| 2 vs. 0 | __ | __ | −0.08±0.11 | 0.50 | ||
| Younger women | ||||||
| (N’=883; N=526; k=1.7) | ||||||
| 1 vs. 0 | +6.42±0.43 | <0.001 | −0.14±0.09 | 0.12 | +0.12±0.10 | 0.24 |
| 2 vs. 0 | __ | __ | −0.08±0.11 | 0.50 | ||
| Younger men | ||||||
| (N’=627; N=386; k=1.6) | ||||||
| 1 vs. 0 | +7.62±0.57 | <0.001 | +0.03±0.13 | 0.81 | +0.22±0.10 | 0.034 |
| 2 vs. 0 | __ | __ | +0.03±0.08 | 0.68 | ||
| Digits Span, Backward | ||||||
| (N’=2,733; N=1,663 ; k=1.6) | ||||||
| 1 vs. 0 | 1.24±4.55 | 0.79 | +0.78±1.06 | 0.46 | −0.02±0.05 | 0.66 |
| 2 vs. 0 | __ | __ | +0.07±0.04 | 0.06 | ||
| Clock, command | ||||||
| Total population | ||||||
| (N’=2,878; N=1,668 ; k=1.7) | ||||||
| 1 vs. 0 | +8.83±0.13 | <0.001 | −0.09±0.04 | 0.011 | +0.02±0.04 | 0.52 |
| 2 vs. 0 | __ | __ | −0.03±0.03 | 0.28 | ||
| Older women | ||||||
| (N’=741; N=425; k=) | ||||||
| 1 vs. 0 | +8.71±0.28 | <0.001 | −0.20±0.08 | 0.009 | +0.17±0.07 | 0.019 |
| 2 vs. 0 | __ | __ | −0.07±0.05 | 0.16 | ||
| Older men | ||||||
| (N’=554; N=326; k=1.7) | ||||||
| 1 vs. 0 | +9.00±0.27 | <0.001 | −0.06±0.09 | 0.45 | +0.01±0.09 | 0.91 |
| 2 vs. 0 | __ | __ | −0.06±0.06 | 0.30 | ||
| Younger women | ||||||
| (N’=926; N=527; k=1.8) | ||||||
| 1 vs. 0 | +9.20±0.24 | <0.001 | −0.11±0.06 | 0.08 | −0.12±0.06 | 0.06 |
| 2 vs. 0 | __ | __ | +0.13±0.07 | 0.06 | ||
| Younger men | ||||||
| (N’=657; N=390; k=1.7) | ||||||
| 1 vs. 0 | −0.00±0.10 | 0.97 | −0.00±0.10 | 0.97 | +0.10±0.08 | 0.17 |
| 2 vs. 0 | __ | __ | −0.03±0.06 | 0.65 | ||
| Trailmaking test, Part A | ||||||
| Total population | ||||||
| (N’=2,771; N=1,634; k=1.7) | ||||||
| 1 vs. 0 | +34.52±3.95 | <0.001 | +2.14±1.14 | 0.06 | +0.08±1.16 | 0.94 |
| 2 vs. 0 | __ | __ | −1.59±0.97 | 0.10 | ||
| Older women | ||||||
| (N’=720; N=420; k=1.7) | ||||||
| 1 vs. 0 | +10.40±11.5 | 0.37 | +7.06±4.02 | 0.08 | −1.09±3.50 | 0.76 |
| 2 vs. 0 | __ | __ | +0.39±2.48 | 0.88 | ||
| Older men | ||||||
| (N’=514; N=311; k=1.7) | ||||||
| 1 vs. 0 | +33.39±8.23 | <0.001 | +3.69±2.35 | 0.12 | −0.28±2.37 | 0.91 |
| 2 vs. 0 | __ | __ | −6.91±1.73 | <0.001 | ||
| Younger women | ||||||
| (N’=906 ; N=522; k=1.7) | ||||||
| 1 vs. 0 | +35.11±4.03 | <0.001 | −0.46±0.8 | 0.59 | +1.43±0.61 | 0.020 |
| 2 vs. 0 | __ | __ | +0.15±0.72 | 0.71 | ||
| Younger men | ||||||
| (N’=631)c | ||||||
| 1 vs. 0 | 36.34±10.28 | <0.001 | +1.27±3.45 | 0.37 | −0.50±2.87 | 0.86 |
| 2 vs. 0 | __ | __ | −1.08±2.28 | 0.80 | ||
| Trailmaking test, Part B | ||||||
| (N’=2,674 ; N=1,620 ; k=1.7) | ||||||
| 1 vs. 0 | +202.0±53.4 | <0.001 | +2.95±12.50 | 0.82 | +4.80±3.41 | 0.16 |
| 2 vs. 0 | __ | __ | +5.60±2.66 | 0.035 | ||
Key: BVRT=Benton Visual Retention Test; CES-D=Center for Epidemiologic Studies-Depression; CVLT=California Verbal Learning Test; HANDLS=Healthy Aging in Neighborhoods of Diversity across the Life Span; MMSE=Mini-Mental State Examination; N=number of participants; N’=number of visits; k=mean visits/person; WRAT=Wide Range Achievement Test.
Multiple mixed-effects linear regression models adjusted for baseline age, sex, race/ethnicity, marital status, education, WRAT total score, poverty income ratio, current smoking status, current use of illicit drugs, body mass index, CES-D total score and 2010-HEI. Models are stratified and presented by sex and age group when in a separate model, the four-way interaction Time×exposure×sex×Age had at least one term that is statistically significant at the type I error level of 0.05.
Most cognitive test scores were in the direction of higher score=better performance, except for BVRT (total errors), and Trailmaking Test both parts (expressed in seconds).
Mixed-effects regression model for younger men when outcome was Trailmaking test, Part A did not converge. Thus, an OLS model was conducted.
FIGURE 2.
Stable, decreased and Increased serum uric acid (ΔSUA) and their association with longitudinal cognitive change over-time on Trailmaking, Part A (sec.), (older men): mixed-effects regression model , HANDLS, 2004–2013
DISCUSSION
Using data from a large bi-racial cohort study of middle-aged adult men and women, our present study revealed that a higher SUAbase was associated with faster annual rate of decline on the Benton Visual Retention Test (i.e. visual memory/visuo-construction ability) by γ=0.07 with a standard error of 0.02, P<0.001 in the total population. Among older men, a significant increase in SUA was associated with slower decline on a test of attention/processing speed, namely Trailmaking test, Part A, measured in seconds to completion (γ=−6.91±1.73, p<0.001).
Uric acid is a substance that accumulates in the kidney as a result of purine metabolism, specifically when xanthine is degraded enzymatically. The paradoxical relationship between UA and neurodegenerative diseases is complex and may involve its dual antioxidant (primarily in plasma) and pro-oxidant (primarily intracellular) function in neurons [31]. UA is a natural antioxidant aiding the removal of superoxide (O2−) by preventing the degradation of superoxide dismutase, the enzyme responsible for its clearing.[32] Removal of O2− helps to prevent its reaction with nitric oxide (NO), blocking the formation of peroxynitrite (ONOO−), [33] a biological oxidant associated with many pathologies, [33], including neurodegenerative diseases (e.g. multiple sclerosis (MS) [34, 35], optic neuritis [36], Parkinson’s disease [37] and Alzheimer’s disease [38]). In these conditions, a low level or over-time reduction in SUA may not be able to prevent the toxicity generated by peroxynitrite, resulting in nitration of amino acids such as tyrosine and cysteine [39], DNA damage and mitochondrial dysfunction leading to cell death, necrosis and apoptosis [32]. Therefore, despite the fact that chronic elevations in SUA are associated with increased risk of stroke mortality or outcomes after stroke [40, 41], acute elevations of SUA can provide anti-oxidant protection by scavenging ONOO-and acting upon astroglia, upregulating protein levels of EAAT-1, a glutamate transporter which can protect spinal cord and cortical neurons against focal ischemic brain injury [42, 43].
Despite the evidence of an antioxidant effect, each UA molecule produced through enzymatic degradation of xanthine generates O2−, which when produced in acute conditions such as ischemia[44] can overwhelm ONOO- production and override UA’s neuro-protective effects. [12].
Our findings are in line with previous studies reporting that higher SUAbase concentrations are associated with poorer performance on several domains of cognitive function, a decline over time in performance as well as dementia and mild cognitive impairment. [5–13] Most recently, a cohort study of 423 cognitively healthy community-dwelling older women participating in the Women’s Health and Aging Study (WHAS II) observed that a higher SUAbase was associated with poorer working memory, with a trend toward slower manual speed and dexterity, after adjusting for several potential demographic and health confounders.[5] This pattern of association was replicated when a study showed that higher SUAbase correlated with greater white matter atrophy [13] and cerebral ischemic burden using volume of hyperintense signal on T2-weighted brain MRI scans as a marker among older adults.[12] The latter studies indicated that the relationship between higher SUA and cognitive dysfunction may be mediated by white matter atrophy and cerebral ischemia.[7] Similarly, a recent cross-sectional study in 288 healthy elderly subjects found that SUA was linked to poorer performance on MMSE.[11] This finding was replicated in another cross-sectional study of 247 subjects with chronic kidney disease and showed that SUA is a stronger predictor of cognitive dysfunction independently of age, educational status and presence of cerebrovascular disease.[9] Similarly, Ruggiero et al. concluded that SUA concentration among a sample of 1,016 community-dwelling older adults was positively related with the prevalence of dementia, independently of other potential confounders.[8] Finally, a case-control study that included Mild Cognitive Impairment (MCI, N=103), AD (N=89) and vascular dementia (VaD, N=54) cases that were compared to 48 controls, found that individuals with simultaneously high levels of homocysteine and SUA had a high probability to be affected by VaD (OR=10.50; 95% CI: 2.33–47.2) but not AD compared to normal controls. [10] The association between a higher SUAbase and a decline in visual memory performance over time may be mediated by increased brain infarction which was shown to occur only in women as a response to elevated SUA. [23] However, further studies are needed to elucidate the potential brain-level mediating factors. Nevertheless, some of our sex- and age-specific findings suggested an increasing SUA over-time is a protective factor against decline in certain domains, particularly attention among older men. This is consistent with studies suggesting that higher levels of SUA had beneficial cognitive effects, [14–19] ranging from a small case-control study of AD (N=41) vs. controls (N=40) comparing SUA between the two groups [17], to a large prospective cohort study of 4,618 participants 55y or older followed-up for 11.1 years for dementia that found an inverse relationship between SUA and risk of dementia after controlling for several cardiovascular risk factors.[14] The remaining 4 studies were cross-sectional in design with the exception of one that was a cohort study of 446 men which found that the lowest quintile of SUA was associated with poorer global cognitive performance as well as poorer performance in domains of memory, executive function, visuo-spatial and attention. These associations were slightly attenuated when adjusting for cerebrovascular and cardiovascular measures. [19] This study [19] replicated our findings with respect to the protective effect of SUA on the domain of attention in particular, and among older men. Similarly, Li and colleagues found that only among men higher SUA showed an inverse correlation with the risk of cognitive impairment.[15] Genetic studies add evidence of an association between UA transporter gene (SLC2A9) and memory performance. In fact, the Lothian Birth Cohort supports a genetic mechanism behind a possible association between higher SUA concentrations and better cognitive performance in later life.[45]
Thus, the relationships between SUA and various domains of cognition in our study were mixed. Specifically a deleterious effect of SUA was seen in the case of visual memory in the total population as opposed to a potentially beneficial effect in the case of attention among older men. Cerebrovascular and cardiovascular factors, including white matter atrophy, cerebral ischemia or infarction may be at play in both cases. [7, 12, 13, 19] As stated earlier, recent evidence suggests that SUA may have a beneficial or no significant cognitive effect among men, while having a potential deleterious effect among women, particularly older women. [5, 20–24] The deleterious effect observed between SUA at baseline and decline in visual memory overall, may be driven by brain infarction occurring mainly among women in specific regions of the brain related to visual memory. [23] Despite the lack of effect modification by sex or by sex and age group, this association was in fact restricted to women (p<0.05 for older women, p<0.01 for younger women). However, more studies are needed to replicate those findings. Antioxidant effects of SUA through the efficient removal of O2− and blocking of the formation of peroxynitrite (ONOO−) [33] may have a major role on the attention domain only in older men. [38] Future human neuroimaging studies among others should shed some light as to the effect of SUA on various regions of the brain that are linked to those cognitive domains. Genetic studies may also uncover UA transporter gene effects on various domains of cognition.
Our study has several important strengths. The large sample size of the HANDLS cohort and its symmetry by age, sex, race and poverty status, allows for adequate power when examining relationship within demographic strata, including age group and sex. The study’s prospective cohort design allows ascertaining temporality of associations with a rich battery of cognitive tests available spanning key domains of cognition. Our analyses also controlled for important potentially confounding covariates, namely key socio-demographic, lifestyle and health-related factors. Since SUA can be influenced by diet, particularly meat consumption,[46, 47] it is important to control for overall dietary quality as was done in the present study. Advanced multivariable techniques were used including mixed-effects regression models which took into account sample selectivity. Moreover, the descriptive part of the analysis accounted for unequal probability of sampling by including sampling weights in order to obtain means and proportions that are representative of Baltimore city.
Nevertheless, our findings should be interpreted with caution in light of some important limitations. First, despite control for major confounding factors, residual confounding cannot be ruled out given that this was an observational non-randomized study. Second, SUA is affected not only by diet and body mass index among other factors that were controlled for, but also by physical activity which was not measured at baseline in the HANDLS cohort. Third, due to lack of factorial invariance across race, gender and poverty status with respect to the structure of the cognitive test battery, we were not able to compute valid cognitive domains from the available test scores. Finally, availability of two cognitive test scores at baseline and the follow-up visit vs. having only 1 test score may be dependent on unmeasured selection factors related to health status of participants.
In sum, a higher SUAbase was associated with faster cognitive decline over-time in a visual memory/visuo-construction ability test. ΔSUA exhibited mixed associations with cognition. After correction of multiple testing, an increasing ΔSUA was potentially beneficial for the domain of attention only among older men, compared to no change over-time. More longitudinal studies are needed to examine cognitive domain-specific effects of over-time change in SUA within sex and age groups.
Supplementary Material
Acknowledgments
This study was supported in part by the NIA/NIH/IRP. The authors would like to thank Megan Williams and Danielle Shaked, NIA/NIH/IRP for the internal review of the manuscript. The authors declare no conflict of interest.
ABBREVIATIONS
- AD
Alzheimer’s Disease
- AF
Animal Fluency test
- BTA
Brief Test of Attention
- BVRT
Benton Visual Retention Test
- CDT
Clock Drawing Test
- CES-D
Center for Epidemiologic Studies-Depression
- CR
Card Rotations
- CVLT-DFR
California Verbal Learning Test, Delayed Free Recall; (List A)
- CVLT-List A
California Verbal Learning Test, immediate recall (List A)
- DS-B
Digit Span Backwards
- DS-F
Digit Span Forward
- EDS
Elevated Depressive Symptoms
- HANDLS
Healthy Aging in Neighborhoods of Diversity Across the Life Span
- HS
High School
- ICMA
Immunochemiluminometric assays
- IP
Identical Pictures
- OLS
Ordinary Least Square
- PIR
Poverty Income Ratio
- SUAbase
Baseline serum uric acid
- SUA
Serum Uric Acid
- ΔSUA
Change in serum uric acid
- Trails A
Trailmaking test, Part A
- Trails B
Trailmaking test, Part B
- WRAT
Wide Range Achievement Tes
Footnotes
ONLINE SUPPLEMENTAL MATERIALS
Supplemental Figure 1. Flow chart of selected participants from HANDLS 2004-2013
APPENDIX I: Description of cognitive tests, literacy and the CES-D
APPENDIX II: Description of mixed-effects regression models
REFERENCES
- 1.Johnson RJ, Kang DH, Feig D, Kivlighn S, Kanellis J, Watanabe S, Tuttle KR, Rodriguez-Iturbe B, Herrera-Acosta J, Mazzali M. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension. 2003;41:1183–1190. doi: 10.1161/01.HYP.0000069700.62727.C5. [DOI] [PubMed] [Google Scholar]
- 2.Mene P, Punzo G. Uric acid: bystander or culprit in hypertension and progressive renal disease? J Hypertens. 2008;26:2085–2092. doi: 10.1097/HJH.0b013e32830e4945. [DOI] [PubMed] [Google Scholar]
- 3.Guasch-Ferre M, Bullo M, Babio N, Martinez-Gonzalez MA, Estruch R, Covas MI, Warnberg J, Aros F, Lapetra J, Serra-Majem L, Basora J, Salas-Salvado J. Mediterranean diet and risk of hyperuricemia in elderly participants at high cardiovascular risk. J Gerontol A Biol Sci Med Sci. 2013;68:1263–1270. doi: 10.1093/gerona/glt028. [DOI] [PubMed] [Google Scholar]
- 4.Chen X, Guo X, Huang R, Chen Y, Zheng Z, Shang H. Serum uric acid levels in patients with Alzheimer’s disease: a meta-analysis. PLoS One. 2014;9:e94084. doi: 10.1371/journal.pone.0094084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vannorsdall TD, Kueider AM, Carlson MC, Schretlen DJ. Higher baseline serum uric acid is associated with poorer cognition but not rates of cognitive decline in women. Exp Gerontol. 2014;60:136–139. doi: 10.1016/j.exger.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schretlen DJ, Inscore AB, Jinnah HA, Rao V, Gordon B, Pearlson GD. Serum uric acid and cognitive function in community-dwelling older adults. Neuropsychology. 2007;21:136–140. doi: 10.1037/0894-4105.21.1.136. [DOI] [PubMed] [Google Scholar]
- 7.Vannorsdall TD, Jinnah HA, Gordon B, Kraut M, Schretlen DJ. Cerebral ischemia mediates the effect of serum uric acid on cognitive function. Stroke. 2008;39:3418–3420. doi: 10.1161/STROKEAHA.108.521591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruggiero C, Cherubini A, Lauretani F, Bandinelli S, Maggio M, Di Iorio A, Zuliani G, Dragonas C, Senin U, Ferrucci L. Uric acid and dementia in community-dwelling older persons. Dement Geriatr Cogn Disord. 2009;27:382–389. doi: 10.1159/000210040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Afsar B, Elsurer R, Covic A, Johnson RJ, Kanbay M. Relationship between uric acid and subtle cognitive dysfunction in chronic kidney disease. Am J Nephrol. 2011;34:49–54. doi: 10.1159/000329097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cervellati C, Romani A, Seripa D, Cremonini E, Bosi C, Magon S, Passaro A, Bergamini CM, Pilotto A, Zuliani G. Oxidative balance, homocysteine, and uric acid levels in older patients with Late Onset Alzheimer’s Disease or Vascular Dementia. J Neurol Sci. 2014;337:156–161. doi: 10.1016/j.jns.2013.11.041. [DOI] [PubMed] [Google Scholar]
- 11.Cicero AF, Desideri G, Grossi G, Urso R, Rosticci M, D’Addato S, Borghi C, Brisighella Heart Study G. Serum uric acid and impaired cognitive function in a cohort of healthy young elderly: data from the Brisighella Study. Intern Emerg Med. 2015;10:25–31. doi: 10.1007/s11739-014-1098-z. [DOI] [PubMed] [Google Scholar]
- 12.Schretlen DJ, Inscore AB, Vannorsdall TD, Kraut M, Pearlson GD, Gordon B, Jinnah HA. Serum uric acid and brain ischemia in normal elderly adults. Neurology. 2007;69:1418–1423. doi: 10.1212/01.wnl.0000277468.10236.f1. [DOI] [PubMed] [Google Scholar]
- 13.Verhaaren BF, Vernooij MW, Dehghan A, Vrooman HA, de Boer R, Hofman A, Witteman JC, Niessen WJ, Breteler MM, van der Lugt A, Ikram MA. The relation of uric acid to brain atrophy and cognition: the Rotterdam Scan Study. Neuroepidemiology. 2013;41:29–34. doi: 10.1159/000346606. [DOI] [PubMed] [Google Scholar]
- 14.Euser SM, Hofman A, Westendorp RG, Breteler MM. Serum uric acid and cognitive function and dementia. Brain. 2009;132:377–382. doi: 10.1093/brain/awn316. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Dong BR, Lin P, Zhang J, Liu GJ. Association of cognitive function with serum uric acid level among Chinese nonagenarians and centenarians. Exp Gerontol. 2010;45:331–335. doi: 10.1016/j.exger.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Wu Y, Zhang D, Pang Z, Jiang W, Wang S, Tan Q. Association of serum uric acid level with muscle strength and cognitive function among Chinese aged 50–74 years. Geriatr Gerontol Int. 2013;13:672–677. doi: 10.1111/j.1447-0594.2012.00962.x. [DOI] [PubMed] [Google Scholar]
- 17.Al-khateeb E, Althaher A, Al-khateeb M, Al-Musawi H, Azzouqah O, Al-Shweiki S, Shafagoj Y. Relation between uric acid and Alzheimer’s disease in elderly Jordanians. J Alzheimers Dis. 2015;44:859–865. doi: 10.3233/JAD-142037. [DOI] [PubMed] [Google Scholar]
- 18.Mendez-Hernandez E, Salas-Pacheco J, Ruano-Calderon L, Tellez-Valencia A, Cisneros-Martinez J, Barraza-Salas M, Arias-Carrion O. Lower Uric Acid Linked with Cognitive Dysfunction in the Elderly. CNS Neurol Disord Drug Targets. 2015 doi: 10.2174/1871527314666150430161659. [DOI] [PubMed] [Google Scholar]
- 19.Molshatzki N, Weinstein G, Streifler JY, Goldbourt U, Tanne D. Serum uric Acid and subsequent cognitive performance in patients with pre-existing cardiovascular disease. PLoS One. 2015;10:e0120862. doi: 10.1371/journal.pone.0120862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alderman MH, Cohen H, Madhavan S, Kivlighn S. Serum uric acid and cardiovascular events in successfully treated hypertensive patients. Hypertension. 1999;34:144–150. doi: 10.1161/01.hyp.34.1.144. [DOI] [PubMed] [Google Scholar]
- 21.Fang J, Alderman MH. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971–1992. National Health and Nutrition Examination Survey. JAMA. 2000;283:2404–2410. doi: 10.1001/jama.283.18.2404. [DOI] [PubMed] [Google Scholar]
- 22.Park JS, Kang S, Ahn CW, Cha BS, Kim KR, Lee HC. Relationships between serum uric acid, adiponectin and arterial stiffness in postmenopausal women. Maturitas. 2012;73:344–348. doi: 10.1016/j.maturitas.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Heo SH, Lee SH. High levels of serum uric acid are associated with silent brain infarction. J Neurol Sci. 2010;297:6–10. doi: 10.1016/j.jns.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 24.O’Reilly EJ, Gao X, Weisskopf MG, Chen H, Schwarzschild MA, Spiegelman D, Ascherio A. Plasma urate and Parkinson’s disease in women. Am J Epidemiol. 2010;172:666–670. doi: 10.1093/aje/kwq195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gephardt MC, Hanlon TJ, Matson CF. Blood Uric Acid Values as Related to Sex and Age. JAMA. 1964;189:1028–1029. doi: 10.1001/jama.1964.03070130048019. [DOI] [PubMed] [Google Scholar]
- 26.Evans MK, Lepkowski JM, Powe NR, LaVeist T, Kuczmarski MF, Zonderman AB. Healthy aging in neighborhoods of diversity across the life span (HANDLS): overcoming barriers to implementing a longitudinal, epidemiologic, urban study of health, race, and socioeconomic status. Ethn Dis. 2010;20:267–275. [PMC free article] [PubMed] [Google Scholar]
- 27.Desideri G, Castaldo G, Lombardi A, Mussap M, Testa A, Pontremoli R, Punzi L, Borghi C. Is it time to revise the normal range of serum uric acid levels? Eur Rev Med Pharmacol Sci. 2014;18:1295–1306. [PubMed] [Google Scholar]
- 28.Beydoun MA, Beydoun HA, Kitner-Triolo MH, Kaufman JS, Evans MK, Zonderman AB. Thyroid hormones are associated with cognitive function: moderation by sex, race, and depressive symptoms. J Clin Endocrinol Metab. 2013;98:3470–3481. doi: 10.1210/jc.2013-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hochberg Y, Tamhane AC. Multiple comparison procedures. New York: Wiley; 1987. [Google Scholar]
- 30.Selvin S. Statistical Analysis of Epidemiologic Data. Oxford University Press; 2004. [Google Scholar]
- 31.Sautin YY, Johnson RJ. Uric acid: the oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids. 2008;27:608–619. doi: 10.1080/15257770802138558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Veen RC, Hinton DR, Incardonna F, Hofman FM. Extensive peroxynitrite activity during progressive stages of central nervous system inflammation. J Neuroimmunol. 1997;77:1–7. doi: 10.1016/s0165-5728(97)00013-1. [DOI] [PubMed] [Google Scholar]
- 34.Drulovic J, Dujmovic I, Stojsavljevic N, Mesaros S, Andjelkovic S, Miljkovic D, Peric V, Dragutinovic G, Marinkovic J, Levic Z, Mostarica Stojkovic M. Uric acid levels in sera from patients with multiple sclerosis. J Neurol. 2001;248:121–126. doi: 10.1007/s004150170246. [DOI] [PubMed] [Google Scholar]
- 35.Toncev G, Milicic B, Toncev S, Samardzic G. Serum uric acid levels in multiple sclerosis patients correlate with activity of disease and blood-brain barrier dysfunction. Eur J Neurol. 2002;9:221–226. doi: 10.1046/j.1468-1331.2002.00384.x. [DOI] [PubMed] [Google Scholar]
- 36.Knapp CM, Constantinescu CS, Tan JH, McLean R, Cherryman GR, Gottlob I. Serum uric acid levels in optic neuritis. Mult Scler. 2004;10:278–280. doi: 10.1191/1352458504ms1042oa. [DOI] [PubMed] [Google Scholar]
- 37.Church WH, Ward VL. Uric acid is reduced in the substantia nigra in Parkinson’s disease: effect on dopamine oxidation. Brain Res Bull. 1994;33:419–425. doi: 10.1016/0361-9230(94)90285-2. [DOI] [PubMed] [Google Scholar]
- 38.Kim TS, Pae CU, Yoon SJ, Jang WY, Lee NJ, Kim JJ, Lee SJ, Lee C, Paik IH, Lee CU. Decreased plasma antioxidants in patients with Alzheimer’s disease. Int J Geriatr Psychiatry. 2006;21:344–348. doi: 10.1002/gps.1469. [DOI] [PubMed] [Google Scholar]
- 39.Ischiropoulos H, Zhu L, Chen J, Tsai M, Martin JC, Smith CD, Beckman JS. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch Biochem Biophys. 1992;298:431–437. doi: 10.1016/0003-9861(92)90431-u. [DOI] [PubMed] [Google Scholar]
- 40.Mazza A, Pessina AC, Pavei A, Scarpa R, Tikhonoff V, Casiglia E. Predictors of stroke mortality in elderly people from the general population. The CArdiovascular STudy in the ELderly. Eur J Epidemiol. 2001;17:1097–1104. doi: 10.1023/a:1021216713504. [DOI] [PubMed] [Google Scholar]
- 41.Weir CJ, Muir SW, Walters MR, Lees KR. Serum urate as an independent predictor of poor outcome and future vascular events after acute stroke. Stroke. 2003;34:1951–1956. doi: 10.1161/01.STR.0000081983.34771.D2. [DOI] [PubMed] [Google Scholar]
- 42.Du Y, Chen CP, Tseng CY, Eisenberg Y, Firestein BL. Astroglia-mediated effects of uric acid to protect spinal cord neurons from glutamate toxicity. Glia. 2007;55:463–472. doi: 10.1002/glia.20472. [DOI] [PubMed] [Google Scholar]
- 43.Yu ZF, Bruce-Keller AJ, Goodman Y, Mattson MP. Uric acid protects neurons against excitotoxic and metabolic insults in cell culture, and against focal ischemic brain injury in vivo. J Neurosci Res. 1998;53:613–625. doi: 10.1002/(SICI)1097-4547(19980901)53:5<613::AID-JNR11>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 44.Phillis JW. A “radical” view of cerebral ischemic injury. Prog Neurobiol. 1994;42:441–448. doi: 10.1016/0301-0082(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 45.Houlihan LM, Wyatt ND, Harris SE, Hayward C, Gow AJ, Marioni RE, Strachan MW, Price JF, Starr JM, Wright AF, Deary IJ. Variation in the uric acid transporter gene (SLC2A9) and memory performance. Hum Mol Genet. 2010;19:2321–2330. doi: 10.1093/hmg/ddq097. [DOI] [PubMed] [Google Scholar]
- 46.Loenen HM, Eshuis H, Lowik MR, Schouten EG, Hulshof KF, Odink J, Kok FJ. Serum uric acid correlates in elderly men and women with special reference to body composition and dietary intake (Dutch Nutrition Surveillance System) J Clin Epidemiol. 1990;43:1297–1303. doi: 10.1016/0895-4356(90)90095-7. [DOI] [PubMed] [Google Scholar]
- 47.Lee SJ, Terkeltaub RA, Kavanaugh A. Recent developments in diet and gout. Curr Opin Rheumatol. 2006;18:193–198. doi: 10.1097/01.bor.0000209434.82096.1f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.