Abstract
The kynurenine pathway (KP), the major catabolic route of the essential amino acid L-tryptophan (L-TRP), contains several neuroactive compounds, including kynurenic acid (KYNA), 3-hydroxykynurenine (3-HK) and quinolinic acid (QUIN). The role of the D-enantiomer (D-TRP) in KP metabolism has received little attention so far. D-TRP can be converted to L-TRP by D-amino acid oxidase (D-AAO), and the same enzyme can produce D-kynurenine, a known bioprecursor of KYNA. To analyze these complex metabolic events systematically in vivo, we injected mice with D-TRP (300 mg/kg, i.p.) and examined KP metabolism in the absence or presence of the D-AAO inhibitor 3-methylpyrazole-5-carboxylic acid (MPC; 100 mg/kg, i.p.,). After 90 min, newly formed L-TRP was recovered in plasma, liver, forebrain and cerebellum, and MPC prevented its neosynthesis in all tissues. In the same animals, de novo production of D-kynurenine from D-TRP was also observed but was much higher in the periphery than in the brain. D-TRP administration raised KYNA, 3-HK, and QUIN levels in all tissues examined, and KYNA production from D-TRP was significantly reduced after pre-treatment with MPC. These results indicate that catabolic routes other than those classically ascribed to L-TRP and L-kynurenine can account for the synthesis of KYNA, 3-HK and QUIN in vivo.
Keywords: 3-Hydroxykynurenine, Kynurenic acid, Quinolinic acid
Introduction
The kynurenine pathway (KP) of tryptophan degradation contains several neuroactive compounds, including kynurenic acid (KYNA), 3-hydroxykynurenine (3-HK) and quinolinic acid (QUIN), which have been proposed to play important roles in brain physiology and pathology (Schwarcz et al. 2012). Although the essential amino acid L-tryptophan (L-TRP) is usually considered the main precursor of these KP metabolites, studies in various mammalian species, including humans, showed that D-tryptophan (D-TRP), too, can be metabolized along the KP. Thus, D-TRP is degraded to D-kynurenine (D-KYN; Higuchi & Hayaishi 1967, Kotake & Ito 1937, Langner & Berg 1955, Loh & Berg 1971a) and further to KYNA (Ishii et al. 2010, Ishii et al. 2011, Loh & Berg 1971b).
Interestingly, D-TRP can also be enzymatically converted to L-TRP (Iizuka et al. 2011, Ohara et al. 1980, Yoshihara et al. 2012), and this process occurs in two steps. First, D-amino acid oxidase (D-AAO) metabolizes D-TRP to the corresponding α-keto acid, indole-3-pyruvic acid (Loh & Berg 1971a, Sylianco & Berg 1959), which is then reversibly converted to L-TRP by aspartate aminotransferase (Bittinger et al. 2003) (Fig. 1). Notably, D-AAO also catalyzes the conversion of D-KYN to KYNA (Fukushima et al. 2009, Ishii et al. 2010, Ogaya et al. 2010) (Fig. 1). This transformation is especially effective in the cerebellum (Blanco Ayala et al. 2015, Wang et al. 2012) where the enzyme is highly expressed (Horiike et al. 1994, Moreno et al. 1999, Verrall et al. 2007).
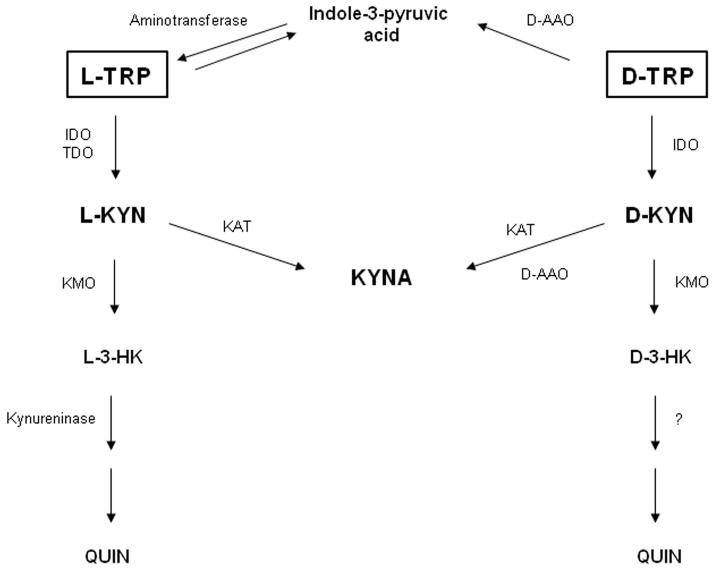
Figure 1.
Enzymatic degradation of D-TRP and L-TRP in vivo. The schematic diagram illustrates enzymatic processes involved in the degradation of L-TRP and D-TRP in mice under physiological conditions. IDO: indoleamine-2,3-dioxygenase; TDO: tryptophan-2,3-dioxygenase; D-AAO: D-amino acid oxidase; KAT: kynurenine aminotransferase; L:TRP: L-tryptophan; D-TRP: D-tryptophan; L-KYN: L-kynurenine; D-KYN: D-kynurenine; KYNA: kynurenic acid, 3-HK: 3-hydroxykynurenine; QUIN: quinolinic acid; KMO: kynurenine 3-monooxygenase.
As D-TRP can be introduced to mammals by microorganisms (Lam et al. 2009), it may contribute to the significant increases in brain KYNA, 3-HK and QUIN levels that are seen in psychiatric and neurological diseases (Schwarcz et al. 2012, Mandi & Vécsei 2012). This could be of particular relevance in psychiatric diseases such as schizophrenia and autism, where maternal exposure to bacterial pathogens and maternal immune activation are associated with the emergence of pathophysiology (Brown & Derkits 2010, Patterson 2011).
The present study was designed to elucidate the fate of systemically applied D-TRP in vivo in greater detail and with special emphasis on cerebral KP metabolism. To this end, we injected mice with D-TRP or L-TRP (300 mg/kg, i.p.) and compared the levels of both enantiomers in plasma, liver, forebrain and cerebellum. We then investigated the production of kynurenine, KYNA, 3-HK and QUIN from D-TRP, and studied the involvement of D-AAO in D-TRP metabolism by treating the animals with the D-AAO inhibitor 3-methyl-pyrazole-5-carboxylic acid (MPC; Adage et al. 2008). Our results provided substantive new information regarding the conversion of D-TRP to KP metabolites in vivo and demonstrated that D-AAO plays essential roles in the catabolic processes involved.
Materials and methods
Chemicals
D-Tryptophan (D-TRP), L-tryptophan (L-TRP), D-kynurenine (D-KYN), kynurenic acid (KYNA), 3-hydroxy-DL-kynurenine (3-HK), quinolinic acid (QUIN), D-amino acid oxidase (D-AAO, purified from porcine kidney; product number A5222) and 3-methylpyrazole-5-carboxylic acid (MPC) were purchased from Sigma-Aldrich (St. Louis, MO, USA). L-Kynurenine (L-KYN) was obtained from Sai Advantium (Hyderabad, India), and [2H3]-QUIN was purchased from Synfine Research (Richmond Hill, Ontario, Canada).
All other chemicals were purchased from various commercial suppliers and were of the highest available purity.
Animals and in vivo treatments
Adult (2–3 months) FVB/N wild-type mice (20–30 g; Taconic, Hudson, NY, USA) of either sex were used in all experiments (n = 5–7 per group). Animals were housed in a temperature-controlled, Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-approved animal facility and were kept on a 12 h/12 h-light/dark cycle with free access to food and water. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Maryland, in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Mice were injected intraperitoneally (i.p.) with 300 mg/kg D-TRP or L-TRP, dissolved in sterile saline. Controls received i.p. saline. Ninety minutes later, the animals were euthanized (CO2), and plasma (supernatant of blood centrifuged at 5,200 × g, 10 min), liver, forebrain (whole brain without cerebellum) and cerebellum were collected immediately, frozen rapidly on dry ice, and stored at −80°C until analysis.
MPC was dissolved in sterile saline (titrated to pH 7.7 with NaOH) and administered i.p. (100 mg/kg) 30 min before D-TRP administration. Plasma and tissues were collected as described above.
KYNA and kynurenine measurement
Tissues were weighed while frozen, and forebrain (1:5, w/v), cerebellum (1:5, w/v for KYNA, 1:10, w/v for kynurenine) and liver (1:20, w/v) were homogenized by sonication in ultrapure water. Plasma was diluted 1:10 (v/v) in ultrapure water. Twenty-five μL of perchloric acid (6%) were then added to 100 μL of the sample preparation, and precipitated proteins were removed by centrifugation (16,000 × g, 15 min). Twenty μL of the resulting supernatant were analyzed by HPLC as follows.
KYNA was eluted from a 3 μm C18 reverse phase HPLC column (80 mm × 4.6 mm; ESA, Chelmsford, Massachusetts, USA), using a mobile phase containing 250 mM zinc acetate, 50 mM sodium acetate, and 3% acetonitrile (pH adjusted to 6.2 with glacial acetic acid) at a flow rate of 1.0 mL/min. In the eluate, KYNA was quantitated fluorimetrically (excitation: 344 nm, emission: 398 nm; S200a fluorescence detector; Perkin Elmer, Waltham, Massachusetts, USA). The retention time was approximately 7 min.
Kynurenine was processed as described above for KYNA and was also detected fluorimetrically (excitation: 365 nm; emission: 480 nm). The retention time was approximately 6 min.
3-HK measurement
Brain (1:5, w/v) and liver (1:5, w/v) were homogenized, and plasma was diluted (1:2, v/v), in ultrapure water. Twenty-five μL of perchloric acid (6%) were then added to 100 μL of the preparation, and precipitated proteins were removed by centrifugation (16,000 × g, 15 min). Twenty μL of the resulting supernatant were injected onto a 3 μM HPLC column (80 mm × 4.6 mm; ESA), using a mobile phase consisting of 1.5% acetonitrile, 0.9% triethylamine, 0.59% phosphoric acid, 0.27 mM EDTA and 8.9 mM sodium heptanesulfonic acid, and a flow rate of 0.5 mL/min. In the eluate, 3-HK was detected electrochemically using an HTEC 500 detector (Eicom, San Diego, CA, USA; oxidation potential: + 0.5 V). The retention time of 3-HK was approximately 11 min.
QUIN measurement
Brain and liver were homogenized (1:20, w/v) and plasma was diluted (1:10, v/v) in 0.1% ascorbic acid. Fifty μL of a solution containing internal standard ([2H3]QUIN) were added to 50 μL of the sample, and proteins were precipitated with 50 μL of acetone. After centrifugation (13,700 × g, 5 min), 50 μL of methanol:chloroform (20:50, v/v) were added to the supernatant, and the samples were centrifuged (13,700 × g, 10 min). The upper layer was added to a glass tube and evaporated to dryness (90 min). The samples were then derivatized with 120 μL of 2,2,3,3,3-pentafluoro-1-propanol and 130 μL of pentafluoropropionic anhydride at 75°C for 30 min, dried down again and reconstituted in 50 μL of ethyl acetate. One μL was injected into the gas chromatograph. GC/MS analysis was carried out with a 7890A GC coupled to a 7000B MS/MS (Agilent Technologies), using electron capture negative chemical ionization (Notarangelo et al. 2012).
D-KYN measurement
D-KYN was measured by the enzyme-based assay described by Wang et al. (2013). Briefly, 50 μL of purified D-AAO (1 mg/mL) were added to 80 μL of the tissue preparation (1:5 w/v for forebrain, 1:10 w/v for cerebellum, 1:20 w/v for liver, 1:10 v/v for plasma; all in 100 mM borate buffer, pH 9.0) and water in a total volume of 200 μL. Routinely, the reactive solution was incubated at 37°C for 3 h, and the reaction was terminated by adding 20 μL of 50% trichloroacetic acid and 200 μL of 0.1 N HCl. After centrifugation, 20 μL of the resulting supernatant were used for KYNA measurement (see above).
D-TRP and L-TRP measurement
TRP enantiomers were measured by a slight modification of the chiral HPLC methodology described by Yan and Row (2008). To this end, the tissue was homogenized (1:100, w/v for brain, 1:200, w/v for liver) by sonication in ultrapure water. Plasma was diluted 1:200 (v/v) in ultrapure water. Twenty-five μL of perchloric acid (6%) were then added to 100 μL of the sample preparation, and precipitated proteins were removed by centrifugation (16,000 × g, 15 min). Twenty μL of the resulting supernatant were injected onto a 5 μm C18 reverse-phase HPLC column (Adsorbil; 150 mm × 4.6 mm; Grace, Deerfield, IL, USA), using a mobile phase containing 3 mM L-phenylalanine, 0.4 mM cupric sulfate, 16% methanol (pH adjusted to 4.7) at a flow rate of 1 mL/min. In the eluate, D-TRP and L-TRP were quantified fluorimetrically (excitation: 285 nm, emission: 365 nm; S200a fluorescence detector; Perkin Elmer). The retention times for D-TRP and L-TRP were approximately 33 and 38 min, respectively.
Data analysis
Results are expressed as the mean ± SEM. Student’s t-test or one-way Anova followed by Bonferroni’s post-hoc test were used to determine significance in all experiments. A p value of <0.05 was considered significant.
Results
D-KYN production from D-TRP in the brain
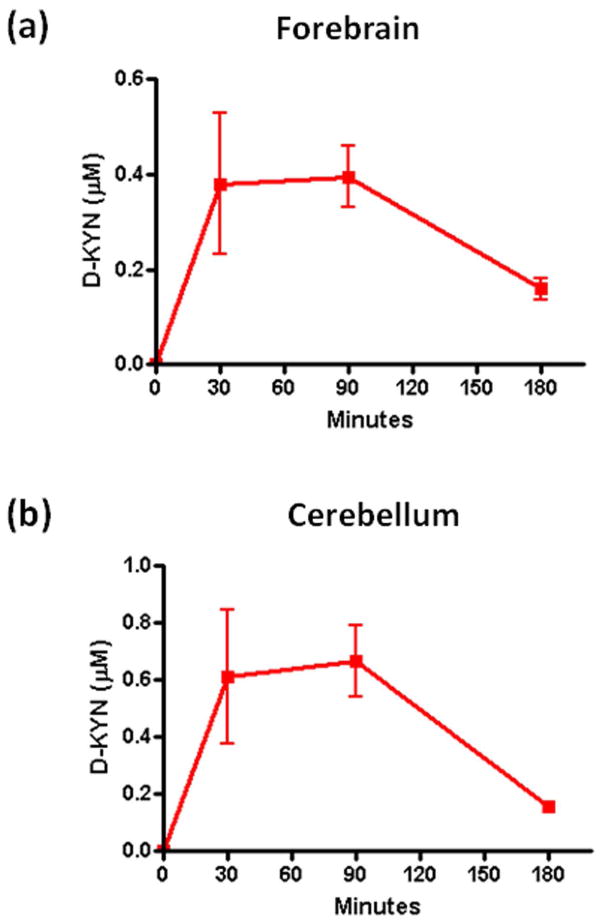
In a pilot experiment, we examined the emergence of D-KYN in the brain following the i.p. injection of D-TRP (300 mg/kg). As illustrated in Fig. 2, D-KYN was detected in both forebrain and cerebellum 30, 90 and 180 min after the systemic administration of D-TRP. In both tissues, D-KYN levels reached a maximum at 30 min, remained constant for another hour, and then decreased. Based on these results, the 90 min time point was selected for analyses in subsequent experiments.
Figure 2.
Time course of D-KYN levels after systemic D-TRP administration in mice. D-KYN was determined in forebrain (a) and cerebellum (b) 30, 90 or 180 min after the D-TRP injection (300 mg/kg, i.p.). Data are the mean ± SEM (n = 4–5).
D-TRP and L-TRP levels following systemic administration
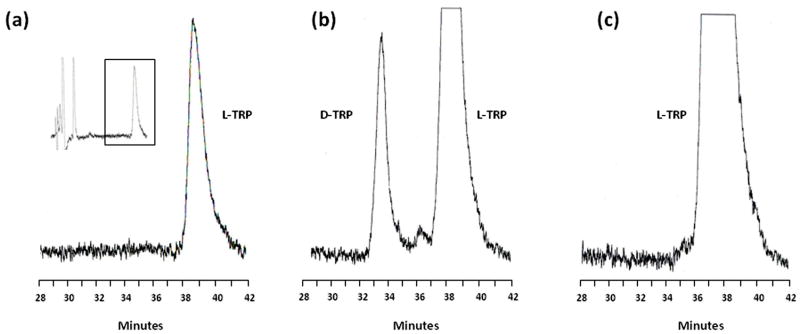
To characterize the metabolism of D-TRP in vivo and compare it to L-TRP, we optimized a procedure for the separation of TRP enantiomers in biological samples by HPLC using a chiral mobile phase (Fig. 3; see Methods for experimental details). This method allowed us to detect D-TRP and L-TRP in the same run in all samples with a detection limit of 1 pmol.
Figure 3.
Representative chromatograms of D-TRP and L-TRP in mouse cerebellar homogenate (1:100, w/v). The enantiomers were detected by HPLC with chiral mobile phase in mice injected i.p. with saline (a), D-TRP (300 mg/kg; b) or L-TRP (300 mg/kg; c). See text for experimental details. Inset: view of the entire chromatogram.
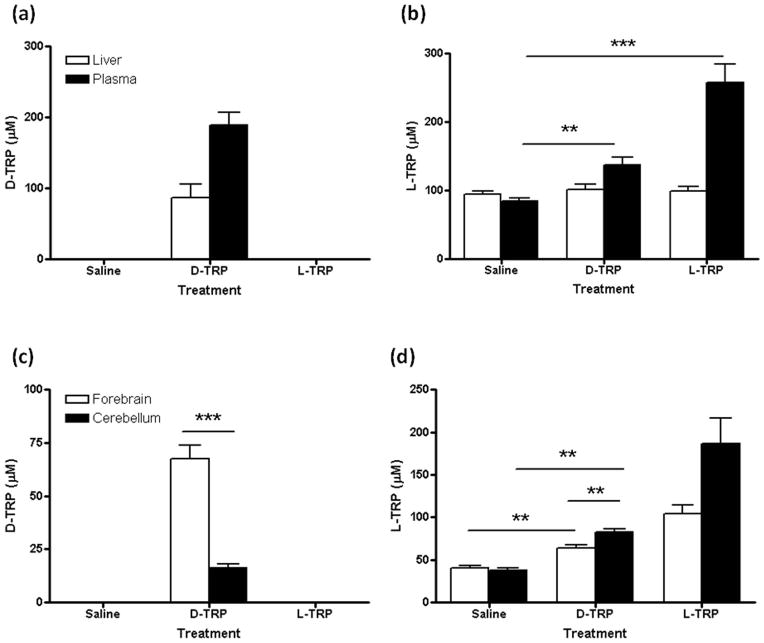
No D-TRP was measurable in saline-injected control animals. Next, we compared the distribution of D-TRP and L-TRP 90 min after each enantiomer was applied at 300 mg/kg. After D-TRP administration, the compound was detected in both liver (86.4 ± 19.7 μM) and plasma (188.8 ± 17.7 μM) (Fig. 4a). In contrast, no D-TRP was observed in the periphery after the injection of L-TRP (Fig. 4a). L-TRP levels significantly increased in the plasma after the administration of D-TRP and, naturally, also after the application of L-TRP itself (p<0.01 each) (Fig. 4b). This confirmed that D-TRP is converted to L-TRP in the mouse in vivo. However, no significant differences in L-TRP levels were detected in the liver after administration of either enantiomer (Fig. 4b).
Figure 4.
D-TRP and L-TRP levels in liver and plasma (a,b), and forebrain and cerebellum (c,d), 90 min after an i.p. injection of saline, D-TRP (300 mg/kg) or L-TRP (300 mg/kg). Data are the mean ± SEM (n = 4–5). **p<0.01; ***p<0.001 (Student’s t-test).
D-TRP was also detected in the brain of mice injected with the compound (Fig. 4c), and its levels were significantly higher in the forebrain (67.3 ± 6.7 μM) than in the cerebellum (16.3 ± 1.9 μM) (p<0.001). As in the plasma, L-TRP levels also increased significantly in both forebrain and cerebellum after the administration of D-TRP (p<0.01 in both tissues) or L-TRP (p<0.01 in both tissues) (Fig. 4d).
After D-TRP injection, higher levels of L-TRP were measured in the cerebellum (80.4 ± 2.5 μM) than in the forebrain (63.8 ± 3.4 μM) (p<0.01), indicating a preferential conversion of D-TRP to L-TRP in the cerebellum (Fig. 4d). In contrast, no D-TRP was detected in the brain after an i.p. injection of L-TRP (Fig. 4c).
In line with the conversion of D-TRP to L-TRP in tissue homogenates (Loh & Berg 1971a) and in vivo (Yoshihara et al. 2012, Iizuka et al. 2011), these results confirm that systemically administered D-TRP is converted to L-TRP in vivo.
Effect of the D-AAO inhibitor MPC on D-TRP and L-TRP levels
Using separate animals, we next investigated the involvement of D-AAO in D-TRP metabolism (Fig. 1; Iizuka et al. 2011) by treating animals with the D-AAO inhibitor MPC (Adage et al., 2008) 30 min prior to D-TRP.
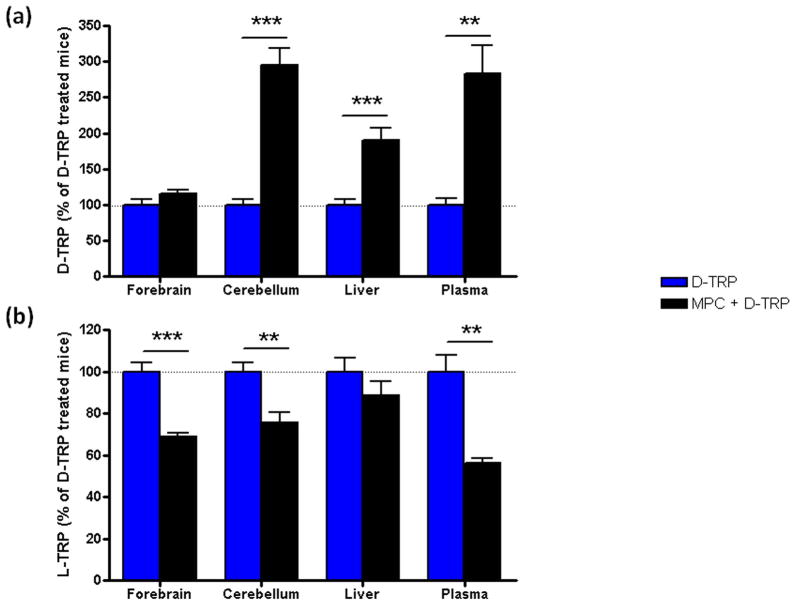
No differences in TRP levels were observed when MPC alone was injected (data not shown). However, D-TRP levels were significantly increased in cerebellum, liver and plasma of mice treated with MPC and D-TRP (MPC + D-TRP group) compared to D-TRP alone (D-TRP group) (Fig. 5a; p<0.01 in plasma, p<0.001 in liver and cerebellum), confirming that D-AAO is involved in D-TRP metabolism. A modest increase in D-TRP levels was also observed in the forebrain of MPC + D-TRP mice, but the effect did not attain statistical significance (Fig. 5a).
Figure 5.
Effect of D-AAO inhibition on D-TRP (a) and L-TRP (b) levels 90 min after the i.p. administration of D-TRP (300 mg/kg), alone or 30 min after pre-treatment with the D-AAO inhibitor MPC (100 mg.kg, i.p.). Data are expressed as the percentage of the levels of D-TRP or L-TRP in D-TRP-treated mice. Data are the mean ± SEM (n = 6–7). **p<0.01; ***p<0.001 (Student’s t-test).
Compared to animals receiving D-TRP alone, MPC + D-TRP mice showed significantly reduced L-TRP levels in forebrain, cerebellum and plasma (Fig 5b; p<0.01 in cerebellum and plasma, p<0.001 in forebrain), confirming that D-AAO is actively involved in the conversion of D-TRP to L-TRP in vivo. In contrast, we observed no significant effect of MPC pre-treatment on L-TRP levels in the liver. After 90 min, L-TRP levels in MPC + D-TRP mice were indistinguishable from endogenous values in plasma and forebrain (data not shown) but were still significantly elevated compared to controls in the cerebellum (61.1 ± 7.1 μM, p<0.05; Student’s t-test).
These results demonstrate the involvement of D-AAO in the conversion of D-TRP to L-TRP in mice in vivo.
De novo synthesis of D-KYN in D-TRP-treated mice: effect of MPC
Next, we determined the levels of D-KYN and L-KYN in mice injected with D-TRP – with or without pre-treatment with MPC – and compared them to total KYN (i.e. both enantiomers combined) levels measured by conventional HPLC. [In a pilot experiment, we confirmed that MPC is ineffective as an inhibitor of kynurenine aminotransferase; data not shown]. As illustrated in Table 1, essentially all KYN produced from D-TRP after 90 min in the plasma was identified as the D-enantiomer. In contrast, D-KYN amounted to 84.4% of total KYN in the liver, 41.6% in the forebrain (p<0.05 vs. total KYN) and only 18.6% in the cerebellum (p<0.01 vs. total KYN), indicating that a proportion of D-TRP was converted to L-KYN (defined as total KYN minus D-KYN) in vivo.
Table 1.
D-KYN production from D-TRP and effect of the D-AAO inhibitor MPC
| D-TRP | MPC + D-TRP | |||||
|---|---|---|---|---|---|---|
| Total KYN (Δ μM) | D-KYN (μM) | (%) | Total KYN (Δ μM) | D-KYN (μM) | (%) | |
| Plasma | 6.8 ± 0.5 | 7.0 ± 0.6 | 100.0 | 6.2 ± 0.5 | 6.6 ± 0.3 | 100.0 |
| Liver | 46.3 ± 3.2 | 33.7 ± 4.5 | 84.4 | 49.3± 5.5 | 40.0 ± 2.2 | 96.7 |
| Forebrain | 1.2 ± 0.1 | 0.5 ± 0.0* | 41.6 | 0.7 ± 0.2 | 0.4 ± 0.0 | 64.0 |
| Cerebellum | 3.8 ± 0.7 | 0.6 ± 0.1** | 18.6 | 1.4 ± 0.3# | 0.6 ± 0.0* | 39.1 |
D-Kynurenine (D-KYN) levels were determined in D-TRP-treated mice (300 mg/kg i.p., 90 min) with or without pre-treatment with MPC (100 mg/kg i.p.) 30 min earlier. Total kynurenine (i.e. L-KYN + D-KYN) levels were measured in the same samples. “Total KYN” represents the increase in kynurenine levels over endogenous content. “%” indicates the percentage of D-KYN compared to total kynurenine in the same samples. Data are the mean ± SEM (n=4–5).
p<0.05,
p<0.01 vs. total kynurenine;
p<0.05 vs. total kynurenine in the absence of MPC (Student’s t-test in all cases).
Compared to D-KYN production from D-TRP alone, MPC pre-treatment slightly increased D-KYN levels in the liver (p>0.05) but not in the plasma. In the forebrain, MPC pre-treatment did not affect D-KYN levels; however, total KYN levels were reduced (from 1.2 ± 0.1 μM to 0.7 ± 0.2 μM; p=0.053), and D-KYN increased to 64.0% of total KYN (p>0.05).
In the cerebellum, pre-treatment with MPC decreased total KYN levels from 3.8 ± 0.7 μM to 1.4 ± 0.3 μM (p<0.05), and D-KYN increased from 18.6% to 39.1% of total KYN. Interestingly, MPC did not completely prevent the production of L-KYN from D-TRP in the cerebellum. Thus, total KYN levels were still significantly higher than D-KYN levels in the MPC + D-TRP group (p<0.05).
These results show that both D-KYN and L-KYN are formed after D-TRP administration in vivo in both periphery and brain, and that the levels of both metabolites are significantly affected by pre-treatment with the D-AAO inhibitor MPC.
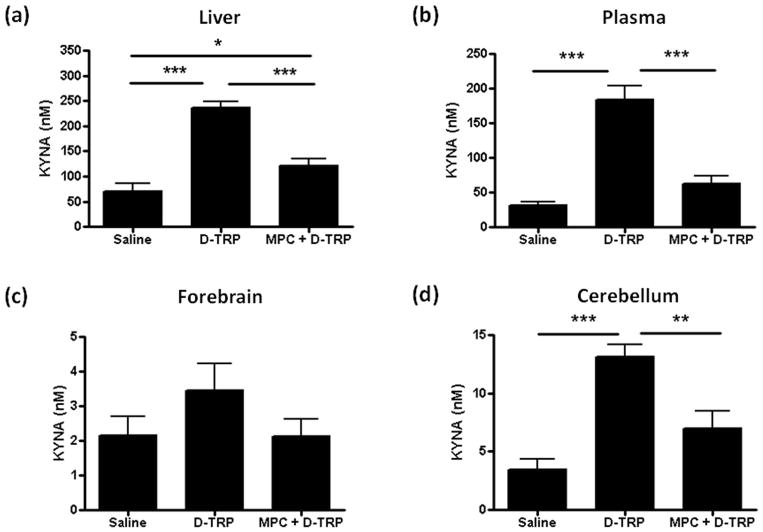
Effect of D-AAO inhibition on KYNA production from D-TRP
We next studied the production of KYNA after the systemic injection of D-TRP and also assessed the effect of an additional pre-treatment with MPC (Fig. 1). As shown in Fig. 6, D-TRP administration caused significant increases in KYNA levels in liver (p<0.001), plasma (p<0.001) and cerebellum (p<0.001), but raised levels in the forebrain only slightly (p>0.05). Interestingly, D-TRP-induced KYNA formation in the cerebellum (13.1 ± 1.1 nM) was significantly more pronounced than in the forebrain (3.5 ± 0.8 nM; p<0.001, Student’s t-test), as also reported after systemic D-KYN injection (Wang et al., 2012).
Figure 6.
Effect of D-AAO inhibition on KYNA production from D-TRP. KYNA was measured in liver (a), plasma (b), forebrain (c) and cerebellum (d) 90 min after the i.p. administration of saline, D-TRP (300 mg/kg) alone or with (MPC + D-TRP) pre-treatment (30 min) with the D-AAO inhibitor MPC (100 mg/kg i.p.). Data are the mean ± SEM (n = 5–7). *p<0.05, **p<0.01, ***p<0.001 (one-way Anova, followed by Bonferroni‘s post-hoc test).
Pre-treatment with MPC reduced the increase in KYNA levels in all tissues (Fig. 6). Levels were significantly decreased in liver (p<0.001 vs. D-TRP), plasma (p<0.001 vs. D-TRP) and cerebellum (p<0.01 vs. D-TRP). However, KYNA levels did not completely return to endogenous levels in the three tissues and were still significantly higher than in saline-injected controls in the liver (p<0.05).
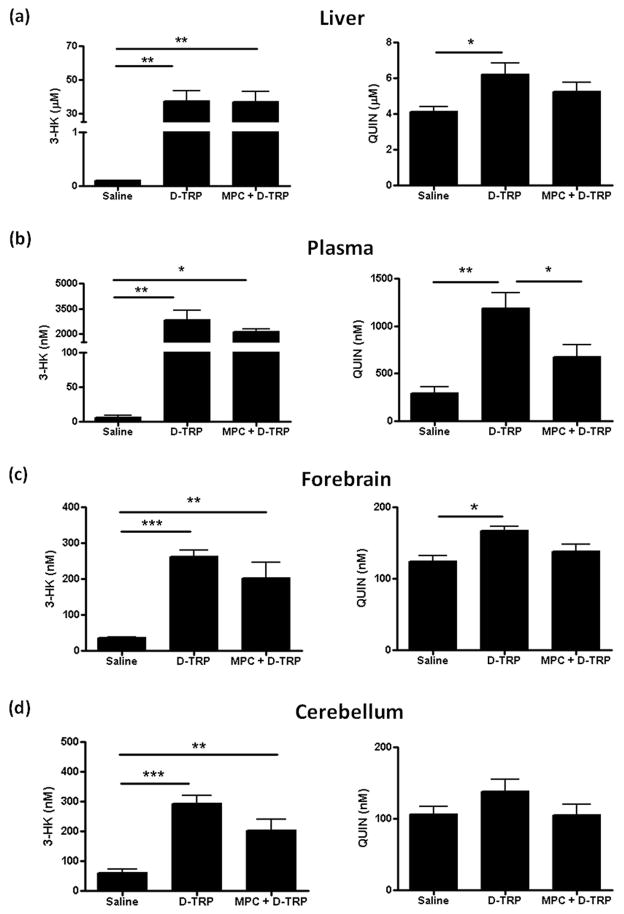
Effect of D-AAO inhibition on 3-HK and QUIN production from D-TRP
Finally, we evaluated the effect of D-TRP, and pre-treatment with MPC, on 3-HK and QUIN levels. Following administration of D-TRP, 3-HK levels increased in liver (p<0.01), plasma (p<0.01), forebrain (p<0.001) and cerebellum (p<0.001). Pre-treatment with MPC did not significantly affect 3-HK levels, with only a slight decrease (p>0.05) seen in most tissues, and 3-HK levels remained consistently higher than endogenous levels (Fig. 7).
Figure 7.
Effect of D-AAO inhibition on 3-HK and QUIN production from D-TRP. Total 3-HK (i.e. L-3-HK + D-3-HK) and QUIN were measured in liver (a), plasma (b), forebrain (c) and cerebellum (d) 90 min after the i.p. administration of saline, D-TRP (300 mg/kg) alone or with (MPC + D-TRP) pre-treatment (30 min) with the D-AAO inhibitor MPC (100 mg/kg i.p.). Data are the mean ± SEM (n = 5–7). *p<0.05, **p<0.01, ***p<0.001 (one-way Anova, followed by Bonferroni‘s post-hoc test).
In the same animals, QUIN levels also increased after D-TRP administration, and the effect reached statistical significance in liver (p<0.05), plasma (p<0.01) and forebrain (p<0.05) (Fig. 7). Pre-treatment with MPC reduced QUIN levels in all tissues, but the effect reached statistical difference only in the plasma (p<0.05 vs. D-TRP; Fig. 7b). After MPC injection, QUIN levels were not significantly different from endogenous values.
Discussion
The present study was designed to provide a comprehensive view of the fate of D-TRP following its systemic administration under physiological conditions, and, specifically, to investigate the role of D-AAO in the metabolism of D-TRP in vivo. Based on the measurement of D-KYN in the brain at several time points after an i.p. injection, we limited our experiments to mice that were euthanized after 90 min. Using an analytical method that allowed us to identify D-TRP and L-TRP in the same run with a detection limit of 1 pmol, we first verified the unidirectional conversion of D-TRP to L-TRP. Next, we demonstrated the de novo production of D-KYN from D-TRP in plasma, liver, forebrain and cerebellum, observing that D-KYN levels were much higher in the periphery than in the brain. Finally, we studied the de novo production of KYNA, 3-HK and QUIN from D-TRP in all tissues. MPC, a specific inhibitor of D-AAO (Adage et al. 2008; Gong et al. 2011), was used to examine the involvement of D-AAO in all these metabolic processes.
Separation of the two TRP enantiomers with an improved chiral HPLC method (Yan & Row 2008) allowed us to reliably quantitate both D-TRP and L-TRP in plasma, liver, forebrain and cerebellum. In agreement with earlier studies in rats (Yoshihara et al. 2012, Iizuka et al. 2011), we demonstrated that D-TRP can penetrate the blood-brain barrier (BBB) in mice and is readily detectable in several tissues 90 min after its systemic administration. Notably, D-TRP levels in the cerebellum were significantly lower than in the forebrain, suggesting greater local degradation by D-AAO, which recognizes D-TRP as a substrate (Iizuka et al. 2011) and is abundant in the cerebellum compared to other brain regions (Horiike et al. 1994, Moreno et al. 1999, Verrall et al. 2007). As D-AAO catalyzes the conversion of D-TRP to L-TRP (Iizuka et al. 2011, Yoshihara et al. 2012, Rodden & Berg 1974), systemic D-TRP administration raised L-TRP levels in plasma, forebrain and cerebellum, and the increase was significantly more pronounced in the cerebellum than in the forebrain.
The role of D-AAO was also tested directly by pre-treating the animals with the enzyme inhibitor MPC 30 min prior to a systemic injection of D-TRP. As expected, MPC increased D-TRP levels and reduced the neosynthesis of L-TRP in the brain as well as in the periphery (cf. Fig. 1). However, as MPC crosses the BBB relatively poorly (Adage et al. 2008), we cannot rule out that some of the effects seen in the brain were influenced by peripheral D-AAO inhibition. Considering the ready transport of L-TRP through the BBB, L-TRP reduction in the brain following D-TRP + MPC treatment could therefore be a consequence of reduced plasma L-TRP levels.
No increase in hepatic L-TRP levels was observed after the administration of either D-TRP or L-TRP. Since the mouse liver lacks D-AAO (Konno et al. 1997), the increase in hepatic D-TRP content following MPC administration is likely a consequence of changes in plasma levels and enhanced transport of circulating D-TRP into the liver (see also below). As for L-TRP administration, it appears that L-TRP was rapidly metabolized by tryptophan-2,3-dioxygenase (TDO), which has long been known to be highly expressed in the liver (Knox & Mehler 1950).
In line with previous studies (Ishii et al. 2010, Kotake & Ito 1937, Langner & Berg 1955, Loh & Berg 1971b, Triebwasser et al. 1976), animals treated with D-TRP readily formed D-KYN. Since D-TRP is a very poor substrate of TDO (Batabyal & Yeh 2007, Watanabe et al. 1980), D-KYN production was probably catalyzed by indoleamine-2,3-dioxygenase (IDO), which recognizes both L-TRP and D-TRP (Shimizu et al. 1978, Tashiro et al. 1961). As no D-KYN was detected in mice injected with L-TRP (data not shown; cf. also Iizuka et al. 2011), conversion of L-KYN to D-KYN was ruled out. D-KYN levels after D-TRP administration were much higher in plasma and liver than in the brain and, interestingly, less than half of neosynthesized kynurenine in the brain – in contrast to the periphery – was recovered as D-KYN (Table 1). As the proportion of D-KYN, compared to total newly formed kynurenine, was even lower (<20%) in the cerebellum, these results suggest that brain uptake of D-KYN and/or synthesis of D-KYN from D-TRP in the brain is restricted. Pre-treatment with MPC decreased cerebral L-KYN levels (i.e. total kynurenine minus D-KYN) by blocking the conversion of D-TRP to L-TRP in the brain and the periphery. However, MPC pre-treatment does not completely prevent L-KYN production from D-TRP in the cerebellum, presumably because of low penetration of MPC into the brain (Adage et al., 2008).
In our study, we paid special attention to the possible role of D-TRP as a bioprecusor of KYNA, 3-HK and QUIN, three KP metabolites with distinct neuroactive properties. KYNA is an antagonist of the α7 nicotinic acetylcholine receptor (Hilmas et al. 2001) and the glycine co-agonist site of the N-methyl-D-aspartate (NMDA) receptor (Parsons et al. 1997), as well as an agonist of the aryl hydrocarbon receptor (DiNatale et al. 2010) and the G protein-coupled receptor GPR35 (Wang et al. 2006). Alone or jointly, these actions likely account for the neuromodulatory effects of endogenous KYNA in the mammalian brain and for its presumed role in the pathophysiology of several neurological and psychiatric diseases (Pocivavsek et al., in press). As illustrated in Fig. 1, KYNA neosynthesis from D-TRP may occur via two mechanisms, both of which require D-AAO. Thus, KYNA can be produced either via D-KYN and subsequent conversion by kynurenine aminotransferase (KAT) and D-AAO (Pérez-de la Cruz et al. 2012, Wang et al. 2012) or, more indirectly, from L-KYN that is derived from D-TRP via the aryl hydrocarbon receptor agonist indole-3-pyruvate (Nguyen et al. 2009) and L-TRP (Fig. 1). In the present study, KYNA levels in liver and plasma were significantly elevated in response to a systemic D-TRP injection, and the de novo formation of the metabolite was greatly reduced by pre-treatment with the D-AAO inhibitor MPC. As mouse liver does not contain D-AAO (Konno et al. 1997), the significant lowering of hepatic KYNA levels (Fig. 6a) following MPC administration was likely a consequence of changes in plasma levels and, subsequently, enhanced transport of circulating KYNA into the liver. A similar pattern was observed in the cerebellum and, to a lesser degree, in the forebrain (Fig. 6). The fact that KYNA crosses the BBB very poorly (Fukui et al. 1991) and that far more KYNA was recovered in the cerebellum than in the forebrain favors the idea that the metabolite originated locally from the direct pathway, i.e. via D-KYN. Notably, this process may also involve the irreversible transamination of D-KYN by KAT (Pérez-de la Cruz et al. 2012).
Both 3-HK, which can generate toxic free radicals (Eastman & Guilarte 1989), and QUIN, an excitotoxic NMDA receptor agonist (Stone & Perkins 1981), may play causative roles in neurodegenerative disorders (Schwarcz et al. 2012). D-TRP administration increased the levels of both neurotoxins in all tissues, in some cases substantially, but pre-treatment with MPC had only modest, and in most cases not statistically significant, effects on the neosynthesis of these two metabolites. In agreement with recent in vivo studies by Wang et al. (2012), who reported the formation of 3-HK (and KYNA) from systemically administered D-KYN, and Maeta et al. (2014), who described the conversion of D-TRP to nicotinamide, these data demonstrate that D-TRP degradation in vivo causes the formation of KP metabolites along the QUIN branch of the pathway. Moreover, our results show that the importance of D-AAO in the formation of downstream metabolites begins to fade as D-AAO-dependent processes are not directly involved in the biosynthesis (cf. Fig. 1). Future experiments will need to elaborate the intricacies of these metabolic events in greater depth by establishing detailed time- and dose-relationships and by comparing the sequelae of acute and chronic D-TRP administration. Pharmacological agents targeting downstream KP enzymes, as well as the use of enantioselective metabolites such as L-3-HK and D-3-HK, too, can be expected to provide valuable new insights in this context (Hankes et al. 1966, Schwarcz & Pellicciari 2002, Tanizawa & Soda 1979, Wang et al. 2012, Vécsei et al. 2013).
The present study may have implications for both physiology and pathology. Thus, D-TRP is present in plants (Zenk & Scherf 1963) and occurs also as a constituent of a bioactive tripeptide (Jimenez et al. 1996). Although not verified experimentally so far, D-TRP in eukaryotic systems likely derives mainly from microorganisms, which can neosynthesize the enantiomer for incorporation into the cell wall (Kolodkin-Gal et al. 2010, Lam et al. 2009, Lupoli et al. 2011). Notably, the gut microbiota produces a range of small molecules which have distinct targets in the host and, more specifically, participate actively in the communication between the enteric nervous system and the brain (Borre et al. 2014, Donia & Fischbach 2015). Indeed, the small amounts of D-TRP detected in humans might originate from microorganisms that populate the digestive tract (Armstrong et al. 1993, Hatanaka et al. 2002, Visser et al. 2011, Zhao & Liu 2001). Of special relevance for pathology, D-TRP production in mammals may therefore be enhanced during bacterial infections.
It follows that D-TRP and D-KYN may be at least in part responsible for the increases in brain KYNA, 3-HK and/or QUIN seen in human brain diseases such as depression, schizophrenia and autism, which have been plausibly linked to genetic or environmental immunogenic risk factors (Brown & Derkits 2010, Mandi & Vécsei 2012, Raison et al. 2010, Schwarcz et al. 2001). Of interest in this context, D-TRP can modulate stress-induced phenomena, which are often associated with psychiatric manifestations (Yoshida et al. 2015). As elaborated here, all these pathological events may be further exacerbated in individuals with a genetic defect leading to increased D-AAO activity, which is believed to enhance vulnerability to a variety of psychiatric diseases (Burnet et al. 2008, Madeira et al. 2008, Prata et al. 2008, Verrall et al. 2007).
To conclude with a specific example of the possible ramifications of the present study for a major psychiatric disease, the preferential D-TRP-induced increase in cerebellar KYNA levels (cf. Figs. 6c and d) may play a distinct role in persons with schizophrenia, who show elevated KYNA levels in brain and cerebrospinal fluid (Erhardt et al. 2001, Schwarcz et al. 2001) as well as increased brain D-AAO activity (Burnet et al. 2008, Madeira et al. 2008, Verrall et al. 2007). Thus, even a relatively small increase in cerebellar KYNA levels raises extracellular glutamate and dopamine levels in the distant medial prefrontal cortex (Wu and Schwarcz, 2013) and may therefore account for the dysfunctional connectivity between cerebellum and medial prefrontal cortex, which has been proposed to be critically involved in the pathophysiology of the disease (Andreasen and Pierson, 2011). This idea and other novel hypotheses linking D-TRP to major human brain diseases are currently being tested in our laboratory.
Acknowledgments
This study was supported in part by NIH grant P50 MH10322.
Abbreviations
- D-AAO
D-Amino acid oxidase
- 3-HK
3-Hydroxykynurenine
- GC/MS
Gas chromatography/mass spectrometry
- HPLC
High performance liquid chromatography
- KYNA
Kynurenic acid
- KP
Kynurenine pathway
- MPC
3-Methylpyrazole-5-carboxylic acid
- QUIN
Quinolinic acid
- TRP
Tryptophan
Footnotes
Conflicts of interest: none
References
- Adage T, Trillat AC, Quattropani A, et al. In vitro and in vivo pharmacological profile of AS057278, a selective d-amino acid oxidase inhibitor with potential anti-psychotic properties. European neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology. 2008;18:200–214. doi: 10.1016/j.euroneuro.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Pierson A. The role of the cerebellum in schizophrenia. Biol Psychiatry. 2008;64:81–88. doi: 10.1016/j.biopsych.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong DW, Gasper M, Lee SH, Zukowski J, Ercal N. D-amino acid levels in human physiological fluids. Chirality. 1993;5:375–378. doi: 10.1002/chir.530050519. [DOI] [PubMed] [Google Scholar]
- Batabyal D, Yeh SR. Human tryptophan dioxygenase: a comparison to indoleamine 2,3-dioxygenase. J Am Chem Soc. 2007;129:15690–15701. doi: 10.1021/ja076186k. [DOI] [PubMed] [Google Scholar]
- Bittinger MA, Nguyen LP, Bradfield CA. Aspartate aminotransferase generates proagonists of the aryl hydrocarbon receptor. Molecular pharmacology. 2003;64:550–556. doi: 10.1124/mol.64.3.550. [DOI] [PubMed] [Google Scholar]
- Blanco Ayala T, Lugo Huitron R, Carmona Aparicio L, et al. Alternative kynurenic acid synthesis routes studied in the rat cerebellum. Frontiers in cellular neuroscience. 2015;9:178. doi: 10.3389/fncel.2015.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borre YE, O’Keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF. Microbiota and neurodevelopmental windows: implications for brain disorders. Trends in molecular medicine. 2014;20:509–518. doi: 10.1016/j.molmed.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167:261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnet PW, Eastwood SL, Bristow GC, Godlewska BR, Sikka P, Walker M, Harrison PJ. D-amino acid oxidase activity and expression are increased in schizophrenia. Mol Psychiatry. 2008;13:658–660. doi: 10.1038/mp.2008.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNatale BC, Murray IA, Schroeder JC, Flaveny CA, Lahoti TS, Laurenzana EM, Omiecinski CJ, Perdew GH. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicological sciences: an official journal of the Society of Toxicology. 2010;115:89–97. doi: 10.1093/toxsci/kfq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donia MS, Fischbach MA. HUMAN MICROBIOTA. Small molecules from the human microbiota. Science. 2015;349:1254766. doi: 10.1126/science.1254766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman CL, Guilarte TR. Cytotoxicity of 3-hydroxykynurenine in a neuronal hybrid cell line. Brain Res. 1989;495:225–231. doi: 10.1016/0006-8993(89)90216-3. [DOI] [PubMed] [Google Scholar]
- Erhardt S, Blennow K, Nordin C, Skogh E, Lindström LH, Engberg G. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci Lett. 2001;313:96–98. doi: 10.1016/s0304-3940(01)02242-x. [DOI] [PubMed] [Google Scholar]
- Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J Neurochem. 1991;56:2007–2017. doi: 10.1111/j.1471-4159.1991.tb03460.x. [DOI] [PubMed] [Google Scholar]
- Fukushima T, Sone Y, Mitsuhashi S, Tomiya M, Toyo’oka T. Alteration of kynurenic acid concentration in rat plasma following optically pure kynurenine administration: a comparative study between enantiomers. Chirality. 2009;21:468–472. doi: 10.1002/chir.20620. [DOI] [PubMed] [Google Scholar]
- Gong N, Gao ZY, Wang Y-C, Li X-Y, Huang J-L, Hashimoto K, Wang YX. A series of D-amino acid oxidase inhibitors specifically prevents and reverses formalin induced tonic pain in rats. J Pharmacol Exp Ther. 2011;336:282–293. doi: 10.1124/jpet.110.172353. [DOI] [PubMed] [Google Scholar]
- Hankes LV, Brown RR, Schmaeler M. Metabolism of isomers of 3-hydroxykynurenine-C14 to quinolinic acid, niacin metabolites and carbon dioxide. Proc Soc Exp Biol Med. 1966;121:253–259. doi: 10.3181/00379727-121-30750. [DOI] [PubMed] [Google Scholar]
- Hatanaka T, Huang W, Nakanishi T, Bridges CC, Smith SB, Prasad PD, Ganapathy ME, Ganapathy V. Transport of D-serine via the amino acid transporter ATB(0,+) expressed in the colon. Biochem Biophys Res Commun. 2002;291:291–295. doi: 10.1006/bbrc.2002.6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi K, Hayaishi O. Enzymic formation of D-kynurenine from D-tryptophan. Arch Biochem Biophys. 1967;120:397–403. doi: 10.1016/0003-9861(67)90256-1. [DOI] [PubMed] [Google Scholar]
- Hilmas C, Pereira EF, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. J Neurosci. 2001;21:7463–7473. doi: 10.1523/JNEUROSCI.21-19-07463.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiike K, Tojo H, Arai R, Nozaki M, Maeda T. D-amino-acid oxidase is confined to the lower brain stem and cerebellum in rat brain: regional differentiation of astrocytes. Brain Res. 1994;652:297–303. doi: 10.1016/0006-8993(94)90240-2. [DOI] [PubMed] [Google Scholar]
- Iizuka H, Ishii K, Hirasa Y, Kubo K, Fukushima T. Fluorescence determination of D- and L-tryptophan concentrations in rat plasma following administration of tryptophan enantiomers using HPLC with pre-column derivatization. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:3208–3213. doi: 10.1016/j.jchromb.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Ishii K, Iizuka H, Ogaya T, Song Z, Fukushima T. Comparative study on kynurenic acid production in the rat striatum by tryptophan enantiomers: an in vivo microdialysis study. Chirality. 2011;23(Suppl 1):E12–15. doi: 10.1002/chir.20938. [DOI] [PubMed] [Google Scholar]
- Ishii K, Ogaya T, Song Z, Iizuka H, Fukushima T. Changes in the plasma concentrations of D-kynurenine and kynurenic acid in rats after intraperitoneal administration of tryptophan enantiomers. Chirality. 2010;22:901–906. doi: 10.1002/chir.20850. [DOI] [PubMed] [Google Scholar]
- Jimenez EC, Olivera BM, Gray WR, Cruz LJ. Contryphan is a D-tryptophan-containing Conus peptide. J Biol Chem. 1996;271:28002–28005. doi: 10.1074/jbc.271.45.28002. [DOI] [PubMed] [Google Scholar]
- Knox WE, Mehler AH. The conversion of tryptophan to kynurenine in liver. I. The coupled tryptophan peroxidase-oxidase system forming formylkynurenine. J Biol Chem. 1950;187:419–430. [PubMed] [Google Scholar]
- Kolodkin-Gal I, Romero D, Cao S, Clardy J, Kolter R, Losick R. D-amino acids trigger biofilm disassembly. Science. 2010;328:627–629. doi: 10.1126/science.1188628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konno R, Sasaki M, Asakura S, Fukui K, Enami J, Niwa A. D-amino-acid oxidase is not present in the mouse liver. Biochim Biophys Acta. 1997;1335:173–181. doi: 10.1016/s0304-4165(96)00136-5. [DOI] [PubMed] [Google Scholar]
- Kotake JY, Ito N. Studien über den intermediären Stoffwechsel des Tryptophans. XXV. Isolierung des d-Kynurenins. J Biochem. 1937;25:71–77. [Google Scholar]
- Lam H, Oh DC, Cava F, Takacs CN, Clardy J, de Pedro MA, Waldor MK. D-amino acids govern stationary phase cell wall remodeling in bacteria. Science. 2009;325:1552–1555. doi: 10.1126/science.1178123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner RR, Berg CP. Metabolism of D-tryptophan in the normal human subject. J Biol Chem. 1955;214:699–707. [PubMed] [Google Scholar]
- Loh HH, Berg CP. Inversion in the metabolism of D-tryptophan in the rabbit and the rat. J Nutr. 1971a;101:1351–1358. doi: 10.1093/jn/101.10.1351. [DOI] [PubMed] [Google Scholar]
- Loh HH, Berg CP. Production of D-kynurenine and other metabolites from D-tryptophan by the intact rabbit and by rabbit tissue. J Nutr. 1971b;101:465–475. doi: 10.1093/jn/101.4.465. [DOI] [PubMed] [Google Scholar]
- Lupoli TJ, Tsukamoto H, Doud EH, Wang TS, Walker S, Kahne D. Transpeptidase-mediated incorporation of D-amino acids into bacterial peptidoglycan. J Am Chem Soc. 2011;133:10748–10751. doi: 10.1021/ja2040656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira C, Freitas ME, Vargas-Lopes C, Wolosker H, Panizzutti R. Increased brain D-amino acid oxidase (DAAO) activity in schizophrenia. Schizophr Res. 2008;101:76–83. doi: 10.1016/j.schres.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Maeta A, Sano M, Fukuwatari T, Funakoshi H, Nakamura T, Shibata K. Contributions of tryptophan 2,3-dioxygenase and indoleamine 2,3-dioxygenase to the conversion of D-tryptophan to nicotinamide analyzed by using tryptophan 2,3-dioxygenase-knockout mice. Biosci Biotechnol Biochem. 2014;78:878–881. doi: 10.1080/09168451.2014.905185. [DOI] [PubMed] [Google Scholar]
- Mandi Y, Vécsei L. The kynurenine system and immunoregulation. Journal of neural transmission. 2012;119:197–209. doi: 10.1007/s00702-011-0681-y. [DOI] [PubMed] [Google Scholar]
- Moreno S, Nardacci R, Cimini A, Ceru MP. Immunocytochemical localization of D-amino acid oxidase in rat brain. J Neurocytol. 1999;28:169–185. doi: 10.1023/a:1007064504007. [DOI] [PubMed] [Google Scholar]
- Nguyen LP, Hsu EL, Chowdhury G, Dostalek M, Guengerich FP, Bradfield CA. D-amino acid oxidase generates agonists of the aryl hydrocarbon receptor from D-tryptophan. Chemical research in toxicology. 2009;22:1897–1904. doi: 10.1021/tx900043s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notarangelo FM, Wu HQ, Macherone A, Graham DR, Schwarcz R. Gas chromatography/tandem mass spectrometry detection of extracellular kynurenine and related metabolites in normal and lesioned rat brain. Anal Biochem. 2012;421:573–581. doi: 10.1016/j.ab.2011.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogaya T, Song Z, Ishii K, Fukushima T. Changes in extracellular kynurenic acid concentrations in rat prefrontal cortex after D-kynurenine infusion: an in vivo microdialysis study. Neurochem Res. 2010;35:559–563. doi: 10.1007/s11064-009-0099-1. [DOI] [PubMed] [Google Scholar]
- Ohara I, Otsuka SI, Yugari Y, Ariyoshi S. Inversion of D-tryptophan to L-tryptophan and excretory patterns in the rat and chick. J Nutr. 1980;110:641–648. doi: 10.1093/jn/110.4.641. [DOI] [PubMed] [Google Scholar]
- Parsons CG, Danysz W, Quack G, et al. Novel systemically active antagonists of the glycine site of the N-methyl-D-aspartate receptor: electrophysiological, biochemical and behavioral characterization. J Pharmacol Exp Ther. 1997;283:1264–1275. [PubMed] [Google Scholar]
- Patterson PH. Maternal infection and immune involvement in autism. Trends in molecular medicine. 2011;17:389–394. doi: 10.1016/j.molmed.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-de la Cruz V, Amori L, Sathyasaikumar KV, Wang XD, Notarangelo FM, Wu HQ, Schwarcz R. Enzymatic transamination of D-kynurenine generates kynurenic acid in rat and human brain. J Neurochem. 2012;120:1026–1035. doi: 10.1111/j.1471-4159.2012.07653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocivavsek A, Notarangelo FM, Wu HQ, Bruno JP, Schwarcz R. Astrocytes as pharmacological targets in the treatment of schizophrenia: focus on kynurenic acid. In: Pletnikov M, Waddington J, editors. Modeling the Psychopathological Dimension of Schizophrenia. Elsevier; 2015. in press. [Google Scholar]
- Prata D, Breen G, Osborne S, Munro J, St Clair D, Collier D. Association of DAO and G72(DAOA)/G30 genes with bipolar affective disorder. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:914–917. doi: 10.1002/ajmg.b.30682. [DOI] [PubMed] [Google Scholar]
- Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G, Spivey JR, Saito K, Miller AH. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol Psychiatry. 2010;15:393–403. doi: 10.1038/mp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodden FA, Berg CP. Enzymatic conversion of L- and D-tryptophan to kynurenine by rat liver. J Nutr. 1974;104:227–238. doi: 10.1093/jn/104.2.227. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. 2012;13:465–477. doi: 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R, Pellicciari R. Manipulation of brain kynurenines: glial targets, neuronal effects, and clinical opportunities. J Pharmacol Exp Ther. 2002;303:1–10. doi: 10.1124/jpet.102.034439. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Rassoulpour A, Wu HQ, Medoff D, Tamminga CA, Roberts RC. Increased cortical kynurenate content in schizophrenia. Biol Psychiatry. 2001;50:521–530. doi: 10.1016/s0006-3223(01)01078-2. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Nomiyama S, Hirata F, Hayaishi O. Indoleamine 2,3-dioxygenase. Purification and some properties. J Biol Chem. 1978;253:4700–4706. [PubMed] [Google Scholar]
- Stone TW, Perkins MN. Quinolinic acid: a potent endogenous excitant at amino acid receptors in CNS. Eur J Pharmacol. 1981;72:411–412. doi: 10.1016/0014-2999(81)90587-2. [DOI] [PubMed] [Google Scholar]
- Sylianco CY, Berg CP. The effect of riboflavin deficiency upon the metabolism of tryptophan by liver and kidney tissue. J Biol Chem. 1959;234:912–917. [PubMed] [Google Scholar]
- Tanizawa K, Soda K. Purification and properties of pig liver kynureninase. J Biochem. 1979;85:901–906. doi: 10.1093/oxfordjournals.jbchem.a132421. [DOI] [PubMed] [Google Scholar]
- Tashiro M, Tsukada K, Kobayashi S, Hayaishi O. A new pathway of D-tryptophan metabolism: enzymic formation of kynurenic acid via D-kynurenine. Biochem Biophys Res Commun. 1961;6:155–160. doi: 10.1016/0006-291x(61)90120-6. [DOI] [PubMed] [Google Scholar]
- Triebwasser KC, Swan PB, Henderson LM, Budny JA. Metabolism of D- and L-tryptophan in dogs. J Nutr. 1976;106:642–652. doi: 10.1093/jn/106.5.642. [DOI] [PubMed] [Google Scholar]
- Vécsei L, Szalardy L, Fülop F, Toldi J. Kynurenines in the CNS: recent advances and new questions. Nature reviews Drug discovery. 2013;12:64–82. doi: 10.1038/nrd3793. [DOI] [PubMed] [Google Scholar]
- Verrall L, Walker M, Rawlings N, Benzel I, Kew JN, Harrison PJ, Burnet PW. d-Amino acid oxidase and serine racemase in human brain: normal distribution and altered expression in schizophrenia. Eur J Neurosci. 2007;26:1657–1669. doi: 10.1111/j.1460-9568.2007.05769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser WF, Verhoeven-Duif NM, Ophoff R, Bakker S, Klomp LW, Berger R, de Koning TJ. A sensitive and simple ultra-high-performance-liquid chromatography-tandem mass spectrometry based method for the quantification of D-amino acids in body fluids. J Chromatogr A. 2011;1218:7130–7136. doi: 10.1016/j.chroma.2011.07.087. [DOI] [PubMed] [Google Scholar]
- Wang J, Simonavicius N, Wu X, Swaminath G, Reagan J, Tian H, Ling L. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J Biol Chem. 2006;281:22021–22028. doi: 10.1074/jbc.M603503200. [DOI] [PubMed] [Google Scholar]
- Wang XD, Horning KJ, Notarangelo FM, Schwarcz R. A method for the determination of D-kynurenine in biological tissues. Analytical and bioanalytical chemistry. 2013;405:9747–9754. doi: 10.1007/s00216-013-7399-7. [DOI] [PubMed] [Google Scholar]
- Wang XD, Notarangelo FM, Wang JZ, Schwarcz R. Kynurenic acid and 3-hydroxykynurenine production from D-kynurenine in mice. Brain Res. 2012;1455:1–9. doi: 10.1016/j.brainres.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Fujiwara M, Yoshida R, Hayaishi O. Stereospecificity of hepatic L-tryptophan 2,3-dioxygenase. Biochem J. 1980;189:393–405. doi: 10.1042/bj1890393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HQ, Schwarcz R. Modulation of glutamate and dopamine levels in the prefrontal cortex by intracerebellar kynurenic acid infusion in the rat. Society for Neuroscience Abstracts. 2013;38:231.03. [Google Scholar]
- Yan H, Row KH. Enantioseparation condition of D,L-tryptophan using ligand exchange chromatography. Indian Journal of Chemistry. 2008;47A:75–80. [Google Scholar]
- Yoshida J, Erwan E, Chowdhury VS, Ogino Y, Shigemura A, Denbow DM, Furuse M. Comparison of centrally injected tryptophan-related substances inducing sedation in acute isolation stress-induced neonatal chicks. Pharmacol Biochem Behav. 2015;129:1–6. doi: 10.1016/j.pbb.2014.11.016. [DOI] [PubMed] [Google Scholar]
- Yoshihara S, Otani H, Tsunoda M, Ishii K, Iizuka H, Ichiba H, Fukushima T. Alterations in extracellular tryptophan and dopamine concentrations in rat striatum following peripheral administration of D- and L-tryptophan: an in vivo microdialysis study. Neurosci Lett. 2012;526:74–78. doi: 10.1016/j.neulet.2012.07.046. [DOI] [PubMed] [Google Scholar]
- Zenk MH, Scherf H. D-Tryptophan in höheren Pflanzen. Biochim Biophys Acta. 1963;71:737–738. [Google Scholar]
- Zhao S, Liu YM. Electrophoretic separation of tryptophan enantiomers in biological samples. Electrophoresis. 2001;22:2769–2774. doi: 10.1002/1522-2683(200108)22:13<2769::AID-ELPS2769>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]