Abstract
Wasp venom characterization is of interest across multiple disciplines such as medicinal chemistry and evolutionary biology. A simple method is described herein to milk wasp venom without undue risks to the researcher. The wasps were immobilized by cooling for safe handling, restrained, and their venom was collected on parafilm. Bradykinin from Hemipepsis ustulata was identified by LC-MS/MS during method verification.
Keywords: Wasp, bradykinin, venom, milking, Hemipepsis ustulata, LC-MS/MS
Wasp venoms have been affected by evolutionary processes based on their changing molecular targets and contain a variety of biologically active substances (Lee et al., 2016) that help define metazoan taxa. In particular, solitary wasps contain neurotoxins such as bradykinins and pompilidotoxins that attack sodium channels, as well as a myriad of other diverse functioning compounds (Palma, 2006). Exploration of venom across families is important for biomedical research (lead drug candidates), although only a few species of solitary wasps have been studied (Konno et al., 2002; Baek et al., 2015). Methods of venom collection for social Hymenopterans (Benton et al.,1963) cannot be applied to solitary wasps. This short communication focuses on a novel milking technique suitable for large-sized, solitary wasps.
The method was tested on three individuals of Hemipepsis ustulata-large wasps (up to 5 cm) with rust colored wings and iridescent blue bodies. Hemipepsis ustulata are ectoparasites that use paralyzed spiders as a food source for their young. These wasps are not aggressive, in general, but their sting has a fierce reputation (Schmidt, 2004) causing extreme, but short-lived pain. Although they are distributed worldwide, they are particularly well known in the southwest U.S., where they enjoy the status of being the state insect of New Mexico.
The safest method for venom collection involves killing the insect and removing the venom sac (Piek, 1986). This method has the disadvantage of producing a more complex sample than that acquired from an external secretion. Three other methods in the literature describe external venom collection, but all of these unnecessarily subject the researcher to the risk of being stung. For instance, one method has the researcher using a piece of tape on their finger to try to immobilize the active wasp by contact with its wings and then crushing the head with a pin (Deyrup and Matthews, 2003). This method also carries the limitation of a one-time milking. Another method suggests small wasps, such as jewel wasps (Ampulex compressa), can be milked by forcing them stinger first into a narrow tube with a hole at the end and then confined with a piston that serves also as a source of blunt trauma to induce venom secretion (Mukhopadhyay, 2014). Other researchers suggest picking up the wasp between the thumb and forefinger (Sahayaraj et al., 2006). Most recently, an agar block-based method has been published (Bhagavathula, et al., 2016), but collecting the venom in agar is unnecessary and introduces an extra extraction step. The novel method described here uses a reduced-temperature incubation to induce a state of immobilization to limit the exposure of the researcher to being stung. Parafilm is used to receive the venom droplets.
Specimens were collected in Grant County, NM in the City of Rocks State Park and from the campus of the Chiricahua Desert Museum in Rodeo, NM. The motor function of the wasps was reduced by placing the cage in a refrigerator at 4 °C. The wasp was checked on every 2 minutes until immobilized and then removed from the refrigerator. At this point, the wasp was handled with tongs and its wings clipped (the wing venation was preserved for identification purposes), an operation which facilitates ease of handling after the wasp reinvigorates. Narrow strips of duct tape were used to secure the wasp in a supine position on a suitably-sized tube. The platform was securely taped down to the work surface to avoid the unexpected movement of the stinger during milking. The wasp was ready for milking after 5-10 min.
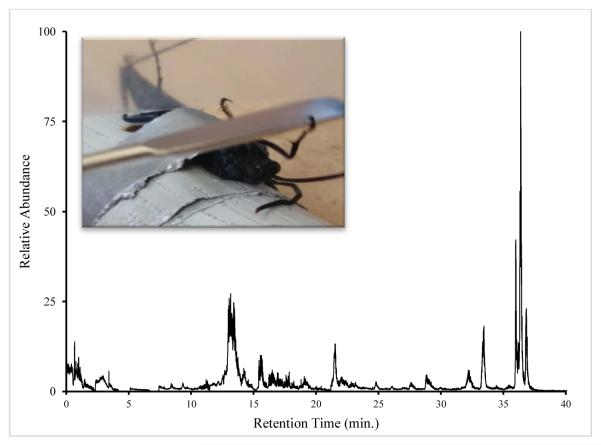
At this point, a piece of parafilm (4×6 cm) was held over the end of the abdomen to collect venom while the head of the wasp was pressed on without crushing to induce secretion (Fig.1). At this point, the stinger (up to 6 mm long) was easily observed and the parafilm was moved in contact with it. The stinger punctured the parafilm and deposited venom on the top side. The droplets were easily observed and were collected rapidly using a micropipetter and a small amount of water (5-10 μL) to help dissolve the venom. After milking, the wasp was immobilized again using the refrigeration protocol. The tape was then carefully removed and the wasp returned to its cage. Cut grapes and wet paper towels were provided to sustain the wasps during captivity. This process was repeated for 2-4 days before the cumulative effect of the procedure became fatal.
Figure 1.
Total ion chromatogram for Hemipepsis ustulata venom after trypsinolysis. Wasps were restrained in a supine position and agitated to sting parafilm.
The droplets secreted by Hemipepsis were around 1 μL with about 4-8 droplets able to be collected in a single session. Venom samples were purified and preconcentrated with C-18 spin columns, lyophilized, and stored frozen until the time of analysis. Thawed venom was redissolved in 20 μL of 0.1 % formic acid solution and a trypsin digestion was performed to reduce coelution issues with proteins. A sample injection of 2 μL was then analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) on a Q Exactive mass spectrometer with an Easy NanoLC-1000 system (Thermo Fisher Scientific, U.S.A). Separation was conducted using a C18 column (75 μm x 150 mm). Reverse phase chromatography was performed with (A) 0.1 % formic acid/water and (B) 0.1 % formic acid/acetonitrile. A four-step, linear gradient was used for the LC separation (column pre-equilibration with 2% B for 10 min; 2% to 30% B in the first 47 min.; followed by 80% B for 13 min.). Bradykinin was targeted in the data sets since bradykinin and related compounds have been found in other solitary wasp species (Konno et al., 2002; Picolo et al., 2010).
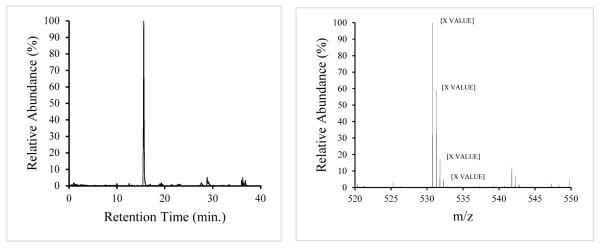
These samples were concentrated enough to generate a large number of peaks in the main chromatogram (Fig. 1). A mass range filter from 530.77-530.79 amu was applied to the total ion chromatogram (Fig. 2a) to search for bradykinin (RPPGFSPFR), resulting in a strong peak at retention time 15.6 min. A match for the parent ion (MH 2+2) at monoisotopic mass 530.787 amu was found (Fig. 2b) as the base peak in the mass spectrum (the online resource from University of California San Francisco, Protein Prospector, was used to calculate the theoretical value of the bradykinin peak at 530.788 amu). The doubly-charged state was deduced from the 0.5 amu spacing of the envelope.
Figure 2.
a Selected ion chromatogram for bradykinin at mass 530.79 (±0.5) amu.
b. Mass spectrum for retention time 15.6 min. (bradykinin) showing the isotopic envelope for the doubly-charged, parent MH 2+2 peak.
This paper presents a novel, effective (validated by LC-MS/MS) approach for milking large wasps safely without the need for an agar block receiver. In addition, this method can provide multiple samples from a single individual over time.
Supplementary Material
Highlights.
A novel method of venom collection from wasps is presented
The method is safe and can be used multiple times on a single individual
Bradykinin is identified in Hemipepsis ustulata by LC-MS/MS
Acknowledgements
This work was supported by funding from NSF grant # IOS-1146875 (PI: Manda Clair Jost) and the New Mexico Idea Networks for Biomedical Research Excellence (NM-INBRE), NIH Prime Award No.: 2P20GM103451-14. The authors would also like to thank Dr. Sucharita Dutta (Eastern Virginia Medical School), Dr. Bill Toth and the Natural Sciences Department at WNMU. We are grateful to Justin Lewis, James Sanders, and John Briggs for assistance in capturing wasp specimens and to Diana Sanchez and Kay Lindsey for their assistance in milking wasps.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baek JH, Lee SH, Kim WY, Kim MG. An insulin-binding protein from the venom of a solitary wasp Eumenes pomiformis binds to apolipophorin III in lepidopteran hemolymph. Toxicon. 2015;111:62–64. doi: 10.1016/j.toxicon.2015.12.019. [DOI] [PubMed] [Google Scholar]
- Benton AW, Morse RA, Stewart JD. Venom collection from honey bees. Science. 1963;142:228–230. doi: 10.1126/science.142.3589.228. [DOI] [PubMed] [Google Scholar]
- Bhagavathula NC, Kumar M, Krishnappa C. A simple non-invasive technique for venom milking from a solitary wasp Delta conoideum Gmelin (Hymenoptera: Vespidae) Toxicon. 2016;109:4–6. doi: 10.1016/j.toxicon.2015.10.023. [DOI] [PubMed] [Google Scholar]
- Deyrup LD, Matthews RW. A simple technique for milking the venom of a small parasitic wasp, Melittobia digitata (Hymenoptera: Eulophidae) Toxicon. 2003;42:217–218. doi: 10.1016/s0041-0101(03)00132-6. [DOI] [PubMed] [Google Scholar]
- Konno K, Palma MS, Hitara IY, Juliano MA, Juliano L, Yasuhara T. Identification of bradykinins in solitary wasp venoms. Toxicon. 2002;40:309–312. doi: 10.1016/s0041-0101(01)00230-6. [DOI] [PubMed] [Google Scholar]
- Lee SH, Baek JH, Yoon KA. Differential properties of venom peptides and proteins in solitary vs. social hunting wasps. Toxins. 2016;8:1–29. doi: 10.3390/toxins8020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay R. Under the spell of the cockroach hunter. ASBMB today. 2014;13:32–39. [Google Scholar]
- Palma MS. Insect Venom Peptides. In: Kastin AJ, editor. Handbook of Biologically Active Peptides. first ed Elsevier; London: 2006. pp. 409–416. [Google Scholar]
- Picolo G, Hisada M, Moura AB, Machado MF, Sciani JM, Conceicao IM, Melo RL, Oliveira V, Lima-Landman MT, Cury Y, Kono K, Hayashi MA. Bradykinin-related peptides in the venom of the solitary wasp Cyphononyx fulvognathus. Biochem. Pharmacol. 2010;79:478–486. doi: 10.1016/j.bcp.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Piek T. Methods for the collection of venoms. In: Piek T, editor. Venoms of the Hymenoptera. first ed Academic Press; London: 1986. pp. 45–61. [Google Scholar]
- Sahayaraj K, Kumar SM, Arandh GP. Evaluation of milking and electric shock methods for venom collection from hunter reduviids. Entomon. 2006;31:65–68. [Google Scholar]
- Schmidt JO. Venom and the good life in tarantula hawks (Hymenoptera: Pompilidae): how to eat, not be eaten, and live long. J. Kansas Entomol. Soc. 2004;77:402–413. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.