Abstract
Small interfering RNA (siRNA) is a promising drug candidate, expected to have broad therapeutic potentials toward various diseases including viral infections and cancer. With recent advances in bioconjugate chemistry and carrier technology, several siRNA-based drugs have advanced to clinical trials. However, most cases address local applications or diseases in the filtering organs, reflecting remaining challenges in systemic delivery of siRNA. The difficulty in siRNA delivery is in large part due to poor circulation stability and unfavorable pharmacokinetics and biodistribution profiles of siRNA. This review describes the pharmacokinetics and biodistribution of siRNA nanomedicines, focusing on those reported in the past 5 years, and their pharmacological effects in selected disease models such as hepatocellular carcinoma, liver infections, and respiratory diseases. The examples discussed here will provide an insight into the current status of the art and unmet needs in siRNA delivery.
Keywords: RNA interference, small interfering RNA, delivery, pharmacokinetics, biodistribution
Graphical abstract
1. Introduction
RNA interference (RNAi) is an endogenous post-transcriptional regulation process, which involves small regulatory RNAs such as small interfering RNAs (siRNAs) or microRNA (miRNAs) that silence target messenger RNAs in a sequence-specific manner. Ever since the discovery of RNAi in C. elegans [1] and the demonstration of siRNA activity in mammalian cells [2], RNAi has gained significant attention as a potential therapeutic for various diseases including viral infections and cancer, especially for those lacking ‘druggable’ targets. The efforts to develop siRNA therapeutics resulted in the first trial in human [3] in less than a decade since the discovery. However, realizing the clinical potential of siRNA therapeutics has found to be a daunting task, in large part due to the unfavorable pharmacokinetics (PK) and biodistribution (BD) profiles of systemically administered siRNA [4–6]. This challenge has been tackled in various ways, including chemical modification of siRNAs and/or the use of nanoparticulate delivery systems based on lipids, polymers, and inorganic platforms, which aim to protect the siRNAs from serum proteins and renal clearance and help cross target cell membranes. These approaches have improved the bioavailability of siRNA and enabled at least 30 siRNA-based drugs to enter clinical trials [7]. Nevertheless, 70% of them address ocular diseases, where siRNA is administered locally, or target the liver, lung, or kidney, the filtering organs the formulations are naturally captured, which indicates that the systemic delivery of RNAi therapeutics to other organs remains a critical challenge. To overcome this challenge and translate the broad potential of RNAi-therapeutics to clinical benefits, it is important to understand the level of PK and BD control achieved by current delivery approaches. The purpose of this review is to provide an overview the current status of the art in siRNA delivery with respect to the effects of carriers on PK, BD, and pharmacological effects of systemically administered siRNA. Due to the large volume of literature, we mainly discuss the studies published in past 5 years.
2. Therapeutic applications of siRNA and target genes
2.1 Ocular diseases
RNAi have been found effective in the treatment of ocular diseases [8]. In early studies, local injection of siRNA targeting vascular endothelial growth factor (VEGF) was shown to reduce neovascularization in several animal models of eye injuries, such as laser-induced photocoagulation [9], suture-induced corneal angiogenesis [10], and CpG oligodeoxynucleotide-or herpes simplex virus-induced neovascularization [11]. Neovascularization is a critical pathological event in age-related macular degeneration (AMD) [12] and diabetic retinopathy [13, 14]. Therefore, siRNAs suppressing the expression of VEGF, receptors, and/or its regulation have been explored as a potential therapy and tested in human for the treatment of AMD (Bevasiranib silencing VEGF) [15] and diabetic macular edema (PF-04523655 silencing hypoxia inducible gene) [16].
Fibrotic eye diseases are significant complications of eye surgeries. Transforming growth factor β (TGF-β) is identified as a main culprit of postoperative ocular scarring; thus, siRNA targeting TGF-β or its receptor is used to inhibit fibrotic responses to wounding. For example, siRNA targeting type II receptor of TGF-β was shown to reduce inflammatory responses and collagen deposition in a mouse model of subconjunctival inflammation and fibrosis [17]. Similarly, siRNA-mediated downregulation of IκB kinase beta (IKKβ), an activator of NF-κB-mediated inflammation and cell proliferation, reduced subconjunctival scarring in a monkey model of glaucoma filtration surgery [18]. siRNA is also pursued for glaucoma therapy. siRNA targeting β2-adrenoceptors (SYL040012) was shown to reduce the expression of β2 adrenergic receptor and the production of aqueous humor, thereby reducing intraocular pressure [19].
Based on promising preclinical results, several siRNA therapeutics entered clinical trials for ocular disease therapy [8, 20]. Due to the accessibility and the blood ocular barrier, most siRNAs targeting ocular diseases are administered via local routes, such as intravitreal injection, subconjunctival injection, or topical instillation [8, 20]. Therefore, ocular application of siRNA will not be covered in the following PK/BD discussion.
2.2 Cancer
Due to the high selectivity and specificity, siRNA has been widely explored as a new therapeutic agent to replace or supplement traditional cytotoxic chemotherapy [21–23]. Targets often considered for siRNA-based cancer therapy include genes promoting uninhibited cell growth, such as VEGFs [24], c-myc [25], EphA2 [26], Raf-1 [27], polo-like kinase 1 (Plk1) [28], cyclin-dependent kinases (CDKs) [29], and those helping cancer cells survive or resist chemotherapy such as survivin and multidrug resistance (MDR) genes [30] (Table 1).
Table 1.
Target genes for siRNA therapeutics in cancer
| Target genes | Function of target genes |
Diseases | References |
|---|---|---|---|
| VEGF | Angiogenesis | Lung cancer and metastasis |
[31] |
| VEGFR | Angiogenesis | Lung adenocarcinoma |
[32] |
| c-myc | Cell proliferation | Melanoma cancer | [33] |
| c-myc, MDM2, VEGF |
Cell proliferation; p53 inhibition; angiogenesis |
Melanoma cancer | [34] |
| EphA2 | Cell-cell interactions | Ovarian cancer | [35] |
| Raf-1 | RAS/MAPK signaling pathway |
Melanoma cancer | [36] |
| PLK1 | Cell division | Renal cell carcinoma | [37] |
| CDK1 | Cell cycle progression |
Breast cancer | [38] |
| Survivin | Drug resistance | Drug resistant Lung cancer and cervical cancer |
[39] |
| MDR-1 | Drug resistance | Drug resistant ovarian tumor |
[40] |
VEGF and corresponding receptors (VEGFRs) participate in the regulation of blood vessel development during early embryogenesis [41]. Binding of VEGF to VEGFR activates multiple cellular pathways important for angiogenesis, an essential component of tumor growth and metastasis. Therefore, siRNAs targeting VEGF/VEGFR are explored as potential anti-cancer therapeutics. c-myc is an oncogene overexpressed in various human tumors, which promotes cell growth, transformation, and angiogenesis [25]. In particular, c-myc expression in melanoma is indispensable for nucleotide metabolism and proliferation of tumor cells [42]. Downregulation of c-myc inhibits tumor growth and sensitizes cancer cells to chemotherapy, possibly by induction of p53 and inhibition of Bcl-2 proteins, which trigger cell apoptosis [43].
EphA2 is a well-known receptor tyrosine kinase belonging to the Eph family, overexpressed in many cancers including breast cancers and ovarian cancers, implicated in poor clinical outcomes [44]. Contact-dependent cell-cell interactions controlled by Eph receptors and ephrins (ligand of Eph receptors) signaling are tightly regulated in normal embryonic development and maintenance of homeostasis [45]. During oncogenesis, normal EphA2-EphrinA1 signaling is disrupted due to the loss of cell contacts, leading to overexpression of EphA2 and oncogenic signal transduction [45]. This dysregulated signaling is implicated in several critical aspects of oncogenesis such as cytoskeleton modulation, cell adhesion, migration, metastasis, proliferation and angiogenesis [45]. A Plk1, known as serine/threonine-protein kinase, is responsible for cell mitosis in mammalian cells. It is overexpressed in various human cancers as a proto-oncogene, which inactivates tumor suppressor proteins like p53 [46, 47]. CDKs are also serine/threonine kinases and essential for the regulation of the cell cycle progression [48]. The abnormal expression or activity of distinct CDK complexes causes cells escape from a well-controlled cell cycle, resulting in malignant transformation [49–51]. Palbociclib, an inhibitor of CDK4/6, received Breakthrough Therapy designation from the FDA in April 2013, for the initial treatment of patients with breast cancer [52].
Survivin is an inhibitor of programmed cell death (apoptosis), expressed in various types of malignant tumor cells, especially in drug resistant cells [53]. Survivin was initially identified as an inhibitor of caspase-9 and also found to be involved in the regulation of the mitotic spindle checkpoint and the promotion of angiogenesis and chemoresistance [54]. Therefore, siRNA targeting survivin is pursued as a way of potentiating the activity of chemotherapeutics. Another main cause of failure in chemotherapy is multi-drug resistance (MDR) related P-glycoprotein (Pgp). Pgp, a typical ATP-binding cassette membrane transporter, causes efflux of a broad range of drugs from a cell, reducing effective accumulation of the drugs in the cell [55]. Overexpression of Pgp, also knowns as MDR 1 protein 1 (MDR-1), and up-regulation of its functional activity in cancer cells lead to reduced sensitivity to chemotherapy, thus making an attractive target for siRNA therapy [56].
2.3 Liver diseases
As a filtering organ where most nanoparticulate delivery systems are naturally captured, the liver has been the main target for most siRNA therapeutics currently in clinical evaluation stages [57]. Target diseases include hepatocellular carcinoma (HCC), viral hepatitis, liver fibrosis, and hypercholesterolemia [58].
2.3.1 HCC
HCC occurs by cumulative generic mutations, which lead to the dysfunctional regulation of cellular machinery and proliferation [59]. RNAi therapy has been pursued to modulate the mutated genes involved in the oncogenesis of HCC, such as adenomatous polyposis coli (APC), VEGF-A, fibroblast growth factor substrate 2 (FRS2), and phosphoinositide 3-kinase (PIK3) [60–63] (Table 2). These genes play an essential role in the signal transduction pathways in HCC pathogenesis, including Wnt/β-catenin, VEGF, FGF, and PI3K/AKT/mTOR pathways [64–67]. For example, HCC is a highly vascularized tumor, where pro-angiogenic factors like VEGF-A, the major VEGF responsible for tumor angiogenesis and pathogenesis, are frequently over-expressed [65]. Therefore, several studies explored siRNA targeting the VEGF pathway for HCC therapy. The epidermal growth factor receptor (EGFR) signaling pathway is another important target [68], with its overexpression detected in 40–70% of the tumors in pre-neoplastic HCC [69].
Table 2.
Target genes for siRNA therapeutics in hepatocellular carcinoma
| Target Genes | Function of Target Genes | References |
|---|---|---|
| VEGF | Tumor angiogenesis and pathogenesis |
[65] |
| EGFR | Cell survival, proliferation, and differentiation |
[68] |
| NIK | Cell proliferation | [71] |
| ApoB | Formation of LDL, metabolism of dietary and endogenous cholesterol |
[72] |
| Notch-1 | Tumorigenesis; epithelial- mesenchymal transition |
[73] |
In addition to siRNA, several miRNAs have been identified as a potential therapy of HCC. For example, miR-122, a liver-specific tumor suppressor miRNA frequently down-regulated in HCC, has drawn increasing attention over the years [70]. Due to the similarity in structure, miRNAs are delivered in similar ways as siRNA.
2.3.2 Hepatic viral infections (Table 3)
Table 3.
Target genes for siRNA therapeutics in hepatic viral infections
| Target genes | Function of target genes | Diseases | References |
|---|---|---|---|
| HBx | Regulates HBV transcription and translation |
HBV | [78, 93] |
| Core | Regulates viral DNA replication |
HBV | [81, 82] |
| IRES in 5’-UTR | Facilitates RNA translation | HCV | [86–88] |
| Core | Triggers activation of multiple signaling pathway in HCV |
HCV | [89] |
| E1, E2 | Viral attachment to cells | HCV | [90] |
| L protein, VP24 and VP35 |
L protein: RNA polymerase activity; VP24/VP35: inhibitory effects on host type 1 interferon response |
Ebola virus | [91] |
Hepatitis virus infection accounts for most cases of liver infections. When left untreated, patients infected by hepatitis B, C, and D viruses are chronically disturbed and further develop liver cirrhosis and HCC [74]. Currently, 90% hepatitis B vaccine is effective in preventing hepatitis B virus (HBV) infection, but >700,000 deaths still occur worldwide as a consequence of HBV infection [75]. Patients with chronic HBV infection are currently treated with antiviral agents such as tenofovir and entecavir together with immunomodulators like IFN-α 2b, but side effects and viral resistance limit the effectiveness of these therapies [76]. In this regard, RNAi is considered a potentially attractive treatment for HBV infection. Hepatitis B virion is composed of circular double stranded DNA, which contains four overlapping open reading frames (ORFs: S, Pol, X and C) that encode essential proteins like pre-core protein (also known as HBeAg), core protein (HBcAg), envelope protein (HBsAg), X protein and viral polymerase [77]. Among them, the X protein, encoded by ORF X gene, was known to regulate transcription and translation by transactivation of viral and cellular promoters, and several studies showed that HBx-specific siRNAs could suppress HBV viral replication [78, 79]. ORF C is another valid target. It encodes polyadenylation region that plays important function in all the transcripts [80] and contains sequences that can encode nuclear localization signal (NLS) needed for transporting covalently closed circular DNA (cccDNA), which serves as the template for viral transcription [81, 82].
Hepatitis C virus (HCV) comprises seven different genotypes with more than 50 subtypes and billions of quasi-species [83]. Current treatment by PEGylated interferons and nucleoside inhibitors like ribavirin is effective in 70–80% of patients with genotype 2 or 3 but less effective in patients with other genotypes [84]. Side effects are also common among patients receiving this combination treatment [84]. HCV genome sequences are highly variable as compared to HBV, but 5’-untranslated region (5’-UTR) contains conserved internal ribosome entering site (IRES), essential for virus replication, providing a useful target for RNAi therapy [85–88]. Moreover, siRNA targeting HCV-core gene [89] or E1, E2 [90] resulted in dramatic reduction in virus RNA by interfering with multiple signaling pathways in HCV or inhibiting the viral attachment to the cells. In addition to hepatitis virus infections, the liver is also one of the primary sites for Ebola virus replication, and RNAi has been pursued as a potential treatment [91, 92].
2.4 Respiratory diseases
siRNA as a potential treatment of respiratory diseases has been reviewed extensively in recent literature [94, 95]. A number of studies have demonstrated the use of siRNA for the treatment of lung cancer, viral infection, cystic fibrosis, and inflammatory lung diseases (Table 4). Knockdown of G proteins (Ga12 and Ga13) was shown to abolish H69 tumorigenicity in mice for the treatment of small cell lung cancer (SCLC) [96]. Silencing of the Wilms’ tumor gene (WT1) was shown to be effective in treating melanoma lung metastases [97]. Administration of siRNA targeting the conserved regions of influenza virus genes or nucleocapsid protein gene resulted in prevention and treatment of influenza infection [98, 99]. The effectiveness of siRNA was also demonstrated in respiratory syncytial virus (RSV) and parainfluenza virus (PIV) infections [100]. siRNA therapeutics have been pursued for the therapy of cystic fibrosis, a genetic disorder that fatally affects the lung functions. Epithelial sodium channel (ENaC) was identified as a potential target for RNAi therapy of cystic fibrosis [101]. For asthma-associated inflammatory reactions, the knockdown of IL-4 alleviated airway inflammations [102], and siRNA targeting IL-13 reduced airway resistance [103] in allergen-challenged mice.
Table 4.
Target genes for siRNA therapeutics in respiratory diseases
| Target genes | Function of target genes | Disease | References |
|---|---|---|---|
| Gα12 & Gα13 | Promotes the growth and oncogenic transformation |
Small cell lung cancer |
[96] |
| Wilms’ tumor gene (WT1) |
Encodes a transcription factor involved in gonadal development |
Melanoma lung metastasis |
[97] |
| Nucleocapsid protein (NP) gene |
Produces nucleocapsid proteins | Influenza infection | [98, 99] |
| P protein | Essential for RNA-dependent RNA polymerase (RdRP) holoenzyme to exit the promoter and to form a closed complex capable of sustained elongation |
Respiratory syncytial virus and parainfluenza virus |
[100] |
| Epithelial sodium channel (ENaC) |
Stimulates sodium and water absorption, counteracting cystic fibrosis transmembrane regulator (CFTR) activity |
Cystic fibrosis | [101] |
| Interleukin-4 (IL-4) | Promotes the differentiation and proliferation of Th2 cells and facilitates the antibody class switching of B cells to IgE |
Asthma-associated inflammatory reactions |
[102] |
| IL-13 | Induces vascular cell adhesion zolecule-1 and chemokines (e.g. eotaxin, MIP-1a) to recruit and activates inflammatory cells; directly affects airway smooth muscle |
Airway resistance in allergen exposure |
[103] |
3. Pharmacokinetics of siRNA therapeutics
3.1 Preclinical studies
For systemic delivery of siRNA, it is critical to maintain siRNA stable with a long half-life (t1/2) in circulation. To investigate the effect of carriers on the circulation t1/2 of siRNA, the siRNA is often labeled with radioactive elements or fluorescent dyes, and the radioactivity or fluorescence intensity of blood is measured at regular time points. siRNA complexed with nanoparticles (NPs) typically show a longer t1/2 (the time required to reduce the plasma concentration of a drug to one half its initial value), higher area under the curve (AUC) (the overall amount of a drug in the bloodstream after a dose), lower plasma clearance (Cl) (the volume of plasma in the vascular compartment cleared of a drug per unit time), and longer mean retention time (MRT) (the average time a drug molecule stays in the body) than free (naked) siRNA, provided that the complex remains stable in circulation.
Xia et al used magnetic mesoporous silica NPs (M-MSN) as a carrier of siRNA [31]. Here, siRNA was loaded in mesopores of the carrier, which was capped with polyethyleneimine (PEI) and surface-modified with polyethylene glycol (PEG) and a fusogenic peptide. Fluorescence dye (Cy3) was conjugated to the 5’ end of the sense strand for detection of siRNA in blood. The blood level of free Cy3-siRNA was reduced to 1% of the injected dose per gram of blood within 20 min. In contrast, Cy3-siRNA loaded in M-MSN maintained ~5% of the injected dose per gram of blood in the first hour [31]. This difference translated to significantly increased bioavailability of siRNA. Free Cy3-siRNA showed an AUC of 40.13 ± 30.49 min mg/mL, a plasma Cl of 57.96 ± 58.07 mL/min/kg, and a MRT of 70.67 ± 20.39 min, whereas Cy3-siRNA packaged in M-MSN showed AUC, Cl, and MRT of 573.34 ± 145.12 min mg/mL, 2.41 ± 0.51 mL/min/kg, and 310.15 ± 21.68 min, respectively [31].
As with other nanomedicine, surface modification of carriers with PEG helps extend the t1/2 and increase the AUC. Kissel et al developed amphiphilic biodegradable non-viral polymeric siRNA carrier, based on PEI, polycaprolactone (PCL), and PEG [104]. To study PK of siRNA complexed with the polymer, they used 111In-radiolabeled siRNA and measured the radioactivity of the blood samples over time. Upon intravenous (IV) injection, siRNA/PEI-g-PCL-b-PEG complexes showed longer circulation times, less steep α and β elimination phases, and higher AUC values as compared to free siRNA or siRNA/PEI complexes (Fig. 1) [104]. On the other hand, siRNA/PEI complex showed a similar PK as free siRNA due to the instability of the complex (i.e., early dissociation of the complex in blood). Similarly, Harashima et al compared PK parameters of a liposomal siRNA carrier, called a multifunctional envelope-type nanodevice (MEND), with and without PEGylation, corroborating the significance of PEG modification [37]. siRNA and lipid envelope were labeled with radioisotopes, 32P and 3H, respectively, and traced in normal mice after IV injection. The siRNA encapsulated in the MEND was eliminated from the blood stream as rapidly as free siRNA, suggesting rapid clearance via the mononuclear phagocyte system (Fig. 2). On the other hand, siRNA formulated in the PEG-MEND showed a longer t1/2β (16.9h vs. 1.35h of free siRNA) and higher AUC (344 µg·h vs 2.1 µg·h of free siRNA). The 3H-labeled lipid component showed a similar blood concentration profile as 32P-labeled siRNA, suggesting that the complex was stable during circulation.
Fig. 1.
PK of siRNA polyplexes and free siRNA as measured by gamma scintillation counting of blood samples. PPP indicates PEI-g-(PCL-b-PEG); 3 and 5 indicate graft density of PCL-PEG to PEI. Reprinted with permission from Reference [104]. Copyright (2012) Elsevier.
Fig. 2.
Blood concentration profile of systemically injected MENDs. The lipid envelope and siRNA were labeled with RIs 3H and 32P, respectively. MENDs and free siRNA were injected via the tail vein of ICR mice, and then at 0.17, 1, 3, 6, and 24h after injection, the radioactivity in plasma was measured by liquid scintillation counting. The data are represented as the mean ± SD (n = 3). MEND, multifunctional envelope-type nanodevice; RI, radio isotope. Reprinted with permission from Reference [37]. Copyright (2013) Nature Publishing Group.
Another approach to increase the stability of siRNA/carrier complex involves covalent crosslinking of the carrier and introduction of hydrophobic interactions [105]. Kataoka et al developed a polyion complex micelle system based on cholesterol-conjugated siRNA (chol-siRNA) and PEG-poly(L-lysine) block-co-polymer (PEG-PLL) modified with 1-(3-mercaptopropyl)amidine (MPA), where the micelles were stabilized by disulfide cross-linking of the polymer and hydrophobic association of cholesterol groups [105]. siRNA was fluorescently labeled by conjugating Cy5 dye to 5’ end of the antisense strand, and the blood level of siRNA was measured by intravital real-time confocal laser scanning imaging (IVRT-CLSM) [105, 106]. Unmodified siRNA (chol-free siRNA) loaded in PEG-PLL(MPA) micelles were rapidly eliminated from circulation, similar to naked siRNA, with a blood t1/2 less than 5 min, indicating that disulfide crosslinking of polymer alone did not stabilize the siRNA/micelle complexes. In contrast, micelles containing chol-siRNA showed a t1/2 longer than 20 min, which indicated the contribution of cholesterol to the stability of the complex (Fig. 3) [105].
Fig. 3.
In vivo performance of siRNA encapsulated in PEG-PLL(MPA) micelles. Blood circulation profiles, determined by IVRT-CLSM after intravenous injection (3.6 nmol siRNA/mouse) into BALB/c nude mice (open triangle: naked Chol-free/Cy5-siRNA, closed triangle: naked Chol/Cy5-siRNA, open circle: Chol-free/Cy5-siRNA micelles and closed circle: Chol/Cy5-siRNA micelles). Data represent the average value (n = 3). Reprinted with permission from Reference [105]. Copyright (2014) Elsevier.
One caveat of using labeled siRNA in PK studies is that the labeled siRNA can be catabolized and misrepresent siRNA. Moreover, the labeling can cause structural modification in siRNA and change its PK and BD. For example, siRNA labeled with 111In via conjugation to 3’ end [107] and siRNA labeled with 3H through internal substitution [108] showed different PK and BD profiles. The former showed predominantly high distribution in the kidneys and low distribution in the liver compared to other organs, but the latter showed high distribution in the salivary gland, spleen, and liver, in addition to the kidneys 1 hour after administration [107, 108].
To overcome such challenges, the amount of siRNA circulating or accumulating in tissues of interest has been determined by other analytical means such as Northern blotting [109], mass spectrometry, and qRT-PCR [37], which can selectively detect full-length siRNA. Swart et al compared PK profiles of 3H-labeled siRNA with [110] and without a lipid nanoparticle (LNP) vehicle [108] after IV injection. Both free siRNA and LNP-complexed siRNA showed a multi-exponential decrease in the concentration of total radiolabeled components with, however, vastly different elimination half-lives: 10 min for free siRNA and 162h for siRNA/LNP complex [108, 110]. Mass spectrometric profiling of metabolites in plasma, urine, and tissues found that free siRNAs were rapidly metabolized and distributed to tissues as low molecular weight metabolites [108]. In contrast, when injected as a siRNA/LNP complex, the guide strand of 3H-siRNA was detectable in plasma up to 48h and 168h post dose, as measured by LC-MS-radioactivity (RA) and RT-qPCR, respectively, with a Cmax of 1.04 µM (LC-MS-RA) or 2.56 µM (RT-qPCR) after 1hour [110]. LC-MS-RA analysis found siRNA degradation products (14–18 mers) as the predominant component of total radioactivity in plasma but did not detect complete degradation products, which indicates the LNP formulation protected siRNA from metabolism at least partly [110].
3.2 Clinical studies
QPI-1002 was the first siRNA therapeutic systemically delivered to human [111]. QPI-1002 was a siRNA targeting proapoptotic protein p53, developed for the prevention of delayed graft function in patients receiving kidney transplantation. Since the target organ (kidneys) was the natural destination of siRNA, it was administered as free siRNA without a carrier [111]. QPI-1002 completed Phase II trials in 2014, but its PK results are not available.
Clinical PK data for siRNA-lipid complex have been reported recently. ALN-VSP was LNPs (also known as SNALPs, stable nucleic acid-lipid particles) containing VEGF-A and kinesin spindle protein (KSP) siRNAs, with a size of 80 to 100 nm and a near neutral zeta potential, developed for the treatment of advanced cancer and liver metastases [112]. ALN-VSP was administered as 15-min IV infusion, and blood levels of total and unencapsulated siRNA were determined by the hybridization ELISA method and analyzed by noncompartmental method. The Cmax and AUC were similar for both siRNAs and dose-dependent. Most siRNA circulated as LNP according to comparison between total and free siRNA, and the level declined to a plateau in 6–7h [112]. Another lipid-based siRNA tested in human was Atu027, a siRNA targeting protein kinase N3 (PKN3) carried by cationic LNPs, developed for the therapy of primary tumors and metastases [113]. Atu027 was given as IV infusion for 4h and analyzed with ELISA detecting single strand RNA. The blood level increased during the infusion period and declined after the end of infusion. The siRNA level declined more rapidly than lipid components, which suggests potential dissociation of the complex during circulation.
CALAA-01 was the first polymer-based siRNA therapeutic tested in human for cancer therapy [22]. CALAA-01 consisted of two components: siRNA designed to reduce the expression of the M2 subunit of ribonucleotide reductase (RRM2), a well-established target for cancer therapy, and the carrier component made of cationic cyclodextrin-based polymer (CDP), PEG modified with a terminal adamantane group (AD-PEG), and AD-PEG conjugated to human transferrin (AD-PEG-Tf). NPs targeted to transferrin receptors were produced by mixing siRNA and CDP, to which the AD-conjugated polymers were attached via inclusion complex formation [114]. PK studies from Phase I clinical trials with 24 patients were recently reported and compared with data obtained from multispecies animal studies [115]. siRNA in plasma was analyzed by the hybridization-ligation assay, which detected the oligonucleotide via hybridization of complementary template and ligation of signaling probe to the template [116]. The plasma concentrations of siRNA component of CALAA-1 rapidly declined to below the detection limit by 30 min after the end of infusion in most patients, with no apparent accumulation upon multiple dosing (Fig. 4) [115]. There was a good correlation between AUC and Cmax of siRNA, which increased linearly with the dose (in mg/kg) [115]. Similar trends were observed in preclinical studies with different animal species including mice, rats, dogs, and monkeys. It is worthwhile to note that CALAA-1 was cleared through the kidneys [117], unlike other NP-based therapeutics that undergo accumulation in the mononuclear phagocytic systems (MPS). The authors attributed it to disassembly of siRNA/polymer complex at the kidney glomerular basement membrane but excluded the possibility of disassembly in circulation due to the stability demonstrated in the gel mobility shift assay [117].
Fig. 4.
PK assessment of CALAA-01 in patients. (a) Time course of average plasma concentration of the siRNA component of CALAA-01 following the end of infusion for all dosing cohorts. (b) Time course of plasma CALAA-01 siRNA following the end of infusion from cycles 1–3 of CALAA-01 from one patient who received 30 mg/m2 CALAA-01. Lines connecting data points are guides for the eye only. Reprinted with permission from Reference [115].
4. Biodistribution of siRNA therapeutics
Similar to PK studies, siRNA BD is studied by tracking surrogate signals of fluorescent dyes or radioactive elements incorporated in siRNA or siRNA itself via PCR. Less frequently, NP carriers are tracked in lieu of siRNA under an assumption that the NPs represent the siRNA BD. Many BD studies are performed in animal models with solid xenograft tumors. siRNA stably encapsulated in NPs show increased tumor accumulation as compared to naked siRNA to some extent, due to the increased circulation t1/2 and the enhanced permeability and retention (EPR) effect common in solid tumors. However, the majority of the injected dose ends up in organs where the MPS is located, including the spleen, liver and lungs.
4.1 Tracking siRNA labeled with fluorescent dyes
Huang et al used a liposome-polycation-hyaluronic acid (LPH) NP system decorated with GC4-single chain variable fragment (scFv) for systemic delivery of a combination of siRNAs [34]. FITC-labeled siRNA was first complexed with protamine in the presence of hyaluronic acid (HA), combined with cationic liposomes, and injected IV to mice bearing experimental lung metastasis. At 4h after IV injection, major organs were collected and lysed to yield supernatants, of which fluorescence intensity was determined to represent the level of siRNA. With free siRNAs, most fluorescence signals were seen in the liver. siRNA carried by LPH NPs showed similar BD as free siRNA, except for 3 times stronger intensity in tumors [34]. This group used a similar NP system based on a liposome-polycation-DNA (LPD) with PEGylated Asn-Gly-Arg peptide (PEG-NGR) as a targeting ligand for the delivery of doxorubicin and siRNA combination [118]. At 4h post-injection, the fluorescent signals of siRNA and doxorubicin delivered as LPD-PEG-NGR NPs were mainly detected in tumor, followed by the liver and kidney, whereas free siRNA was mainly seen in the liver and kidney and free doxorubicin was broadly seen in the tumor, liver, kidney, spleen, and heart, respectively (Fig. 5) [118].
Fig. 5.
Tissue distribution of FITC-siRNA (a) and DOX (b) delivered with LPD-PEG-NGR (NGR). Data = mean ± SD, n = 3. *P < 0.05 compared with free siRNA or DOX (Free). Reprinted with permission from Reference [118]. Copyright (2010) Nature Publishing Group.
A CD44-targeted, inorganic siRNA delivery system was prepared by combining calcium phosphate (CaP)-siRNA complex and 3,4-dihydroxy-L-phenylalanine (dopa)-conjugated HA [119]. This system took advantage of the ability of CaP to co-precipitate with siRNA and form condensed NPs, dopa to bind to the CaP crystals, and HA to prevent the growth of crystal size and to target CD44 and CD168 (also known as Receptor for Hyaluronan Mediated Motility, RHAMM) overexpressed in various cancer cells [119]. The CaP/Cy5.5-labeled siRNA/dopa–HA NPs were administered IV in nude mice bearing HT29-luc tumors. Ex vivo imaging after 4h showed relatively high accumulation of the NPs in the tumor and the liver but no significant signals in the spleen and the lungs (Fig. 6). The accumulation of CaP/siRNA/dopa–HA in the liver was attributed to the presence of the HA receptor in hepatocytes. Without dopa-HA, CaP/siRNA formed particles as large as >4 µm, which were captured predominantly by the liver, most likely due to the size [119].
Fig. 6.
Normalized organ distribution of Cy5.5-siRNA delivered by CAP/siRNA and CAP/siRNA/dopa–HA. Normalized organ fluorescence intensity was obtained by dividing the fluorescence intensity of the organ by the area of the organ using image analysis software. Reprinted with permission from Reference [119]. Copyright (2014) Elsevier.
CaP was also used to stabilize polymeric micelles [120]. siRNA was first encapsulated in micelles of PEG-conjugated polyaspartamide derivative (poly[N’-[N-(20aminoethyl)-2-aminoethyl]aspartamide]) and stabilized with CaP complexation [120]. BD of siRNA was examined by tracing the fluorescence of Alexa Fluor 647-labeled siRNA in transgenic mice with spontaneous pancreatic tumors. At 6h after injection of the hybrid micelles, 0.9% and 1.5% of the injected siRNA were found in each gram of pancreas/tumor and the kidneys, respectively, which corresponded to 6 times (pancreas/tumor) and half (kidneys) the levels of naked siRNA, with no significant difference in other organs [120]. The siRNA/micelles accumulated in tumors achieved 61% reporter gene silencing at 24h after injection, whereas scrambled siRNA/micelles had no effect [120].
A hybrid of cationic lipid and polymer NPs was used for the delivery of CDK1 siRNA for the therapy of triple negative breast cancer (TNBC) [38]. siRNA was encapsulated in PLA-PEG block-co-polymer by the double emulsion method. Here, cationic cholesterol derivative was included in the organic phase to help condense siRNA in the NPs. Upon IV administration to SUM149 tumor bearing mice, Cy5-siRNA encapsulated in NPs showed persistent signals in the tumor, liver, spleen and kidneys up to 72h post injection, whereas free Cy5-siRNA was barely seen at 12h after injection [38]. At a dose of 2 mg/kg every other day, CDK siRNA/NP system suppressed the growth of SUM149 and BT549 xenograft tumors, an effect comparable to 50 mg/kg dinaciclib (a known CDK1 inhibitor), whereas neither free CDK1 siRNA nor scrambled siRNA in NPs was effective. Real-time PCR and western blot of tumor tissues found that CDK1 siRNA-loaded NPs suppressed the levels of CDK1 mRNA and CDK1 protein expression levels, respectively, confirming that the tumor suppression was mediated by CDK1 silencing in tumors [38].
To enhance the formation and stability of siRNA-carrier complex, Kim et al used a polymerized siRNA (poly-siRNA), where several siRNA molecules were linked to one another via disulfide bond, and thiolated human serum albumin (tHSA), which formed a stable complex with the poly-siRNA via intermolecular disulfide crosslinking [121]. The poly-siRNA/tHSA complex was systemically delivered to mice bearing squamous cell carcinoma (SCC7) and human prostatic carcinoma (PC-3) tumors. siRNA was labeled with Cy5.5 fluorecence dye, and the fluorescence intensity of each organ was measured by ex vivo imaging [121]. Overall, poly-siRNA/tHSA complex showed a stronger fluorescence signal throughout the whole body than free poly-siRNA, indicating relatively good stability of poly-siRNA/tHSA NPs [121]. Ex vivo imaging of organs at 24h after intravenous injection revealed that animals treated with naked poly-siRNA had relatively high fluorescence intensity in the liver, kidney, and tumor, whereas those treated with poly-siRNA-tHSA complex did predominantly in tumors [121]. Consistently, poly-VEGF siRNA/tHSA showed superior anti-cancer effects to that of a complex containing poly-scrambled siRNA through inhibition of tumor angiogenesis [121]. This group later replaced tHSA with thiolated glycol chitosan (tGC) for the delivery of poly-siRNA and observed a similar pattern – relatively high fluorescence level in tumor as compared to that of naked poly-siRNA [40].
4.2 Tracking radiolabeled siRNA
BD of the CDP-based siRNA NPs (discussed in section 3.2) was evaluated using siRNA labeled with 64Cu and positron emission tomography (PET)-computer tomography (CT) in vivo imaging [114]. Free 64Cu-DOTA-conjugated siRNA showed rapid blood clearance through liver accumulation (23% ID/cm3 at 60 min) and kidney filtration followed by the bladder accumulation (73% ID/cm3 at 60 min). 64Cu-DOTA-siRNA packaged in transferrin receptor (TfR)-targeted CDP NPs showed similar BD to that of naked 64Cu- DOTA-siRNA, except for slightly higher liver accumulation (26% ID/cm3 at 60 min) and a delayed peak in kidney accumulation [114]. In both cases, % injected dose per cm3 tumor was no higher than 2%.
Swart et al used 3H-labeled siRNA to study BD of siRNA delivered with LNPs [110]. BD of siRNA/LNPs was estimated by quantitative whole-body autoradiography (QWBA) and matrix-assisted laser desorption ionization mass spectrometry imaging (MALDI-MSI) techniques, where QWBA measured the radiolabeled siRNA and MALDI-MSI measured the cationic lipid component. Following a single intravenous administration, animals treated with free siRNA showed the highest radioactivity in kidneys and salivary gland, and the radioactivity in different organs declined in a similar pattern (Fig. 7a) [108]. In contrast, animals treated with siRNA/LNPs showed the highest radioactivity of siRNA in the spleen and liver, which lasted over 168h, whereas the blood level radioactivity gradually decreased (Fig. 7b) [110]. MALDI-MSI found similar distribution of the cationic lipid as that of siRNA radioactivity, suggesting that siRNA and lipid circulated together and co-distributed as a LNP complex (Figs. 7c and 7d).
Fig. 7.
(a) Concentration-time profiles of radiolabeled naked 3H-SSB siRNA in dried blood, plasma, and selected tissues [108]; (b) concentration-time profiles of radiolabeled 3H-SSB siRNA in LNP in dried blood, plasma, and selected tissues; (c) selected whole-body autoradioluminographs and (d) selected whole-body MALDI-MS images at 1, 48, and 168h [110] after a single intravenous administration of free 3H-SSB siRNA (5 mg/kg siRNA) or [3H]-SBB siRNA in LNP vehicle (2.5 mg/kg siRNA) to male CD-1 mice. In (c), the whitest area corresponds to the highest concentration of radiolabeled siRNA. In (d), the whitest area corresponds to the highest concentration of DLin-KC2-DMA (one of the main components of the LNP vehicle). Reprinted from References [108, 110]. Copyright (2013, 2014) The American Society for Pharmacology and Experimental Therapeutics.
Harashima et al evaluated the BD of siRNA delivered with MEND by tracking siRNA and the carrier separately [37]. Here, siRNA was labeled with 32P and the carrier with 3H. Both siRNA and the carrier showed significant increase in tumor accumulation with PEGylation of the MEND. According to the radioactivity measurement, the levels of tumor accumulation of siRNA delivered with MEND and PEG-MEND were 3.0% ID/g of tumor and 5.1% ID/g of tumor, respectively. On the other hand, when measured by the stem-loop primer-mediated qRT-PCR, which measured full-length siRNA, the values were much lower: 0.0079% ID/g tumor (siRNA by MEND) and 1.9% ID/g tumor (siRNA by PEG-MEND). 32P-labeled siRNA showed differential organ distribution according to the delivery methods (free, MEND, PEG-MEND) (Fig. 8a). It is worthy to note that the radioactivity of free siRNA were detected in the liver, spleen, kidney, and lung 6h after injection, but the stem-loop qRT-PCR did not detect siRNA in any of these organs (Fig. 8b). This discrepancy strongly suggests that the radioactivity measurement may have overestimated the amount of siRNA by counting catabolites of the labeled siRNA as well. BD of siRNA indeed coincided better with that of carriers when measured with the stem-loop qRT-PCR [37].
Fig. 8.
Comparison of the methodology between radioisotope and stem-loop qRT-PCR methods. Accumulations in liver, spleen, kidney and lung 6h after injection were measured by the two methodologies. Free siRNA and formulated siRNA in these organs were determined by using radioisotope (a) and stem-loop qRT-PCR method (b). N.D.: not detected. Reprinted from Reference [37]. Copyright (2013) Nature Publishing Group.
4.3 Tracking siRNA itself
With the notion that the labeled siRNA may be degraded to inactive catabolites, alternative methods are used to detect siRNA itself instead of labels. For example, Northern blotting was used to determine the integrity of siRNA and complement quantitative measurement based on radioactivity counting [109]. Various formulations including chitosan, liposome, or JetPEI NPs were injected intravenously into mice as complexes with 32P-labeled siRNA. The radioactivity measurement found that liposomal formulation increased the accumulation of siRNA in the lungs five- to tenfold higher than other organs (Fig. 9a), with 5% of 30-minute levels still present at 24h (Fig. 9b). Similarly, JetPEI/siRNA complex showed greater levels in the lung (two- to tenfold) than in the other organs (Fig. 9a). On the other hand, chitosan/siRNA complex showed different organ distribution patterns with pronounced accumulation in the kidney (Fig. 9a). The Northern blotting analysis with total RNA extracted from tissues showed consistent patterns over 24h, confirming the validity of radioactive label as a marker of intact siRNA [109].
Fig. 9.
Organ distribution of siRNA delivered with different carriers at 30 min (a) or 24h (b) after IV injection, analyzed with Northern blotting. Lane 1: 1.5-ng duplexes (Ctrl 1); lane 2: 1 ng siRNA/NP complex (Ctrl 2); lane 3: blank; lanes 4–13: duplexes or siRNA/NP complex in organs. Ctrl, control; LNA, locked nucleic acid; siLNA, LNA-modified siRNA; siLNA-light, lightly LNA–modified siRNA. Reprinted with permission from Reference [109]. Copyright (2009) Nature Publishing Group.
Alternatively, qPCR was employed to study BD of siRNA delivered with LNP [122]. Seven organs of interest were collected at different time points, and the level of siRNA was measured with the stem-loop qPCR. At 0.5 and 2h post IV injection, the siRNA levels were highest in the liver, followed by spleen and kidney, with little in lung and heart, and almost none in duodenum and brain. Reflecting the siRNA distribution, the greatest level of target mRNA knockdown was observed in the liver (85%) and spleen (25%), with no detectable knockdown in lung, kidney, heart, duodenum, or brain [122].
4.4 Tracking carriers
Carrier concentrations in tissues are sometimes measured as an indirect measure of siRNA distribution. Chen et al developed a new carrier of siRNA based on HA, hydrophobically modified for self-assembly formation, conjugated with a phosphate receptor Zn (II)-dipicolylamine (DPA/Zn) for RNA binding, and stabilized with CaP surface layer [123]. BD of the siRNA/carrier assembly (CaP-HDz/siLuc-NFs) was studied by tracking Cy5.5-labeled carriers in a human colon cancer (HCT116) xenograft mouse model. From 3h post-administration, tumors showed strong fluorescence, which reached the maximum at 6h and lasted until 48h post-injection (Fig. 10a). Ex vivo images exhibited consistently strong fluorescence intensities in tumors. Noticeable fluorescence signal was also found in the liver, lung, and kidneys at 48h after injection (Fig. 10b). As an indirect measure of the siRNA integrity, a model siRNA silencing the luciferase-expressing gene (siLuc) was delivered with the CaP-HDz carrier, showing 79.4% reduction of luminescence signal in tumor (Figs. 10c and 10d).
Fig. 10.
In vivo monitoring of tumor-targeting and gene silencing effect of CaP-HDz/siRNA-NFs. (a) In vivo near-infrared fluorescence (NIRF) imaging of HCT116 tumor-bearing mice systemically administered with CaP-HDz/siLuc-NFs labeled with Cy5.5. (b) Quantitative analysis of fluorescence intensities at the major organs and tumor tissues of mice treated with CaP-HDz/siLuc-NFs labeled with Cy5.5 48h after IV injection. (c) In vivo bioluminescence imaging (BLI) of fLuc gene expression in fLuc-expressing HCT116 tumor-bearing mice intravenously injected with PBS, free siLuc, or CaP-HDz/siLuc-NFs (280 µL; RNA = 700 pmol). (d) Quantitative analysis of fLuc expression at tumors after intravenous injection of CaP-HDz/siLuc-NFs into the fLuc-expressing HCT116 tumor mice (n = 4). Red arrow indicates tumor site; *p < 0.005 vs control or siNC. Reprinted with permission from Reference [123].
Mirkin et al developed spherical nucleic acid (SNA) gold NP conjugates as a carrier of siRNA, where siRNA duplexes were conjugated to an inorganic gold NP core via thiol-gold bond, forming densely packed, highly oriented nucleic acid corona around the core [124]. BD of SNA particles was evaluated in glioblastoma multiforme (GBM)-bearing mice based on the level of Au in the tissue by ICP-MS. Similar to other siRNA delivery systems, most Au was detected in the liver and spleen after 24h, and 1% of the total SNA injected was found in the brain tumor. The delivery of intact siRNA was confirmed by evaluating the silencing effect of siRNA targeting Bcl2Like12 (Bcl2L12) oncogene and survival of GBM-bearing mice [124].
BD of M-MSN (discussed in Section 3.1), containing iron in the core, was studied by measuring the iron level in organs by atomic absorption analysis (AAS) [31]. M-MSN NPs loaded with siRNA was injected to A549 lung cancer bearing mice. One day after injection, 10% of the total administered NPs accumulated in the tumor, and ~4% remained after 3 days. The highest iron concentration was found in liver (~27%ID/g), followed by lung (~15%ID/g), spleen (~14%ID/g) and kidney (~4%ID/g) after 24h (Fig. 11a). Separate tracking of the fluorescently labeled siRNA loaded in M-MSN revealed that the carrier distribution was consistent with siRNA distribution, which was significantly higher than that of free siRNA. Fluorescence images of excised mouse organs (brain, tumor, spleen, lung, kidney, heart, stomach and liver) obtained 24h after injection of the NPs showed relatively high siRNA accumulation in liver, lung, spleen, kidneys as well as in tumor. In contrast, organs from the animals receiving free siRNA showed little fluorescence except for the liver and kidneys (Fig. 11b) [31].
Fig. 11.
(a) Distribution profile of M-MSN_NC siRNA@PEI-PEG-KALA in tumor and various organs of tumor-bearing mice after tail vein administration. The % of NP distribution was determined from measurement of Fe element content at different time points (1 day, 3 days, 7 days after particle injection), subtracting the basal Fe content of tumor bearing mice without particle injection (n =3). (b) Fluorescence images of excised mouse organs (heart, liver, spleen, lung, kidney, brain, tumor and stomach) 24h after injection of M-MSN_Cy3-siRNA@PEI-PEG-KALA (left), naked-Cy3-siRNA (right) using an IVIS spectrum imaging system with excitation and emission wavelengths of 554/568 nm. Reprinted with permission from Reference [31]. Copyright (2014) Elsevier.
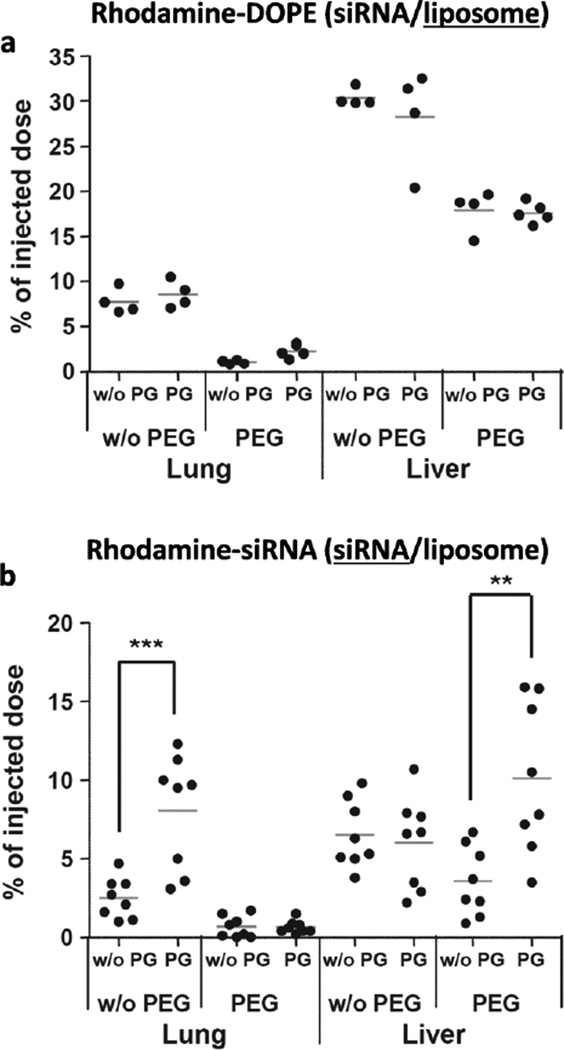
Tracking carriers may serve as a surrogate of siRNA in a BD study if siRNA and carriers remain stable in circulation, but this may not be always a valid assumption. Escriou et al tracked carriers and siRNA, separately labeled with distinct fluorescent dyes, and found different BD patterns [125]. They used liposomes containing cationic lipid (DOPE) and an anionic polymer (polyglutamate, PG) as a complexation aid and studied the effect of PG on BD of the complexes in the lung and liver. The tissue level of lipid remained constant irrespective of the presence of PG (Fig. 12a). However, siRNA level increased by 2–3 folds with PG (Fig. 12b), which was interpreted as a consequence of the enhanced stability of the PG-containing complexes [125]. The conclusion would have been different if they measured the level of carriers only.
Fig. 12.
Lipoplexes recovery after IV injection. siRNA lipoplexes, formed with or without PG at a charge ratio of 8, were labeled with fluorescent lipid (a, 5% rhodamine-DOPE incorporated in cationic liposome) or with fluorescent siRNA (b, 5′ rhodamine-labeled siRNA used to form siRNA lipoplexes) and IV injected (10 µg siRNA/mouse). Two hours post injection, lung and liver were removed and fluorescent lipid (a) or fluorescent siRNA (b) were extracted and quantified; n=4 (a) or n=8 (b), **p<0.005; ***p<0.002. Reprinted with permission from Reference [125]. Copyright (2011) Elsevier.
4.5 Targeted vs. non-targeted
One notable trend observed with the so-called ‘targeted’ gene carriers is that the targeting ligands seemed to play a minimal role in changing initial BD of siRNA. The dual-stabilized polymeric micelles, discussed in section 3.1, were further decorated with cyclic RGD (cRGD) peptide ligands for tumor-targeting [105]. Ex vivo organ imaging at 4h after IV injection found that BD of Cy5-labeled siRNA fluorescence was similar between non-targeted and targeted micelles, with the majority of signals found in the kidneys and the liver. A difference attributable to cRGD was seen as slight increase in tumor accumulation (Fig. 13) [105]. A comparable observation was made in an earlier study with TfR-targeted CDP-based NPs (discussed in section 3.2), where both non-targeted and targeted NPs showed similar BD and tumor localization of siRNA (Fig. 14) [114]. Despite the similarity in BD, siRNA delivered with TfR-targeted NPs showed 50% greater gene silencing effect than non-targeted NPs, indicating more efficient entry of the targeted NPs into tumor cells [114]. This study suggests that the contribution of a targeting molecule is to facilitate cellular uptake of the NPs rather than to actively guide the NPs to the target tissues as the name implies [114]. On the other hand, other studies show the increase in overall tumor localization of targeted NPs [126, 127]. In interpreting those studies, it will be worthwhile to consider that the increased tumor accumulation is likely a net result of the EPR-driven extravasation of NPs (initial BD: targeted ≈ non-targeted) and efficient cellular uptake and retention at target cells (targeted > non-targeted).
Fig. 13.
BD of non-targeted (gray bars) or actively-targeted (black bars) Chol/Cy5 -siRNA micelles (1.8 nmol siRNA/mouse) at 4h after IV injection via tail vein of BALB/c nude mice. Reprinted with permission from Reference [105]. Copyright (2014) Elsevier.
Fig. 14.
Tissue distribution of 64Cu-DOTA-siRNA delivered by targeted (Tf) and non-targeted (PEG) NPs. (a) Fused micro-PET/CT images of mice at 1, 10, and 60 min after injection. (b) Blood clearance and tumor localization of Tf-targeted and non-targeted siRNA NPs. Reprinted with permission from Reference [114]. Copyright (2007) National Academy of Sciences, USA.
5. Pharmacological effects of siRNA therapeutics
Due to the favorable BD of siRNA delivery systems, the liver and lungs are among the primary targets of siRNA therapeutics. This section provides a brief overview of recent preclinical studies on pharmacological effects of siRNA therapeutics in liver and lung diseases.
5.1 Liver diseases
5.1.1 HCC
Initial efforts to treat HCC with siRNA involved free siRNA injection. siRNA targeting VEGF expression in hepatoma and endothelial cells with no specific carrier system was tested in a murine orthotopic hepatoma model [61]. Animals were injected with 200 µg/kg of siRNA-VEGF intraperitoneally, every 2 days for 14 days starting from 24h prior to tumor cell inoculation. When determined terminally, tumor burden was reduced by 63% [61]. The antitumor efficacy was attributed to the inhibition of tumor angiogenesis, corresponding to intratumoral microvessel density [61]. Similarly, siRNA targeting antiapoptotic protein heme oxygenase 1 (HO-1) was intraperitoneally injected to immune-competent mice bearing HO-1+ Hepa129 tumors in the liver and reduced tumor growth by 50% with no damages in normal organs [128]. The authors attributed this effect to proapoptotic and possibly antiangiogenic activity of siHO-1 [128].
For improving siRNA delivery to the liver and HCC, NPs based on cationic lipid such as 1,2-dioleyloxy-3-trimethylammonium propane (DOTAP) are widely used as a carrier of siRNA. Schmitz et al made a complex of VEGF-targeting siRNA with DOTAP and injected the complex intraperitoneally multiple times to mice with pre-existing liver fibrosis as well as orthotopic HCC (Hepa129 cells injected to the left liver lobe) [129]. The VEGF-siRNA/DOTAP complexes resulted in significant reduction in the number of HCC satellites in the liver (82% mice had <30 satellites), whereas free VEGF-siRNA and control siRNA had a minimal effect (22.1% and 12.5% mice with <30 satellites, respectively). DOTAP helped VEGF-siRNA enter HCC satellites and silence VEGF expression in hepatic tissues. However, the effect of the internalized VEGF-siRNA-DOTAP complex on tumors was more likely through immune activation via interferon-1 upregulation than VEGF silencing [129]. In fact, intratumoral VEGF expression was not reduced by VEGF-siRNA/DOTAP complexes but rather increased [129]. More recently, Shen et al screened several lipid NPs for optimal siRNA delivery to HCC and identified a combination of lipids that showed significant anti-tumor effect with cancer-targeting siRNA’s [130]. An interesting note in this study is that good in vitro transfection efficiency did not necessarily translate to good in vivo silencing effect in orthotopic HCC tumors, which indicates the significance of circulation stability of gene-carrier complexes [130].
To further improve cell specificity of siRNA delivery, cell-interactive ligands have been added to the carrier. For example, a model siRNA was encapsulated in PEGylated DOTAP-based immunoliposomes decorated with anti-EGFR Fab’ [131]. A BD study in an orthotopic mouse model of HCC showed that the immunoliposomes accumulated in the liver and were taken up by HCC cells to greater extents than non-targeted liposomes, achieving higher silencing effect on the model luciferase gene expression [131]. This system was later used for the co-delivery of doxorubicin and siRNA targeting RRM2, a gene important for DNA synthesis and repair and highly expressed in HCC [126]. The siRNA and drug delivered with immunoliposomes showed 50–60% ID/g in the HCC tumors, compared to 20–30% ID/g of non-targeted liposomes, 8h after injection. Accordingly, animals treated with siRNA-RRM2 and doxorubicin in immunoliposomes showed the most significant retardation of tumor growth, compared to those receiving each treatment or dual loads in non-targeted liposomes [126].
5.1.2 Liver infections
Due to the preferable BD in the liver, hepatitis infection like HBV and HCV were one of the earliest targets of siRNA therapeutics. For example, siRNA targeting the S region of HBV RNA was delivered with SNALPs based on a mixture of cationic and fusogenic lipids [132, 133]. Here, 2’-OH of siRNA was chemically modified to increase the resistance against nucleases [132]. IV-injected SNALP particles were accumulated predominantly in the liver (28%) with minimal accumulation in other organs (8.2% in spleen and 0.3% in the lung) by 24h after injection [132]. Reflecting the high liver accumulation, siRNA delivered with SNALPs achieved a significant reduction of the level of HBV DNA copies and HBsAg proteins for 7 days after dosing [132]. In another case, siRNAs targeting HBV transcript sites 1407 or 1794 were delivered with lipid assemblies based on three different lipids, including one conjugated to PEG2000 by oxime linkages, which could be cleaved at pH lower than 5.5 [78]. A BD study with the radiolabeled assemblies found that regardless of the PEG concentration in the formulation, most of the dose (>50%) were detected in the liver after 1h post-injection, and other organs such as spleen, kidneys, blood and lung showed minimal accumulation of the particles. Accordingly, HBV transgenic mice receiving the siRNA-lipid assemblies showed significant reduction in HBV replication, indicated by the reduced levels of HBV mRNA and HBeAg proteins in the liver and HBsAg in serum, equivalent to daily intraperitoneal treatment of Lamivudine, a licensed drug for HBV treatment [78]. Later, this group introduced a new cationic lipid DODAG (N’,N’-dioctadecyl-N-4,8-diaza-10-aminodecanoyl-glycylamide) and achieved a similarly effective knock-down of HBV infection [134].
siRNA targeting 5’-UTR of HCV genome was encapsulated in liposomes composed of a cationic lipid, phosphatidylcholine (PC) and lactosylated phosphatidylethanol, which targeted the asialoglycoprotein receptor on hepatocytes [86]. A BD study with a fluorescently labeled (Alexa-568) siRNA in BALB/c mice showed preferential accumulation of siRNA in the liver in 30 min after injection with the lactosylated liposomes, whereas those delivered with non-lactosylated liposomes showed equally strong signals in the spleen and liver [86]. In a pharmacodynamics study with an HCV transgenic mouse model, siRNA targeting 325–344 nucleotides of HCV genome, delivered with the lactosylated liposomes, efficiently suppressed HCV protein expression in a dose-dependent manner 48h after a single treatment [86]. Similarly, siRNA was incorporated in cationic liposomes (‘DTC-apo’) composed of DOTAP and cholesterol decorated with apolipoprotein A-1, the protein component of high density lipoprotein (HDL) targeting hepatocytes. A BD study using fluorescently labeled 21-mer dsDNA encapsulated in DTC-apo showed relatively high fluorescence intensity in the liver compared to other organs like kidneys, lung, and spleen [89]. Without the apolipoprotein ligand, fluorescence was preferentially seen in the lung and spleen [89]. In vivo silencing effect was tested in a HCV mouse model created with hydrodynamic injection of plasmid DNA expressing viral structural proteins. HCV-specific siRNA/DTC-apo complex inhibited viral gene expression by 65% in the liver at 2 mg/kg siRNA on day 2 post administration. The extent and duration of siRNA were further improved by 2’-O-methyl modification of siRNA [89]. Apolipoprotein A-1 was later replaced with a recombinant form of apolipoprotein (rhApo) derived from E.coli to avoid the safety concern (e.g., pathogen contamination) related to the origin (plasma) [135]. In addition to siRNA, synthetic shRNA targeting the IRES of the HCV genome was incorporated in a mixture of lipids to form lipid NPs, showing long lasting potent efficacy against HCV in mice with minimal toxicity [87].
More recently, it was demonstrated that simple co-injection of a hepatocyte-targeted peptide could help deliver siRNA targeting conserved HBV sequences to the liver [77]. The peptide consisted of N-acetylgalatosamin (NAG), a ligand with high affinity for asialoglycoprotein receptors highly expressed on the hepatocytes, and melittin-like peptide (MLP), an endosomolytic agent to facilitate endolysosomal escape of siRNA [77]. A co-injection of NAG-MLP with liver-tropic cholesterol-conjugated siRNA increased the hepatic gene silencing effect in a dose-dependent manner, resulting in multilog repression of viral RNA, proteins, and viral DNA with long duration of effect in mouse models of HBV infection. This formulation is unique in that it does not require preformulation of the complex like typical siRNA delivery systems, thus reducing the complexity in large-scale manufacturing. It has recently entered phase IIa clinical trial for the treatment of chronic HBV infection (NCT02065336 ) [136].
As one of the primary sites for Ebola virus replication, the liver also serves as a valid target for siRNA therapy of Ebola virus infection. siRNA targeting individual regions of Zaire Ebola virus (ZEBOV) L gene was delivered with SNALPs [92]. Despite the evidence suggesting premature release of siRNA in circulation (differential BD of labeled SNALPs and siRNA), siRNA/SNALP provided post-exposure protection to guinea pig challenged with a lethal Ebola virus. Animals challenged with ZEBOV received siRNA treatment at 1, 24, 48, 72, 96, 120, 144h after the infection. On day 30, 100% survival rate (n=5) was observed in the group treated with the siRNA/SNALP formulation, while the control group treated with scrambled siRNA/SNALPs presented 20% survival rate. Consistently, the plague titer analysis on day 7 showed a significantly reduced blood level in animals treated with siRNA/SNALPs compared to the control group. Later this group encapsulated a cocktail of three siRNA that each targeted L protein, VP24 and VP35 genes in ZEBOV in SNALPs and showed complete post-exposure protection against ZEBOV in monkeys [91]. Tekmira Pharmaceuticals commenced a Phase I clinical trial for the cocktail siRNA-SNALP formulation (TKM-100802) in Jan 2014 (NCT02041715) [137]. Two patients transported from West Africa to the United States during the 2014 outbreak of Ebola virus disease were treated with TKM-100802 and other supportive measures and recovered without serious long-term sequelae, although the actual role the siRNA formulation played in the recovery of these patients remains to be investigated [138].
5.2 Respiratory diseases
For systemic delivery of siRNA to the lungs, cationic polymers such as PEI are frequently used as carriers [139]. In an early study by Klibanov et al, deacylated PEI was used as a carrier of siRNA to the lungs [99]. Here, deacylation increased the number of protonatable nitrogens of PEI, thereby facilitating complexation with siRNA and transfection of target cells. Luc-siRNA-deacylated PEI complexes were administered to mice transfected with luciferase-encoding DNA by retroobrital injection, and the luciferase activities in different tissues were measured at 24h after the treatment. The Luc-siRNA/polymer complexes showed 77 or 93% suppression of the gene expression in the lungs, depending on the molecular weight of the polymer. Consistently, siRNA targeting influenza viral genes, as complexed with deacylated PEI, led to significant reduction in the virus titer in the lungs of influenza-infected mice 24h after injection [99]. More recently, Bolcato-Bellemin et al used PEI as a carrier of siRNA with sticky overhangs (ssiRNA) targeting cyclin B1 or survivin to suppress tumor progression in a lung metastasis model [140]. Here, ssiRNA formed a larger linear nucleic acid structure via hybridization of the complementary overhangs and made a relatively more stable complex with PEI, which survived better than a classic siRNA/PEI complex in circulation [141].
Cationic liposomes tend to accumulate in the liver and hence are popular in the delivery to the liver, but they are also used for lung delivery. McMillan et al used cationic liposomes containing DOTAP and DOPE as a carrier of siRNA targeting lung endothelium [142]. A BD study with the liposomes labeled with a non-covalently incorporated fluorescent dye found the highest fluorescence intensity was found in the liver, followed by lung and spleen. The liposomes transfected various cell populations in the lung, including CD146+ endothelial cells and CD326+ epithelial cells. Accordingly, mice treated with lamin A/C-specific siRNA-liposomes (2 mg/kg siRNA) showed 80% knockdown of lamin A/C in the lung at 48h after a single IV injection, with the majority silencing occurring in the endothelial and epithelial cells [142]. A new choice of cationic lipids and combinations improved lung-specific BD of liposomes [143]. Kaufmann et al used “DACC” liposomes containing AtuFECT01 (β-L-arginyl-2,3-L-diaminopropionic acid-N-palmityl-N-oleyl-amide trihydrochloride) as a cationic lipid for systemic administration of siRNA. When combined with cholesterol and mPEG2000-DSPE in optimal ratios, DACC liposomes delivered the most siRNA to the lung tissues at 1h after systemic application, followed by the spleen and the liver. On the other hand, other liposomes containing AtuFECT01 and different colipids and/or PEG-lipids showed typical BD pattern of cationic liposomes (high liver and spleen distribution) [143]. In accordance with the BD profile, DACC lipoplexes with siRNA specific for Tie-2 gene showed over 80% reduction in Tie-2 mRNA in lung tissue of mice but no significant Tie-2 knockdown in liver, kidney and heart tissues after 3 daily injections of 2.8 mg siRNA/kg [143]. DACC lipoplexes also helped deliver siRNAs targeting other genes in the lung vasculature including CD31, responsible for multiple processes of tumorigenesis, increasing the survival rate in an experimental lung metastasis mouse model [143]. Geick et al also found BD favorable for lung delivery of siRNA with a lipopolyamine called Staramine [144]. siRNA level in each tissue was measured by the stem-loop qRT PCR over 96h post intravenous injection. Liver accumulation was dominant immediately after the injection, but lung accumulation occurred over time, exceeding the levels in the liver, spleen, and kidneys in 24h. Consistently, siRNA/staramine complexes showed ~60% reduction of target mRNA in the lungs over 96h after a single administration, whereas the level of target mRNA in the liver was not significantly different from the one treated with control siRNA [144]. The siRNA retention in the lungs was attributed to the affinity of nanocomplexes for the endothelial lining in the capillary bed and relatively low exposure to phagocytic cells [144].
Other types of siRNA carriers to the lung include hybrids of lipid and polymer or lipid and inorganic components. Polymeric NPs made of conjugates of low molecular weight polycation (PEI) and lipids were used for delivery of siRNA to lung endothelial cells [145]. Unlike lipid-based carriers, the lipid-polymer hybrid NPs achieved the gene silencing effect at a dose that did not affect hepatocytes or immune cells. The biological effects of siRNAs delivered with the NPs were confirmed in the lungs, as an inducer of an emphysema-like phenotype (by silencing VEGFR-2 gene) or a suppressor of primary and metastatic Lewis lung carcinoma (by silencing VEGFR-1 and Dll4) [145].
In addition to systemic administration, siRNA has been directly delivered to the lungs as an aerosol (intratracheal or intranasal administration in experimental studies). One of the advantages of airway delivery is the potential to achieve high local concentration of siRNA in the lungs without going through blood circulation, which can compromise the stability of the siRNA/carrier complexes or siRNA itself and subject them to distribution in other MPS organs. Minko et al administered siRNA/cationic liposome complexes via intravenous injection or intratracheal instillation to mice bearing tumors in the lung to compare the BD [146]. At 24h post-administration, both the carrier and siRNA showed substantially high peak concentrations and long retentions in the lungs compared to other organs after intratracheal administration. On the other hand, IV injection resulted in broad distribution in other organs with relatively low levels of liposomes and siRNA in the lungs [146]. Kissel et al also delivered siRNA with PEI or PEG-grafted PEI (PEG-PEI) as carriers via intratracheal instillation and observed the BD of siRNA and the polymer by single photon emission computed tomography (SPECT) and radioactivity counting of organs [147]. Both siRNA and PEG-PEI were detectable in the lungs at 48h after instillation. Without PEG, siRNA/PEI complexes showed differential lung accumulation of each component: negligible level of siRNA and high level of PEI (even higher than that of PEG-PEI), which suggested dissociation of the complex in the lung and preferential association of PEI with mucus and cell membranes [147]. The locally delivered EGFP-specific siRNA/PEG-PEI complexes reached bronchial cells and alveolar cells and knocked down the EGFP expression in the lungs by 42% after 5 days post-instillation [147]. Airway administration is potentially a promising way of delivering siRNA to the lungs; however, studies to date suggest remaining challenges in airway delivery of siRNA, including the destructive interaction with anionic components in the lungs [147] and local irritation that can lead to inflammatory consequences [148].
5.3 Potential toxicity of siRNA therapeutics
siRNA with suboptimal potency can result in unintended side effects such as downregulation of off-target genes or activation of innate immune responses [149]. Efforts to mitigate these off-target effects include chemical modification of siRNA, redesign of the sequence, and the use of siRNA cocktails [149–151]. Toxicity may also come from the delivery systems. For example, intravenously injected lipoplexes have shown to induce the production of pro-inflammatory cytokines with signs of hepatocyte injury [152, 153]. These pro-inflammatory effects were initially attributed to the polynucleotide part of the complex rather than the carrier [152, 153]; however, later studies have reported intrinsic pro-inflammatory properties of some cationic lipids [154–156]. PEI, the most commonly used polymeric gene carrier, shows molecular weight, structure, and dose-dependent toxicity [157]. This challenge is currently tackled by developing degradable polycations, which can be degraded into less toxic small molecules [158].
6. Closing Remarks
We discussed several siRNA delivery approaches focusing on their contributions to the control of PK and BD of siRNA. Solid tumors are obviously one of the most anticipated targets, and many studies show PK/BD profiles favorable for siRNA delivery to tumors. Despite the optimism prevalent in the literature, one may recognize that most clinical translations are made in the applications targeting the liver and the lungs, where the majority of siRNA/NP systems naturally accumulate. This indicates that systemic delivery of siRNA in a therapeutic dose to tissues other than MPS organs remains suboptimal. Given the discrepancy, some of the current experimental practices may be worth reconsideration. First, many studies rely on labeling siRNA for PK and BD studies. As mentioned earlier, these approaches are valid only when the labeled components represent the full-length, bioactive siRNA; otherwise, inactive catabolites retaining the label may be mistaken for siRNA in target tissues. In addition, one should also consider that structural changes due to labeling may alter the PK/BD profiles of siRNA. To avoid overestimation of siRNA in PK/BD studies, it is desirable to complement with hybridization-based methods that can detect full-length siRNA, such as Northern blot analysis, hybridization-ligation assay, or PCR-based assay. Second, BD of siRNA is often presented as % of injected dose per unit mass of tissue at the final time point of the study. While this presentation offers a useful means to compare the relative concentrations of siRNA in different tissues, it rarely reports the amount of siRNA collected in the feces and urine, making it difficult to judge the fraction of siRNA that was immediately excreted. A caveat of this practice is that relative siRNA distribution in selected tissues can mislead to an overrated optimism for target-specific siRNA delivery, when in reality the absolute amount of siRNA in target tissue may be only a small fraction of the injected dose. Such misinterpretation may be prevented by considering the concentration in each tissue in addition to the relative quantity. Finally, it is not unusual to see studies tracking carriers in lieu of siRNA in PK/BD studies. This approach may be valid when the siRNA/carrier complex remains stable throughout the study, which, however, should not be taken for granted without proof. It is possible that siRNA/carrier complexes disassemble prematurely during circulation but still show favorable pharmacological effects due to other biological events related to the carrier itself. In order to identify actual contribution of carriers to the siRNA delivery, it is desirable to track siRNA and carrier separately through distinct labeling and/or detection techniques.
Supplementary Material
Acknowledgments
This work was supported by Global Innovative Research Center program (2012K1A1A2A01055811) of the National Research Foundation of Korea and by the Intramural Research Program (Global RNAi Carrier Initiative) of KIST.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 2.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;24:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 3. https://clinicaltrials.gov/show/NCT00363714. A Dose Escalation Trial of an Intravitreal Injection of Sirna-027 in Patients With Subfoveal Choroidal Neovascularization (CNV) Secondary to Age-Related Macular Degeneration (AMD)
- 4.Haussecker D. Current issues of RNAi therapeutics delivery and development. J. Control. Release. 2014;195:49–54. doi: 10.1016/j.jconrel.2014.07.056. [DOI] [PubMed] [Google Scholar]
- 5.Seth S, Johns R, Templin MV. Delivery and biodistribution of siRNA for cancer therapy: challenges and future prospects. Ther. Deliv. 2012;3:245–261. doi: 10.4155/tde.11.155. [DOI] [PubMed] [Google Scholar]
- 6.Kanasty R, Dorkin JR, Vegas A, Anderson D. Delivery materials for siRNA therapeutics. Nat. Mater. 2013;12:967–977. doi: 10.1038/nmat3765. [DOI] [PubMed] [Google Scholar]
- 7. https://clinicaltrials.gov/ct2/show/NCT02065336. Study of ARC-520 in Patients With Chronic Hepatitis B Virus
- 8.Guzman-Aranguez A, Loma P, Pintor J. Small-interfering RNAs (siRNAs) as a promising tool for ocular therapy. Br. J. Pharmacol. 2013;170:730–747. doi: 10.1111/bph.12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reich SJ, Fosnot J, Kuroki A, Tang W, Yang X, Maguire AM, Bennett J, Tolentino MJ. Small interfering RNA (siRNA) targeting VEGF effectively inhibits ocular neovascularization in a mouse model. Mol. Vis. 2003;9:210–216. [PubMed] [Google Scholar]
- 10.Murata M, Takanami T, Shimizu S, Kubota Y, Horiuchi S, Habano W, Ma J-x, Sato S. Inhibition of ocular angiogenesis by diced small interfering RNAs (siRNAs) specific to vascular endothelial growth factor (VEGF) Curr. Eye Res. 2006;31:171–180. doi: 10.1080/02713680500514636. [DOI] [PubMed] [Google Scholar]
- 11.Kim B, Tang Q, Biswas PS, Xu J, Schiffelers RM, Xie FY, Ansari AM, Scaria PV, Woodle MC, Lu P, Rouse BT. Inhibition of ocular angiogenesis by siRNA targeting vascular endothelial growth factor pathway genes. Am. J. Pathol. 2004;165:2177–2185. doi: 10.1016/S0002-9440(10)63267-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaiser PK, Symons RC, Shah SM, Quinlan EJ, Tabandeh H, Do DV, Reisen G, Lockridge JA, Short B, Guerciolini R, Nguyen QD, Sirna-027 Study I. RNAi-based treatment for neovascular age-related macular degeneration by Sirna-027. Am. J. Ophthalmol. 2010;150:33–39. e32. doi: 10.1016/j.ajo.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Frank RN. Diabetic retinopathy. N. Engl. J. Med. 2004;350:48–58. doi: 10.1056/NEJMra021678. [DOI] [PubMed] [Google Scholar]
- 14.Adamis AP, Miller JW, Bernal MT, D’Amico DJ, Folkman J, Yeo TK, Yeo KT. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am. J. Ophthalmol. 1994;118:445–450. doi: 10.1016/s0002-9394(14)75794-0. [DOI] [PubMed] [Google Scholar]
- 15.Garba AO, Mousa SA. Bevasiranib for the Treatment of Wet, Age-Related Macular Degeneration. Ophthalmol. Eye Dis. 2010;2:75–83. doi: 10.4137/OED.S4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen QD, Schachar RA, Nduaka CI, Sperling M, Basile AS, Klamerus KJ, Chi-Burris K, Yan E, Paggiarino DA, Rosenblatt I, Aitchison R, Erlich SS, Group DCS. Dose-ranging evaluation of intravitreal siRNA PF-04523655 for diabetic macular edema (the DEGAS study) Invest. Ophthalmol. Vis. Sci. 2012;53:7666–7674. doi: 10.1167/iovs.12-9961. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura H, Siddiqui SS, Shen X, Malik AB, Pulido JS, Kumar NM, Yue BY. RNA interference targeting transforming growth factor-beta type II receptor suppresses ocular inflammation and fibrosis. Mol. Vis. 2004;10:703–711. [PubMed] [Google Scholar]
- 18.Ye H, Qian Y, Lin M, Duan Y, Sun X, Zhuo Y, Ge J. Cationic nano-copolymers mediated IKKbeta targeting siRNA to modulate wound healing in a monkey model of glaucoma filtration surgery. Mol. Vis. 2010;16:2502–2510. [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez T, Gonzalez MV, Roehl I, Wright N, Paneda C, Jimenez AI. In vitro and in vivo efficacy of SYL040012, a novel siRNA compound for treatment of glaucoma. Mol. Ther. 2014;22:81–91. doi: 10.1038/mt.2013.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thakur A, Fitzpatrick S, Zaman A, Kugathasan K, Muirhead B, Hortelano G, Sheardown H. Strategies for ocular siRNA delivery: Potential and limitations of non-viral nanocarriers. J. Biol. Eng. 2012;6:7. doi: 10.1186/1754-1611-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez-Aguayo C, Chavez-Reyes A, Lopez-Berestein G, Sood AK. RNAi in cancer therapy. In: Cheng K, Mahato RI, editors. Advanced Delivery and Therapeutic Applications of RNAi. John Wiley and Sons, Ltd; 2013. pp. 271–307. [Google Scholar]
- 22.Davis ME, Zuckerman JE, Choi CHJ, Seligson D, Tolcher A, Alabi CA, Yen Y, Heidel JD, Ribas A. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464:1067–1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gandhi NS, Tekade RK, Chougule MB. Nanocarrier mediated delivery of siRNA/miRNA in combination with chemotherapeutic agents for cancer therapy: Current progress and advances. J. Control. Release. 2014;194:238–256. doi: 10.1016/j.jconrel.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 25.Cole MD. The myc oncogene: its role in transformation and differentiation. Annu Rev Genet. 1986;20:361–384. doi: 10.1146/annurev.ge.20.120186.002045. [DOI] [PubMed] [Google Scholar]
- 26.Sulman EP, Tang XX, Allen C, Biegel JA, Pleasure DE, Brodeur GM, Ikegaki N. ECK, a human EPH-related gene, maps to 1p36.1, a common region of alteration in human cancers. Genomics. 1997;40:371–374. doi: 10.1006/geno.1996.4569. [DOI] [PubMed] [Google Scholar]
- 27.Rapp UR, Goldsborough MD, Mark GE, Bonner TI, Groffen J, Reynolds FH, Jr, Stephenson JR. Structure and biological activity of v-raf, a unique oncogene transduced by a retrovirus. Proc. Natl. Acad. Sci. U. S. A. 1983;80:4218–4222. doi: 10.1073/pnas.80.14.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holtrich U, Wolf G, Brauninger A, Karn T, Bohme B, Rubsamen-Waigmann H, Strebhardt K. Induction and down-regulation of PLK, a human serine/threonine kinase expressed in proliferating cells and tumors. Proc. Natl. Acad. Sci. U. S. A. 1994;91:1736–1740. doi: 10.1073/pnas.91.5.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee MG, Nurse P. Complementation used to clone a human homologue of the fission yeast cell cycle control gene cdc2. Nature. 1987;327:31–35. doi: 10.1038/327031a0. [DOI] [PubMed] [Google Scholar]
- 30.Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim. Biophys. Acta. 1976;455:152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y, Gu H, Zhang DS, Li F, Liu T, Xia W. Highly effective inhibition of lung cancer growth and metastasis by systemic delivery of siRNA via multimodal mesoporous silica-based nanocarrier. Biomaterials. 2014;35:10058–10069. doi: 10.1016/j.biomaterials.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Liu X, Wang W, Samarsky D, Liu L, Xu Q, Zhang W, Zhu G, Wu P, Zuo X, Deng H, Zhang J, Wu Z, Chen X, Zhao L, Qiu Z, Zhang Z, Zeng Q, Yang W, Zhang B, Ji A. Tumor-targeted in vivo gene silencing via systemic delivery of cRGD-conjugated siRNA. Nucleic Acids Res. 2015;42:11805–11817. doi: 10.1093/nar/gku831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y, Bathula SR, Yang Q, Huang L. Targeted nanoparticles deliver siRNA to melanoma. J Invest Dermatol. 2010;130:2790–2798. doi: 10.1038/jid.2010.222. [DOI] [PubMed] [Google Scholar]
- 34.Chen Y, Zhu X, Zhang X, Liu B, Huang L. Nanoparticles modified with tumor-targeting scFv deliver siRNA and miRNA for cancer therapy. Mol. Ther. 2010;18:1650–1656. doi: 10.1038/mt.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Landen CN, Jr, Chavez-Reyes A, Bucana C, Schmandt R, Deavers MT, Lopez-Berestein G, Sood AK. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Res. 2005;65:6910–6918. doi: 10.1158/0008-5472.CAN-05-0530. [DOI] [PubMed] [Google Scholar]
- 36.Chou ST, Leng Q, Scaria P, Kahn JD, Tricoli LJ, Woodle M, Mixson AJ. Surface-modified HK:siRNA nanoplexes with enhanced pharmacokinetics and tumor growth inhibition. Biomacromolecules. 2013;14:752–760. doi: 10.1021/bm3018356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakurai Y, Hatakeyama H, Sato Y, Hyodo M, Akita H, Harashima H. Gene silencing via RNAi and siRNA quantification in tumor tissue using MEND, a liposomal siRNA delivery system. Mol. Ther. 2013;21:1195–1203. doi: 10.1038/mt.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y, Zhu YH, Mao CQ, Dou S, Shen S, Tan ZB, Wang J. Triple negative breast cancer therapy with CDK1 siRNA delivered by cationic lipid assisted PEG-PLA nanoparticles. J. Control. Release. 2014;192:114–121. doi: 10.1016/j.jconrel.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 39.Zhu L, Perche F, Wang T, Torchilin VP. Matrix metalloproteinase 2-sensitive multifunctional polymeric micelles for tumor-specific co-delivery of siRNA and hydrophobic drugs. Biomaterials. 2014;35:4213–4222. doi: 10.1016/j.biomaterials.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yhee JY, Song S, Lee SJ, Park SG, Kim KS, Kim MG, Son S, Koo H, Kwon IC, Jeong JH, Jeong SY, Kim SH, Kim K. Cancer-targeted MDR-1 siRNA delivery using self-cross-linked glycol chitosan nanoparticles to overcome drug resistance. J. Control. Release. 2015;198:1–9. doi: 10.1016/j.jconrel.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 41.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- 42.Zhuang D, Mannava S, Grachtchouk V, Tang WH, Patil S, Wawrzyniak JA, Berman AE, Giordano TJ, Prochownik EV, Soengas MS, Nikiforov MA. C-MYC overexpression is required for continuous suppression of oncogene-induced senescence in melanoma cells. Oncogene. 2008;27:6623–6634. doi: 10.1038/onc.2008.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108:153–164. doi: 10.1016/s0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 44.Thaker PH, Deavers M, Celestino J, Thornton A, Fletcher MS, Landen CN, Kinch MS, Kiener PA, Sood AK. EphA2 expression is associated with aggressive features in ovarian carcinoma. Clin. Cancer. Res. 2004;10:5145–5150. doi: 10.1158/1078-0432.CCR-03-0589. [DOI] [PubMed] [Google Scholar]
- 45.Nakamoto M, Bergemann AD. Diverse roles for the Eph family of receptor tyrosine kinases in carcinogenesis. Microsc. Res. Tech. 2002;59:58–67. doi: 10.1002/jemt.10177. [DOI] [PubMed] [Google Scholar]
- 46.Eckerdt F, Yuan J, Strebhardt K. Polo-like kinases and oncogenesis. Oncogene. 2005;24:267–276. doi: 10.1038/sj.onc.1208273. [DOI] [PubMed] [Google Scholar]
- 47.Ando K, Ozaki T, Yamamoto H, Furuya K, Hosoda M, Hayashi S, Fukuzawa M, Nakagawara A. Polo-like kinase 1 (Plk1) inhibits p53 function by physical interaction and phosphorylation. J. Biol. Chem. 2004;279:25549–25561. doi: 10.1074/jbc.M314182200. [DOI] [PubMed] [Google Scholar]
- 48.Malumbres M, Barbacid M. Mammalian cyclin-dependent kinases. Trends Biochem. Sci. 2005;30:630–641. doi: 10.1016/j.tibs.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 49.Malumbres M, Barbacid M. To cycle or not to cycle: a critical decision in cancer. Nat. Rev. Cancer. 2001;1:222–231. doi: 10.1038/35106065. [DOI] [PubMed] [Google Scholar]
- 50.Malumbres M. Cyclin-dependent kinases. Genome biology. 2014;15:122. doi: 10.1186/gb4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bonelli P, Tuccillo FM, Borrelli A, Schiattarella A, Buonaguro FM. CDK/CCN and CDKI alterations for cancer prognosis and therapeutic predictivity. Biomed. Res. Int. 2014;2014:361020. doi: 10.1155/2014/361020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. http://www.focr.org/breakthrough-therapies. Breakthrough Therapies
- 53.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat. Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 54.Altieri DC, Survivin versatile modulation of cell division and apoptosis in cancer. Oncogene. 2003;22:8581–8589. doi: 10.1038/sj.onc.1207113. [DOI] [PubMed] [Google Scholar]
- 55.Bradley G, Ling V. P-glycoprotein, multidrug resistance and tumor progression. Cancer Metastasis Rev. 1994;13:223–233. doi: 10.1007/BF00689638. [DOI] [PubMed] [Google Scholar]
- 56.Abbasi M, Lavasanifar A, Uludag H. Recent attempts at RNAi-mediated P-glycoprotein downregulation for reversal of multidrug resistance in cancer. Med. Res. Rev. 2013;33:33–53. doi: 10.1002/med.20244. [DOI] [PubMed] [Google Scholar]
- 57.Lorenzer C, Dirin M, Winkler AM, Baumann V, Winkler J. Going beyond the liver: Progress and challenges of targeted delivery of siRNA therapeutics. J. Control. Release. 2015;203:1–15. doi: 10.1016/j.jconrel.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 58.Arbuthnot P, Ely A, Weinberg MS. Hepatic delivery of RNA interference activators for therapeutic application. Curr. Gene Ther. 2009;9:91–103. doi: 10.2174/156652309787909517. [DOI] [PubMed] [Google Scholar]
- 59.Erez A, Shchelochkov OA, Plon SE, Scaglia F, Lee B. Insights into the pathogenesis and treatment of cancer from inborn errors of metabolism. Am. J. Hum. Genet. 2011;88:402–421. doi: 10.1016/j.ajhg.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.AlixScholer-Dahirel MRS, Loo Alice, Bagdasarian Linda, Meyer Ronald, Guo Ribo, Woolfenden Steve, Kristine JM, Yu K, Killary Karen, Dmitry Sonkin, Yao Yung-Mae, Warmuth Markus, Sellers William R, Schlegel F. S. Robert, Mosher Rebecca E, McLaughlin Margaret E. Maintenance of adenomatous polyposis coli(APC)-mutant colorectal cancer is dependent on Wnt/β-catenin signaling. Proc. Natl. Acad. Sci. U. S. A. 2011;108:17135–17140. doi: 10.1073/pnas.1104182108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Raskopf E, Vogt A, Sauerbruch T, Schmitz V. siRNA targeting VEGF inhibits hepatocellular carcinoma growth and tumor angiogenesis in vivo. J. Hepatol. 2008;49:977–984. doi: 10.1016/j.jhep.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 62.Zhang K, Chu K, Wu X, Gao H, Wang J, Yuan YC, Loera S, Ho K, Wang Y, Chow W, Un F, Chu P, Yen Y. Amplification of FRS2 and activation of FGFR/FRS2 signaling pathway in high-grade liposarcoma. Cancer Res. 2013;73:1298–1307. doi: 10.1158/0008-5472.CAN-12-2086. [DOI] [PubMed] [Google Scholar]
- 63.Rychahou PG, Jackson LN, Silva SR, Rajaraman S, Evers BM. Targeted molecular therapy of the PI3K pathway: therapeutic significance of PI3K subunit targeting in colorectal carcinoma. Ann. Surg. 2006;243:833–842. doi: 10.1097/01.sla.0000220040.66012.a9. discussion 843-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 65.Kaseb AO, Hanbali A, Cotant M, Hassan MM, Wollner I, Philip PA. Vascular endothelial growth factor in the management of hepatocellular carcinoma: a review of literature. Cancer. 2009;115:4895–4906. doi: 10.1002/cncr.24537. [DOI] [PubMed] [Google Scholar]
- 66.Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 67.Fruman DA, Rommel C. PI3K and cancer: lessons. challenges and opportunities. Nat. Rev. Drug Discov. 2014;13:140–156. doi: 10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang P, Xu X, Wang L, Zhu B, Wang X, Xia J. The role of EGF-EGFR signalling pathway in hepatocellular carcinoma inflammatory microenvironment. J. Cell. Mol. Med. 2014;18:218–230. doi: 10.1111/jcmm.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Whittaker S, Marais R, Zhu AX. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene. 2010;29:4989–5005. doi: 10.1038/onc.2010.236. [DOI] [PubMed] [Google Scholar]
- 70.Szabo G, Bala S. MicroRNAs in liver disease. Nat. Rev. Gastroenterol Hepatol. 2013;10:542–552. doi: 10.1038/nrgastro.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Azam F, Koulaouzidis A. Hepatitis B virus and Hepatocarcinogenesis. Ann. Hepatol. 2008;7:125–129. [PubMed] [Google Scholar]
- 72.Cefalu AB, Pirruccello JP, Noto D, Gabriel S, Valenti V, Gupta N, Spina R, Tarugi P, Kathiresan S, Averna MR. A novel APOB mutation identified by exome sequencing cosegregates with steatosis, liver cancer, and hypocholesterolemia. Arterioscler. Thromb. Vasc. Biol. 2013;33:2021–2025. doi: 10.1161/ATVBAHA.112.301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang XQ, Zhang W, Lui EL, Zhu Y, Lu P, Yu X, Sun J, Yang S, Poon RT, Fan ST. Notch1-Snail1-E-cadherin pathway in metastatic hepatocellular carcinoma. Int. J. Cancer. 2012;131:E163–E172. doi: 10.1002/ijc.27336. [DOI] [PubMed] [Google Scholar]
- 74.Riaz M, Idrees M, Kanwal H, Kabir F. An overview of triple infection with hepatitis B, C and D viruses. Virol. J. 2011;8:368. doi: 10.1186/1743-422X-8-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. http://www.cdc.gov/hepatitis/B/bFAQ.htm. Hepatitis B information for public
- 76.Halegoua-De Marzio D, Hann H-W. Then and now: The progress in hepatitis B treatment over the past 20 years. World J. Gastroenterol. 2014;20:401–413. doi: 10.3748/wjg.v20.i2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wooddell CI, Rozema DB, Hossbach M, John M, Hamilton HL, Chu Q, Hegge JO, Klein JJ, Wakefield DH, Oropeza CE, Deckert J, Roehl I, Jahn-Hofmann K, Hadwiger P, Vornlocher H-P, McLachlan A, Lewis DL. Hepatocyte-targeted RNAi Therapeutics for the Treatment of Chronic Hepatitis B Virus Infection. Mol. Ther. 2013;21:973–985. doi: 10.1038/mt.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Carmona S, Jorgensen MR, Kolli S, Crowther C, Salazar FH, Marion PL, Fujino M, Natori Y, Thanou M, Arbuthnot P, Miller AD. Controlling HBV replication in vivo by intravenous administration of triggered PEGylated siRNA-nanoparticles. Mol. Pharm. 2009;6:706–717. doi: 10.1021/mp800157x. [DOI] [PubMed] [Google Scholar]
- 79.Kim SI, Shin D, Choi TH, Lee JC, Cheon G-J, Kim K-Y, Park M, Kim M. Systemic and specific delivery of small interfering RNAs to the liver mediated by apolipoprotein A-I. Mol. Ther. 2007;15:1145–1152. doi: 10.1038/sj.mt.6300168. [DOI] [PubMed] [Google Scholar]
- 80.Konishi M, Wu CH, Wu GY. Inhibition of HBV replication by siRNA in a stable HBV-producing cell line. Hepatology. 2003;38:842–850. doi: 10.1053/jhep.2003.50416. [DOI] [PubMed] [Google Scholar]
- 81.Li G, Jiang G, Lu J, Chen S, Cui L, Jiao J, Wang Y. Inhibition of hepatitis B virus cccDNA by siRNA in transgenic mice. Cell Biochem. Biophys. 2014;69:649–654. doi: 10.1007/s12013-014-9847-1. [DOI] [PubMed] [Google Scholar]
- 82.Li G-Q, Gu H-X, Li D, Xu W-Z. Inhibition of Hepatitis B virus cccDNA replication by siRNA. Biochem. Biophys. Res. Commun. 2007;355:404–408. doi: 10.1016/j.bbrc.2007.01.163. [DOI] [PubMed] [Google Scholar]
- 83.Grimm D. All for One, One for All: New Combinatorial RNAi therapies combat hepatitis C virus evolution. Mol. Ther. 2012;20:1661–1663. doi: 10.1038/mt.2012.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liang TJ, Ghany MG. Current and future therapies for hepatitis C virus infection. N. Engl. J. Med. 2013;368:1907–1917. doi: 10.1056/NEJMra1213651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Korf M, Jarczak D, Beger C, Manns MP, Kruger M. Inhibition of hepatitis C virus translation and subgenomic replication by siRNAs directed against highly conserved HCV sequence and cellular HCV cofactors. J. Hepatol. 2005;43:225–234. doi: 10.1016/j.jhep.2005.02.046. [DOI] [PubMed] [Google Scholar]
- 86.Watanabe T, Umehara T, Yasui F, Nakagawa S-i, Yano J, Ohgi T, Sonoke S, Satoh K, Inoue K, Yoshiba M, Kohara M. Liver target delivery of small interfering RNA to the HCV gene by lactosylated cationic liposome. J. Hepatol. 2007;47:744–750. doi: 10.1016/j.jhep.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 87.Ma H, Dallas A, Ilves H, Shorenstein J, MacLachlan I, Klumpp K, Johnston BH. Formulated minimal-length synthetic small hairpin RNAs are potent inhibitors of hepatitis C virus in mice with humanized livers. Gastroenterology. 2014;146:63–66.e65. doi: 10.1053/j.gastro.2013.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chandra PK, Kundu AK, Hazari S, Chandra S, Bao L, Ooms T, Morris GF, Wu T, Mandal TK, Dash S. Inhibition of hepatitis C virus replication by intracellular delivery of multiple siRNAs by nanosomes. Mol. Ther. 2012;20:1724–1736. doi: 10.1038/mt.2012.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim SI, Shin D, Lee H, Ahn BY, Yoon Y, Kim M. Targeted delivery of siRNA against hepatitis C virus by apolipoprotein A-I-bound cationic liposomes. J. Hepatol. 2009;50:479–488. doi: 10.1016/j.jhep.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 90.Ansar M, Ashfaq UA, Shahid I, Sarwar MT, Javed T, Rehman S, Hassan S, Riazuddin S. Inhibition of full length hepatitis C virus particles of 1a genotype through small interference RNA. Virol. J. 2011;8:8–203. doi: 10.1186/1743-422X-8-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Geisbert TW, Lee ACH, Robbins M, Geisbert JB, Honko AN, Sood V, Johnson JC, de Jong S, Tavakoli I, Judge A, Hensley LE, MacLachlan I. Postexposure protection of non-human primates against a lethal Ebola virus challenge with RNA interference: a proof-of-concept study. Lancet. 2010;375:1896–1905. doi: 10.1016/S0140-6736(10)60357-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Geisbert TW, Hensley LE, Kagan E, Yu EZ, Geisbert JB, Daddario-DiCaprio K, Fritz EA, Jahrling PB, McClintock K, Phelps JR, Lee ACH, Judge A, Jeffs LB, MacLachlan I. Postexposure protection of guinea pigs against a lethal ebola virus challenge is conferred by RNA interference. J. Infect. Dis. 2006;193:1650–1657. doi: 10.1086/504267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shlomai A, Shaul Y. Inhibition of hepatitis B virus expression and replication by RNA interference. Hepatology. 2003;37:764–770. doi: 10.1053/jhep.2003.50146. [DOI] [PubMed] [Google Scholar]
- 94.Merkel OM, Rubinstein I, Kissel T. siRNA delivery to the lung: what’s new? Adv. Drug Deliv. Rev. 2014;75:112–128. doi: 10.1016/j.addr.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kelly C, Yadav AB, McKiernan PJ, Greene CM, Cryan SA. RNAi in Respiratory Diseases. In: Cheng K, Mahato RI, editors. Advanced Delivery and Therapeutic Applications of RNAi. John Wiley and Sons, Ltd; 2013. pp. 391–416. [Google Scholar]
- 96.Grzelinski M, Pinkenburg O, Buch T, Gold M, Stohr S, Kalwa H, Gudermann T, Aigner A. Critical role of G(alpha)12 and G(alpha)13 for human small cell lung cancer cell proliferation in vitro and tumor growth in vivo. Clin. Cancer Res. 2010;16:1402–1415. doi: 10.1158/1078-0432.CCR-09-1873. [DOI] [PubMed] [Google Scholar]
- 97.Zamora-Avila DE, Zapata-Benavides P, Franco-Molina MA, Saavedra-Alonso S, Trejo-Avila LM, Resendez-Perez D, Mendez-Vazquez JL, Isaias-Badillo J, Rodriguez-Padilla C. WT1 gene silencing by aerosol delivery of PEI-RNAi complexes inhibits B16-F10 lung metastases growth. Cancer Gene Ther. 2009;16:892–899. doi: 10.1038/cgt.2009.35. [DOI] [PubMed] [Google Scholar]
- 98.Ge Q, Filip L, Bai A, Nguyen T, Eisen HN, Chen J. Inhibition of influenza virus production in virus-infected mice by RNA interference. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8676–8681. doi: 10.1073/pnas.0402486101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Thomas M, Lu JJ, Ge Q, Zhang C, Chen J, Klibanov AM. Full deacylation of polyethylenimine dramatically boosts its gene delivery efficiency and specificity to mouse lung. Proc. Natl. Acad. Sci. U. S. A. 2005;102:5679–5684. doi: 10.1073/pnas.0502067102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bitko V, Musiyenko A, Shulyayeva O, Barik S. Inhibition of respiratory viruses by nasally administered siRNA. Nat. Med. 2005;11:50–55. doi: 10.1038/nm1164. [DOI] [PubMed] [Google Scholar]
- 101.Clark KL, Hughes SA, Bulsara P, Coates J, Moores K, Parry J, Carr M, Mayer RJ, Wilson P, Gruenloh C, Levin D, Darton J, Weber WM, Sobczak K, Gill DR, Hyde SC, Davies LA, Pringle IA, Sumner-Jones SG, Jadhav V, Jamison S, Strapps WR, Pickering V, Edbrooke MR. Pharmacological Characterization of a Novel ENaCalpha siRNA (GSK2225745) With Potential for the Treatment of Cystic Fibrosis. Mol. Ther. Nucleic Acids. 2013;2:e65. doi: 10.1038/mtna.2012.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Seong JH, Lee KM, Kim ST, Jin SE, Kim CK. Polyethylenimine-based antisense oligodeoxynucleotides of IL-4 suppress the production of IL-4 in a murine model of airway inflammation. J. Gene. Med. 2006;8:314–323. doi: 10.1002/jgm.848. [DOI] [PubMed] [Google Scholar]
- 103.Lively TN, Kossen K, Balhorn A, Koya T, Zinnen S, Takeda K, Lucas JJ, Polisky B, Richards IM, Gelfand EW. Effect of chemically modified IL-13 short interfering RNA on development of airway hyperresponsiveness in mice. J. Allergy Clin. Immunol. 2008;121:88–94. doi: 10.1016/j.jaci.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zheng M, Librizzi D, Kilic A, Liu Y, Renz H, Merkel OM, Kissel T. Enhancing in vivo circulation and siRNA delivery with biodegradable polyethylenimine-graft-polycaprolactone-block-poly(ethylene glycol) copolymers. Biomaterials. 2012;33:6551–6558. doi: 10.1016/j.biomaterials.2012.05.055. [DOI] [PubMed] [Google Scholar]
- 105.Oe Y, Christie RJ, Naito M, Low SA, Fukushima S, Toh K, Miura Y, Matsumoto Y, Nishiyama N, Miyata K, Kataoka K. Actively-targeted polyion complex micelles stabilized by cholesterol and disulfide cross-linking for systemic delivery of siRNA to solid tumors. Biomaterials. 2014;35:7887–7895. doi: 10.1016/j.biomaterials.2014.05.041. [DOI] [PubMed] [Google Scholar]
- 106.Matsumoto Y, Nomoto T, Cabral H, Matsumoto Y, Watanabe S, Christie RJ, Miyata K, Oba M, Ogura T, Yamasaki Y, Nishiyama N, Yamasoba T, Kataoka K. Direct and instantaneous observation of intravenously injected substances using intravital confocal micro-videography. Biomed. Opt. Express. 2010;1:1209–1216. doi: 10.1364/BOE.1.001209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.van de Water FM, Boerman OC, Wouterse AC, Peters JG, Russel FG, Masereeuw R. Intravenously administered short interfering RNA accumulates in the kidney and selectively suppresses gene function in renal proximal tubules. Drug Metab. Dispos. 2006;34:1393–1397. doi: 10.1124/dmd.106.009555. [DOI] [PubMed] [Google Scholar]
- 108.Christensen J, Litherland K, Faller T, van de Kerkhof E, Natt F, Hunziker J, Krauser J, Swart P. Metabolism studies of unformulated internally [3H]-labeled short interfering RNAs in mice. Drug Metab. Dispos. 2013;41:1211–1219. doi: 10.1124/dmd.112.050666. [DOI] [PubMed] [Google Scholar]
- 109.Gao S, Dagnaes-Hansen F, Nielsen EJ, Wengel J, Besenbacher F, Howard KA, Kjems J. The effect of chemical modification and nanoparticle formulation on stability and biodistribution of siRNA in mice. Mol. Ther. 2009;17:1225–1233. doi: 10.1038/mt.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Christensen J, Litherland K, Faller T, van de Kerkhof E, Natt F, Hunziker J, Boos J, Beuvink I, Bowman K, Baryza J, Beverly M, Vargeese C, Heudi O, Stoeckli M, Krauser J, Swart P. Biodistribution and metabolism studies of lipid nanoparticle-formulated internally [3H]-labeled siRNA in mice. Drug Metab. Dispos. 2014;42:431–440. doi: 10.1124/dmd.113.055434. [DOI] [PubMed] [Google Scholar]
- 111.Ozcan G, Ozpolat B, Coleman RL, Sood AK, Lopez-Berestein G. Preclinical and clinical development of siRNA-based therapeutics. Adv. Drug Deliv. Rev. 2015;87:108–119. doi: 10.1016/j.addr.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tabernero J, Shapiro GI, LoRusso PM, Cervantes A, Schwartz GK, Weiss GJ, Paz-Ares L, Cho DC, Infante JR, Alsina M, Gounder MM, Falzone R, Harrop J, White AC, Toudjarska I, Bumcrot D, Meyers RE, Hinkle G, Svrzikapa N, Hutabarat RM, Clausen VA, Cehelsky J, Nochur SV, Gamba-Vitalo C, Vaishnaw AK, Sah DW, Gollob JA, Burris HA., 3rd First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 2013;3:406–417. doi: 10.1158/2159-8290.CD-12-0429. [DOI] [PubMed] [Google Scholar]
- 113.Schultheis B, Strumberg D, Santel A, Vank C, Gebhardt F, Keil O, Lange C, Giese K, Kaufmann J, Khan M, Drevs J. First-in-human phase I study of the liposomal RNA interference therapeutic Atu027 in patients with advanced solid tumors. J. Clin. Oncol. 2014;32:4141–4148. doi: 10.1200/JCO.2013.55.0376. [DOI] [PubMed] [Google Scholar]
- 114.Bartlett DW, Su H, Hildebrandt IJ, Weber WA, Davis ME. Impact of tumor-specific targeting on the biodistribution and efficacy of siRNA nanoparticles measured by multimodality in vivo imaging. Proc. Natl. Acad. Sci. U. S. A. 2007;104:15549–15554. doi: 10.1073/pnas.0707461104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zuckerman JE, Gritli I, Tolcher A, Heidel JD, Lim D, Morgan R, Chmielowski B, Ribas A, Davis ME, Yen Y. Correlating animal and human phase Ia/Ib clinical data with CALAA-01, a targeted, polymer-based nanoparticle containing siRNA. Proc. Natl. Acad. Sci. U. S. A. 2014;111:11449–11454. doi: 10.1073/pnas.1411393111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yu RZ, Baker B, Chappell A, Geary RS, Cheung E, Levin AA. Development of an ultrasensitive noncompetitive hybridization-ligation enzyme-linked immunosorbent assay for the determination of phosphorothioate oligodeoxynucleotide in plasma. Anal. Biochem. 2002;304:19–25. doi: 10.1006/abio.2002.5576. [DOI] [PubMed] [Google Scholar]
- 117.Zuckerman JE, Choi CH, Han H, Davis ME. Polycation-siRNA nanoparticles can disassemble at the kidney glomerular basement membrane. Proc. Natl. Acad. Sci. U. S. A. 2012;109:3137–3142. doi: 10.1073/pnas.1200718109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen Y, Wu JJ, Huang L. Nanoparticles targeted with NGR motif deliver c-myc siRNA and doxorubicin for anticancer therapy. Mol. Ther. 2010;18:828–834. doi: 10.1038/mt.2009.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lee MS, Lee JE, Byun E, Kim NW, Lee K, Lee H, Sim SJ, Lee DS, Jeong JH. Target-specific delivery of siRNA, by stabilized calcium phosphate nanoparticles using dopa-hyaluronic acid conjugate. J. Control. Release. 2014;192:122–130. doi: 10.1016/j.jconrel.2014.06.049. [DOI] [PubMed] [Google Scholar]
- 120.Pittella F, Cabral H, Maeda Y, Mi P, Watanabe S, Takemoto H, Kim HJ, Nishiyama N, Miyata K, Kataoka K. Systemic siRNA delivery to a spontaneous pancreatic tumor model in transgenic mice by PEGylated calcium phosphate hybrid micelles. J. Control. Release. 2014;178:18–24. doi: 10.1016/j.jconrel.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 121.Son S, Song S, Lee SJ, Min S, Kim SA, Yhee JY, Huh MS, Chan Kwon I, Jeong SY, Byun Y, Kim SH, Kim K. Self-crosslinked human serum albumin nanocarriers for systemic delivery of polymerized siRNA to tumors. Biomaterials. 2013;34:9475–9485. doi: 10.1016/j.biomaterials.2013.08.085. [DOI] [PubMed] [Google Scholar]
- 122.Shi B, Keough E, Matter A, Leander K, Young S, Carlini E, Sachs AB, Tao W, Abrams M, Howell B, Sepp-Lorenzino L. Biodistribution of small interfering RNA at the organ and cellular levels after lipid nanoparticle-mediated delivery. J. Histochem. Cytochem. 2011;59:727–740. doi: 10.1369/0022155411410885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Choi KY, Silvestre OF, Huang X, Min KH, Howard GP, Hida N, Jin AJ, Carvajal N, Lee SW, Hong JI, Chen X. Versatile RNA interference nanoplatform for systemic delivery of RNAs. ACS Nano. 2014;8:4559–4570. doi: 10.1021/nn500085k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jensen SA, Day ES, Ko CH, Hurley LA, Luciano JP, Kouri FM, Merkel TJ, Luthi AJ, Patel PC, Cutler JI, Daniel WL, Scott AW, Rotz MW, Meade TJ, Giljohann DA, Mirkin CA, Stegh AH. Spherical nucleic acid nanoparticle conjugates as an RNAi-based therapy for glioblastoma. Sci. Transl. Med. 2013;5 doi: 10.1126/scitranslmed.3006839. 209ra152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schlegel A, Largeau C, Bigey P, Bessodes M, Lebozec K, Scherman D, Escriou V. Anionic polymers for decreased toxicity and enhanced in vivo delivery of siRNA complexed with cationic liposomes. J. Control. Release. 2011;152:393–401. doi: 10.1016/j.jconrel.2011.03.031. [DOI] [PubMed] [Google Scholar]
- 126.Gao J, Chen H, Yu Y, Song J, Song H, Su X, Li W, Tong X, Qian W, Wang H, Dai J, Guo Y. Inhibition of hepatocellular carcinoma growth using immunoliposomes for co-delivery of adriamycin and ribonucleotide reductase M2 siRNA. Biomaterials. 2013;34:10084–10098. doi: 10.1016/j.biomaterials.2013.08.088. [DOI] [PubMed] [Google Scholar]
- 127.Xiong XB, Lavasanifar A. Traceable multifunctional micellar nanocarriers for cancer-targeted co-delivery of MDR-1 siRNA and doxorubicin. ACS Nano. 2011;5:5202–5213. doi: 10.1021/nn2013707. [DOI] [PubMed] [Google Scholar]
- 128.Sass G, Leukel P, Schmitz V, Raskopf E, Ocker M, Neureiter D, Meissnitzer M, Tasika E, Tannapfel A, Tiegs G. Inhibition of heme oxygenase 1 expression by small interfering RNA decreases orthotopic tumor growth in livers of mice. Int. J. Cancer. 2008;123:1269–1277. doi: 10.1002/ijc.23695. [DOI] [PubMed] [Google Scholar]
- 129.Kornek M, Lukacs-Kornek V, Limmer A, Raskopf E, Becker U, Klockner M, Sauerbruch T, Schmitz V. 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP)-formulated, immune-stimulatory vascular endothelial growth factor a small interfering RNA (siRNA) increases antitumoral efficacy in murine orthotopic hepatocellular carcinoma with liver fibrosis. Mol. Med. 2008;14:365–373. doi: 10.2119/2008-00003.Kornek. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li L, Wang R, Wilcox D, Sarthy A, Lin X, Huang X, Tian L, Dande P, Hubbard RD, Hansen TM, Wada C, Zhao X, Kohlbrenner WM, Fesik SW, Shen Y. Developing lipid nanoparticle-based siRNA therapeutics for hepatocellular carcinoma using an integrated approach. Mol. Cancer Ther. 2013;12:2308–2318. doi: 10.1158/1535-7163.MCT-12-0983-T. [DOI] [PubMed] [Google Scholar]
- 131.Gao J, Yu Y, Zhang Y, Song J, Chen H, Li W, Qian W, Deng L, Kou G, Chen J, Guo Y. EGFR-specific PEGylated immunoliposomes for active siRNA delivery in hepatocellular carcinoma. Biomaterials. 2012;33:270–282. doi: 10.1016/j.biomaterials.2011.09.035. [DOI] [PubMed] [Google Scholar]
- 132.Morrissey DV, Lockridge JA, Shaw L, Blanchard K, Jensen K, Breen W, Hartsough K, Machemer L, Radka S, Jadhav V, Vaish N, Zinnen S, Vargeese C, Bowman K, Shaffer CS, Jeffs LB, Judge A, MacLachlan I, Polisky B. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat. Biotechnol. 2005;23:1002–1007. doi: 10.1038/nbt1122. [DOI] [PubMed] [Google Scholar]
- 133.Morrissey DV, Blanchard K, Shaw L, Jensen K, Lockridge JA, Dickinson B, McSwiggen JA, Vargeese C, Bowman K, Shaffer CS, Polisky BA, Zinnen S. Activity of stabilized short interfering RNA in a mouse model of hepatitis B virus replication. Hepatology. 2005;41:1349–1356. doi: 10.1002/hep.20702. [DOI] [PubMed] [Google Scholar]
- 134.Mével M, Kamaly N, Carmona S, Oliver MH, Jorgensen MR, Crowther C, Salazar FH, Marion PL, Fujino M, Natori Y, Thanou M, Arbuthnot P, Yaouanc J-J, Jaffrès PA, Miller AD. DODAG; a versatile new cationic lipid that mediates efficient delivery of pDNA and siRNA. J. Control. Release. 2010;143:222–232. doi: 10.1016/j.jconrel.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 135.Lee H, Kim SI, Shin D, Yoon Y, Choi TH, Cheon G-J, Kim M. Hepatic siRNA delivery using recombinant human apolipoprotein A-I in mice. Biochem. Biophys. Res. Commun. 2009;378:192–196. doi: 10.1016/j.bbrc.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 136. https://clinicaltrials.gov/show/NCT02065336. Study of ARC-520 in Patients With Chronic Hepatitis B Virus
- 137. https://clinicaltrials.gov/show/NCT02041715. Safety, Tolerability and Pharmacokinetic First in Human (FIH) Study for Intravenous (IV) TKM-100802
- 138.Kraft CS, Hewlett AL, Koepsell S, Winkler AM, Kratochvil CJ, Larson L, Varkey JB, Mehta AK, Lyon GM, 3rd, Friedman-Moraco RJ, Marconi VC, Hill CE, Sullivan JN, Johnson DW, Lisco SJ, Mulligan MJ, Uyeki TM, McElroy AK, Sealy T, Campbell S, Spiropoulou C, Stroher U, Crozier I, Sacra R, Connor MJ, Jr, Sueblinvong V, Franch HA, Smith PW, Ribner BS. The Use of TKM-100802 and Convalescent Plasma in 2 Patients With Ebola Virus Disease in the United States. Clin. Infect. Dis. 2015;61:496–502. doi: 10.1093/cid/civ334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gunther M, Lipka J, Malek A, Gutsch D, Kreyling W, Aigner A. Polyethylenimines for RNAi-mediated gene targeting in vivo and siRNA delivery to the lung. Eur. J. Pharm. Biopharm. 2011;77:438–449. doi: 10.1016/j.ejpb.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 140.Bonnet ME, Gossart JB, Benoit E, Messmer M, Zounib O, Moreau V, Behr JP, Lenne-Samuel N, Kedinger V, Meulle A, Erbacher P, Bolcato-Bellemin AL. Systemic delivery of sticky siRNAs targeting the cell cycle for lung tumor metastasis inhibition. J. Control. Release. 2013;170:183–190. doi: 10.1016/j.jconrel.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 141.Bolcato-Bellemin A-L, Bonnet M-E, Creusat G, Erbacher P, Behr J-P. Sticky overhangs enhance siRNA-mediated gene silencing. Proc. Natl. Acad. Sci. U. S. A. 2007;104:16050–16055. doi: 10.1073/pnas.0707831104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.McCaskill J, Singhania R, Burgess M, Allavena R, Wu S, Blumenthal A, McMillan NA. Efficient Biodistribution and Gene Silencing in the Lung epithelium via Intravenous Liposomal Delivery of siRNA. Mol. Ther. Nucleic Acids. 2013;2:e96. doi: 10.1038/mtna.2013.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fehring V, Schaeper U, Ahrens K, Santel A, Keil O, Eisermann M, Giese K, Kaufmann J. Delivery of therapeutic siRNA to the lung endothelium via novel Lipoplex formulation DACC. Mol. Ther. 2014;22:811–820. doi: 10.1038/mt.2013.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Polach KJ, Matar M, Rice J, Slobodkin G, Sparks J, Congo R, Rea-Ramsey A, McClure D, Brunhoeber E, Krampert M, Schuster A, Jahn-Hofmann K, John M, Vornlocher HP, Fewell JG, Anwer K, Geick A. Delivery of siRNA to the mouse lung via a functionalized lipopolyamine. Mol. Ther. 2012;20:91–100. doi: 10.1038/mt.2011.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dahlman JE, Barnes C, Khan OF, Thiriot A, Jhunjunwala S, Shaw TE, Xing Y, Sager HB, Sahay G, Speciner L, Bader A, Bogorad RL, Yin H, Racie T, Dong Y, Jiang S, Seedorf D, Dave A, Singh Sandhu K, Webber MJ, Novobrantseva T, Ruda VM, Lytton-Jean AK, Levins CG, Kalish B, Mudge DK, Perez M, Abezgauz L, Dutta P, Smith L, Charisse K, Kieran MW, Fitzgerald K, Nahrendorf M, Danino D, Tuder RM, von Andrian UH, Akinc A, Panigrahy D, Schroeder A, Koteliansky V, Langer R, Anderson DG. In vivo endothelial siRNA delivery using polymeric nanoparticles with low molecular weight. Nat. Nanotechnol. 2014;9:648–655. doi: 10.1038/nnano.2014.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Garbuzenko OB, Saad M, Betigeri S, Zhang M, Vetcher AA, Soldatenkov VA, Reimer DC, Pozharov VP, Minko T. Intratracheal versus intravenous liposomal delivery of siRNA antisense oligonucleotides and anticancer drug. Pharm. Res. 2009;26:382–394. doi: 10.1007/s11095-008-9755-4. [DOI] [PubMed] [Google Scholar]
- 147.Merkel OM, Beyerle A, Librizzi D, Pfestroff A, Behr TM, Sproat B, Barth PJ, Kissel T. Nonviral siRNA Delivery to the Lung: Investigation of PEG-PEI Polyplexes and Their In Vivo Performance. Mol. Pharm. 2009;6:1246–1260. doi: 10.1021/mp900107v. [DOI] [PubMed] [Google Scholar]
- 148.Gutbier B, Kube SM, Reppe K, Santel A, Lange C, Kaufmann J, Suttorp N, Witzenrath M. RNAi-mediated suppression of constitutive pulmonary gene expression by small interfering RNA in mice. Pulm. Pharmacol. Ther. 2010;23:334–344. doi: 10.1016/j.pupt.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 149.Wittrup A, Lieberman J. Knocking down disease: a progress report on siRNA therapeutics. Nat. Rev. Genet. 2015;16:543–552. doi: 10.1038/nrg3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jackson AL, Burchard J, Leake D, Reynolds A, Schelter J, Guo J, Johnson JM, Lim L, Karpilow J, Nichols K, Marshall W, Khvorova A, Linsley PS. Position-specific chemical modification of siRNAs reduces “off-target” transcript silencing. RNA. 2006;12:1197–1205. doi: 10.1261/rna.30706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Huang L, Jin J, Deighan P, Kiner E, McReynolds L, Lieberman J. Efficient and specific gene knockdown by small interfering RNAs produced in bacteria. Nat. Biotechnol. 2013;31:350–356. doi: 10.1038/nbt.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Loisel S, Le Gall C, Doucet L, Ferec C, Floch V. Contribution of plasmid DNA to hepatotoxicity after systemic administration of lipoplexes. Hum. Gene Ther. 2001;12:685–696. doi: 10.1089/104303401300057405. [DOI] [PubMed] [Google Scholar]
- 153.Tousignant JD, Gates AL, Ingram LA, Johnson CL, Nietupski JB, Cheng SH, Eastman SJ, Scheule RK. Comprehensive analysis of the acute toxicities induced by systemic administration of cationic lipid:plasmid DNA complexes in mice. Hum. Gene Ther. 2000;11:2493–2513. doi: 10.1089/10430340050207984. [DOI] [PubMed] [Google Scholar]
- 154.Yan W, Chen W, Huang L, Mechanism of adjuvant activity of cationic, liposome: phosphorylation of a MAP kinase. ERK and induction of chemokines. Mol. Immunol. 2007;44:3672–3681. doi: 10.1016/j.molimm.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 155.Vangasseri DP, Cui Z, Chen W, Hokey DA, Falo LD, Jr, Huang L. Immunostimulation of dendritic cells by cationic liposomes. Mol. Membr. Biol. 2006;23:385–395. doi: 10.1080/09687860600790537. [DOI] [PubMed] [Google Scholar]
- 156.Tanaka T, Legat A, Adam E, Steuve J, Gatot JS, Vandenbranden M, Ulianov L, Lonez C, Ruysschaert JM, Muraille E, Tuynder M, Goldman M, Jacquet A. DiC14-amidine cationic liposomes stimulate myeloid dendritic cells through Toll-like receptor 4. Eur. J. Immunol. 2008;38:1351–1357. doi: 10.1002/eji.200737998. [DOI] [PubMed] [Google Scholar]
- 157.Fischer D, Bieber T, Li Y, Elsässer H-P, Kissel T. A novel non-viral vector for DNA delivery based on low molecular weight, branched polyethylenimine: effect of molecular weight on transfection efficiency and cytotoxicity. Pharm. Res. 1999;16:1273–1279. doi: 10.1023/a:1014861900478. [DOI] [PubMed] [Google Scholar]
- 158.Islam MA, Park TE, Singh B, Maharjan S, Firdous J, Cho MH, Kang SK, Yun CH, Choi YJ, Cho CS. Major degradable polycations as carriers for DNA and siRNA. J. Control. Release. 2014;193:74–89. doi: 10.1016/j.jconrel.2014.05.055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.