Abstract
A variety of multidimensional anthropogenic activities, especially of industrial level, are contaminating our aquatic and terrestrial environments with a variety of metallic and non-metallic pollutants. The metallic and non-metallic pollutants addressed specifically in this review are heavy metals and various compound forms of sulfates, respectively. Direct and indirect deleterious effects of the both types of pollutants to all forms of life are well-known. The treatment of such pollutants is therefore much necessary before their final discharge into the environment. This review summarizes the productive utility of sulfate-reducing bacteria (SRB) for economical and concomitant treatment of the above mentioned wastes. Utilization of agro-industrial wastes and some environmental contaminants including hydrocarbons, as economical growth substrates for SRB, is also suggested and proved efficient in this review. Mechanistically, SRB will utilize sulfates as their terminal electron acceptors during respiration while utilizing agro-industrial and/or hydrocarbon wastes as electron donors/carbon sources and generate H2S. The biogenic H2S will then react vigorously with dissolved metals present in the wastewaters thus forming metal sulfide. The metal sulfide being water insoluble and heavier than water will settle down in the water as precipitates. In this way, three types of pollutants i.e., metals, sulfates and agro-industrial and/or hydrocarbon wastes will be treated simultaneously.
Keywords: Agro-industrial wastes, Beneficial microorganisms, Bioprecipitation, Economical bioremediation, Sulfate-reducing bacteria, Toxic metals
Introduction
No doubt chemical as well as biotechnological industrial units supply us with a number of inevitable products. But pollution from industries cannot be ignored in addition to their usefulness. It is a nuisance causing the degradation of the environment by affecting the air, water and soil (Govindarajalu 2003). Industrial wastes and emissions contain toxic and hazardous substances, of which mostly are detrimental to human health as well as to the environment. Human health and environmental quality are being affected negatively by the perpetual production of industrial wastes (Adebisi and Fayemiwo 2011). In the context of the environmental pollution and its impact on health and global climatic change, the present time necessitates the importance of environmental remediation. This review emphasizes the importance of an economical bioremediation strategy, especially for developing countries like Pakistan that cannot afford much budget in the protection of their local environments. This notion is exemplified in this review by the synchronous bioremediation of three categories of pollutants, i.e., metals, sulfates and agro-industrial and/or hydrocarbon wastes originating from different industries. Sulfate-reducing bacteria (SRB) are advocated as remedial agents. A variety of organic wastes produced in agricultural lands like sugarcane bagasse, rice and wheat straw, animal manure, etc. are described as economical growth substrates in addition to some hydrocarbon contaminants, for the propagation of SRB. The proposed model is likely to solve the problems of metals and sulfate toxicity, as well as to improve the concerned soil and water habitats’ biology. This review falls into following subtopics:
Metal pollutants of industrial origin and their detrimental health effects
Heavy metal pollution is becoming a significant concern in many countries because of its being non-biodegradable, persistent and thus bioaccumulative and continuous generation nature (Armitage et al. 2007; Sakan et al. 2009; Wang et al. 2013). Besides the dominant source of industrial origin, sewage water may also contain significant amounts of heavy metals such as zinc, iron, copper, manganese, lead, cadmium, chromium, nickel and cobalt, etc. (Idris et al. 2007; Malla et al. 2007; Zhang et al. 2016a). It is well known that all types of metals including radioactive ones are transferred to animals and human beings through food chains and exert harmful effects (Gall et al. 2015; Meena et al. 2016). According to WHO (1984) metals of the most immediate concern are aluminium, chromium, manganese, iron, cobalt, nickel, copper, zinc, cadmium, mercury and lead. Health effects of some commoner encountering heavy metals are given in the Table 1.
Table 1.
Health effects of most commonly encountering heavy metals and their industrial sources of generation
| Metal | Generation sources | Principal health hazards |
|---|---|---|
| Aluminium | Aluminium alloys’ production, packaging units, pharmaceutical industries | Aerial occupational exposure may produce lung fibrosis in humans In uremic patients, osteomalacia can occur due to aluminium in dialysis fluid May alter intestinal functions and metabolism of calcium in several organ systems |
| Cadmium | Alloys’ production, automotive and air craft industries, electroplating/galvanizing, metallurgical processing, mining, nickel–cadmium battery manufacturing industries, paint industries, plastic industries, textile printing | Affects the activity of alcohol dehydrogenase, arylsulfatase, delta-aminolevulinic acid dehydratase, delta-aminolevulinic acid synthetase, lipoamide dehydrogenase, pyruvate decarboxylase and pyruvate dehydrogenase Ingestion may result in disturbances in the gastrointestinal tract, vomiting, proteinuria, osteomalacia, liver dysfunction, kidney dysfunction/damage manifested by anemia and hypertension Long term low-level exposure leads to chronic obstructive pulmonary and renal tubular diseases and emphysema |
| Chromium | Cement manufacturing, chemical and refractory processing, chrome-plating, combustion of fossil fuels, ferrochrome production, metal-finishing industries, ore refineries, tanneries, textile plants | Low-level chronic exposure leads to kidney damage while occupational exposure may leads to asthma as well as cancer of the respiratory tract especially in the chrome production and chrome pigment industries May cause allergic dermatitis in humans |
| Cobalt | Cemented tungsten carbide industry, high temperature alloys’ manufacturing, paint industry | Exposure to low concentrations (0.002 to 0.01 mg/m3) causes respiratory irritation while to higher concentrations (0.1 mg/m3 or higher) can lead to “hard metal” pneumoconiosis Ingestion in excessive amounts can cause erythropiotic effects and cardiomyopathy Intravenous administration can cause deafness due to nerve damage, flushing of the face, giddiness, increased blood pressure, slowed respiration and tinnitus |
| Copper | Copper mining, metal fumes from smelting operations, welding | Excessive accumulation leads to Wilson’s disease Higher doses can cause anaemia, liver and kidney damage and irritation in stomach and intestine Ingestion of large amounts of copper sulfate may lead to hepatic necrosis and death |
| Iron | Hematite mining industries, metal industries, welding | Inhalation of iron oxide fumes or dust may leads to deposition of iron particles in lungs which produces an X-ray appearance like silicosis |
| Lead | Combustion of lead containing industrial emissions, glass polishing, hand loading of ammunition, jewelry making, lead-glazed pottery, painting, plastic industry, rubber industry, stained glass crafting | Deleterious effects include abdominal cramps, anorexia, insomnia, muscle aches, nausea, serious injuries to brain and kidneys, weakness of joints and weight loss It can pass the placental barriers and may reach the fetus resulting in miscarriages, abortions and still births In severe cases coma and death may occur |
| Manganese | Iron industry, welding | Chronic poisoning leads to a neuropsychiatric disorder characterized by difficulty in walking, irritability, speech disturbances and compulsive behaviour which may include fighting, running and singing |
| Mercury | Chlor-alkali industry, extraction of gold, in dentistry as amalgam tooth filling, paper industry, pulp manufacturing industry, smelting operations | Associated with kidney damage and its chronic poisoning may cause anemia, excessive irritation of tissues, gingivitis, loss of appetite, nutritional disturbances and salivation Inhalation of vapours at extremely high concentrations may lead to an acute, corrosive bronchitis and interstitial pneumonitis |
| Nickel | Combustion of fossil fuels, electroplating, fumes from alloys used in welding and brazing, metal plating industries, nickel mining, nickel-refining industries | Acts as a respiratory tract carcinogen |
| Zinc | Coal and waste combustion, mining, steel processing | Acute zinc toxicity leads to gastrointestinal distress and diarrhoea while inhalation of freshly formed fumes of zinc may cause metal fume fever |
Sulfates containing industrial effluents and their deleterious effects
Among non-metal pollutants, various compound forms of sulfates deserve special attention. Continuous intrusion, especially from the wastes of industrial origins, of which effluents from edible oil production plants, food processing industries, paper mills, petroleum refineries, potato starch factories, pulp manufacturing industries, solid waste processing plants, tanneries and textile wastewaters make presence of different sulfur species in soils and waters at varying levels (Boshoff et al. 2004; Vaiopoulou et al. 2005; Huang et al. 2006). In ecologically viable locations, such pollutants are recycled by the microbes of sulfur cycle (Madsen 2008). However, in the situations where its presence behaves as a pollutant, many deteriorative processes like acidogenesis, corrosion of metals and H2S altered toxicological effects occurs (Lin and Hsiu 1997; Muyzer and Stams 2008; Lim et al. 2016; Zhao et al. 2016). In addition, human health is being affected negatively due to an exposure to sulfates. The most commonly encountering adverse health effects in human beings include acute renal failure, coma, confusion, cough, dyspnea, hepatotoxicity, increase in hippocampus superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) activities, loss of consciousness, late sequelae of interstitial fibrosis, metabolic acidosis, myocardial necrosis, prolonged apnea, pulmonary edema, seizures, severe intravascular hemolysis, severe neurological impairment and shocks (Duong et al. 2001; Mbaye et al. 2003; Christia-Lotter et al. 2006; Kucukatay et al. 2007; Mortazavi and Jafari-Javid 2009).
Remediation of metals and sulfates: scope and types
Treatment of metals and sulfates from industrial effluents is very much necessary before discharging them to the environment. There are a number of physicochemical treatment methods for the removal of metal ions from aqueous solutions. These include mainly electrodialysis, reduction, reverse osmosis, solvent extraction (Zhang et al. 1998) adsorption (Aguado et al. 2009) coagulation (El-Samrani et al. 2008) electrochemical precipitation (Chen and Lim 2005) filtration (Fatin-Rouge et al. 2006) and ion exchange, etc. (Dizge et al. 2009). However, such efforts require the use of energy and implication of more chemicals. In this way a pollutant can be recovered from the environment usually at the expense of adding more new chemical(s) to the scene. However, the chemical treatment methods have been declared environmentally non-compatible owing to their low treatment efficiency, complicated operation, high operational cost and the possible generation of secondary pollutions (Rocha et al. 2009; Ihsanullah et al. 2016). On the other hand, biological methods of the metals’ removal have gained importance for their better performance, low cost and environmentally compatible natures (Malik 2004; Okoh and Trejo-Hernandez 2006; Gillespie and Philp 2013). Bioremediation of metals’ containing effluents has experienced various shades including phytoremediation (Jadia and Fulekar 2009; Tauqeer et al. 2016) and biosorption (Hussein et al. 2004; Gupta et al. 2015; García et al. 2016). Although, both of the mentioned ways make the toxic metals generally non-available to the environment. But as regards their bioavailability, the plants and microorganisms may concentrate pollutants at various levels and being a component of food chains they may become the toxicants’ transferring agents in greater amounts at higher trophic levels (Peralta-Videaa et al. 2009).
The most recent and attractive approach for the treatment of metallic wastes is the precipitation of metal ions in the form of their respective sulfides. The counter reactant (hydrogen sulfide) of the metals needed for this process may be provided by the activity of SRB removing metals as well as sulfates concomitantly. However, the treatment of sulfates at larger scale has not been described by researchers still now.
Biosulfidogenesis
Generation of hydrogen sulfide by microorganisms is known as biosulfidogenesis. It may occur via desulfhydration, sulfate reduction, sulfur respiration and sulfur disproportionation (inorganic ‘S’ fermentation). Due to its (1) proton consuming reaction (2) precipitating many metals and metalloids efficiently and (3) lowering the concentrations of sulfates (Jameson et al. 2010), the H2S has been employed for bioremediation of selected pollutant sites. Many different bacterial groups have the ability to reduce sulfate, thiosulfate, elemental sulfur and even can break down the sulfur containing amino acids in proteins to produce sulfide (Magot et al. 1997). However, SRB are the most widely studied biosulfidogens having the potential of remediating metal-rich contaminated wastewaters (Koschorreck 2008).
Ecology and biotechnology of SRB
SRB make morphologically and physiologically a diverse group of obligatory anaerobes which share the ability to dissimilate sulfate to sulfide while oxidizing various growth substrates (Willis et al. 1997). These prokaryotic microorganisms are much versatile in their metabolism as well as in the environmental conditions in which they thrive and particularly make their importance in specific ecosystems such as acid mine drainages, cyanobacterial microbial mats, deep-sea hydrothermal vents, hypersaline microbial mats, marine and freshwater sediments, methane zone of marine sediments, oil fields’ environments, polluted environments such as anaerobic purification plants, rhizosphere of plants and rice fields (Fauque 1995; Dhillon et al. 2003; Rabus et al. 2006; Foti et al. 2007; Leloup et al. 2007; Ollivier et al. 2007; Muyzer and Stams 2008; Hussain and Qazi 2016; Wissuwa et al. 2016). In the above mentioned ecosystems, SRB have to cope with drastic physicochemical conditions including high temperature and high pressure, etc. SRB may represent the first respiring microorganisms and contribute to the complete oxidation of organic matter. They also play a key role in the overall biogeochemistry of various environments where they inhabit by the production of sulfide and/or metal reduction. Due to their key role in the marine carbon and sulfur cycles, the significance of SRB in high and low-sulfate environments is highly appealing for understanding the factors that influence their distribution, population size and metabolic activities in the seabed.
Diversity of SRB
In the last few decades, through the use of 16S rRNA or dsrAB (dissimilatory sulfite reductase) genes as molecular markers, many SRB species have been reported. The dsrAB gene fingerprinting methods such as t-RFLP, DGGE and gel-retardation analyses have been used for rapid determination of SRB diversity in different environments (Wagner et al. 2005; Geets et al. 2006). More than 220 species of 60 genera of SRB have been described still now. They belong to five divisions (phyla) within the bacteria that are the Deltaproteobacteria, Firmicutes, Nitrospira and two phyla represented by Thermodesulfobium narugense and Thermodesulfobacterium/Thermodesulfatator species) and two divisions within the archaea (the euryarchaeotal genus Archaeoglobus and the two crenarchaeotal genera Thermocladium and Caldivirga, affiliated with the Thermoproteales) (Mori et al. 2003; Rabus et al. 2006; Ollivier et al. 2007; Muyzer and Stams 2008; Leloup et al. 2009). Rabus and Strittmatter (2007) reported that the complete genome sequences of nine SRB have been deposited in public databases. These include Archaeoglobu fulgidus (Euryarchaeota), Caldivirga maquilingensis (Crenarchaeota), the Gram-positive Desulfotomaculum reducens (Firmicutes) and six Gram negative Deltaproteobacteria: Desulfobacterium autotrophicum, Desulfovibrio vulgaris Hildenborough, Desulfovibrio vulgaris subsp. vulgaris DP4, Desulfovibrio desulfuricans G20, Desulfotalea psychrophila and Syntrophobacter fumaroxidans. Owing to bioremedial potential, it is important to know the nutritional requirements of the sulfidogenic bacteria for both strengthening the remedial processes as well as widening their applications in this regard.
Nutritional aspects of SRB
SRB may have an autotrophic, litho-autotrophic, or heterotrophic respiration-type of life under anaerobiosis. While their possible microaerophilic natures have also been reported (Fauque and Ollivier 2004). Heterotrophic SRB utilize organic compounds as substrates, while autotrophic use CO2 as the carbon source and obtain electrons from the oxidation of H2 (Lens and Kuennen 2001). The latest biochemical and microbiological studies suggest that SRB can utilize a wide variety of substrates as electron acceptors and donors (Rabus et al. 2006; Hussain and Qazi 2012, 2014; Hussain et al. 2014a, b). In addition to different sulfur species (sulfite, sulfate, thiosulfate and tetrathionate) various other organic and inorganic compounds serve as terminal electron acceptors for these bacteria (Fauque et al. 1991; Fauque 1995; Fauque and Ollivier 2004; Rabus et al. 2006; Muyzer and Stams 2008). More than one hundred different compounds including sugars (fructose, glucose, etc.), amino acids (alanine, glycine, serine, etc.), alcohols (methanol, ethanol, etc.), monocarboxylic acids (acetate, butyrate, propionate, etc.), dicarboxylic acids (fumarate, malate, succinate, etc.) and aromatic compounds (benzoate, phenol, etc.) serve as potential electron donors for SRB (Fauque et al. 1991; Rabus et al. 2006; Liamleam and Annachhatre 2007; Huang and Kao 2015; Stasik et al. 2015; Meckenstock et al. 2016). In general, SRB prefer low-molecular weight organic compounds as carbon and energy sources.
Cultivation of SRB using various environmental contaminants as growth substrates
Dissimilatory sulfate reducers have been reported to utilize lactate as a preferred carbon source and thus most widely employed for cultivating DSRB at laboratory scale (Barnes 1998; El-Bayoumy et al. 1999). However, lactate is too much expensive for a large scale practice. Hydrogen gas can also be used as an energy source by some DSRB (Lens et al. 2003). Although hydrogen is a relatively inexpensive substrate, yet it cannot be considered an acceptable energy source because of engineering and safety measures on a commercial scale while ethanol has been reported as a cost-effective substrate (Huisman et al. 2006). Several different natural sources of organic materials such as animal manure, sugarcane bagasse, leaf mulch, molasses, mushroom compost, fruit wastes, sawdust, sewage sludge, vegetal compost, whey and wood chips have been described as electron donors and carbon sources for the cultivation of SRB (Annachhatre and Suktrakoolvait 2001; Costa and Duarte 2005; Coetser et al. 2006; Hussain and Qazi 2012, 2014; Hussain et al. 2014a, b). Researchers have also demonstrated tannery effluents and wastes from the wine industry for supporting growth of dissimilatory SRB to economize certain bioremediation strategies (Boshoff et al. 2004; Martins et al. 2009a). SRB can utilize a range of different other environmental contaminants such as petroleum hydrocarbon constituents (e.g. alkanes, benzene, ethylbenzene, polycyclic aromatic hydrocarbons, toluene, xylenes) or halogenated compounds directly as a source of carbon and energy (Fauque et al. 1991; Hao et al. 1996; Harms et al. 1999; Morasch et al. 2004; Huang and Kao 2015; Stasik et al. 2015; Meckenstock et al. 2016). Recent data on SRB report that they can grow on long-chain alkanes (Davidova et al. 2006; Kleindienst et al. 2014; Herath et al. 2016), alkenes (Grossi et al. 2007; Fullerton et al. 2013) and short-chain alkanes (Kniemeyer et al. 2007). The above mentioned metabolic diversity and versatility of SRB in terms of their potential of using the range of carbon and energy sources is highly promising for designing strategies addressing bioremediation of metals and sulfates.
Applications of SRB
The exploitation of SRB for the treatment of industrial wastewaters is of great interest. A number of studies, based on the applications of SRB have been carried out for the treatment of simulated and real wastewaters contaminated with a range of pollutants. The latest advancement in the applications of SRB have shown that SRB are used to treat various environmental contaminants including metals (Hussain and Qazi 2016; Mothe et al. 2016; Zhang et al. 2016b), metalloids (Battaglia-Brunet et al. 2012; Altun et al. 2014; Sahinkaya et al. 2015) sulfates (Hussain and Qazi 2014, 2016; Hussain et al. 2014a), methane (Krukenberg et al. 2016), various non-methane hydrocarbons e.g. alkanes (Callaghan et al. 2012; Khelifi et al. 2014; Kleindienst et al. 2014; Herath et al. 2016) and alkenes (Fullerton et al. 2013), alicyclic hydrocarbons e.g. cyclohexane (Jaekel et al. 2015), aromatic hydrocarbons e.g. benzene (Huang and Kao 2015; Aüllo et al. 2016; Meckenstock et al. 2016), naphthalene (Kümmel et al. 2015; Meckenstock et al. 2016), phenanthrene (Sayara et al. 2015; Meckenstock et al. 2016), toluene (Huang and Kao 2015; Stasik et al. 2015; Aüllo et al. 2016), xylene (Huang and Kao 2015; Stasik et al. 2015), ethylbenzene (Stasik et al. 2015; Aüllo et al. 2016) and 2-methylnaphthalene (Folwell et al. 2015) and nitroaromatic compounds e.g. trinitrotoluene (Boopathy 2014; Mulla et al. 2014).
Almost all of the mentioned investigations were carried out at laboratory scale; however, no data are available on the commercial-scale applications of SRB. Only two patented technological applications, based on the microbially mediated sulfate reduction in bioreactor systems, have been developed and operated as pilot-, demonstration- and full-scale plants for the treatment of acidic wastewater from metal mines and related sites (Johnson 2000): Thiopaq® by Paques, The Netherlands (Boonstra et al. 1999; Buisman et al. 2007) and BioSulphide® by BioteQ, Canada (Rowley et al. 1997; Ashe et al. 2008). The most probable reason of poor applicability of SRB at commercial scale may be the uncontrolled generation of H2S exhaust. According to Martins et al. (2009b) additionally produced H2S (unreacted) easily escapes as a gas being some of it not accessible to the pollutants and thus the treatment of pollutants can never be quantitative. The escaped H2S my pose severe environmental impacts as well. This information necessitates optimization of sulfidogenesis and the wastes to be treated within tangibly designed bioreactors allowing maximum contact area and time for the H2S and waste(s) to react (Hussain and Qazi 2016). The H2S exhaust can also be controlled by making the entire remedial setup closed. Some sort of bio/technical control of unwanted (additionally produced) H2S is, therefore, recommended to make the commercial-utility of SRB feasible.
Economical and concomitant treatment of metals and sulfates
Big cities of developing countries represent one of the major sources of water pollution. Wherein untreated domestic and industrial effluents are ultimately thrown into streams and rivers. The biota of the concerned lotic environments has been changing its composition rapidly. While the withstanding populations are being affected negatively in terms of their population density and biochemical alterations. Recalcitrant pollutants, especially metals are transferring through the food chains to humans. Untreated sewage effluents cause high BOD and COD levels tremendously and the resultant anaerobiosis may escalate the growth of sulfidogenic bacteria yielding H2S in selected locations. Such situations have contaminated the environment with increasing populations of harmful microorganisms and their byproducts.
Keeping in view the above mentioned facts, metal-tolerant sulfidogenic bacteria have been perceived an appealing condition for precipitating metals from effluents in the form of their sulfides (Gadd 2000; Hussain and Qazi 2016). SRB play an important role in metal sulfide immobilization in anaerobic environments that contain high concentrations of metals (Kaksonen et al. 2004; Van Roy et al. 2006). This remedy, however, requires the provision of physical factors and nutrients which can support the growth of relevant microbes as well as production of H2S. Domestic and industrial sewages rich in organic contents, themselves pollutants in the environment, may provide the nutritional requirements of sulfidogenic bacteria. Other nutritional requirements of sulfidogenic bacteria can also be accomplished from different agro-industrial wastes on low/no cost basis. Blending of suitable carbon-sources (electron donors) may support indigenous or inoculant microbial communities capable of reducing sulfates to sulfides by precipitating metal contaminants.
The above discussed facts suffice to advocate detrimental effects of metals and sulfates as well as potential of suflidogenic bacteria for remediating these pollutants concomitantly. For this purpose, a biphasic model is proposed in Fig. 1 which also shows the routes of untreated effluents. The figure route 4A employs H2S exhaust, produced from sewage effluents by sulfidogenic bacteria under anaerobic conditions to precipitate metals from diverse industrial effluents. While the route 4B illustrates the importance of metal-resistant and heterotrphic sulfidogens for single-chambered bioremediation process development addressing the two categories of the pollutants concurrently.
Fig. 1.
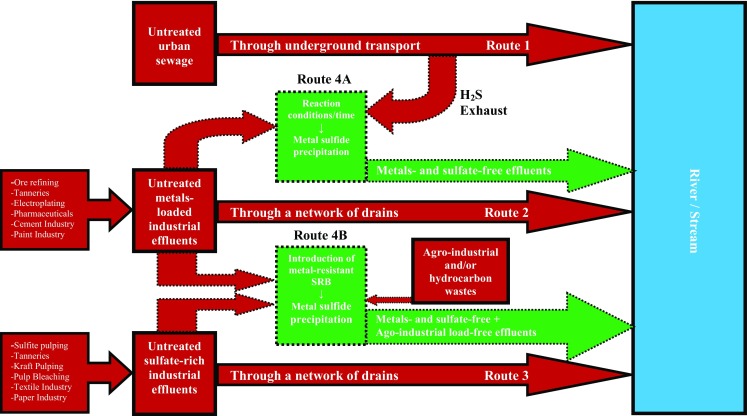
Routes 1, 2 and 3 represent the present situation of untreated sewage, metal and sulfate containing industrial effluents, respectively while routes 4A and 4B indicate two different possible bioremediating fates of metals and H2S and metals and other sulfate pollutants, respectively. In route 4B SRB growth is accomplished by agro-industrial and/or hydrocarbon wastes
Concluding remarks
This review arrives at the conclusion that mixed industrial effluents, loaded with metals and sulfates, can be treated concomitantly using metal-resistant SRB. In addition, different types of agro-industrial and/or hydrocarbon wastes can be used as growth substrates for the efficient propagation of SRB. Infact, this approach leads to the treatment of three categories of pollutants i.e., metals, sulfates and agro-industrial/hydrocarbon wastes. Practical work on these lines at is likely to identify more suitable wastes which may resume the status of ingredients of a suitable medium for the cultivation of desired microorganisms capable of remediating the diverse pollutants. The authors of this review in addition to other researchers have also worked on different carbon sources for biological sulfate reduction. However, more work is required to identify the suitable SRB and the environmental wastes for their growth to meet the desired goal of economical bioremediation. In addition, exploitation of SRB on pilot and commercial scales is also necessarily required to further investigate the effectivess of closed and tangibly designed bioreactors and to improve the process(s) accordingly.
Compliance with ethical standards
Conflict of interest
The authors report no conflicts of interest.
References
- Adebisi SA, Fayemiwo K. Physiochemical properties of industrial effluent in Ibadan, Nigeria. EJEAFChe. 2011;10:2026–2031. [Google Scholar]
- Aguado J, Arsuaga JM, Arencibia A, Lindo M, Gascon V. Aqueous heavy metals removal by adsorption on aminefunctionalized mesoporous silica. J Hazard Mater. 2009;163:213–221. doi: 10.1016/j.jhazmat.2008.06.080. [DOI] [PubMed] [Google Scholar]
- Altun M, Sahinkaya E, Durukan I, Bektas S, Komnitsas K. Arsenic removal in a sulfidogenic fixed-bed column bioreactor. J Hazard Mater. 2014;269:31–37. doi: 10.1016/j.jhazmat.2013.11.047. [DOI] [PubMed] [Google Scholar]
- Annachhatre AP, Suktrakoolvait S. Biological sulfate reducing using molasses as a carbon source. Water Environ Res. 2001;73:118–126. doi: 10.2175/106143001X138778. [DOI] [PubMed] [Google Scholar]
- Armitage PD, Bowes MJ, Vincent HM. Long-term changes in macroinvertebrate communities of a heavy metal polluted stream: the river Nent (Cumbria, UK) after 28 years. River Res Appl. 2007;23:997–1015. doi: 10.1002/rra.1022. [DOI] [Google Scholar]
- Ashe NL, McLean I, Nodwell M (2008) Review of operations of the biosulphide process plant at the copper Queen mine, Bisbee, Arizona. In: Young CA, Taylor PR, Anderson CG and Choi Y (eds) Hydrometallurgy 2008, proceedings of the 6th international symposium on hydrometallurgy, Society for Mining, Metallurgy and Exploration, Inc., Phoenix, Arizona, pp 98–107
- Aüllo T, Berlendis S, Lascourrèges JF, Dessort D, Duclerc D, Saint-Laurent S, Schraauwers B, Mas J, Patriarche D, Boesinger C, Magot M, Ranchou-Peyruse A. New bio-indicators for long term natural attenuation of monoaromatic compounds in deep terrestrial aquifers. Front Microbiol. 2016;7:122. doi: 10.3389/fmicb.2016.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes LJ. Removal of heavy metals and sulphate from contaminated grounwater using sulphate-reducing bacteria: development of a commercial process. In: Sikdar SK, Irvine RL, editors. Bioremediation technologies. USA: Lancaster; 1998. pp. 577–619. [Google Scholar]
- Battaglia-Brunet F, Crouzet C, Burnol A, Coulon S, Morin D, Joulian C. Precipitation of arsenic sulphide from acidic water in a fixed-film bioreactor. Water Res. 2012;46:3923–3933. doi: 10.1016/j.watres.2012.04.035. [DOI] [PubMed] [Google Scholar]
- Becker M, Edwards S, Massey RI. Toxic chemicals in toys and children’s products: limitations of current responses and recommendations for government and industry. Environ Sci Technol. 2010;44:7986–7991. doi: 10.1021/es1009407. [DOI] [PubMed] [Google Scholar]
- Boonstra J, Van Lier R, Janssen G, Dijkman H, Buisman CJN. Biological treatment of acid mine drainage. In: Amils R, Ballester A, editors. Biohydrometallurgy and the environment toward the mining of the 21st century, proceedings of the international biohydrometallurgy symposium IBS’99. Madrid: Elsevier; 1999. pp. 559–567. [Google Scholar]
- Boopathy R. Biodegradation of 2, 4, 6-trinitrotoluene (TNT) under sulfate and nitrate reducing conditions. Biologia. 2014;69:1264–1270. doi: 10.2478/s11756-014-0441-1. [DOI] [Google Scholar]
- Boshoff G, Duncan J, Rose PD. Tannery effluent as a carbon source for biological sulphate reduction. Water Res. 2004;38:2651–2658. doi: 10.1016/j.watres.2004.03.030. [DOI] [PubMed] [Google Scholar]
- Buisman CJN, Huisman J, Dijkman H, Bijmans MFM (2007) Trends in application of industrial sulfate reduction for sulfur and metal recycling. In: Horizons of sustainable growth of the non-ferrous metals production. Proceedings of the European Metallurgical Conference EMC 2007, GDMB Medienverlag, Dusseldorf, Germany, pp 383–387
- Callaghan AV, Morris BE, Pereira IA, McInerney MJ, Austin RN, Groves JT, Kukor JJ, Suflita JM, Young LY, Zylstra GJ, Wawrik B. The genome sequence of Desulfatibacillum alkenivorans AK-01: a blueprint for anaerobic alkane oxidation. Environ Microbiol. 2012;14:101–113. doi: 10.1111/j.1462-2920.2011.02516.x. [DOI] [PubMed] [Google Scholar]
- Chen JP, Lim LL. Recovery of precious metals by an electrochemical deposition method. Chemosphere. 2005;60:1384–1392. doi: 10.1016/j.chemosphere.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Christia-Lotter A, Bartoli C, Piercecchi-Marti M-D, Demory D, Pelissier-Alicot A-L, Sanvoisin A, Leonetti G. Fatal occupational inhalation of hydrogen sulfide. Forensic Sci Int. 2006;169:206–209. doi: 10.1016/j.forsciint.2006.02.043. [DOI] [PubMed] [Google Scholar]
- Coetser S, Pulles W, Heath R, Cloete T. Chemical characterization of organic electron donors for sulfate reduction for potential use in acid mine drainage treatment. Biodegradation. 2006;17:67–77. doi: 10.1007/s10532-005-7567-3. [DOI] [PubMed] [Google Scholar]
- Costa MC, Duarte JC. Bioremediation of acid mine drainage using acidic soil and organic wastes for promoting sulphate-reducing bacteria activity on a column reactor. Water Air Soil Pollut. 2005;165:325–345. doi: 10.1007/s11270-005-6914-7. [DOI] [Google Scholar]
- Davidova IA, Duncan KE, Choi OK, Suflita JM. Desulfoglaeba alkanexedens gen. nov., sp. nov., an n-alkane-degrading, sulphate-reducing bacterium. Int J Syst Evol Microbiol. 2006;56:2737–2742. doi: 10.1099/ijs.0.64398-0. [DOI] [PubMed] [Google Scholar]
- Dhillon A, Teske A, Dillon J, Stahl DA, Sogin ML. Molecular characterization of sulfate-reducing bacteria in the Guaymas basin. Appl Environ Microbiol. 2003;69:2765–2772. doi: 10.1128/AEM.69.5.2765-2772.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dizge N, Keskinler B, Barlas H. Sorption of Ni(II) ions from aqueous solution by Lewatit cation-exchange resin. J Hazard Mater. 2009;167:915–926. doi: 10.1016/j.jhazmat.2009.01.073. [DOI] [PubMed] [Google Scholar]
- Duong TX, Suruda AJ, Maier LA. Interstitial fibrosis following hydrogen sulfide exposure. Am J Ind Med. 2001;40:221–224. doi: 10.1002/ajim.1091. [DOI] [PubMed] [Google Scholar]
- El-Bayoumy MA, Bewtra JK, Ali HI, Biswas N. Sulfide production by sulfate reducing bacteria with lactate as feed in an upflow anaerobic fixed film reactor. Water Air Soil Pollut. 1999;112:67–84. doi: 10.1023/A:1005016406707. [DOI] [Google Scholar]
- El-Samrani AG, Lartiges BS, Villiéras F. Chemical coagulation of combined sewer overflow: heavy metal removal and treatment optimization. Water Res. 2008;42:951–960. doi: 10.1016/j.watres.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Fatin-Rouge N, Dupont A, Vidonne A, Dejeu J, Fievet P, Foissy A. Removal of some divalent cations from water by membrane-filtration assisted with alginate. Water Res. 2006;40:1303–1309. doi: 10.1016/j.watres.2006.01.026. [DOI] [PubMed] [Google Scholar]
- Fauque GD. Ecology of sulfate-reducing bacteria. In: Barton LL, editor. Biotechnology handbooks. New York: Plenum Press; 1995. pp. 217–241. [Google Scholar]
- Fauque G, Ollivier B. Anaerobes: the sulfate-reducing bacteria as an example of metabolic diversity. In: Bull AT, editor. Microbial diversity and bioprospecting. Washington DC: ASM Press; 2004. pp. 169–176. [Google Scholar]
- Fauque G, Legall J, Barton LL. Sulfate-reducing and sulfur-reducing bacteria. In: Shively JM, Barton LL, editors. Variations in autotrophic life. London: Academic Press Limited; 1991. pp. 271–337. [Google Scholar]
- Folwell BD, McGenity TJ, Price A, Johnson RJ, Whitby C. Exploring the capacity for anaerobic biodegradation of polycyclic aromatic hydrocarbons and naphthenic acids by microbes from oil-sands-process-affected waters. Int Biodeter Biodegr. 2015;108:214–221. doi: 10.1016/j.ibiod.2014.12.016. [DOI] [Google Scholar]
- Foti M, Sorokin DY, Lomans B, Mussman M, Zacharova EE, Pimenov NV, Kuenen GJ, Muyzer G. Diversity, activity and abundance of sulfate-reducing bacteria in saline and hypersaline soda lakes. Appl Environ Microbiol. 2007;73:2093–2100. doi: 10.1128/AEM.02622-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullerton H, Crawford M, Bakenne A, Freedman DL, Zinder SH. Anaerobic oxidation of ethene coupled to sulfate reduction in microcosms and enrichment cultures. Environ Sci Technol. 2013;47:12374–12381. doi: 10.1021/es4029765. [DOI] [PubMed] [Google Scholar]
- Gadd GM. Bioremedial potential of microbial mechanisms of metal mobilization and immobiliozation. Curr Opin Biotechnol. 2000;11:271–279. doi: 10.1016/S0958-1669(00)00095-1. [DOI] [PubMed] [Google Scholar]
- Gall JE, Boyd RS, Rajakaruna N. Transfer of heavy metals through terrestrial food webs: a review. Environ Monit Assess. 2015;187(4):1–21. doi: 10.1007/s10661-015-4436-3. [DOI] [PubMed] [Google Scholar]
- García R, Campos J, Cruz JA, Calderón ME, Raynal ME, Buitrón G. Biosorption of Cd, Cr, Mn, and Pb from aqueous solutions by Bacillus sp strains isolated from industrial waste activate sludge. TIP. 2016;19:5–14. doi: 10.1016/j.recqb.2016.02.001. [DOI] [Google Scholar]
- Geets J, Borremans B, Diels L, Springael D, Vangronsveld J, Van Der Lelie D, Vanbroekhoven K. DsrB gene-based DGGE for community and diversity surveys of sulfate-reducing bacteria. J Microbiol Methods. 2006;66:194–205. doi: 10.1016/j.mimet.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Gillespie IMM, Philp JC. Bioremediation, an environmental remediation technology for the bioeconomy. Trends Biotechnol. 2013;31:329–332. doi: 10.1016/j.tibtech.2013.01.015. [DOI] [PubMed] [Google Scholar]
- Govindarajalu K (2003) Industrial effluents and health status—a case study of Noyyal river basin. In: Proceedings of the 3rd international conference on environment and health. Chennai, India, pp 150–157
- Goyer RA, Clarkson TW. Toxic effects of metals. In: Klaassen CD, editor. Casarett and Doull’s toxicology: the basic science of poisons. New York: McGraw-Hill; 2001. pp. 811–867. [Google Scholar]
- Grossi V, Cravo-Laureau C, Méou A, Raphel D, Garzino F, Hirschler-Réa A. Anaerobic 1-alkene metabolism by the alkane- and alkene-degrading sulfate-reducer Desulfatibacillum aliphaticivorans strain CV2803T. Appl Environ Microbiol. 2007;73:7882–7890. doi: 10.1128/AEM.01097-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta VK, Nayak A, Agarwal S. Bioadsorbents for remediation of heavy metals: current status and their future prospects. Environ Eng Res. 2015;20:1–8. doi: 10.4491/eer.2015.018. [DOI] [Google Scholar]
- Hao OJ, Chen JM, Huang LJ, Buglass RL. Sulfate-reducing bacteria. Crit Rev Environ Sci Technol. 1996;26:155–187. doi: 10.1080/10643389609388489. [DOI] [Google Scholar]
- Harms G, Zengler K, Rabus R, Aeckersberg F, Minz D, Rossello-Mora R, Widdel F. Anaerobic oxidation of o-xylene, m-xylene, and homologous alkylbenzenes by new types of sulfate-reducing bacteria. Appl Environ Microbiol. 1999;65:999–1004. doi: 10.1128/aem.65.3.999-1004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herath A, Wawrik B, Qin Y, Zhou J, Callaghan AV. Transcriptional response of Desulfatibacillum alkenivorans AK-01 to growth on alkanes: insights from RT-qPCR and microarray analyses. FEMS Microbiol Ecol. 2016 doi: 10.1093/femsec/fiw062. [DOI] [PubMed] [Google Scholar]
- Huang WH, Kao CM. Bioremediation of petroleum-hydrocarbon contaminated groundwater under sulfate-reducing conditions: effectiveness and mechanism study. J Environ Eng. 2015 [Google Scholar]
- Huang CC, Chen CH, Chu SM. Effect of moisture on H2S adsorption by copper impregnated activated carbon. J Hazard Mater. 2006;136:866–873. doi: 10.1016/j.jhazmat.2006.01.025. [DOI] [PubMed] [Google Scholar]
- Huisman JL, Schouten G, Dijkman H. Biotechnological solutions for the treatment of pickle liquors. In: Dutrizac J, Riveros PA, editors. Iron control technologies. Montreal: Metallurgical Society; 2006. pp. 805–814. [Google Scholar]
- Hussain A, Qazi JI. Biological sulphate reduction using watermelon rind as a carbon source. Biologia (Pakistan) 2012;58:85–92. [Google Scholar]
- Hussain A, Qazi JI. Application of sugarcane bagasse for passive anaerobic biotreatment of sulphate rich wastewaters. Appl Water Sci. 2014 [Google Scholar]
- Hussain A, Qazi JI. Metals-induced functional stress in sulphate-reducing thermophiles. 3Biotech. 2016;6(1):1–8. doi: 10.1007/s13205-015-0342-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain A, Shakir HA, Qazi JI. Anaerobic biodegradation of sulphate employing animal manure as a cost effective growth substrate. J Anim Plant Sci. 2014;24:913–918. [Google Scholar]
- Hussain A, Shakir HA, Qazi JI. Implication of molasses as electron donor for biological sulphate reduction. Am J Environ Eng. 2014;4:7–10. [Google Scholar]
- Hussein H, Ibrahim SF, Kandeel K, Moawad H. Biosorption of heavy metals from waste water using Pseudomonas sp. Electron J Biotechnol. 2004;7:38–46. doi: 10.2225/vol7-issue1-fulltext-2. [DOI] [Google Scholar]
- Idris AM, Eltayeb MAH, Potgieter-Vermaak SS, Van-Grieken R, Potgieter JH. Assessment of heavy metals pollution in Sudanese harbours along the Red sea coast. Microchem J. 2007;87:103–112. doi: 10.1016/j.microc.2007.06.004. [DOI] [Google Scholar]
- Ihsanullah Abbas A, Al-Amer AM, Laoui T, Al-Marri MJ, Nasser MS, Khraisheh M, Atieh MA. Heavy metal removal from aqueous solution by advanced carbon nanotubes: critical review of adsorption applications. Sep Purif Technol. 2016;157:141–161. doi: 10.1016/j.seppur.2015.11.039. [DOI] [Google Scholar]
- Jadia CD, Fulekar MH. Phytoremediation of heavy metals: recent techniques. Afr J Biotechnol. 2009;8:921–928. [Google Scholar]
- Jaekel U, Zedelius J, Wilkes H, Musat F. Anaerobic degradation of cyclohexane by sulfate-reducing bacteria from hydrocarbon-contaminated marine sediments. Front Microbiol. 2015;6:116. doi: 10.3389/fmicb.2015.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson E, Rowe OF, Hallberg KB, Johnson DB. Sulfidogenesis and selective precipitation of metals at low pH mediated by Acidithiobacillus spp. and acidophilic sulfate-reducing bacteria. Hydrometallurgy. 2010;104:488–493. doi: 10.1016/j.hydromet.2010.03.029. [DOI] [Google Scholar]
- Johnson DB. Biological removal of sulfurous compounds from inorganic wastewaters. In: Lens PNL, Hulshoff Pol LW, editors. Environmental technologies to treat sulfur pollution: principles and engineering. London: IWA Publishing; 2000. pp. 175–205. [Google Scholar]
- Kaksonen AH, Plumb JJ, Franzman PD, Puhakka JA. Simple organic electron donors support diverse sulfate-reducing communities in fluidized-bed reactors treating acidic metal- and sulfate-containing wastewater. FEMS Microbiol Ecol. 2004;47:279–289. doi: 10.1016/S0168-6496(03)00284-8. [DOI] [PubMed] [Google Scholar]
- Khelifi N, Ali OA, Roche P, Grossi V, Brochier-Armanet C, Valette O, Ollivier B, Dolla A, Hirschler-Réa A. Anaerobic oxidation of long-chain n-alkanes by the hyperthermophilic sulfate-reducing archaeon, Archaeoglobus fulgidus. ISME J. 2014;8:2153–2166. doi: 10.1038/ismej.2014.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleindienst S, Herbst FA, Stagars M, von Netzer F, von Bergen M, Seifert J, Peplies J, Amann R, Musat F, Lueders T, Knittel K. Diverse sulfate-reducing bacteria of the Desulfosarcina/Desulfococcus clade are the key alkane degraders at marine seeps. ISME J. 2014;8:2029–2044. doi: 10.1038/ismej.2014.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniemeyer O, Musat F, Sievert SM, Knittel K, Wilkes H, Blumenberg M, Michaelis W, Classen A, Bolm C, Joye SB, Widdel F. Anaerobic oxidation of short-chain hydrocarbons by marine sulphate-reducing bacteria. Nature. 2007;449:898–901. doi: 10.1038/nature06200. [DOI] [PubMed] [Google Scholar]
- Koschorreck M. Microbial sulfate reduction at a low pH. FEMS Microbiol Ecol. 2008;64:329–342. doi: 10.1111/j.1574-6941.2008.00482.x. [DOI] [PubMed] [Google Scholar]
- Krukenberg V, Harding K, Richter M, Glöckner FO, Gruber-Vodicka HR, Adam B, Berg JS, Knittel K, Tegetmeyer HE, Boetius A, Wegener G. Candidatus Desulfofervidus auxilii, a hydrogenotrophic sulfate-reducing bacterium involved in the thermophilic anaerobic oxidation of methane. Environ Microbiol. 2016 doi: 10.1111/1462-2920.13283. [DOI] [PubMed] [Google Scholar]
- Kucukatay V, Bor-Kucukatay M, Atsak P, Ağar A. Effect of ingested sulfite on hippocampus antioxidant enzyme activities in sulfite oxidase competent and deficient rats. Int J Neurosci. 2007;117:971–983. doi: 10.1080/00207450600934085. [DOI] [PubMed] [Google Scholar]
- Kümmel S, Herbst FA, Bahr A, Duarte M, Pieper DH, Jehmlich N, Seifert J, von Bergen M, Bombach P, Richnow HH, Vogt C. Anaerobic naphthalene degradation by sulfate-reducing Desulfobacteraceae from various anoxic aquifers. FEMS Microbiol Ecol. 2015 doi: 10.1093/femsec/fiv006. [DOI] [PubMed] [Google Scholar]
- Landis WG, Yu M-H. Introduction to environmental toxicology. USA: Lewis; 2004. [Google Scholar]
- Leloup J, Loy A, Knab NJ, Borowski C, Wagner M, Jorgensen BB. Diversity and abundance of sulfate-reducing microorganisms in the sulfate and methane zones of a marine sediment, Black Sea. Environ Microbiol. 2007;9:131–142. doi: 10.1111/j.1462-2920.2006.01122.x. [DOI] [PubMed] [Google Scholar]
- Leloup J, Fossing H, Kohls K, Holmkvist L, Borowski C, Jorgensen BB. Sulfate-reducing bacteria in marine sediment (Aarhus Bay, Denmark): abundance and diversity related to geochemical zonation. Environ Microbiol. 2009;11:1278–1291. doi: 10.1111/j.1462-2920.2008.01855.x. [DOI] [PubMed] [Google Scholar]
- Lens PNL, Kuennen JG. The biological sulfur cycle: novel opportunities for environmental biotechnology. Water Sci Technol. 2001;44:57–66. [PubMed] [Google Scholar]
- Lens PNL, Gastesi R, Lettinga G. Use of sulfate reducing cell suspension bioreactors for the treatment of SO2 rich flue gases. Biodegradation. 2003;14:229–240. doi: 10.1023/A:1024222020924. [DOI] [PubMed] [Google Scholar]
- Liamleam W, Annachhatre AP. Electron donors for biological sulfate reduction. Biotechnol Adv. 2007;25:452–463. doi: 10.1016/j.biotechadv.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Lim E, Mbowe O, Lee AS, Davis J. Effect of environmental exposure to hydrogen sulfide on central nervous system and respiratory function: a systematic review of human studies. Int J Occup Environ Health. 2016 doi: 10.1080/10773525.2016.1145881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C-Y, Hsiu M-W. Effects of sulfide, sulfite and sulfate on acidogenesis in upflow anaerobic sludge blanket process. J Environ Sci Health. 1997;32:1171–1184. [Google Scholar]
- Madsen EL. Environmental microbiology. UK: Blackwell; 2008. [Google Scholar]
- Magot M, Ravot G, Campaignolle X, Ollivier B, Patel BK, Fardeau ML, Thomas P, Crolet JL, Garcia JL. Dethiosulfovibrio peptidovorans gen. nov., sp. nov., a new anaerobic, slightly halophilic, thiosulfate-reducing bacterium from corroding offshore oil wells. Int J Syst Bacteriol. 1997;47:818–824. doi: 10.1099/00207713-47-3-818. [DOI] [PubMed] [Google Scholar]
- Malik A. Metal bioremediation through growing cells. Environ Int. 2004;30:261–278. doi: 10.1016/j.envint.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Malla R, Tanaka Y, Mori K, Totawat KL. Short term effect of sewage irrigation on chemical buildup in soil and vegetables. Agric Eng Int. 2007;9:1–14. [Google Scholar]
- Martins M, Faleiro LM, Barros RJ, Veri´Ssimo AR, Costa CM. Biological sulphate reduction using food industry wastes as carbon sources. Biodegradation. 2009;20:559–567. doi: 10.1007/s10532-008-9245-8. [DOI] [PubMed] [Google Scholar]
- Martins M, Faleirob ML, Barros RJ, Veríssimo AR, Barreirosd MA, Costa MC. Characterization and activity studies of highly heavy metal resistant sulphate-reducing bacteria to be used in acid mine drainage decontamination. J Hazard Mater. 2009;166:706–713. doi: 10.1016/j.jhazmat.2008.11.088. [DOI] [PubMed] [Google Scholar]
- Mbaye I, Ntunzimbona I, Soumah M, Sow ML. Acute hydrogen sulfite intoxication: report of three lethal cases occurred at a Senegalese chemical plant. Dakar Med. 2003;48:128–130. [PubMed] [Google Scholar]
- Meckenstock RU, Boll M, Mouttaki H, Koelschbach JS, Cunha Tarouco P, Weyrauch P, Dong X, Himmelberg AM. Anaerobic degradation of benzene and polycyclic aromatic hydrocarbons. J Mol Microbiol Biotechnol. 2016;26:92–118. doi: 10.1159/000441358. [DOI] [PubMed] [Google Scholar]
- Meena R, Datta SP, Golui D, Dwivedi BS, Meena MC. Long-term impact of sewage irrigation on soil properties and assessing risk in relation to transfer of metals to human food chain. Environ Sci Pollut Res. 2016 doi: 10.1007/s11356-016-6556-x. [DOI] [PubMed] [Google Scholar]
- Morasch B, Schink B, Tebbe CC, Meckenstock RU. Degradation of o-xylene and m-xylene by a novel sulphate-reducer belonging to the genus Desulfotomaculum. Arch Microbiol. 2004;181:407–417. doi: 10.1007/s00203-004-0672-6. [DOI] [PubMed] [Google Scholar]
- Mori K, Kim H, Kakegawa T, Hanada S. A novel lineage of sulfate-reducing microorganisms: thermodesulfobiaceae fam. nov., Thermodesulfobium narugense, gen. nov., sp. nov, a new thermophilic isolate from a hot spring. Extremophiles. 2003;7:283–290. doi: 10.1007/s00792-003-0320-0. [DOI] [PubMed] [Google Scholar]
- Mortazavi F, Jafari-Javid A. Acute renal failure due to copper sulfate poisoning; a case report. Iran J Pediatr. 2009;19:75–78. [Google Scholar]
- Mothe GK, Pakshirajan K, Das G. Heavy metal removal from multicomponent system by sulfate reducing bacteria: mechanism and cell surface characterization. J Hazard Mater. 2016 doi: 10.1016/j.jhazmat.2015.12.042. [DOI] [PubMed] [Google Scholar]
- Mulla SI, Talwar MP, Ninnekar HZ. Bioremediation of 2, 4, 6-trinitrotoluene explosive residues. In: Singh SN, editor. Biological remediation of explosive residues. Switzerland: Springer; 2014. pp. 201–233. [Google Scholar]
- Muyzer G, Stams AJM. The ecology and biotechnology of sulphate-reducing bacteria. Nat Rev Microbiol. 2008;6:441–454. doi: 10.1038/nrmicro1892. [DOI] [PubMed] [Google Scholar]
- Okoh AI, Trejo-Hernandez MR. Remediation of petroleum polluted systems: exploiting the bioremediation strategies. Afr J Biotechnol. 2006;5:2520–2525. [Google Scholar]
- Ollivier B, Cayol JL, Fauque G. Sulphate-reducing bacteria from oil fields environments and deep-sea hydrothermal vents. In: Barton LL, Hamilton WA, editors. Sulphate-reducing bacteria: environmental and engineered systems. Cambridge: Cambridge University Press; 2007. pp. 305–328. [Google Scholar]
- Peralta-Videaa JR, Lopeza ML, Narayana M, Saupea G, Gardea-Torresdey J. The biochemistry of environmental heavy metal uptake by plants: implications for the food chain. Int J Biochem Cell Biol. 2009;41:1665–1677. doi: 10.1016/j.biocel.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Rabus R, Strittmatter A. Functional genomics of sulphate-reducing prokaryotes. In: Barton LL, Hamilton WA, editors. Sulphate-reducing bacteria: environmental and engineered system. Cambridge: Cambridge University Press; 2007. pp. 117–140. [Google Scholar]
- Rabus R, Hansen TA, Widdel F. Dissimilatory sulfate- and sulfur-reducing prokaryotes. In: Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E, editors. The prokaryotes. Berlin: Springer; 2006. pp. 659–768. [Google Scholar]
- Rocha GG, Zaia DAM, Alfaya RVD, Alfaya AAD. Use of rice straw as biosorbent for removal of Cu(II), Zn(II), Cd(II) and Hg(II) ions in industrial effluents. J Hazard Mater. 2009;166:383–388. doi: 10.1016/j.jhazmat.2008.11.074. [DOI] [PubMed] [Google Scholar]
- Rowley MV, Warkentin DD, Sicotte V (1997) Site demonstration of the Biosulphide process at the former Britannia mine. In: Proceedings of the 4th international conference on acid rock drainage, Vancouver, Canada, pp 1533–1547
- Sahinkaya E, Yurtsever A, Toker Y, Elcik H, Cakmaci M, Kaksonen AH. Biotreatment of As-containing simulated acid mine drainage using laboratory scale sulfate reducing upflow anaerobic sludge blanket reactor. Miner Eng. 2015;75:133–139. doi: 10.1016/j.mineng.2014.08.012. [DOI] [Google Scholar]
- Sakan S, Dordevic DS, Manojlovic DD, Predrag PS. Assessment of heavy metal pollutants accumulation in the Tisza river sediments. J Environ Manage. 2009;90:3382–3390. doi: 10.1016/j.jenvman.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Sayara T, Čvančarová M, Cajthaml T, Sarrà M, Sánchez A. Anaerobic bioremediation of PAH-contaminated soil: assessment of the degradation of contaminants and biogas production under thermophilic and mesophilic conditions. Environ Eng Manag J. 2015;14:153–165. [Google Scholar]
- Scragg A. Environmental biotechnology. UK: Oxford University Press; 2006. [Google Scholar]
- Stasik S, Wick LY, Wendt-Potthoff K. Anaerobic BTEX degradation in oil sands tailings ponds: impact of labile organic carbon and sulfate-reducing bacteria. Chemosphere. 2015;138:133–139. doi: 10.1016/j.chemosphere.2015.05.068. [DOI] [PubMed] [Google Scholar]
- Tauqeer HM, Ali S, Rizwan M, Ali Q, Saeed R, Iftikhar U, Ahmad R, Farid M, Abbasi GH. Phytoremediation of heavy metals by Alternanthera bettzickiana: growth and physiological response. Ecotoxicol Environ Safety. 2016;126:138–146. doi: 10.1016/j.ecoenv.2015.12.031. [DOI] [PubMed] [Google Scholar]
- Vaiopoulou E, Melidis P, Aivasidis A. Sulfide removal in wastewater from petrochemical industries by autotrophic denitrification. Water Res. 2005;39:4101–4109. doi: 10.1016/j.watres.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Van Roy S, Vanbroekhoven K, Diels L. Immobilization of heavy metals in the saturated zone by sorption and in situ bioprecipitation processes. Hydrometallurgy. 2006;83:195–203. doi: 10.1016/j.hydromet.2006.03.024. [DOI] [Google Scholar]
- Wagner M, Loy A, Klein M, Lee N, Ramsing NB, Stahl DA, Friedrich MW. Functional marker genes for identification of sulfate-reducing prokaryotes. Methods Enzymol. 2005;397:469–489. doi: 10.1016/S0076-6879(05)97029-8. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu RH, Fang DJ, Yu P, Wang JY, Tang AK. Distribution and accumulation characteristics of heavy metals in sediments in southern sea area of Huludao City, China. Chin Geogra Sci. 2013;23:194–202. doi: 10.1007/s11769-012-0579-0. [DOI] [Google Scholar]
- WHO: World Health Organization (1984) Guidelines for drinking water quality. Geneva
- Willis CL, Cummings JH, Neale G, Gibson GR. Nutritional aspects of dissimilatory sulfate reduction in the human large intestine. Curr Microbiol. 1997;35:294–298. doi: 10.1007/s002849900257. [DOI] [PubMed] [Google Scholar]
- Wissuwa J, Stokke R, Fedøy AE, Lian K, Smalås AO, Steen IH (2016) Isolation and complete genome sequence of the thermophilic Geobacillus sp. 12AMOR1 from an Arctic deep-sea hydrothermal vent site. Stand Genomic Sci 11:16. doi:10.1186/s40793-016-0137-y [DOI] [PMC free article] [PubMed]
- Zhang L, Zhao L, Yu Y, Chen C. Removal of lead from aqueous solution by non-living Rhizopus nigricans. Water Res. 1998;32:1437–1444. doi: 10.1016/S0043-1354(97)00348-5. [DOI] [Google Scholar]
- Zhang D, Wang J, Zhao J, Cai Y, Lin Q. Comparative study of nickel removal from synthetic wastewater by a sulfate-reducing bacteria filter and a zero valent iron—sulfate-reducing bacteria filter. Geomicrobiol J. 2016;15:318–324. doi: 10.1080/01490451.2015.1052116. [DOI] [Google Scholar]
- Zhang J, Tian Y, Zhang J, Li N, Kong L, Yu M, Zuo W. Distribution and risk assessment of heavy metals in sewage sludge after ozonation. Environ Sci Pollut Res. 2016 doi: 10.1007/s11356-016-6313-1. [DOI] [PubMed] [Google Scholar]
- Zhao W, Zou Y, Matsuda K, Zou Z. Characterization of the effect of hydrogen sulfide on the corrosion of X80 pipeline steel in saline solution. Corros Sci. 2016;102:455–468. doi: 10.1016/j.corsci.2015.10.038. [DOI] [Google Scholar]


