Abstract
Surgical guidance with fluorescence has been demonstrated in individual clinical trials for decades, but the scientific and commercial conditions exist today for a dramatic increase in clinical value. In the past decade, increased use of indocyanine green based visualization of vascular flow, biliary function, and tissue perfusion has spawned a robust growth in commercial systems that have near-infrared emission imaging and video display capabilities. This recent history combined with major preclinical innovations in fluorescent-labeled molecular probes, has the potential for a shift in surgical practice toward resection guidance based upon molecular information in addition to conventional visual and palpable cues. Most surgical subspecialties already have treatment management decisions partially based upon the immunohistochemical phenotype of the cancer, as assessed from molecular pathology of the biopsy tissue. This phenotyping can inform the surgical resection process by spatial mapping of these features. Further integration of the diagnostic and therapeutic value of tumor metabolism sensing molecules or immune binding agents directly into the surgical process can help this field mature. Maximal value to the patient would come from identifying the spatial patterns of molecular expression in vivo that are well known to exist. However, as each molecular agent is advanced into trials, the performance of the imaging system can have a critical impact on the success. For example, use of pre-existing commercial imaging systems are not well suited to image receptor targeted fluorophores because of the lower concentrations expected, requiring orders of magnitude more sensitivity. Additionally the imaging system needs the appropriate dynamic range and image processing features to view molecular probes or therapeutics that may have nonspecific uptake or pharmacokinetic issues which lead to limitations in contrast. Imaging systems need to be chosen based upon objective performance criteria, and issues around calibration, validation, and interpretation need to be established before a clinical trial starts. Finally, as early phase trials become more established, the costs associated with failures can be crippling to the field, and so judicious use of phase 0 trials with microdose levels of agents is one viable paradigm to help the field advance, but this places high sensitivity requirements on the imaging systems used. Molecular-guided surgery has truly transformative potential, and several key challenges are outlined here with the goal of seeing efficient advancement with ideal choices. The focus of this vision 20/20 paper is on the technological aspects that are needed to be paired with these agents.
Keywords: surgery, molecular imaging, molecular probe, surgical guidance, imaging
1. INTRODUCTION—HISTORICAL DEVELOPMENTS TO CURRENT STATUS
In the past decade, there has been rapid growth in clinical use in fluorescence to guide surgical procedures, and recent FDA 510(k) clearance of four new systems in the last two years. This growth has been from the use surgical imaging of indocyanine green (ICG) for tissue perfusion and vascular function.1–4 Now it is possible to take advantage of this fluorescence imaging approach through development of targeted agents which could be advanced into clinical use for truly molecular-guided surgery, where the fluorescence signal is both a molecular diagnostic as well as part of the intraoperative decision making process,5–10 or even used to determine when to activate molecular based therapy.11–17 In principle, imaging signals for as many pertinent information streams as is feasible could be possible, but this currently includes just blood flow and tissue perfusion. Ideally signals such as metabolic proteins and ions could be imaged or immunologic receptors and cytokines with appropriately accurate molecular probes. This paradigm would be analogous to the codiagnostic imaging promoted by nuclear medicine18 or providing the specificity of gene analysis,19 where a targeted imaging agent reports on the metabolic or immunologic functions that are the therapeutic biological target. The biomarker can be either measurement of receptor activity or reporting on molecules upstream/downstream from it, but the key feature is that the imaging informs the potential efficacy of the therapeutic choice. In the case of optical imaging, the natural utility is to directly guide surgical oncology decisions at the point of care,20 but there is also an extension of this where the fluorescent agent is also packaged as a therapeutic, either directly as a photodynamic agent or indirectly as part of a nanoparticle delivery system. While this new paradigm is emerging, it is inhibited by the costs of how agents are developed and tested, and concerns lie around the appropriate imaging guidance tools and advancing the right molecules for testing.21–23 In any emerging field, it is possible that inappropriate choices made early could encumber the development to the point where the paradigm is viewed as a failure, even if other combination approaches might have been more successful. As such, this paper describes the key issues of imaging technologies, molecule evaluation plans, and choices of when to advance into human trials, and some of the economics needed to develop a viable paradigm for molecular-guided surgery.
Basic research and development in fluorescence imaging has been ongoing for decades,24–31 with major contributions to the concepts and even clinical trials happening as early at the 1940s. Most of the basic technology work has been a continual evolution from clinical reflectance imaging systems in endoscopy, colonoscopy, and colposcopy, as illustrated in Fig. 1, with individual feedback from each subspecialty into the development of new systems and molecules. The steps in imaging system evolution are discussed in Sec. 2 of this paper. Key differences for molecular guidance are the needs to make the signal specific to the molecules of interest and the expected concentration and contrast ratio, and to present the information in a manner which is customized to the medical specialty. Specialty areas in surface imaging in the skin, oral cavity, esophagus, colon, bladder and cervix have each seen innovations in technology and contrast agents, which led to clinical trials. Laparoscopic32–37 and endoscopic38–43 methods also have a natural synergy with fluorescence imaging because of the common workflow of viewing the procedure on a display screen, and the ease of shifting to different excitation light fields with little background room light interference. Neurosurgery is one of the early adopters of high technological influence, because of the need for microprecision specificity and the fact that neurosurgeons are already used to high technological guidance in their procedures.2,9,44–49 Urology has led the way in robotic surgery,50–52 which has now adopted fluorescence guidance with an optional camera as one feature in the system.53–57 Now that more probes become established and systems have been optimized for their detection, adoption into higher risk procedures is occurring, especially where there is significant added value to the procedure, such as in complex vascular procedures or surgical margin detection.
FIG. 1.
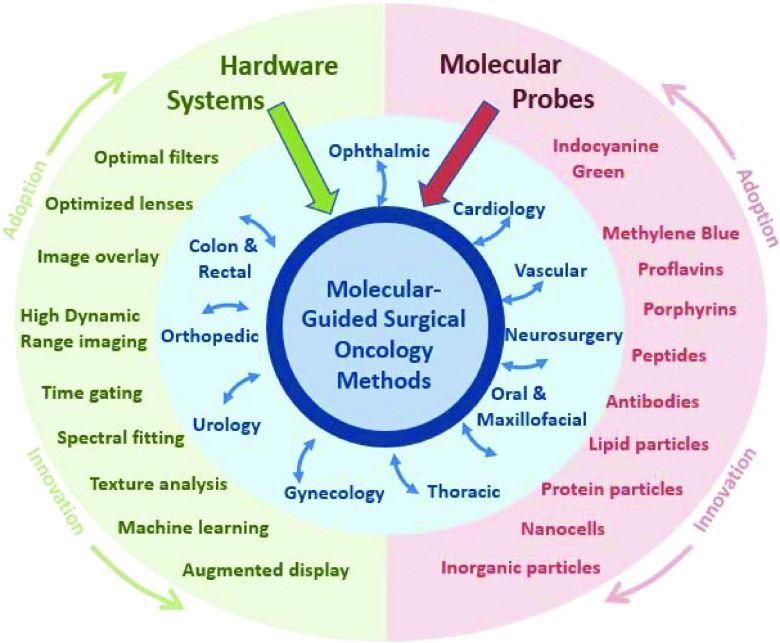
The two essential components of hardware systems and molecular probes are illustrated feeding to the development process of molecular-guided surgery (center). The technological innovations feed to the center, but these also feed to and from the individual surgical subspecialties, around the central core ideas. The most well developed and readily adopted system features and molecular probes are at top, with R&D pushing innovations downward, yet adoption of both generally comes from the most well developed systems and probes first (top of outer circle). Commercial adoption tends to be at the more conservative end of this (top of figure), and in many cases is the opposite of research innovations (bottom of figure). Additionally interaction through the surgical subspecialties generally comes from the manufacturer outward, without significant interspecialty cross-fertilization.
Clinical imaging with ICG has caused a doubling in the number of published papers in the last ten years, and much higher growth in approved procedures.1,58–64 This use has expanded from simple vascular tracer imaging to tissue perfusion viability, kidney function, biliary tract function, lymph node detection, as well as many others under investigation. Some alternative fluorescent agents being tested have been aminolevulinic acid to produce protoporphyrin IX (ALA-PpIX) for photodynamic therapy of skin lesions, or proflavins as oral antiseptic agents,65,66 both of which also work as fluorescent contrast agents. ALA-PpIX is perhaps one of the most successful examples of a metabolic probe, first advanced as a clinical tool almost twenty five years ago.67 PpIX is produced in the heme synthesis cycle,68 which is upregulated in many cancers and has been shown to be a robust guide in neurosurgery9,69–71 and bladder cancer detection,72–75 with approvals for use in both diagnostic settings in several European countries. There has been explosive growth in basic and applied research in more complex proteins, biologicals, vesicles, and nanoparticles which can be used as optical tracers, however with most of these waiting for translation into human trials, with uncertain evidence that this could be funded. The key issues around advancing these exogenous molecular contrast agents into human use need study, as is detailed in Sec. 3 of this paper.
The components needed for growth in this diverging field are agents with true specificity to the disease, as shown by maximized contrast to background, and the imaging system used must be optimized for this expected use, with customized display/guidance tools that easily facilitate use. Producers try to differentiate their systems based upon customized features that address the intended indication. Each of these is discussed in this review. Figure 1 illustrates that as innovation progresses, the drive to more specialized probes and to more specific features of the systems can be independent of the surgical specialties, yet can penetrate them as needs arise. Interestingly there appears to be less interaction between the surgical subspecialties in terms of procedures than there is feedback to and from the technological and molecular probe advances, and it is these latter areas that then relay advances to other specialty areas. However one other critical part of development is to ensure that clinical trials are funded with an economic model which realizes how transformative this type of imaging could become, but also how risk prone it is at the early stage. This tricky issue is discussed in Sec. 4 of the paper, outlining one possible model using microdose studies, to lower the economic risk such that commercial investment might be enticed. While the upfront risks of this new procedure are high, the commercial and human benefits could be profound in surgical oncology. Yet this paradigm uses very low concentration doses, and so it requires even more stringent demands upon the sensitivity of the imaging system used.
2. IMAGING SYSTEMS—PERFORMANCE, DIFFERENTIATION, MAXIMIZING FEATURE CONTRAST, AND DISPLAY
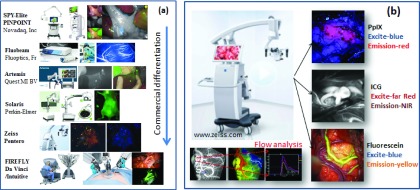
Optical systems to image fluorescence in surgery have been in constant evolution, with a very wide range of systems from microscopy to macroscopy being developed. In terms of adoption in surgery though, there have been relatively few successfully marketed systems to date, with the Novadaq SPY being the most widely used [Fig. 2(a), top], but with now several new systems emerging into this space. These new systems present with a greater diversity in capabilities, such as superior ergonomics, white light suppression, and adaptive gain for higher sensitivity and dynamic range. The spike in usage of ICG for perfusion imaging of tissue has driven this growth in the market place, with several of the competing systems shown in Fig. 2(a). One example of a highly differentiated system is the Zeiss Pentero neurosurgical station, Fig. 2(b), which has been customized to allow imaging options with all of the major dyes used in neurosurgery, including indocyanine green, fluorescein, and now aminolevulinic acid induced protoporphyrin IX.76–78 Each has its approved use, and even the kinetics analysis of flow can be done in the software tool. This system, with all of these fluorescence options, illustrates how advanced the field has become within the neurosurgery specialty. The use of ALA-PpIX is approved in Germany and has been carried out in a growing number of clinical trials in the United States.
FIG. 2.
Emerging commercial fluorescence surgical systems are differentiating themselves from simple NIR fluorescence of ICG, with additional wavelength bands, optimized background rejection, kinetics analysis, and integration into higher level functionality systems. Some of the leading and newly introduced systems are shown in (a) with more differentiation being a commercial driving factor. In (b), the Zeiss Pentero is highlighted, which has capabilities for 3 fluorescence channel options and a kinetics analysis of blood flow, integrated into a standard neurosurgical system. (See color online version.)
Despite rapid growth in systems, the factors that make a fluorescence system truly successful are not well established. Performance goals and specifications are somewhat lacking, since this is being driven by the needs of surgical specialties, rather than a concerted technological drive, and without consensus studies or top-down oversight. As a result, it is unfortunately quite easy for systems to vary in sensitivity by orders of magnitude because of the range of components and uses that make the up the systems.79 Key factors are many, and currently there are working groups in several societies fostering needed discussion of these issues, and work toward a wider understanding of the pertinent issues. There is convergence happening in some community documents and guidelines, and these may inform regulatory and standards in the future. All current systems have a single highly specialized use, being driven toward the singular application of ICG perfusion and flow imaging. The technical demands of this application are relatively straight forward because of the high concentration of fluorescent agents being used, but the application to lower concentration probes will seriously test the lower limits of systems. A good example of this is in ALA-PpIX imaging, where concentrations can be present in tissue at the 1.0–0.01 μM level in vivo. The numbers expected are important, because current imaging-based systems can fail to detect this, despite this being a needed goal for the surgical indication of neurosurgery.46 As a result, direct probe measurements on the tissue have been developed to detect the lower levels, simply as a way to gain more optical sensitivity to the tissue concentration. Increased detection sensitivity or the ability to discriminate the specific signal from the background is likely required for growth of the field into specific molecular reporters. The need to push detection sensitivity to the lowest levels is also intertwined with the issue of background signal levels being one of the main limiting performance functions.30 The nature of the background signal is often a compelling feature which can dominate the technical choice of system.
A key part of most fluorescence guidance systems, which is different than reflectance imaging systems, is that the effective background dominates the performance and can limit the lower sensitivity of the system.30 The factors affecting this background are several, but the most important are excitation or ambient light leakage into the fluorescence detector and getting mixed up with true fluorescence signal.80 Thus, methods to limit leakage of this contribution to background from getting into the detector or to remove it from the processed signal are needed, as illustrated in Fig. 3(a). There are three primary methods for optimization of the signal to background ratio. The most important is optimization of the lens, filter, and camera design, which limits the original signal from being detected. The second method commonly employed is temporal gating of the signal [Fig. 3(b)], to remove ambient light and gate to the excitation pulse.81 The third method employed is some kind of wavelength filtering or fitting in the detected signal [Fig. 3(c)], which can remove the broad background which evaded the previous two methods, and contaminated the detected signal anyway.82–86 The combination of all three approaches to narrowing the bandwidth in time, spectrum, and space can minimize the background signal. There are several optional technological approaches to each of these three, but their implementation is essential to gaining maximum background removal. One of the more problematic issues in wavelength filtering has always been use of suitably effective wavelength filters, as illustrated in Fig. 3(c). These must be custom produced for the application when rejection of more than a few orders of magnitude optical density (OD) is required. Essentially all fluorescence systems today use custom filters, and sometimes multiple layers of dielectric and then absorbance filters are used to further reduce unwanted signals, and the optical design to maximize a parallel path of light through the dielectric filter is essential for proper filtering efficiency. Without these careful designs, it has been all too common for fluorescence systems to fundamentally underperform in the area of excitation light contamination into the detected fluorescence signal.
FIG. 3.
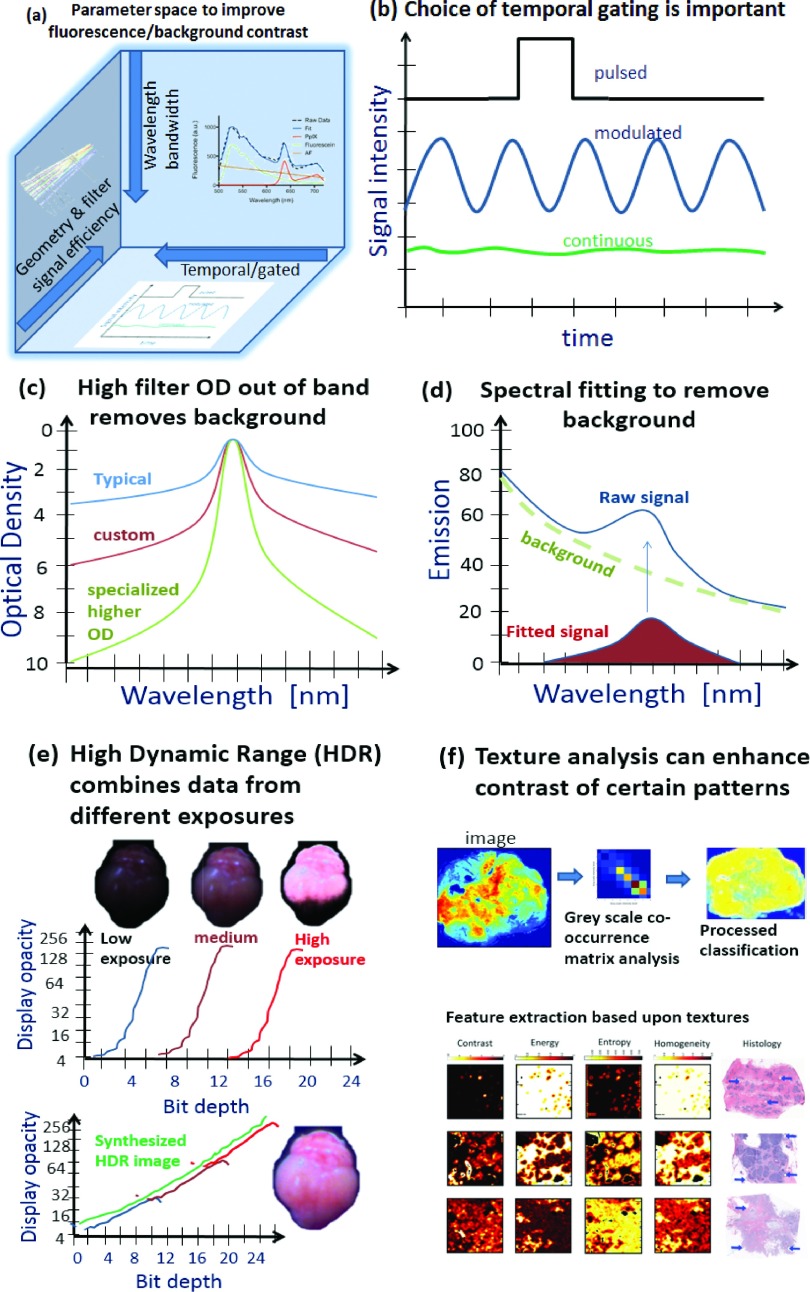
The main hardware methods to increase signal specificity before camera capture are shown with (i) geometry and filter optimization, (ii) wavelength or bandwidth restrictions, or (iii) temporal signal processing with mechanical or electronic shuttering. The temporal gating approaches are illustrated in (b) as single shot pulsed, modulated relative to continuous sampling. Filtering and wavelength processing are illustrated in (c) with typical, custom and specially designed bandpass filters for detection, and (d) spectral fitting which might be implemented to remove background. The use of HDR principles (e) can allow superior detection by better background subtraction without loss of pertinent dynamic range. Finally, image texture analysis (f) might be used for improved diagnostic performance based upon the morphology of the contrast.
In the last few years, an important extra layer of background removal which has been added to some systems is the idea of spectral fitting of the detected signal, even if strong optical filtering is done at the input to the detector. This is often required, because optical filters are known to be imperfect in all cases, with typical values being about 3 ODs of rejection for out of band wavelengths, and the best filters being 5 ODs of rejection. Since the background contamination has different wavelength dependence, spectral detection and fitting can be used to remove it. This requires spectrometer detection or multispectral imaging capability, which is becoming more achievable with high speed filter wheels or with liquid crystal tunable filters. As shown in Fig. 3(d) at right, when the background is a large fraction of the signal, the process of spectral fitting can lower the detection threshold by over an order of magnitude. This design has only been implemented in preclinical or research grade systems to date, but emergence of this in clinical systems is likely just a matter of a few years. However since the spectral fitting is specific to the molecule and the type of background, this development is a layer of specialization which needs to be adapted to the needs of the molecule used to image, but also must be developed in a manner which is time efficient.
One of the least discussed but critically important parts of a fluorescence imaging system is the digitized dynamic range and performance of the camera and lens system used. This issue is well appreciated in medical imaging, such as in CT scanners which produce 12-bit images but are displayed on standard 8-bit monitors, with the appropriate window/level adjustment to the useful dynamic range.87 Typical optical CMOS cameras operate on 8-bit output, and color cameras have three channels of 8-bit color output. Yet fluorescence signals can easily vary by many orders of magnitude, making the 255 gray scale values of an 8-bit digitization to have little value. This can be addressed by moving the field of view in or out, which varies the irradiance of the excitation source by the square of the distance to the tissue. This approach, while practical, can easily limit quantitative interpretation of the observed intensity, corresponding to the concentration of fluorophore. A better way to deal with this situation is the utilization of high dynamic range (HDR) imaging approaches, with calibrated display of a windowed region [see Fig. 3(e)]. HDR imaging is now widely implemented in commercial camera systems and provides a logical alternative to the high overhead needed for high analog to digital conversion bit depth for single images. The logistic advantage of HDR is that two exposures are taken of the same imaging field, at low and high excitation intensities or integration time. Thus the images can be combined with some calibration to ensure that a much wider dynamic range is achieved (>16 bit images) than traditional 8-bit images. In this paradigm, there is no need for the dramatically higher electronic overhead of 16-bit readout, but rather is can be simply reading out two 8-bit images. This approach to high dynamic range imaging is ideally suited for fast imaging which requires higher contrast capability.
The related issue to increased dynamic range imaging is how to display the full dynamic range such that it can be usefully interpreted by the surgical team. While window and level methods are ubiquitous in radiology, they have little value in video rate imaging because of the logistical limitation of changing the values on the fly. But automated window-level methods combined with automated histogram normalization can be implanted if done carefully to the application. Alternatively, logarithmic transformation can help to adjust the dynamic range to the viewable depth of the display, avoiding much need for intensive mathematical processing.87
Methods to view the display of fluorescence in a manner which is intuitive and logically differentiated from the surgical field are also growing in importance. There is certainly no consensus on colors nor methods for display, yet the need to match the field of view of white light imaging with computed or segmented emission is critically important. A recent study by Elliott et al.88 demonstrates how analysis of the observed color ranges in a view can be used to predict the maximum color contrast or range available for the added information of molecular fluorescence. This type of feature display improvement in either the surgical view or the surgical monitor will improve the ability to detect and discriminate the imaged signals, and this becomes even more important when the dynamic ranges of intensity exceed the viewable dynamic range of current display monitors. This is an area of major need, yet little development.
Finally, while much of the molecular imaging world has focused on raw uptake values, a major shift in imaging is toward texture analysis of image data, where the structural features of the tissue are used with machine learning algorithms, to identify more suspicious areas. It is quite feasible that the texture analysis of fluorescence images will provide this type of information as well, since vascular patterns and nodular cancer regions have specific morphologies that could be learned by an imaging system. This type of classification is largely undeveloped in vivo at a surgical decision tool. But it is well known that structural patterns of vascular density and size exist in cancer, and that structural patterns of stroma and epithelial growth exist, as well. It should be expected that these type of structural features will appear in the fluorescence image data as well, as affected by the spatial distribution mechanisms and the tissue features that provide or inhibit transport. The heterogeneity of these features is high, and so validation of these biomarkers would require substantial development and testing. Similar algorithmic classification looks promising in the related fields of radiological image analysis89–91 and pathology image analysis92–95 of cancer.
The systems being advanced into human surgery are largely macroscopic imaging tools, with fields of view on the order of centimeters across. While research focus in microscopy is widespread in academic medicine, there are very few commercial products being advanced to guide surgery. This is largely because surgeons tend to be working on the removal of macroscopic volumes of tissue, and so they require tools which inform decisions on this size similar scale. Similarly, tomographic technologies have been advanced in a range of academic research trials, yet few systems have ever even entered a surgical trial, because of the steep resolution loss after just a millimeter of tissue, from optical scattering.96–98 While surgical systems based upon photoacoustic tomography could likely advance this field productively, because of the fields of view and sensitivity range, these tools retrieve their contrast based upon absorption of molecules which typically must be present in the millimolar concentration range. Since metabolic and immunologic probes largely exist in tissue at the micromolar to nanomolar concentration ranges, photoacoustic tomography imaging may result in limited value. Still, research into these devices continues, and it may be that innovations in the contrast agents make these more attractive for commercial advancement into clinical trials. The devices which are now commercially available for fluorescence molecular imaging are largely based upon traditional methods of broad field optical excitation using LEDs or laser illumination, coupled with optical filtering of the remitted light, focused onto a lower cost imaging sensor. These workhorse systems have advanced significantly in the past decade, to the point where there are easily now a dozen companies competing with FDA approved systems for this emerging clinical surgery market.
3. MOLECULAR PROBES—GOING BEYOND VASCULAR PERFUSION AND ASSESSING MOLECULAR SPECIFICITY
The earliest Food and Drug Administration (FDA) approval for fluorescence guided imaging happened in 1959, when IC-green was approved for ophthalmology, to visualize vessels in the retina.99–101 Later in 1991, it was approved for additional indications in cardiac output, hepatic function, and liver blood flow, and this application continues today as a mainstream of the field, with major advances in the technology used for imaging, to image faster and at higher resolution. Fluorescein was approved for ophthalmology use in 1976102,103 and continues as a staple tool within the field. While these flow and perfusion probes are the cause of the recent explosion in systems and use of fluorescence guided surgery, they are also inherently limiting the potential value to vascular/perfusion imaging. These agents are not well suited to site specific binding to proteins, and while they can be packaged into nanoparticles,104–106 their use a molecular-specific imaging tools is limited by low emission yield and biochemical instability issues. As a result, many fluorescence technology companies have put forth more stable near-infrared dyes which can be linked to specific proteins. The first to enter a range of clinical trials has been IRDye® 800CW, which has been linked to Erbitux, Herceptin, and deoxyglucose to name a few targeted approaches. Others have made customized fluorophores which inherently bind to targets, as a novel single tracer.
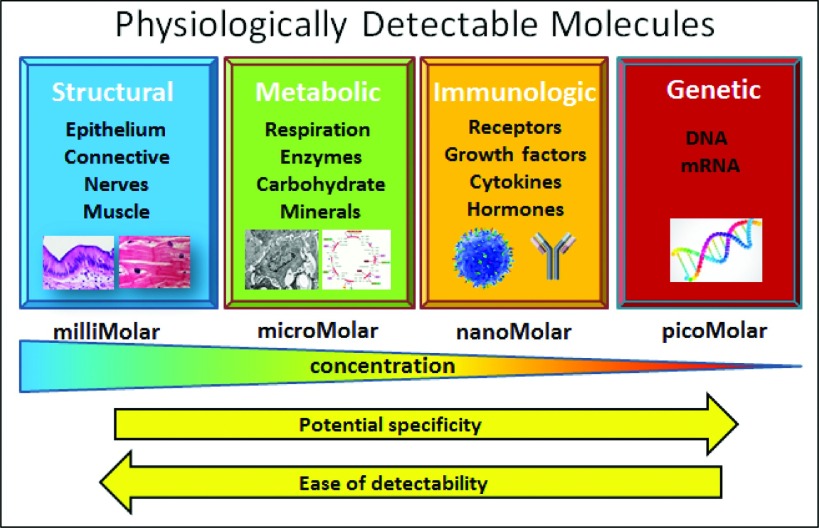
A systematic review of the types of targets available and their relative concentrations illustrates that, as shown in Fig. 4, physiological molecules can be classified into four broad categories, including (i) structural features, (ii) metabolic systems, (iii) immunologic systems, and (iv) genetic material.107 These features each have many molecular targets within them, and for the majority of the imaging systems, structural features have been targeted, simply because they exist in abundance, with typical concentrations in the millimolar range. While genetic material might be the ideally specific goal for targeted imaging, but it exists in such low concentrations, near picomolar, that it cannot be imaged with most detection methods. The vast majority of molecular imaging approaches in oncology have targeted metabolic features or immunologic features of tumors,108–113 with these typically existing in the range of micromolar quantities, whereas immunologic molecules exist in the range of nanomolar concentrations. As such, imaging the lower concentrations likely has more specificity, but also becomes a much harder signal to capture. For example, mRNA based sensor probes have been studied for a long time, yet have been challenging to utilize in vivo at sufficiently low concentrations, and so would have toxicological effects if used at sufficiently high concentrations to do useful detection.11,114,115 Still recent work has shown solid potential for imaging based upon genetic material in vitro,116 and this may eventually work well for larger tissue sensing in vivo if able to be achieved with modest toxicity. However, the most important feature to recognize from Fig. 4 is that if fluorescence imaging is likely to succeed with robust signals, the metabolic information will likely have to be detected with probes in the millimolar concentration range, which is very achievable for most agents and imaging systems. However, immunologic sensing will require detection of signals as low as the nanomolar range, pushing signal detection to the limits of what is achievable today with the very best fluorescence imaging systems. Most commercial systems today could not reach robust nanomolar sensitivity of their target fluorescent molecule, so only those which have designed their system to the most stringent specifications will likely complete in this latter space. This observation is critically important when choosing a system to match a targeting agent for clinical trial use.
FIG. 4.
The possible range of molecules which could be used for increased tumor specificity are in the categories of (i) structural, (ii) metabolic, (iii) immunologic, or (iv) genetic features of the tissue. Generally the most potential for high specificity is to the right in this illustration, but the concentrations available for sensing also decrease dramatically in these categories, and so the availability of the signal decreases.
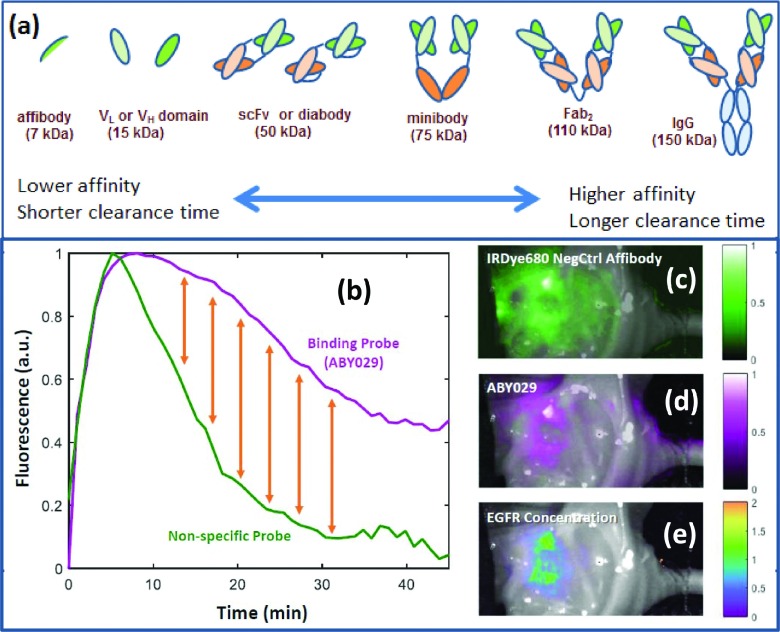
At present, there is an enormous amount of development going on in the metabolic and immunologic imaging realms,117–121 and clinical trials have started on cell surface receptor based imaging with antibodies and antibody fragments.20,122–124 Figure 5(a) illustrates the range of protein components of antibodies which have been explored for specific binding to cell receptors, ranging from the smallest binding domain, Affibody® molecules, through binding domain components of heavy chain and light chain (VH and VL), fractions, single chain fragments (scFv), to minibodies, fragments of antibodies (Fab), and full immunoglobulin proteins (Ig). Generally as the protein size increases, it retains the full affinity of the binding domain and has the highest potential for specificity; however, these larger proteins also can have longer plasma retention times.125–127 The increase in plasma lifetime leads to more nonspecific signals in vivo because there is a continuous feed of new dye to the tumor interstitium. Higher specificity of bound dye usually occurs in the time sequence when the plasma clearance is dominated by clearance, as illustrated in Fig. 5(b).
FIG. 5.
The range of immunological probes which could be utilized for cell surface receptors are shown (a), with the trade-off that comes with variation in size, being affinity for metabolic clearance time (Refs. 127 and 133). In (b) the problem of binding versus nonbinding probes is illustrated, in that even a specifically binding probe has significant signal simply from the wash in and wash out kinetics of perfusion. A nonspecific probe could be utilized to provide a reference, with the difference between the two being the bound fraction of specific probe (yellow arrows) (Ref. 132). In (c)–(e), an example tumor image is shown where EGFR-binding affibody labeled with IRDye 800CW (called ABY-029) is shown localizing (d) and a nonspecific affibody control (c). These were imaged simultaneously after a simultaneous injection, and the fitted difference signal between the two images shows the smaller EGFR + bound region (e).
One of the major problems in molecular imaging is the issue that perfusion supply and clearance of probes tend to dominante the influence of the probe concentration.128–130 A recent study by Tichauer et al. explored the extent of this confounder in lymph node imaging with Cetuximab labeled with IRDye® 800CW. Another example summarized in Fig. 5(b) shows the time-dependent fluorescent signal measured in a mouse glioma subcutaneous xenograft after injection of ABY029 and negative control Affibody molecules labeled with IRDye680RD. The shape differences between these two curves are attributed to specific binding activity in the tumor,131 but spatial variation in the intensity of the signal can obscure these differences and lead to high background enhancement [Fig 5(c), top]. In this case, the data were fit along with image-derived arterial input functions using a graphical approach to obtain available receptor concentration map [Fig. 5(c), bottom]. A key conclusion from these studies is that specific dyes by themselves can be affected by perfusion and transport processes to the point where they do not accurately report on specific binding by itself. Some secondary method to reduce the nonspecific signal is needed, which could be a physiochemical effect such as activation of the fluorescence by enzymes, or change in lifetime or fluorescence intensity by factors such as pH or membrane breakage effects. The use of combination cocktails of fluorescent reporters is one proposed methodology to get a better measure of binding.130,132 No matter which method is used, it is essential to appreciate that the single molecule reporters do not always report on specific binding, and so their use must be interpreted carefully so that the information is not misinterpreted.
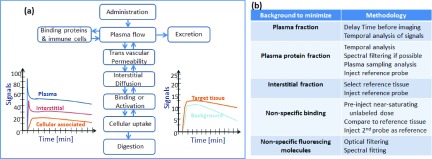
Further probe or probe combination use is emerging in the clinic, albeit slower than the introduction of new hardware imaging systems. This issue is primarily one of economics, which will be discussed in Sec. 4. The methods to enhance signal from the temporal dynamics of the contrast agent is a complex one though, and one in which there has been considerable preclinical study. A summary of methods has recently been published recently,130 and a brief illustration of the compartmental flow kinetics is illustrated in Fig. 6(a). In Fig. 6(b), there is a tabulated list of ways to maximize the contrast of the tracer, based upon the particular background that needs to be minimized.
FIG. 6.
The compartment flow of intravenous tracers are illustrated in (a) along with example kinetics curves, illustrating how the different compartments of plasma, interstitium, and cell parenchyma contribute to the overall signals in the target and background tissues, as a function of time. In (b), methods for maximizing the signal relative to background are tabulated, to maximize signal specificity, mostly based around which background signal needs to be minimized.
4. FUTURE GROWTH AND MEDICAL ADOPTION FOR SUCCESS OF MOLECULAR-GUIDED SURGERY
The metrics for success of any new surgical practice should ideally be improved patient outcomes or reduced costs for the same outcome, and in the current climate of cost management, this latter issue will likely be a key metric of success going forward. However, there are secondary factors that are less financially tangible, which could easily have just as much influence on the adoption, such as device market position, patient preference, medical center competition, and litigation activity.134 The current climate for adoption is being driven predominantly by the device companies, making systems available through the 510 K clearance route at the FDA, to be used for ICG imaging in vivo. The use for other molecular dyes could come as a later indication approval, for these existing devices. However, this pathway is the crux of the technology problem which could occur, and in that devices designed for ICG, imaging may not perform well for other molecular probes which have shifted wavelengths or present in tissue at significantly lower concentrations, and most importantly higher background tissue levels. The advancements discussed in Sec. 2 are needed in order to detect the probes discussed in Sec. 3.
Thus, metrics for adoption and guidelines for industry need to be made available from professional societies which are in the space of quantifying interventional procedures. Several stages of development should precede wider clinical trials, including (i) calibration approach, (ii) performance verification, (iii) tissue simulating phantom value, and (iv) professional society scrutiny. The role of quantification is still not obviously needed yet, and so is not well defined in most procedures, and the role that medical physicists will have in this field is still emerging. Currently device companies and biomedical engineering research are driving much of the activity, but as clinical adoption grows, the shift from company driven practice guidelines to medical community driven guidelines should occur, and the value in the stages of system calibration, verification, and quantitative interpretation will become more apparent. Diagnostic imaging QA of these devices will likely need to be established, as will user guidelines, based upon experiences in initial adopting academic centers. Medical physicists involved in surgical guidance or fluorescence imaging development will need to be involved in this pathway toward professional society consensus.
5. ECONOMICS—DEVELOPMENT MODELS THAT SPAN THE VALLEY OF DEATH
One of the reasons that new molecular probes are not emerging quickly is because of the lack of an immediate economic model in molecular-guided surgery.23 Much of the focus in molecular probe imaging with nuclear medicine has been to pair the development of targeted agents to medical oncology biologic agents, with the thematic goal of personalized medicine, customized to the disease phenotype. This goal still has major cost limitations in the diagnostic probe development which can only be mitigated if the therapeutic works well in this subpopulation of identified patients.22 The other major paradigm utilized in the nuclear medicine world is that of microdose studies, where imaging is done at subpharmacodynamic dose levels (FDA specifies this as <30 nM for synthetic drugs such as proteins, or <100 μg for most imaging agents).135 Both the paired diagnostic-therapeutic model and the microdose model need to be taken advantage of in fluorescence molecular-guided surgery.
The concept of optical targeted agents is a similar one, but the agent is both a diagnostic and a component of the therapy, because it guides the surgery. This new paradigm is not approved in the vast majority of settings today, inhibiting development because of the classic “chicken and egg?” problem. The question is how does one finance a new contrast agent to guide surgery when this paradigm does not exist, and the reimbursement codes and structure for implementing them do not exist. This means that all new agents and imaging systems must pass through a premarket approval (PMA) pathway at the FDA with no existing predicate approach, and will need to be done for the combination product of the device and the contrast agent. The lack of an economic pull of an existing market in surgery is the direct reason for a lack of development in this field (see Fig. 7). However the value proposition must be large enough for a company to invest in, and given the historical modest economic value of a contrast agent, it means that most major companies would view this as a moderately poor investment, unless the value of the contrast was higher. Classic contrast agents are used for vascular or compartment visualization by a radiologist and are not by themselves considered definitive. This is despite the large amount of data supporting that fact that challenging cancers such as glioma or pancreas have the best outcomes from complete surgical removal, based upon contrast CT or contrast MRI exam. Still, use of contrast agents during surgery has the potential to help decisions in the process to visualize flow, tissue perfusion, and molecular expression, if developed and applied carefully. However, the first step in this process is developing the pipeline of useful active molecular contrast agents.
FIG. 7.
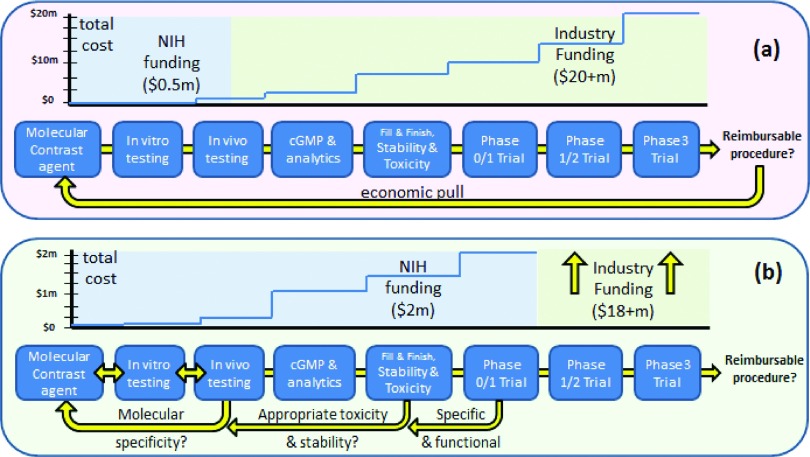
The classic drug discovery approach to new target therapy is shown in (a) with the economic input of the NIH being almost negligible and restricted to the early embryonic phase of discovery going up to limited animal testing. In the paradigm of creation of a new molecular contrast agent, in the absence of an economic pull, NIH funding must have a larger role in creation of a pipeline of testing and evaluation at several levels, going all the way up to pilot phase 0/1 clinical trials, in order to derisk the investment of industry involvement (Ref. 136).
With the current paradigm, where there is no established molecular-guided surgery approved, the lack of an economic pull to develop the pipeline is quite stark and largely has completely inhibited the pipeline. The drivers and costs are illustrated in Fig. 7(a), with pilot activity always ongoing in the range of well below 1 × 106 dollars/prospective agent, and being completed in individual labs with neither sufficient resources nor expertise to carry testing beyond the single animal model stage. However, going beyond testing in a single animal model is critical in this particular application, because the predominant fraction of the signal contrast is from vascular leakage in tumors, and so the choice of animal model to test has an inordinately high impact on the results of the study.136 Thus, one of the major benefits of molecular targeting of receptors (which are present in the 0–100 nM concentration in vivo) is that only nanomolar levels of the imaging agent are really required. The realization of the synergy here in low dose use together with lower trial costs is critically important.
The alternative development paradigm that uses this microdose model is illustrated in Fig. 7(b), where for highly ambitious research programs, it becomes imperative to advance the compound through an early phase studies with dye produced under FDA approved good manufacturing practice (GMP) production. These are ideally tested in a phase 0/1 trial in humans, under pilot funding. A Phase 0 trial as defined by the FDA is one where a single microdose concentration of the imaging agent is used, and the study therefore is not about safety, but rather just efficacy of the imaging and binding to the receptors. The peripheral benefit of microdose imaging is that only a single species toxicity testing is required, without the need for large animal work, because the dose is expected to be well below any pharmacodynamic or toxicity effect. But this study can be used to collect data on specificity of uptake and binding to the molecules of interest if designed carefully, and further, if the agents are highly fluorescent in the near-infrared where there is low background, then it is feasible to image this low concentration in vivo. This process allows the demonstration of specificity of uptake in humans, and then merits further investment into full human trials on efficacy. The maximum commercial benefit would come if microdose imaging could be utilized routinely, because then the requirements for drug production and toxicity testing are permanently reduced, as is seen in the pipeline for nuclear medicine agents, as compared to therapeutic agents. This latter design is almost essential in the absence of an economic pull of an approve procedure, as is the case for oncology therapeutics.
6. CONCLUSIONS
This review paper outlines key issues in future system development as related to molecular probe development and the costs around advancing them. These dominating issues each have different levels of success, with the hardware systems significantly outpacing the molecular probes at this time. FDA approved hardware systems are almost entirely cleared for use with a single agent, ICG, at this time, which is used at a high concentration, and its use in vascular imaging requires little background suppression. However future systems to be utilized for molecular-specific probes will need to have superior background suppression, and several preclinical systems and pending approval systems have implemented the hardware improvements of temporal gating, wavelength bandwidth improvements, and spectral fitting of the detected signals needed for these new applications. These types of hardware fixes, combined with corrections for tissue optics and calibration, will improve the potential for accurate use at lower concentration and will make it possible to detect molecular signals without disturbing the workflow by lighting changes. However the application of existing hardware tools to future molecular probe imaging may not be successful without these important modifications to maximize the signal to background imaged.
The molecular probe development is lagging the development of hardware and software tools; however, preclinical development is ongoing for a number of innovative molecules and delivery systems. The key issues which need to be solved are complicated though, including both nonspecific signal issues, and the financial issues of how to motivate R&D and carefully test new agents.
ACKNOWLEDGMENT
This work has been supported by National Institutes of Health research Grants Nos. R01CA109558, R01CA167403, R01CA160998, and P01CA084203.
CONFLICT OF INTEREST DISCLOSURE
The authors (BWP, KDP, KSS, JTE, TH, TVS) have no financial conflicts to declare related to this paper. Author Dan Draney was employed by LI-COR Biosciences at the time of the writing of the paper, developing commercial applications of IRDyes. Author Joachim Feldwisch is employed by Affibody AB, developing commercial applications of affibody molecules.
REFERENCES
- 1.Namikawa T., Sato T., and Hanazaki K., “Recent advances in near-infrared fluorescence-guided imaging surgery using indocyanine green,” Surg. Today 45, 1467–1474 (2015). 10.1007/s00595-015-1158-7 [DOI] [PubMed] [Google Scholar]
- 2.Hide T., Yano S., Shinojima N., and Kuratsu J., “Usefulness of the indocyanine green fluorescence endoscope in endonasal transsphenoidal surgery,” J. Neurosurg. 122, 1185–1192 (2015). 10.3171/2014.9.JNS14599 [DOI] [PubMed] [Google Scholar]
- 3.Boni L., David G., Mangano A., Dionigi G., Rausei S., Spampatti S., Cassinotti E., and Fingerhut A., “Clinical applications of indocyanine green (ICG) enhanced fluorescence in laparoscopic surgery,” Surg. Endosc. 29, 2046–2055 (2014). 10.1007/s00464-014-3895-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marano A., Priora F., Lenti L. M., Ravazzoni F., Quarati R., and Spinoglio G., “Application of fluorescence in robotic general surgery: Review of the literature and state of the art,” World J. Surg. 37, 2800–2811 (2013). 10.1007/s00268-013-2066-x [DOI] [PubMed] [Google Scholar]
- 5.Mitchell C. R. and Herrell S. D., “Image-guided surgery and emerging molecular imaging: Advances to complement minimally invasive surgery,” Urol. Clin. North Am. 41, 567–580 (2014). 10.1016/j.ucl.2014.07.011 [DOI] [PubMed] [Google Scholar]
- 6.Chi C., Du Y., Ye J., Kou D., Qiu J., Wang J., Tian J., and Chen X., “Intraoperative imaging-guided cancer surgery: From current fluorescence molecular imaging methods to future multi-modality imaging technology,” Theranostics 4, 1072–1084 (2014). 10.7150/thno.9899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laguna M. P., “Are we ready for molecular imaging-guided surgery?,” Eur. Urol. 65, 965–966 (2014). 10.1016/j.eururo.2013.08.014 [DOI] [PubMed] [Google Scholar]
- 8.Nguyen Q. T. and Tsien R. Y., “Fluorescence-guided surgery with live molecular navigation–a new cutting edge,” Nat. Rev. Cancer 13, 653–662 (2013). 10.1038/nrc3566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts D. W., Valdes P. A., Harris B. T., Fontaine K. M., Hartov A., Fan X., Ji S., Lollis S. S., Pogue B. W., Leblond F., Tosteson T. D., Wilson B. C., and Paulsen K. D., “Coregistered fluorescence-enhanced tumor resection of malignant glioma: Relationships between delta-aminolevulinic acid-induced protoporphyrin IX fluorescence, magnetic resonance imaging enhancement, and neuropathological parameters. Clinical article,” J. Neurosurg. 114, 595–603 (2011). 10.3171/2010.2.JNS091322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bogaards A., Sterenborg H. J., Trachtenberg J., Wilson B. C., and Lilge L., “In vivo quantification of fluorescent molecular markers in real-time by ratio imaging for diagnostic screening and image-guided surgery,” Lasers Surg. Med. 39, 605–613 (2007). 10.1002/lsm.20525 [DOI] [PubMed] [Google Scholar]
- 11.Stefflova K., Chen J., and Zheng G., “Using molecular beacons for cancer imaging and treatment,” Front. Biosci. 12, 4709–4721 (2007). 10.2741/2420 [DOI] [PubMed] [Google Scholar]
- 12.Chen J., Lovell J. F., Lo P. C., Stefflova K., Niedre M., Wilson B. C., and Zheng G., “A tumor mRNA-triggered photodynamic molecular beacon based on oligonucleotide hairpin control of singlet oxygen production,” Photochem. Photobiol. Sci. 7, 775–781 (2008). 10.1039/b800653a [DOI] [PubMed] [Google Scholar]
- 13.Lo P. C., Chen J., Stefflova K., Warren M. S., Navab R., Bandarchi B., Mullins S., Tsao M., Cheng J. D., and Zheng G., “Photodynamic molecular beacon triggered by fibroblast activation protein on cancer-associated fibroblasts for diagnosis and treatment of epithelial cancers,” J. Med. Chem. 52, 358–368 (2009). 10.1021/jm801052f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu T., Wu L. Y., Choi J. K., and Berkman C. E., “Targeted photodynamic therapy for prostate cancer: Inducing apoptosis via activation of the caspase-8/-3 cascade pathway,” Int. J. Oncol. 36, 777–784 (2010). 10.3892/ijo_00000644 [DOI] [PubMed] [Google Scholar]
- 15.Spring B. Q., Abu-Yousif A. O., Palanisami A., Rizvi I., Zheng X., Mai Z., Anbil S., Sears R. B., Mensah L. B., Goldschmidt R., Erdem S. S., Oliva E., and Hasan T., “Selective treatment and monitoring of disseminated cancer micrometastases in vivo using dual-function, activatable immunoconjugates,” Proc. Natl. Acad. Sci. U. S. A. 111, E933–E942 (2014). 10.1073/pnas.1319493111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruger R., Tansi F. L., Rabenhold M., Steiniger F., Kontermann R. E., Fahr A., and Hilger I., “In vivo near-infrared fluorescence imaging of FAP-expressing tumors with activatable FAP-targeted, single-chain Fv-immunoliposomes,” J. Controlled Release 186, 1–10 (2014). 10.1016/j.jconrel.2014.04.050 [DOI] [PubMed] [Google Scholar]
- 17.Mallidi S., Spring B. Q., Chang S., Vakoc B., and Hasan T., “Optical imaging, photodynamic therapy and optically triggered combination treatments,” Cancer J. 21, 194–205 (2015). 10.1097/PPO.0000000000000117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ilovich O., Natarajan A., Hori S., Sathirachinda A., Kimura R., Srinivasan A., Gebauer M., Kruip J., Focken I., Lange C., Carrez C., Sassoon I., Blanc V., Sarkar S. K., and Gambhir S. S., “Development and validation of an immuno-PET tracer as a companion diagnostic agent for antibody-drug conjugate therapy to target the CA6 epitope,” Radiology 276, 191–198 (2015). 10.1148/radiol.15140058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ji J., Yu L., Yu Z., Forgues M., Uenishi T., Kubo S., Wakasa K., Zhou J., Fan J., Tang Z. Y., Fu S., Zhu H., Jin J. G., Sun H. C., and Wang X. W., “Development of a miR-26 companion diagnostic test for adjuvant interferon-alpha therapy in hepatocellular carcinoma,” Int. J. Biol. Sci. 9, 303–312 (2013). 10.7150/ijbs.6214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Boer E., Harlaar N. J., Taruttis A., Nagengast W. B., Rosenthal E. L., Ntziachristos V., and van Dam G. M., “Optical innovations in surgery,” Br. J. Surg. 102, e56–e72 (2015). 10.1002/bjs.9713 [DOI] [PubMed] [Google Scholar]
- 21.Nunn A. D., “The cost of bringing a radiopharmaceutical to the patient’s bedside,” J. Nucl. Med. 48, 169 (2007). [PubMed] [Google Scholar]
- 22.Nunn A. D., “The uncertain path to the future of imaging biomarkers,” Q. J. Nucl. Med. Mol. Imaging 51, 96–98 (2007). [PubMed] [Google Scholar]
- 23.Nunn A. D., “The cost of developing imaging agents for routine clinical use,” Invest. Radiol. 41, 206–212 (2006). 10.1097/01.rli.0000191370.52737.75 [DOI] [PubMed] [Google Scholar]
- 24.Schaefer D. T. and Baldwin L., “The photography of fluorescein-dye fluorescence in surgery,” J. Biol. Photogr. Assoc. 38, 70–74 (1970). [PubMed] [Google Scholar]
- 25.Andersson-Engels S., Johansson J., and Svanberg S., “Medical diagnostic system based on simultaneous multispectral fluorescence imaging,” Appl. Opt. 33, 8022–8029 (1994). 10.1364/AO.33.008022 [DOI] [PubMed] [Google Scholar]
- 26.Baumgartner R., Fisslinger H., Jocham D., Lenz H., Ruprecht L., Stepp H., and Unsold E., “A fluorescence imaging device for endoscopic detection of early stage cancer–instrumental and experimental studies,” Photochem. Photobiol. 46, 759–763 (1987). 10.1111/j.1751-1097.1987.tb04844.x [DOI] [PubMed] [Google Scholar]
- 27.Andersson-Engels S., Canti G., Cubeddu R., Eker C., af Klinteberg C., Pifferi A., Svanberg K., Svanberg S., Taroni P., Valentini G., and Wang I., “Preliminary evaluation of two fluorescence imaging methods for the detection and the delineation of basal cell carcinomas of the skin,” Lasers Surg. Med. 26, 76–82 (2000). [DOI] [PubMed] [Google Scholar]
- 28.Leunig A., Mehlmann M., Betz C., Stepp H., Arbogast S., Grevers G., and Baumgartner R., “Fluorescence staining of oral cancer using a topical application of 5-aminolevulinic acid: Fluorescence microscopic studies,” J. Photochem. Photobiol. B 60, 44–49 (2001). 10.1016/S1011-1344(01)00117-8 [DOI] [PubMed] [Google Scholar]
- 29.De Grand A. M. and Frangioni J. V., “An operational near-infrared fluorescence imaging system prototype for large animal surgery,” Technol. Cancer Res. Treat. 2, 553–562 (2003). 10.1177/153303460300200607 [DOI] [PubMed] [Google Scholar]
- 30.Frangioni J. V., “In vivo near-infrared fluorescence imaging,” Curr. Opin. Chem. Biol. 7, 626–634 (2003). 10.1016/j.cbpa.2003.08.007 [DOI] [PubMed] [Google Scholar]
- 31.Okuda T., Kataoka K., Yabuuchi T., Yugami H., and Kato A., “Fluorescence-guided surgery of metastatic brain tumors using fluorescein sodium,” J. Clin. Neurosci. 17, 118–121 (2010). 10.1016/j.jocn.2009.06.033 [DOI] [PubMed] [Google Scholar]
- 32.Glatz J., Varga J., Garcia-Allende P. B., Koch M., Greten F. R., and Ntziachristos V., “Concurrent video-rate color and near-infrared fluorescence laparoscopy,” J. Biomed. Opt. 18, 101302 (2013). 10.1117/1.JBO.18.10.101302 [DOI] [PubMed] [Google Scholar]
- 33.Verbeek F. P., van der Vorst J. R., Schaafsma B. E., Hutteman M., Bonsing B. A., van Leeuwen F. W., Frangioni J. V., van de Velde C. J., Swijnenburg R. J., and Vahrmeijer A. L., “Image-guided hepatopancreatobiliary surgery using near-infrared fluorescent light,” J. Hepatobiliary Pancreatic Sci. 19, 626–637 (2012). 10.1007/s00534-012-0534-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ashitate Y., Stockdale A., Choi H. S., Laurence R. G., and Frangioni J. V., “Real-time simultaneous near-infrared fluorescence imaging of bile duct and arterial anatomy,” J. Surg. Res. 176, 7–13 (2012). 10.1016/j.jss.2011.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tajima Y., Murakami M., Yamazaki K., Masuda Y., Kato M., Sato A., Goto S., Otsuka K., Kato T., and Kusano M., “Sentinel node mapping guided by indocyanine green fluorescence imaging during laparoscopic surgery in gastric cancer,” Ann. Surg. Oncol. 17, 1787–1793 (2010). 10.1245/s10434-010-0944-0 [DOI] [PubMed] [Google Scholar]
- 36.Hillemanns P., Weingandt H., Stepp H., Baumgartner R., Xiang W., and Korell M., “Assessment of 5-aminolevulinic acid-induced porphyrin fluorescence in patients with peritoneal endometriosis,” Am. J. Obstet. Gynecol. 183, 52–57 (2000). 10.1016/s0002-9378(00)90793-2 [DOI] [PubMed] [Google Scholar]
- 37.Hornung R., Major A. L., McHale M., Liaw L. H., Sabiniano L. A., Tromberg B. J., Berns M. W., and Tadir Y., “In vivo detection of metastatic ovarian cancer by means of 5-aminolevulinic acid-induced fluorescence in a rat model,” J. Am. Assoc. Gynecol. Laparosc. 5, 141–148 (1998). 10.1016/S1074-3804(98)80080-7 [DOI] [PubMed] [Google Scholar]
- 38.Segal E., Prestwood T. R., van der Linden W. A., Carmi Y., Bhattacharya N., Withana N., Verdoes M., Habtezion A., Engleman E. G., and Bogyo M., “Detection of intestinal cancer by local, topical application of a quenched fluorescence probe for cysteine cathepsins,” Chem. Biol. 22, 148–158 (2015). 10.1016/j.chembiol.2014.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakuma S., Yu J. Y., Quang T., Hiwatari K., Kumagai H., Kao S., Holt A., Erskind J., McClure R., Siuta M., Kitamura T., Tobita E., Koike S., Wilson K., Richards-Kortum R., Liu E., Washington K., Omary R., Gore J. C., and Pham W., “Fluorescence-based endoscopic imaging of Thomsen-Friedenreich antigen to improve early detection of colorectal cancer,” Int. J. Cancer 136, 1095–1103 (2015). 10.1002/ijc.29093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roy H. K., Goldberg M. J., Bajaj S., and Backman V., “Colonoscopy and optical biopsy: Bridging technological advances to clinical practice,” Gastroenterology 140, 1863–1867 (2011). 10.1053/j.gastro.2011.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DaCosta R. S., Wilson B. C., and Marcon N. E., “Optical techniques for the endoscopic detection of dysplastic colonic lesions,” Curr. Opin. Gastroenterol. 21, 70–79 (2005). [PubMed] [Google Scholar]
- 42.Brand S., Stepp H., Ochsenkuhn T., Baumgartner R., Baretton G., Holl J., von Ritter C., Paumgartner G., and Sackmann M., “Detection of colonic dysplasia by light-induced fluorescence endoscopy: A pilot study,” Int. J. Colorectal Dis. 14, 63–68 (1999). 10.1007/s003840050186 [DOI] [PubMed] [Google Scholar]
- 43.Cothren R. M. et al. , “Gastrointestinal tissue diagnosis by laser-induced fluorescence spectroscopy at endoscopy,” Gastrointest. Endosc. 36, 105–111 (1990). 10.1016/S0016-5107(90)70961-3 [DOI] [PubMed] [Google Scholar]
- 44.D’Amico R. S., Kennedy B. C., and Bruce J. N., “Neurosurgical oncology: Advances in operative technologies and adjuncts,” J. Neuro-Oncol. 119, 451–463 (2014). 10.1007/s11060-014-1493-3 [DOI] [PubMed] [Google Scholar]
- 45.Li Y., Rey-Dios R., Roberts D. W., Valdes P. A., and Cohen-Gadol A. A., “Intraoperative fluorescence-guided resection of high-grade gliomas: A comparison of the present techniques and evolution of future strategies,” World Neurosurg. 82, 175–185 (2014). 10.1016/j.wneu.2013.06.014 [DOI] [PubMed] [Google Scholar]
- 46.Valdes P. A., Jacobs V., Harris B. T., Wilson B. C., Leblond F., Paulsen K. D., and Roberts D. W., “Quantitative fluorescence using 5-aminolevulinic acid-induced protoporphyrin IX biomarker as a surgical adjunct in low-grade glioma surgery,” J. Neurosurg. 123, 771–780 (2015). 10.3171/2014.12.jns14391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stummer W., Tonn J. C., Goetz C., Ullrich W., Stepp H., Bink A., Pietsch T., and Pichlmeier U., “5-Aminolevulinic acid-derived tumor fluorescence: The diagnostic accuracy of visible fluorescence qualities as corroborated by spectrometry and histology and postoperative imaging,” Neurosurgery 74, 310–319 (2014), discussion 319–320. 10.1227/NEU.0000000000000267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu J. T., Meza D., and Sanai N., “Trends in fluorescence image-guided surgery for gliomas,” Neurosurgery 75, 61–71 (2014). 10.1227/NEU.0000000000000344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okuda T., Yoshioka H., and Kato A., “Fluorescence-guided surgery for glioblastoma multiforme using high-dose fluorescein sodium with excitation and barrier filters,” J. Clin. Neurosci. 19, 1719–1722 (2012). 10.1016/j.jocn.2011.12.034 [DOI] [PubMed] [Google Scholar]
- 50.Hashizume M. and Tsugawa K., “Robotic surgery and cancer: The present state, problems and future vision,” Jpn. J. Clin. Oncol. 34, 227–237 (2004). 10.1093/jjco/hyh053 [DOI] [PubMed] [Google Scholar]
- 51.Herrell S. D., Kwartowitz D. M., Milhoua P. M., and Galloway R. L., “Toward image guided robotic surgery: System validation,” J. Urol. 181, 783–789 (2009), discussion, pp. 789–790. 10.1016/j.juro.2008.10.022 [DOI] [PubMed] [Google Scholar]
- 52.Tan G. Y., Goel R. K., Kaouk J. H., and Tewari A. K., “Technological advances in robotic-assisted laparoscopic surgery,” Urol. Clin. North Am. 36, 237–249 (2009). 10.1016/j.ucl.2009.02.010 [DOI] [PubMed] [Google Scholar]
- 53.Angell J. E., Khemees T. A., and Abaza R., “Optimization of near infrared fluorescence tumor localization during robotic partial nephrectomy,” J. Urol. 190, 1668–1673 (2013). 10.1016/j.juro.2013.04.072 [DOI] [PubMed] [Google Scholar]
- 54.Autorino R., Zargar H., White W. M., Novara G., Annino F., Perdona S., De Angelis M., Mottrie A., Porpiglia F., and Kaouk J. H., “Current applications of near-infrared fluorescence imaging in robotic urologic surgery: A systematic review and critical analysis of the literature,” Urology 84, 751–759 (2014). 10.1016/j.urology.2014.05.059 [DOI] [PubMed] [Google Scholar]
- 55.Hassan M., Kerdok A., Engel A., Gersch K., and Smith J. M., “Near infrared fluorescence imaging with ICG in TECAB surgery using the da Vinci Si surgical system in a canine model,” J. Card. Surg. 27, 158–162 (2012). 10.1111/j.1540-8191.2011.01411.x [DOI] [PubMed] [Google Scholar]
- 56.Hellan M., Spinoglio G., Pigazzi A., and Lagares-Garcia J. A., “The influence of fluorescence imaging on the location of bowel transection during robotic left-sided colorectal surgery,” Surg. Endosc. 28, 1695–1702 (2014). 10.1007/s00464-013-3377-6 [DOI] [PubMed] [Google Scholar]
- 57.Hockenberry M. S., Smith Z. L., and Mucksavage P., “A novel use of near-infrared fluorescence imaging during robotic surgery without contrast agents,” J. Endourol. 28, 509–512 (2014). 10.1089/end.2013.0606 [DOI] [PubMed] [Google Scholar]
- 58.Yuen K., Miura T., Sakai I., Kiyosue A., and Yamashita M., “Intraoperative fluorescence imaging for detection of sentinel lymph nodes and lymphatic vessels during open prostatectomy using indocyanine green,” J. Urol. 194, 371–377 (2015). 10.1016/j.juro.2015.01.008 [DOI] [PubMed] [Google Scholar]
- 59.van Driel P. B., van de Giessen M., Boonstra M. C., Snoeks T. J., Keereweer S., Oliveira S., van de Velde C. J., Lelieveldt B. P., Vahrmeijer A. L., Lowik C. W., and Dijkstra J., “Characterization and evaluation of the artemis camera for fluorescence-guided cancer surgery,” Mol. Imaging Biol. 17, 413–423 (2015). 10.1007/s11307-014-0799-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van den Berg N. S., Brouwer O. R., Schaafsma B. E., Matheron H. M., Klop W. M., Balm A. J., van Tinteren H., Nieweg O. E., van Leeuwen F. W., and Valdes Olmos R. A., “Multimodal surgical guidance during sentinel node biopsy for Melanoma: Combined gamma tracing and fluorescence imaging of the sentinel node through use of the hybrid tracer indocyanine green–99mTc-nanocolloid,” Radiology 275, 521–529 (2015). 10.1148/radiol.14140322 [DOI] [PubMed] [Google Scholar]
- 61.Tummers Q. R., Verbeek F. P., Prevoo H. A., Braat A. E., Baeten C. I., Frangioni J. V., van de Velde C. J., and Vahrmeijer A. L., “First experience on laparoscopic near-infrared fluorescence imaging of hepatic uveal melanoma metastases using indocyanine green,” Surg. Innovation 22, 20–25 (2015). 10.1177/1553350614535857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schols R. M., Connell N. J., and Stassen L. P., “Near-infrared fluorescence imaging for real-time intraoperative anatomical guidance in minimally invasive surgery: A systematic review of the literature,” World J. Surg. 39, 1069–1079 (2015). 10.1007/s00268-014-2911-6 [DOI] [PubMed] [Google Scholar]
- 63.Okusanya O. T., Madajewski B., Segal E., Judy B. F., Venegas O. G., Judy R. P., Quatromoni J. G., Wang M. D., Nie S., and Singhal S., “Small portable interchangeable imager of fluorescence for fluorescence guided surgery and research,” Technol. Cancer Res. Treat. 14, 213–220 (2015). 10.7785/tcrt.2012.500400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nishigori N., Koyama F., Nakagawa T., Nakamura S., Ueda T., Inoue T., Kawasaki K., Obara S., Nakamoto T., Fujii H., and Nakajima Y., “Visualization of lymph/blood flow in laparoscopic colorectal cancer surgery by ICG fluorescence imaging (Lap-IGFI),” Ann. Surg. Oncol. 23, 266–274 (2015). 10.1245/s10434-015-4509-0 [DOI] [PubMed] [Google Scholar]
- 65.Thekkek N., Muldoon T., Polydorides A. D., Maru D. M., Harpaz N., Harris M. T., Hofstettor W., Hiotis S. P., Kim S. A., Ky A. J., Anandasabapathy S., and Richards-Kortum R., “Vital-dye enhanced fluorescence imaging of GI mucosa: Metaplasia, neoplasia, inflammation,” Gastrointest. Endosc. 75, 877–887 (2012). 10.1016/j.gie.2011.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vila P. M., Park C. W., Pierce M. C., Goldstein G. H., Levy L., Gurudutt V. V., Polydorides A. D., Godbold J. H., Teng M. S., Genden E. M., Miles B. A., Anandasabapathy S., Gillenwater A. M., Richards-Kortum R., and Sikora A. G., “Discrimination of benign and neoplastic mucosa with a high-resolution microendoscope (HRME) in head and neck cancer,” Ann. Surg. Oncol. 19, 3534–3539 (2012). 10.1245/s10434-012-2351-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kennedy J. C. and Pottier R. H., “Endogenous protoporphyrin IX, a clinically useful photosensitizer for photodynamic therapy,” J. Photochem. Photobiol. B 14, 275–292 (1992). 10.1016/1011-1344(92)85108-7 [DOI] [PubMed] [Google Scholar]
- 68.Schoenfeld N., Mamet R., Nordenberg Y., Shafran M., Babushkin T., and Malik Z., “Protoporphyrin biosynthesis in Melanoma B16 cells stimulated by 5-aminolevulinic acid and chemical inducers: Characterization of photodynamic inactivation,” Int. J. Cancer 56, 106–112 (1994). 10.1002/ijc.2910560119 [DOI] [PubMed] [Google Scholar]
- 69.Stummer W., Stocker S., Wagner S., Stepp H., Fritsch C., Goetz C., Goetz A. E., Kiefmann R., and Reulen H. J., “Intraoperative detection of malignant gliomas by 5-aminolevulinic acid-induced porphyrin fluorescence,” Neurosurgery 42, 518–525 (1998), discussion, pp. 516–525. 10.1097/00006123-199803000-00017 [DOI] [PubMed] [Google Scholar]
- 70.Stummer W., Pichlmeier U., Meinel T., Wiestler O. D., Zanella F., and Reulen H. J., “Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial,” Lancet Oncol. 7, 392–401 (2006). 10.1016/S1470-2045(06)70665-9 [DOI] [PubMed] [Google Scholar]
- 71.Valdes P. A., Leblond F., Kim A., Harris B. T., Wilson B. C., Fan X., Tosteson T. D., Hartov A., Ji S., Erkmen K., Simmons N. E., Paulsen K. D., and Roberts D. W., “Quantitative fluorescence in intracranial tumor: Implications for ALA-induced PpIX as an intraoperative biomarker,” J. Neurosurg. 115, 11–17 (2011). 10.3171/2011.2.jns101451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jichlinski P., Guillou L., Karlsen S. J., Malmstrom P. U., Jocham D., Brennhovd B., Johansson E., Gartner T., Lange N., van den Bergh H., and Leisinger H. J., “Hexyl aminolevulinate fluorescence cystoscopy: New diagnostic tool for photodiagnosis of superficial bladder cancer–a multicenter study,” J. Urol. 170, 226–229 (2003). 10.1097/01.ju.0000060782.52358.04 [DOI] [PubMed] [Google Scholar]
- 73.Frimberger D., Zaak D., and Hofstetter A., “Endoscopic fluorescence diagnosis and laser treatment of transitional cell carcinoma of the bladder,” Semin. Urol. Oncol. 18, 264–272 (2000). [PubMed] [Google Scholar]
- 74.Riedl C. R., Plas E., and Pfluger H., “Fluorescence detection of bladder tumors with 5-amino-levulinic acid,” J. Endourol. 13, 755–759 (1999). 10.1089/end.1999.13.755 [DOI] [PubMed] [Google Scholar]
- 75.Koenig F., McGovern F. J., Larne R., Enquist H., Schomacker K. T., and Deutsch T. F., “Diagnosis of bladder carcinoma using protoporphyrin IX fluorescence induced by 5-aminolaevulinic acid,” BJU Int. 83, 129–135 (1999). 10.1046/j.1464-410x.1999.00917.x [DOI] [PubMed] [Google Scholar]
- 76.Acerbi F., Broggi M., Eoli M., Anghileri E., Cuppini L., Pollo B., Schiariti M., Visintini S., Orsi C., Franzini A., Broggi G., and Ferroli P., “Fluorescein-guided surgery for grade IV gliomas with a dedicated filter on the surgical microscope: Preliminary results in 12 cases,” Acta Neurochir. (Wien) 155, 1277–1286 (2013). 10.1007/s00701-013-1734-9 [DOI] [PubMed] [Google Scholar]
- 77.Kamp M. A., Slotty P., Turowski B., Etminan N., Steiger H. J., Hanggi D., and Stummer W., “Microscope-integrated quantitative analysis of intraoperative indocyanine green fluorescence angiography for blood flow assessment: First experience in 30 patients,” Neurosurgery 70, 65–73 (2012), discussion pp. 64–73. 10.1227/NEU.0b013e31822f7d7c [DOI] [PubMed] [Google Scholar]
- 78.Murakami T., Koyanagi I., Kaneko T., Iihoshi S., and Houkin K., “Intraoperative indocyanine green videoangiography for spinal vascular lesions: Case report,” Neurosurgery 68, 241–245 (2011), discussion, p. 245. 10.1227/NEU.0b013e318217161a [DOI] [PubMed] [Google Scholar]
- 79.Zhu B., Rasmussen J. C., and Sevick-Muraca E. M., “A matter of collection and detection for intraoperative and noninvasive near-infrared fluorescence molecular imaging: To see or not to see?,” Med. Phys. 41(2), 022105 (11pp.) (2014). 10.1118/1.4862514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hwang K., Houston J. P., Rasmussen J. C., Joshi A., Ke S., Li C., and Sevick-Muraca E. M., “Improved excitation light rejection enhances small-animal fluorescent optical imaging,” Mol. Imaging 4, 194–204 (2005). [DOI] [PubMed] [Google Scholar]
- 81.Sexton K., Davis S. C., McClatchy D. III, Valdes P. A., Kanick S. C., Paulsen K. D., Roberts D. W., and Pogue B. W., “Pulsed-light imaging for fluorescence guided surgery under normal room lighting,” Opt. Lett. 38, 3249–3252 (2013). 10.1364/OL.38.003249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tauber S., Stepp H., Meier R., Bone A., Hofstetter A., and Stief C., “Integral spectrophotometric analysis of 5-aminolaevulinic acid-induced fluorescence cytology of the urinary bladder,” BJU Int. 97, 992–996 (2006). 10.1111/j.1464-410X.2006.06094.x [DOI] [PubMed] [Google Scholar]
- 83.Tunnell J. W., Desjardins A. E., Galindo L., Georgakoudi I., McGee S. A., Mirkovic J., Mueller M. G., Nazemi J., Nguyen F. T., Wax A., Zhang Q., Dasari R. R., and Feld M. S., “Instrumentation for multi-modal spectroscopic diagnosis of epithelial dysplasia,” Technol. Cancer Res. Treat. 2, 505–514 (2003). 10.1177/153303460300200603 [DOI] [PubMed] [Google Scholar]
- 84.Cardenas-Turanzas M., Freeberg J. A., Benedet J. L., Atkinson E. N., Cox D. D., Richards-Kortum R., MacAulay C., Follen M., and Cantor S. B., “The clinical effectiveness of optical spectroscopy for the in vivo diagnosis of cervical intraepithelial neoplasia: Where are we?,” Gynecol. Oncol. 107, S138–S146 (2007). 10.1016/j.ygyno.2007.08.082 [DOI] [PubMed] [Google Scholar]
- 85.DaCosta R. S., Wilson B. C., and Marcon N. E., “Fluorescence and spectral imaging,” Sci. World J. 7, 2046–2071 (2007). 10.1100/tsw.2007.308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Comelli D., Valentini G., Nevin A., Farina A., Toniolo L., and Cubeddu R., “A portable UV-fluorescence multispectral imaging system for the analysis of painted surfaces,” Rev. Sci. Instrum. 79, 086112 (2008). 10.1063/1.2969257 [DOI] [PubMed] [Google Scholar]
- 87.DSouza A., Lin H., Gunn J., and Pogue B. W., “Logarithmic intensity compression in fluorescence guided surgery applications,” J. Biomed. Opt. 20, 80504 (2015). 10.1117/1.JBO.20.8.080504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Elliott J. T., Dsouza A. V., Davis S. C., Olson J. D., Paulsen K. D., Roberts D. W., and Pogue B. W., “Review of fluorescence guided surgery visualization and overlay techniques,” Biomed. Opt. Express 6, 3765–3782 (2015). 10.1364/BOE.6.003765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Parmar C., Grossmann P., Bussink J., Lambin P., and Aerts H. J., “Machine learning methods for quantitative radiomic biomarkers,” Sci. Rep. 5, 13087 (2015). 10.1038/srep13087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Parmar C., Leijenaar R. T., Grossmann P., Rios Velazquez E., Bussink J., Rietveld D., Rietbergen M. M., Haibe-Kains B., Lambin P., and Aerts H. J., “Radiomic feature clusters and prognostic signatures specific for lung and head and neck cancer,” Sci. Rep. 5, 11044 (2015). 10.1038/srep11044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Coroller T. P., Grossmann P., Hou Y., Rios Velazquez E., Leijenaar R. T., Hermann G., Lambin P., Haibe-Kains B., Mak R. H., and Aerts H. J., “CT-based radiomic signature predicts distant metastasis in lung adenocarcinoma,” Radiother. Oncol. 114, 345–350 (2015). 10.1016/j.radonc.2015.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wei L., Lin S. A., Fan K., Xiao D., Hong J., and Wang S., “Relationship between pituitary adenoma texture and collagen content revealed by comparative study of MRI and pathology analysis,” Int. J. Clin. Exp. Med. 8, 12898–12905 (2015). [PMC free article] [PubMed] [Google Scholar]
- 93.Dong F., Irshad H., Oh E. Y., Lerwill M. F., Brachtel E. F., Jones N. C., Knoblauch N. W., Montaser-Kouhsari L., Johnson N. B., Rao L. K., Faulkner-Jones B., Wilbur D. C., Schnitt S. J., and Beck A. H., “Computational pathology to discriminate benign from malignant intraductal proliferations of the breast,” PLoS One 9, e114885 (2014). 10.1371/journal.pone.0114885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang F., Kong J., Cooper L., Pan T., Kurc T., Chen W., Sharma A., Niedermayr C., Oh T. W., Brat D., Farris A. B., Foran D. J., and Saltz J., “A data model and database for high-resolution pathology analytical image informatics,” J. Pathol. Inf. 2, 32 (2011). 10.4103/2153-3539.92037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cordon-Cardo C., Kotsianti A., Verbel D. A., Teverovskiy M., Capodieci P., Hamann S., Jeffers Y., Clayton M., Elkhettabi F., Khan F. M., Sapir M., Bayer-Zubek V., Vengrenyuk Y., Fogarsi S., Saidi O., Reuter V. E., Scher H. I., Kattan M. W., Bianco F. J., Wheeler T. M., Ayala G. E., Scardino P. T., and Donovan M. J., “Improved prediction of prostate cancer recurrence through systems pathology,” J. Clin. Invest. 117, 1876–1883 (2007). 10.1172/JCI31399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gruber J. D., Paliwal A., Krishnaswamy V., Ghadyani H., Jermyn M., O’Hara J. A., Davis S. C., Kerley-Hamilton J. S., Shworak N. W., Maytin E. V., Hasan T., and Pogue B. W., “System development for high frequency ultrasound-guided fluorescence quantification of skin layers,” J. Biomed. Opt. 15, 026028 (2010). 10.1117/1.3374040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kepshire D., Davis S. C., Dehghani H., Paulsen K. D., and Pogue B. W., “Fluorescence tomography characterization for sub-surface imaging with protoporphyrin IX,” Opt. Express 16, 8581–8593 (2008). 10.1364/OE.16.008581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kepshire D. S., Davis S. C., Dehghani H., Paulsen K. D., and Pogue B. W., “Subsurface diffuse optical tomography can localize absorber and fluorescent objects but recovered image sensitivity is nonlinear with depth,” Appl. Opt. 46, 1669–1678 (2007). 10.1364/AO.46.001669 [DOI] [PubMed] [Google Scholar]
- 99.Polom K., Murawa D., Rho Y. S., Nowaczyk P., Hunerbein M., and Murawa P., “Current trends and emerging future of indocyanine green usage in surgery and oncology: A literature review,” Cancer 117, 4812–4822 (2011). 10.1002/cncr.26087 [DOI] [PubMed] [Google Scholar]
- 100.Marshall M. V., Rasmussen J. C., Tan I. C., Aldrich M. B., Adams K. E., Wang X., Fife C. E., Maus E. A., Smith L. A., and Sevick-Muraca E. M., “Near-infrared fluorescence imaging in humans with indocyanine green: A review and update,” Open Surg. Oncol. J. 2, 12–25 (2010). 10.2174/1876504101002020012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stanescu-Segall D. and Jackson T. L., “Vital staining with indocyanine green: A review of the clinical and experimental studies relating to safety,” Eye (London) 23, 504–518 (2009). 10.1038/eye.2008.249 [DOI] [PubMed] [Google Scholar]
- 102.Ffytche T. J., Shilling J. S., Chisholm I. H., and Federman J. L., “Indications for fluorescein angiography in disease of the ocular fundus: A review,” J. R. Soc. Med. 73, 362–365 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wykes W. N. and Livesey S. J., “Review of fluorescein angiograms performed in one year,” Br. J. Ophthalmol. 75, 398–400 (1991). 10.1136/bjo.75.7.398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xu L., Su H. R., Sun G. R., Wang Y., Guo S. J., Zhang X. R., Zhang S. S., and Xing S. C., “Fluorescein-labeled ‘arch-like’ DNA probes for electrochemical detection of DNA on gold nanoparticle-modified gold electrodes,” J. Biotechnol. 168, 388–393 (2013). 10.1016/j.jbiotec.2013.09.022 [DOI] [PubMed] [Google Scholar]
- 105.Tsujimoto H., Morimoto Y., Takahata R., Nomura S., Yoshida K., Horiguchi H., Hiraki S., Ono S., Miyazaki H., Saito D., Hara I., Ozeki E., Yamamoto J., and Hase K., “Photodynamic therapy using nanoparticle loaded with indocyanine green for experimental peritoneal dissemination of gastric cancer,” Cancer Sci. 105, 1626–1630 (2014). 10.1111/cas.12553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Navarro F. P., Berger M., Guillermet S., Josserand V., Guyon L., Neumann E., Vinet F., and Texier I., “Lipid nanoparticle vectorization of indocyanine green improves fluorescence imaging for tumor diagnosis and lymph node resection,” J. Biomed. Nanotechnol. 8, 730–741 (2012). 10.1166/jbn.2012.1430 [DOI] [PubMed] [Google Scholar]
- 107.Pogue B. W., “Optics in the molecular imaging race,” Opt. Photonics News 26, 25–31 (2015). 10.1364/opn.26.9.000024 [DOI] [Google Scholar]
- 108.Lee H., Kim J., Kim H., Kim Y., and Choi Y., “A folate receptor-specific activatable probe for near-infrared fluorescence imaging of ovarian cancer,” Chem. Commun. (Cambridge) 50, 7507–7510 (2014). 10.1039/c4cc02301c [DOI] [PubMed] [Google Scholar]
- 109.Mitra S., Modi K. D., and Foster T. H., “Enzyme-activatable imaging probe reveals enhanced neutrophil elastase activity in tumors following photodynamic therapy,” J. Biomed. Opt. 18, 101314 (2013). 10.1117/1.JBO.18.10.101314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nguyen Q. T., Olson E. S., Aguilera T. A., Jiang T., Scadeng M., Ellies L. G., and Tsien R. Y., “Surgery with molecular fluorescence imaging using activatable cell-penetrating peptides decreases residual cancer and improves survival,” Proc. Natl. Acad. Sci. U. S. A. 107, 4317–4322 (2010). 10.1073/pnas.0910261107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Olson E. S., Jiang T., Aguilera T. A., Nguyen Q. T., Ellies L. G., Scadeng M., and Tsien R. Y., “Activatable cell penetrating peptides linked to nanoparticles as dual probes for in vivo fluorescence and MR imaging of proteases,” Proc. Natl. Acad. Sci. U. S. A. 107, 4311–4316 (2010). 10.1073/pnas.0910283107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Urano Y., Asanuma D., Hama Y., Koyama Y., Barrett T., Kamiya M., Nagano T., Watanabe T., Hasegawa A., Choyke P. L., and Kobayashi H., “Selective molecular imaging of viable cancer cells with pH-activatable fluorescence probes,” Nat. Med. 15, 104–109 (2009). 10.1038/nm.1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hama Y., Urano Y., Koyama Y., Choyke P. L., and Kobayashi H., “Activatable fluorescent molecular imaging of peritoneal metastases following pretargeting with a biotinylated monoclonal antibody,” Cancer Res. 67, 3809–3817 (2007). 10.1158/0008-5472.CAN-06-3794 [DOI] [PubMed] [Google Scholar]
- 114.Chang E., Zhu M. Q., and Drezek R., “Novel siRNA-based molecular beacons for dual imaging and therapy,” Biotechnol. J. 2, 422–425 (2007). 10.1002/biot.200600257 [DOI] [PubMed] [Google Scholar]
- 115.Yang L., Cao Z., Lin Y., Wood W. C., and Staley C. A., “Molecular beacon imaging of tumor marker gene expression in pancreatic cancer cells,” Cancer Biol. Ther. 4, 561–570 (2005). 10.4161/cbt.4.5.1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pan W., Yang H., Li N., Yang L., and Tang B., “Simultaneous visualization of multiple mRNAs and matrix metalloproteinases in living cells using a fluorescence nanoprobe,” Chemistry 21, 6070–6073 (2015). 10.1002/chem.201500365 [DOI] [PubMed] [Google Scholar]
- 117.Hiroshima Y., Maawy A., Metildi C. A., Zhang Y., Uehara F., Miwa S., Yano S., Sato S., Murakami T., Momiyama M., Chishima T., Tanaka K., Bouvet M., Endo I., and Hoffman R. M., “Successful fluorescence-guided surgery on human colon cancer patient-derived orthotopic xenograft mouse models using a fluorophore-conjugated anti-CEA antibody and a portable imaging system,” J. Laparoendosc. Adv. Surg. Tech. A 24, 241–247 (2014). 10.1089/lap.2013.0418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jin T., Tiwari D. K., Tanaka S., Inouye Y., Yoshizawa K., and Watanabe T. M., “Antibody-protein A conjugated quantum dots for multiplexed imaging of surface receptors in living cells,” Mol. Biosyst. 6, 2325–2331 (2010). 10.1039/c0mb00056f [DOI] [PubMed] [Google Scholar]
- 119.Nakajima T., Mitsunaga M., Bander N. H., Heston W. D., Choyke P. L., and Kobayashi H., “Targeted, activatable, in vivo fluorescence imaging of prostate-specific membrane antigen (PSMA) positive tumors using the quenched humanized J591 antibody-indocyanine green (ICG) conjugate,” Bioconjugate Chem. 22, 1700–1705 (2011). 10.1021/bc2002715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.van Brussel A. S., Adams A., Vermeulen J. F., Oliveira S., van der Wall E., Mali W. P., van Diest P. J., and van Bergen En Henegouwen P. M., “Molecular imaging with a fluorescent antibody targeting carbonic anhydrase IX can successfully detect hypoxic ductal carcinoma in situ of the breast,” Breast Cancer Res. Treat. 140, 263–272 (2013). 10.1007/s10549-013-2635-6 [DOI] [PubMed] [Google Scholar]
- 121.Xu H., Eck P. K., Baidoo K. E., Choyke P. L., and Brechbiel M. W., “Toward preparation of antibody-based imaging probe libraries for dual-modality positron emission tomography and fluorescence imaging,” Bioorg. Med. Chem. 17, 5176–5181 (2009). 10.1016/j.bmc.2009.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.van Dam G. M., Themelis G., Crane L. M., Harlaar N. J., Pleijhuis R. G., Kelder W., Sarantopoulos A., de Jong J. S., Arts H. J., van der Zee A. G., Bart J., Low P. S., and Ntziachristos V., “Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-alpha targeting: First in-human results,” Nat. Med. 17, 1315–1319 (2011). 10.1038/nm.2472 [DOI] [PubMed] [Google Scholar]
- 123.Korb M. L., Hartman Y. E., Kovar J., Zinn K. R., Bland K. I., and Rosenthal E. L., “Use of monoclonal antibody-IRDye800CW bioconjugates in the resection of breast cancer,” J. Surg. Res. 188, 119–128 (2014). 10.1016/j.jss.2013.11.1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Heath C. H., Deep N. L., Beck L. N., Day K. E., Sweeny L., Zinn K. R., Huang C. C., and Rosenthal E. L., “Use of panitumumab-IRDye800 to image cutaneous head and neck cancer in mice,” Otolaryngol. Head Neck Surg. 148, 982–990 (2013). 10.1177/0194599813482290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Watanabe R., Sato K., Hanaoka H., Harada T., Nakajima T., Kim I., Paik C. H., Wu A. M., Choyke P. L., and Kobayashi H., “Minibody-indocyanine green based activatable optical imaging probes: The role of short polyethylene glycol linkers,” ACS Med. Chem. Lett. 5, 411–415 (2014). 10.1021/ml400533y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sano K., Nakajima T., Ali T., Bartlett D. W., Wu A. M., Kim I., Paik C. H., Choyke P. L., and Kobayashi H., “Activatable fluorescent cys-diabody conjugated with indocyanine green derivative: Consideration of fluorescent catabolite kinetics on molecular imaging,” J. Biomed. Opt. 18, 101304 (2013). 10.1117/1.JBO.18.10.101304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Olafsen T. and Wu A. M., “Antibody vectors for imaging,” Semin. Nucl. Med. 40, 167–181 (2010). 10.1053/j.semnuclmed.2009.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Samkoe K. S., Tichauer K. M., Gunn J. R., Wells W. A., Hasan T., and Pogue B. W., “Quantitative in vivo immunohistochemistry of epidermal growth factor receptor using a receptor concentration imaging approach,” Cancer Res. 15, 7465–7474 (2014). 10.1158/0008-5472.CAN-14-0141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tichauer K. M., Samkoe K. S., Sexton K. J., Hextrum S. K., Yang H. H., Klubben W. S., Gunn J. R., Hasan T., and Pogue B. W., “In vivo quantification of tumor receptor binding potential with dual-reporter molecular imaging,” Mol. Imaging Biol. 14, 584–592 (2011). 10.1007/s11307-011-0534-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tichauer K. M., Wang Y., Pogue B. W., and Liu J. T., “Quantitative in vivo cell–surface receptor imaging in oncology: Kinetic modeling and paired-agent principles from nuclear medicine and optical imaging,” Phys. Med. Biol. 60, R239–R269 (2015). 10.1088/0031-9155/60/14/R239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Elliott J. T., Samkoe K. S., Davis S. C., Gunn J. R., Paulsen K. D., Roberts D. W., and Pogue B. W., “Image-derived arterial input function for quantitative fluorescence imaging of receptor-drug binding in vivo,” J Biophotonics 9, 282–295 (2016). 10.1002/jbio.201500162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pogue B. W., Samkoe K. S., Hextrum S., O’Hara J. A., Jermyn M., Srinivasan S., and Hasan T., “Imaging targeted-agent binding in vivo with two probes,” J. Biomed. Opt. 15, 030513 (2010). 10.1117/1.3449109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Orlova A., Tolmachev V., Pehrson R., Lindborg M., Tran T., Sandstrom M., Nilsson F. Y., Wennborg A., Abrahmsen L., and Feldwisch J., “Synthetic affibody molecules: A novel class of affinity ligands for molecular imaging of HER2-expressing malignant tumors,” Cancer Res. 67, 2178–2186 (2007). 10.1158/0008-5472.CAN-06-2887 [DOI] [PubMed] [Google Scholar]
- 134.Herndon J. H., Hwang R., and Bozic K. J., “Healthcare technology and technology assessment,” Eur. Spine J. 16, 1293–1302 (2007). 10.1007/s00586-007-0369-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.FDA, Guidance for Industry, Investigators, and Reviewers: Exploratory IND Studies (FDA, Washington, DC, 2006). [Google Scholar]
- 136.Pogue B. W., Paulsen K. D., Hull S. M., Samkoe K. S., Gunn J., Hoopes J., Roberts D. W., Strong T. V., Draney D., and Feldwisch J., “Advancing molecular-guided surgery through probe development and testing in a moderate cost evaluation pipeline,” Proc. SPIE 9311, 931112 (2015). 10.1117/12.2083224 [DOI] [PMC free article] [PubMed] [Google Scholar]