Abstract
Clostridium difficile is an important nosocomial pathogen in adults. Its significance in children is less well defined, but cases of C. difficile infection (CDI) appear to be increasingly prevalent in paediatric patients. This review aims to summarize reported Clostridium difficile carriage rates across children of different age groups, appraise the relationship between CDI and factors such as method of delivery, type of infant feed, antibiotic use, and co-morbidities, and review factors affecting the gut microbiome in children and the host immune response to C. difficile. Searches of PubMed and Google Scholar using the terms ‘Clostridium difficile neonates’ and ‘Clostridium difficile children’ were completed, and reference lists of retrieved publications screened for further papers. In total, 88 papers containing relevant data were included. There was large inter-study variation in reported C. difficile carriage rates. There was an association between CDI and recent antibiotic use, and co-morbidities such as immunosuppression and inflammatory bowel disease. C. difficile was also found in stools of children with diarrhoea attributed to other pathogens (e.g. rotavirus). The role of C. difficile in the paediatric gut remains unclear; is it an innocent bystander in diarrhoeal disease caused by other organisms, or a pathogen causing subclinical to severe symptoms? Further investigation of the development of serological and local host response to C. difficile carriage may shed new light on disease mechanisms. Work is underway on defining a framework for diagnosis and management of paediatric CDI.
Electronic supplementary material
The online version of this article (doi:10.1007/s10096-016-2639-3) contains supplementary material, which is available to authorized users.
Background
Clostridium difficile (C. difficile) is a Gram-positive, anaerobic spore-forming bacillus, which can exist as both toxigenic and non-toxigenic forms [1]. It has become a significant cause of nosocomial infection with high mortality rates, particularly in the elderly. There is increasing interest in the changing epidemiology of C. difficile, as mortality rates have risen in association with emergence of hypervirulent strains such as the toxinotype group V, PCR ribotype 078 (NAP7/BK/078) and North American toxinotype III, PCR ribotype 027 (NAP1/BI/027), and there have been increasing rates of community-associated disease in recent years [2]. Approximately 4–5 % of non-hospitalised healthy adults carry the organism in their intestinal flora [3]. In hospitalised adults and those in long-term care facilities, the rate of asymptomatic carriage is estimated to be 20–50 % [4, 5], and varying carriage rates of up to 70 % have been reported in healthy newborns [6]. In children, there is a decreasing trend in carriage rate with increasing age; with colonisation falling to adult levels of around 5 % by the age of 2 years.
C. difficile colonisation results in a spectrum of clinical conditions ranging from asymptomatic carrier state to fulminant colitis. The pathophysiology of C. difficile-associated diarrhoea requires alteration of the colonic microflora, colonisation by C. difficile, and the release of enterotoxins from the toxigenic strains (typically toxin A and toxin B and in some instances a binary toxin) [1]. The use of broad-spectrum antibiotics disturbs the indigenous intestinal microbiota, which eliminates competing microbes and allows C. difficile overgrowth and toxin production in the colon.
Researchers have tried to identify the differences in host mechanism between adult and paediatric populations, as C. difficile has traditionally been viewed as non-pathogenic in young infants, given that they may carry both toxigenic and non-toxigenic strains without overt clinical symptoms. One theory is that infants lack the mechanism for cellular internalization of the large clostridial toxins owing to their presumed lack of toxin receptors, which purportedly reach adult levels after weaning [7]. Some studies have considered the protective mechanisms of breast milk in C. difficile colonisation in comparison to artificial formula [8, 9]. An in-vitro and in-vivo study showed that human colostrum contains neutralizing antibodies to toxins A and B [6, 10]. A study examining the association between serum IgG antitoxin A levels and development of clinical symptoms found that adults with low or absent antibody levels were more likely to develop diarrhoea or colitis, whereas those with higher titres were more likely to exhibit asymptomatic carriage [11]. Similarly, relapse/recurrence of CDI occurred more frequently in individuals with lower levels of IgG/IgM to Toxin A [12], but there are no reported data on when infants develop seropositivity to C. difficile antigens, and whether this correlates with the clearing of the organism from the bowel flora or with symptomatic C. difficile infection.
Concern about C. difficile disease in children has resurfaced due to the higher rates of infections and recurrence found in specific groups of children, such as children with haematological malignancies, inflammatory bowel disease (IBD), and cystic fibrosis following lung transplantation [13]. Although there have been a number of epidemiological studies performed in the United States [14] and Canada, large gaps in our knowledge remain as to the role of C. difficile and its interaction with other bowel flora in neonates and children. There is also controversy over whom to test for C. difficile, with the American Academy of Pediatrics releasing a policy statement in 2013 outlining when C. difficile testing should be considered in children — recommending avoidance of routine testing in children under 1 year of age, due to their higher carriage rates. Between 1–3 years, testing may be considered, but testing for other pathogens (especially viral pathogens) should be prioritized. Over 3 years, it is advised that testing should be performed in the same circumstances as it would be in adults (i.e., acute diarrhoea and recent history of antibiotic use) [14].
First-line treatments for C. difficile disease are vancomycin or metronidazole, although in 22–38 % of cases (particularly in severe disease), failure of treatment has been reported with metronidazole. Disease relapse/recurrence is also a concern with both drugs [15]. More recently, fidaxomicin, the first in a new class of macrocylic antimicrobials against C. difficile, has been introduced with greater efficacy in patients with recurrent disease, though data is lacking in use for patients below 18 years of age [16, 17]. Pharmacokinetic study of the drug in children 6 months–18 years is underway in the USA (Clinicaltrials.gov: NCT01591863), but expert panel has suggested that there is no unmet need for a new treatment in children under 2 years, given the lack of a clear case definition in this population [18].
In recent years, with rapid advances in genetic sequencing techniques, there has been increasing interest in the human gut microbiome. The microbiome constitutes the many and varied microbes (including bacteria, viruses, archaea, and fungi) that colonize the skin, oral cavity, and gut shortly after birth in all humans [19]. These microbes are generally thought to be commensals; however, their particular composition is thought to play a role in certain illnesses (e.g., IBD) [20, 21]. There is an association between reduced diversity of the gut microbiome, intestinal dysbiosis, C. difficile carriage [22], and C. difficile disease/recurrence in adults [23]. Analysis of C. difficile-infected mice found that the microbiota consistently contained (in addition to C. difficile) opportunistic pathogens that have been identified within the microbiota of humans with CDI. These pathogens include: Klebsiella pneumoniae, Proteus mirabilis, and Enterococcus faecalis [22]. Klebsiella pneumoniae and Ruminococcus gnavus were noted to be associated with C. difficile carriage in an infant study, with Bifidobacterium longum appearing to have a protective role [24]. In addition, administration of targeted bacteriotherapy (with a mixture including Lactobacillus reuteri and Bacteroidetes sp. nov.) to mice with chronic CDI was able to eliminate disease and shedding by restoring a more diverse intestinal microbiota [22].
Objectives
The objectives of this review are:
To summarise current available evidence on prevalence and distribution of C. difficile in neonates, infants, and children.
To ascertain the relationship between C. difficile infection (CDI) and factors such as delivery method, infant feed type, environmental exposure (e.g., time spent on NICU), antibiotic use, and co-morbidities.
To summarise risk factors for relapse of CDI, and review factors affecting the gut microbiome in children and the immunological response to C. difficile in childhood.
Methods
Search methods for identification of studies
Electronic searches
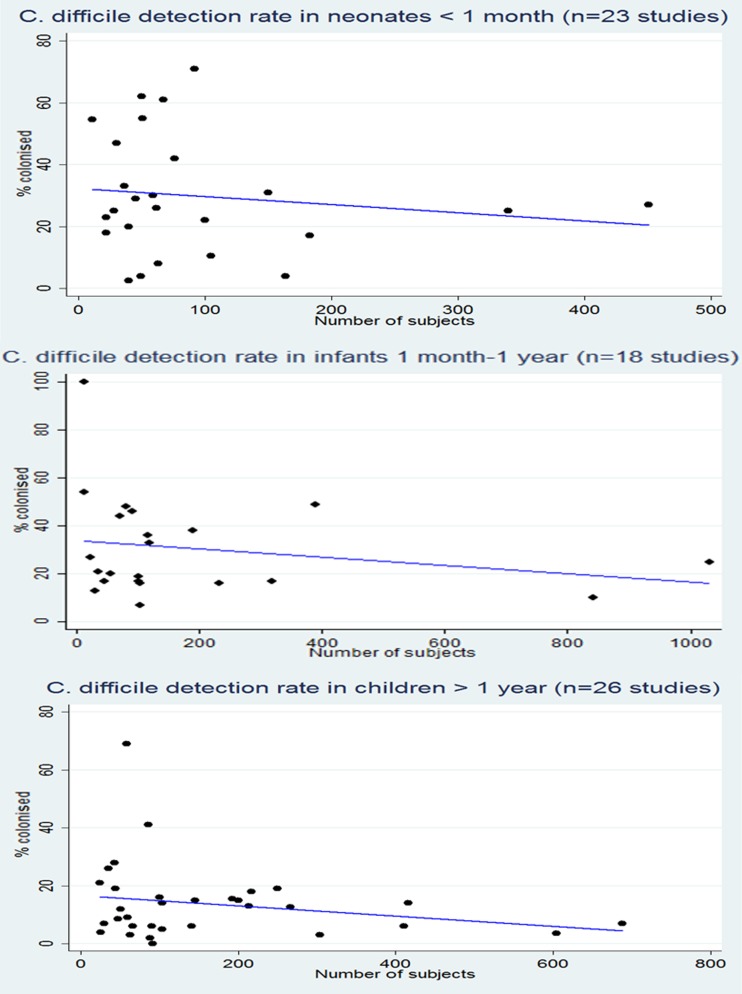
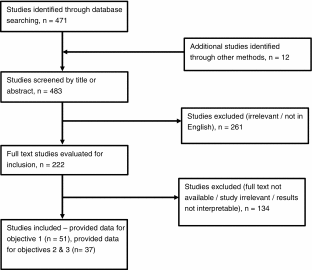
Searches of PubMed and Google Scholar were completed, using the terms ‘Clostridium difficile neonate/newborn/infant’ and ‘Clostridium difficile child/children’. The Cochrane Library and the Public Health England (formerly Health Protection Agency) websites were searched for current UK guidance on C. difficile infection. Reference lists of retrieved publications were screened for further papers. A total of 51 articles containing epidemiological data on C. difficile were reviewed and included (see appendix 1 and Supplement 1). Of these, 23 studies contained data for participants 0 to 1 month old, 18 for participants 1 month to 1 year old, and 26 studies contained data for participants >1 year of age. Reported studies were conducted between 1981 and 2013, in diverse locations worldwide and in both high- and low-resource settings. Sample size ranged from 12 to 1032 and a wide range of participants were involved, including: inpatients on Neonatal Intensive Care Unit (NICU) and post-natal wards, healthy outpatients, nursery attendees, hospitalised children with and without diarrhoea, and immunocompromised children. In addition to these studies, 37 articles were identified that also provided data relevant to issues addressed in objectives 2 and 3.
Data collection and analysis
Selection of studies
Studies were included if they were written in English and offered data on C. difficile prevalence in children, regardless of setting (i.e., inpatient/outpatient/healthy volunteer). Results of epidemiological studies were split into three age groups: neonates < 1 month, infants 1 month to 1 year, and children >1 year of age. If the age range of patients was not specified, results were included in the category that contained the median and mean age (where recorded). If median and mean were not specified and the exact number of participants in each age group was not determined from the figures in each study, data were included in the group into which the greatest number of patients appeared to fall (this occurred for four studies, and these are highlighted in online supplement 1). The initial intention had been to determine rates specifically in the age group of children >2 years, to correspond with the current accepted lower age limit for C. difficile testing in most UK paediatric centres; however, few studies included data on this age group separately from those >1 year of age.
Given the heterogeneous nature of the study populations, and lack of measurable health outcomes (given that some studies assessed C. difficile prevalence, whereas others treated those with C. difficile as having disease), it was felt that a meta-analysis was not appropriate for these data. Data analysis was performed using STATA statistical software (release 14.0, STATA Corp., College Station, TX, USA) and Microsoft Excel.
Results
Carriage rates in different age groups
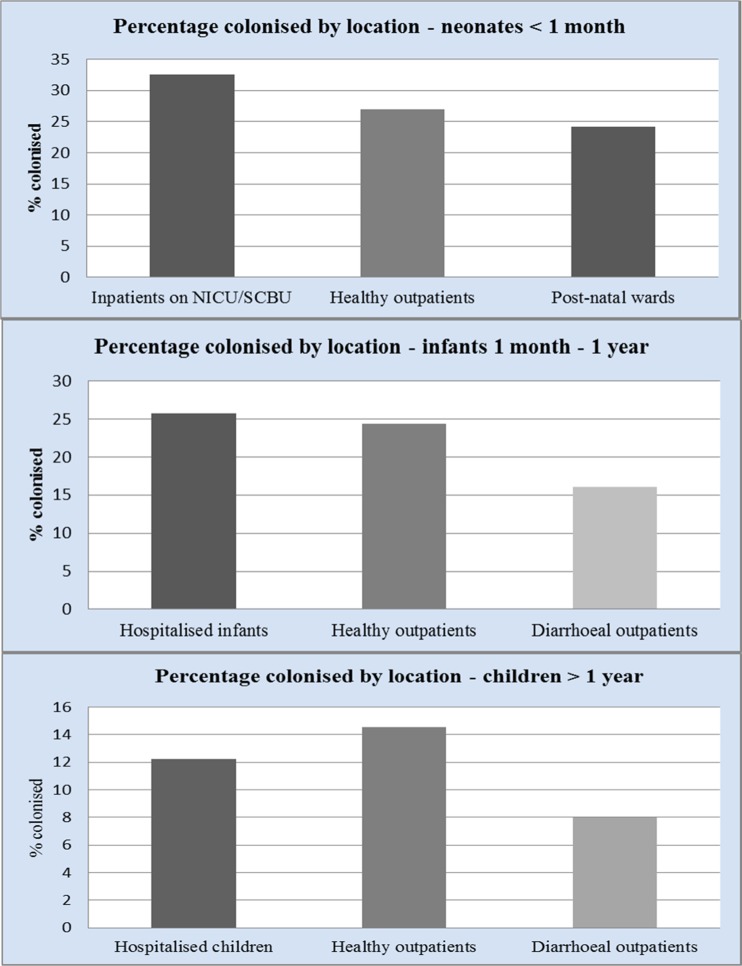
For each age group, there was a large disparity in rate of C. difficile colonisation (Fig. 1); a volume effect was seen in larger studies, suggesting rates of 25–30 % in neonates under 1 month of age, 10–25 % in infants 1 month to 1 year of age, and 5–10 % in children >1 year old; the latter value approaches the rate seen in adult studies. We also analysed data by location of patients tested; for neonates, the highest rates of colonisation were seen in patients on NICU (33 %), suggesting persistence of C. difficile in the NICU environment, but high rates were also seen in healthy outpatients (27 %) and on the postnatal ward (24 %) (Fig. 2).
Fig. 1.
C. difficile detection rate in populations by age
Fig. 2.
Percentage of subjects colonised by location
For infants 1 month to 1 year of age and those over 1 year of age, comparable rates of C. difficile were seen in hospitalised infants (26 % and 12 % respectively) and healthy outpatients (24 % and 15 % respectively). Interestingly, C. difficile was isolated least frequently in diarrhoeal outpatients (16 % and 7 % respectively).
Association between environment, antibiotic use, infant feed type, delivery method, and colonisation in neonates
Environmental exposure
Rates of C. difficile detection have been shown to increase with length of stay on the neonatal unit; some studies have also shown clear patterns of environmental contamination [25, 26]. Kato et al. reported a 61 % colonisation rate of infants in a NICU, with 53 of 55 isolates from 30 patients being identical and non-toxigenic, suggesting nosocomial spread on the NICU [27], whereas Merida et al. found no carriage of C. difficile in term neonates born on a recently opened maternity unit, where it is likely that environmental contamination had not yet occurred [28].
Antibiotic use
In a previous US study, antibiotic treatment for neonates on NICU was associated with a lower C. difficile colonisation rate [29], but colonisation with C. difficile occurred rapidly after cessation of antibiotics. The presence of C. difficile colonisation and other faecal microflora is delayed in infants who receive antibiotic treatment [30, 31]. There was an inverse correlation between gut diversity index (number and distribution of bacterial species in the gut) and number of days of antibiotics received in infants born between 27 and 29 weeks’ gestation, and weight gain increased with increased diversity scores [32].
None of the neonates in the studies reviewed were treated for C. difficile, with the exception of the study by Han et al. [33], where neonates admitted to NICU who were found to carry C. difficile were treated with vancomycin due to an outbreak of necrotizing enterocolitis (NEC) attributed to C. difficile. However, since other bacterial gut pathogens were not investigated, a causal link here between C. difficile and NEC cannot be inferred.
Method of infant feeding
Penders et al. [34], found that in healthy 1-month-old infants who had not received antibiotics, twice as many formula-fed infants were colonised by C. difficile than those who were exclusively breast fed. Also, those breast-fed infants who were colonised by C. difficile had significantly lower colony counts than formula-fed infants. These findings were replicated with a study of infants up to 1 year of age, demonstrating colonisation rates of C. difficile to be 4 times higher in formula-fed infants than in those who were exclusively breast-fed. Those receiving both breast and formula feeds had intermediate colonisation rates [8]. A large Swedish study also found significantly more formula-fed infants under 6 months to be colonised with C. difficile [9]. Ruminococcus (which is more commonly found in the gut of breast-fed infants), is thought to inhibit growth of Clostridia, thereby preventing colonisation by C. difficile [35].
Mode of delivery
A small UK study found 22 % women had vaginal colonisation by C. difficile either pre- or post-delivery; of these, 89 % delivered infants whose stools tested positive for C. difficile within 4 days of birth, compared to a 56 % detection rate in infants born to swab-negative mothers [36]. These findings have not been replicated in other maternal studies.
A delay in colonisation and alteration in composition of microbiota (with lower counts of Bifidobacteria) has been noted in babies delivered by caesarean section (CS) relative to those born by vaginal delivery [37, 38]. Another study found lower bacterial counts until day 7 of life in infants delivered by planned caesarean (i.e., amniotic membranes intact and no exposure to vaginal/bowel flora) [39]. This study, which sampled neonatal stools regularly over the period of 1 year, also demonstrated that whilst the broad groups of bacteria found in the GI tract were similar for each participant, there was a great degree of diversity and individuality in the combination of species that each infant acquired and their change over time. The microbiome was more stable than anticipated, even in the neonatal period, with certain colonizing bacteria being found in the stool repeatedly over a period of weeks or months. Fraternal twins had similar microbiomes, indicating the important influence of environment on colonisation. By 1 year of age, the microbiome was starting to resemble an adult profile, though interestingly, there were no great similarities between infants and their own parents.
A longitudinal study of term infants born by CS (with prophylactic antibiotics) or vaginal delivery noted significantly lower bacterial counts in the CS group, even at 6 months of age and delayed colonisation by Bacteroides. They were also significantly more likely to be colonised by Clostridium perfringens. No differences up to this point were noted in intestinal signs and symptoms, such as diarrhoea or colic [40]. A study recruiting healthy Finnish children at 7 years of age found significantly higher numbers of Clostridia in the stool of children delivered vaginally than in those delivered by CS. This study suggests that method of delivery may have persistent effects on microbiota well beyond infancy [41].
C. difficile burden in children over 1 year
In England, Wales, & N. Ireland in 2014, 257 cases of CDI were reported in children <15 years, representing a 5.9 % decrease on the previous year’s figures [42]. When analysed by age, rates of CDI were (expressed as cases per 100,000 population): <2 years = 2.7, 2–4 years = 4.1, 5–9 years = 1.8 and 10–14 year s = 2.0.
In a recent US-based study, the incidence of CDI in children had increased remarkably, from 2.6 to 32.6 per 100,000 person-years between the periods 1991–1997 and 2004–2009 [43]. Cases were defined as diarrhoea with positive C. difficile enzyme immunoassay (EIA), or PCR and no other identifiable cause for diarrhoea. There was a sharp increase in cases from 2006 to 2008, but the C. difficile detection method was altered from EIA to PCR in July 2007, suggesting increased case ascertainment with use of PCR. Of all cases, 75 % were community-attributable, but interestingly, 85.5 % of these patients had reported an ED or outpatient visit in the 3 months prior to disease onset.
A 2014 Polish study reports rates of 13.5 cases of CDI per 1,000 children hospitalised with diarrhoea; however, not all children were tested for viral/alternative bacterial pathogens, and those who were co-infected with C. difficile and another pathogen were recorded as being a case of CDI [44]. Indeed, a recent literature review on co-infections in children with C. difficile notes a rate of 20.7 % (range 0–100 %) for reported co-infections (predominantly viral infections), but found it difficult to draw any meaningful conclusions, given the heterogeneity between studies as to which organisms were tested for (virus/bacteria/parasite) and the difficulty in describing what constitutes CDI in the paediatric population [45].
Where data on treatment of cases was available for C. difficile positive subjects in the studies reviewed here of infants 1 month to 1 year or children >1 year, 23/968 cases (2.4 %) aged 1 month to 1 year and 86/368 (23.4 %) >1 year received treatment with metronidazole and/or vancomycin for their suspected CDI.
A recent study of adults in Oxfordshire (UK) noted a decrease in prevalence of the hypervirulent ST1/NAP/027 strain, which was proposed to be due to improved antibiotic stewardship and infection control measures. Interestingly, this study also found a fairly diverse reservoir of C. difficile subtypes (with 45 % of CDI cases being genetically distinct from one another), and a further 36 % patients with CDI from genetically similar strains having no hospital or community contact with one another [46]. This suggests the existence of other reservoirs for infection, possibly asymptomatically colonised infants. Contact with children under 2 years of age has also been linked with CDI in adults with community-acquired disease, with 14 % of cases vs 2 % of controls having reported contact with a child under 2 [47].
Risk factors for C. difficile infection (CDI)
In the US, 57 % to 75 % of paediatric patients with community-acquired CDI reported antibiotic use in the 3 months prior to admission [48]. In children hospitalised with CDI, exposure to three antibiotic classes in the month prior to admission was associated with severe disease, as was malignancy [49]. No association was found with age, prematurity (delivery <37 weeks’ gestation), GI surgery, or steroid/immunosuppressant use for 2 weeks in the month preceding diagnosis. Other studies have shown a strong link between immunosuppression and severe illness with CDI [50, 51]. Inflammatory bowel disease (IBD) [52], intestinal stasis (Hirschsprung’s disease) [13], organ transplant and gastrostomy/jejunostomy and cystic fibrosis [53] have all been implicated in the development of CDI [48]. Indeed, the first presentation of IBD could appear clinically similar to that of C. difficile enterocolitis. A US study found a significantly increased incidence of C. difficile in children >3 years with previous antibiotic exposure (35 % exposed vs 2 % unexposed) [54]. Another study reported association between community-acquired CDI and cephalosporin use within 30 days (OR 3.32; 95 % CI: 1.10–10.01) and presence of a gastrointestinal feeding device (OR 2.59; 95 % CI: 1.07–6.30) [55].
Elevated gastric pH has been hypothesized to influence CDI risk by facilitating bacterial colonisation of the upper gastrointestinal tract and/or survival of the vegetative phase of C. difficile in the stomach. An Italian paediatric retrospective case–control study found a significant association between proton-pump inhibitor (PPI) usage and CDI (PPI use in 22.1 % C. difficile-positive vs 5.9 % C. difficile-negative patients) and a non-significant association between H2-receptor antagonists and CDI (10.3 % vs 2.9 %) [56].
There have also been reports of CDI associated with viral gastroenteritis (norovirus and caliciviruses), with a suggested mechanism of inflammation of the intestinal epithelium following gastroenteritis facilitating adherence and colonisation by C. difficile and the attachment of its toxins [57, 58]. In an Italian study, co-infection with multiple pathogens was seen in 27 of 151 patients (18 %), with the most common co-infections being rotavirus and toxin-producing C. difficile, accounting for 63 % of co-infections [59]. Children with co-infection had a higher incidence of severe disease and were more likely to be dehydrated on presentation. There was no significant age difference between those with and without co-infection. All cases of C. difficile occurred in children under 6 years, with the majority being concentrated in the youngest group (under 2 years). Conversely, another study noted that diarrhoeal children with viral co-infections tended to have higher C. difficile bacterial burden (median difference = 565,957 cfu/ml; p = 0.011), but were clinically indistinguishable from those with C. difficile alone [60]. Similarly, an Italian study found no significant difference in clinical presentation in children with prolonged/mucohaemorrhagic diarrhoea by C. difficile status [61]. The significance of detecting C. difficile in the presence of known pathogenic viruses remains unclear.
Relapsing infection in children
Relapses and/or recurrences have been reported in up to 25 % of paediatric CDI cases [62], which is in line with 20–30 % seen in adult studies [63]. In adults, relapse has been associated with lower blood concentrations of anti-toxin A and B antibody and with the presence of strain BI/NAP1/027 [11, 64]. This has led to use of pooled intravenous immunoglobulins (IVIg) and anti-toxin monoclonal antibodies in recurrent, refractory, or severe disease. There has been little work done in children, but a small study by Leung et al. reported lower serum levels of IgG for TcdA in children with relapsing C. difficile-associated colitis than in healthy children, with symptoms ameliorating following IVIg infusions [65]. There was, however, no control group in this study so it is difficult to infer causation, although a study of CDI in children found that transient hypogammaglobulinaemia of infancy was significantly associated with recurrent disease, adding credence to this theory [66].
The reported incidence of complications with CDI in children (e.g., renal failure, bowel perforation, death) is lower than that in adults, varying between 0–12 % [67]. However, it is difficult to classify C. difficile disease in children, as there is no validated paediatric tool and current guidance comes from the adult classification systems. One retrospective paediatric study found that although 76 % of the cases seen in their hospital would be classified as severe using adult guidelines, most cases experienced a fairly mild illness with low morbidity and mortality [68]. The majority of patients in this study had not been treated or were given probiotics or metronidazole with good recovery, despite their ‘severe’ classification. The authors proposed new disease classification criteria for children which are currently undergoing prospective validation.
Faecal biomarkers and paediatric CDI
Faecal C. difficile bacterial load does not appear to differ between symptomatic and asymptomatic children and does not correlate with outcome [69]. In this study, faecal levels of lactoferrin and cytokines (CXCL-5, IL-8) were elevated in C. difficile-positive compared to C. difficile-negative children with diarrhoea, and time to diarrhoea resolution after treatment was significantly longer in those with elevated faecal CXCL-5 mRNA, and IL-8 mRNA at diagnosis (medians of 7 vs 2 days and 5 vs 3 days respectively). A relatively small sample size of 102 may limit further generalization from this study’s results. Similarly, immunosuppressed CDI patients had lower IL-8 mRNA expression than immunocompetent patients.
Serological response to exposure to C. difficile
A study of infants under 6 months of age found that 11 % and 33 % had detectable levels of serum IgG against toxins A and B respectively. Prevalence of serum anti-toxin IgG to both toxins increased throughout childhood, reaching 25 % (toxin A) and 53 % (toxin B) by 2 years [70]. Likelihood of strongly reactive antibodies (as measured by ELISA values) also increased with age over 2 years. Interestingly, those who produced a strong antibody response against toxin A were less likely to produce an equivalent response against toxin B and vice versa.
Conclusions
It is accepted that C. difficile is present relatively frequently in neonates, though its significance and effects on the microbiota in later life have yet to be determined. Possible hypotheses for lack of C. difficile disease in this population include: immaturity of bowel mucosa with a lack of receptors for C. difficile toxins, immunoglobulin fractions present in breast milk preventing binding of toxins to their receptors, as well as the nature and composition of infant gut microflora being protective against C. difficile overgrowth [71]. Further studies are needed to determine the significance of asymptomatic C. difficile colonisation and consequent changes in the microbiota throughout infancy and childhood and into later life.
There is a huge body of literature on C. difficile infection in adults, and now an expanding body of work on its role in children. There remains a great deal of disagreement on what constitutes paediatric C. difficile infection, and the differentiation between symptomatic manifestation and what is believed to be presence of the organism as a bystander in diarrheal disease caused by other organisms. A collaborative policy document published by the Society for Healthcare Epidemiology of America supports the view that in the setting of high prevalence of asymptomatic carriage, C. difficile cannot be assumed to be the causative agent of diarrhoea prior to adolescence (particularly in younger children) [72].
Defining paediatric CDI is further complicated by the lack of a standardized scoring system for paediatric infection, making it more difficult to quantify disease burden in those thought to have CDI and thus to know whom to treat. Crews et al. [55] suggest a framework as to how severity of disease may be defined in children, and clearly consideration of the presence of risk factors should play an additional role in ascertaining the likelihood of CDI versus incidental finding. Given the consensus that children who do have CDI run a much milder disease course than adults, it is appropriate to tailor treatment as such, with the first steps being supportive care (rehydration) and discontinuation of unnecessary antibiotics, or at least narrowing spectrum and reviewing course length, prior to considering active treatment with metronidazole/vancomycin.
Longitudinal exploration of the role of the intestinal microbiota and the development of serological host response to C. difficile during carriage and disease and the age at which this occurs would prove valuable approaches to this issue. This, alongside more detailed work on local gut response to C. difficile in diarrheal children, would provide a firm basis for the mechanistic understanding of pathogenesis of CDI in early life. Further work is also warranted on the hypothesis that children are a major community reservoir for community-attributable CDI cases in adults, as this would have important public health implications.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(PDF 664 kb)
Acknowledgments
EAL is an Academic Clinical Fellow supported by the National Institute for Health Research.
Appendix 1: study selection
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Footnotes
Please note that references 73 onwards in the reference list below are cited not in the main text, but in the electronic supplementary material.
References
- 1.Lamont JT, Theodore E. Woodward award. How bacterial enterotoxins work: insights from in vivo studies. Trans Am Clin Climatol Assoc. 2002;113:167–180. [PMC free article] [PubMed] [Google Scholar]
- 2.O'Donoghue C, Kyne L. Update on Clostridium difficile infection. Curr Opin Gastroenterol. 2011;27(1):38–47. doi: 10.1097/MOG.0b013e3283411634. [DOI] [PubMed] [Google Scholar]
- 3.Miyajima F, Roberts P, Swale A, et al. Characterisation and carriage ratio of Clostridium difficile strains isolated from a community-dwelling elderly population in the United Kingdom. PLoS One. 2011;6(8) doi: 10.1371/journal.pone.0022804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riggs MM, Sethi AK, Zabarsky TF, Eckstein EC, Jump RL, Donskey CJ. Asymptomatic carriers are a potential source for transmission of epidemic and nonepidemic Clostridium difficile strains among long-term care facility residents. Clin Infect Dis. 2007;45(8):992–998. doi: 10.1086/521854. [DOI] [PubMed] [Google Scholar]
- 5.McFarland LV, Mulligan ME, Kwok RY, Stamm WE. Nosocomial Acquisition of Clostridium difficile infection. N Engl J Med. 1989;320(4):204–210. doi: 10.1056/NEJM198901263200402. [DOI] [PubMed] [Google Scholar]
- 6.Jangi S, Lamont JT. Asymptomatic colonization by Clostridium difficile in infants: implications for disease in later life. J Pediatr Gastroenterol Nutr. 2010;51(1):2–7. doi: 10.1097/MPG.0b013e3181d29767. [DOI] [PubMed] [Google Scholar]
- 7.Eglow R, Pothoulakis C, Itzkowitz S, et al. Diminished Clostridium difficile toxin A sensitivity in newborn rabbit ileum is associated with decreased toxin A receptor. J Clin Invest. 1992;90(3):822–829. doi: 10.1172/JCI115957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooperstock M, Riegle L, Woodruff CW, Onderdonk A. Influence of age, sex, and diet on asymptomatic colonization of infants with Clostridium difficile. J Clin Microbiol. 1983;17(5):830–833. doi: 10.1128/jcm.17.5.830-833.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tullus K, Aronsson B, Marcus S, Mollby R. Intestinal colonization with Clostridium difficile in infants up to 18 months of age. Eur J Clin Microbiol Infect Dis. 1989;8(5):390–393. doi: 10.1007/BF01964052. [DOI] [PubMed] [Google Scholar]
- 10.Rolfe RD, Song W. Immunoglobulin and non-immunoglobulin components of human milk inhibit Clostridium difficile toxin A-receptor binding. J Med Microbiol. 1995;42(1):10–19. doi: 10.1099/00222615-42-1-10. [DOI] [PubMed] [Google Scholar]
- 11.Kyle L, Warny M, Qamar A, et al. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N Engl J Med. 2000;342:390–397. doi: 10.1056/NEJM200002103420604. [DOI] [PubMed] [Google Scholar]
- 12.Kyne L, Warny M, Qamar A, Kelly CP. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet. 2001;357(9251):189–193. doi: 10.1016/S0140-6736(00)03592-3. [DOI] [PubMed] [Google Scholar]
- 13.Enoch DA, Butler MJ, Pai S, Aliyu SH, Karas JA. Clostridium difficile in children: colonisation and disease. J Infect. 2011;63(2):105–113. doi: 10.1016/j.jinf.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 14.Schutze GE, Willoughby RE. Clostridium difficile infection in infants and children. Pediatrics. 2013;131(1):196–200. doi: 10.1542/peds.2012-2992. [DOI] [PubMed] [Google Scholar]
- 15.Kuipers EJ, Surawicz CM. Clostridium difficile infection. Lancet. 2008;371(9623):1486–1488. doi: 10.1016/S0140-6736(08)60635-2. [DOI] [PubMed] [Google Scholar]
- 16.National Institute for Health and Care Excellence (2012) Evidence summary: Clostridium difficile infection: fidaxomicin. Available from: https://www.nice.org.uk/advice/esnm1/chapter/Overview
- 17.Mullane K. Fidaxomicin in Clostridium difficile infection: latest evidence and clinical guidance. Ther Adv Chronic Dis. 2014;5(2):69–84. doi: 10.1177/2040622313511285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faust SN, Wilcox MH, Banaszkiewicz A, Bouza E, Raymond J, Gerding DN. Lack of evidence for an unmet need to treat Clostridium difficile infection in infants aged <2 years: expert recommendations on how to address this issue. Clin Infect Dis. 2015;60(6):912–918. doi: 10.1093/cid/ciu936. [DOI] [PubMed] [Google Scholar]
- 19.Morgan XC, Huttenhower C. Chapter 12: human microbiome analysis. PLoS Comput Biol. 2012;8(12) doi: 10.1371/journal.pcbi.1002808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rigsbee L, Agans R, Shankar V, et al. Quantitative profiling of gut microbiota of children with diarrhea-predominant irritable bowel syndrome. Am J Gastroenterol. 2012;107(11):1740–1751. doi: 10.1038/ajg.2012.287. [DOI] [PubMed] [Google Scholar]
- 21.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9(5):313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawley TD, Clare S, Walker AW, et al. Targeted restoration of the intestinal microbiota with a simple, defined bacteriotherapy resolves relapsing Clostridium difficile disease in mice. PLoS Pathog. 2012;8(10) doi: 10.1371/journal.ppat.1002995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang JY, Antonopoulos DA, Kalra A, et al. Decreased diversity of the fecal microbiome in recurrent Clostridium difficile-associated diarrhea. J Infect Dis. 2008;197(3):435–438. doi: 10.1086/525047. [DOI] [PubMed] [Google Scholar]
- 24.Rousseau C, Levenez F, Fouqeray C, Dore J, Collignon A, Lepage P. Clostridium difficile colonisation in early infancy is accompanied by changes in intestinal microbiotal composition. J Clin Microbiol. 2011;49(3):858–865. doi: 10.1128/JCM.01507-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larson HE, Barclay FE, Honour P, Hill ID. Epidemiology of Clostridium difficile in infants. J Infect Dis. 1982;146(6):727–733. doi: 10.1093/infdis/146.6.727. [DOI] [PubMed] [Google Scholar]
- 26.Delmee M, Verellen G, Avesani V, Francois G. Clostridium difficile in neonates: serogrouping and epidemiology. Eur J Pediatr. 1988;147(1):36–40. doi: 10.1007/BF00442608. [DOI] [PubMed] [Google Scholar]
- 27.Kato H, Kato N, Watanabe K, et al. Application of typing by pulsed-field gel electrophoresis to the study of Clostridium difficile in a neonatal intensive care unit. J Clin Microbiol. 1994;32(9):2067–2070. doi: 10.1128/jcm.32.9.2067-2070.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merida V, Moerman J, Colaert J, Lemmens P, Vandepitte J. Significance of Clostridium difficile and its cytotoxin in children. Eur J Pediatr. 1986;144(5):494–496. doi: 10.1007/BF00441746. [DOI] [PubMed] [Google Scholar]
- 29.Donta ST, Myers MG. Clostridium difficile toxin in asymptomatic neonates. J Pediatr. 1982;100(3):431–434. doi: 10.1016/S0022-3476(82)80454-X. [DOI] [PubMed] [Google Scholar]
- 30.Blakey JL, Lubitz L, Barnes GL, Bishop RF, Campbell NT, Gillam GL. Development of gut colonisation in pre-term neonates. J Med Microbiol. 1982;15(4):519–529. doi: 10.1099/00222615-15-4-519. [DOI] [PubMed] [Google Scholar]
- 31.Holton AF, Hall MA, Lowes JA. Antibiotic exposure delays intestinal colonization by Clostridium difficile in the newborn. J Antimicrob Chemother. 1989;24(5):811–817. doi: 10.1093/jac/24.5.811. [DOI] [PubMed] [Google Scholar]
- 32.Jacquot A, Neveu D, Aujoulat F, et al. Dynamics and clinical evolution of bacterial gut microflora in extremely premature patients. J Pediatr. 2011;158(3):390–396. doi: 10.1016/j.jpeds.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 33.Han VK, Sayed H, Chance GW, Brabyn DG, Shaheed WA. An outbreak of Clostridium difficile necrotizing enterocolitis: a case for oral vancomycin therapy? Pediatrics. 1983;71(6):935–941. [PubMed] [Google Scholar]
- 34.Penders J, Vink C, Driessen C, London N, Thijs C, Stobberingh EE. Quantification of Bifidobacterium spp., Escherichia coli and Clostridium difficile in faecal samples of breast-fed and formula-fed infants by real-time PCR. FEMS Microbiol Lett. 2005;243(1):141–147. doi: 10.1016/j.femsle.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 35.Morelli L. Postnatal development of intestinal microflora as influenced by infant nutrition. J Nutr. 2008;138(9):1791S–1795S. doi: 10.1093/jn/138.9.1791S. [DOI] [PubMed] [Google Scholar]
- 36.Tabaqchali S, O'Farrell S, Nash JQ, Wilks M. Vaginal carriage and neonatal acquisition of Clostridium difficile. J Med Microbiol. 1984;18(1):47–53. doi: 10.1099/00222615-18-1-47. [DOI] [PubMed] [Google Scholar]
- 37.Penders J, Thijs C, Vink C, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118(2):511–521. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- 38.Bennet R, Nord CE. Development of the faecal anaerobic microflora after caesarean section and treatment with antibiotics in newborn infants. Infection. 1987;15(5):332–336. doi: 10.1007/BF01647733. [DOI] [PubMed] [Google Scholar]
- 39.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5(7) doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gronlund MM, Lehtonen OP, Eerola E, Kero P. Fecal microflora in healthy infants born by different methods of delivery: permanent changes in intestinal flora after cesarean delivery. J Pediatr Gastroenterol Nutr. 1999;28(1):19–25. doi: 10.1097/00005176-199901000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Salminen S, Gibson GR, McCartney AL, Isolauri E. Influence of mode of delivery on gut microbiota composition in seven year old children. Gut. 2004;53(9):1388–1389. doi: 10.1136/gut.2004.041640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Public Health England (2014) Voluntary surveillance of Clostridium difficile in England, Wales and Northern Ireland. Infection Report 9(21), published 19/6/2015
- 43.Khanna S, Baddour LM, Huskins WC, et al. The epidemiology of Clostridium difficile infection in children: a population-based study. Clin Infect Dis. 2013;56(10):1401–1406. doi: 10.1093/cid/cit075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duleba K, Pawlowska M, Wietlicka-Piszcz M. Clostridium difficile infection in children hospitalized due to diarrhea. Eur J Clin Microbiol Infect Dis. 2014;33(2):201–209. doi: 10.1007/s10096-013-1946-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Graaf H, Pai S, Burns DA, Karas JA, Enoch DA, Faust SA. Co-infection as a confounder for the role of Clostridium difficile infection in children with diarrhoea: a summary of the literature. Eur J Clin Microbiol Infect Dis. 2015;34:1281–1287. doi: 10.1007/s10096-015-2367-0. [DOI] [PubMed] [Google Scholar]
- 46.Eyre DW, Cule ML, Wilson DJ, et al. Diverse sources of C. difficile infection identified on whole-genome sequencing. N Engl J Med. 2013;369(13):1195–1205. doi: 10.1056/NEJMoa1216064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilcox MH, Mooney L, Bendall R, Settle CD, Fawley WN. A case–control study of community-associated Clostridium difficile infection. J Antimicrob Chemother. 2008;62(2):388–396. doi: 10.1093/jac/dkn163. [DOI] [PubMed] [Google Scholar]
- 48.Sandora TJ, Fung M, Flaherty K, et al. Epidemiology and risk factors for Clostridium difficile infection in children. Pediatr Infect Dis J. 2011;30(7):580–584. doi: 10.1097/INF.0b013e31820bfb29. [DOI] [PubMed] [Google Scholar]
- 49.Kim J, Shaklee JF, Smathers S, et al. Risk factors and outcomes associated with severe Clostridium difficile infection in children. Pediatr Infect Dis J. 2012;31(2):134–138. doi: 10.1097/INF.0b013e3182352e2c. [DOI] [PubMed] [Google Scholar]
- 50.Wolfhagen MJ, Meijer K, Fluit AC, et al. Clinical significance of Clostridium difficile and its toxins in faeces of immunocompromised children. Gut. 1994;35(11):1608–1612. doi: 10.1136/gut.35.11.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.El-Mahallawy HA, El-Din NH, Salah F, El-Arousy M, El-Naga SA. Epidemiologic profile of symptomatic gastroenteritis in pediatric oncology patients receiving chemotherapy. Pediatr Blood Cancer. 2004;42(4):338–342. doi: 10.1002/pbc.10394. [DOI] [PubMed] [Google Scholar]
- 52.Pascarella F, Martinelli M, Miele E, Del Pezzo M, Roscetto E, Staiano A. Impact of Clostridium difficile infection on pediatric inflammatory bowel disease. J Pediatr. 2009;154(6):854–858. doi: 10.1016/j.jpeds.2008.12.039. [DOI] [PubMed] [Google Scholar]
- 53.Pohl JF, Patel R, Zobell JT, et al. Clostridium difficile infection and proton pump inhibitor use in hospitalized pediatric cystic fibrosis patients. Gastroenterol Res Pract. 2011;2011:345012. doi: 10.1155/2011/345012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klein EJ, Boster DR, Stapp JR, et al. Diarrhea etiology in a children's hospital emergency department: a prospective cohort study. Clin Infect Dis. 2006;43(7):807–813. doi: 10.1086/507335. [DOI] [PubMed] [Google Scholar]
- 55.Crews JD, Anderson LR, Waller DK, Swartz MD, DuPont HL, Starke JR. Risk factors for community-associated Clostridium difficile-associated diarrhea in children. Pediatr Infect Dis J. 2015;34(9):919–923. doi: 10.1097/INF.0000000000000767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Turco R, Martinelli M, Miele E, et al. Proton pump inhibitors as a risk factor for paediatric Clostridium difficile infection. Aliment Pharmacol Ther. 2010;31(7):754–759. doi: 10.1111/j.1365-2036.2009.04229.x. [DOI] [PubMed] [Google Scholar]
- 57.Lukkarinen H, Eerola E, Ruohola A, et al. Clostridium difficile ribotype 027-associated disease in children with norovirus infection. Pediatr Infect Dis J. 2009;28(9):847–848. doi: 10.1097/INF.0b013e31819d1cd9. [DOI] [PubMed] [Google Scholar]
- 58.Pokorn M, Radsel A, Cizman M, et al. Severe Clostridium difficile-associated disease in children. Pediatr Infect Dis J. 2008;27(10):944–946. doi: 10.1097/INF.0b013e3181723d32. [DOI] [PubMed] [Google Scholar]
- 59.Valentini D, Vittucci AC, Grandin A, et al. Coinfection in acute gastroenteritis predicts a more severe clinical course in children. Eur J Clin Microbiol Infect Dis. 2013;32(7):909–915. doi: 10.1007/s10096-013-1825-9. [DOI] [PubMed] [Google Scholar]
- 60.El Feghaly RE, Stauber JL, Tarr PI, Haslam DB. Viral co-infections are common and are associated with higher bacterial burden in children with Clostridium difficile infection. J Pediatr Gastroenterol Nutr. 2013;57(6):813–816. doi: 10.1097/MPG.0b013e3182a3202f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Borali E, Ortisi G, Moretti C, et al. Community-acquired Clostridium difficile infection in children: a retrospective study. Dig Liver Dis. 2015;47(10):842–846. doi: 10.1016/j.dld.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 62.Aslam S, Hamill RJ, Musher DM. Treatment of Clostridium difficile-associated disease: old therapies and new strategies. Lancet Infect Dis. 2005;5(9):549–557. doi: 10.1016/S1473-3099(05)70215-2. [DOI] [PubMed] [Google Scholar]
- 63.Deshpande A, Pasupuleti V, Thota P, et al. Risk factors for recurrent Clostridium difficile infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2015;36(4):452–460. doi: 10.1017/ice.2014.88. [DOI] [PubMed] [Google Scholar]
- 64.Leav BA, Blair B, Leney M, et al. Serum anti-toxin B antibody correlates with protection from recurrent Clostridium difficile infection (CDI) Vaccine. 2010;28(4):965–969. doi: 10.1016/j.vaccine.2009.10.144. [DOI] [PubMed] [Google Scholar]
- 65.Leung DY, Kelly CP, Boguniewicz M, Pothoulakis C, LaMont JT, Flores A. Treatment with intravenously administered gamma globulin of chronic relapsing colitis induced by Clostridium difficile toxin. J Pediatr. 1991;118(4 Pt 1):633–637. doi: 10.1016/S0022-3476(05)83393-1. [DOI] [PubMed] [Google Scholar]
- 66.Gryboski JD, Pellerano R, Young N, Edberg S. Positive role of Clostridium difficile infection in diarrhea in infants and children. Am J Gastroenterol. 1991;86(6):685–689. [PubMed] [Google Scholar]
- 67.Sammons JS, Toltzis P, Zaoutis TE. Clostridium difficile Infection in children. JAMA Pediatr. 2013;167(6):567–573. doi: 10.1001/jamapediatrics.2013.441. [DOI] [PubMed] [Google Scholar]
- 68.Pai S, Aliyu SH, Enoch DA, Karas JA. Five years experience of Clostridium difficile infection in children at a UK tertiary hospital: proposed criteria for diagnosis and management. PLoS One. 2012;7(12) doi: 10.1371/journal.pone.0051728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.El Feghaly RE, Stauber JL, Tarr PI, Haslam DB, et al. Intestinal inflammatory biomarkers and outcome in pediatric Clostridium difficile infections. J Pediatr. 2013;163(6):1697.e2–1704.e2. doi: 10.1016/j.jpeds.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Viscidi R, Laughon BE, Yolken R, et al. Serum antibody response to toxins A and B of Clostridium difficile. J Infect Dis. 1983;148(1):93–100. doi: 10.1093/infdis/148.1.93. [DOI] [PubMed] [Google Scholar]
- 71.Pant C, Deshpande A, Altaf MA, Minocha A, Sferra TJ. Clostridium difficile infection in children: a comprehensive review. Curr Med Res Opin. 2013;29(8):967–984. doi: 10.1185/03007995.2013.803058. [DOI] [PubMed] [Google Scholar]
- 72.Dubberke ER, Carling P, Carrico R, et al. Strategies to prevent Clostridium difficile infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(6):628–645. doi: 10.1086/522262. [DOI] [PubMed] [Google Scholar]
- 73.Ferraris L, Butel MJ, Campeotto F, Vodovar M, Roze JC, Aires J. Clostridia in premature Neonates’ Gut: incidence, antibiotic susceptibility and perinatal determinants influencing colonization. PLoS One. 2012;7(1) doi: 10.1371/journal.pone.0030594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rousseau C, Lemee L, Le Monnier A, Poilane I, Pons JL, Colignon A. Prevalence and diversity of Clostridium difficile strains in infants. J Med Microbiol. 2011;60(8):1112–1118. doi: 10.1099/jmm.0.029736-0. [DOI] [PubMed] [Google Scholar]
- 75.Tonooka T, Sakata S, Kitahara M, et al. Detection and quantification of four species of the genus Clostridium in infant feces. Microbiol Immunol. 2005;49(11):987–992. doi: 10.1111/j.1348-0421.2005.tb03694.x. [DOI] [PubMed] [Google Scholar]
- 76.Matsuki S, Ozaki E, Shozu M, et al. Colonization by Clostridium difficile of neonates in a hospital, and infants and children in three day-care facilities of Kanazawa, Japan. Int Microbiol. 2005;8(1):43–48. [PubMed] [Google Scholar]
- 77.Martirosian G, Kuipers S, Verbrugh H, van Belkum A, Meisel-Mikolajczyk F. PCR ribotyping and arbitrarily primed PCR for typing strains of Clostridium difficile from a Polish maternity hospital. J Clin Microbiol. 1995;33(8):2016–2021. doi: 10.1128/jcm.33.8.2016-2021.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rotimi VO, Olowe SA, Ahmed I. The development of bacterial flora of premature neonates. J Hyg (Lond) 1985;94(3):309–318. doi: 10.1017/S0022172400061532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bolton RP, Tait SK, Dear PR, Losowsky MS. Asymptomatic neonatal colonsiation by Clostridium difficile. Arch Dis Child. 1984;59(5):466–472. doi: 10.1136/adc.59.5.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Al-Jumaili IJ, Shibley M, Lishman AH, Record CO. Incidence and origin of Clostridium difficile in neonates. J Clin Microbiol. 1984;19(1):77–78. doi: 10.1128/jcm.19.1.77-78.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Malamou-Ladas H, O'Farrell S, Nash JQ, Tabaqchali S. Isolation of Clostridium difficile from patients and the environment of hospital wards. J Clin Pathol. 1983;36(1):88–92. doi: 10.1136/jcp.36.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Richardson SA, Alcock PA, Gray J. Clostridium difficile and its toxin in healthy neonates. Br Med J (Clin Res Ed) 1983;287(6396):878. doi: 10.1136/bmj.287.6396.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Viscidi R, Willey S, Bartlett JG. Isolation rates and toxigenic potential of Clostridium difficile isolates from various patient populations. Gastroenterology. 1981;81(1):5–9. [PubMed] [Google Scholar]
- 84.Holst E, Helin I, Mårdh PA. Recovery of Clostridium difficile from children. Scand J Infect Dis. 1981;13(1):41–45. doi: 10.1080/00365548.1981.11690365. [DOI] [PubMed] [Google Scholar]
- 85.Alrifai SB, Alsaadi A, Mahmood YA, Ali AA, Al-Kaisi LA. Prevalence and etiology of nosocomial diarrhea in children < 5 years in Tikrit teaching hospital. East Mediterr Health J. 2009;15(5):1111–1118. [PubMed] [Google Scholar]
- 86.Denno DM, Stapp JR, Boster DR, et al. Etiology of diarrhea in pediatric outpatient settings. Pediatr Infect Dis J. 2005;24(2):142–148. doi: 10.1097/01.inf.0000151031.47761.6d. [DOI] [PubMed] [Google Scholar]
- 87.Tang P, Roscoe M, Richardson SE. Limited clinical utility of Clostridium difficile toxin testing in infants in a pediatric hospital. Diagn Microbiol Infect Dis. 2005;52(2):91–94. doi: 10.1016/j.diagmicrobio.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 88.Naaber P, Klaus K, Sepp E, Björksten B, Mikelsaar M. Colonization of infants and hospitalized patients with Clostridium difficile and lactobacilli. Clin Infect Dis. 1997;25(S2):189–190. doi: 10.1086/516183. [DOI] [PubMed] [Google Scholar]
- 89.Kim KH, Suh IS, Kim JM, Kim CW, Cho YJ. Etiology of childhood diarrhea in Korea. J Clin Microbiol. 1989;27(6):1192–1196. doi: 10.1128/jcm.27.6.1192-1196.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brady MT, Pacini DL, Budde CT, Connell MJ. Diagnostic studies of nosocomial diarrhea in children: assessing their use and value. Am J Infect Control. 1989;17(2):77–82. doi: 10.1016/0196-6553(89)90021-7. [DOI] [PubMed] [Google Scholar]
- 91.Torres JF, Cedillo R, Sanchez J, Dillman C, Giono S, Munoz O. Prevalence of Clostridium difficile and its cytotoxin in infants in Mexico. J Clin Microbiol. 1984;20(2):274–275. doi: 10.1128/jcm.20.2.274-275.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ellis ME, Mandal BK, Dunbar EM, Bundell KR. Clostridium difficile and its cytotoxin in infants admitted to hospital with infectious gastroenteritis. Br Med J (Clin Res Ed) 1984;288(6416):524–526. doi: 10.1136/bmj.288.6416.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stark PL, Lee A, Parsonage BD. Colonization of the large bowel by Clostridium difficile in healthy infants: quantitative study. Infect Immun. 1982;35(3):895–899. doi: 10.1128/iai.35.3.895-899.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nash JQ, Chattopadhyay B, Honeycombe J, Tabaqchali S. Clostridium difficile and cytotoxin in routine faecal specimens. J Clin Pathol. 1982;35(5):561–565. doi: 10.1136/jcp.35.5.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Svedhem A, Kaijser B, MacDowall I. Intestinal occurrence of Campylobacter fetus subspecies jejuni and Clostridium difficile in children in Sweden. Eur J Clin Microbiol. 1982;1(1):29–32. doi: 10.1007/BF02014137. [DOI] [PubMed] [Google Scholar]
- 96.Lamousé-Smith ES, Weber S, Rossi RF, Neinstedt LJ, Mosammaparast N, Sandora TJ. Polymerase chain reaction test for Clostridium difficile toxin B gene reveals similar prevalence rates in children with and without inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2013;57(3):293–297. doi: 10.1097/MPG.0b013e3182999990. [DOI] [PubMed] [Google Scholar]
- 97.Banaszkiewicz A, Wultanska D, Pituch H, Radzikowski A. Prevalence of Clostridium difficile infection in Polish pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2010;16(4):554. doi: 10.1002/ibd.21056. [DOI] [PubMed] [Google Scholar]
- 98.Castagnola E, Battaglia T, Bandettini R, et al. Clostridium difficile-associated disease in children with solid tumors. Support Care Cancer. 2009;17(3):321–324. doi: 10.1007/s00520-008-0507-0. [DOI] [PubMed] [Google Scholar]
- 99.Simon A, Ammann R, Bode U, et al. Healthcare-associated infections in pediatric cancer patients: results of a prospective surveillance study from university hospitals in Germany and Switzerland. BMC Infect Dis. 2008;8:70. doi: 10.1186/1471-2334-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vernacchio L, Vezina RM, Mitchell AA, Lesko SM, Plaut AG, Acheson DW. Diarrhea in American infants and young children in the community setting: incidence, clinical presentation and microbiology. Pediatr Infect Dis J. 2006;25(1):2–7. doi: 10.1097/01.inf.0000195623.57945.87. [DOI] [PubMed] [Google Scholar]
- 101.Gogate A, De A, Nanivadekar R, et al. Diagnostic role of stool culture & toxin detection in antibiotic associated diarrhoea due to Clostridium difficile in children. Indian J Med Res. 2005;122(6):518–524. [PubMed] [Google Scholar]
- 102.Ferreira CE, Nakano V, Durigon EL, Avila-Campos MJ. Prevalence of Clostridium spp. and Clostridium difficile in children with acute diarrhea in Sao Paulo city, Brazil. Mem Inst Oswaldo Cruz. 2003;98(4):451–454. doi: 10.1590/S0074-02762003000400003. [DOI] [PubMed] [Google Scholar]
- 103.Pinto LJ, Alcides AP, Ferreira EO, et al. Incidence and importance of Clostridium difficile in paediatric diarrhoea in Brazil. J Med Microbiol. 2003;52(12):1095–1099. doi: 10.1099/jmm.0.05308-0. [DOI] [PubMed] [Google Scholar]
- 104.Langley JM, LeBlanc JC, Hanakowski M, Goloubeva O. The role of Clostridium difficile and viruses as causes of nosocomial diarrhea in children. Infect Control Hosp Epidemiol. 2002;23(11):660–664. doi: 10.1086/501990. [DOI] [PubMed] [Google Scholar]
- 105.Oguz F, Uysal G, Dasdemir S, Oskovi H, Vidinlisan S. The role of Clostridium difficile in childhood nosocomial diarrhea. Scand J Infect Dis. 2001;33(10):731–733. doi: 10.1080/003655401317074509. [DOI] [PubMed] [Google Scholar]
- 106.Shastri S, Doane AM, Gonzales J, Upadhyayula U, Bass DM. Prevalence of astroviruses in a children’s hospital. J Clin Microbiol. 1998;36:2571–2574. doi: 10.1128/jcm.36.9.2571-2574.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Burgner D, Siarakas S, Eagles G, McCarthy A, Bradbury R, Stevens M. A prospective study of Clostridium difficile infection and colonization in pediatric oncology patients. Pediatr Infect Dis J. 1997;16(12):1131–1134. doi: 10.1097/00006454-199712000-00006. [DOI] [PubMed] [Google Scholar]
- 108.Schuller I, Saha V, Lin L, Kingston J, Eden T, Tabaqchali S. Investigation and management of Clostridium difficile colonisation in a paediatric oncology unit. Arch Dis Child. 1995;72(3):219–222. doi: 10.1136/adc.72.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Uhnoo I, Wadell G, Svensson L, Olding-Stenkvist E, Ekwall E, Mölby R. Aetiology and epidemiology of acute gastroenteritis in Swedish children. J Infect. 1986;13:73–89. doi: 10.1016/S0163-4453(86)92348-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 664 kb)