Abstract
Drug discovery for neurodegenerative diseases is particularly challenging because of the discrepancies in drug effects between in vitro and in vivo studies. These discrepancies occur in part because current cell culture systems used for drug screening have many limitations. First, few cell culture systems accurately model human aging or neurodegenerative diseases. Second, drug efficacy may differ between dividing and stationary cells, the latter resembling nondividing neurons in the CNS. Brain aggregates (BrnAggs) derived from embryonic day 15 gestation mouse embryos may represent neuropathogenic processes in prion disease and reflect in vivo drug efficacy. Here, we report a new method for the production of BrnAggs suitable for drug screening and suggest that BrnAggs can model additional neurological diseases such as tauopathies. We also report a functional assay with BrnAggs by measuring electrophysiological activities. Our data suggest that BrnAggs could serve as an effective in vitro cell culture system for drug discovery for neurodegenerative diseases.
Keywords: Brain aggregates, Dendritic degeneration, Drug screening, Neurodegeneration, Tau.
INTRODUCTION
The need and efforts to prevent or cure neurodegenerative diseases have increased, but developing effective therapeutics has proven to be particularly challenging. There are many obstacles to developing effective drugs for neurological diseases. Pathobiological mechanisms of these diseases are not fully understood, and diagnoses are often made too late to treat affected patients. Drugs for neurodegenerative diseases should also be able to cross the blood–brain barrier. At this time, there are no efficient in vitro and in vivo models suitable for translation to patient treatments.
Most in vitro culture systems in current drug screening assays use proliferating cells of neuronal or nonneuronal origin (1–6). To be effective in vivo, however, drugs most often need to target nonproliferating neurons. Therefore, current in vitro cell cultures used in drug screening assays may not accurately model critical neuronal properties; this may be one reason for discrepancies in drug efficacy between in vitro and in vivo systems. For example, several studies showed that quinacrine (Qa) is an effective antiprion compound in vitro (7, 8), but it was not effective in vivo (9, 10). We found that Qa showed different pharmacokinetics and efficacies in dividing and stationary cells (10).
Recently, we have used the brain aggregate (BrnAgg) in vitro culture system to study prion diseases (11). BrnAggs are prepared from embryonic day 15 (E15) mouse brains. Dissociated brain cells form spheres measuring 750–1000 μm in diameter in 10–15 days. BrnAggs differ from neurospheres or embryonic neural stem cells because they do not proliferate; therefore, it is not possible to passage and maintain them in culture. On the other hand, BrnAggs contain all CNS components including differentiated neurons, astrocytes, oligodendrocytes, and microglia (11–13), and they can be maintained in culture for over 40–50 days. BrnAggs show senescence of neurons over time; for example, dendritic densities increase up to 25 days in culture, but the dendrites slowly age after 35 days in culture and undergo pruning back (11).
Rocky Mountain Laboratory (RML) prion isolate-infected BrnAggs show similar pathobiological features to those of prion diseases in vivo (11). We observed progressive accumulation of scrapie-associated prion protein (PrPSc) in RML-infected BrnAggs (indicating prion replication and accumulation) over time. As PrPSc propagates in cells, dendritic degeneration is rapid, that is, dendritic densities of RML-infected BrnAggs at 25 and 35 days in culture were lower compared to the age-matched, uninfected control BrnAggs (11). In addition, BrnAggs from prion-knockout mice showed no PrPSc accumulation, as in in vivo studies (11, 14, 15). More importantly, RML-infected BrnAggs treated with either γ-secretase inhibitor (GSI) or Qa alone showed dendritic degeneration similar to untreated controls, but dual treatment with GSI and Qa prevented dendritic degeneration; these effects were very similar to those in RML-infected CD1 mice (11, 16). Because BrnAggs show similar progressive neurodegeneration and drug responses shown in in vivo studies, they are beneficial to study prion pathobiology and develop effective drug screening assays for prion disease.
Here, we report a new preparation method allowing control of cell density and size of a single BrnAgg in a 96-well plate format. The new method also increases the yield of BrnAggs by 3- to 4-fold. With this new methodology, variability among BrnAggs is minimized, and culture conditions are suitable for drug screening purposes. We also demonstrate that BrnAggs form functional electrophysiological networks. Lastly, we examine possible uses of BrnAggs as models for tauopathies.
MATERIALS AND METHODS
All experiments were carried out in accordance with the Institutional Animal Care and Use Committee/Laboratory Animal Research Center (IACUC) protocols of the University of California San Francisco (UCSF) and the McLaughlin Research Institute (MRI). The protocol was approved by the UCSF IACUC Committee (UCSF Authorization protocol number AB091167-02, MRI protocol #2013-GAC-10). Euthanization was performed under isofluorane followed by cervical dislocation; all efforts were made to minimize animal suffering.
Animals
E15-days-gestation FVB mouse embryos were used to prepare BrnAggs for prion infection. Timed-pregnant FVB mice were purchased from Charles River Laboratories (Wilmington, MA). Timed-pregnant FVB-Tg(tetO-HuTauP301L)4510 (rTg4510) or FVB-Tg(tetO-HuTauwt)21221 (rTg21221) female mice that had been mated with 129.Tg(CK-tTA) males were shipped from MRI to UCSF. One quarter of the embryos are expected to carry both the responder and transactivator transgenes and to express human P301 mutant or wild-type tau (17).
Genotyping of rTg4510 and rTg21221 Embryos
Embryos from rTg4510 and rTg21221 mice (18) were genotyped by PCR analysis of tail-snip lysates using transgene-specific primers. Briefly, embryonic tails were collected and lysed with 50 μl of lysis buffer containing 200 μg/ml proteinase K. They were digested at 55°C for 30 minutes and incubated at 95°C for 15 minutes for deactivation. The samples were then diluted 1:40, and 10 μl of diluted samples were run by PCR (cycle 1: 94°C for 3 minutes; cycle 2: 94°C for 15 seconds, 60°C for 30 seconds, 72°C for 45 seconds [35X]; cycle 3: 72°C for 7 minutes, 4°C). Isolated embryonic brains were kept on ice while genotyping. After confirming by PCR, positive embryonic brains were combined and processed for BrnAgg preparation.
BrnAggs Culture
Brain cells from E15-days-gestation FVB embryos were dissociated through 2 nylon meshes (250/130 μm). Following 2 washes with a wash medium (Dulbecco’s Modified Eagle’s Medium (DMEM H21) [UCSF cell-culture facility]) containing glucose (12 g/L), fungizone (2.5 mg/L), and gentamycin (50 mg/L), the dissociated cells were then resuspended in the growth culture medium (DMEM H21 supplemented with glucose [6 g/L], fungizone, and gentamycin) and 10% fetal bovine serum at densities of 1 x 107 cells per milliliter. About 150 µl of cells were placed in each well of a 96-well plate at constant rotation (76 rpm) at 37°C, 10% CO2. The next day, 150 µl of exchange medium (DMEM H21 supplemented with glucose, gentamycin, and 15% fetal bovine serum) was added to each well. BrnAggs were fed every other day by removing 200 µl of conditioned medium and replacing with 200 µl of fresh exchange medium.
Immunohistochemistry Reagents
Antibodies to the following were used in this study: anti-microtubule-associated protein 2 (MAP2) (AB5622, Millipore, Billerica, MA), anti-glial fibrillary acidic protein (GFAP, 20334, Dako, Carpinteria, CA), R2 antibody (gift from Dr S. Prusiner, UCSF, San Francisco, CA), anti-dopamine (MAB369, Millipore), anti-serotonin (MAB352, Millipore), anti-NeuN (MAB377, Millipore), anti-Iba1 (190-1974, Wako, Richmond, VA), anti-γ-aminobutyric acid (GABA, MAB316, Millipore), anti-choline acyltransferase (ChAT) (AB143, Millipore), anti-glutamate transporter (AB1520, Millipore), anti-PHF-Tau (MN1020, Thermo Scientific, Waltham, MA), anti-beta tubulin (H-235, Santa Cruz Biotechnology, Dallas, TX), and goat anti-rabbit IgG conjugated with Alexa 633, goat anti-mouse IgG2a conjugated with Alexa 488, and goat anti-mouse IgG1 conjugated with Alexa 568 (all from Jackson ImmunoResearch Laboratory, West Grove, PA). Slides were mounted with Vectashield hardset mounting medium (H-1500, Vector Laboratories, Burlingame, CA).
Immunohistochemistry and Confocal Microscopy
BrnAggs were washed with phosphate-buffered saline (PBS) for 10 minutes (2x), fixed in 4% formaldehyde for 3 days at 4°C, and washed with PBS for 60 minutes (3x). For PrPSc staining, BrnAggs were treated with 3M guanidine isothiocyanate for 10 minutes, washed with wash buffer (PBS containing 0.3% Trion-X100 and 0.1% Tween20), and blocked with blocking buffer (PBS containing 0.3% Triton-X100, 0.1% Tween20, 2% bovine serum albumin, and 10% normal goat serum) for 3 days at 4°C. For nonprion staining, PBS-washed BrnAggs were blocked with blocking buffer overnight at 4°C. Following the incubation with primary antibodies (1:100 except for the anti-GABA antibody, 1:200, and anti-dopamine and anti-glutamate transporter antibodies, 1:1000) for 1–3 days at 4°C, they were washed with wash buffer for 60 minutes (3x) and incubated with the secondary antibodies (1:800) overnight at 4°C. All antibodies were diluted in the blocking buffer. BrnAggs were washed with wash buffer for 60 minutes (3x), incubated with Hoechst (1:5000 diluted in PBS) for 30 minutes at room temperature, and washed with PBS buffer for 30 minutes (2x). BrnAggs were placed in glycerol containing 2% n-propyl gallate onto a slide and covered with a cover glass. Fluorescent confocal images were taken with a Leica SP8 confocal microscope. Quantification of neural composition or dendritic density was determined by measuring the mean of the integrated density of 20–30 z-stack confocal images of each BrnAgg with Image J. P values for statistical analyses were calculated with the t test.
Electrophysiology
To prevent movement of BrnAggs in these experiments, recording wells were built by fixing a block of agarose gel on a poly-D-lysine-coated coverslip with grease. The gel had a 3-mm-diameter hole in the center. For each experiment, 1 BrnAgg was transferred to a well and allowed to settle on the bottom coverslip for 15 minutes. The well was subsequently submerged in the bath chamber of a Kerr Tissue Recording System (Kerr Scientific Instruments, Christchurch, New Zealand). The bath was continuously perfused with 5 ml/minute normal artificial cerebrospinal fluid (nACSF), high-K solution, or tetrodotoxin (TTX) + high-K solution. All the solutions were bubbled with carbogen (95% O2/5% CO2). The nACSF was composed of the following (in mM): 125 NaCl, 1.25 NaH2PO4, 26 NaHCO3, 3 KCl, 10 glucose, 2 MgCl2, and 2 CaCl2. The high-K solution contained (in mM): 125 NaCl, 1.25 NaH2PO4, 26 NaHCO3, 5 KCl, 10 glucose, 1 MgCl2, and 0.2 CaCl2. Extracellular field potential activity was recorded using similar methods as previously reported by Voss et al (19, 20). A 50-µm Teflon-coated silver electrode was positioned in the well under a digital microscope. A silver/silver-chloride reference electrode was located at the bottom of the bath chamber. Recordings were conducted in a Faraday cage to minimize electrical background noise. The signal was acquired at 20 KHz, amplified at 250X (Kerr Scientific Instruments), and band-filtered between 1 and 100 Hz using Labchart 8.0 software (PowerLab, AD Instruments, Inc., Colorado Springs, CO).
RESULTS
A New Preparation Method of BrnAggs Is Developed for Drug Screening
One complication with the original BrnAgg preparation was that the size and cell density of single BrnAggs are variable. Dissociated brain cells are incubated in relatively large volumes for the first day and the first week (5 or 25 ml, respectively). During the first week, aggregates/layers of cells are formed in a different size by randomly encountering each other. When they are larger than at least 5 mm, each of them is transferred into a well of a 24-well plate to form a single BrnAgg, and they are cultured in a 24-well plate. To be useful in drug screening assays, at least a 96-well plate format is necessary.
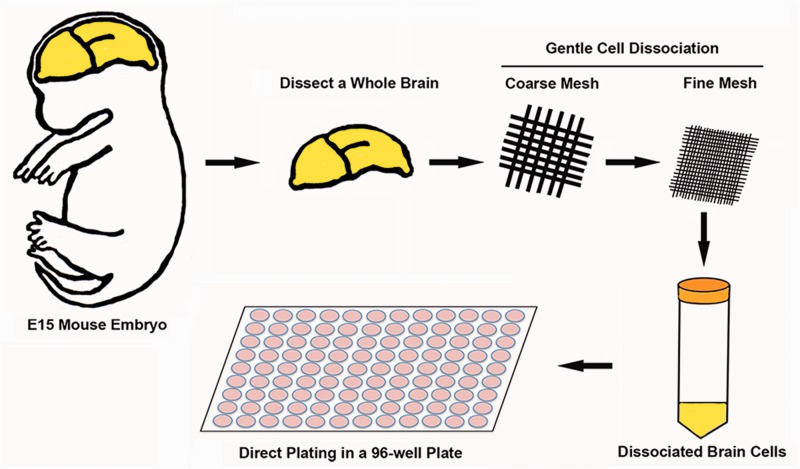
To control the cell density and size of a single BrnAgg, we developed a new preparation method that allows direct plating into a 96-well plate at a controlled cell density after dissociating brain cells of E15-day embryos (Fig. 1). It removes 2 steps of the original preparation before plating into the 24-well plate, where a single BrnAgg is formed. The variability in cell density and size between BrnAggs are minimized by direct plating of a constant number of dissociated embryonic brain cells. Within 1 day, a layer of cells is formed in each well that matches the size of the well (∼6.8 mm diameter) and forms a single BrnAgg in a few days. This new method shortens the BrnAgg formation time with a controlled cell density and also increases the yield of BrnAgg by 3- to 4-fold compared with the original method. Lastly, the volume of media in a 96-well plate format (∼200 µl/well) would be more favorable than a 24-well plate for drug screening purposes. Approximately 10 BrnAggs can be prepared from a single fetus.
FIGURE 1.
Schematic drawing of preparation method. Whole brains from E15-day mouse embryos are pooled and passed gently through coarse and fine mesh by pressing with pipette tips. Dissociated cells are washed and resuspended in growing media. Cell density is measured and cells are directly plated in 96-well plates and incubated with constant agitation. A sheet of cells is formed overnight, 100 μl of fresh exchange media is added into each well, and 50% of media is changed with fresh exchange media every day. Within a few days, a sheet of cells is forming a single sphere-shaped BrnAgg per well (n > 1000).
BrnAggs Are Composed of Excitatory/Inhibitory Neurons and Astrocytes, and They Show Normal Aging
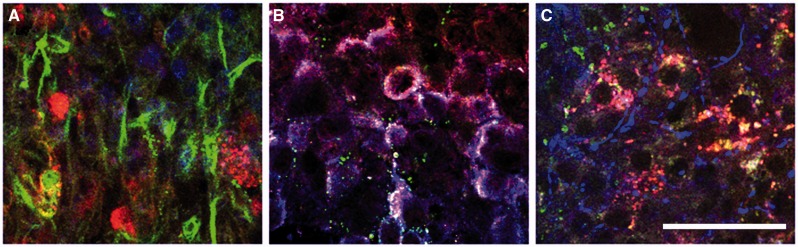
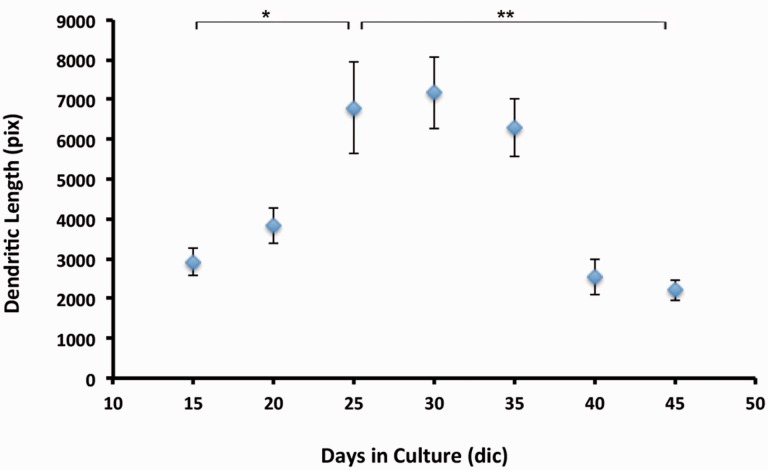
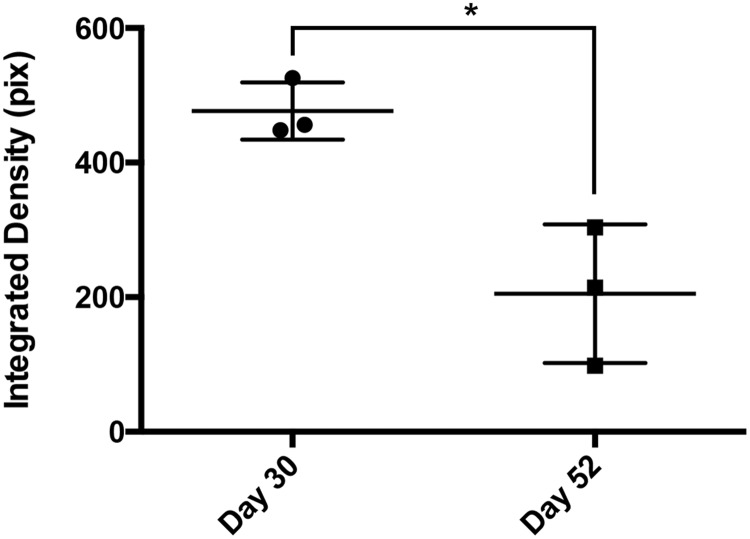
BrnAggs prepared by the new method possess all CNS components and show signs of normal aging. We examined them from 15 days in culture (dic) to 45 dic. When they mature around 25 dic, BrnAggs are composed of CNS components such as inhibitory and excitatory neurons, astrocytes, and microglia (Fig. 2, Table). They begin to show neural senescence over time. BrnAggs exhibit neuronal development up to 30 dic and slow neurodegeneration thereafter (Fig. 3). We also observed that serotonergic neurons degenerate with aging BrnAggs (Fig. 4). Serotonergic neuronal degeneration has been reported in aging brains and in the brains of patients with Alzheimer disease (21). Our observations suggest that BrnAggs model normal brain aging in 40–50 days.
FIGURE 2.
Neural composition of BrnAggs. BrnAggs are prepared from wild-type FVB mice and harvested at 25 days in culture. They are fixed and immunostained for neuronal marker proteins and examined by confocal microscopy. (A) NeuN (red), Iba-1 (green), DAPI (blue); (B) synaptophysin (red), serotonin (green), choline acetyltransferase (blue); (C) GABA (red), dopamine (green), MAP2 (blue). Data are representative of n = 9–12. Bar = 50 μm.
TABLE.
Neuronal Composition of BrnAggs at 25–30 Days in Culturea
| Mean ± SE | Number of BrnAggs | |
|---|---|---|
| Dopamine-positive neurons | 58.7 ± 3.2 | 10 |
| GABA-positive neurons | 49.2 ± 7.2 | 12 |
| ChAT-positive neurons | 56.2 ± 5.6 | 9 |
| 5HT-positive neurons | 56.8 ± 10.1 | 9 |
ChAT, choline acetyltransferase; GABA, γ-aminobutyric acid; 5HT, serotonin. SE, standard error.
The z-stacks of 20–30 confocal images of a whole BrnAgg were used to calculate the mean integrated density with ImageJ.
FIGURE 3.
Dendritic length of aging BrnAggs. BrnAggs were prepared from wild-type FVB mice and harvested from 15 to 45 days in culture. Dendritic density of BrnAggs was measured by MAP2 signals. Stacks of confocal images were taken from each BrnAggs and the dendritic length was measured with ImageJ. Data are representative of n = 6–9. *p = 0.002; **p = 0.0008. Bar = SEM.
FIGURE 4.
Serotonergic neurons in aging BrnAggs. BrnAggs harvested at 30 and 52 days in culture were stained with an anti-serotonin antibody. Serotonergic neurons were measured with ImageJ at 30 and 52 days in culture. *p = 0.0135. Data are representative of n = 3.
BrnAggs From Transgenic Mice Can Be Utilized for Various Disease Models Such as of Frontotemporal Lobar Degeneration
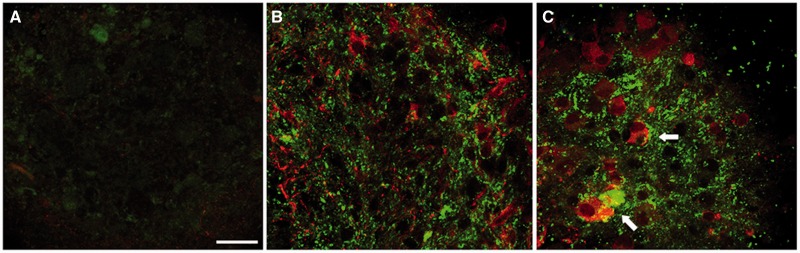
Previously, we have shown that BrnAggs are infectable with RML and that they undergo prion pathobiological processes and antiprion drug responses similar to those that occur in vivo (11). Because BrnAggs can be prepared from virtually any transgenic (Tg) mice, BrnAggs may be suitable models for various brain diseases. We tested whether BrnAggs show pathobiological characteristics in a model for frontotemporal lobar degeneration. We prepared BrnAggs from rTg4510 mice expressing human tau with a P301L mutation. We measured hyperphosphorylated tau (p-tau, CP13) and tau tangles in huTauP301L BrnAggs at 35 dic (Fig. 5). We observed intense p-tau and Thioflavin S (Thio-S)-positive tau aggregates in huTauP301L BrnAggs compared with nontransgenic FVB BrnAggs (Fig. 5B, A, respectively). RML-infected huTauP301L BrnAggs showed more intense signals of p-tau and Thio-S, suggesting that RML infection exaggerates tau phosphorylation and protein aggregation (Fig. 5C, white arrow).
FIGURE 5.
Increased levels of phosphorylated tau and Thio-S in aged Tg(huTauP301L) BrnAggs. BrnAggs were harvested at 35 days in culture. (A) Wild-type FVB mouse; (B) Tg(huTauP301L); (C) Tg(huTauP301L) infected with RML for 10 days at 15 days in culture. Thio-S (green) and p-tau (red; CP13, a p-tau antibody recognizing pSer202). Data are representative of n = 3. Bar = 50 μm.
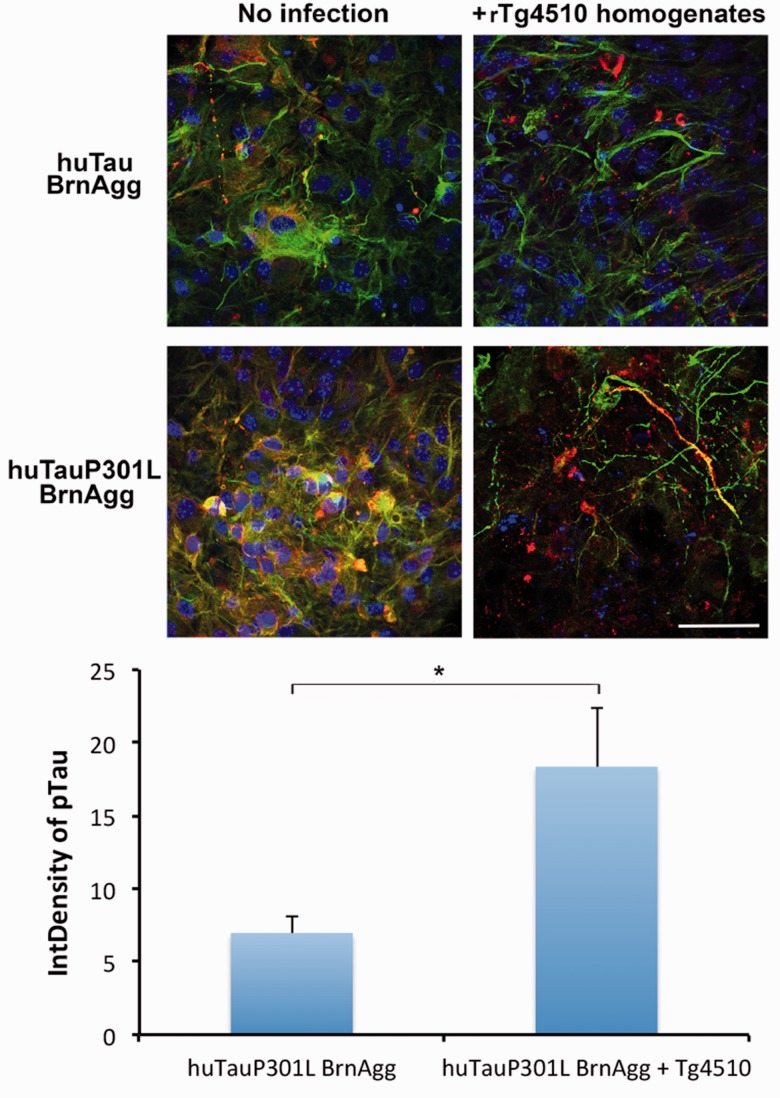
We also exposed huTauP301L BrnAggs with diseased approximately 1-year-old rTg4510 brain homogenates to determine whether misfolded tau aggregates exacerbate the pathological changes seen in rTg4510 BrnAggs. rTg4510 brain-homogenate infection induced p-tau levels and disruption of microtubules in huTauP301L BrnAggs compared with the uninfected control (Fig. 6). The rTg4510 homogenate infection did not cause microtubule disruption in wild-type huTau BrnAggs prepared from rTg21221 mice, although there were inclusions of p-tau aggregates in some cells (Fig. 6).
FIGURE 6.
Tau pathology induced in Tg(huTauP301L) BrnAggs infected with rTg4510 brain homogenates. BrnAggs prepared from rTg4510 and rTg21221 embryonic brains were infected with ∼360 days-old diseased rTg4510 mouse-brain homogenates from 15 to 25 days in culture and harvested at 35 days in culture. They were fixed with 4% paraformaldehyde and stained with PHF-1 (a p-tau antibody recognizing pSer396 and pSer404, red), anti-β tubulin (green) antibodies, and DAPI (blue). Data are representative of n = 6–8. *p = 0.03. Bar = 50 µm.
A Functional Assay in BrnAggs
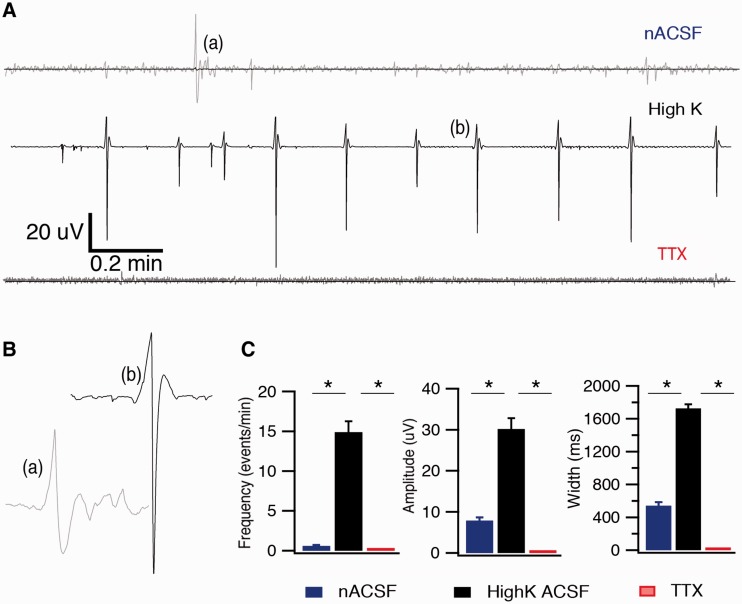
To examine whether neurons in BrnAggs formed functional networks, extracellular spontaneous records of field potential activity in wild-type FVB BrnAggs were collected using the Kerr Tissue Recording System (Fig. 7). Spontaneous waves of field potential activity were observed from BrnAggs when perfused with nACSF (Fig. 7A[a], B[a]). In the presence of high potassium (5 mM) nACSF, spontaneous seizure-like sharp waves of field potential activity were observed (Fig. 7A[b], B[b]). The field potential activity was blocked with TTX (Fig. 7A). The parameters (amplitude, width, and frequency) of these events increased substantially when BrnAggs were exposed to high-K solution (Fig. 7C). In turn, these amplified events were sharply attenuated in the presence of TTX (Fig. 7C). These field-potential records were similar to those of neuronal networks in acute brain slice preparations (19, 20, 22). They suggest that neurons in BrnAggs preparations build functional networks capable of generating spontaneous and synchronized activity.
FIGURE 7.
Neural-network activity in BrnAggs. (A) Examples of typical field-potential recording from BrnAgg cultures perfused with normal artificial CSF (ACSF), high-K ACSF, and tetrodotoxin (TTX) + high-K ACSF, illustrating the change in network activity in each condition. Gray traces are magnification of superimposed black traces to visualize the field activity. (B) Magnification of individual events marked in (A). (C) Graphs of field potential activity parameters in different recording conditions. These parameters were increased in high-K ACSF and dramatically reduced in the presence of TTX (100 nM). The frequency was 0.6 ± 0.11 events/minute versus 14.9 ± 1.35 events/minute in high-K ACSF, and these events were suppressed in the presence of TTX. The amplitude was 7.90 ± 0.75 µV in ACSF versus 30.2 ± 2.61 µV in high-K solution. The width was 543.3 ± 41.3 ms in normal ACSF versus 1726.8 ± 47.7 ms in high-K solution. Data are representative of = 6–10. *p < 0.05 (t test).
DISCUSSION
Developing drugs for neurodegenerative diseases for such a complex system as the brain is very challenging. There are excitatory and inhibitory neurons activated by different neurotransmitters such as glutamate, dopamine, serotonin, and GABA. Therefore, to screen drugs for neurological disorders, a cell-culture model should be simple enough to prepare and assay but contain all CNS components forming the functional networks in the brain.
BrnAgg culture is a 3-dimensional (3D) culture with all of the major components of the brain. BrnAggs may be described as “mini-brains,” although they differ from other 3D neural culture systems. Our culture system provides a more practical environment to study neurological diseases. It is likely that its 3D structure may be why BrnAggs are infectable with prions, unlike other primary cultures. In neurodegenerative diseases, transcellular propagation of a variety of protein aggregates involves their release into the extracellular space for disease progression to take place. Free protein aggregates released from neurons in BrnAggs may be captured in the extracellular space between neural cells and then enter neighboring cells to seed further abnormal fibrillization, as occurs in the brain. We have demonstrated prion infection of BrnAggs previously (11) and also in this study by infecting huTauP301L BrnAggs with rTg4510 homogenates.
BrnAggs also show aging progresses that are similar to those in the brain in vivo. Dendritic density became denser up to 25 dic; then progressive dendritic degeneration was observed up to 50 dic. Exposure to prions such as RML or tau aggregates exaggerates neurodegeneration in BrnAggs (Figs. 5, 6). More importantly, BrnAggs can be used to assess antiprion drug efficacy in vivo. We previously showed that an effective antiprion compound, Qa, had no effect on cell growth-arrested neuroblastoma cells while it did in dividing neuroblastoma cells (10). This may explain why many drug trials for neurodegenerative diseases fail in in vivo studies or clinical trials because most drug candidates are selected from screening assays in dividing cells. Therefore, establishing an effective in vitro cell culture system for neurodegenerative diseases is essential to screen and develop drugs effective in vivo.
The availability of various transgenic mice is an additional benefit because BrnAggs from these transgenic mice (Tg BrnAggs) can be prepared to study and screen drug candidates for many neurological diseases. Tg BrnAggs expressing human tau(P301L) show similar neuropathological characteristics: higher phosphorylated tau and Thio-S-positive aggregates to those that occur in vivo (Fig. 5). Infection with rTg4510 brain homogenates induced tau phosphorylation and astrocytic gliosis (Fig. 6). BrnAgg from prion-knockout mice are not infectable with RML (11, 15), as prion knockout mice are resistant to prion disease (14). These results support that Tg BrnAggs show neuronal pathobiology similar to corresponding disease models.
In addition, BrnAggs produce a relatively high quantity of a homogeneous population (∼500 BrnAggs per preparation from 50 fetuses) for drug screening assays, which is an advantage over other culture systems such as brain slice culture. Although brain slice culture has its own distinct advantages, such as retaining the brain structure in culture and allowing for histological analysis, BrnAggs may be more suitable for effective drug screening purposes.
BrnAggs may also be useful for studies of neural networks. Our electrophysiological recordings of BrnAggs show low levels of spontaneous synchronized burst of neural-network activity when the culture is infused with nACSF, an increase of this baseline activity in the presence of high potassium nACSF, and saturated concentration of TTX completely suppresses all the network activity (Fig. 7). These findings suggest that these neurons in BrnAggs form a functional network and could, therefore, be used in studies of the pathophysiology of prion and other neurological diseases. This new additional functional assay in BrnAggs may widen the use for this culture system for research in neuroscience and can be very useful to exclude drug candidates causing neurotoxicity. It will also allow screening drug candidates for other neurological diseases such as epilepsy.
In summary, BrnAgg culture is a reliable 3D neuronal cell culture system to study the neural network, monitor neural toxicity, and measure drug efficacy accurately. BrnAggs will serve as a novel effective in vitro culture system to develop effective therapeutics for many neurological disorders.
REFERENCES
- 1.Choi SH, Kim YH, Hebisch M, et al. A three-dimensional human neural cell culture model of Alzheimer's disease. Nature 2014;515:274–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hou XQ, Yan R, Yang C, et al. A novel assay for high-throughput screening of anti-Alzheimer's disease drugs to determine their efficacy by real-time monitoring of changes in PC12 cell proliferation. Int J Mol Med 2014;33:543–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loffler T, Flunkert S, Taub N, et al. Stable mutated tau441 transfected SH-SY5Y cells as screening tool for Alzheimer's disease drug candidates. J Mol Neurosci 2012;47:192–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yahata N, Asai M, Kitaoka S, et al. Anti-Aβ drug screening platform using human iPS cell-derived neurons for the treatment of Alzheimer's disease. PLoS One 2011;6:e25788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dehdashti SJ, Zheng W, Gever JR, et al. A high-throughput screening assay for determining cellular levels of total tau protein. Curr Alzheimer Res 2013;10:679–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skibinski G, Finkbeiner S. Drug discovery in Parkinson's disease-update and developments in the use of cellular models. Int J High Throughput Screen 2011;2011:15–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korth C, May BC, Cohen FE, et al. Acridine and phenothiazine derivatives as pharmacotherapeutics for prion disease. Proc Natl Acad Sci U S A 2001;98:9836–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klingenstein R, Lober S, Kujala P, et al. Tricyclic antidepressants, quinacrine and a novel, synthetic chimera thereof clear prions by destabilizing detergent-resistant membrane compartments. J Neurochem 2006;98:748–59 [DOI] [PubMed] [Google Scholar]
- 9.Collins SJ, Lewis V, Brazier M, et al. Quinacrine does not prolong survival in a murine Creutzfeldt-Jakob disease model. Ann Neurol 2002;52:503–6 [DOI] [PubMed] [Google Scholar]
- 10.Ghaemmaghami S, Ahn M, Lessard P, et al. Continuous quinacrine treatment results in the formation of drug-resistant prions. PLoS Pathog 2009;5:e1000673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bajsarowicz K, Ahn M, Ackerman L, et al. A brain aggregate model gives new insights into the pathobiology and treatment of prion diseases. J Neuropathol Exp Neurol 2012;71:449–66 [DOI] [PubMed] [Google Scholar]
- 12.Pulliam L, Dix RD, Panitch HS, et al. Use of aggregating brain cultures to study the replication of herpes simplex virus types 1 and 2 in central nervous system tissue. J Virol Methods 1984;9:301–16 [DOI] [PubMed] [Google Scholar]
- 13.Pulliam L. Cytomegalovirus preferentially infects a monocyte derived macrophage/microglial cell in human brain cultures: Neuropathology differs between strains. J Neuropathol Exp Neurol 1991;50:432–40 [DOI] [PubMed] [Google Scholar]
- 14.Bueler H, Aguzzi A, Sailer A, et al. Mice devoid of PrP are resistant to scrapie. Cell 1993;73:1339–47 [DOI] [PubMed] [Google Scholar]
- 15.Ahn M, Bajsarowicz K, Oehler A, et al. Convection-enhanced delivery of AAV2-PrPshRNA in prion-infected mice. PLoS One 2014;9:e98496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spilman P, Lessard P, Sattavat M, et al. A gamma-secretase inhibitor and quinacrine reduce prions and prevent dendritic degeneration in murine brains. Proc Natl Acad Sci U S A 2008;105:10595–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santacruz K, Lewis J, Spires T, et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science 2005;309:476–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orr ME, Pitstick R, Canine B, et al. Genotype-specific differences between mouse CNS stem cell lines expressing frontotemporal dementia mutant or wild type human tau. PLoS One 2012;7:e39328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voss LJ, Brock M, Carlsson C, et al. Investigating paradoxical hysteresis effects in the mouse neocortical slice model. Eur J Pharmacol 2012;675: 26–31 [DOI] [PubMed] [Google Scholar]
- 20.Voss LJ, George SA, Sleigh JW. Testing neocortical slice viability in non-perfused no-magnesium artificial cerebrospinal fluid solutions. J Neurosci Methods 2012;204:273–5 [DOI] [PubMed] [Google Scholar]
- 21.Meltzer CC, Smith G, DeKosky ST, et al. Serotonin in aging, late-life depression, and Alzheimer's disease: The emerging role of functional imaging. Neuropsychopharmacology 1998;18:407–30 [DOI] [PubMed] [Google Scholar]
- 22.Jiruska P, Csicsvari J, Powell AD, et al. High-frequency network activity, global increase in neuronal activity, and synchrony expansion precede epileptic seizures in vitro. J Neurosci 2010;30:5690–701 [DOI] [PMC free article] [PubMed] [Google Scholar]