Abstract
Background
GLP-1 agonists, including liraglutide, have emerged as effective therapies for type 2 diabetes (DM) and obesity. Here, we attempted to delineate how liraglutide, at doses approved for DM, may impact circulating hormones influencing energy homeostasis in diabetics.
Basic Procedures
Using a randomized, placebo-controlled, double-blind, cross-over trial of 20 patients with type 2 diabetes, we examined the effects of liraglutide as compared to placebo on fasting levels of circulating hormones important to energy homeostasis, including leptin, ghrelin, PYY, and GIP. After 17 days (0.6 mg for 7 days, 1.2 mg for 7 days and 1.8 mg for 3 days) of treatment, we also studied changes in fMRI responses to food cues.
Main Findings
By design, to avoid any confounding by weight changes, subjects were studied for 17 days, i.e. before body weight changed. Participants on liraglutide had significantly increased GLP-1 levels (p<0.001), decreased percent change in leptin levels (p<0.01) and increased GIP levels (p<0.03) in comparison to placebo treated subjects. Whole brain regressions of functional activity in response to food cues reveal that increased GIP levels were associated with deactivation of the attention- and reward-related insula. Decreases in leptin levels were associated with activations in the reward-related midbrain, precuneus, and dorsolateral prefrontal cortex (DLPFC), and sensorimotor-related motor cortex and with deactivations in the attention-related parietal cortex and the cognitive control-related thalamus and pre-SMA.
Principal Conclusions
We demonstrate herein short-term changes to circulating levels of GIP and leptin in response to GLP-1 agonist liraglutide therapy. These findings suggest that liraglutide may alter the circulating levels of hormones important in energy homeostasis that, in turn, influence CNS perception of food cues. This could possibly lead to compensatory changes in energy homeostasis that would over time limit the efficacy of liraglutide to decrease body weight. These novel findings, which, pointing to the potential advantages of combination therapies, may have therapeutic implications, will need to be confirmed by larger and longer-term trials.
Obesity and its comorbidies, including type 2 diabetes, are rapidly increasing problems in need of effective therapies [1]. Multiple circulating hormones, including glucagon-like peptide-1 (GLP-1), that are secreted from the gut are known to convey information about nutritional status to the brain, regulating satiety and food intake, thus providing a crucial link between peripheral metabolic processes and the central nervous system. GLP-1 agonists, such as liraglutide, are becoming an increasingly attractive option for patients with type 2 diabetes whose symptoms would also be improved by weight loss and for whom glycated hemoglobin levels are moderately elevated [2]. Through mechanisms not fully understood, liraglutide in doses approved for type 2 diabetes (1.8 mg) is associated with a significant reduction in weight (treatment difference −1.4 to −3.5 kg when compared with placebo and/or other anti-diabetes medications such as the dipeptidyl-peptidase-4 inhibitor sitagliptin, insulin, or sulfonylureas such as glimepiride) [3].
Although GLP-1 slows gastric emptying, thereby promoting gastric distention and sensation of satiety [4], increasing evidence points to central mechanisms of action, demonstrating that GLP-1 acts in the brain of mice and limited number of human studies [5–7]. Enterohormonal signals may also mediate how the brain responds to food cues, providing an indirect mechanism for central actions of GLP-1 agonists [8–11]. We have recently shown decreased attention-related parietal cortex activations to highly desirable food cues during a fasting-state fMRI study with a short course of liraglutide, pointing to a central mechanism of action [12]. However, whether GLP-1 analog administration interacts with peripheral signals, i.e. alters levels of circulating hormones in the fasting state, and these changes lead, in turn, to altered functional brain activation, remains to be studied.
We sought to explore whether GLP-1 related weight loss in humans is linked to altered levels of hormones important in energy homeostasis using the maximum daily dose (1.8 mg) of liraglutide approved for diabetics in the context of a dose escalation, randomized, placebo-controlled, cross-over study for 17 days, i.e. a time period that allows for participants to escalate to the maximum dose to eliminate side effects but which does not allow for weight loss. We first studied how circulating biomarkers and hormones which are important in energy homeostasis and weight loss may be changed in response to treatment with liraglutide and thus mediate the effects of liraglutide in the short-term, i.e. before actual weight loss had occurred. We also analyzed how these hormonal changes in turn related to functional changes in the brain, thus determining whether liraglutide’s actions on peripheral cues may mediate a central (brain) response.
Research Design and Methods
As part of a larger study, twenty men and women with type 2 diabetes mellitus (DM; defined as fasting plasma glucose > 125 mg/dL and/or HbA1c > 6.5%) provided written informed consent to participate in a randomized, cross-over, placebo-controlled, double-blind study, approved by the Beth Israel Deaconess Medical Center (BIDMC) Institutional Review Board (full study details in [12]). Patients were being treated for their DM with metformin or with lifestyle modification (diet and exercise); patients receiving other treatments were excluded from the study.
Briefly, participants were randomized to receive liraglutide or placebo for their first phase; they then received the opposite for the second phase after a three week to three month wash-out period. Doses of liraglutide were escalated during the phase, which consisted of four visits. The first three visits were each a week apart where daily doses of 0.6mg and 1.2mg were begun at the first two and 1.8mg began at the third and continued for 3 days before participants returned for their 4th visit of the phase, which consisted of an overnight visit after which they underwent MRI (as previously described [12]). Detailed anthropometric data (e.g. dual energy x-ray absorptiometry or DEXA) and resting metabolic rate (RMR) were collected at the first and fourth visit of each phase.
Biochemical measurements and analysis
Fasting blood was drawn by venipuncture by a registered nurse between 8 and 10 am. Nurses took vital signs in the morning before fasting blood draws. Samples were immediately processed for plasma and serum isolation according to standard operating procedures and stored at −80°C until analysis as previously described [12–14]. All samples and standards were assayed in duplicate and only results with a CV <15% were used.
Amylin, gastric inhibitory peptide (GIP), and pancreatic polypeptide (PP) were measured in serum samples by commercially available enzyme-linked immunosorbent assay (ELISA; Millipore, Billerica, MA, USA). The GIP ELISA does not cross-react with GLP-1 or GLP-2. Fibroblast growth factor 21 (FGF-21) was measured by commercially available ELISA (R&D Systems, Inc. Minneapolis, MN). Irisin was measured by ELISA (Phoenix Pharmaceuticals. Burlingame, CA). GLP-1, leptin, ghrelin, peptide YY (PYY), and adiponectin in serum was measured by commercially available radioimmunoassay (Millipore Co. Billerica, MA USA). Fructosamine was analyzed in serum samples by the Roche cobas c311 clinical chemistry analyzer using a standardized kit (cat#04537939-190). All assays were performed as previously described [12–14].
Fasting serum glucose, total cholesterol, triglycerides, high-density lipoprotein (HDL), low-density lipoprotein (LDL), amylase, and lipase were measured by LabCorp (Raritan, NJ), a CLIA-certified laboratory. Fasting blood samples were sent directly to LabCorp by the BIDMC CRC as per standard protocols.
Data were analyzed using the Statistical Package for Social Sciences (SPSS), v.19 and were first summarized with descriptive statistics. For example, continuous variables are presented as mean ± standard error of the mean (SE). Data for categorical variables are presented as numbers and/or percentages. Kolmogorov-Smirnov test and frequency histograms were used to check the normality of distribution of the continuous variables. We obtained the skew statistic and standard error of skew (using the descriptive command in SPSS) to identify variables that are non-normal. The treatment effect was assessed using a general linear mixed model. The variables of treatment, visit, and sequence were included in the model as fixed effects and patient-within-sequence was included as a random effect. Baseline values were included as a covariate. The values of anthropometric, clinical, and laboratory variables in visit 4 and visit 8 were compared and used as a dependent variables. The dependent variables which did not fulfill the normality assumptions were log-transformed before analysis. The difference in values between visit 4 and visit 1 and visit 8 and visit 5 as well as the percent change between each period [(value in visit 4 – value in visit 1)/value in visit 1*100 vs. (value in visit 8 – value in visit 5)/value in visit 5 *100] was also compared and assessed as dependent variables. Visual analog scale ratings were correlated using a Spearman correlation with hormones that showed a significant change with liraglutide, and those with P<.025 were considered significant.
fMRI protocol and analysis
MRI scanning occurred at the Center for Biomedical Imaging, Boston University School of Medicine, using a 3-T Philips Intera whole-body MRI (Philips Medical Systems, Best, The Netherlands), as previously described [12]. The fMRI protocol consisted of six runs during both the fasting and fed scans, during which subjects viewed blocks of images and provided responses on how much they like each image (on a 1–3 scale) using a fiberoptic response box held in their right hand.
BOLD data was preprocessed using the SPM8 (Statistical Parametric Mapping; The Wellcome Trust Centre of Neuroimaging; London, UK). First-level processing occurred as previously described [12]. The contrast images of the first-level analysis were used for the second-level group statistics. Whole brain regressions were run with hormone levels or change in hormone levels for fasting state images with the highly desirable- less desirable food cues contrast. Activations significant at p<.001, uncorrected and p<.025, Family-wise Error (FWE) corrected for voxel are reported. We chose the stringent criteria of p<.025 since we tested 2 hormones (leptin and GIP; 0.05/2=0.025).
Results
Metabolic Impacts of liraglutide
20 participants completed the study (11 males; aged 49.7±2.4 years). As previously reported, during the short course of liraglutide therapy, body weight and weight circumference did not change by design (i.e. duration of the study; Table 1) to avoid any confounding effects of weight loss. As previously reported, participants consumed less kcal per day while on 1.8mg liraglutide than while on placebo (placebo(averaged): 1782±127; 0.6mg: 1723±150; 1.2mg: 1640±93; 1.8mg: 1424±127; t(7)= 2.11; p<.07, two-tailed; p<.03, one-tailed).
Table 1.
Body Composition and Hormonal Measurements after liraglutide or placebo. Data shown as means ± standard error (SE) of the mean.
| Placebo (mean ± SE) | Liraglutide (mean ± SE) | P | P | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Visit 1 | Visit 4 | percent change | Visit 1 | Visit 4 | percent change | mixed model | percent change | |
| Anthropometry | ||||||||
|
| ||||||||
| BMI (kg/m2) | 31.2 ± 1.7 | 30.5 ± 1.4 | −0.5 ± 0.9 | 31.9 ± 1.7 | 31.5 ± 1.7 | −1.2 ± 0.9 | 0.95 | 0.59 |
| Body weight (kg) | 93.4 ± 4.8 | 91.1 ± 1.0 | −0.4 ± 0.7 | 94.7 ± 4.7 | 91.0 ± 1.0 | −1.2 ± 0.7 | 0.43 | 0.46 |
| WC iliac (cm) | 105.1 ± 3.5 | 104.1 ± 3.4 | −0.5 ± 0.7 | 106.8 ± 3.5 | 105.3 ± 3.4 | −1.3 ± 0.6 | 0.34 | 0.34 |
| Fat body mass (kg) | 30.9 ± 3.1 | 29.2 ± 1.1 | −1.2 ± 1.1 | 31.9 ± 3.1 | 29.2 ± 1.1 | −0.1 ± 1.1 | 0.70 | 0.51 |
| Fat body mass (percentage) | 32.3 ± 2.0 | 32.9 ± 2.0 | −1.0 ± 1.1 | 32.8 ± 2.1 | 33.2 ± 2.0 | 1.3 ± 1.0 | 0.14 | 0.14 |
| Bone mass density (g/cm2) | 1.27 ± 0.03 | 1.2 ± 1.0 | −0.7 ± 0.7 | 1.26 ± 0.03 | 1.2 ± 1.0 | 0.0 ± 0.6 | 0.59 | 0.47 |
| Lean body mass (kg) | 62.9 ± 2.9 | 62.4 ± 2.6 | 0.3 ± 0.7 | 63.5 ± 2.8 | 61.6 ± 2.6 | −1.9 ± 0.7 | 0.07 | 0.03 |
|
| ||||||||
| SBP (mmHg) | 134.4 ± 2.4 | 130.8 ± 2.3 | −2.4 ± 2.4 | 136.6 ± 3.8 | 132 ± 3.0 | −2.4 ± 2.4 | 0.83 | 0.99 |
| DBP (mmHg) | 78.9 ± 2.6 | 78.8 ± 2.0 | 1.3 ± 3.1 | 84.6 ± 245 | 83.8 ± 2.6 | −1.0 ± 3.0 | 0.44 | 0.55 |
| VO2 (L/min) | ND | 0.23 ± 0.01 | N/A | ND | 0.24 ± 0.01 | N/A | 0.27 | N/A |
| VO2/kg (mL/kg/min) | ND | 2.5 ± 1.0 | N/A | ND | 2.6 ± 1.0 | N/A | 0.22 | N/A |
| VCO2 (L/min) | ND | 0.19 ± 0.01 | N/A | ND | 0.19 ± 0.01 | N/A | 0.87 | N/A |
| Respiratory quotient | ND | 0.84 ± 0.02 | N/A | ND | 0.81 ± 0.02 | N/A | 0.23 | N/A |
| Resting energy expenditure (Kcal/day) | ND | 1583.2 ± 52.9 | N/A | ND | 1622.6 ± 52.9 | N/A | 0.31 | N/A |
| Predicted basal metabolic rate (Kcal/day) | ND | 1746.3 ± 62.6 | N/A | ND | 1738.1 ± 63.5 | N/A | 0.72 | N/A |
| Total energy intake (Kcal/day) | ND | 1782±542 | N/A | ND | 1434±537 | N/A | 0.07 | N/A |
|
| ||||||||
| Metabolic Profile | ||||||||
|
| ||||||||
| GLP-1 (pM) | 49.8 ± 8.6 | 38.2 ± 1.2 | 50.4 ± 44.6 | 89.3 ± 12.1 | 912.3 ± 1.2 | 1674.2 ± 212.3 | <0.001 | <0.001 |
| Glucose (mg/dL) | 119 ± 6.3 | 122.3 ± 6.3 | 5.8 ± 5.4 | 124.7 ± 6.2 | 95.4 ± 4.1 | −22.4 ± 5.4 | <0.001 | 0.001 |
| Fructosamine (umol/L) | 296.8 ± 9.9 | 273.8 ± 7.9 | −6.8 ± 2.5 | 300.3 ± 8.8 | 271.7 ± 7.9 | −9.5 ± 2.4 | 0.36 | 0.35 |
| HbA1c (g/dL) | ND | 6.7 ± 1.2 | N/A | ND | 6.6 ± 1.2 | N/A | 0.82 | N/A |
| Insulin (uIU/mL) | 16.2 ± 2.8 | 11.5 ± 2.6 | −19.4 ± 9.9 | 15.6 ± 2.3 | 11.2 ± 1.4 | −10.8 ± 9.9 | 0.48 | 0.54 |
| C-peptide (ng/mL) | 2.1 ± 0.2 | 1.7 ± 0.1 | −13.3 ± 9.7 | 2.4 ± 0.2 | 2.2 ± 0.2 | −4.4 ± 9.3 | 0.48 | 0.51 |
| Glucagon (pg/mL) | 181.0 ± 27.6 | 97.4 ± 1.1 | −19.3 ± 11.7 | 135.6 ± 18.2 | 84.9 ± 1.1 | −12.5 ± 11.5 | 0.41 | 0.68 |
| Total cholesterol (mg/dL) | 177.1 ± 5.7 | 167.9 ± 5.3 | −4.5 ± 3.5 | 186.8 ± 7.5 | 170.6 ± 5.6 | 0.5 ± 3.7 | 0.18 | 0.34 |
| Triglycerides (mg/dL) | 115.6 ± 20 | 89.8 ± 12.6 | −17.0 ± 11.8 | 118.1 ± 18.3 | 87.4 ± 12.6 | −16.6 ± 12.6 | 0.23 | 0.06 |
| HDL (mg/mL) | 53.3 ± 2.7 | 52.4 ± 2.3 | −0.8 ± 4.5 | 53.5 ± 2.6 | 50.8 ± 2.5 | −7.3 ± 4.8 | 0.22 | 0.30 |
| LDL (mg/mL) | 100.8 ± 4.8 | 99.3 ± 4.7 | 1.2 ± 5.7 | 107.0 ± 5.4 | 100.8 ± 4.6 | 6.1 ± 6.2 | 0.22 | 0.56 |
|
| ||||||||
| Key Hormones in Energy Homeostasis | ||||||||
|
| ||||||||
| Leptin (ng/mL) | 19.8 ± 3.5 | 23.0 ± 4.4 | 16.6 ± 7.1 | 25.1 ± 4.3 | 20.8 ± 4.3 | −10.4 ± 7.1 | 0.12 | 0.01 |
| Adiponectin (ug/mL) | 8.8 ± 1.3 | 6.7 ± 1.2 | −18.4 ± 12.2 | 16.1 ± 8.7 | 8.4 ± 2.3 | −9.3 ± 10.2 | 0.37 | 0.20 |
| Irisin (ng/mL) | 166.8 ± 14.9 | 164.1 ± 20.9 | −1.7 ± 3.8 | 167.1 ± 15.3 | 168.3 ± 20.9 | 0.2 ± 3.8 | 0.63 | 0.73 |
| PP (pg/mL) | 277.0 ± 48.9 | 206.4 ± 43.2 | −37.0 ± 11.6 | 230.2 ± 47.9 | 244.4 ± 45.7 | 12.9 ± 6.9 | 0.09 | 0.21 |
| GIP (pg/mL) | 72.6 ± 12.0 | 42.1 ± 15.6 | −34.5 ± 24.6 | 62.6 ± 10.6 | 91.0 ± 14.8 | 53.2 ± 24.6 | 0.03 | 0.02 |
| Amylin (pM) | 11.4 ± 1.7 | 11.6 ± 1.9 | −11.3 ± 8.6 | 11.6 ± 1.8 | 10.5 ± 1.8 | −21.9 ± 9.3 | 0.45 | 0.92 |
| Ghrelin (pg/mL) | 868.6 ± 89.3 | 698.5 ± 90.6 | −13.8 ± 11.3 | 696.3 ± 103.1 | 671.4 ± 91.6 | −3.2 ± 3.8 | 0.72 | 0.16 |
| TSH (uIU/mL) | 2.3 ± 0.4 | 1.9 ± 0.4 | −8.2 ± 16.0 | 2.4 ± 0.7 | 1.9 ± 0.4 | −15.2 ± 15.3 | 0.81 | 0.75 |
| Free T3 (pg/mL) | 3.4 ± 0.3 | 2.9 ± 0.1 | −10.3 ± 11.5 | 3.5 ± 0.1 | 2.9 ± 0.2 | −15.5 ± 11.5 | 0.99 | 0.76 |
| Free T4 (ng/dL) | 1.1 ± 0.0 | 1.1 ± 0.0 | −2.2 ± 3.4 | 1.1 ± 0.0 | 1.1 ± 0.0 | 1.7 ± 3.4 | 0.43 | 0.43 |
| TBG (ug/mL) | 18.7 ± 1.7 | 16.0 ± 1.2 | −11.3 ± 3.0 | 18.0 ± 1.4 | 17.0 ± 1.3 | −3.8 ± 3.0 | 0.18 | 0.09 |
| FGF21 (pg/mL) | 164.3 ± 24.8 | 88.5 ± 1.3 | −7.4 ± 17.5 | 165.1 ± 36.5 | 97.6 ± 1.3 | −1.0 ± 17.5 | 0.49 | 0.71 |
| IGF-1 (ng/mL) | 181.1 ± 16.3 | 192.6 ± 14.7 | 13.1 ± 10.5 | 201.4 ± 19.2 | 213.5.4 ± 15.8 | 10.7 ± 10.5 | 0.77 | 0.88 |
| FFA (mEq/L) | 1.0 ± 0.1 | 1.0 ± 1.1 | 42.9 ± 35.0 | 1.1 ± 0.1 | 1.0 ± 1.1 | 29.8 ± 33.6 | 0.57 | 0.79 |
| PYY (pg/mL) | 183.7 ± 21.5 | 177.6 ± 23.2 | −4.8 ± 13.4 | 174.7 ± 22.9 | 146.9 ± 11.3 | −6.3 ± 13.9 | 0.48 | 0.46 |
SE, standard error; ND, no data available; N/A, not applicable (due to missing visit 1 values); SBP, systolic blood pressure; DBP, diastolic blood pressure; GLP-1, glucagon-like peptide-1; HbA1c, hemoglobin A1c; HDL, high density lipoproteins; LDL, low density lipoproteins; PP, pancreatic polypeptide; GIP, gastric inhibitory peptide; TSH, thyroid-stimulating hormone; T3, triiodothyronine; T4, thyroxine; TBG, thyroxine-binding globulin; FGF21, fibroblast growth factor 21; IGF-1, insulin-like growth factor-1; FFA, free fatty acids; PYY, peptide YY
There was a significant decrease in the percentage change of leptin while on liraglutide therapy (−10.4 ± 7.1%) compared to the placebo (16.6 ± 7.1%; p<.010), and this remained significant when adjusted for BMI (p<.014). Furthermore, this percentage change in leptin while on liraglutide inversely correlates with feelings of fullness from visual analog scale (VAS) ratings (rho=-.544, p=0.016). There were no changes in adiponectin (p<.371), irisin (p<.630), ghrelin (p<.723), amylin (p<.448), or thyroid hormone levels. Gastric inhibitory peptide (GIP) showed a significant increase with liraglutide treatment (p<.030). However, many other gut and neural related hormones showed no significant changes (Table 1).
Neurocognitive impacts of liraglutide
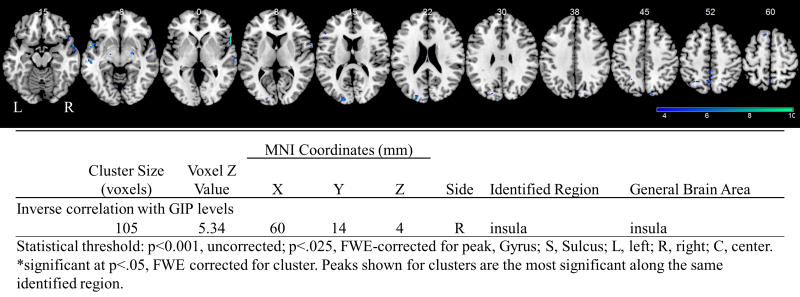
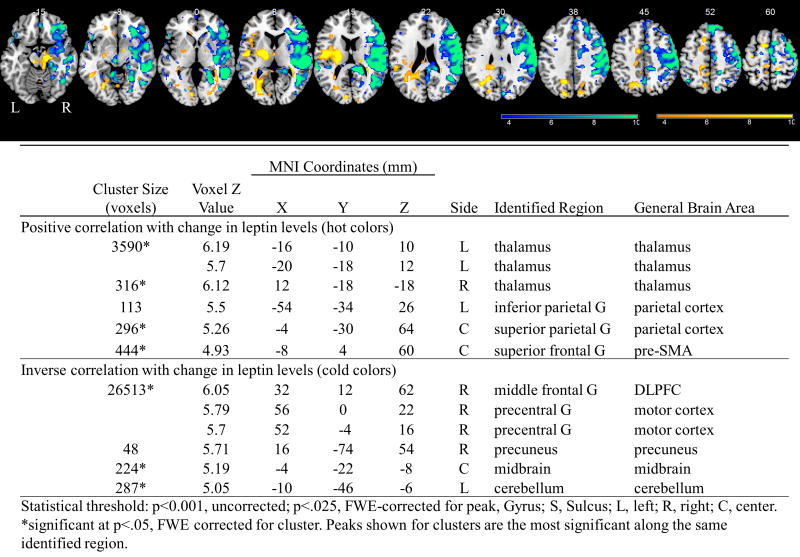
There were no changes in brain activations to food cues during the fed state. Whole brain regressions of the fMRI scans in the fasting state with significant hormones after 17 days of liraglutide therapy, reveal that GIP levels inversely correlate with activation of the insula while participants viewed highly desirable as compared to less desirable food images (Figure 1). Additionally, for the same contrast and time point, the change in leptin levels correlates positively with activations in the thalamus, parietal cortex, and pre-SMA, while it correlates inversely with activations in the dorsolateral prefrontal cortex (DLPFC), motor cortex, precuneus, midbrain, and cerebellum (Figure 2).
Figure 1.
Results of whole brain regressions while participants were on liraglutide with GIP levels at 17 days during the fasting state while viewing highly desirable as compared to less desirable food cues at p<.001. BOLD contrasts are superimposed on a T1 structural image in neurological orientation. The color bar represents voxel T value. Table shown below figure for activations significant at P<.025, FWE-corrected for peak.
Figure 2.
Results of whole brain regressions while participants were on liraglutide with the change in leptin levels from baseline to 17 days during the fasting state while viewing highly desirable as compared to less desirable food cues at p<.001. BOLD contrasts are superimposed on a T1 structural image in neurological orientation. The color bar represents voxel T value. Table shown below figure for activations significant at P<.025, FWE-corrected for peak.
Discussion
This study gives insights for the first time into how GLP-1 agonists influence hormones important to energy homeostasis in the short-term (17 days) and how these changes in turn influence central nervous system response to food cues in the fasting (but not the fed) state 17 days after initiation of therapy. These changes, observed using the ideal randomized, cross-over, double-blind design, if they persist, may influence weight loss outcomes indirectly and in a compensatory manner over time.
We have reported a significant decrease of caloric intake with liraglutide during the 17 days of treatment (using a one-tailed t-test consistent with our a priori hypothesis) [12]. In contrast, we do not observe any changes in resting energy expenditure. Of note, we did not measure 24-hour energy expenditure and thus cannot state whether activity/energy expenditure changed with liraglutide therapy. In another study, 24-hour energy expenditure, as measured in a respiratory chamber, was reduced after incremental doses of liraglutide (1.8 mg/d and 3.0 mg/d) for 5 weeks in apparently healthy, obese participants by 350kJ and 581kJ (~3–5%), respectively [15]. Longer-term studies have found no effects of liraglutide administration (0.6, 1.2 or 1.8 mg/day) either alone or in combination with metformin on energy expenditure and/or RQ acutely [16], after 4 weeks [17] or 1 year [18] compared to control and/or other medication in obese patients with type 2 diabetes, suggesting that falling leptin levels with prolonged therapy may be responsible for the discrepant results.
We then focused on peripheral hormones potentially mediating or otherwise modifying the weight loss induced by liraglutide. We observed changes in GIP and leptin levels. Leptin is the prototypical adipokine which signals energy homeostasis to the brain by circulating at levels proportional to the amount of body fat as well as to acute changes in caloric intake [19]. We observed acute changes in leptin without changes in fat mass, which remained significantly different after controlling for BMI. These changes may reflect either the effect of decreasing caloric intake and/or a direct effect of GLP-1 to decrease leptin levels. The possibility that GLP-1 may decrease leptin production by adipocytes remains to be confirmed by dedicated molecular studies in humans, but this effect has already been observed in two studies of liraglutide with rodents [20, 21]. Other GLP-1 analogs have resulted in similar decreases of leptin levels in rodents [20–29]. However, other studies with rodents have not showed decreases in leptin with GLP-1 analogs [30–33]. Similarly, one study has shown that GLP-1 analogs decrease levels of leptin in humans [34], while most have shown no changes in leptin levels [35–40].Such a decrease of leptin levels could possibly be expected to be even more pronounced in response to long-term weight loss resulting in decreases of both main predictors of leptin levels i.e. energy intake and fat mass.
Greater liraglutide-induced decreases of fasting leptin levels result in increased activation of the reward-related midbrain, precuneus, and DLPFC and sensorimotor-related motor cortex (inverse correlation with the change in leptin levels) and less activation of the attention-related parietal cortex and cognitive control-related thalamus and pre-SMA (positive correlation with the change in leptin levels) to highly desirable as compared to less desirable food cues. Leptin receptors are expressed throughout the brain [41, 42], and thus may mediate the central response to GLP-1 analogs, leading to changes in energy intake and/or expenditure. These findings, if confirmed, could be interpreted as decreased leptin leading to altered brain activations to food cues and consequent weight changes.
Increased activity of the DLPFC, midbrain, and precuneus with decreased leptin may indicate altered control and reward-related circuitry induced by leptin in response to GLP-1 administration. Indeed, decreased control- and reward-related DLPFC activation after food consumption has been associated with obesity [43–45]. Additionally, the midbrain, including the ventral tegmental area, is well-known to be involved in the rewarding aspect of food [46]. The precuneus is more involved in attention and saliency processing but has also been shown to be impacted by rewarding stimuli, as these are highly salient [47–50]. The motor cortex activation encompasses a large area of sensorimotor cortex and may indicate greater sensory and motor processing of highly desirable food cues with greater decreases in leptin while on liraglutide, indicating that they may be more appetitive or salient [51]. Altogether, these results support the notion that lower leptin levels may be counteracting the effects of liraglutide, by increasing reward and salience related brain activations and decreasing cortical and control related activations to highly palatable food cues during the fasting state.
Furthermore, we observed greater changes in leptin levels with liraglutide correlating with decreased activity in the thalamus, parietal cortex, and pre-SMA while viewing highly desirable food cues in the fasting state. The thalamus and pre-SMA are involved in cognitive and motor-related control [52–54], while the parietal cortex is a part of the attention system and shows activation to salient stimuli [55–57]. This may suggest that these higher cortical attention- and cognitive-related systems are attempting to counteract the appetite-reducing effects of liraglutide in the brain.
Taken altogether, these results suggest a number of systems related to the control of eating are impacted in relation to leptin levels during liraglutide therapy. The role of leptin in feeding is known to be complex. One recent study has shown that communication between frontal and parietal regions plays a role in value-based choices [58]. The relationship of leptin with pre-SMA, motor cortex, parietal cortex, and precuneus that we observed in this study could suggest a potential role for leptin in those complex neuronal circuits which evaluate food choices. The decreases in leptin before weight loss may be a compensatory mechanism which counteracts the appetite-reducing effects of liraglutide peripherally and centrally. Thus, a future study combining leptin in replacement doses with liraglutide therapy could be warranted since it could potentially offer additional weight loss.
GIP is secreted in response to food intake and regulates glucose postprandially by increasing the secretion of insulin, similar to GLP-1 [59]. It has been repeatedly observed that individuals with DM have a diminished insulin response to GIP [59]. Furthermore, GIP has been shown to increase GLP-1 levels in animal models [60] and DPP-4 inhibitors which increase GLP-1 also increase GIP [61]. Altogether, these data support the existence of a feedback loop between the incretins GLP-1 and GIP [59, 60], which may be activated by the GLP-1 analog, liraglutide, as it increases GIP in our study. In the past, others have found no effect of GLP-1 analogs given intravenously on GIP levels in humans [62–66]. In contrast, another group found that intravenous GLP-1 decreased GIP in type 1 diabetics and healthy participants [67], and another found that intravenous GLP-1 attenuated the breakfast-induced increase of GIP [68]. Confirming our findings, another study of intravenous GLP-1 found that although GIP decreased over the first hour of GLP-1 administration, it then increased [69]. Considering our blood samples are obtained more than an hour after liraglutide administration, we may be capturing this same effect. Regarding the potential liraglutide-mediating effects of GIP on the brain, we found an inverse correlation between GIP levels and insula activation while participants are on liraglutide, demonstrating that higher GIP levels observed with liraglutide decrease insular activation. Insula is known to be involved in reward and saliency processing [70–74], indicating the higher levels of GIP with liraglutide decrease the rewarding value and saliency of highly desirable food cues. In addition, GIP per se has been found to be expressed widely throughout the brain. A role for GIP has been identified in adult hippocampal progenitor cell proliferation, as well as one of neurotransmitter or neuromodulator [75, 76]. This may support a role for GIP in mediating and augmenting, in part, the activity of liraglutide on the central nervous system and consequently on weight loss.
In summary, an increase in GIP may promote the anorexigenic actions of liraglutide, whereas compensatory decreases of leptin may counteract the effects of liraglutide. Of note, these hormonal changes may reflect short-term effects in response to early liraglutide therapy and may be attenuated or altered with longer term therapy pointing to a need for more long term studies. Indeed, findings in longer-term studies demonstrate that there are long-term differences in hormone levels induced by liraglutide that are not captured in our study. For instance, in a 48-week trial with liraglutide, increases in glucagon levels were observed with liraglutide [77], while we do not observe any changes in glucagon in the 17 days of our trial. Future longitudinal studies are needed to determine short-term versus long-term changes with liraglutide therapy both at low-dose 1.8mg daily and high-dose 3.0mg daily administration. Additionally, a relatively small sample size might account for a lack of differences and larger studies should confirm our results. If our data are confirmed by future independent studies, combination therapies with leptin analogues would be warranted for additional weight loss effects.
Acknowledgments
Funding
The project was supported by Harvard Clinical and Translational Science Center Grant UL1 RR025758 from the National Center for Research Resources. Novo Nordisk supported the study through an Investigator-Initiated Study grant and supplied liraglutide/placebo. They approved the design of the study, but had no role in study design; conduct of the study; collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript. Olivia M. Farr is supported by a training grant through the NICHD 5T32HD052961.
Abbreviations
- DM
type 2 diabetes
- GLP-1
glucagon-like peptide-1
- fMRI
functional magnetic resonance imaging
- CNS
central nervous system
- DLPFC
dorsolateral prefrontal cortex
- pre-SMA
pre-supplementary motor area
- BMI
body mass index
- BIDMC
Beth Israel Deaconess Medical Center
- DEXA
dual energy x-ray absorptiometry
- RMR
resting metabolic rate
- PP
pancreatic polypeptide
- GIP
gastric inhibitory peptide
- FGF21
fibroblast growth factor 21
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- HbA1c
hemoglobin A1c
- HDL
high density lipoproteins
- LDL
low density lipoproteins
- TSH
thyroid-stimulating hormone
- T3
triiodothyronine
- T4
thyroxine
- TBG
thyroxine-binding globulin
- IGF-1
insulin-like growth factor-1
- FFA
free fatty acids
- PYY
peptide YY
- SE
standard error
- SPSS
statistical package for the social sciences
- BOLD
blood oxygenation level dependent
- FWE
family-wise error
Footnotes
CT Registration: ClinicalTrials.gov NCT01562678
Conflicts of Interest
CSM has served on the scientific advisory board and is a shareholder of Novo Nordisk. All other authors have nothing to declare.
Contributions
CSM designed the study. OMF, MAT, FD, AF, and CSM collected the data. OMF and BJK analyzed the data. OMF wrote the manuscript. All authors (OMF, MAT, GT, FD, AF, BJK, and CSM) reviewed and edited the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hotamisligil G. Inflammation and metabolic disorders. Nature. 2006;444 doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 2.Aronis KN, Tsoukas MA, Mantzoros CS. Potential cardioprotective action of glp-1: From bench to bedside. Metabolism. 2014;63(8):979–88. doi: 10.1016/j.metabol.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Vilsboll T, Zdravkovic M, Le-Thi T, Krarup T, Schmitz O, Courreges JP, et al. Liraglutide, a long-acting human glucagon-like peptide-1 analog, given as monotherapy significantly improves glycemic control and lowers body weight without risk of hypoglycemia in patients with type 2 diabetes. Diabetes Care. 2007;30(6):1608–10. doi: 10.2337/dc06-2593. [DOI] [PubMed] [Google Scholar]
- 4.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3(3):153–65. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Hunter K, Holscher C. Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci. 2012;13:33. doi: 10.1186/1471-2202-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sisley S, Gutierrez-Aguilar R, Scott M, D’Alessio DA, Sandoval DA, Seeley RJ. Neuronal glp1r mediates liraglutide’s anorectic but not glucose-lowering effect. J Clin Invest. 2014;124(6):2456–63. doi: 10.1172/JCI72434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Bloemendaal L, RGIJ, Ten Kulve JS, Barkhof F, Konrad RJ, Drent ML, et al. Glp-1 receptor activation modulates appetite- and reward-related brain areas in humans. Diabetes. 2014;63(12):4186–96. doi: 10.2337/db14-0849. [DOI] [PubMed] [Google Scholar]
- 8.Farr OM, Fiorenza C, Papageorgiou P, Brinkoetter M, Ziemke F, Koo BB, et al. Leptin therapy alters appetite and neural responses to food stimuli in brain areas of leptin-sensitive subjects without altering brain structure. J Clin Endocrinol Metab. 2014;99(12):E2529–38. doi: 10.1210/jc.2014-2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konturek SJ, Pepera J, Zabielski K, Konturek PC, Pawlik T, Szlachcic A, et al. Brain-gut axis in pancreatic secretion and appetite control. J Physiol Pharmacol. 2003;54(3):293–317. [PubMed] [Google Scholar]
- 10.Konturek SJ, Zabielski R, Konturek JW, Czarnecki J. Neuroendocrinology of the pancreas; role of brain-gut axis in pancreatic secretion. Eur J Pharmacol. 2003;481(1):1–14. doi: 10.1016/j.ejphar.2003.08.042. [DOI] [PubMed] [Google Scholar]
- 11.Orr J, Davy B. Dietary influences on peripheral hormones regulating energy intake: Potential applications for weight management. J Am Diet Assoc. 2005;105(7):1115–24. doi: 10.1016/j.jada.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Farr OM, Sofopoulos M, Tsoukas MA, Dincer F, Thakkar B, Sahin-Efe A, et al. Glp-1 receptors exist in the parietal cortex, hypothalamus and medulla of human brains and the glp-1 analogue liraglutide alters brain activity related to highly desirable food cues in individuals with diabetes: A crossover, randomised, placebo-controlled trial. Diabetologia. 2016 doi: 10.1007/s00125-016-3874-y. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, et al. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med. 2004;351(10):987–97. doi: 10.1056/NEJMoa040388. [DOI] [PubMed] [Google Scholar]
- 14.Farr OM, Ko BJ, Joung KE, Zaichenko L, Usher N, Tsoukas M, et al. Posttraumatic stress disorder, alone or additively with early life adversity, is associated with obesity and cardiometabolic risk. Nutr Metab Cardiovasc Dis. 2015;25(5):479–88. doi: 10.1016/j.numecd.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WH. Effects of the once-daily glp-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes (Lond) 2014;38(6):784–93. doi: 10.1038/ijo.2013.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harder H, Nielsen L, Tu DT, Astrup A. The effect of liraglutide, a long-acting glucagon-like peptide 1 derivative, on glycemic control, body composition, and 24-h energy expenditure in patients with type 2 diabetes. Diabetes Care. 2004;27(8):1915–21. doi: 10.2337/diacare.27.8.1915. [DOI] [PubMed] [Google Scholar]
- 17.Horowitz M, Flint A, Jones KL, Hindsberger C, Rasmussen MF, Kapitza C, et al. Effect of the once-daily human glp-1 analogue liraglutide on appetite, energy intake, energy expenditure and gastric emptying in type 2 diabetes. Diabetes Res Clin Pract. 2012;97(2):258–66. doi: 10.1016/j.diabres.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 18.Beiroa D, Imbernon M, Gallego R, Senra A, Herranz D, Villarroya F, et al. Glp-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic ampk. Diabetes. 2014;63(10):3346–58. doi: 10.2337/db14-0302. [DOI] [PubMed] [Google Scholar]
- 19.Chan JL, Heist K, DePaoli AM, Veldhuis JD, Mantzoros CS. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. Journal of Clinical Investigation. 2003;111(9):1409–21. doi: 10.1172/JCI17490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larsen PJ, Fledelius C, Knudsen LB, Tang-Christensen M. Systemic administration of the long-acting glp-1 derivative nn2211 induces lasting and reversible weight loss in both normal and obese rats. Diabetes. 2001;50(11):2530–9. doi: 10.2337/diabetes.50.11.2530. [DOI] [PubMed] [Google Scholar]
- 21.Clemmensen C, Chabenne J, Finan B, Sullivan L, Fischer K, Kuchler D, et al. Glp-1/glucagon coagonism restores leptin responsiveness in obese mice chronically maintained on an obesogenic diet. Diabetes. 2014;63(4):1422–7. doi: 10.2337/db13-1609. [DOI] [PubMed] [Google Scholar]
- 22.Al-Barazanji KA, Arch JR, Buckingham RE, Tadayyon M. Central exendin-4 infusion reduces body weight without altering plasma leptin in (fa/fa) zucker rats. Obes Res. 2000;8(4):317–23. doi: 10.1038/oby.2000.38. [DOI] [PubMed] [Google Scholar]
- 23.Aziz A, Anderson GH, Giacca A, Cho F. Hyperglycemia after protein ingestion concurrent with injection of a glp-1 receptor agonist in rats: A possible role for dietary peptides. Am J Physiol Regul Integr Comp Physiol. 2005;289(3):R688–94. doi: 10.1152/ajpregu.00850.2004. [DOI] [PubMed] [Google Scholar]
- 24.He M, Su H, Gao W, Johansson SM, Liu Q, Wu X, et al. Reversal of obesity and insulin resistance by a non-peptidic glucagon-like peptide-1 receptor agonist in diet-induced obese mice. PLoS One. 2010;5(12):e14205. doi: 10.1371/journal.pone.0014205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iepsen EW, Lundgren J, Dirksen C, Jensen JE, Pedersen O, Hansen T, et al. Treatment with a glp-1 receptor agonist diminishes the decrease in free plasma leptin during maintenance of weight loss. Int J Obes (Lond) 2015;39(5):834–41. doi: 10.1038/ijo.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel V, Joharapurkar A, Dhanesha N, Kshirsagar S, Patel K, Bahekar R, et al. Co-agonist of glucagon and glp-1 reduces cholesterol and improves insulin sensitivity independent of its effect on appetite and body weight in diet-induced obese c57 mice. Can J Physiol Pharmacol. 2013;91(12):1009–15. doi: 10.1139/cjpp-2013-0189. [DOI] [PubMed] [Google Scholar]
- 27.Patel V, Joharapurkar A, Gandhi T, Patel K, Dhanesha N, Kshirsagar S, et al. Omeprazole improves the anti-obesity and antidiabetic effects of exendin-4 in db/db mice (-4 db/db)*. J Diabetes. 2013;5(2):163–71. doi: 10.1111/j.1753-0407.2012.00227.x. [DOI] [PubMed] [Google Scholar]
- 28.Pocai A, Carrington PE, Adams JR, Wright M, Eiermann G, Zhu L, et al. Glucagon-like peptide 1/glucagon receptor dual agonism reverses obesity in mice. Diabetes. 2009;58(10):2258–66. doi: 10.2337/db09-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su H, He M, Li H, Liu Q, Wang J, Wang Y, et al. Boc5, a non-peptidic glucagon-like peptide-1 receptor agonist, invokes sustained glycemic control and weight loss in diabetic mice. PLoS One. 2008;3(8):e2892. doi: 10.1371/journal.pone.0002892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Claret M, Corominola H, Canals I, Nadal B, Chavanieu A, Pfeiffer B, et al. S 23521 decreases food intake and body weight gain in diet-induced obese rats. Obes Res. 2004;12(10):1596–603. doi: 10.1038/oby.2004.199. [DOI] [PubMed] [Google Scholar]
- 31.Liu R, Ma D, Li Y, Hu R, Peng Y, Wang Q. The anorexic effect of ex4/fc through glp-1 receptor activation in high-fat diet fed mice. Acta Biochim Biophys Sin (Shanghai) 2014;46(8):675–81. doi: 10.1093/abbs/gmu044. [DOI] [PubMed] [Google Scholar]
- 32.Malendowicz LK, Macchi C, Nussdorfer GG, Nowak KW, Zyterska A, Ziolkowska A. Effects of prolonged exendin-4 administration on entero-insular axis of normal and streptozotocin-induced diabetic rats. Int J Mol Med. 2003;11(6):763–6. [PubMed] [Google Scholar]
- 33.Perez-Tilve D, Gonzalez-Matias L, Alvarez-Crespo M, Leiras R, Tovar S, Dieguez C, et al. Exendin-4 potently decreases ghrelin levels in fasting rats. Diabetes. 2007;56(1):143–51. doi: 10.2337/db05-0996. [DOI] [PubMed] [Google Scholar]
- 34.Hogan AE, Gaoatswe G, Lynch L, Corrigan MA, Woods C, O’Connell J, et al. Glucagon-like peptide 1 analogue therapy directly modulates innate immune-mediated inflammation in individuals with type 2 diabetes mellitus. Diabetologia. 2014;57(4):781–4. doi: 10.1007/s00125-013-3145-0. [DOI] [PubMed] [Google Scholar]
- 35.Daousi C, Pinkney JH, Cleator J, Wilding JP, Ranganath LR. Acute peripheral administration of synthetic human glp-1 (7–36 amide) decreases circulating il-6 in obese patients with type 2 diabetes mellitus: A potential role for glp-1 in modulation of the diabetic pro-inflammatory state? Regul Pept. 2013;183:54–61. doi: 10.1016/j.regpep.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 36.Diaz-Soto G, de Luis DA, Conde-Vicente R, Izaola-Jauregui O, Ramos C, Romero E. Beneficial effects of liraglutide on adipocytokines, insulin sensitivity parameters and cardiovascular risk biomarkers in patients with type 2 diabetes: A prospective study. Diabetes Res Clin Pract. 2014;104(1):92–6. doi: 10.1016/j.diabres.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 37.Drewes C, Nauck MA, Horn R, Holst J, Schmiegel W, Brabant G. A liquid mixed meal or exogenous glucagon-like peptide 1 (glp-1) do not alter plasma leptin concentrations in healthy volunteers. Acta Diabetol. 1997;34(3):230–4. doi: 10.1007/s005920050079. [DOI] [PubMed] [Google Scholar]
- 38.Gutzwiller JP, Goke B, Drewe J, Hildebrand P, Ketterer S, Handschin D, et al. Glucagon-like peptide-1: A potent regulator of food intake in humans. Gut. 1999;44(1):81–6. doi: 10.1136/gut.44.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jeibmann A, Zahedi S, Simoni M, Nieschlag E, Byrne MM. Glucagon-like peptide-1 reduces the pulsatile component of testosterone secretion in healthy males. Eur J Clin Invest. 2005;35(9):565–72. doi: 10.1111/j.1365-2362.2005.01542.x. [DOI] [PubMed] [Google Scholar]
- 40.Shalev A, Vosmeer S, Keller U. Absence of short-term effects of glucagon-like peptide-1 and of hyperglycemia on plasma leptin levels in man. Metabolism. 1997;46(7):723–5. doi: 10.1016/s0026-0495(97)90112-8. [DOI] [PubMed] [Google Scholar]
- 41.Wada N, Hirako S, Takenoya F, Kageyama H, Okabe M, Shioda S. Leptin and its receptors. J Chem Neuroanat. 2014;61–62:191–9. doi: 10.1016/j.jchemneu.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 42.Farr OM, Tsoukas MA, Mantzoros CS. Leptin and the brain: Influences on brain development, cognitive functioning and psychiatric disorders. Metabolism. 2015;64(1):114–30. doi: 10.1016/j.metabol.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 43.Le DS, Pannacciulli N, Chen K, Del Parigi A, Salbe AD, Reiman EM, et al. Less activation of the left dorsolateral prefrontal cortex in response to a meal: A feature of obesity. Am J Clin Nutr. 2006;84(4):725–31. doi: 10.1093/ajcn/84.4.725. [DOI] [PubMed] [Google Scholar]
- 44.Le DS, Pannacciulli N, Chen K, Salbe AD, Del Parigi A, Hill JO, et al. Less activation in the left dorsolateral prefrontal cortex in the reanalysis of the response to a meal in obese than in lean women and its association with successful weight loss. Am J Clin Nutr. 2007;86(3):573–9. doi: 10.1093/ajcn/86.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rapuano KM, Huckins JF, Sargent JD, Heatherton TF, Kelley WM. Individual differences in reward and somatosensory-motor brain regions correlate with adiposity in adolescents. Cereb Cortex. 2015 doi: 10.1093/cercor/bhv097. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berridge KC. Food reward: Brain substrates of wanting and liking. Neurosci Biobehav Rev. 1996;20(1):1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- 47.Rosen M, Stern C, Devaney K, Somers D. Functional mri reveals a cognitive control subnetwork supporting long-term memory-guided visual attention. J Vis. 2015;15(12):1247. [Google Scholar]
- 48.Neale C, Johnston P, Hughes M, Scholey A. Functional activation during the rapid visual information processing task in a middle aged cohort: An fmri study. PLoS One. 2015;10(10):e0138994. doi: 10.1371/journal.pone.0138994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mason L, Trujillo-Barreto NJ, Bentall RP, El-Deredy W. Attentional bias predicts increased reward salience and risk taking in bipolar disorder. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 50.Hosseini AH, Holroyd CB. Reward feedback stimuli elicit high-beta eeg oscillations in human dorsolateral prefrontal cortex. Sci Rep. 2015;5:13021. doi: 10.1038/srep13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carnell S, Gibson C, Benson L, Ochner CN, Geliebter A. Neuroimaging and obesity: Current knowledge and future directions. Obes Rev. 2012;13(1):43–56. doi: 10.1111/j.1467-789X.2011.00927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Irlbacher K, Kraft A, Kehrer S, Brandt SA. Mechanisms and neuronal networks involved in reactive and proactive cognitive control of interference in working memory. Neurosci Biobehav Rev. 2014;46(Pt 1):58–70. doi: 10.1016/j.neubiorev.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 53.Bari A, Robbins TW. Inhibition and impulsivity: Behavioral and neural basis of response control. Prog Neurobiol. 2013;108:44–79. doi: 10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 54.Prevosto V, Sommer MA. Cognitive control of movement via the cerebellar-recipient thalamus. Front Syst Neurosci. 2013;7:56. doi: 10.3389/fnsys.2013.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Corbetta M, Kincade JM, Shulman GL. Neural systems for visual orienting and their relationships to spatial working memory. J Cogn Neurosci. 2002;14(3):508–23. doi: 10.1162/089892902317362029. [DOI] [PubMed] [Google Scholar]
- 56.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–15. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 57.McFadden KL, Cornier MA, Melanson EL, Bechtell JL, Tregellas JR. Effects of exercise on resting-state default mode and salience network activity in overweight/obese adults. Neuroreport. 2013;24(15):866–71. doi: 10.1097/WNR.0000000000000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Polania R, Moisa M, Opitz A, Grueschow M, Ruff CC. The precision of value-based choices depends causally on fronto-parietal phase coupling. Nat Commun. 2015;6:8090. doi: 10.1038/ncomms9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Deacon CF, Ahren B. Physiology of incretins in health and disease. Rev Diabet Stud. 2011;8(3):293–306. doi: 10.1900/RDS.2011.8.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Deacon CF. What do we know about the secretion and degradation of incretin hormones? Regul Pept. 2005;128(2):117–24. doi: 10.1016/j.regpep.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 61.El-Ouaghlidi A, Rehring E, Holst JJ, Schweizer A, Foley J, Holmes D, et al. The dipeptidyl peptidase 4 inhibitor vildagliptin does not accentuate glibenclamide-induced hypoglycemia but reduces glucose-induced glucagon-like peptide 1 and gastric inhibitory polypeptide secretion. J Clin Endocrinol Metab. 2007;92(11):4165–71. doi: 10.1210/jc.2006-1932. [DOI] [PubMed] [Google Scholar]
- 62.Woerle HJ, Carneiro L, Derani A, Goke B, Schirra J. The role of endogenous incretin secretion as amplifier of glucose-stimulated insulin secretion in healthy subjects and patients with type 2 diabetes. Diabetes. 2012;61(9):2349–58. doi: 10.2337/db11-1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mentis N, Vardarli I, Kothe LD, Holst JJ, Deacon CF, Theodorakis M, et al. Gip does not potentiate the antidiabetic effects of glp-1 in hyperglycemic patients with type 2 diabetes. Diabetes. 2011;60(4):1270–6. doi: 10.2337/db10-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lund A, Vilsboll T, Bagger JI, Holst JJ, Knop FK. The separate and combined impact of the intestinal hormones, gip, glp-1, and glp-2, on glucagon secretion in type 2 diabetes. Am J Physiol Endocrinol Metab. 2011;300(6):E1038–46. doi: 10.1152/ajpendo.00665.2010. [DOI] [PubMed] [Google Scholar]
- 65.Salehi M, Aulinger B, Prigeon RL, D’Alessio DA. Effect of endogenous glp-1 on insulin secretion in type 2 diabetes. Diabetes. 2010;59(6):1330–7. doi: 10.2337/db09-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meier JJ, Kemmeries G, Holst JJ, Nauck MA. Erythromycin antagonizes the deceleration of gastric emptying by glucagon-like peptide 1 and unmasks its insulinotropic effect in healthy subjects. Diabetes. 2005;54(7):2212–8. doi: 10.2337/diabetes.54.7.2212. [DOI] [PubMed] [Google Scholar]
- 67.Kielgast U, Holst JJ, Madsbad S. Antidiabetic actions of endogenous and exogenous glp-1 in type 1 diabetic patients with and without residual beta-cell function. Diabetes. 2011;60(5):1599–607. doi: 10.2337/db10-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Flint A, Raben A, Ersboll AK, Holst JJ, Astrup A. The effect of physiological levels of glucagon-like peptide-1 on appetite, gastric emptying, energy and substrate metabolism in obesity. Int J Obes Relat Metab Disord. 2001;25(6):781–92. doi: 10.1038/sj.ijo.0801627. [DOI] [PubMed] [Google Scholar]
- 69.Toft-Nielsen M, Madsbad S, Holst JJ. Exaggerated secretion of glucagon-like peptide-1 (glp-1) could cause reactive hypoglycaemia. Diabetologia. 1998;41(10):1180–6. doi: 10.1007/s001250051049. [DOI] [PubMed] [Google Scholar]
- 70.Rolls ET. Taste, olfactory, and food reward value processing in the brain. Prog Neurobiol. 2015;127–128:64–90. doi: 10.1016/j.pneurobio.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 71.Rolls ET. Functions of the anterior insula in taste, autonomic, and related functions. Brain Cogn. 2015 doi: 10.1016/j.bandc.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 72.Val-Laillet D, Aarts E, Weber B, Ferrari M, Quaresima V, Stoeckel LE, et al. Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity. Neuroimage Clin. 2015;8:1–31. doi: 10.1016/j.nicl.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang L, Yu H, Hu J, Theeuwes J, Gong X, Xiang Y, et al. Reward breaks through center-surround inhibition via anterior insula. Hum Brain Mapp. 2015 doi: 10.1002/hbm.23004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wijngaarden MA, Veer IM, Rombouts SA, van Buchem MA, Willems van Dijk K, Pijl H, et al. Obesity is marked by distinct functional connectivity in brain networks involved in food reward and salience. Behav Brain Res. 2015;287:127–34. doi: 10.1016/j.bbr.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 75.Nyberg J, Anderson MF, Meister B, Alborn AM, Strom AK, Brederlau A, et al. Glucose-dependent insulinotropic polypeptide is expressed in adult hippocampus and induces progenitor cell proliferation. J Neurosci. 2005;25(7):1816–25. doi: 10.1523/JNEUROSCI.4920-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nyberg J, Jacobsson C, Anderson MF, Eriksson PS. Immunohistochemical distribution of glucose-dependent insulinotropic polypeptide in the adult rat brain. J Neurosci Res. 2007;85(10):2099–119. doi: 10.1002/jnr.21349. [DOI] [PubMed] [Google Scholar]
- 77.Kramer CK, Zinman B, Choi H, Connelly PW, Retnakaran R. The impact of chronic liraglutide therapy on glucagon secretion in type 2 diabetes: Insight from the libra trial. J Clin Endocrinol Metab. 2015;100(10):3702–9. doi: 10.1210/jc.2015-2725. [DOI] [PubMed] [Google Scholar]