Abstract
Background:
Cigarettes and other forms of tobacco contain the addictive drug nicotine. Other components, either naturally occurring in tobacco or additives that are intentionally added during the manufacturing process, may add to the addictiveness of tobacco products. As such, these components can make cigarette smokers more easily and heavily dependent.
Efforts to regulate tobacco product dependence are emerging globally. Additives that increase tobacco dependence will be prohibited under the new European Tobacco Product Directive.
Objective:
This article provides guidelines and recommendations for developing a regulatory strategy for assessment of increase in tobacco dependence due to additives. Relevant scientific literature is summarized and criteria and experimental studies that can define increased dependence of tobacco products are described.
Conclusions:
Natural tobacco smoke is a very complex matrix of components, therefore analysis of the contribution of an additive or a combination of additives to the level of dependence on this product is challenging. We propose to combine different type of studies analyzing overall tobacco product dependence potential and the functioning of additives in relation to nicotine. By using a combination of techniques, changes associated with nicotine dependence such as behavioral, physiological, and neurochemical alterations can be examined to provide sufficient information.
Research needs and knowledge gaps will be discussed and recommendations will be made to translate current knowledge into legislation. As such, this article aids in implementation of the Tobacco Product Directive, as well as help enable regulators and researchers worldwide to develop standards to reduce dependence on tobacco products.
Implications:
This article provides an overall view on how to assess tobacco product constituents for their potential contribution to use and dependence. It provides guidelines that help enable regulators worldwide to develop standards to reduce dependence on tobacco products and guide researches to set research priorities on this topic.
Introduction
Tobacco use is wide spread and the major preventable cause of cancer and respiratory diseases worldwide.1 The majority of tobacco related diseases is attributable to combustible products in particular cigarettes.2 For tobacco smoking the rate of initiation and progression to dependence are high. This can be explained by accessibility to, and availability of, the product and factors influencing the product appeal such as social influences and marketing. Tobacco comprises of many substances of which nicotine is the most characteristic and addictive component.3 Tobacco is usually not present in tobacco products in unprocessed form; chemical substances other than naturally occurring in tobacco are added to the eventual tobacco products to make them more palatable and attractive to consumers. These substances that are intentionally added to tobacco products during the manufacturing process are referred to as additives. Additives may increase the addictiveness, attractiveness, and toxicity of tobacco products and are therefore starting point for regulation.
The term “addiction” is commonly referred to, but the term “dependence” is used globally as the technical term for substance use disorder.4,5 Therefore we will refer to this term throughout this article.
Currently, efforts to regulate tobacco product dependence are emerging. The WHO FCTC includes a strategy for regulating tobacco products to reduce their attractiveness, but does not yet provide any guidance for reducing either the dependence potential or toxicity of tobacco products.6 In the United States, efforts are ongoing to evaluate possibilities to reduce nicotine content of cigarettes to nondependence levels and to restrict sales of menthol cigarettes.7,8 In Brazil, some additives suspected to influence the action of nicotine were banned following flavor regulation.9
In 2014, the European Union (EU) has set up a new Tobacco Product Directive (TPD) to regulate tobacco products.10 One of the aspects by which the EU aims to regulate tobacco products is by influencing the dependence potential. The TPD prohibits tobacco products with increased dependence potential, thereby specifically focusing on the role of additives or a combination of additives in increasing the dependence potential of cigarettes and roll-your-own tobacco.
To assess the effects of additives on the level of dependence potential of a product, the TPD states that “Member States shall require manufacturers and importers of cigarettes and roll-your-own tobacco containing an additive that is included in the priority list,11 to carry out comprehensive studies, which shall examine for each additive whether it: contributes to the addictiveness of the products concerned, and whether this has the effect of increasing the addictiveness of any of the products concerned to a significant or measurable degree.” This requires standardized methods that allow for the assessment of increased dependence potential of tobacco products, due to additives.
The aim of this article is to define scientific criteria and to describe experimental methods to assess the effect of (individual) additives on the (increase in) dependence potential of tobacco products. As such, this article aids in implementation of the TPD, as well as in providing a guideline for global tobacco regulation and research priorities on this topic. This article is part of a series of three articles on criteria to assess the three key dimensions for tobacco product control: dependence potential, toxicity, and attractiveness.12,13
In chapter 2, the TPD regulations regarding tobacco product dependence potential and its definition will be described. Natural tobacco contains several components with dependence potential of which nicotine is one of them. The mode of action of these components will be discussed in chapter 3. The different paragraphs of chapter 4 represent the criteria that can be used to determine if a tobacco additive contributes to the overall dependence potential of the tobacco product. In these paragraphs the current state of knowledge on methods used to assess (increased) dependence potential of tobacco will be reviewed.
To conclude, advantages and drawbacks of current methods will be evaluated, knowledge gaps will be discussed and recommendation will be made.
The EU Tobacco Product Directive on Regulating Addictiveness
In 2001 the EU set up the TPD (2001/37/EC) with the aim of developing internationally agreed rules and standards on tobacco product presentation, production and manufacture. With regard to the dependence promoting properties of tobacco products the TPD called for example for the establishment of a common list of tobacco ingredients “which takes into account inter alia their addictiveness.”14 In particular “member states may provide for the prohibition of the use of ingredients which have the effect of increasing the addictive properties of tobacco products.”14
In 2010 the Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) provided scientific advice to the European Commission on the role of tobacco ingredients in the dependence potential and attractiveness of tobacco products.15
The SCENIHR recommendations were taken along to update the TPD (2014/40/EU). The overall objective of the revision is to further protect human health and improve the functioning of the internal market. This new TPD strengthens existing rules and introduces novel rules for certain tobacco-related products.10
For the purpose of the TPD, dependence potential was defined as: “the pharmacological potential of a substance to cause addiction, a state which affects an individual’s ability to control behavior typically by instilling a reward or a relief from withdrawal symptoms, or both.”10
The revised TPD states that “additives that increase addictiveness should be prohibited” whereby it is mentioned that this includes “additives that facilitate inhalation or nicotine uptake.” An increase in dependence potential of a product is defined as: “to a significant or measurable degree.”
In order for the member states to assess increased dependence potential and carry out their regulatory tasks, information on the ingredients and emissions from tobacco products is needed. Therefore “…the existing reporting obligations for ingredients and emissions should be reinforced. Particular in respect of adopting and adapting maximum yields for emissions and their measurement methods, setting maximum levels for additives that increase addictiveness (and toxicity or attractiveness).” Also “additives necessary for the manufacture of tobacco products, for example sugar to replace sugar that is lost during the curing process” should not result in an increase of dependence potential of the product.10
Addictive Components in Natural Tobacco
In the following paragraphs, components of natural tobacco causing dependence will be described. Knowledge on underlying mechanisms of tobacco dependence is necessary to understand how tobacco components and/or tobacco additives may increase dependence on products as cigarettes and roll-your-own tobacco.
Nicotine is the Major Addictive Component of Natural Tobacco
Multiple studies in both human and animal subjects have shown the major component of natural tobacco, nicotine, to be the main component responsible for the dependence on tobacco products.16 Nicotine dependence does not happen after using tobacco once or twice; it develops over time. The first symptoms of nicotine dependence can appear within days to weeks after tobacco smoking started, often before the onset of smoking daily.17 Initially the stimulant effects predominate followed by a domination of reward effects.
The amount of nicotine absorbed from the smoke into the lungs is high. Upon adsorption into the lungs nicotine enters the blood and reaches the brain in about 10 seconds after inhalation.3 Once in the brain, nicotine activates and desensitizes nicotinic acetylcholine receptors (nAChRs).18 Activation of the receptors results in activation of the mesolimbic pathway and release of several neurotransmitters such as dopamine (DA). The mesolimbic pathway is associated with feelings of compulsion, pleasure, euphoria, and reward that plays a critical role in the reinforcement for continued tobacco use.19 Nicotine administration increases DA activity in the nucleus accumbens (NAc) and other limbic structures by direct stimulation of nicotinic acetylcholine receptors subunits within the ventral tegmental area.20 The activity of DA neurons in the ventral tegmental area is additionally influenced by the activity of multiple neurotransmitter pathways. Release of neurotransmitters as DA, glutamate, and GABA is particularly important in the development of nicotine dependence. The release is mediated by activation of receptors such as glutamate and serotonergic receptors and the endocannabinoid system.21,22 The systems are thereby all involved in the rewarding/reinforcing effects of nicotine. While a reward effect is exerted in the limbic system a stimulating effect is exerted mainly in the cortex via the locus ceruleus. The availability of mesolimbic DA can be increased by inhibition of monoamine oxidases A and B (MAO-A and MAO-B).23
Nicotine or tobacco withdrawal is a separately diagnosable disorder, from “dependence” or “substance use disorder” according to the International Classification of Diseases4 and the American Psychiatric Association,24 respectively. Withdrawal is also used by pharmacologists as the primary indicator that physiological dependence and tolerance have developed. Withdrawal and physiological dependence may occur in the absence of dependence (eg, as in the case neonates delivered from dependent cigarette smokers who show signs of withdrawal but would not meet any other criteria for dependence); conversely many people meeting criteria for dependence or “substance use disorder”24 do not exhibit withdrawal symptoms whether the substance is tobacco, opiods, or stimulants.25
Nicotine withdrawal can be an important manifestation of dependence and motivates relapse. Withdrawal is associated with downregulation of the production of DA and other stimulatory neurotransmitters as the brain attempts to compensate for artificial stimulation. These somatic manifestations of withdrawal symptoms are mediated by the different nAChR expressed in the medial habenula brain area.26–28
Other Addictive Components in Natural Tobacco
Accumulating evidence suggests non-nicotine tobacco components to be involved in determining the dependent nature of natural tobacco.29–31 A self-administration study in rats shows roll-your-tobacco to have a higher dependence potential than cigarettes irrespective of nicotine levels.32
Minor Alkaloids
Minor alkaloids present in the tobacco leaf share a chemical structure closely related to nicotine. In most tobacco strains, nornicotine and anatabine are the most abundant of minor alkaloids, followed by anabasine. Nornicotine is a minor metabolite of nicotine and its effects are enantioselective.33 Testing of these components for nicotine-induced locomotor activity in rats shows anatabine, cotinine and myosmine to increase these effects suggesting they can increase the motivation for nicotine.34
Due to their nicotine-like structure, the minor alkaloids are likely to act in similar way as nicotine does. Components with affinity for the nAChRs have either synergistic effects with nicotine or reinforcing effects of their own.
Nornicotine is indeed able to act as an agonist on nicotine acetylcholine receptors, but with about tenfold lower potency.35,36 Self-administration studies show nornicotine to have reinforcing effects while anabasine and anatabine do not. This indicates it has dependence potential.37
Anabasine was also shown to have affinity for nAChRs, induce desensitization of nAChRs and evoke DA release in the striatum.38,39 Lower doses of anabasine on the level typically found in cigarette smoke likely significantly contribute to the reinforcing effects of tobacco.40
Anatabine dose-dependently reduces nicotine self-administration in rats and rhesus monkeys suggesting that anatabine act as an agonist.41 Monkeys did not self-administer anatabine above control (saline) levels indicating it does not have reinforcing effects nicotine. Recently, using in vitro binding studies it has been shown that the effects of anatabine on reinforcement and craving may be mediated through activity at nAChR subtypes.42
The minor tobacco alkaloid beta-nicotyrine have been reported to inhibit the major enzyme involved in nicotine metabolism.43
Natural Sugars
Sugars are natural tobacco components, and are frequently added to tobacco during the manufacturing process as well. Sugars are present in significant amounts in Virginia tobacco or are added in high quantities to Burley based tobacco products. Added sugars are typically among the main ingredients in major commercial cigarette brands and likely contribute to smoking pleasure and dependence potential by several mechanisms including making the smoke more palatable and contributing to the formation of numerous aldehydes upon combustion. Aldehydes are sometimes also added to natural tobacco and are reported to enhance nicotine self-administration in young, but not in adult rats at low doses.44,45 The observed reinforcing effects of acetaldehyde seem to be caused by condensation products of acetaldehyde and with small molecules such as amino acids (eg, tryptophan and tryptamine), which are present in tobacco as well as with other molecules present throughout the body. The beta carboline, harman, is an inhibitor of MAO and is formed from the reaction of acetaldehyde with tryptophan and tryptamine.46,47 MAO-A inhibition dramatically increases the motivation to self-administer nicotine in rats.48,49 In a recent study examining the effect of beta-carbolines on nicotine self-administration it was shown that pretreatment with harmane or norharmane did not show significant differences.40 Another study however shows reinforcing and neural activating effects of norharmane alone and in combination with nicotine.50 A recent study suggests that a combination of cigarette smoke constituents including acetaldehyde, beta carbolines, and minor alkaloids does not alter the reinforcing effects on nicotine. However cigarette smoke constituents that inhibit MAO may increase the effects of nicotine.51
Naturally Occurring MAO Inhibitors
Brain analysis of tobacco smokers showed a significant reduction in MAO levels relative to nonsmokers or former smokers.52 MAO inhibition is associated with enhanced DA activity leading to increased reinforcement behaviors. The release in the bloodstream of MAO inhibitors (MAOIs) along with nicotine upon inhalation of tobacco smoke is thought to be responsible for most of its dependence properties.53 Examples of MAO inhibitors isolated from tobacco leaves and present in tobacco smoke are 2,3,6-trimethyl-benzoquinone and 2-naphthylamine.54 Preliminary findings suggest that replacement of the effects of MAO inhibitors contained in cigarette smoke may enhance quit rates during smoking cessation.55
Drugs taken for mental disorders often exert MAO inhibitory effects. Suggesting the higher prevalence rates for tobacco dependence among individuals with anxiety disorders, depression, or schizophrenia56 could be related to shared co-occurring mechanisms involving nicotine and MAO inhibition in the brain. Evidence for increased dependence related to medication intake in these individuals is however lacking.
Tobacco additives exerting MAO inhibitory effects are described in the section “Exerting additive effects on DA signaling by inhibition of its degradation”.
Additives and Tobacco Addiction
In this chapter experimental studies are described that are performed for some additives to assess dependence potential. Overall assessment of increase in dependence potential can be performed using both human and animal studies. In addition to psychological state, metabolites, brain activity, and DA turnover can be analyzed.
Assessment of the Overall Increase in Tobacco Product Dependence Potential
Comparing the product with and without the additive, allows for assessing the contribution of the additive to the overall dependence potential of the tobacco product. By setting up panels of human subjects psychological analyses can be performed. When a significant difference is scored this suggests the possibility that the additive leads to an increase or decrease in dependence compared to the product without the additive.
Several measures can be applied to assess dependence. Recently, revisions for DSM-V were proposed in order to increase the predictive value of these criteria for tobacco dependence assessment.5,24 Dependence characteristic for nicotine and smoke(less) tobacco can be self-assessed using the Fagerström Test for Nicotine Dependence (FTND), a 12-item cigarette dependence scale and the cigarette withdrawal scale (CWS-21).57–59 Twelve-item cigarette dependence scale covers the main definitions of dependence: compulsion, withdrawal symptoms, loss of control, neglect of other activities, time allocation, and persistence despite harm. Tolerance is however not measured using 12-item cigarette dependence scale. The FTND can assess the degree or severity of tobacco dependence using a scale indicative for the level of dependence. The higher the score the more dependent on nicotine the individual performing the test is. The FTND does measure physical dependence and tolerance by assessing control over use and the urgency for use. The test does not assess other salient dimensions of dependence such as craving, compulsion, or withdrawal and several measured items are more difficult to apply to moderate smokers.60 The cigarette withdrawal scale (CWS-21) is a 21-item multidimensional self-administered scale that measures withdrawal symptoms and predicts relapse to smoking.58
Indicators of nicotine dependence were assessed in menthol and non-menthol cigarette smokers using the FTND. Differences were observed in time to first cigarette of the day (TTF) suggesting greater urgency to smoke. Other indicators such as amount of cigarettes smoked on a day (CPD) did not differ between the two groups of smokers.61,62
An important limitation of the foregoing is that these are diagnostic instruments for assessing dependence in people and not necessarily the dependence potential of the given substance or product type. Those methods are discussed below and are important complements to the foregoing.
Methods of assessing dependence potential of products and product variants in humans what is commonly referred to as “dependence potential” assessment methods internationally is more typically referred to as “abuse potential” or “abuse liability” assessment by the United States Food and Drug Administration (US FDA). Accepted methods used in participant studies of dependence potential include measuring responses using visual analogue scales (VAS) for drug liking and response to a drug. Other measures include assessment of likelihood to take the drugs again. Human laboratory studies can be used to detect signals suggestive of dependence potential, however the ability of these studies to make distinctions of relative dependence potential is limited.25,63,64
Tobacco product dependence potential cannot only be tested in human subjects but also using laboratory animals. Current animal models for tobacco product dependence are based on assessing nicotine dependence and not dependence of tobacco products as a whole. These models aim to deliver nicotine independently from cigarette smoke, with similar pharmacokinetics of inhaled nicotine. A few studies do propose animal models for tobacco smoke exposure that could potentially be used to evaluate tobacco addiction.62,65
The experimental animal models investigating nicotine dependence are mainly models of nicotine reward and reinforcement. Current tests to analyze dependence potential can monitor self-administration, speed of acquisition, conditioned rewarding effects, and drug discrimination.38,66,67 Severity of withdrawal can also be measured.68
Self-administration of nicotine has been repeatedly demonstrated in laboratory animal models. This effect is observed in a reliable manner using an intravenous self-administration paradigm despite the fact that nicotine itself is regarded as a relatively weak reinforcer.69
A recent animal study shows the sensory properties of menthol can serve as a conditioned reinforcer for nicotine.70 Results of this study also suggest that during withdrawal smokers of menthol cigarettes are likely to experience a stronger craving for nicotine, which could result in lower smoking cessation rates.71
Beside behavioral responses also neurobiological effects can be analyzed using animal models (see section “Exerting additive effects on nicotine dependent activation of mesolimbic pathways”).
Acting on Nicotine Duration and Concentration in Blood Circulation
Optimizing dosing characteristics including speed and delivery of nicotine is, as well as for most other dependence producing drugs, an important determinant of its dependence potential.72 The concentration as well as the time nicotine is present in the blood circulation of cigarette smokers determines the effects of the compound in the body. Concentration and duration of nicotine can be increased by improving the uptake and bioavailability of nicotine as discussed in the following paragraphs.
Exerting an Increase Nicotine Uptake
It has been shown that inhalation results in a rapid brain increase of nicotine in the brain thereby contributing to nicotine dependence in smokers. The rate of rise of nicotine concentration in the brain can be measured using positron emission tomography scans of brain regions.73,74
Inhalation can be facilitated by certain additives leading to deeper and more frequent inhalation by the cigarette smoker resulting in an increase in lung exposure and nicotine uptake. Additives can achieve this by enhancing sensory properties such as cooling effects or by having local anesthetic and bronchodilating properties. Menthol, theobromine and eucalyptol are described to have bronchodilator and antitussive (relieves coughs) effects.75 This allows for more air flow through the lungs and inhalation of larger volumes of smoke resulting in an increased bioavailability of nicotine and thereby enhancing its dependence promoting properties.
Menthol is a commonly used additive by activating cold sensitive ion channels, such as TRPM8, thereby inducing cooling effects in a dose dependent manner.76 The tobacco additive eucalyptol can also activate similar ion channels involved in sensory sensation.77 Menthol has both a cooling effect on mucosal surfaces and a local anesthetic effect.78
The efficiency of nicotine uptake in the blood stream via the lung is difficult to measure. Engineered 3D lung tissue constructs and mathematical computer models can be used to provide predictive information on lung uptake and particle deposition.79,80
Exerting an Increase Nicotine Bioavailability
Nicotine bioavailability is defined by an optimal rate of adsorption and distribution from the lungs into the bloodstream.
Upon uptake in the lungs the bioavailability of nicotine in the body is determined by properties such as its hydrophobicity and solubility. It has been proposed that the use of alkalizing compounds (such as ammonia) as tobacco additive increases the absorption of nicotine in the lungs. Biomarker analysis of nicotine in blood samples from smokers of cigarettes with different ammonia yields was performed to evaluate the effects on nicotine bioavailability. Different ammonia yields in cigarettes did not increase the rate or amount of nicotine absorption from the lungs to the arterial blood circulation.81,82 From these studies it is not excluded that other ingredients than ammonium salts influence nicotine adsorption in a similar way.
Nicotine is metabolized in the liver by cytochrome P450 (CYP) enzymes. The main enzymes involved in the metabolic pathway of nicotine are CYP2A6 and CYP2B6.35 Inhibition of nicotine metabolism enhances its bioavailability and alters the behavioral effects in mice.68,83 Additives which can modulate the activity of metabolic pathways are therefore likely to effect the dependence potential of nicotine.
The effectiveness of an additive in inhibiting nicotine metabolism is expressed as relative CYP inhibitor ratio and is represented in a 50% inhibitory concentration (IC50 value).84 This inhibitory concentration of human and mouse CYP2A can be tested in an in vitro assay using recombinant enzyme.85 Inhibition of CYP can also be analyzed taking human liver microsomal preparations as enzyme source.
An example of mild to weak inhibitor of CYP2A6 are several lactones added to tobacco. The high IC50 of lactones indicates their effectiveness in inhibiting nicotine metabolism.86 The concentration of lactones added to tobacco products is much lower making its contribution to inhibit nicotine metabolism less likely. However the inhibitory effect of these compounds on CYP2A6, can be relatively weak in isolation, but might be greater when the chemicals act in combination.86
By using an in vitro liver CYP activity assay derivatives of phenylethylamine and benzaldehyde were confirmed to be inhibitors of the metabolic enzyme mouse CYP2A5 (human CYP2A6).85 Benzaldehydes are present as a natural tobacco component, but are also added to tobacco as flavorant.
When an additive inhibits CYP2A6 activity it doesn’t necessarily mean exposure to nicotine will increase. Flavonoids or furanocoumarins have been shown to inhibit nicotine metabolism, but at the same time increases renal clearance of nicotine and cotinine resulting in no significant effect on overall exposure.87
A study using smokers whereby nicotine and its metabolite cotinine were analyzed after smoking cigarette with or without menthol showed inhibition of nicotine metabolism upon mentholated cigarette smoking.88,89
Several studies showed menthol to be a weak inhibitor of human CYP2A6.43
Acting on the Nicotine Mode of Action in the Brain
Additives can exert effects that influence the ability of nicotine to activate mesolimbic pathways and/or influence DA signaling by inhibition of its degradation
Exerting Additive Effects on Nicotine Dependent Activation of Mesolimbic Pathways
Activation of the mesolimbic DA system is analyzed in various ways. Neuroimaging techniques are used to study in vivo activity of mesolimbic brain areas upon nicotine use. Neuronal activity in these regions can be measured as well as activation of nACh receptors, neurotransmitter release and transcriptional activation of specific mRNAs.
To provide brain images indicative of nicotine dependence positron emission tomography, functional magnetic resonance imaging (fMRI) and single-photon emission computed tomography (SPECT) techniques are used.90
Using neuroimaging studies nicotine, MAO or nAChR can be labeled and traced to demonstrate nicotine occupancy at nAChRs, nAChR availability and upregulation of nAChRs induced by chronic smoking.90–92 A recent study used positron emission tomography scan to provide evidence that in brains of female menthol cigarette smokers nicotine accumulated fast thereby contributing to dependence. However a role of menthol in enhancing brain nicotine accumulation was not supported by this study.93 Neuroimaging studies using labeled nAChR subunits showed an upregulation of these receptors in the brain of menthol smokers. Indicating a higher nicotine exposure in smokers of menthol cigarettes, although other mechanisms for menthol influencing receptor density are possible.94 Analysis of nicotinic acetylcholine receptor activity in vitro shows that menthol inhibits nAChR subtypes in a noncompetitive manner.95,96 Activity of neurons in the mesolimbic DA brain area is not only measured by nACh receptor activation but also by measuring the result of this receptor activation, a change in the release or turnover of DA. DA release and turnover can be measured either ex vivo or in vivo via isolation of specific brain tissue or microdialysis techniques.30,39,97,98
A study in mice showed up-regulation of nAChR subtypes in various brain regions upon exposure to nicotine and menthol using western blots. A significant increase in nicotine plasma levels was observed, which was accompanied by an increase of withdrawal intensity.99
Exerting Additive Effects on DA Signaling by Inhibition of its Degradation
Additives may influence the dependence potential of nicotine by interacting with the neural responses to the drug. For example MAO inhibitors that are not leading to dependence on their own slow the breakdown of monoamines such as DA thereby affecting the overall motivational impact of nicotine.
Beta-carbolines as harman and norharmane are MAO inhibitors present in tobacco products. As mentioned in the section “Natural sugars,” sugars are a source of these alkaloids and are present in natural tobacco but are also frequently added during the manufacturing process. Furthermore coffee, a tobacco additive, has been shown to be a source of beta-carbolines and other MAO inhibitors.100 Another example of additives showing MAO inhibition are synthesized coumarin derivatives. Coumarin has been used as an aroma enhancer in pipe tobaccos.101
Inhibition of the enzymatic activity of MAO can be measured in vitro using peroxidase-linked spectrophotometric assay. Enzymes can be isolated from rat liver microsomes or recombinantly generated. Using recombinant human MAO-A and MAO-B, IC50 values for enzyme inhibition by can be experimentally determined.102 In vivo MAO activity can be analyzed using a radiotracer, which can irreversibly bind MAO followed by a positron emission tomography scan of the brain.52
How to Translate Test Results Into Legislation?
An overview of additives that could influence tobacco product dependence potential is provided in Table 1. Several studies assessed the potential of menthol to enhance nicotine dependence and clarified the mechanisms behind this. The contribution of menthol to ease of inhalation, absorbability, and sensory cueing effects may all contribute to increasing the dependence potential of cigarettes.103 Therefore the information provided on menthol may provide a model example of how to assess the effect of other additives on tobacco dependence. Although multiple studies have been performed on the impact of menthol, evidence for other additives increasing tobacco dependence is limited. Menthol is intentially added, but often additives are also present in natural tobacco such as sugars, benzaldehyde and acetaldehyde making it more challenging to study effects of their addition. Additives can exert additive effects on nicotine bioavailability, duration, and concentration in the blood circulation or nicotine dependent activation of mesolimbic pathways in the brain.
Table 1.
Additives Described to Have Properties That Could Contribute to Dependence of Tobacco Products
| Tobacco additive | Chemical structure | Source | Main properties |
|---|---|---|---|
| Menthol |
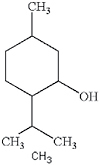
|
Additive, (pepper)mint | Sensory response (cooling), effects on central nervous system, local anaesthetic, nicotine metabolism, relieve throat irritation, flavorant |
| Eucalyptol |
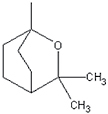
|
Additive, eucalyptus | Sensory reponse (cooling), Bronchodilating properties, flavorant |
| Theobromine |
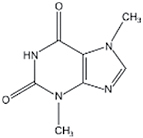
|
Cocoa | Bronchodilating properties, vasodilator |
| Ammonia |
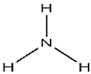
|
Additive, ammonium salt | Alkalizing (increased lung absorption), neurotoxin |
| Lactones |
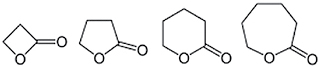
|
Additive | Nicotine metabolism |
| Benzaldehyde |
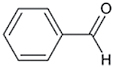
|
Additive | Nicotine metabolism, flavorant |
| Acetaldehyde |
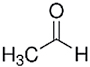
|
Additive and/or sugars | Flavorant, probable human carcinogen, preservative |
| (Nor)Harman |
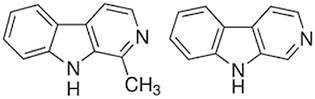
|
Sugars, coffee | MAO inhibition |
| Coumarin |
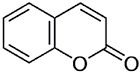
|
Additive, cinnamon | MAO inhibiting properties, flavorant |
MAO = monoamine oxidase. Chemical structure, source and main properties are indicated.
Guidelines to assess the impact of tobacco product contents on dependence potential could be similar to those already established for testing the dependence potential of pharmaceutical products. Special challenges include product complexity and the diverse range of tobacco products.104,105 For example, the US FDA has issued guidance that cover dependence potential assessment for a range of different substances, formulations, and product types in which factors such as additives and product design features may act to either promote or deter dependence potential.63,72
To accurately assess tobacco dependence potential for regulatory purposes, it is necessary to use multiple evaluation methods whereby several factors associated with tobacco addiction are analyzed. Combinations of techniques examining behavioral, physiological and neurochemical changes occurring in specific brain sites with nicotine dependence will provide sufficient information. Correlations between responses and convergence of studies will lead to evidence based conclusions.
Since natural tobacco smoke is a very complex matrix of components and differs significantly among different brands it is challenging to analyze the contribution of an additive or a combination of additives to the level of dependence on this product. We propose to combine studies analyzing overall tobacco product dependence potential and the functioning of additives in relation to nicotine. Proposed analysis would involve a combination of in vivo (in awake or anesthetized animals), ex vivo (in brain slices or cell cultures), and/or in vitro studies as well as studies using human subjects whereby exposure to mainstream smoke can be measured.
Simplified experiments in animal models analyzing the mode of action of additives on nicotine addiction without making use of the tobacco matrix are well developed. The self-administration paradigm has been widely accepted as a reliable animal model with high predictive value for the dependence potential of a drug and can be used to support findings observed in humans.
Animal models allow to control for factors that can affect study outcome such as environmental factors, genetic background and prior drug exposure. In human studies gender, age and inter individual difference in genetic susceptibility to drug dependence might have an impact on receptor response or nicotine metabolism.106–108 Persons with a genetic basis for slow metabolism smoke fewer cigarettes daily than persons with faster metabolism, show a lower level of dependence and experience less severe withdrawal symptoms.109
The results of psychological/behavioral tests or more laborious neuroimaging studies analyzing different aspects or stages of nicotine dependence in vivo can be represented as a measureable degree of dependence caused by an additive. However, several different tests exist measuring slightly different dependence parameters that are given different levels of importance. For regulatory purposes, consensus needs to be established on the (combination of) tests that are preferred.
Ex vivo and in vitro studies are less time consuming and cheaper than in vivo studies. They are an important first step in the analysis of the dependence potential of an additive. In in vitro test systems additives can be analyzed either a separate compound or combined with nicotine.
Effects on the activity of enzymes specific for nicotine metabolism (CYP2A6) and DA breakdown (MAO) as well as DA turnover and/or nAChR specific subunit regulations can be measured accurately. These tests allow for establishment of dose response relationships and definition of IC50 and EC50 values.
This type of analysis could give an indication of the concentration at which an additive could have an effect on nicotine dependence potential and is therefore suitable for regulatory purposes. However, one has to keep in mind that these types of tests may have limited relevance to human dependence potential; inhibition potency can be different in vitro versus in vivo or between mouse and human. Dependence potential is determined by multiple factors and should be tested in the tobacco matrix. The effect of separate compounds might be weak, but combined maybe enough to exert an effect.
Research Needs and Knowledge Gaps
Further investigation on the relationship between MAO inhibition and nicotine reward is recommended. MAO inhibiting properties are described for several additives. Sources of MAO inhibitors are sugars but also coffee and coumarin contribute directly or indirectly via their reaction products to such effects. Studies are needed to define the concentration of additives whereby clear MAO inhibition is observed.
Knowledge gaps that can be answered by performing research as indicated on this topic by SCENIHR include a focus on the importance and concentration of different sugars. Several aspects can be analyzed such as efficacy of various sugars to generate aldehydes and the capacity of different tobacco blends to form aldehydes and inhibit MAO. When the sugar level in natural tobacco is determined the concentration of sugars added on top of that can be defined and the relationship with increased dependence potential of tobacco products can be determined.
In general further research is needed to select and standardize cellular- or biochemical tests to analyze in vitro activation of nAChR subunits or MAO or CYP2A5/6 inhibition that can act as predictors for increased nicotine dependence in vivo. Using standardized tests multiple additives can be tested to analyze if they contain properties as MAO or CYP2A5/6 inhibition or nAChR binding.
Most research performed to assess the role of additives in potentiating tobacco dependence is specific for combustible products like cigarettes. This data can therefore not directly be translated to products whereby tobacco is heated or to products that do not contain tobacco but do contain nicotine such as electronic nicotine delivery systems (ENDS). The contribution of other ways of nicotine administration (such as via vaporization) to the dependence potential of the product are not well known. Studies can be performed to assess the effect of different nicotine containing products and inhalation techniques on tobacco or nicotine dependence.
Funding
This work was supported by the Dutch Ministry of Health, Welfare and Sport (Project V/050057).
Declaration of Interests
None declared.
Acknowledgments
SvdN performed literature research and authored the manuscript. AK and RT critically reviewed the manuscript. All authors have given final approval of the manuscript to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- 1. WHO. WHO Report on the Global Tobacco Epidemic: Enforcing Bans on Tobacco Advertising, Promotion and Sponsorship. Geneva: World Health Organization (WHO); 2013. http://apps.who.int/iris/bitstream/10665/85380/1/9789241505871_eng.pdf Accessed January 15, 2016. [Google Scholar]
- 2. U.S. Dept. of Health and Human Services. The Health Consequences of smoking - 50 years of Progress: A report of the surgeon general 2014. www.surgeongeneral.gov/library/reports/50-years-of-progress/ Accessed January 15, 2016.
- 3.U.S. Department of Health and Human Services. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2010. www.ncbi.nlm.nih.gov/books/NBK53018/ Accessed January 15, 2016. [Google Scholar]
- 4. WHO. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva, Switzerland: World Health Organization; 1992. www.who.int/classifications/icd/en/bluebook.pdf Accessed January 15, 2016. [Google Scholar]
- 5. Baker TB, Breslau N, Covey L, Shiffman S. DSM criteria for tobacco use disorder and tobacco withdrawal: a critique and proposed revisions for DSM-5. Addiction. 2012;107(2):263–275. doi:10.1111/j.1360-0443.2011.03657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. FCTC WHO Framework Concention on Tobacco Control. Regulation of the contents of tobacco products and of tobacco product disclosures. Art. 9/10 guidelines for implementation 2013. http://apps.who.int/iris/bitstream/10665/80510/1/9789241505185_eng.pdf Accessed January 15, 2016.
- 7. U.S. Food and Drug Administration FDA. Family smoking prevention and tobacco control act 2009. www.fda.gov/tobaccoproducts/labeling/rulesregulationsguidance/ucm246129.htm#ingredients Accessed January 15, 2016.
- 8. Bolcic-Jankovic D, Biener L. Public opinion about FDA regulation of menthol and nicotine. Tob Control. 2014;24(e4):e241–e245. doi:10.1136/tobaccocontrol-2013-051392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Anvisa Brazilian Health Surveillance Agency. RDC 14/2012. Anvisa resolution that regulates the use of additives in tobacco products 2012. http://portal.anvisa.gov.br/wps/wcm/connect/d50d8f804d44b146bcecfe4031a95fac/Resolucao_RDC_14_Teores_e_Aditivos_16Mar12.pdf?MOD=AJPERES Accessed January 15, 2016.
- 10. EU Tobacco Product Directive TPD. Tobacco product directive 2014/40/EU of the European parliament and of the council 2014. http://ec.europa.eu/health/tobacco/docs/dir_201440_en.pdf Accessed January 15, 2016.
- 11. SCENIHR. Preliminary opinion on additives used in tobacco products (opinion 1). European commission, scientific committee 2015. http://ec.europa.eu/health/scientific_committees/consultations/public_consultations/scenihr_consultation_29_en.htm Accessed January 15, 2016.
- 12.Talhout R, van de Nobelen S, Kienhuis AS. An inventory of methods suitable to assess additive-induced characterising flavours of tobacco products [published online ahead of print December 30, 2015]. Drug Alcohol Depend. doi:10.1016/j.drugalcdep.2015.12.019. [DOI] [PubMed]
- 13.Kienhuis AS, Staal Y, Soeteman-Hernández LG,, van de Nobelen S, Talhout R. A test strategy for the assessment of additive attributed toxicity of tobacco products. 2016. Submitted. [DOI] [PubMed]
- 14. EU Tobacco Product Directive TPD. Tobacco product directive 2001/37/EU of the European parliament and of the council 2001. http://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32001L0037&from=EN Accessed January 15, 2016.
- 15. SCENIHR. Addictiveness and attractiveness of tobacco additives. European commission, scientific committee 2010. http://ec.europa.eu/health/scientific_committees/emerging/docs/scenihr_o_029.pdf Accessed January 15, 2016.
- 16. Le Foll B, Goldberg SR. Nicotine as a typical drug of abuse in experimental animals and humans. Psychopharmacology (Berl). 2006;184(3–4):367–381. doi:10.1007/s00213-005-0155-8. [DOI] [PubMed] [Google Scholar]
- 17. DiFranza JR, Rigotti NA, McNeill AD, et al. Initial symptoms of nicotine dependence in adolescents. Tob Control. 2000;9(3):313–319. doi:10.1136/tc.9.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Papke RL. Merging old and new perspectives on nicotinic acetylcholine receptors. Biochem Pharmacol. 2014;89(1):1–11. doi:10.1016/j.bcp.2014.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dani JA, Ji D, Zhou FM. Synaptic plasticity and nicotine addiction. Neuron. 2001;31(3):349–352. doi:10.1016/S0896-6273(01)00379-8. [DOI] [PubMed] [Google Scholar]
- 20. Nisell M, Nomikos GG, Svensson TH. Infusion of nicotine in the ventral tegmental area or the nucleus accumbens of the rat differentially affects accumbal dopamine release. Pharmacol Toxicol. 1994;75(6):348–352. doi:10.1111/j.1600-0773.1994.tb00373.x. [DOI] [PubMed] [Google Scholar]
- 21. Watkins SS, Koob GF, Markou A. Neural mechanisms underlying nicotine addiction: acute positive reinforcement and withdrawal. Nicotine Tob Res. 2000;2(1):19–37. doi:10.1080/14622200050011277. [DOI] [PubMed] [Google Scholar]
- 22. Kishioka S, Kiguchi N, Kobayashi Y, Saika F. Nicotine effects and the endogenous opioid system. J Pharmacol Sci. 2014;125(2):117–124. doi:10.1254/jphs.14R03CP. [DOI] [PubMed] [Google Scholar]
- 23. Lewis A, Miller JH, Lea RA. Monoamine oxidase and tobacco dependence. Neurotoxicology. 2007;28(1):182–195. doi:10.1016/j.neuro.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 24. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. 5th ed. Arlington, VA: American Psychiatric Association; 2013:571–574. http://dx.doi.org/10.1176/appi.books.9780890425596 Accessed January 15, 2016. [Google Scholar]
- 25. Carter LP, Stitzer ML, Henningfield JE, et al. Abuse liability assessment of tobacco products including potential reduced exposure products. Cancer Epidemiol Biomarkers Prev. 2009;18(12):3241–3262. doi:10.1158/1055-9965.epi-09-0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dani JA, De Biasi M. Mesolimbic dopamine and habenulo-interpeduncular pathways in nicotine withdrawal. Cold Spring Harb Perspect Med. 2013;3(6):1–14. doi:10.1101/cshperspect.a012138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Salas R, Sturm R, Boulter J, De Biasi M. Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice. J Neurosci. 2009;29(10):3014–3018. doi:10.1523/jneurosci.4934-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Piper ME. Withdrawal: expanding a key addiction construct. Nicotine Tob Res. 2015;17(12):1405–1415. doi:10.1093/ntr/ntv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Addicott MA, Froeliger B, Kozink RV, et al. Nicotine and non-nicotine smoking factors differentially modulate craving, withdrawal and cerebral blood flow as measured with arterial spin labeling. Neuropsychopharmacology. 2014;39(12):2750–2759. doi:10.1038/npp.2014.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Khalki H, Navailles S, Piron CL, De Deurwaerdere P. A tobacco extract containing alkaloids induces distinct effects compared to pure nicotine on dopamine release in the rat. Neurosci Lett. 2013;544:85–88. doi:10.1016/j.neulet.2013.03.047. [DOI] [PubMed] [Google Scholar]
- 31. Ambrose V, Miller JH, Dickson SJ, et al. Tobacco particulate matter is more potent than nicotine at upregulating nicotinic receptors on SH-SY5Y cells. Nicotine Tob Res. 2007;9(8):793–799. doi:10.1080/14622200701485117. [DOI] [PubMed] [Google Scholar]
- 32. Brennan KA, Crowther A, Putt F, et al. Tobacco particulate matter self-administration in rats: differential effects of tobacco type. Addict Biol. 2015;20(2):227–235. doi:10.1111/adb.12099. [DOI] [PubMed] [Google Scholar]
- 33. Stairs DJ, Neugebauer NM, Wei X, et al. Effects of nornicotine enantiomers on intravenous S(-)-nicotine self-administration and cardiovascular function in rats. Psychopharmacology (Berl). 2007;190(2):145–155. doi:10.1007/s00213-006-0610-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Clemens KJ, Caille S, Stinus L, Cador M. The addition of five minor tobacco alkaloids increases nicotine-induced hyperactivity, sensitization and intravenous self-administration in rats. Int J Neuropsychopharmacol. 2009;12(10):1355–1366. doi:10.1017/s1461145709000273. [DOI] [PubMed] [Google Scholar]
- 35. Hukkanen J, Jacob P, III, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57(1):79–115. doi:10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- 36. Dwoskin LP, Teng LH, Crooks PA. Nornicotine, a nicotine metabolite and tobacco alkaloid: desensitization of nicotinic receptor-stimulated dopamine release from rat striatum. Eur J Pharmacol. 2001;428(1):69–79. doi:10.1016/S0014-2999(01)01283-3. [DOI] [PubMed] [Google Scholar]
- 37. Caine SB, Collins GT, Thomsen M, et al. Nicotine-like behavioral effects of the minor tobacco alkaloids nornicotine, anabasine, and anatabine in male rodents. Exp Clin Psychopharmacol. 2014;22(1):9–22. doi:10.1037/a0035749. [DOI] [PubMed] [Google Scholar]
- 38. Hoffman AC, Evans SE. Abuse potential of non-nicotine tobacco smoke components: acetaldehyde, nornicotine, cotinine, and anabasine. Nicotine Tob Res. 2013;15(3):622–632. doi:10.1093/ntr/nts192. [DOI] [PubMed] [Google Scholar]
- 39. Lu Y, Marks MJ, Collins AC. Desensitization of nicotinic agonist-induced [3H]gamma-aminobutyric acid release from mouse brain synaptosomes is produced by subactivating concentrations of agonists. J Pharmacol Exp Ther. 1999;291(3):1127–1134. doi:0022-3565/99/2913-1127$03.00/0. [PubMed] [Google Scholar]
- 40. Hall BJ, Wells C, Allenby C, et al. Differential effects of non-nicotine tobacco constituent compounds on nicotine self-administration in rats. Pharmacol Biochem Behav. 2014;120:103–108. doi:10.1016/j.pbb.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mello NK, Fivel PA, Kohut SJ, Caine SB. Anatabine significantly decreases nicotine self-administration. Exp Clin Psychopharmacol. 2014;22(1):1–8. doi:10.1037/a0035409. [DOI] [PubMed] [Google Scholar]
- 42. Lanier RK, Cohen AE, Loescher JL, et al. Effects of anatabine at relevant human nicotinic receptor subtypes in vitro and on craving and C-reactive protein in heavy smokers. Drug Alcohol Depend. 2015;146:e167. doi:10.1016/j.drugalcdep.2014.09.371. [Google Scholar]
- 43. Kramlinger VM, von Weymarn LB, Murphy SE. Inhibition and inactivation of cytochrome P450 2A6 and cytochrome P450 2A13 by menthofuran, beta-nicotyrine and menthol. Chem Biol Interact. 2012;197(2–3):87–92. doi:10.1016/j.cbi.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sershen H, Shearman E, Fallon S, et al. The effects of acetaldehyde on nicotine-induced transmitter levels in young and adult brain areas. Brain Res Bull. 2009;79(6):458–462. doi:10.1016/j.brainresbull.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 45. Talhout R, Opperhuizen A, van Amsterdam JG. Sugars as tobacco ingredient: effects on mainstream smoke composition. Food Chem Toxicol. 2006;44(11):1789–1798. doi:10.1016/j.fct.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 46. Talhout R, Opperhuizen A, van Amsterdam JG. Role of acetaldehyde in tobacco smoke addiction. Eur Neuropsychopharmacol. 2007;17(10):627–636. doi:10.1016/j.euroneuro.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 47. Herraiz T, Chaparro C. Human monoamine oxidase is inhibited by tobacco smoke: beta-carboline alkaloids act as potent and reversible inhibitors. Biochem Biophys Res Commun. 2005;326(2):378–386. doi:10.1016/j.bbrc.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 48. Guillem K, Vouillac C, Azar MR, et al. Monoamine oxidase inhibition dramatically increases the motivation to self-administer nicotine in rats. J Neurosci. 2005;25(38):8593–8600. doi:10.1523/jneurosci.2139-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Guillem K, Vouillac C, Azar MR, et al. Monoamine oxidase A rather than monoamine oxidase B inhibition increases nicotine reinforcement in rats. Eur J Neurosci. 2006;24(12):3532–3540. doi:10.1111/j.1460-9568.2006.05217.x. [DOI] [PubMed] [Google Scholar]
- 50. Arnold MM, Loughlin SE, Belluzzi JD, Leslie FM. Reinforcing and neural activating effects of norharmane, a non-nicotine tobacco constituent, alone and in combination with nicotine. Neuropharmacology. 2014;85c:293–304. doi:10.1016/j.neuropharm.2014.05.035. [DOI] [PubMed] [Google Scholar]
- 51. Smith TT, Schaff MB, Rupprecht LE, et al. Effects of MAO inhibition and a combination of minor alkaloids, beta-carbolines, and acetaldehyde on nicotine self-administration in adult male rats. Drug Alcohol Depend. 2015;155:243–252. doi:10.1016/j.drugalcdep.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fowler JS, Volkow ND, Wang GJ, et al. Inhibition of monoamine oxidase B in the brains of smokers. Nature. 1996;379(6567):733–736. doi:10.1038/379733a0. [DOI] [PubMed] [Google Scholar]
- 53. Villegier AS, Blanc G, Glowinski J, Tassin JP. Transient behavioral sensitization to nicotine becomes long-lasting with monoamine oxidases inhibitors. Pharmacol Biochem Behav. 2003;76(2):267–274. doi:10.1016/S0091-3057(03)00223-5. [DOI] [PubMed] [Google Scholar]
- 54. Fowler JS, Logan J, Wang GJ, Volkow ND. Monoamine oxidase and cigarette smoking. Neurotoxicology. 2003;24(1):75–82. doi:10.1016/S0161-813X(02)00109-2. [DOI] [PubMed] [Google Scholar]
- 55. Rose JE. Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacology (Berl). 2006;184(3–4):274–285. doi:10.1007/s00213-005-0250-x. [DOI] [PubMed] [Google Scholar]
- 56. Ziedonis D, Hitsman B, Beckham JC, et al. Tobacco use and cessation in psychiatric disorders: National Institute of Mental Health report. Nicotine Tob Res. 2008;10(12):1691–1715. doi:10.1080/14622200802443569. [DOI] [PubMed] [Google Scholar]
- 57. Fagerstrom K. Determinants of tobacco use and renaming the FTND to the Fagerstrom Test for Cigarette Dependence. Nicotine Tob Res. 2012;14(1):75–78. doi:10.1093/ntr/ntr137. [DOI] [PubMed] [Google Scholar]
- 58. Etter JF. A self-administered questionnaire to measure cigarette withdrawal symptoms: the Cigarette Withdrawal Scale. Nicotine Tob Res. 2005;7(1):47–57. doi:10.1080/14622200412331328501. [DOI] [PubMed] [Google Scholar]
- 59. Etter JF, Le Houezec J, Perneger TV. A self-administered questionnaire to measure dependence on cigarettes: the cigarette dependence scale. Neuropsychopharmacology. 2003;28(2):359–370. doi:10.1038/sj.npp.1300030. [DOI] [PubMed] [Google Scholar]
- 60. Etter JF, Duc TV, Perneger TV. Validity of the Fagerstrom test for nicotine dependence and of the Heaviness of Smoking Index among relatively light smokers. Addiction. 1999;94(2):269–281. doi:10.1046/j.1360-0443.1999.94226910.x. [DOI] [PubMed] [Google Scholar]
- 61. Collins CC, Moolchan ET. Shorter time to first cigarette of the day in menthol adolescent cigarette smokers. Addict Behav. 2006;31(8):1460–1464. doi:10.1016/j.addbeh.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 62. Hoffman AC, Simmons D. Menthol cigarette smoking and nicotine dependence. Tob Induc Dis. 2011;9(suppl 1):S5. doi:10.1186/1617-9625-9-s1-s5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. U.S. Dept. of Health and Human Services, FDA, Center for Drug Evaluation and Research (CDER). Guidance for Industry Assessment of Abuse Potential of Drugs 2010. www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm198650.pdf Accessed January 15, 2016.
- 64. Health Canada. Clinical Assessment of Abuse Liability for Drugs with Central Nervous System Activity 2007. www.hc-sc.gc.ca/dhp-mps/alt_formats/hpfb-dgpsa/pdf/prodpharma/abuse_liability_abusif_usage_clin-eng.pdf Accessed January 15, 2016.
- 65. Small E, Shah HP, Davenport JJ, et al. Tobacco smoke exposure induces nicotine dependence in rats. Psychopharmacology (Berl). 2010;208(1):143–158. doi:10.1007/s00213-009-1716-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wilkinson JL, Bevins RA. Intravenous nicotine conditions a place preference in rats using an unbiased design. Pharmacol Biochem Behav. 2008;88(3):256–264. doi:10.1016/j.pbb.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yararbas G, Keser A, Kanit L, Pogun S. Nicotine-induced conditioned place preference in rats: sex differences and the role of mGluR5 receptors. Neuropharmacology. 2010;58(2):374–382. doi:10.1016/j.neuropharm.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 68. Bagdas D, Muldoon PP, Zhu AZ, Tyndale RF, Damaj MI. Effects of methoxsalen, a CYP2A5/6 inhibitor, on nicotine dependence behaviors in mice. Neuropharmacology. 2014;85:67–72. doi:10.1016/j.neuropharm.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Caille S, Clemens K, Stinus L, Cador M. Modeling nicotine addiction in rats. Methods Mol Biol. 2012;829:243–256. doi:10.1007/978-1-61779-458-2_15. [DOI] [PubMed] [Google Scholar]
- 70. Wang T, Wang B, Chen H. Menthol facilitates the intravenous self-administration of nicotine in rats. Front Behav Neurosci. 2014;8:437. doi:10.3389/fnbeh.2014.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Okuyemi KS, Faseru B, Sanderson Cox L, Bronars CA, Ahluwalia JS. Relationship between menthol cigarettes and smoking cessation among African American light smokers. Addiction. 2007;102(12):1979–1986. doi:10.1111/j.1360-0443.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- 72. U.S. Dept. of Health and Human Services, FDA, Center for Drug Evaluation and Research (CDER). Abuse-Deterrent Opioids — Evaluation and Labeling Guidance for Industry 2015. www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm334743.pdf Accessed January 15, 2016.
- 73. Henningfield JE, London ED, Benowitz NL. Arterial-venous differences in plasma concentrations of nicotine after cigarette smoking. JAMA. 1990;263(15):2049–2050. doi:10.1001/jama.1990.03440150053020. [PubMed] [Google Scholar]
- 74. Berridge MS, Apana SM, Nagano KK, et al. Smoking produces rapid rise of [11C]nicotine in human brain. Psychopharmacology (Berl). 2010;209(4):383–394. doi:10.1007/s00213-010-1809-8. [DOI] [PubMed] [Google Scholar]
- 75. Usmani OS, Belvisi MG, Patel HJ, et al. Theobromine inhibits sensory nerve activation and cough. FASEB J. 2005;19(2):231–233. doi:10.1096/fj.04-1990fje. [DOI] [PubMed] [Google Scholar]
- 76. Yerger VB. Menthol’s potential effects on nicotine dependence: a tobacco industry perspective. Tob Control. 2011;20(suppl 2):ii29–36. doi:10.1136/tc.2010.041970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Frasnelli J, Albrecht J, Bryant B, Lundstrom JN. Perception of specific trigeminal chemosensory agonists. Neuroscience. 2011;189:377–383. doi:10.1016/j.neuroscience.2011.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Galeotti N, Ghelardini C, Mannelli L, et al. Local anaesthetic activity of (+)- and (-)-menthol. Planta Med. 2001;67(2):174–176. doi:10.1055/s-2001-11515. [DOI] [PubMed] [Google Scholar]
- 79. Nichols JE, Niles JA, Vega SP, Cortiella J. Novel in vitro respiratory models to study lung development, physiology, pathology and toxicology. Stem Cell Res Ther. 2013;4(suppl 1):S7. doi:10.1186/scrt368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Asgharian B, Price O, McClellan G, et al. Development of a rhesus monkey lung geometry model and application to particle deposition in comparison to humans. Inhal Toxicol. 2012;24(13):869–899. doi:10.3109/08958378.2012.725782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. McKinney DL, Gogova M, Davies BD, et al. Evaluation of the effect of ammonia on nicotine pharmacokinetics using rapid arterial sampling. Nicotine Tob Res. 2012;14(5):586–595. doi:10.1093/ntr/ntr257. [DOI] [PubMed] [Google Scholar]
- 82. van Amsterdam J, Sleijffers A, van Spiegel P, et al. Effect of ammonia in cigarette tobacco on nicotine absorption in human smokers. Food Chem Toxicol. 2011;49(12):3025–3030. doi:10.1016/j.fct.2011.09.037. [DOI] [PubMed] [Google Scholar]
- 83. Alsharari SD, Siu EC, Tyndale RF, Damaj MI. Pharmacokinetic and pharmacodynamics studies of nicotine after oral administration in mice: effects of methoxsalen, a CYP2A5/6 inhibitor. Nicotine Tob Res. 2014;16(1):18–25. doi:10.1093/ntr/ntt105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rahnasto M, Wittekindt C, Juvonen RO, et al. Identification of inhibitors of the nicotine metabolising CYP2A6 enzyme--an in silico approach. Pharmacogenomics J. 2008;8(5):328–338. doi:10.1038/sj.tpj.6500481. [DOI] [PubMed] [Google Scholar]
- 85. Rahnasto M, Raunio H, Poso A, Juvonen RO. More potent inhibition of human CYP2A6 than mouse CYP2A5 enzyme activities by derivatives of phenylethylamine and benzaldehyde. Xenobiotica. 2003;33(5):529–539. doi:10.1080/0049825031000085979. [DOI] [PubMed] [Google Scholar]
- 86. Juvonen RO, Gynther J, Pasanen M, Alhava E, Poso A. Pronounced differences in inhibition potency of lactone and non-lactone compounds for mouse and human coumarin 7-hydroxylases (CYP2A5 and CYP2A6). Xenobiotica. 2000;30(1):81–92. doi:10.1080/004982500237848. [DOI] [PubMed] [Google Scholar]
- 87. Hukkanen J, Benowitz NL. Grapefruit juice inhibits CYP2A6 and nicotine metabolism[ast]. Clin Pharmacol Ther. 2005;77(2):P75–P75. doi:10.1016/j.clpt.2004.12.178. [Google Scholar]
- 88. Kabbani N. Not so Cool? Menthol’s discovered actions on the nicotinic receptor and its implications for nicotine addiction. Front Pharmacol. 2013;4:95. doi:10.3389/fphar.2013.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Benowitz NL, Herrera B, Jacob P., III Mentholated cigarette smoking inhibits nicotine metabolism. J Pharmacol Exp Ther. 2004;310(3):1208–1215. doi:10.1124/jpet.104.066902. [DOI] [PubMed] [Google Scholar]
- 90. Jasinska AJ, Zorick T, Brody AL, Stein EA. Dual role of nicotine in addiction and cognition: a review of neuroimaging studies in humans. Neuropharmacology. 2014;84c:111–122. doi:10.1016/j.neuropharm.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Brody AL, Mukhin AG, Mamoun MS, et al. Brain nicotinic acetylcholine receptor availability and response to smoking cessation treatment: a randomized trial. JAMA Psychiatry. 2014;71(7):797–805. doi:10.1001/jamapsychiatry.2014.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Volkow ND, Fowler JS, Ding YS, Wang GJ, Gatley SJ. Imaging the neurochemistry of nicotine actions: studies with positron emission tomography. Nicotine Tob Res. 1999;1(suppl 2):S127–132; discussion S139-140. doi:10.1080/14622299050011941. [DOI] [PubMed] [Google Scholar]
- 93. Zuo Y, Mukhin AG, Garg S, et al. Sex-specific effects of cigarette mentholation on brain nicotine accumulation and smoking behavior. Neuropsychopharmacology. 2014;40(4):884–892. doi:10.1038/npp.2014.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Brody AL, Mukhin AG, La Charite J, et al. Up-regulation of nicotinic acetylcholine receptors in menthol cigarette smokers. Int J Neuropsychopharmacol. 2013;16(5):957–966. doi:10.1017/s1461145712001022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hans M, Wilhelm M, Swandulla D. Menthol suppresses nicotinic acetylcholine receptor functioning in sensory neurons via allosteric modulation. Chem Senses. 2012;37(5):463–469. doi:10.1093/chemse/bjr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ashoor A, Nordman JC, Veltri D, et al. Menthol binding and inhibition of alpha7-nicotinic acetylcholine receptors. PLoS One. 2013;8(7):e67674. doi:10.1371/journal.pone.0067674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zhang T, Zhang L, Liang Y, et al. Dopamine signaling differences in the nucleus accumbens and dorsal striatum exploited by nicotine. J Neurosci. 2009;29(13):4035–4043. doi:10.1523/jneurosci.0261-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Rollema H, Chambers LK, Coe JW, et al. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52(3):985–994. doi:10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 99. Alsharari SD, King JR, Nordman JC, et al. Effects of menthol on nicotine pharmacokinetic, pharmacology and dependence in mice. PLoS One. 2015;10(9):1–10. doi:10.1371/journal.pone.0137070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Herraiz T, Chaparro C. Human monoamine oxidase enzyme inhibition by coffee and beta-carbolines norharman and harman isolated from coffee. Life Sci. 2006;78(8):795–802. doi:10.1016/j.lfs.2005.05.074. [DOI] [PubMed] [Google Scholar]
- 101. He X, Chen YY, Shi JB, et al. New coumarin derivatives: design, synthesis and use as inhibitors of hMAO. Bioorg Med Chem. 2014;22(14):3732–3738. doi:10.1016/j.bmc.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 102. Lewis AJ, Truman P, Hosking MR, Miller JH. Monoamine oxidase inhibitory activity in tobacco smoke varies with tobacco type. Tob Control. 2012;21(1):39–43. doi:10.1136/tc.2010.040287. [DOI] [PubMed] [Google Scholar]
- 103. Committee, TPSAC. Menthol Cigarettes and Public Health: Review of the Scientific Evidence and Recommendations 2011. www.fda.gov/downloads/advisorycommitteees/committeesmeetingmaterials/tobaccoproductsscientificadvisorycommittee/ucm269697.pdf Accessed January 15, 2016.
- 104. Henningfield JE, Hatsukami DK, Zeller M, Peters E. Conference on abuse liability and appeal of tobacco products: conclusions and recommendations. Drug Alcohol Depend. 2011;116(1–3):1–7. doi:10.1016/j.drugalcdep.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. WHO Study Group on Tobacco Product Regulation TobReg. Report on the Scientific Basis of Tobacco Product Regulation, WHO Technical Report Series, no. 967 2012. www.who.int/tobacco/publications/prod_regulation/trs_967/en/ Accessed January 15, 2016.
- 106. Li CY, Zhou WZ, Zhang PW, et al. Meta-analysis and genome-wide interpretation of genetic susceptibility to drug addiction. BMC Genomics. 2011;12:508. doi:10.1186/1471-2164-12-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Perkins KA, Karelitz JL. Sex differences in acute relief of abstinence-induced withdrawal and negative affect due to nicotine content in cigarettes. Nicotine Tob Res. 2015;17(4):443–448. doi:10.1093/ntr/ntu150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Hoegberg BG, Lomazzo E, Lee NH, Perry DC. Regulation of alpha4beta2alpha5 nicotinic acetylcholinergic receptors in rat cerebral cortex in early and late adolescence: sex differences in response to chronic nicotine. Neuropharmacology. 2015;99:347–355. doi:10.1016/j.neuropharm.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Rubinstein ML, Benowitz NL, Auerback GM, Moscicki AB. Rate of nicotine metabolism and withdrawal symptoms in adolescent light smokers. Pediatrics. 2008;122(3):e643–647. doi:10.1542/peds.2007-3679. [DOI] [PMC free article] [PubMed] [Google Scholar]


