Abstract
Introduction:
Tobacco harm reduction aims to provide reduced risk alternatives to adult smokers who would otherwise continue smoking combustible cigarettes (CCs). This randomized, open-label, three-arm, parallel-group, single-center, short-term confinement study aimed to investigate the effects of exposure to selected harmful and potentially harmful constituents (HPHCs) of cigarette smoke in adult smokers who switched to a carbon-heated tobacco product (CHTP) compared with adult smokers who continued to smoke CCs and those who abstained from smoking for 5 days.
Methods:
Biomarkers of exposure to HPHCs, including nicotine and urinary excretion of mutagenic material, were measured in 24-hour urine and blood samples in 112 male and female Caucasian smokers switching from CCs to the CHTP ad libitum use. Puffing topography was assessed during product use.
Results:
Switching to the CHTP or smoking abstinence (SA) resulted in marked decreases from baseline to Day 5 in all biomarkers of exposure measured, including carboxyhemoglobin (43% and 55% decrease in the CHTP and SA groups, respectively). The urinary excretion of mutagenic material was also markedly decreased on Day 5 compared with baseline (89% and 87% decrease in the CHTP and SA groups, respectively). No changes in biomarkers of exposure to HPHCs or urinary mutagenic material were observed between baseline and Day 5 in the CC group.
Conclusions:
Our results provide clear evidence supporting a reduction in the level of exposure to HPHCs of tobacco smoke in smokers who switch to CHTP under controlled conditions, similar to that observed in SA.
Implications:
The reductions observed in biomarkers of exposure to HPHCs of tobacco smoke in this short-term study could potentially also reduce the incidence of cancer, cardiovascular and respiratory diseases in those smokers who switch to a heated tobacco product.
Introduction
Tobacco harm reduction aims to provide reduced risk alternatives to adult smokers who would otherwise continue smoking combustible cigarettes (CCs). From snus, the popular smokeless tobacco product in Sweden, to electronic cigarettes, alternatives to CCs are increasingly being recognized as important public health tools to complement policies for preventing smoking initiation and promoting smoking cessation. An emerging platform technology to lower the level of harmful and potentially harmful constituents (HPHCs) in smoke is based on heating tobacco rather than burning it. This technology avoids pyrolysis/combustion by distillation of tobacco, with less formation of HPHCs.
Previously, a range of innovative products were developed, which were referred to as “potential reduced-exposure products.”1 In 1989, RJ Reynolds released “Premier,” a product similar in size and appearance to a CC, but hosting an aluminum canister containing alumina beads impregnated with tobacco extract and vaporization based on a carbon heat source.2 Based on a similar technology, RJ Reynolds introduced a successor product in 1996, Eclipse, which according to Pederson and Nelson,3 produced tar levels similar to reduced tar-level CCs. In 1999, Phillip Morris released Accord, a smoking system that ignited a special low-tar cigarette only when puffed, producing less smoke and no ashes.4 While, these devices appeared to diminish the exposure to carcinogens, the lack of data to support their effective harm reduction5,6 and design flaws (potentially harmful nicotine carriers, glass fiber parts, and bulky casing,6 among others7) hampered their widespread use.
Devices aimed at reducing HPHCs and risk for smoking-related disease are currently referred to as modified-risk tobacco products.8 Herein, we report on the assessment of a novel, candidate modified-risk tobacco product, the carbon-heated tobacco product (CHTP, version MD2-E7). The CHTP contains a column of tobacco that is connected to a carbon heating source and is lit with a specifically designed electric lighter. The aerosol created by gentle and controlled heating of the tobacco is composed primarily of water, a humectant (eg, glycerol), and reduced concentrations of HPHCs, including aldehydes and polycyclic aromatic hydrocarbons.
In a 28-day, repeated-dose, inhalation toxicity rat study, the biological effects of mainstream aerosol from the CHTP were compared with the mainstream smoke from the University of Kentucky Reference Cigarette (3R4F). The biological effects of the CHTP aerosol were consistently lower in the respiratory tract, especially in the nose and lungs, with significantly fewer adaptive and inflammatory changes compared with the typical inhalation effects from the 3R4F at equal nicotine concentrations.9 Gene expression data regarding biological network disruption indicated a lesser impact of the CHTP on cell stress and inflammation compared with CCs.10 A study in a C57BL/6 mouse model investigated the chronic obstructive pulmonary disease risk reduction potential of a CHTP compared with CCs. Mice were exposed to CC smoke (3R4F), fresh air (sham), or CHTP aerosol for up to 7 months. The CC smoke induced typical adaptive airway changes and lung inflammation associated with emphysema, impaired pulmonary function and alveolar damage. Conversely, there were no signs of lung inflammation and emphysema after CHTP aerosol exposure at nicotine-matched concentrations.11 Furthermore, we have reported that CHTP aerosol has 20 times less impact on cytotoxicity, inflammation, chemotaxis and transendothelial migration in vitro than 3R4F smoke.12
The exposure to toxicants is a function of cigarette composition and burning or heating temperature in addition to individual smoking behavior (eg, puff rate and inhalation depth). It is thus necessary to measure the smoker’s actual exposure to determine the effects of different cigarettes.13 This study aimed to determine whether adult smokers who switch to the CHTP reduce their levels of the biomarkers of exposure to HPHCs, specifically carboxyhemoglobin (COHb), S-phenylmercapturic acid (S-PMA), and total 4-methylnitrosamino-1-(3-pyridyl)-1-butanol (total NNAL), compared with adult smokers who continue to smoke CCs and those who abstain from smoking for 5 days.
Methods
Study Design
This controlled, randomized, open-label, three-arm parallel group, single-center confinement study was conducted at the premises of MTZ Clinical Research Ltd, Warsaw, Poland and was approved by the independent ethics committee of the Regional Medical Chamber of Physicians in Warsaw, Poland. It is posted on ClinicalTrials.gov under the identifier NCT00812279.
A sample size of 28 subjects in each group for a two-arm comparison is sufficient for detecting a relative effect of 0.8 at type I and II error rates of 5% and 20%, respectively. Because of the lack of information for CHTP on the variability in biomarkers of exposure measurements and product use in an ad libitum setting, the group size in the CHTP arm was doubled to 56 subjects.
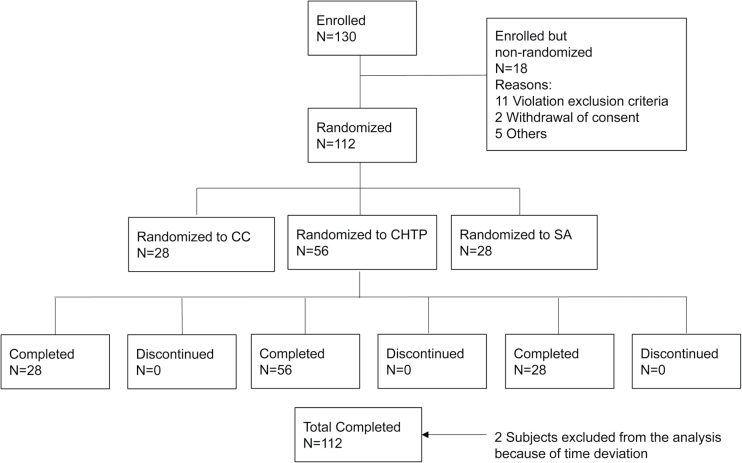
A total of 130 eligible subjects were enrolled on Day −2 (Admission), 18 subjects were disqualified during selection criteria verification (Figure 1) prior to randomization. A total of 112 subjects were randomly assigned into one of the three study arms by an interactive voice response system. The study was conducted in four successive cohorts. All subjects of a cohort were randomly assigned in the evening of Day 0 (D0). Randomization was stratified by sex and self-reported daily CC consumption (10−19 CCs per day [cpd] and 20−30 cpd).
Figure 1.
Subject disposition. CC: combustible cigarettes; SA: smoking abstinence; CHTP: carbon-heated tobacco product; N: number of subjects.
During the 2-day baseline period (D −1 and D0), subjects smoked their own brand of CCs ad libitum with each subject’s maximum daily CC consumption limited to 120% of the median daily CC consumption, derived from a 7-day self-reported CC consumption diary recorded prior to admission. The exposure period lasted 5 days (D1−D5). During the exposure period, CC smoking or CHTP use was allowed ad libitum in separate rooms until 11:00 PM. Subjects using the CHTP did not have access to CCs and vice versa. Participants in the smoking abstinence (SA) group were denied access to these rooms and underwent counseling on smoking cessation, but no pharmacotherapy. Subjects were discharged during the morning of D6 after undergoing all safety examination procedures. They entered a 7-day passive adverse event (AEs) follow-up period.
Subjects
Male and female Caucasian adult smokers that met the following criteria were eligible for this study: body mass index between 18.5−27.5kg/m2, aged between 23−55 years, smoking habit of 10−30 cpd (maximum International Organization for Standardization [ISO] tar yield of 10mg), smoking history of at least 5 consecutive years, and acceptable health conditions based on clinical and laboratory parameters (spirometry, vital signs, physical examination, electrocardiogram, chest radiograph, and medical history). Women of childbearing potential were excluded if they were pregnant or breastfeeding or if they did not use an effective contraception method. After full explanation of the study, subjects signed an informed consent form prior to any assessments.
Assessments
A self-administered smoking history questionnaire was completed by the subjects on D1, including the Fagerström Test for Nicotine Dependence (FTND).14
Biomarkers of exposure assessed as part of this study were COHb (biomarker for carbon monoxide), MHBMA (biomarker for 1,3-butadiene), 3-HPMA (biomarker for acrolein), total 1-OHP (biomarker for pyrene), O-toluidine (biomarker for ortho-toluidine), 2-NA (biomarker for 2-aminonaphthalene), 4-ABP (biomarker for 4-aminobiphenyl), S-PMA (biomarker for benzene), total NNAL (biomarker for NNK) and nicotine equivalents (biomarker of exposure for nicotine) and cotinine (biomarker of exposure for nicotine). These biomarkers were selected because: (1) the related HPHCs are relatively specific to tobacco smoke, (2) the HPHCs cover both the vapor and particulate phases of tobacco smoke, (3) the levels of these biomarkers are well described in smokers and non-smokers as well as with SA, and (4) there were validated methods to assess these biomarkers. In order to avoid inflation of the false positive rate the primary objective was restricted, a priori, to a subset of three biomarkers of exposure (COHb, S-PMA, and total NNAL), the complete set of the biomarkers assessed descriptively as a secondary objective.
COHb and cotinine were measured (spectrophotometrically) in blood, while S-PMA and total NNAL were measured in 24-hour urine. Further biomarkers of exposure to various HPHCs were measured in 24-hour urine. Seven 24-hour urine samples were collected for each subject during the 7-day study period. Blood samples were drawn daily between 5:00 PM to 7:00 PM from D1 to D5.
The biomarkers of exposure in urine were assayed by liquid chromatography using tandem mass spectrometry techniques at Philip Morris Research Laboratories, Cologne, Germany. Urine mutagenicity was measured at D0 and D5 in 24-hour urine samples. The urinary evaluation was performed on one bacterial strain (Salmonella typhimurium strain YG1024, an O-acetyltransferase-overproducing derivative of strain TA98) with high sensitivity for detecting aromatic amine compound mutagenicity.15 Four doses of each urine extract, and S9 metabolic activation were used in three replications. Arithmetic means of the replicates were used to evaluate the dose-response of the mutagenicity potential in each urine sample. The urine mutagenicity index (the number of revertants/24h) was calculated by multiplying the number of revertants/24h/ml by the total 24-hour urine volume.
All biomarkers of exposure assessed in this study exhibit, on average, an elimination half-life of up to 24 hours, except for total NNAL that has an estimated half-life range of 10–18 days.15 Therefore, 5 days of product use were sufficient to observe the optimal decrease in biomarker levels, except for total NNAL.
All subjects underwent human puffing topography (HPT) assessments at baseline (D −1 and D0), for their usual brand of CC, and after randomization, on D1 and D5, for each CHTP and CC used. A portable SODIM device (model SPA-M, Sodim SAS, Fleury-les-Aubrais, France) was assigned to each subject and used throughout HPT assessment days to record puffing behavior: frequency, puff duration, inhaled smoke volume and pressure drop. The CC brands of 7 subjects were incompatible with the SODIM device (slim CCs); thus, they were excluded from this analysis. Another four subjects were excluded because of device-handling issues. Data for 45 CHTP users are reported.
Tobacco Products
We tested the CHTP prototype MD2-E7 (3mg tar, 2mg glycerol, 0.4mg nicotine, and 1mg CO yield; aerosol chemistry determined under ISO conditions, 12 puffs). It consists of a carbon heat source, a tobacco plug wrapped in paper, an empty tube (to allow aerosol transfer), and a filter (a strip of aluminum foil that attaches the carbon heat source to the tobacco plug). Its appearance is similar to that of a CC, but the CHTP is based on technology that avoids pyrolysis/combustion of tobacco. The aerosol chemistry of the CHTP was previously reported.9 The test product and a specifically designed electric lighter were provided to the subjects.
Reference and baseline products were commercially available non-menthol CCs, with an ISO tar yield of up to 10mg. All subjects purchased the anticipated amount of their usual CC brand required for the confinement period and handed them over to the site staff at admission. All products were stored at room temperature in a locked room with restricted access. Used CCs and CHTPs were returned to the site for product accountability.
Data Analysis
For all biomarkers of exposure and all three study arms, means and standard deviations were calculated for baseline (average of D0 and D –1), end of exposure (average of D4 and D5), and for the change from baseline to end of exposure, along with 95% confidence intervals. Differences of the means between the CHTP and CC groups and their 95% confidence intervals were calculated. To demonstrate reduced levels of the primary biomarkers of exposure (COHb, S-PMA, and total NNAL) in the CHTP compared with the CC arm at the end of the exposure, we conducted an analysis of covariance comparing differences between the two arms at an unadjusted alpha level of 5% each. Covariates considered in this analysis were sex, usual daily CC consumption and the baseline level of the analyzed biomarker of exposure. In addition, terms for interactions between study arm and sex, and between study arm and usual daily CC consumption were included, whenever the corresponding interaction test resulted in a P value ≤ 10%. Data were analyzed using SAS V8.2 (SAS Institute Inc, Cary, NC).
Results
In total, 112 subjects were randomly assigned into one of the three study groups (CHTP: 56 subjects, CC: 28 subjects, SA: 28 subjects; full analysis set). All randomly assigned subjects completed the study. No subjects were prematurely discontinued. Two subjects in the CHTP group were excluded from the analysis of biomarkers of exposure because they underwent assessments outside of the allowed time window. The distributions of age, sex, body mass and usual CC consumption of subjects was comparable across study arms. Subject characteristics are shown in Table 1.
Table 1.
Baseline Characteristics by Study Group
| Variable and statistics | CHTP | CC | Abstinence |
|---|---|---|---|
| Number (N) | 56 | 28 | 28 |
| Age, years (mean [SD]) | 36 (8.2) | 35.4 (7.4) | 37.9 (8.4) |
| Sex, n (%) | |||
| Female | 28 (50) | 14 (50) | 14 (50) |
| Male | 28 (50) | 14 (50) | 14 (50) |
| BMI, kg/m2 (mean [SD]) | 23.6 (2.2) | 23 (2.4) | 23.2 (2.5) |
| Usual cigarette consumption, n (%) | |||
| 10–19 cpd | 28 (50) | 14 (50) | 14 (50) |
| 20–30 cpd | 28 (50) | 14 (50) | 14 (50) |
| ISO tar yield, n (%) | |||
| 3–5 | 11 (19.6) | 3 (10.7) | 3 (10.7) |
| 6–8 | 22 (39.3) | 18 (64.3) | 19 (67.9) |
| 9–10 | 23 (41.1) | 7 (25) | 6 (21.4) |
| Fagerström score (mean [SD]) | 5.5 [2.0] | 5.6 [1.8] | 5.9 [1.1] |
BMI = body mass index; CC = combustible cigarette; CHTP = carbon-heated tobacco product MD2-E7; cpd = combustible cigarettes per day; ISO = International Organization for Standardization; N = number of subjects.
Biomarkers of Exposure
At the end of the exposure period, reduced levels (least squares means) were observed for all three biomarkers of exposure in the CHTP compared with CC study arm (P < .0001 for each of the three comparisons). In the CHTP arm, the COHb level (% hemoglobin saturation) was lower by 3.66% (adjusted for baseline level, sex, usual CC consumption and the interaction between study arm and usual CC consumption), the S-PMA level was lower by 5.66 μg/24h (adjusted for baseline level, sex, usual CC consumption and interactions between study arm and usual CC consumption and study arm and sex), and the total NNAL level was lower by 112ng/24h (adjusted for baseline level, sex and usual CC consumption).
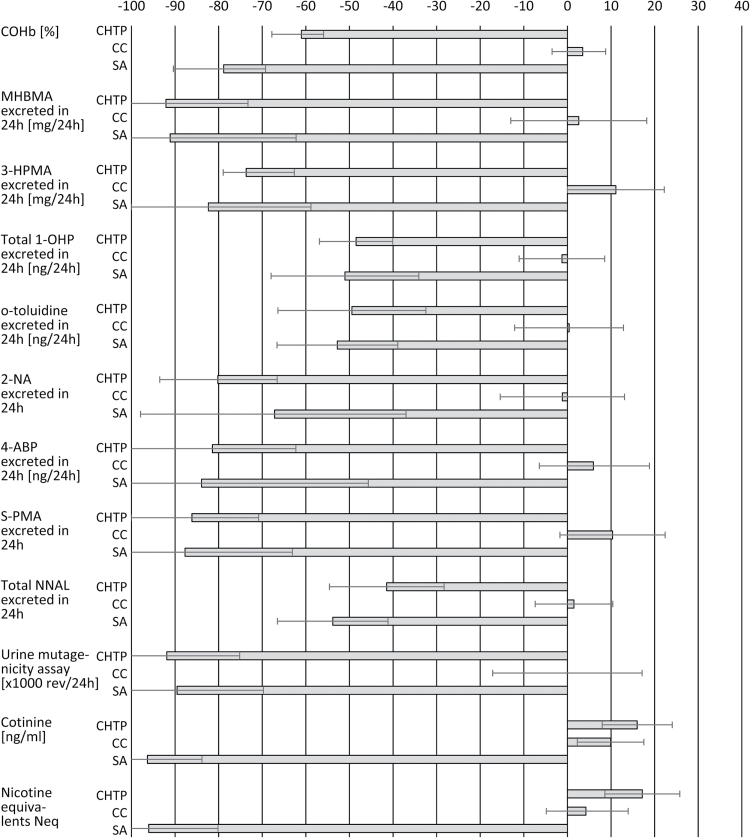
The other biomarkers of exposure to selected cigarette smoke HPHCs (1,3-butadiene, acrolein, pyrene, O-toluidine, 4-aminobiphenyl, 2-aminonaphthalene), and excretion of mutagenic material in urine were also decreased in the CHTP group at D5/6 compared with baseline (Figure 2). At the end of the exposure period, there were no changes in biomarkers of exposure levels in the CC group. All decreased levels were more marked in CHTP users compared with CC smokers (Supplementary Table 1).
Figure 2.
Relative (%) change (mean and 95% CI) from baseline to end of exposure (Day 5/6) per study group. CC: combustible cigarette; SA: smoking abstinence; CHTP: carbon-heated tobacco product MD2-E7; CI: confidence interval; MHBMA: mono-hydroxybutenyl mercapturic acid; HPMA: hydroxypropylmercapturic acid; OHP: hydroxypyrene and its glucuronide and sulfate conjugates; ABP: aminobiphenyl; S-PMA: S-phenylmercapturic acid; NNAL: 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol.
Except for 2-napththylamine, 4-aminobiphenyl and urinary excretion of mutagenic material, the largest decrease in biomarkers of exposure was observed in the SA group. In the SA and CHTP groups, the smallest decrease was for pyrene with 48% and 51%, respectively, while the largest decrease was for 1,3-butadiene in both groups (>90%).
Product Use and Nicotine Exposure
The baseline mean (standard deviation) number of cpd was similar in the CHTP (17.8 [3.2]) and the CC (17.4 [3.4]) groups. Product consumption increased slightly in both groups by D5 (18.8 [4.4] CCs and 19.7 [7.8] CHTPs). The exposure to nicotine, measured by plasma cotinine concentrations and by nicotine equivalent (urinary excretion of nicotine and five nicotine metabolites) increased from D1 to D5 and remained at comparable levels in the CHTP and CC groups (Figure 2; Supplementary Table 1).
Human Puffing Topography
Table 2 summarizes the HPT results, stratified by tar level of brands used prior to switching to the CHTP. At baseline, higher average nicotine levels were found in the full flavor category (9–10mg tar yield), with a similar number of consumed CCs of the usual brand, compared with the 3–5 and 6–8mg tar categories, although the 95% confidence intervals of the point estimates did overlap. The puff volume for CCs ranged from 54 to 74ml.
Table 2.
Puffing Topography in the CHTP Group Stratified by Tar Level of Usual Brand at Baseline (Day 0)
| Tar level 3–5mg N = 7 | Tar level 6–8mg N = 18 | Tar level 9–10mg N = 20 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Visit | Day 0 | Day 1 | Day 5 | Day 0 | Day 1 | Day 5 | Day 0 | Day 1 | Day 5 |
| Puff duration (s) | 2.1 (0.77) | 2.1 (0.60) | 2.2 (0.67) | 1.8 (0.40) | 2.3 (0.65) | 2.4 (0.74) | 1.8 (0.52) | 2.2 (0.46) | 2.2 (0.59) |
| Puff volume (ml) | 73.9 (21.35) | 83.8 (21.23) | 91.6 (21.96) | 57.9 (12.45) | 90.3 (31.03) | 94.0 (44.63) | 53.9 (13.27) | 80.0 (20.41) | 82.4 (21.09) |
| Puff number (n) | 15.7 (2.36) | 22.1 (2.11) | 24.5 (2.50) | 13.3 (2.24) | 21.5 (4.31) | 21.9 (4.53) | 13.8 (3.62) | 22.7 (3.78) | 25.0 (3.13) |
| Total volume (ml) | 1139.0 (218.59) | 1848.0 (526.04) | 2196.6 (451.41) | 767.3 (144.91) | 1846.7 (417.97) | 1920.8 (519.34) | 728.6 (134.99) | 1779.0 (448.22) | 2012.3 (433.97) |
| Cigarettes per day | 17.6 (2.76) | 14.6 (4.16) | 18.9 (5.24) | 17.8 (3.79) | 15.7 (4.03) | 19.8 (5.95) | 17.4 (3.47) | 16.6 (5.56) | 19.6 (8.23) |
| Neq (geometric mean, 95% CI, mg/24h) | 11.5 (8.8–15.1) | 10.7 (7.7–15.0) | 12.9 (7.5–22.3) | 10.0 (8.5–11.7) | 10.2 (8.7–12.1) | 12.6 (10.7–14.8) | 12.5 (9.3–16.6) | 12.0 (9.3–15.6) | 16.2 (13.9–18.9) |
CI = confidence interval; CHTP = carbon-heated tobacco product MD2-E7; M = Mean; Neq = nicotine equivalent. Data in the table are presented as mean (SD), except where indicated.
Switching to the CHTP resulted in an approximately 40% increase in number of puffs and a markedly increased puff volume, with an increased total puff volume on D1. Across all three tar level categories, the users adapted their puffing to achieve comparable and individually adjusted nicotine levels starting on D1. In the following days, participants increased their tobacco consumption, number of sticks used and overall nicotine exposure in the CHTP and the CC groups (Table 2). The topography measures remained stable between D1 and D5 in the CHTP group.
Safety
The frequency of reported AEs was similar in each of the three study groups: CHTP (50%, 47 AEs), SA (46%, 23 AEs), and CC (54%, 22 AEs). Headache was the most common AE, with a total of 13 (23%), 4 and 8 (29%) subjects in the CHTP, SA, and CC groups, respectively.
Discussion
We investigated the changes in biomarkers of exposure in adult smokers in a controlled clinical setting. Randomization and monitoring of subjects together with full control of product distribution in confinement allowed the unbiased evaluation of the maximal induction of exposure achieved by switching to the CHTP for 5 days.
Biomarkers of Exposure to Selected Cigarette Smoke HPHCs
A study evaluating the effects on 1,3-butadiene metabolite in urine, monohydroxy-3-butenyl-mercapturic acid (MHBMA), after switching from cellulose acetate to charcoal filtered CCs found that the MHBMA levels decreased by 18% and decreased by 90% to 95% after smoking cessation.16 Concordantly, in the present study, smokers who switched to the CHTP presented a marked decrease of MHBMA compared with those smoking their usual CC brand. Moreover, the decrease in the CHTP group was similar to that achieved after 5 days of SA.
In a study of smokers that switched from cellulose acetate to charcoal filter CCs, levels of S-(3-hydroxypropyl)-N-acetylcysteine (3-HPMA), the main urinary metabolite of acrolein, decreased by 8% and 17%.17 In a study of smoking cessation, the 3-HPMA levels decreased significantly by 78% in smokers who abstained for 4 weeks.18 Notably, in this study, the mean change of 3-HPMA levels from baseline to end of exposure in subjects who switched to the CHTP was as pronounced as that after SA. Similarly, we measured S-phenylmercapturic acid (S-PMA), the urinary metabolite of benzene, a well-known carcinogenic.19,20 The urinary excretion of S-PMA was markedly decreased in both the CHTP and SA groups compared with the CC group, with that decrease being slightly more pronounced in the CHTP group. O-toluidine, 2-naphthylamine, and 4-aminobiphenyl in urine are also biomarkers of exposure to the HPHCs of CC smoke.21 We found that subjects exposed to the CHTP and SA presented significantly and markedly decreased urinary levels of these compounds.
Although pyrene itself is not considered a HPHC, its urinary metabolite, 1-hydroxypyrene, is a surrogate of exposure to polycyclic aromatic hydrocarbons (PAHs).22 Many PAHs are potent carcinogens in animals and humans.19 In our study, the levels of pyrene decreased in the CHTP and SA groups compared with the CC group. However, the decrease in pyrene was the lowest of all the biomarkers evaluated. Other well-established biomarkers of exposure to CO in tobacco smokers are COHb in blood and exhaled CO.23 As with the other biomarkers, the level of COHb decreased in both the CHTP and SA groups compared with the CC arm. A similar downward trend was seen in the urinary excretion of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and its O-glucuronide conjugate (NNAL-O-Gluc) in urine (total NNAL excretion), which are tobacco-specific biomarkers of exposure to 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone.19 In a previous study, the total NNAL level decreased by 70% after 1 week and by 90% after 4 weeks of smoking cessation.24 Although the maximal total NNAL decrease was probably not observed in this short-term study because of the 10- to 18-day half-life of NNAL,25 total NNAL levels at the end of the exposure period in both the CHTP and SA arms indicate that CHTP use decreased the exposure of total NNAL almost as effectively as SA.
Urine Mutagenicity
CC smoke contains a number of mutagenic and carcinogenic compounds, including nitroso-compounds, PAHs and hetero-cyclic amines that undergo urinary or fecal excretion. Thus, the urinary level of mutagens reflects both the exposure dose and the metabolic state of these carcinogens and mutagens.26 The urine mutagenicity values observed in this study indicate a marked reduction in the exposure to potential mutagens, with matching values between subjects who switched to the CHTP and those in the SA group. These reductions are in agreement with other published data for SA27 and electrically heated cigarettes.28
Puffing Behavior
Cigarette design characteristics affect HPT when smokers first switch by inducing changes in the draw resistance, sensation, and taste. Switching from higher- to lower-yield CCs increases topography parameters such as puff volume and puffs per cigarette. Conversely, when smokers switch to higher-yield CCs, or CCs with constant tar but increased nicotine content, they decrease puffing intensity or take longer to smoke a CC.29 In our short-term study, smokers who switched to the CHTP compensated to their self-adjusted nicotine level by inhaling more frequent and larger puffs. A feature of the CHTP tested was that the nicotine delivery/puff decreased substantially over time during a single-stick experience. With a standard smoking machine, FTC ISO measurement of the CHTP, the product stopped yielding nicotine beyond 16 puffs (data not shown). The observed increase in the number of puffs smokers drew may reflect this fading effect of nicotine delivery. Conversely, the puff volume for CCs was not different to that in a previous report.30
Despite compensatory puffing behavior, the biomarkers of exposure were dramatically reduced in the smokers who switched to the CHTP. Combustible reduced-nicotine-content cigarettes were designed to avoid compensation. These products were tested and were found to contain levels of nicotine close to those found in vegetables like aubergine, an order of magnitude lower (eg, 0.05mg nicotine yield) than the levels in CCs (eg, 1mg). Compensatory smoking behavior was not reported.31,32 However, these modified reduced-nicotine cigarettes harbor the same HPHCs present in CCs, and lack the level of exposure reduction achieved by heated tobacco platforms. Based on the present findings, the CHTP delivers on the request raised by advocates of reduced-nicotine cigarettes,33 namely to dissociate nicotine from tar. Rather than reduced-nicotine cigarettes, the CHTP delivers nicotine and reduces tar, and not the other way around.
Nicotine, cotinine and urinary Neq levels decreased throughout the 5-day exposure in the SA study group as expected, while there was a slight increase in consumption in both the CC and the CHTP groups. This indicates that increased nicotine exposure was a study-related effect of the confinement condition.
Subjects tolerated the CHTP well. There were no apparent group differences in any of the clinical laboratory values, vital signs, electrocardiographic parameters, spirometry, or physical examination during the study. Only minor AEs were reported and these were not attributed to the study product.
The evidence that CHTPs can reduce smoking machine-measured toxicant levels in mainstream aerosol compared with CCs is reflected by the reduced levels of biomarkers of exposure observed in our study. Exposure reduction was demonstrated in this short-term study. However, long-term studies on clinical endpoints are needed to demonstrate risk reduction because dose-response relationships that could be useful for the extrapolation of health-risk reduction based on exposure reduction are currently unknown. However, studies on long latency diseases such as cancer, heart disease, and chronic obstructive pulmonary disease are problematic as very long-term follow-up would be required to measure health effects. Such studies are only feasible after the CHTP has been used by a sufficiently large proportion of previous CC smokers for a sufficiently long period of time. At the current stage, biomarkers of exposure provide useful indications on long-term outcomes within a short time, which can be the basis for estimating specific effects long before direct evidence from epidemiological studies becomes available.34
This study has some limitations, particularly related to the external validity of randomized controlled trials. The study duration was short; thus, some subjects may not have fully adapted their smoking behavior to the CHTP. The study conditions and procedures were tightly controlled, eliminating the possibility of product use behavior as in real-world conditions. Although subjects could smoke ad libitum during the study, they had to ask for each CC or CHTP, which may have affected their smoking behavior; higher consumption may occur under more stressful real-world conditions. However, the daily consumption increased from baseline to the end of the exposure period so the current data do not support this theoretical possibility. The strengths of the study were the tight controls and maximized internal validity to avoid confounding, allowing the assessment of the CHTP performance when used exclusively.
The obtained findings provide clear evidence of reduced exposure to HPHCs in smokers who switched to CHTP, similar in magnitude to the reductions after SA for 5 days. The examined biomarkers of exposure in this study have shown a quantifiable link to tobacco-related diseases and may facilitate the scientific evaluation of alternative, reduced risk tobacco products. Additionally, empirically coherent and mutually supportive data from multiple clinical risk endpoints across several biological processes, physiological systems and mechanisms could be integrated to strengthen the evidentiary basis of modified-risk tobacco products.
Supplementary Material
Supplementary Table 1 can be found online at http://www.ntr.oxfordjournals.org
Funding
The study was supported by Philip Morris Products S.A.
Declaration of Interests
All authors are employees of Philip Morris Products S.A.
Supplementary Material
Acknowledgments
The authors acknowledge with gratitude the work of Dr Katarzyna Jarus-Dziedzic and her staff at MTZ Clinical Research Inc, Warsaw, Poland. We are also very grateful to Dr Michael Peck, an employee of Philip Morris International, and Dr Keyra Martinez Dunn, of Edanz Group Ltd, for their editorial support in the preparation of this manuscript.
References
- 1. IOM (Institute of Medicine). Clearing the Smoke - Assessing the Science Base for Tobacco Harm Reduction. Washington, DC: The National Academies Press; 2001. www.nap.edu/read/10029/chapter/1 Accessed November 26, 2015. [PubMed] [Google Scholar]
- 2. Slade J. Nicotine delivery device. In: Orleans CT, Slade J, eds. Nicotine Addiction: Principles and Management. New York, NY: Oxford University Press; 1993:3–23. [Google Scholar]
- 3. Pederson LL, Nelson DE. Literature review and summary of perceptions, attitudes, beliefs, and marketing of potentially reduced exposure products: communication implications. Nicotine Tob Res. 2007;9(5):525–534. doi:10.1080/14622200701239548. [DOI] [PubMed] [Google Scholar]
- 4. Felter JL, Lee RL, Solanky A, et al.; Philip Morris USA. Electrically heated cigarette smoking system with internal manifolding for puff detection. US6810883 B2. November 8, 2002.
- 5. Buchhalter AR, Eissenberg T. Preliminary evaluation of a novel smoking system: effects on subjective and physiological measures and on smoking behavior. Nicotine Tob Res. 2000;2(1):39–43. doi:10.1080/14622200050011286. [DOI] [PubMed] [Google Scholar]
- 6. Holzman D. Safe cigarette alternatives? Industry critics say ‘not yet’. J Natl Cancer Inst. 1999;91(6):502–504. doi:10.1093/jnci/91.6.502. [DOI] [PubMed] [Google Scholar]
- 7. Caraballo RS, Pederson LL, Gupta N. New tobacco products: do smokers like them? Tob Control. 2006;15(1):39–44. doi:10.1136/tc.2005.012856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. FDA (Food and Drug Administration). Guidance for Industry - Modified Risk Tobacco Product Applications - Draft Guidance 2012. www.fda.gov/downloads/TobaccoProducts/GuidanceComplianceRegulatoryInformation/UCM297751.pdf Accessed November 26, 2015.
- 9. Kogel U, Schlage WK, Martin F, et al. A 28-day rat inhalation study with an integrated molecular toxicology endpoint demonstrates reduced exposure effects for a prototypic modified risk tobacco product compared with conventional cigarettes. Food Chem Toxicol. 2014;68:204–217. doi:10.1016/j.fct.2014.02.034. [DOI] [PubMed] [Google Scholar]
- 10. Talikka M, Sierro N, Ivanov NV, et al. Genomic impact of cigarette smoke, with application to three smoking-related diseases. Crit Rev Toxicol. 2012;42(10):877–889. doi:10.3109/10408444.2012.725244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Phillips B, Veljkovic E, Peck MJ, et al. A 7-month cigarette smoke inhalation study in C57BL/6 mice demonstrates reduced lung inflammation and emphysema following smoking cessation or aerosol exposure from a prototypic modified risk tobacco product. Food Chem Toxicol. 2015;80:328–345. doi:10.1016/j.fct.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 12. van der Toorn M, Frentzel S, Goedertier D, Peitsch M, Hoeng J, De Leon H. A prototypic modified risk tobacco product exhibits reduced effects on chemotaxis and transendothelial migration of monocytes compared with a reference cigarette. Food Chem Toxicol. 2015;80:277–286. doi:10.1016/j.fct.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 13. Benowitz NL, Jacob P, III, Bernert JT, et al. Carcinogen exposure during short-term switching from regular to “light” cigarettes. Cancer Epidemiol Biomarkers Prev. 2005;14(6):1376–1383. doi:10.1158/1055-9965.EPI-04-0667. [DOI] [PubMed] [Google Scholar]
- 14. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. www.dartmouth.edu/~thlab/pubs/91_Heatherton_etal_BJA.pdf Accessed November 26, 2015. [DOI] [PubMed] [Google Scholar]
- 15. Einisto P, Nohmi T, Watanabe M, Ishidate M., Jr Sensitivity of Salmonella typhimurium YG1024 to urine mutagenicity caused by cigarette smoking. Mutat Res. 1990;245(2):87–92. www.sciencedirect.com/science/article/pii/0165799290900055 Accessed November 26, 2015. [DOI] [PubMed] [Google Scholar]
- 16. Scherer G, Urban M, Engl J, Hagedorn HW, Riedel K. Influence of smoking charcoal filter tipped cigarettes on various biomarkers of exposure. Inhal Toxicol. 2006;18(10):821–829. doi:10.1080/08958370600747945. [DOI] [PubMed] [Google Scholar]
- 17. Sarkar M, Kapur S, Frost-Pineda K, et al. Evaluation of biomarkers of exposure to selected cigarette smoke constituents in adult smokers switched to carbon-filtered cigarettes in short-term and long-term clinical studies. Nicotine Tob Res. 2008;10(12):1761–1772. doi:10.1080/14622200802443718. [DOI] [PubMed] [Google Scholar]
- 18. Carmella SG, Chen M, Han S, et al. Effects of smoking cessation on eight urinary tobacco carcinogen and toxicant biomarkers. Chem Res Toxicol. 2009;22(4):734–741. Erratum in Chem Res Toxicol. 2012 Mar 19;25(3):763. doi:10.1021/tx800479s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. IARC (International Agency for Research on Cancer). Monographs on the Evaluation of Carcinogenic Risks to Humans: Chemical Agents and Related Occupations. Vol 100F Geneva, Switzerland: WHO Press; 2012. http://apps.who.int/bookorders/anglais/detart1.jsp?sesslan=1&codlan=1&codcol=72&codcch=6100 Accessed November 26, 2015. [Google Scholar]
- 20. Boogaard PJ, van Sittert NJ. Suitability of S-phenyl mercapturic acid and trans-trans-muconic acid as biomarkers for exposure to low concentrations of benzene. Environ Health Perspect. 1996;104(suppl 6):1151–1157. www.ncbi.nlm.nih.gov/pmc/articles/PMC1469762 Accessed November 26, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Riedel K, Scherer G, Engl J, Hagedorn HW, Tricker AR. Determination of three carcinogenic aromatic amines in urine of smokers and nonsmokers. J Anal Toxicol. 2006;30(3):187–195. doi:10.1093/jat/30.3.187. [DOI] [PubMed] [Google Scholar]
- 22. Jongeneelen FJ, Anzion RB, Scheepers PT, et al. 1-Hydroxypyrene in urine as a biological indicator of exposure to polycyclic aromatic hydrocarbons in several work environments. Ann Occup Hyg. 1988;32(1):35–43. doi:10.1093/annhyg/32.1.35. [DOI] [PubMed] [Google Scholar]
- 23. U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry. Toxicological Profile for Carbon Monoxide 2012. www.atsdr.cdc.gov/toxprofiles/tp201.pdf Accessed November 23, 2014.
- 24. Hecht SS, Carmella SG, Chen M, et al. Quantitation of urinary metabolites of a tobacco-specific lung carcinogen after smoking cessation. Cancer Res. 1999;59(3):590–596. http://cancerres.aacrjournals.org/content/59/3/590.long Accessed November 26, 2015. [PubMed] [Google Scholar]
- 25. Hecht SS. Metabolic activation and detoxification of tobacco-specific nitrosamines-a model for cancer prevention strategies. Drug Metab Rev. 1994;26(1–2):373–390. doi:10.3109/03602539409029803. [DOI] [PubMed] [Google Scholar]
- 26. Mure K, Hayatsu H, Takeuchi T, Takeshita T, Morimoto K. Heavy cigarette smokers show higher mutagenicity in urine. Mutat Res. 1997;373(1):107–111. doi:10.1016/S0027-5107(96)00195-9. [DOI] [PubMed] [Google Scholar]
- 27. Theophilus EH, Coggins CRE, Chen P, Schmidt ER, Borgerding MF. Magnitudes of biomarker reductions in response to controlled reductions in cigarettes smoked per day: a one-week clinical confinement study. Regul Toxicol Pharmacol. 2015;71(2):225–234. doi:10.1016/j.yrtph.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 28. Tricker AR, Jang IJ, Martin Leroy C, Lindner D, Dempsey R. Reduced exposure evaluation of an Electrically Heated Cigarette Smoking System. Part 4: eight-day randomized clinical trial in Korea. Regul Toxicol Pharmacol. 2012;64(suppl 2):S45–53. doi:10.1016/j.yrtph.2012.08. [DOI] [PubMed] [Google Scholar]
- 29. Marian C, O’Connor RJ, Djordjevic MV, Rees VW, Hatsukami DK, Shields PG. Reconciling human smoking behavior and machine smoking patterns: implications for understanding smoking behavior and the impact on laboratory studies. Cancer Epidemiol Biomarkers Prev. 2009;18(12):3305–3320. doi:10.1158/1055-9965.EPI-09-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rees VW, Wayne GF, Connolly GN. Puffing style and human exposure minimally altered by switching to a carbon-filtered cigarette. Cancer Epidemiol Biomarkers Prev. 2008;17(11):2995–3003. doi:10.1158/1055-9965.EPI-07-2533. [DOI] [PubMed] [Google Scholar]
- 31. Benowitz NL, Hall SM, Stewart S, Wilson M, Dempsey D, Jacob P., III Nicotine and carcinogen exposure with smoking of progressively reduced nicotine content cigarette. Cancer Epidemiol Biomarkers Prev. 2007;16(11):2479–2485. doi:10.1158/1055-9965.EPI-07-0393. [DOI] [PubMed] [Google Scholar]
- 32. Hatsukami DK, Kotlyar M, Hertsgaard LA, et al. Reduced nicotine content cigarettes: effects on toxicant exposure, dependence and cessation. Addiction. 2010;105(2):343–355. doi:10.1158/1055-9965.EPI-07-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fiore M, Baker T. Reduced nicotine cigarettes - A promising regulatory pathway. N Engl J Med. 2015;373(14):1289–1291. doi:10.1056/NEJMp1509510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hu Q, Hou H. Tobacco Smoke Exposure Biomarkers. Boca Raton, FL: CRC Press, Taylor & Francis Group; 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.