Abstract
Stress-elicited behavioral and physiologic responses vary widely across individuals and depend on a combination of environmental and genetic factors. Adolescence is an important developmental period when neural circuits that guide emotional behavior and stress reactivity are still maturing. A critical question is whether stress exposure elicits contrasting effects when it occurs during adolescence versus adulthood. We previously found that Sprague Dawley rats selectively-bred for low behavioral response to novelty (bred Low Responders; bLRs) are particularly sensitive to chronic unpredictable mild stress (CMS) exposure in adulthood, which exacerbates their typically high levels of spontaneous depressive- and anxiety- like behavior. Given developmental processes known to occur during adolescence, we sought to determine whether the impact of CMS on bLR rats is equivalent when they are exposed to it during adolescence as compared to adulthood. Young bLR rats were either exposed to CMS or control condition from postnatal day 35-60. As adults we found that CMS-exposed bLRs maintained high levels of sucrose preference and exhibited increased social exploration along with decreased immobility on the forced swim test compared to bLR controls. These data indicate a protective effect of CMS exposure during adolescence in bLR rats.
Keywords: adolescence, rat, chronic stress, depression, anxiety, social interaction
1. Introduction
Inborn differences in emotional reactivity strongly shape how individuals respond to stress and influence vulnerability to depression and anxiety. This biological endowment powerfully interacts with adverse environmental influences, such as chronic stress, to trigger pathological changes in the brain and body (Young et al., 1997). Certain individuals are more vulnerable to the adverse effects of stress than others, due to a combination of genetic predisposition and environmental risks (Caspi et al., 2003); however, neurobiological mechanisms underlying stress vulnerability (or resilience) remain elusive.
The selectively-bred High (bHR)/Low (bLR) novelty responder rats offer a useful tool to study neurobiological factors driving individual differences in emotionality and stress coping. These rats were bred based on novelty-induced exploratory activity, with bLR rats showing low novelty exploration coupled with enhanced depression- and anxiety- like behavior (Flagel et al., 2014). We previously demonstrated that bLR rats are especially susceptible to adverse consequences of chronic stress when exposed during early postnatal life or in adulthood, while bHR rats are generally stress-resilient (Stedenfeld et al., 2011 ; Clinton et al., 2014). Specifically, we found that adult bLR rats were vulnerable to the adverse effects of chronic mild intermittent stress (CMS), where rats are subjected to a variety of stressors in an unpredictable fashion over 3-4 weeks (Willner, 2005). We found that CMS exposure during adulthood exaggerated bLRs anxiety-/depressive-like behavioral phenotype, leading to greater anhedonia and hyponeophagia, while bHRs were unaffected by CMS (Stedenfeld et al., 2011).
Extensive literature illustrates adverse effects of chronic stress, although its effects vary widely depending upon the developmental time point when stress exposure occurs. For instance, infant rats exhibit a stress hyporesponsive period around the second postnatal week when stress exposure fails to activate the hypothalamic-pituitary-adrenal (HPA) system as it does in adulthood (Sapolsky & Meaney, 1986). Similarly, adolescence represents a unique neurodevelopmental period that renders adolescent rats resilient to some chronic stressors (Toth et al., 2008 ; Taliaz et al., 2011). These observations recapitulate uniqueness of pediatric depression, as depressed children and adolescents are less responsive to certain antidepressant medications (Hazell et al., 1995) and are less likely to manifest hypercortisolemia that is common in depressed adults (Badanes et al., 2011). On the other hand, social instability stress during adolescence was found to enhance anxiety-like behavior and impair later sexual behavior (Green et al., 2013 ; McCormick et al., 2013), suggesting that stress vulnerability or resilience during adolescence depends greatly on the nature of stressors. Together these observations indicate that adolescence is a unique developmental period characterized by stress resilience or vulnerability depending on the nature of stress exposure.
Adolescence is characterized by the maturation of social and cognitive processes during the transition from childhood to adulthood, and as such represents a critical developmental period (Suo et al., 2013). In rodents behavioral and neurobiological changes that take place between postnatal days 30 and 60 are reminiscent of adolescent transitioning from childhood to adulthood in humans (Eiland & Romeo, 2013). Stress exposure during this adolescent period alters structural and functional organization specifically within the limbic and cortical regions, and it can have a life-long impact on behavior and physiology (reviewed in (Eiland & Romeo, 2013)). However, the factors that lead to either vulnerability or resiliency later in life are not well understood, with a number of studies reporting that adolescent stress exposure increases anxiety- and/or depressive- like behavior in adulthood (Pohl et al., 2007 ; Tsoory et al., 2007 ; McCormick et al., 2008) while other studies report increased resilience (Kendig et al., 2011 ; Buwalda et al., 2013 ; Suo et al., 2013). These contradicting findings suggest that the adolescent period is complex and dynamic in nature, where multiple factors determine the consequences of stress exposure.
Given bLR rats' propensity to high depression- and anxiety-like behavior along with our observations of their susceptibility to CMS exposure in adulthood (Stedenfeld et al., 2011), the current study asked whether bLRs are susceptible to CMS during adolescence. We found that CMS exposure in adolescence (unlike exposure during adulthood) elicited protective effects in bLRs, leading to diminished depressive-like behavior and augmented social behavior.
2. Methods
2.1 Animals
All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham and conformed to the Guide for the Care and Use of Laboratory Animals (National Research Council 2011). Adult male and female bLR Sprague-Dawley rats from the 30th generation of the University of Michigan bLR/bHR colony were mated to produce male bLR offspring (n=18) for the present experiments. Animal housing rooms and testing facilities were kept at 21-23°C at 50-55% humidity. Male bLR offspring derived from 10 bLR litters were weaned at age postnatal day (P)21. Rats were divided into experimental groups that would later be subjected to a 4-wk CMS procedure (described below), or control handling condition (n=9 male bLRs/condition). Each litter provided two male bLR pups for the study, with one male sibling assigned to control condition and the other male sibling assigned to CMS condition. Therefore, rats from the same litter were not housed together. Rats were housed 3 per cage in a 12:12 light-dark cycle (lights on/off at 6 AM/6 PM) with food and water available ad libitum except when mentioned.
2.2 Chronic Mild Stress (CMS) Paradigm
One group of male bLR rats was exposed to a 4-week CMS paradigm from P35 to P60 (bLR-CMS, n=9), which corresponds to the adolescent period in rats (Eiland & Romeo, 2013). Control rats of the same age (bLR-con, n=9) were handled according to standard animal care protocol (bi-weekly cage changes). The CMS protocol was similar to the one from our previous study (Stedenfeld et al., 2011) and included: damp bedding (300-500 ml lukewarm water added to bedding); intermittent white noise (radio static at ∼85dB, from 2-5 h); continuous overnight lighting; overnight food deprivation; overnight water deprivation occasionally followed by empty water bottle replacement for 1-2 h; 40-degree cage tilt; stroboscopic lighting (10 flashes per second at 20 watts for 3-5 h; Eliminator Lighting E-105 strobe light); and 1 h exposure to predator odor – 10 μl undiluted 2,4,5-trimethylthiazoline (Pherotech International) dropped on a piece of filter paper and placed in cage. All CMS rats were exposed to the same schedule of stressors; multiple stressors occasionally overlapped in varying order across different weeks and were presented in an intermittent fashion with increasing frequency of presentation during the four-week CMS period (Fig. 1).
Figure 1.
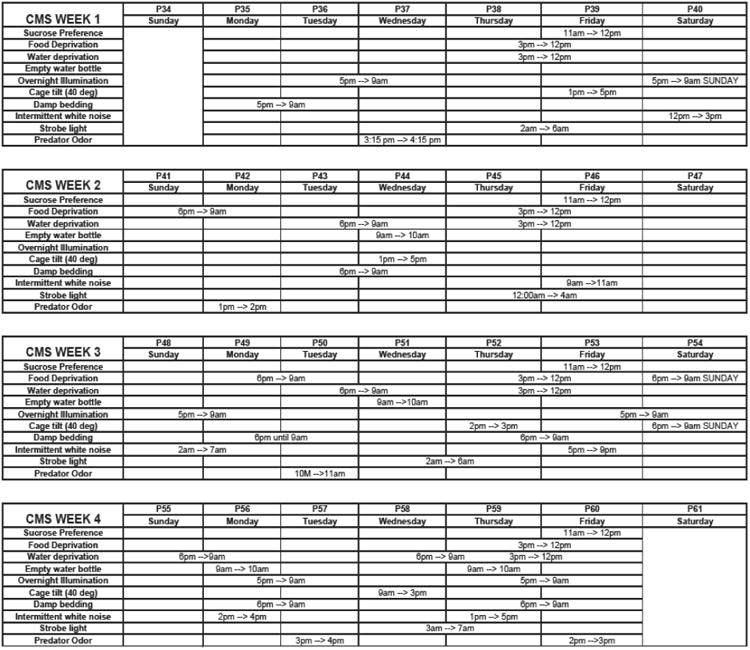
Weekly schedule of stressor exposures. Weekly schedule of stressors are presented chronologically (from top to bottom panel). The stress paradigm lasted from postnatal day (P)35 – P60. The number and intensity of stressors was increased gradually from Week 1 to Week 4. CMS – chronic mild unpredictable stress.
2.3 Behavior Test Battery
Behavior testing began on P66 and was conducted under dim light conditions (30 lux) between 8:30 a.m. – 11:30 a.m. except for the sucrose preference test (SPT), which was conducted between 11:00 a.m. – 12:00 p.m. Rats were assessed on the following tests: novelty suppressed feeding (NSF), social interaction (SI), and forced swim test (FST). Animals were habituated to the testing room overnight in their home cages prior to NSF and SI, but not for FST. The same animals were assessed across all behavioral tests with at least 2 days of resting in between tests.
2.3.1 Sucrose preference test (SPT)
SPT measures anhedonia and is reduced by stress exposure (Willner et al., 1987 ; Stedenfeld et al., 2011). The test was conducted once per week at ages: P39, P46, P53, and P60 between 11:00 a.m. – 12:00 p.m. at the end of each CMS week. Rats were habituated to sucrose solution for 4 days prior to the testing. Rats were deprived of food and water overnight (starting from 3pm previous day) prior to testing as previously described (Stedenfeld et al., 2011). Rats that were group-housed three per cage during the CMS procedure were briefly placed in a new cage individually during the 1 hour testing period. Rats were given choice to drink freely among the two bottles that were placed in the cage, one of which contained water and the other contained 1% sucrose solution during the period of one hour. The bottles were weighed before and after the testing period. During each successive testing day, the sides of the water and sucrose bottle were switched to avoid the placement preference. Sucrose preference was calculated from data obtained from the 4 testing sessions as follows: (volume of sucrose solution consumed/total volume of liquid consumed) × 100%.
2.3.2 Novelty-suppressed feeding (NSF)
NSF test provides an anxiety-related measure that is modifiable by chronic antidepressant treatment (Dulawa & Hen, 2005). NSF testing was performed at P66 when rats were food-deprived overnight, habituated to the testing room in their homecage, and were then tested in a novel environment in the Noldus PhenoTyper® Boxes (45 × 45 × 60 cm clear Plexiglas chambers). A single food pellet of standard rat chow was placed in the center of the chamber before the start of the test. Then a rat was placed in the periphery of the box and permitted to freely explore for 15-min. Latencies to sniff, touch, and eat the food pellet were measured by a trained observer blinded to experimental groups. The animals' movement was assessed by Ethovision® XT 8.0 software. Food pellet weight was manually measured before and after each session.
2.3.3 Social Interaction
To evaluate exploration of novel social stimuli, we utilized our previously published testing paradigm where the test rat was sequentially exposed to a novel object, a novel male, and a novel female (Nam et al., 2014 ; Cohen et al, 2015). The social interaction test was conducted over a 3-day period (P73-75), with each daily session lasting 10-min. The rats were habituated to the testing room in their homecage overnight, and the test was conducted in a Noldus PhenoTyper® Cage (a 45 × 45 × 60 cm clear Plexiglas chamber outfitted with a camera), which contained a smaller interaction box (10 × 10 × 8 cm clear Plexiglas box with breathing holes) placed in the corner. This interaction box was used to house the novel stimulus male or female rat that the experimental animal could interact with during a test session. On Day 1 of the test, experimental rats were placed in the test chamber containing an empty interaction box. On Day 2, experimental rats encountered an interaction box containing a novel male rat. On Day 3, the interaction box contained a female stimulus rat. Stimulus rats were age- and strain-matched, and were previously habituated to the interaction box by 10-15 min. exposure on 3 consecutive days. Each stimulus rat was used for a single test trial per day. In order to assess the experimental animals' level of social interaction, we defined specific zones in the Phenotype chamber as either zones of interaction or avoidance. Thus, a 2-cm-wide zone around the interaction box was designated as the interaction zone, and a 10 × 10 cm zone in the corner diagonal from the interaction box was considered the avoidance zone. Time spent in each zone and number of visits to each zone during the 3 10-min test sessions were quantified with an Ethovision® XT 8.0 videotracking system.
2.3.4 Forced Swim Test (FST)
FST was performed at age P79 (Day 1), and P80 (Day 2), where the testing protocol was performed as described by Cryan et al. (2005) in clear Plexiglas cylinders (40 cm high × 40 cm diameter) containing 30-cm deep water (25°C). On day 1, rats were placed (one rat/cylinder) in the water for 15-min; 24 h later the rats were returned to the water-filled cylinder and tested for 5-min (day 2). Water was changed after every swim session so that every rat was swimming in clean water. Each rat's FST behavior was digitally recorded, and immobility and velocity was scored by the Ethovision® XT 8.0. We focused on the immobility measure since it is classically considered an indicator of behavioral despair and depressive-like behavior (Porsolt et al., 1977), and it can be clearly defined and easily distinguishable from active coping measures such as swimming and climbing, which are sometimes difficult to reliably distinguish across experimental observers (Porsolt et al., 1977; Cryan et al., 2005; Muigg et al., 2007).
2.4 Tissue Collection and Processing
Five days after the final behavioral test, rats were decapitated and trunk blood was collected in EDTA-coated tubes between 8:00 a.m. – 11:30 a.m.. At this time rats were 85 days old. The timeframe between removing the rats from their housing room and homecage to decapitation was less than 2-3 min. Whole blood was allowed to clot, centrifuged at 3,220g for 20 min at 4°C to collect serum, and stored at -80°C. Corticosterone levels were assayed using ELISA (ADI-900-097, Enzo Life Sciences) per manufacturer instructions.
2.5 Statistical Analyses
Data were analyzed using GraphPad Prism 6 (http://www.graphpad.com/); normality was tested with the D'Agostino & Pearson test and presence of potential outliers was tested using the ROUT method with Q set at 1%. Groups were compared with two-tailed Student's t-test, or Mann-Whitney U test if not normally distributed. Sucrose preference and FST data were analyzed using repeated measures two-way ANOVA with time and treatment as factors; Sidak test was used post-hoc where indicated. Data are presented as mean ± SEM.
3. Results
Young bLR male rats exposed to four weeks of CMS were resistant to stress-evoked anhedonia or hyponeophagia. They did not diminish their preference for sucrose during the four weeks of CMS (F (1,16) = 0.58, p > 0.05; Fig. 2A), and did not differ from their non-stressed counterparts in the NSF test (latencies to interact with or consume the food pellet, total exploration, or total food consumption; Table 1). By contrast, CMS-exposed bLRs showed greater drive to interact with female stimulus rats in the SI test compared to bLR controls. We calculated ratios for the frequency of visits to the female zone vs. the novel box (female/novel box ratio) and for the frequency of visits to the female vs. the male zone (female/male ratio). bLR-CMS rats exhibited significant increases in the female/novel box ratio (t(15) = 2.93, p < 0.05; Fig. 2B) as well as in the female/male ratio (U = 14, p < 0.05; Fig. 2C). Time spent within the social interaction zone (a 2 cm-wide zone surrounding the interaction box containing the stimulus rat) was not different when the interaction box was empty or contained a novel stimulus rat. When the interaction box was empty, both groups spent a comparable amount of time in the social interaction zone (bLR-con: 79.8 ±10.5 sec vs. bLR-CMS: 65.0 ±21.9 sec; p = 0.17). When the interaction box contained a male stimulus rat, the groups again spent similar amounts of time in the social interaction zone (bLR-con: 164.3 ± 26.7 sec vs. bLR-CMS: 221.8 ± 62.3 sec; p = 0.41). Finally, when the interaction box contained a female stimulus rat, there were again no group differences in time spent in the social interaction zone (bLR-con: 134.0 ± 15.8 sec vs. bLR-CMS: 145.2 ± 22.0 sec; p = 0.69).
Figure 2.
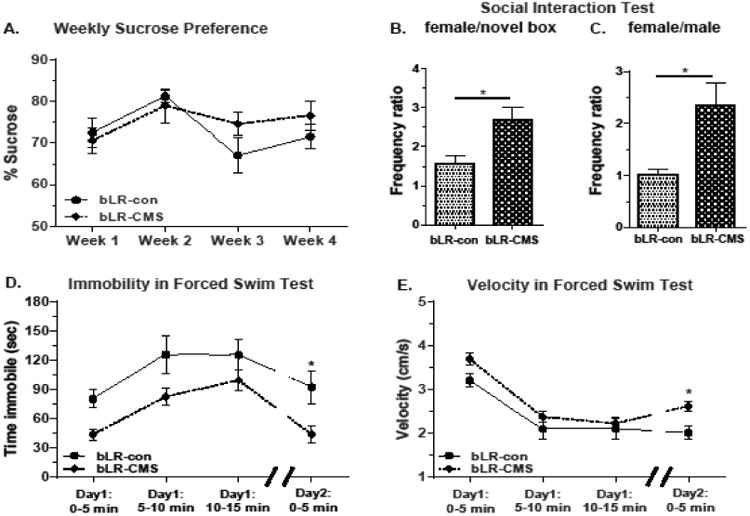
Selectively-bred low responder (bLR) rats exposed to chronic mild stress (CMS) during adolescence did not diminish their preference for sucrose (A). They exhibited increased ratios of transitions to the female zone relative to either: a novel box (B) or a novel male (C). CMS rats also displayed shorter duration of immobility (D) and greater velocity of movement (E) on the forced swim test. * – p < 0.05.
Table 1.
Effects of CMS on bLR behavior in the novelty suppressed feeding test.
| bLR-con | bLR-CMS | p | |
|---|---|---|---|
| Total distance moved (cm) | 1,092.0 ± 89.0 | 1,019.0 ± 166.1 | 0.70 |
| Latency to touch food (sec) | 439.2 ± 93.5 | 607.9 ± 118.8 | 0.28 |
| Latency to eat food (sec) | 610.1 ± 86.0 | 741.4 ± 82.4 | 0.29 |
| Total food consumed (g) | 0.49 ± 0.1 | 0.31 ± 0.2 | 0.27 |
CMS exposure also appeared to improve bLRs' typically high level of depression-like behavior in the FST. CMS-exposed bLRs displayed diminished bLRs' FST immobility relative to control bLRs (F(1,16) = 9.17, p < 0.01; Fig. 2D) and increased velocity of movement (F(1, 16) = 5.93, p < 0.05; Fig. 2E). Post-hoc testing indicated significant (p < 0.05) differences in immobility (Fig. 2D) and velocity (Fig. 2E) on Day 2 of FST. Adolescent CMS exposure did not impact bLRs' circulating corticosterone levels (22.0 ± 3.2 ng/ml [bLR-con] vs. 32.6 ± 6.7 ng/ml [bLR-CMS], p = 0.36).
4. Discussion
The present study was motivated by our previous observations of bLR rats' selective vulnerability to chronic stress exposure during early postnatal life or during adulthood. We found that repeated maternal separation during the first two postnatal weeks (a model of maternal neglect (Ladd et al., 2000)), or CMS exposure in adulthood increased bLRs' depressive- and anxiety- like behaviors, while bHRs were relatively resistant to the stressors (Stedenfeld et al., 2011 ; Clinton et al., 2014). When tested as adults, maternally-separated bLR rats exhibited potentiated stress-induced defecation and augmented stress-induced activation of the HPA axis (Clinton et al., 2014). Similarly, when exposed to CMS as adults, bLR rats developed a dramatic loss of sucrose preference along with increased latencies to eat food in the NSF test (Stedenfeld et al., 2011). In contrast to these previous observations, our current data indicate that CMS exposure in adolescent bLR rats does not elicit these effects, with no CMS-induced changes in sucrose preference, hyponeophagia, or circulating corticosterone levels. Furthermore, CMS appears to be protective for adolescent bLRs, leading to enhanced social interaction and diminished depression-like behavior in the FST.
Other studies indicate that adolescence is a unique developmental period characterized by resilience to CMS. Zangen and co-workers demonstrated that CMS exposure in adult (60 day-old) Sprague-Dawley rats elicited decreased sucrose preference and open field exploration (Toth et al., 2008). However, CMS exposure in young (30 day-old) rats did not affect behavior (Toth et al., 2008 ; Taliaz et al., 2011). These findings are consistent with our previous observations that adult CMS exposure causes bLRs to lose their preference for sucrose (Stedenfeld et al., 2011), as well as our current findings that young CMS-exposed bLRs maintain their sucrose preference. Toth, et al. also reported decreased morning corticosterone levels in rats exposed to CMS as adults, but not those exposed during adolescence (Toth et al., 2008). Our results are consistent with these findings as well, since we found no effect of CMS on bLRs' circulating corticosterone levels. Although adolescent rats seem to be resilient to detrimental effects of CMS, research shows that they may be vulnerable to stressors influencing the social domain and the results further depend on the experimental design, age and sex of the rats (Buwalda et al., 2011).
Beyond resilience to anhedonia and hyponeophagia, CMS exposure in young bLR rats elicited positive effects on social interaction and FST immobility, indicating a decrease in depressive-like behaviors. These observations suggest that CMS exposure in adolescence may “inoculate” susceptible bLR rats against future stressful events. This notion is consistent with studies in rodents and primates demonstrating that consistent exposure to manageable stressors during development leads to decreased stress-evoked behavioral and hormonal responses in adulthood (Meaney & Szyf, 2005 ; Lyons & Parker, 2007).
One limitation of the present study is that we focused solely on the effects of CMS on adolescent bLR rats, but not bHRs. We chose to focus on bLR rats due to our previous observations that bLRs are generally more influenced by environmental factors, including prenatal stress (Clinton et al., 2008), early-life maternal separation stress (Clinton et al., 2014), cross-fostering (Cohen et al., 2015), and CMS exposure during adulthood (Stedenfeld et al., 2011), while bHRs are resilient to such interventions. Nonetheless, it would be useful to determine the effect of CMS exposure in adolescent bHRs, particularly because we found unexpected beneficial effects in bLRs. Moreover, it would be interesting to consider applying different types of stress in adolescent bLR/bHR animals (i.e. CMS vs. social instability stress), since disparate stress paradigms may trigger unique effects in either strain.
Acknowledgments
This study was funded by NIMH grants MH081927 (IAK), MH085859 (SMC), and MH105447 (SMC); NARSAD Young Investigator award (IAK); and American Heart Association Pre-doctoral Fellowship 13PRE16940050 (SR); Office of Naval Research ONR- N00014-09-1-0598 (HA), and NIDA PPG 5P01DA021633-02 (HA).
Footnotes
Conflict of Interest Statement: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Badanes LS, Watamura SE, Hankin BL. Hypocortisolism as a potential marker of allostatic load in children: associations with family risk and internalizing disorders. Development and psychopathology. 2011;23:881–896. doi: 10.1017/S095457941100037X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buwalda B, Geerdink M, Vidal J, Koolhaas JM. Social behavior and social stress in adolescence: a focus on animal models. Neurosci Biobehav Rev. 2011;35:1713–1721. doi: 10.1016/j.neubiorev.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Buwalda B, Stubbendorff C, Zickert N, Koolhaas JM. Adolescent social stress does not ecessarily lead to a compromised adaptive capacity during adulthood: a study on the consequences of social stress in rats. Neuroscience. 2013;249:258–270. doi: 10.1016/j.neuroscience.2012.12.050. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Clinton S, Miller S, Watson SJ, Akil H. Prenatal stress does not alter innate novelty-seeking behavioral traits, but differentially affects individual differences in neuroendocrine stress responsivity. Psychoneuroendocrinology. 2008;33:162–177. doi: 10.1016/j.psyneuen.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton SM, Watson SJ, Akil H. High novelty-seeking rats are resilient to negative physiological effects of the early life stress. Stress. 2014;17:97–107. doi: 10.3109/10253890.2013.850670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JL, Glover ME, Pugh PC, Fant AD, Simmons RK, Akil H, Kerman IA, et al. Maternal Style Selectively Shapes Amygdalar Development and Social Behavior in Rats Genetically Prone to High Anxiety. Dev Neurosci. 2015;37:203–214. doi: 10.1159/000374108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulawa SC, Hen R. Recent advances in animal models of chronic antidepressant effects: the novelty-induced hypophagia test. Neuroscience and biobehavioral reviews. 2005;29:771–783. doi: 10.1016/j.neubiorev.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Eiland L, Romeo RD. Stress and the developing adolescent brain. Neuroscience. 2013;249:162–171. doi: 10.1016/j.neuroscience.2012.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Waselus M, Clinton SM, Watson SJ, Akil H. Antecedents and consequences of drug abuse in rats selectively bred for high and low response to novelty. Neuropharmacology. 2014;76 Pt B:425–436. doi: 10.1016/j.neuropharm.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MR, Barnes B, McCormick CM. Social instability stress in adolescence increases anxiety and reduces social interactions in adulthood in male Long-Evans rats. Dev Psychobiol. 2013;55:849–859. doi: 10.1002/dev.21077. [DOI] [PubMed] [Google Scholar]
- Hazell P, O'Connell D, Heathcote D, Robertson J, Henry D. Efficacy of tricyclic drugs in treating child and adolescent depression: a meta-analysis. Bmj. 1995;310:897–901. doi: 10.1136/bmj.310.6984.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendig MD, Bowen MT, Kemp AH, McGregor IS. Predatory threat induces huddling in adolescent rats and residual changes in early adulthood suggestive of increased resilience. Behavioural brain research. 2011;225:405–414. doi: 10.1016/j.bbr.2011.07.058. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM. Long-term behavioral and neuroendocrine adaptations to adverse early experience. Progress in brain research. 2000;122:81–103. doi: 10.1016/s0079-6123(08)62132-9. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Parker KJ. Stress inoculation-induced indications of resilience in monkeys. J Trauma Stress. 2007;20:423–433. doi: 10.1002/jts.20265. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Green MR, Cameron NM, Nixon F, Levy MJ, Clark RA. Deficits in male sexual behavior in adulthood after social instability stress in adolescence in rats. Horm Behav. 2013;63:5–12. doi: 10.1016/j.yhbeh.2012.11.009. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Smith C, Mathews IZ. Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behavioural brain research. 2008;187:228–238. doi: 10.1016/j.bbr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues in clinical neuroscience. 2005;7:103–123. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam H, Clinton SM, Jackson NL, Kerman IA. Learned helplessness and social avoidance in the Wistar-Kyoto rat. Frontiers in behavioral neuroscience. 2014;8:109. doi: 10.3389/fnbeh.2014.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl J, Olmstead MC, Wynne-Edwards KE, Harkness K, Menard JL. Repeated exposure to stress across the childhood-adolescent period alters rats' anxiety- and depression-like behaviors in adulthood: The importance of stressor type and gender. Behavioral neuroscience. 2007;121:462–474. doi: 10.1037/0735-7044.121.3.462. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain research. 1986;396:64–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- Stedenfeld KA, Clinton SM, Kerman IA, Akil H, Watson SJ, Sved AF. Novelty-seeking behavior predicts vulnerability in a rodent model of depression. Physiology & behavior. 2011;103:210–216. doi: 10.1016/j.physbeh.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo L, Zhao L, Si J, Liu J, Zhu W, Chai B, Zhang Y, et al. Predictable chronic mild stress in adolescence increases resilience in adulthood. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:1387–1400. doi: 10.1038/npp.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taliaz D, Loya A, Gersner R, Haramati S, Chen A, Zangen A. Resilience to chronic stress is mediated by hippocampal brain-derived neurotrophic factor. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:4475–4483. doi: 10.1523/JNEUROSCI.5725-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth E, Gersner R, Wilf-Yarkoni A, Raizel H, Dar DE, Richter-Levin G, Levit O, et al. Age-dependent effects of chronic stress on brain plasticity and depressive behavior. Journal of neurochemistry. 2008;107:522–532. doi: 10.1111/j.1471-4159.2008.05642.x. [DOI] [PubMed] [Google Scholar]
- Tsoory M, Cohen H, Richter-Levin G. Juvenile stress induces a predisposition to either anxiety or depressive-like symptoms following stress in adulthood. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2007;17:245–256. doi: 10.1016/j.euroneuro.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology. 1987;93:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- Young EA, Abelson JL, Curtis GC, Nesse RM. Childhood adversity and vulnerability to mood and anxiety disorders. Depression and anxiety. 1997;5:66–72. [PubMed] [Google Scholar]


