Abstract
Cytomegalovirus (CMV) infection following kidney transplantation is associated with increased morbidity and mortality. In this case report we describe a case of a 23 year-old female with an unusual presentation of diffuse CMV lymphadenitis following kidney transplantation who did not respond to gangiclovir therapy, This case highlights the atypical presentation of CMV disease in a kidney transplant recipient, importance of CMV hypergammaglobulin in the treatment of CMV infection post kidney transplantation, and the difficulties in transitions of care from pediatric to adult transplant programs.
CASE PRESENTATION
A 23 year old Caucasian female presented to our kidney transplant clinic after being referred by her primary nephrologist for persistent lymphadenopathy.
The patient was initially diagnosed with kidney disease at age 14 after presenting with increased lower extremity swelling and nephrotic range proteinuria. She received high dose steroids for a presumptive diagnosis of minimal change disease; however, her course did not improve and she subsequently underwent percutaneous kidney biopsy revealing focal segmental glomerulosclerosis (FSGS). Genetic testing revealed that she was compound heterozygote for NPHS2. The patient was treated with cyclosporine for steroid resistant FSGS. Over the next 3 years the patient received cyclophosphamide, mycophenolate mofetil and tacrolimus, all of which failed to reduce proteinuria. She was then referred to another center where she received rituximab therapy, but developed acute shortness of breath following her fourth dose and the regimen was discontinued. All immunosuppressive medications were discontinued.
Four years after initial presentation, and one year after discontinuation of immunosuppressive therapy, the patient developed renal failure and was started on peritoneal dialysis. She remained on dialysis for nine months before undergoing living related kidney transplant from her father at age 19 in the pediatric transplant program. The patient received ten sessions of plasmapheresis and mycophenolic acid for two weeks prior to transplantation to prevent recurrence of FSGS. She was induced with daclizumab and solumedrol and transitioned to a steroid free protocol with tacrolimus and mycophenolic acid as maintenance therapy by post operative day 3. Her renal function improved from an admission creatinine of 6.8 mg/dl to 1.1 mg/dl by post operative day 4. Urine protein was monitored post transplant for recurrence of FSGS and reached a peak of 1.31 gm/gm before decreasing to 0.20 at time of hospital discharge. She received antimicrobial prophylaxis with Bactrim for one year and valganciclovir for 6 months as her donor was CMV positive and the patient was CMV negative.
The patient continued to do well until post operative month 8 when she was found to have low level CMV viremia on surveillance screening, with a viral load of 8000 copies. She was restarted on valcyte 900 mg twice a day until levels were undetectable and then continued on valcyte 450 mg daily by her local nephrologist. Her mycophenolate mofetil was temporarily reduced to 500 mg twice a day until her CMV viremia resolved.
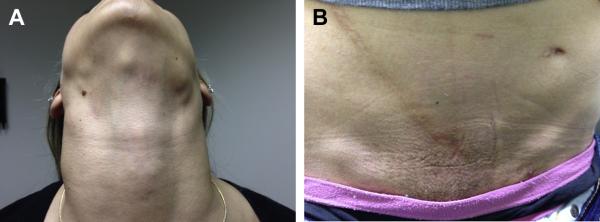
Three years post transplant, and eight months prior to being seen, the patient developed a sore throat with bilateral cervical lymphadenopathy. She was started on oral antibiotics for strep throat and her sore throat resolved, but her lymphadenopathy persisted. The patient underwent cervical lymph node biopsy one month later, which showed lymph node hyperplasia with normal histology. The patient's lymphadenopathy slowly improved over the course of the next three months without therapy, though did not completely resolve. One month prior to presentation the patient developed recurrent night sweats with worsening cervical lymphadenopathy and new inguinal lymphadenopathy as well as fatigue and malaise (figure 1). Physical examination also revealed axillary lymphadenopathy and splenomegaly. By admission, the patient stated that she had minimized her symptoms and had been slow to seek medical attention due to a desire not to interrupt her college education.
FIGURE 1.
1A: Patient with enlargement of multiple lymph nodes (arrows) in the cervical and submandibular chains
1B: Enlarged inguinal lymph nodes (arrows) on examination
Given that she was now considered too old for follow-up in the pediatric kidney transplant clinic, she was referred to our adult kidney transplant program. Initial laboratory testing demonstrated stable kidney function with a creatinine of 1.0 mg/dl, low level CMV PCR at < 137 copies/ml, no detectable EBV on PCR, and negative HHV 8 (see Table 1 for additional laboratory studies). She underwent PET/CT of the head, neck, chest, abdomen and pelvis which revealed extensive bilateral lymphadenopathy with intense FDG activity involving neck levels I through V, bilateral hilar, mediastinal, paratracheal, subcarinal, retroperitoneal, axillary, common iliac, internal and external iliac, and inguinal lymph nodes. Excisional biopsy of the left inguinal lymph node was performed. The specimen contained a single lymph node measuring 4.0 × 1.8 × 1.4 cm that was sectioned for microscopy with immunohistochemistry (figures 2, 3) and flow cytometry (figure 4). Microscopic examination revealed and enlarged lymph node follicular and parafollicular hyperplasia with preserved architecture. Warthrin, AFB and GMS stains were negative for microorganisms. Immunohistochemistry demonstrated positive CMV within scattered large cells and focal EBV staining (figure 3). Flow cytometry of the specimen demonstrated polymorphic B cell proliferation and no T cell aberrancies.
TABLE 1.
Laboratory Data
| 1 year prior | At presentation | Normal Values | |
|---|---|---|---|
| WBC | 3.57 | 3.86 | 4.16-9.95 ×103/ μL |
| Hemoglobin | 11.7 | 12.5 | 11.6-15.2 g/dL |
| Hematocrit | 35.9 | 37.9 | 34-42.1% |
| Platelet Count | 223 | 186 | 143-398 ×103/ μL |
| Sodium | 139 | 140 | 135-145 mmol/L |
| Potassium | 4.4 | 4.4 | 3.6-5.4 mmol/L |
| Chloride | 107 | 106 | 98-108 mmol/L |
| Bicarbonate | 22 | 22 | 20-29 mmol/L |
| BUN | 20 | 22 | 7-23 mg/dL |
| Creatinine | 1.2 | 1.1 | 0.5-1.3 mg/dL |
| Tacrolimus | 7.7 | 6.1 | NO REF RANGE ng/mL |
| CMV PCR | < 500 copies | < 137 copies | Not detectable |
| EBV PCR | Not detectable | Not detectable | Not detectable |
| HIV PCR | n/a | Not detectable | Not detectable |
| HHV 6/8 Ab titer | n/a | < 1:20 | < 1:20 |
| Bartonella Henselae | n/a | Not detectable | Not detectable |
| Bartonella Quintana | n/a | Not detectable | Not detectable |
| Coxsackei A/B Ab | n/a | < 1:8 | <1:8 |
| Echovirus | n/a | < 1:8 | <1:8 |
| Toxoplasmosis Gondii PCR | n/a | Not detectable | Not detectable |
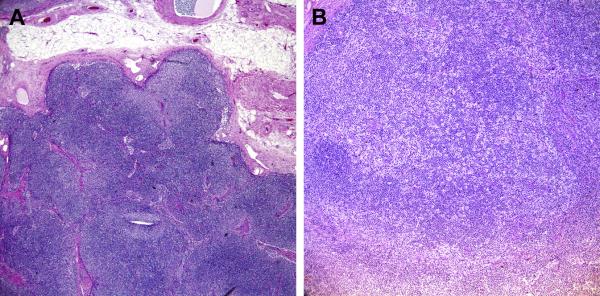
FIGURE 2.
FIGURE 2A: Light microscopy of lymph node
40× Formalin architecture” (Formalin fixation; 40× magnification): Sections demonstrate interfollicular expansion by polymorphous lymphocytes including clusters of “monocytoid B-cells” (paler areas). The background shows increased vascularity.
FIGURE 2B “100X B5 mottled areas” (B5 Fixation; 100X magnificiation): Sections demonstrate increased T-zone histiocytes, imparting a “mottled” appearance to the nodal architecture.
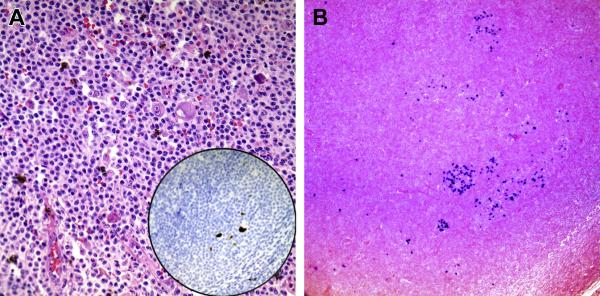
FIGURE 3.
FIGURE 3A: CMV stain
“CMV inset” (B5 Fixation; 400X magnificaition): Sections demonstrate enlarged histiocytes with characteristics of CMV infected cells embedded in a background of monocytoid B-cells. Inset demonstrates the CMV immuohistochemical stain showing positivity in these large cells.
FIGURE 3B: EBV stain
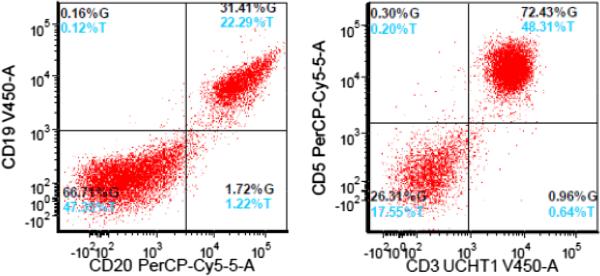
FIGURE 4.
Flow cytometry results. The lymphocyte gate comprised 82% of total cells and consists of T-cells (50%) and polytypic B-cells (32%). T-cells show no discrete phenotypic aberrancies. No monotypic B-cell population detected.
Pathology was reviewed by hematology/oncology specialists and thought to be consistent with CMV lymphadenitis, with no evidence of post-transplant lymphoproliferative disorder. Given the presumptive diagnosis of localized CMV disease of the lymph nodes, her mycophenolate mofetil with discontinued and she was started with IV ganciclovir 5mg/kg every twelve hours for a total of two weeks. However, the patient's lymphadenopathy and night sweats worsened during treatment. Given her locally invasive disease that was unresponsive to ganciclovir she was started on CMV enriched IVIG (Cytogam) at a dose of 150 mg weekly for 6 weeks in conjunction with oral valganciclovir 900 mg by mouth twice daily. After the first dose of IVIG her lymphadenopathy markedly improved and resolved after completing her IVIG course. She received oral valganciclovir for an additional 8 weeks. Repeat quantitative CMV PCR of blood was repeatedly negative or positive at <137 copies/ml. At time of last follow up 9 months after the initiation of antiviral treatment the patient remained on tacrolimus monotherapy and had no signs of recurrent lymphadenopathy. She is now being continued on maintenance therapy with valganciclovir at 450 mg by mouth twice daily.
Discussion
The patient presented in this case developed generalized lymphadenopathy in the setting of immunosuppression. The presence of diffuse lymph node enlargement in multiple regions suggests a systemic cause for her lymphadenopathy, which are most commonly caused by systemic infection or hematologic malignancy. In a post kidney transplant patient post-transplant lymphoproliferative disorder (PTLD) should be suspected. PTLD is the most common malignancy, with the exception of skin cancer, after solid organ transplant in adults and occurs in up to 10% of patients. Infectious causes of diffuse lymphadenopathy in the immunosuppressed individual include: EBV, CMV, tuberculosis, toxoplasmosis, brucellosis, endemic fungi such as cryptococcosis, histoplasmosis, or coccidioidomycosis, primary HIV infection, Castelman's disease, and Kikuchi's disease.
Her presentation was particularly worrisome given the size and extent of her lymph nodes (> 1 cm), the presence of lymphadenopathy for over 1 month, rapidity of node growth in the weeks prior to referral, and the presence of night sweats, so called B symptoms. These are all indications for lymph node biopsy to rule out malignancy. Open lymph node biopsy is the gold standard for diagnosis, as it provides both histology and architectural evaluation, and additional tissue for flow cytometry and cytogenetic screening.
In the case described the biopsy was consistent with CMV lymphadenitis. Cytomegalovirus (CMV) is a major pathogen in the immunocompromised host. Viremia, even without active disease, is associated with increased mortality and allograft failure [1-4]. The virus is a member of the herpesvirus family, and like other members in this family establishes a latent infection following resolution of an acute infection. Seroprevalence increases with age, with 47% of individuals age 10 to 12, 68% age 15 to 35 and 81% of age 36 to 60 years olds being seropositive [5,6]. Approximately two-thirds of transplant donors and recipients are seropositive at time of transplantation [7,8]. Primary infection may occur when a CMV seronegative solid organ transplant recipient receives an organ from a CMV seropositive donor, as was the case in our patient. Alternatively, immunosuppression used for transplantation can lead to reactivation of latent virus [9]. The importance of CMV disease in transplantation has resulted in the implementation of post transplant protocols for prevention and screening utilizing either preemptive therapy if screening CMV PCR testing is positive or prophylactic therapy with oral valganciclovir. There is no consensus at this time as to whether prophylaxis or preemptive therapy is the better approach for prevention of CMV infection in kidney transplant recipients [10-12].
Asymptomatic CMV infection with viremia must de distinguished from clinically active or invasive disease. Asymptomatic CMV infection presents with evidence of CMV on quantitiative blood PCR. Serological and qualititative measurements are insufficient for the diagnosis of CMV transmission or reactivation. Treatment for asymptomatic CMV viremia typically involves discontinuation of the anti-metabolite and initiation of oral valganciclovir 900 mg twice a day for three weeks or until CMV PCR is negative on two consecutive measurements one week apart; this is followed by one month of valganclovir prophylaxis [13]. Our patient had evidence of tissue invasive disease, which necessitated more aggressive therapy with intravenous ganciclovir at a dose of 5 mg/kg for a 2 to 4 week course [14-17].
Patients previously treated with valganciclovir or ganciclovir may develop viral resistance, usually manifesting as continued disease or a persistent elevated viral load after greater than 5 months of therapy, a rising viral load while on appropriate therapy, or rebound in viral load following initial decline [18,19]. When resistance is suspected testing for UL97 kinase and UL54 polymerase is indicated to further to determine therapy. UL97 kinase results in ganciclovir resistance and the UL54 polymerase results in resistance to ganciclovir, foscarnet, and cidofovir. However, in order to test for resistance there must be > 1000 viral copies in the blood. The patient in this case did not have enough viral copies to test for drug resistance despite multiple lymph node involvement. While PCR values of > 10,000 copies predict a high risk for tissue invasive disease, levels may be undetectable or occur at low copy numbers in patients with tissue invasive disease [20]. Additional therapy in these cases includes foscarnet, cidofovir, off label use of leflunamide and CMV-IVIG.
Diffuse lymphadenopathy as seen in this case is rare in the immunocompetent host, and has not been described following solid organ transplantation. In addition, progressive CMV disease is uncommon in the setting of ongoing suppressive therapy, such as our patient was receiving. This unusual case raises the possibility of an immune reconstitution inflammatory syndrome (IRIS) as has been previously described in patients with HIV, perhaps triggered by reduction in immunosuppression and made possible by her young age and active proinflammatory immune response. As has been observed in HIV-positive patients with IRIS, treatment of the underlying infection leads to resolution of the inflammatory reaction.
The patient's young age also raises the issue of transition from pediatric to adult transplant follow-up. This transition may have slowed the process of re-referral to the primary transplant center, requiring referral to a new transplant team without previous knowledge of the patient's post-transplant course. The difficulties of this transition, and the challenges in compliance and timely referral for medical complications in the adolescent and young adult population suggest that a proactive plan for ‘graduation’ from the pediatric to the adult transplant program improves patient outcomes.
Highlights.
We present an unusual presentation of CMV lymphadenitis in a kidney transplant patient
We present a case of CMV disease refractory to ganciclovir therapy with marked improvement on administration of CMV hypergammaglobulin
We highlight the importance of the transition from pediatric to adult medicine
References
- 1.Kanter J, Pallardo L, Gavela E. Cytomegalovirus infection renal transplant recipients: risk factors and outcome. Transplant Proc. 2009;41(6):2156–8. doi: 10.1016/j.transproceed.2009.06.057. [DOI] [PubMed] [Google Scholar]
- 2.Opelz G, Dohler B, Ruhenstroth A. Cytomegalovirus Prophylaxis and Graft Outcome in Solid Organ Transplantation: A Collaborative Transplant Study Report. American Journal of Transplantation. 2004;4(6):928–36. doi: 10.1111/j.1600-6143.2004.00451.x. [DOI] [PubMed] [Google Scholar]
- 3.Borchers AT, Perez R, Kaysen G, Ansari A, Gershwin ME. Role of cytomegalovirus infectionin allograft rejection: a review of possible mechanisms. Transplant Immunol. 1999;7:75–82. doi: 10.1016/s0966-3274(99)80023-9. [DOI] [PubMed] [Google Scholar]
- 4.Sagedal S, Hartmann A, Nordal KP, Osnes K, Leivestad T, Foss A, Degre M, Fauchald P, Rollag H. Impact of early cytomegalovirus infection and disease on long-term recipient and kidney graft survival. Kidney International. 2004;66:329–37. doi: 10.1111/j.1523-1755.2004.00735.x. [DOI] [PubMed] [Google Scholar]
- 5.Colungnati FAB, Staras SAS, Dollard SC, Cannon MJ. Incidence of cytomegalovirus infection among the general population and pregnant women in the United States. BMC Infectious Diseases. 2007;7:71. doi: 10.1186/1471-2334-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. 2010;20(4):202–13. doi: 10.1002/rmv.655. [DOI] [PubMed] [Google Scholar]
- 7.Staras SA, Dollard SC, Radford KE, et al. Seroprevalence of cytomegalovirus infection n the United States, 1988-1994. Clin Infect Dis. 2006;43:1143. doi: 10.1086/508173. [DOI] [PubMed] [Google Scholar]
- 8.Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988-2004. Clin Infeect Dis. 2010;50:1439. doi: 10.1086/652438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cordero E, Casasola C, Ecarma R, et al. Cytomegalovirus disease in kidney transplant recipients: incidence, clinical profile and risk factors. Transplant Proc. 2012;44(3):694–700. doi: 10.1016/j.transproceed.2011.11.053. [DOI] [PubMed] [Google Scholar]
- 10.Zhang LF, Wang YT, TIan JH, et al. Preemptive versus prophylactic protocol to prevent cytomegalovirus infection after renal transplantation: a meta-analysis and systemic review of randomized controlled trials. Transpl Infect Dis. 2011;13(6):622–32. doi: 10.1111/j.1399-3062.2011.00652.x. [DOI] [PubMed] [Google Scholar]
- 11.Witzke O, Hauser IA, Bartels M, et al. Valganciclovir prophylaxis versus preemptive therapy in cytomegalovirus-positive renal allograft recipients: 1-year results of a randomized clinical trial. Transplantation. 2012;93(1):61–8. doi: 10.1097/TP.0b013e318238dab3. [DOI] [PubMed] [Google Scholar]
- 12.Reischig T, Hribova P, Jindra P, et al. Long-term outcome of preemptive valganciclovir compared with valacyclovir prophylaxis for prevention of cytomegalovirus in renal transplantation. J Am Soc Nephrol. 2012;23:1588–97. doi: 10.1681/ASN.2012010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asberg A, Humar A, Rollag H, et al. Oral vanganciclovir is noninferior to intravenous ganciclovir for the treatment of cytomegalovirsus disease in solid organ transplant recipients. Am J Transplant. 2007;7:2106–13. doi: 10.1111/j.1600-6143.2007.01910.x. [DOI] [PubMed] [Google Scholar]
- 14.Jordan ML, Hrebink RL, Jr, Dummer JS, et al. Therapeutic use of ganciclovir for invasive cytomegalovirus infection in cadaveric renal allograft recipients. J Urol. 1992;148(5):1388–92. doi: 10.1016/s0022-5347(17)36918-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nevins TE, Dunn DL. Use of Ganciclovir for Cytomegalovirus infection. J Am Soc Nephrol. 1992;2:S270–73. doi: 10.1681/ASN.V212s270. [DOI] [PubMed] [Google Scholar]
- 16.Harbison MA, De Girolami PC, Jenkins RL, et al. Ganciclovir therapy of severe cytomegalovirus infections in solid-organ transplant recipients. Transplantation. 1988;46:82. doi: 10.1097/00007890-198807000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Snydman Ganciclovir therapy for cytomegalovirus disease associated with renal transpants. Rev Infect Dis. 1988;10S:554. doi: 10.1093/clinids/10.supplement_3.s554. [DOI] [PubMed] [Google Scholar]
- 18.Jabs DA, Enger C, Dunn JP, et al. Cytomegalovirus retinitis and viral resistance: ganciclovir resistance. J Infect Dis. 1998;177(3):770–73. doi: 10.1086/514249. [DOI] [PubMed] [Google Scholar]
- 19.Kotton CN, Kumar D, Caliendo AM, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333. doi: 10.1097/TP.0b013e31829df29d. [DOI] [PubMed] [Google Scholar]
- 20.Humar A, Kumar D, Boivin G, et al. Cytomegalovirus (CMV) Virus Load Kinetics to Predict Recurrent Disease in Solid-Organ Transplant Patients with CMV Disease. J Infect Dis. 2002;186(6):829–33. doi: 10.1086/342601. [DOI] [PubMed] [Google Scholar]