Abstract
Background:
Transcriptional factors (TFs) and microRNAs (miRNAs) have been recognized as 2 classes of principal gene regulators that may be responsible for genome coexpression changes observed in schizophrenia (SZ).
Methods:
This study aims to (1) identify differentially coexpressed genes (DCGs) in 3 mRNA expression microarray datasets; (2) explore potential interactions among the DCGs, and differentially expressed miRNAs identified in our dataset composed of early-onset SZ patients and healthy controls; (3) validate expression levels of some key transcripts; and (4) explore the druggability of DCGs using the curated database.
Results:
We detected a differential coexpression network associated with SZ and found that 9 out of the 12 regulators were replicated in either of the 2 other datasets. Leveraging the differentially expressed miRNAs identified in our previous dataset, we constructed a miRNA–TF–gene network relevant to SZ, including an EGR1–miR-124-3p–SKIL feed-forward loop. Our real-time quantitative PCR analysis indicated the overexpression of miR-124-3p, the under expression of SKIL and EGR1 in the blood of SZ patients compared with controls, and the direction of change of miR-124-3p and SKIL mRNA levels in SZ cases were reversed after a 12-week treatment cycle. Our druggability analysis revealed that many of these genes have the potential to be drug targets.
Conclusions:
Together, our results suggest that coexpression network abnormalities driven by combinatorial and interactive action from TFs and miRNAs may contribute to the development of SZ and be relevant to the clinical treatment of the disease.
Key words: schizophrenia, transcriptional factor, microRNA, miR-124-3p, EGRl
Introduction
Schizophrenia (SZ) is a severe chronic mental disorder affecting approximately 1% of the population worldwide. Generally arising in late adolescence, it profoundly disrupts such key traits of human cognition and personality as language, thought, perception, emotional affect, and sense of self. When the form of SZ manifests before age 18, it is categorized as early-onset SZ (EOS), a subcategory of SZ associated with more familial vulnerability and poor outcomes.1
Microarray-based gene expression profiling has been used to explore peripheral molecular disruptions in SZ patients, through the establishment of biomarkers in a noninvasive manner. The identification of over- and under-expressed genes associated with a disease provides insights into the disease’s underlying mechanisms; however, variance between studies may complicate the efforts related to replication attempts, and construction of an overarching framework.
Often when examining genes for a potential disease association, the individual genes are assumed to be autonomous, without considering interactions with other molecular factors. In fact, the genes’ combinatorial effect reflects the most likely cause of mental health disorders, such as SZ. Previous studies have indicated that coexpressed genes tend to be functionally related;2,3 therefore, the gene coexpression analysis holds promise as an effective tool used for expression data. Gene coexpression networks encapsulate the activity of multiple regulatory systems and highlight specific molecular mechanisms for disease.4 Differential coexpression refers to variations in gene–gene correlation between 2 sets of phenotypically distinct samples.5 Furthermore, the level of gene–gene correlation may change without affecting differential expression, indicating that a gene may alter its regulatory pattern that could be missed by traditional differential expression analysis. Differential coexpression analysis (DCEA) aims to detect gene networks with different connectivity in the disease state, and offers a powerful approach for elucidating transcriptome patterns and dysfunction of gene expression underlying phenotypic changes.6
Several studies have conducted gene coexpression analysis for mental health disorders.7–11 One commonly used method is the weighted gene coexpression network analysis (WGCNA).12 Nevertheless a limitation of methods such as WGCNA is rooted in their failure to explain the molecular mechanisms underlying coexpression. Among many possibilities, transcriptional factors (TFs) and microRNAs (miRNAs) are 2 classes of pivotal gene expression regulators, and both may vary together with their targets.13–15 miRNAs posttranscriptionally repress expression of target genes by mRNA degradation or translational inhibition, using fine-tuned gene expression, while TFs regulate the initiation of transcription by binding promoter regions of target genes, providing an on/off switch for gene expression. Thus, TFs have been recognized as a major driver of gene coexpression.13 In parallel, another framework, DCGL, was proposed to identify differentially co-expressed genes (DRGs) and links (DRLs).6,16 Similar to DCEA, DCGL enables differential regulation analysis (DRA), leveraging TF-target information to probe regulatory forces that account for the changes in coexpression.
To date, a myriad of TFs17–27 or miRNAs28 have been associated with SZ. However, previous studies typically focused on 1 of the 2 classes of molecules, ignoring the reciprocal regulation between TFs and miRNAs. A TF may be posttranscriptionally regulated by miRNAs. Alternatively, the transcription of miRNAs may be regulated by TFs.
The formation of TF-miRNA regulatory networks to modulate transcription has been previously demonstrated.14,29 The TF-miRNA coordination comprises network motifs, including feed-forward loops (FFLs) or feedback loops (FBLs).30,31 The FFL is a motif in which a TF regulates a miRNA or a miRNA represses a TF, and together they co-regulate a common target. FFLs can be divided into 3 types according to the master regulator: TF-FFL, miRNA-FFL and composite FFL (supplementary figure 1).30 Together, these regulatory loops play a critical role in multiple biological processes and disease development.29
In this study, to systematically comprehend molecular mechanisms in SZ, we applied the network coexpression analysis in 3 microarray datasets, compiled coexpression links, protein–protein interactions (PPIs), TF-target binding information, experiment-supported TF-miRNA binding information, and experiment-supported miRNA-target binding information into a comprehensive network, validated the expression levels of some key molecules within the network, and explored the druggability of the network genes.
Methods
Subjects
All participants were unrelated Han Chinese recruited from the north of China. For the real-time quantitative PCR (RT-qPCR) analysis, we recruited 38 SZ patients (15 males and 23 females, aged 34.3±10.6 y) who were drug-free for at least 1 month before the enrollment; among them 30 patients (13 males and 17 females, aged 37.1±11.4 y) were successfully followed-up with a 12-week period of antipsychotic treatment (see supplementary material for detailed clinical medication and assessments). Consensus diagnoses were made by at least 2 experienced psychiatrists independently according to the Diagnosis and Statistical Manual of Mental Disorders Fourth Edition (DSM-IV) criteria for SZ. Patients with unanimous diagnosis were enrolled into the study.
A total of 48 healthy controls (17 males and 31 females, aged 31.6±6.88 y) were recruited from local communities or were undergoing routine health check-ups. All control subjects were assessed using the Chinese Version of the Modified Structured Clinical Interview for DSM-IV TR Axis I Disorders Nonpatient Edition (SCID-I/NP). Subjects with relevant physical diseases or a history of major psychiatric disorders or suicidal behavior were excluded, and those who had a first-degree relative with a history of severe mental disorder or suicidal behavior were also excluded.
No significant difference was observed in gender or age between SZ cases and controls (supplementary table 1). The study was approved by Medical Research Ethics Committee of Shanxi Medical University. Informed consent was signed by both the teenage participants and their parents.
mRNA Dataset
Three mRNA microarray datasets were used, including a blood mRNA microarray dataset from 18 EOS patients (8 males and 10 females, aged 14.78±1.70 y ranging from 10–18 y) and 12 healthy controls (6 males and 6 females, aged 14.75±2.14 y ranging from 10–17 y) (NCBI GEO: GSE54913, referred as YX30, see supplementary material for detailed preprocessing steps, Xu et al submitted), a blood mRNA microarray dataset from 106 SZ patients (30 males and 76 females, aged 39.6±10.7 y) and 96 healthy controls (54 males and 42 females, aged 39.3±14.2 y) (NCBI GEO: GSE38484, referred as SJ202) as described previously,9 and a postmortem brain mRNA microarray dataset from 51 SZ patients (14 males and 37 females, aged 42.6±9.87 y) and 50 healthy controls (15 males and 35 females, aged 45.5±8.99 y) (NCBI GEO: GSE35977, referred as CC101) as described previously.11
Analysis of Gene and miRNA Expression by RT-qPCR
Statistical and in Silico Analysis
R32 was used to perform the data processing and in silico analysis. We used false discovery rate (FDR) for multiple testing correction. Coexpression analysis was conducted using the R package DCGL v2.0.6 Protein–protein interaction networks were retrieved via STRING v9.1.33 Experiment-supported miRNA targets were screened using the R package multiMiR, a comprehensive collection of predicted and validated miRNA–target interactions and their associations with diseases and drugs.34 TF–miRNA bindings were screened using TransmiR, a manually curated database collecting experiment-supported TF–miRNA regulatory relationships from publications.35 The identified networks were plotted using Cytoscape.36 For RT-qPCR analysis, the comparative Ct (2−ΔΔCT) method was used for the quantification of transcripts, the differences of mRNA levels between cases and controls were analyzed using the Wilcoxon rank-sum test, and the differences of mRNA levels between 30 pairs of SZ patients before and after treatment were analyzed using the paired Wilcoxon rank-sum test. The mRNA expression figure was plotted using the R package ggplot2.37 Druggability analysis was conducted using the Drug-Gene Interaction database (DGIdb)38 and the R package dnet.39
Results
Coexpression Analysis
The expression values of the 3 mRNA microarray datasets were analyzed using DCGL with default parameters. For dataset YX30, a total of 8522 unique genes from 17219 valid probes were derived from the mRNA microarray data. expressionBasedfilter40 and varianceBasedfilter41 are 2 functions to filter out genes whose expression values are extremely low or notably invariable across samples. expressionBasedfilter removes genes with Between-Experiment Mean Expression Signal (BEMES) lower than the median BEMES of all genes, resulting in 4261 genes. varianceBasedfilter removes genes not significantly more variable than the median gene, yielding 1339 genes. If a differentially co-expressed link (DCL) happens to be a TF-to-target relation, this type of DCL is called “TF2target DCL.” We highlighted this DCL due to its direct relation to differential regulation. If there are 1 or more common TFs regulating the 2 genes of a DCL, this type of DCL is termed “TF bridged DCLs.” We prioritized the TF bridged DCLs, because the change in the expression correlation of the 2 genes could be attributed to the disruption of their co-regulation by the common TFs.6
A total of 327 DCGs and 89135 DCLs were summarized using DCsum (a function to summarize a final set of DCGs and DCLs) based on results from Differential Co-expression profile (DCp) and Differential Co-expression enrichment (DCe) analysis. DRA analysis yielded 119 TF2target_DCLs (figure 1a, supplementary figure 3, supplementary table 3) and 25632 TF_bridged_DCLs (supplementary figure 2).
Fig. 1.
TF2target_DCL-centered networks. DCG: differentially coexpressed gene. TF: transcriptional factor. Pink nodes denote DCGs; blue nodes denote non-DCGs; square nodes are TFs and circle nodes are non-TF genes. (a) Network of the dataset YX30. (b) Network of the dataset SJ202. (c) Network of the dataset CC101. (d) Overlap diagram of the 3 network transcriptional factors.
For dataset SJ202, a total of 19 930 unique genes from 48803 valid probes were derived. expressionBasedfilter removed half of the genes, resulting in 9965 genes, and 5510 genes were removed by varianceBasedfilter, yielding 4455 genes. After DCGL analysis, a total of 1104 DCGs and 709 286 DCLs were summarized by DCsum based on results from DCp and Dce analysis. DRA analysis yielded 14 823 TF2target_DCLs (figure 1b, supplementary figure 4).
For dataset CC101, a total of 20 044 unique genes from 33 297 valid probes were derived. expressionBasedfilter removed half of the genes, resulting in 10 022 genes, and 6001 genes were removed by varianceBasedfilter, yielding 4021 genes. After DCGL analysis, a total of 984 DCGs and 550 999 DCLs were summarized by DCsum based on results from DCp and Dce analysis. DRA analysis yielded 11 169 TF2target_DCLs (figure 1c, supplementary figure 5).
A significant portion of TFs in the TF2target_DCLs were overlapped across the 3 datasets (figure 1d). There were 12 TFs in dataset YX30, with 5 TFs (FOS, IRF1, LMO2, NFYB, and STAT3) and 6 TFs (EGR1, FOS, JUN, STAT3, SOX9, and CBFB) being overlapped with the SJ202 and CC101 dataset, respectively; especially, FOS and STAT3 were observed across all the 3 datasets (supplementary table 4).
miRNA–TF–Gene–PPI Network
As shown in figure 1a, JUN, MYOD1, EGR1 and FOS are the hub regulators in the network, since they are DCGs by themselves, and they have relatively more predicted targets. There are some interconnections among the 4 TF-target modules. Therefore, these 4 modules constitute a core differential coexpression network, involving 37 DCGs. To further explore the potential interactions among these 37 DCGs, we predicted their interactions using STRING v9.1,33 yielding 21 protein-protein interactions among the 15 proteins (supplementary figure 6). Interestingly, the 4 TFs are well interconnected.
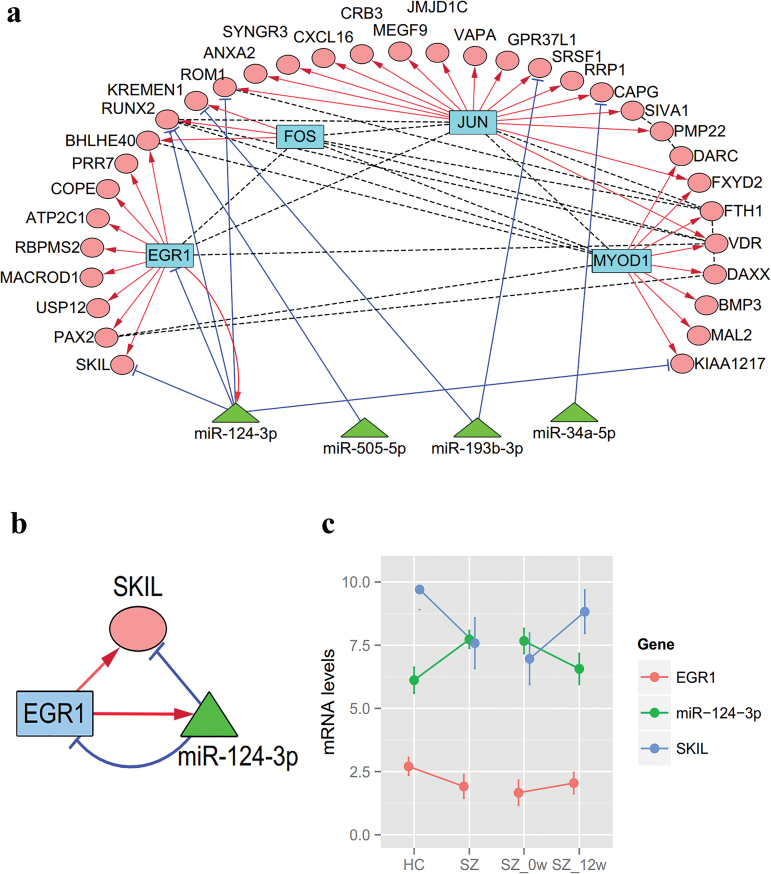
We then selected 63 differentially expressed miRNAs from our peripheral miRNA expression dataset (from 15 EOS patients and 15 healthy controls, GEO: GSE54578)42 and searched for validated targets for the differentially expressed miRNAs among the 37 DCGs. We detected 9 experiment-supported miRNA-target pairs, involving 4 miRNAs (miR-124-3p, miR-193b-3p, miR-34a-5p, and miR-505-5p) and 6 genes (supplementary table 5). Mapping from the 4 TFs to the 63 miRNAs identified a validated EGR1–miR-124-3p binding.43 Furthermore, we integrated coexpression TF-target binding, TF–miRNA binding, PPIs, and miRNA-target binding into a hybrid miRNA–TF–gene–PPI network (figure 2a). The network is composed of 4 TFs, 37 target genes (DCGs), 4 miRNAs, and a myriad of links.
Fig. 2.
microRNA–transcriptional factor (miRNA–TF) network and gene expressions. (a) miRNA–TF–gene–protein–protein interaction (PPI) network. Triangles denote miRNAs, ellipses denote target genes of TFs, and rectangles denote TFs. Blue lines are miRNA-target, red lines are TF-target, and dashed lines are PPIs. (b) A miRNA-TF-gene loop derived from the miRNA–TF–gene–PPI network. (c) Expression levels miR-124-3p, EGR1 and SKIL in 48 healthy controls and 38 schizophrenia (SZ), including 30 SZ patients before (SZ_0w) and after the 12-week treatment (SZ_12w).
Figure 2a indicates that some miRNAs and TFs may co-regulate target genes. Of note, interactions among EGR1, miR-124-3p, and SKIL, form a composite–FFL (figure 2b).
RT-qPCR Analysis of Key Regulators
We evaluated blood expression levels of miR-124-3p, EGR1 and SKIL in 48 healthy controls, 38 SZ patients, and 30 SZ patients completing the 12-week following-up treatment using RT-qPCR. SKIL and EGR1 were underexpressed, while miR-124-3p was overexpressed in SZ cases (FDR < 0.05) compared with controls (supplementary table 6, figure 2c). The expression of miR-124-3p decreased after the 12-week treatment, while SKIL was up-regulated after the 12-week treatment (supplementary table 6, figure 2c).
Druggability Analysis
Forty-one percent of the coexpression network genes belong to the druggable gene categories (including JUN, MYOD1, and SKIL). Not surprisingly, 2 TF-related classes (TF binding and TF complex) were among the top druggable gene categories highlighted by enrichment analysis (overlapped genes in TF binding category encompasses those in TF complex category), and the TF binding category involves 15 genes (DAXX, JUN, ZNF703, GATA6, STAT3, VDR, RUNX2, FOXN3, NKX2-5, TSC22D3, LMO2, MYOD1, NFYB, BHLHE40, and JMJD1C) (table 1, supplementary table 7). Drug-gene interaction analysis revealed a total of 228 drug-gene pairs, involving 24 unique genes (supplementary table 8). Antipsychotic drug targets include FAAH (fatty acid amide hydrolase), RGS2 (regulator of G-protein signaling 2) and HTR4 (5-hydroxytryptamine receptor 4, G protein-coupled).
Table 1.
Top Druggable Gene Categories and Their Annotated Genes
| Gene Set | nA | nO | P | FDR | Members |
|---|---|---|---|---|---|
| TF binding | 403 | 15 | 1.50E-8 | 3.40E-7 | DAXX, JUN, ZNF703, GATA6, STAT3, VDR, RUNX2, FOXN3, NKX2-5, TSC22D3, LMO2, MYOD1, NFYB, BHLHE40, JMJD1C |
| TF complex | 281 | 8 | 1.80E-4 | 2.10E-3 | JUN, GATA6, RUNX2, FOXN3, NKX2-5, LMO2, MYOD1, NFYB |
| Lipase | 10 | 1 | 2.30E-3 | 0.018 | PNLIPRP1 |
| Abc transporter | 101 | 3 | 6.40E-3 | 0.033 | FXYD2, ATP2B3, ATP2C1 |
| Nuclear hormone receptor | 54 | 2 | 7.30E-3 | 0.033 | STAT3, VDR |
Note: nA, number of annotation genes in the category; nO, number of overlap genes in the category; FDR, false discovery rate.
Discussion
In this study, we detected a TF-mediated coexpression network relevant to SZ. The majority of the implicated TFs (8 out of 11, 73%) were replicated in other datasets. Most of TFs observed in brains of the patients (18 out of 30, 60%) were also detected in peripheral blood of the SZ patients. This consistency was rather satisfactory, given the diversity in populations, disease stages and different tissues across the studies. The overlaps of regulators derived from coexpression relationships were more enriched compared with overlaps of individual genes derived from expression changes. For some TFs, they may exert an effect through their regulatory alterations in the lack of overall expression changes.
We constructed a hybrid miRNA–TF–gene–PPI network by integrating coexpressed signatures with other functional networks, such as TF binding, miRNA binding, and PPIs. We sought to identify critical regulatory elements underlying gene coexpression networks as novel biomarkers for the disease state at the systems-level. Node centrality and overall connectivity in a network are presumed to be closely associated with the disease activity. Therefore, our results prioritized 4 TFs (EGR1, JUN, MYOD1, and FOS) and 1 miRNA, miR-124-3p, as the key regulators in the hybrid network. Most of these genes were not highly differentially expressed in the original datasets, and thus were likely overlooked by traditional microarray analysis. However, the ramifications induced by these regulators should be exacerbated through the cascade regulations on their targets.
Some of the identified molecules have previously been implicated in SZ, eg, FOS and JUN were up-regulated in post-mortem brains of SZ patients.20,23 Moreover, it was reported that an allosteric metabotropic glutamate receptor 1 antagonist with antipsychotic activity, CFMTI, may affect FOS expression level in the regions of the various brain related to SZ.24 Finally, a recent genome-wide expression study using skin fibroblasts in SZ cases and controls discovered 5 upregulated genes (JUN, HIST2H2BE, FOSB, FOS, and EGR1), and an overexpression of EGR1 and an underexpression of JUN were detected in the blood of SZ by RT-qPCR analysis.44
Early growth response gene 1 (EGR1) is a member of the immediate early gene (IEG) family of TFs and plays a role in cell development, synaptic plasticity, and memory processing.45 One study revealed that EGR1 controls regulatory networks associated with neurodegeneration.46 EGR1 was found to be under-expressed in the brains of SZ patients,26 consistent with our results. In addition, EGR family genes, EGR3,25 EGR2 and EGR4,22 have also been reported to be associated with SZ.
SKI-like proto-oncogene (SKIL) is a component of the SMAD pathway, which regulates cell growth and differentiation through suppression of the transforming growth factor-beta (TGFB) pathway.47 SKIL is ubiquitously expressed in all embryonic and adult tissues, playing an important role in embryonic development, and normal tissue morphogenesis and homeostasis.47 SKIL is involved in the activation of p53 to induce the cellular stress pathways.48
miR-124 is the most abundant brain miRNA, implying its key function in the central nervous system (CNS).49 Previously considered as neuron-specific, miR-124, is also expressed in peripheral blood samples. Hundreds of targets have been identified for miR-124, suggesting miR-124 as an important regulator of differentiation and maturation of various cell types in the CNS. Furthermore, empirical evidence has established miR-124’s essential roles in neural development and neuronal function.49 Furthermore, miR-124 has been shown to be involved in neurological phenotypes including autism50,51 and Alzheimer’s disease.52
Recent work suggested that TF-miRNA co-regulation is prevalent in animal genomes and can affect development and diseases. Combinatorial regulation by TFs alone or miRNAs alone offers a wide range of regulatory paradigms. Therefore, joint regulation by TFs and miRNAs may provide more sophisticated regulatory mechanisms, enabling the formation of FFLs and FBLs.
TF-miRNA FFLs may promote robustness by its buffering effect. It can cushion the response to stochastic signaling noise to maintain steady-state levels of the target protein by tuning the translation of its target in a direction opposite to that of the signal.53 This robustness conferred by FFLs can protect cells from rapid switches between alternative cell decisions in the development process.53 These regulatory loops may serve as important motifs in gene regulatory networks.
In this study, we detected a SZ-related composite–FFL, miR-124-3p–EGR1–SKIL. In this loop, miR-124-3p and EGR1 regulate each other reciprocally,43,54 and SKIL is a validated target of miR-124-3p43 and a predicted target of EGR1. Altered expressions of the 3 molecules were confirmed in the RT-qPCR analysis, with miR-124-3p upregulation (FC = 2.17), and the 2 other genes being moderately downregulated. Upon treatment, direction changes of miR-124-3p and SKIL expression were reversed, with miR-124-3p being downregulated (FC = 0.60), and SKIL being upregulated (FC = 2.32), indicating a rescue-effect of the antipsychotic treatment. The opposite change directions for miR-124-3p and SKIL were consistent with expectation, since miRNAs may negatively regulate target expression. Results presented here provide evidence for the binding of miR-124-3p and SKIL, suggesting a plausible role for miR-124-3p–SKIL relationship in both disease development and drug treatment.
The transcription factor signal transducer and activator of transcription 3 (STAT3) was 1 of the 2 TFs implicated across all the 3 co-expression networks. STAT3 is a critical regulator of numerous essential and very diverse cellular processes.55 The Janus kinase-STAT (JAK/STAT) pathway has been shown related to the release of various cytokines and inflammatory mediators and implicated in many immune diseases and cancers.56 Given the immune component in the development of SZ, it is plausible that STAT3 may be related to the mechanism of SZ.
Of note, a significant portion of coexpression network genes are either drug targets or potentially druggable, with TF binding being the top enriched category. Among the druggable genes, both FAAH and HTR4 are targeted by multiple drugs, including antipsychotics. For example, FAAH, a regulatory target of STAT3, is targeted by risperidone, clozapine, aripiprazole, olanzapine, quetiapine, haloperidol, and cannabinoids. HTR4 is targeted by antidepressant vilazodone, and RGS2 is another gene targeted by antipsychotics. These data add evidence corroborating the close relevance of the coexpression network in the development of SZ. Also, it is tempting to speculate that new drugs are developed to target these genes, or there may be of auxiliary treatment values for some existent drugs targeting these genes. Pleiotropic effectors such as FAAH, HTR4, RGS2, JUN, FOS, STAT3, MYOD1, and SKIL may hold promise for the development of novel therapeutic avenues. On the other hand, explicit effectors such as VDR (vitamin D receptor) may be of interest for the auxiliary treatment, given the role of vitamin D in mammalian brain development and functioning.57,58
Several limitations of our study should be noted. First, only a small number of experiment-supported targets were available within the dataset used to screen miRNA targets and TF targets. Next, the evidence for miRNA binding and TF binding came from the microarray data. Therefore, some of these targets need to be biologically validated. There was a sex imbalance in the SJ202 dataset. However, there was no option to control co-variants in the DCGL package. We hope the inclusion of 3 different datasets may mitigate the potential bias caused by the sex-imbalance. The mRNA dataset and the miRNA dataset were obtained from 2 similar yet different panels of subjects, and the sample size for each of the datasets was relatively small. A caveat in mind was that our major results were derived from EOS patients; thus, due care should be taken when extrapolating these results into adult or chronic SZ patients.
Compared with previous network studies, our study has several merits. First, with the exception of the coexpression links, network interactions were evidence-based instead of in silico predictions; even for the coexpression links, our study indicated their involvement in the disease. Next, the network involves multi-layer regulatory relationships, especially the reciprocal regulation between TFs and miRNAs. Furthermore, the expression levels of a vital motif were independently validated and dynamically observed during treatment. Finally, the clinical relevance of the network genes was explored.
To sum, our results suggested that genome expression abnormality observed in SZ may arise from transcriptional and posttranscriptional dysregulatory network. Here we detected several disease-related regulators and regulatory motifs. We highlighted a SZ-related composite–FFL, miR-124-3p–EGR1–SKIL, which may be of clinical relevance. Together, our findings may provide insights for more precise SZ treatment by monitoring the TF and miRNA expression levels.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
This work was supported by National Natural Science Foundation of China (81271482, 81471364, 81571319, 81222017), Program for New Century Excellent Talents in University (NCET-12–1036), and Construction Plan for Shanxi Science & Technology Infrastructure Platforms (2015091002-0102). Shugart was supported by the Intramural Research Program of National Institute of Mental Health, National Institutes of Health (MH002929-05).
Supplementary Material
Acknowledgments
We want to take this opportunity to thank all the subjects for their support and participation and all the medical staff involved in collecting blood samples. The views expressed in this presentation do not necessarily represent the views of the NIMH, NIH, HHS, or the United States Government. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Clemmensen L, Vernal DL, Steinhausen HC. A systematic review of the long-term outcome of early onset schizophrenia. BMC Psychiatry. 2012;12:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Oldham MC, Horvath S, Geschwind DH. Conservation and evolution of gene coexpression networks in human and chimpanzee brains. Proc Natl Acad Sci USA. 2006;103:17973–17978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Okamura Y, Aoki Y, Obayashi T, et al. COXPRESdb in 2015: coexpression database for animal species by DNA-microarray and RNAseq-based expression data with multiple quality assessment systems. Nucleic Acids Res. 2015;43:D82–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gaiteri C, Ding Y, French B, Tseng GC, Sibille E. Beyond modules and hubs: the potential of gene coexpression networks for investigating molecular mechanisms of complex brain disorders. Genes Brain Behav. 2014;13:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de la Fuente A. From ‘differential expression’ to ‘differential networking’ - identification of dysfunctional regulatory networks in diseases. Trends Genet. 2010;26:326–333. [DOI] [PubMed] [Google Scholar]
- 6. Yang J, Yu H, Liu BH, et al. DCGL v2.0: an R package for unveiling differential regulation from differential co-expression. PLoS One. 2013;8:e79729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Torkamani A, Dean B, Schork NJ, Thomas EA. Coexpression network analysis of neural tissue reveals perturbations in developmental processes in schizophrenia. Genome Res. 2010;20:403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Voineagu I, Wang X, Johnston P, et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature. 2011;474:380–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Jong S, Boks MP, Fuller TF, et al. A gene co-expression network in whole blood of schizophrenia patients is independent of antipsychotic-use and enriched for brain-expressed genes. PLoS One. 2012;7:e39498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ponomarev I, Wang S, Zhang L, Harris RA, Mayfield RD. Gene coexpression networks in human brain identify epigenetic modifications in alcohol dependence. J Neurosci. 2012;32:1884–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen C, Cheng L, Grennan K, et al. Two gene co-expression modules differentiate psychotics and controls. Mol Psychiatry. 2013;18:1308–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9:559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marco A, Konikoff C, Karr TL, Kumar S. Relationship between gene co-expression and sharing of transcription factor binding sites in Drosophila melanogaster. Bioinformatics. 2009;25:2473–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bandyopadhyay S, Bhattacharyya M. Analyzing miRNA co-expression networks to explore TF-miRNA regulation. BMC Bioinformatics. 2009;10:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gennarino VA, D’Angelo G, Dharmalingam G, et al. Identification of microRNA-regulated gene networks by expression analysis of target genes. Genome Res. 2012;22:1163–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu BH, Yu H, Tu K, Li C, Li YX, Li YY. DCGL: an R package for identifying differentially coexpressed genes and links from gene expression microarray data. Bioinformatics. 2010;26:2637–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stefansson H, Ophoff RA, Steinberg S, et al. Common variants conferring risk of schizophrenia. Nature. 2009;460:744–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Steinberg S, de Jong S, Irish Schizophrenia Genomics Consortium et al. Common variants at VRK2 and TCF4 conferring risk of schizophrenia. Hum Mol Genet. 2011;20:4076–4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Navarrete K, Pedroso I, De Jong S, et al. TCF4 (e2-2; ITF2): a schizophrenia-associated gene with pleiotropic effects on human disease. Am J Med Genet B Neuropsychiatr Genet. 2013;162B:1–16. [DOI] [PubMed] [Google Scholar]
- 20. Todorova VK, Elbein AD, Kyosseva SV. Increased expression of c-Jun transcription factor in cerebellar vermis of patients with schizophrenia. Neuropsychopharmacology. 2003;28:1506–1514. [DOI] [PubMed] [Google Scholar]
- 21. Ahn YM, Seo MS, Kim SH, et al. Reduction in the protein level of c-Jun and phosphorylation of Ser73-c-Jun in rat frontal cortex after repeated MK-801 treatment. Psychiatry Res. 2009;167:80–87. [DOI] [PubMed] [Google Scholar]
- 22. Cheng MC, Chuang YA, Lu CL, et al. Genetic and functional analyses of early growth response (EGR) family genes in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:149–155. [DOI] [PubMed] [Google Scholar]
- 23. Kyosseva SV. Differential expression of mitogen-activated protein kinases and immediate early genes fos and jun in thalamus in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:997–1006. [DOI] [PubMed] [Google Scholar]
- 24. Suzuki G, Satow A, Ohta H. Effect of CFMTI, an allosteric metabotropic glutamate receptor 1 antagonist with antipsychotic activity, on Fos expression in regions of the brain related to schizophrenia. Neuroscience. 2010;168:787–796. [DOI] [PubMed] [Google Scholar]
- 25. Yamada K, Gerber DJ, Iwayama Y, et al. Genetic analysis of the calcineurin pathway identifies members of the EGR gene family, specifically EGR3, as potential susceptibility candidates in schizophrenia. Proc Natl Acad Sci USA. 2007;104:2815–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pérez-Santiago J, Diez-Alarcia R, Callado LF, et al. A combined analysis of microarray gene expression studies of the human prefrontal cortex identifies genes implicated in schizophrenia. J Psychiatr Res. 2012;46:1464–1474. [DOI] [PubMed] [Google Scholar]
- 27. O’Donovan MC, Craddock N, Norton N, et al. Identification of loci associated with schizophrenia by genome-wide association and follow-up. Nat Genet. 2008;40:1053–1055. [DOI] [PubMed] [Google Scholar]
- 28. Mellios N, Sur M. The emerging role of microRNAs in schizophrenia and autism spectrum disorders. Front Psychiatry. 2012;3:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang HM, Kuang S, Xiong X, Gao T, Liu C, Guo AY. Transcription factor and microRNA co-regulatory loops: important regulatory motifs in biological processes and diseases. Brief Bioinform. 2015;16:45–58. [DOI] [PubMed] [Google Scholar]
- 30. Shalgi R, Lieber D, Oren M, Pilpel Y. Global and local architecture of the mammalian microRNA-transcription factor regulatory network. PLoS Comput Biol. 2007;3:e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tsang J, Zhu J, van Oudenaarden A. MicroRNA-mediated feedback and feedforward loops are recurrent network motifs in mammals. Mol Cell. 2007;26:753–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. RCoreTeam. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 33. Franceschini A, Szklarczyk D, Frankild S, et al. STRING v9.1: protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2013;41:D808–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ru Y, Kechris KJ, Tabakoff B, et al. The multiMiR R package and database: integration of microRNA-target interactions along with their disease and drug associations. Nucleic Acids Res. 2014;42:e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang J, Lu M, Qiu C, Cui Q. TransmiR: a transcription factor-microRNA regulation database. Nucleic Acids Res. 2010;38:D119–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wickham H. Ggplot2: Elegant Graphics for Data Analysis. New York: Springer; 2009. [Google Scholar]
- 38. Griffith M, Griffith OL, Coffman AC, et al. DGIdb: mining the druggable genome. Nat Methods. 2013;10:1209–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fang H, Gough J. The ‘dnet’ approach promotes emerging research on cancer patient survival. Genome Med. 2014;6:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Prieto C, Risueño A, Fontanillo C, De las Rivas J. Human gene coexpression landscape: confident network derived from tissue transcriptomic profiles. PLoS One. 2008;3:e3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Simon R. BRB-Array Tools. Bethesda, MD: Biometric Research Branch, National Cancer Institute; 2015. http://linus.nci.nih.gov/BRB-ArrayTools.html. Accessed June 21, 2015. [Google Scholar]
- 42. Zhang F, Xu Y, Shugart YY, et al. Converging evidence implicates the abnormal microRNA system in schizophrenia. Schizophr Bull. 2015;41:728–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang W, Zhou D, Shi X, et al. Global Egr1-miRNAs binding analysis in PMA-induced K562 cells using ChIP-Seq. J Biomed Biotechnol. 2010;2010:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cattane N, Minelli A, Milanesi E, et al. Altered gene expression in schizophrenia: findings from transcriptional signatures in fibroblasts and blood. PLoS One. 2015;10:e0116686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Veyrac A, Besnard A, Caboche J, Davis S, Laroche S. The transcription factor Zif268/Egr1, brain plasticity, and memory. Prog Mol Biol Transl Sci. 2014;122:89–129. [DOI] [PubMed] [Google Scholar]
- 46. Koldamova R, Schug J, Lefterova M, et al. Genome-wide approaches reveal EGR1-controlled regulatory networks associated with neurodegeneration. Neurobiol Dis. 2014;63:107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhu Q, Luo K. SnoN in regulation of embryonic development and tissue morphogenesis. FEBSLett. 2012;586:1971–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pan D, Zhu Q, Conboy MJ, Conboy IM, Luo K. SnoN activates p53 directly to regulate aging and tumorigenesis. Aging Cell. 2012;11:902–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sonntag KC, Woo TU, Krichevsky AM. Converging miRNA functions in diverse brain disorders: a case for miR-124 and miR-126. Exp Neurol. 2012;235:427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tabarés-Seisdedos R, Rubenstein JL. Chromosome 8p as a potential hub for developmental neuropsychiatric disorders: implications for schizophrenia, autism and cancer. Mol Psychiatry. 2009;14:563–589. [DOI] [PubMed] [Google Scholar]
- 51. Vaishnavi V, Manikandan M, Tiwary BK, Munirajan AK. Insights on the functional impact of microRNAs present in autism-associated copy number variants. PLoS One. 2013;8:e56781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fang M, Wang J, Zhang X, et al. The miR-124 regulates the expression of BACE1/beta-secretase correlated with cell death in Alzheimer’s disease. ToxicolLett. 2012;209:94–105. [DOI] [PubMed] [Google Scholar]
- 53. Inui M, Martello G, Piccolo S. MicroRNA control of signal transduction. Nat Rev Mol Cell Biol. 2010;11:252–263. [DOI] [PubMed] [Google Scholar]
- 54. Baek D, Villen J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature. 2008;455:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Levy DE, Darnell JE., Jr Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–662. [DOI] [PubMed] [Google Scholar]
- 56. Vogel TP, Milner JD, Cooper MA. The Ying and Yang of STAT3 in Human Disease. J Clin Immunol. 2015;35:615–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. McGrath JJ, Féron FP, Burne TH, Mackay-Sim A, Eyles DW. Vitamin D3-implications for brain development. J Steroid Biochem Mol Biol. 2004;89–90:557–560. [DOI] [PubMed] [Google Scholar]
- 58. Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29:21–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.