Abstract
Metacognitive training (MCT) is a new, widely used intervention for psychosis. The present meta-analysis examines the efficacy of MCT in schizophrenia. Fifteen studies comparing effects of MCT on positive symptoms, delusions or acceptance of MCT with a control group were included in this meta-analysis. These studies comprised a total of 408 patients in the MCT condition and 399 in the control condition. The moderating effects of masking of outcome assessment, randomization, incomplete outcome data, use of an active control intervention, and individual vs group MCT were investigated. Possible effects of sensitivity analyses and publication bias were also examined. The results show a significant overall effect of MCT for positive symptoms (g = −0.34, 95% CI [−0.53, −0.15]), delusions (g = −0.41, 95% CI [−0.74, −0.07]) and acceptance of the intervention (g = −0.84, 95% CI [−1.37, −0.31]). Using only studies being at low risk for bias regarding randomization, masking and incomplete outcome data reduced effect sizes for positive symptoms and delusions (g = −0.28, 95% CI [−0.50, −0.06] and g = −0.18, 95% CI [−0.43, 0.06]), respectively. This meta-analysis demonstrates that MCT exerts a small to moderate effect on delusions and positive symptoms and a large effect on acceptance of the intervention. The effect on delusions is reduced, but remains significant when potential biases are considered.
Key words: metacognitive training, psychosis, schizophrenia, delusions, acceptance, cognitive bias, rehabilitation
Introduction
Delusions are key symptoms of schizophrenia that are often accompanied by distress, and may result in hazardous decisions including assaulting others or suicide.1 Several studies have shown that delusions arise from cognitive biases2–7 that consist of distortions in the collection, appraisal and processing of information (eg, jumping to conclusions [JTC], overconfidence in errors). Several psychological interventions have been developed in order to reduce the symptoms of schizophrenia, with recent meta-analyses showing small to moderate effect-sizes for cognitive-behavioral therapy, cognitive remediation, and psychoeducation.8–12 Metacognitive training (MCT) is a novel intervention for patients with schizophrenia that has been developed by Moritz and colleagues.13 This program blends elements of psychoeducation, cognitive remediation, and cognitive-behavioral therapy. In contrast to cognitive-behavioral therapy, MCT first addresses cognitive biases before approaching the core symptoms. It aims to “straighten” the cognitive biases associated with delusions, particularly JTC,14,15 problems with taking the perspective of others and deficits in social cognition.16 Furthermore, MCT also tries to foster self-esteem, as people diagnosed with schizophrenia have low self-esteem compared to healthy controls.17
The initial version of MCT consists of a manualized group training and contains interventions addressing attributional style, JTC, problems taking perspectives of others, change of beliefs, low self-esteem and exercises to improve memory and to foster correction of beliefs.13 The modules can be downloaded free of charge from via the following website: http://www.uke.de/mct. A summary of the different modules of MCT is presented in supplementary material S1. Individual MCT (MCT+) is a variant of MCT designed for use in a one to one setting. Important add-ons to group MCT are the generation of an individual illness model and recovery plan and an additional focus on negative symptoms. In addition it is possible to focus more on individual participants’ symptoms than in the group MCT. MCT+ may be particularly useful for severely ill patients who have difficulties taking part in group MCT. Finally, all modules of MCT follow the same structure and combine theoretical explanations with practical elements. This provides the program with substantial unity and coherence, making MCT particularly suitable for meta-analytic reviews.
The efficacy of MCT in patients with schizophrenia spectrum disorder was summarized in a narrative review18 and investigated in 2 meta-analyses.19,20 Both meta-analyses were however limited either by insufficient statistical power (the meta-analysis by Jiang et al19 included only 4 studies measuring positive symptoms and delusions) or by statistical flaws, particularly with respect to the selective exclusion of positive studies.21 The meta-analysis by van Oosterhout et al20 excluded 3 positive studies as a result of using excessively conservative exclusion criteria, particularly when considering the small number of available studies: 2 studies22,23 were excluded given that pre- and post-measures were not available, although the pre-post difference was reported in the article and although statistical methods exist to determine effect size in such cases24; one study25 was excluded because scores of partial subscales (and not of the global scale) were reported, although the complete data were available by contacting the authors of the study. Therefore in the meta-analysis presented here, more suitable inclusion criteria were used to investigate the effect of MCT on positive symptoms and delusions. Given that the level of active engagement differs significantly between those patients with schizophrenia who dropout of cognitive-behavioral therapy and those who finish the therapy,26 acceptance of MCT and of control interventions were compared as higher acceptance of a therapy might foster higher adherence.
It was hypothesized that patients undergoing MCT would display a reduction in positive symptoms and fewer delusions compared to participants in control groups at the end of therapy. Additionally, it was hypothesized that acceptance of MCT was higher than acceptance of control interventions.
Method
Inclusion and Exclusion Criteria
Studies were included in the meta-analysis, if (1) participants had a diagnosis of a schizophrenia spectrum disorder according to DSM-IV-TR criteria27; (2) the intervention group received MCT; (3) a control condition was included; and (4) at least one of the relevant outcomes was measured. Studies evaluating group and individual MCT were considered.
Studies that provided other elements of psychological interventions for the experimental group in addition to MCT were excluded, particularly Reasoning Training28 and combinations of Social Cognition and Interaction Training (SCIT) and MCT,29 as these studies can not differentiate between effects stemming from MCT and those stemming from the addition of other psychological interventions.
Outcomes
Positive Symptoms.
As a measure of positive symptoms the positive subscale of the Positive and Negative Syndrome Scale (PANSS)30 was used. One study31 calculating the positive subscale of the PANSS with slightly different items than in the original version was also included. For another study25 that used multiple algorithms for the positive subscale of the PANSS, one algorithm was chosen by a person not involved in this meta-analysis drawing numbers, with the result being that the algorithm from Knorring32 was used for this meta-analysis. In contrast, the total score of the Psychotic Symptom Rating Scales (PSYRATS)33 was not used as a measure of positive symptoms, because some studies showed that the PSYRATS is not strongly correlated with the positive subscale of the PANSS, such that these instruments may tap into different concepts.34,35
Delusions.
The sum of the delusions subscale of the PSYRATS was used as a measure for delusions. As an alternative, the Peters et al Delusion Inventory (PDI-21)36 was used in one study. Not used was the Brown Assessment of Beliefs Scale (BABS)37 because it focuses more on insight in delusions and is therefore not directly comparable to the PSYRATS and PDI-21.38
Subjective Acceptance of the Intervention.
Subjective acceptance of the intervention was measured with the 10-item acceptance questionnaire39 or similar shorter versions thereof. Answers could be given on a 5-point Likert scale from 1 (fully disagree) to 5 (fully agree). The standard deviations of the means in individual studies only reporting means and standard deviations for the individual items were calculated using the formula from Borenstein40 and imputing the correlations between items in the study by Moritz et al.41 For all calculations pertaining to acceptance, calculated effect sizes of individual studies were recoded, so the direction of effects was the same as for delusions and positive symptoms with lower values indicating an advantage for the group receiving MCT.
For all outcomes only the post measurements were considered for this meta-analysis. Follow-up measurements were not considered as these were only available for a few studies that also used different interval until follow-up so that results were not comparable.
Identification of Studies
Studies about MCT for schizophrenia were searched by C.E. in the following data bases from 2007 until June 2, 2015: PsycINFO, PUBMED, Embase, and the Cochrane central register of controlled trials. 2007 was chosen as a start date as the first study on MCT was published in this year. The search in these data bases was conducted using the following terms which had to be part of the title or keywords: (delusion* or psychosis or psychotic or schizophren*) and (metacogn* or reason* or cognitive bias*) and (training or therap* or intervention). Studies in any language were considered, although all studies included in this meta-analysis were published in English. Additionally, the reference lists of all identified studies were searched for further studies. Prof. Dr Steffen Moritz, one of the developers of MCT, was also consulted for identifying relevant studies. The systematic review was executed according to the PRISMA standard, including evaluation of bias (confounding, overlapping data, publication bias).42
Data Collection and Analysis
Control of Potential Biases.
Data from studies was coded independently by the 2 authors of the article using a coding protocol.
Randomized Group Allocation
To control for potential effects of nonrandomized group, allocation studies that stated that participants were nonrandomly allocated to experimental groups were considered to be at a high risk for bias. Additionally, studies that did not explicitly state that participants were randomly allocated to groups were considered as being at a high risk for bias. It was assumed that study authors would have mentioned randomized group allocation, if they had employed it. Studies stating that they randomly assigned participants to different groups were considered to be at a low risk for bias with regard to randomized group allocation.
Masking
Studies that used interviewers for assessing outcomes, who were not informed about group allocation of the questioned participants, were considered as being at a low risk for bias. Studies using interviewers who knew about group allocation of the tested participants were considered as being at a high risk for bias. Studies making no statement about masking were also considered as having a high risk for bias, as it was assumed that study authors would have provided information about masking if they had employed it. Furthermore, data that was only assessed by self-report of the participants was considered to have a high risk for bias.
Incomplete Outcome Data
Similar to the approach used in the meta-analysis about cognitive-behavioral therapy,8 studies with dropout rates of more than 20% that used no intent-to-treat approach were considered to be at a high risk for bias.
Effect Size Measures.
Effect sizes were calculated using the standardized mean difference Hegdes’ g with Review Manager 5.
Dealing With Missing Data
If variables, necessary for effect size calculations, could not be taken directly from studies responsible authors were contacted in keeping with Cochrane guidelines (Chapter 7).43 In cases where only the standard deviation of posttest scores was missing the standard deviation from the pretest was imputed. In studies that reported mean change scores instead of mean posttest scores, the change scores were used as an estimate for the effect size.24 Change scores were recoded to ensure that the direction of the effect was similar to studies using the posttest mean for the calculation of effect sizes. The required standard deviations of the change scores were calculated with the formula provided by Lipsey and Wilson.24 Moritz et al41 calculated a mean correlation of r = .768 between pretest and posttest scores of the PANSS positive subscale in their study investigating the efficacy of MCT. This correlation was imputed for calculations of missing standard deviations of change scores. In one study,22 only change scores of single items of the delusion subscale of the PSYRATS were reported. The change scores were summed and used as an estimate for the effect size according to the method put forward by Lipsey and Wilson.24 The sum of change scores of single items was recoded to ensure the direction of the effect was similar to studies using the posttest mean for calculation of effect sizes. In one study,44 only 2 of 3 items used to assess acceptance were reported. As mean and standard deviation for the missing item could not be estimated, the acceptance scale in this study was calculated considering just the 2 items for which means and standard deviations were reported.
To assess heterogeneity of effects values for I 2 with confidence intervals, τ with confidence intervals and Q-statistics with significance tests were calculated using R metafor. Publication bias was examined using funnel plots for all relevant outcomes. Missing studies were imputed using trim and fill procedures as proposed by Duval and Tweedie45,46 using R metafor. Random effect models were used for the analysis of all outcome measures as the studies included in this meta-analysis were heterogeneous. As differences between groups at baseline can skew the estimate of the posttest effect size, effect sizes for pretest scores were computed for both delusions and positive symptoms.
Subgroup Analysis and Analysis of Heterogeneity.
Effect of an Active Control Intervention
The aim was to investigate how the use of an active psychological control intervention influenced the effect sizes. Each psychological intervention that exceeds contacts with providers of treatment typically provided in treatment as usual settings was defined as an active control intervention. Q-statistics with significance tests were used to test for subgroup differences.
Effect of Group vs Individual Training
It was examined whether effects of MCT differed depending on the setting, ie, group or individual therapy. Q-statistics with significance tests were used to test for subgroup differences.
Sensitivity Analyses.
Sensitivity analyses were performed for all outcomes to determine whether the results were driven mainly by single studies. Heterogeneity of the remaining studies was assessed with I 2 with confidence intervals, τ with confidence intervals and Q-statistics with significance tests. These were calculated using R metafor.
Results
Description of Studies
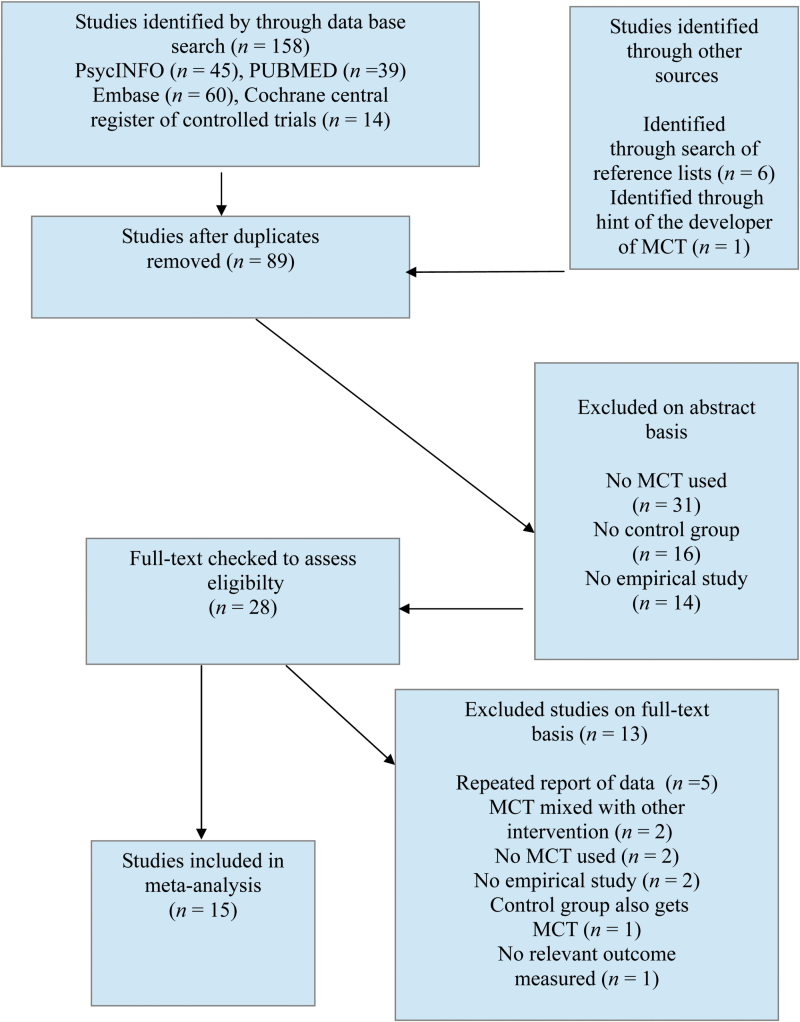
The search in data bases produced 158 articles, another 6 articles were identified by checking reference lists of considered studies. In addition, one study47 was considered that had come to the attention of Prof. Steffen Moritz, one of the developers of MCT. After removing duplicates, 89 studies were screened for title or abstract for fulfilling inclusion criteria. Then, 28 studies were screened on full text basis; 13 studies were excluded on the basis of the full text. Finally, 15 studies were included in the meta-analysis (see figure 1 for a flow chart of the selection process).
Fig. 1.
Flow chart of study selection. Adapted from, Moher et al.42 n = number of studies.
One study48 was excluded on the grounds that both groups received MCT. One study29 was excluded, because the intervention group received social cognition training in addition to MCT. One study28 was excluded, because delusions were measured by self-rated conviction and no other outcome relevant for this meta-analysis was measured. One study49 was excluded because acceptance was only assessed for the group receiving MCT and no other relevant outcome was reported. An overview over all included studies is given in table 1.
Table 1.
Summary Table of Included Studies and Effect Sizes
| Author and Year | Samplea | Mesurementb | RCT | Masking | Dropoutc | Effect Sizes [CI] | Significance | Notes |
|---|---|---|---|---|---|---|---|---|
| Aghotor et al, 2010 | MCT (group): n = 16, vs newspaper discussion group: n = 14 | Baseline, after 4 weeks | yes | yes | 13.3 | Positive: −0.28 [−1.05, 0.50] | Positive: P = .48 | Low statistical power, change scores are used for effect size calculation |
| Acceptance: P = .67 | ||||||||
| Acceptance: −0.57 [−1.41, 0.28] | ||||||||
| Balzan et al, 2014 | MCT (individual single-session training): n = 14, vs TAU: n = 14 | Baseline, 2 weeks after training | no | no | 0 | Positive: 0.05 [−0.69, 0.80] | Positive: P = .89 | Significant group differences favoring control group at pretest, PDI-21 used to assess delusions |
| Delusions: P = .76 | ||||||||
| Delusions: 0.12 [−0.63, 0.86] | ||||||||
| Acceptance: M = −3.92d, SD = 0.46d | ||||||||
| Briki et al, 2014 | MCT (group): n = 35, vs supportive therapy: n = 33 | Baseline, after 8 weeks | yes | yes | 26.5 | Positive: −0.59 [−1.16, −0.02] | Positive: P = .04 | Only 3 items used to assess acceptance, of which only 2 were reported |
| Delusions: P = .55 | ||||||||
| Acceptance: P = .38 | ||||||||
| Delusions: −0.17 [−0.72, 0.39] | ||||||||
| Acceptance: −0.25 [−0.80, 0.31] | ||||||||
| Erawati et al, 2014 | MCT (individual): n = 26, vs TAU: n = 26 | Baseline, after 4 weeks | no | no | 0 | Delusions: −1.72 [−2.37, −1.08] | Delusions: P < .01 | Change scores are used for effect size calculation, pretest standard deviations are imputed |
| Acceptance: M = −3.78d, SD = 0.10d | ||||||||
| Favrod et al, 2014 | MCT (group): n = 26, vs TAU: n = 26 | Baseline, after 8 weeks | yes | yes | 7.7 | Positive: −0.49 [−1.06, 0.09] | Positive: P = .10 | |
| Delusions: P = .07 | ||||||||
| Delusions: −0.54 [−1.12, 0.04] | ||||||||
| Gawęda et al, 2015 | MCT (group): n = 26, vs TAU: n = 24 | Baseline, after 4 to 5 weeks | yes | yes | 12.0 | Delusions: −0.35 [−0.94, 0.25] | Delusions: P = .25 | |
| Kumar et al, 2010 | MCT (group): n = 8, vs TAU: n = 8 | Baseline, after 4 weeks | yes | yes | 0 | Positive: −0.92 [−1.97, 0.13] | Positive: P = .09 | Low statistical power |
| Kuokkanen et al, 2014 | MCT (group): n = 10, vs TAU: n = 10 | Baseline, after 4 weeks | yes | yes | 0 | Positive: −0.16 [−1.03, 0.72] | Positive: P = .73 | SD were imputed from pretest scores, P1+P6+ G12 used for PANSS positive |
| Delusions: P = .57 | ||||||||
| Delusions: −0.25 [−1.13, 0.63] | ||||||||
| Moritz, Kerstan, et al, 2011 | MCT (group): n = 18, vs wait list control n = 18 | Baseline, after 8 weeks | yes | yes | 0 | Positive: −0.03 [−0.68, 0.63] | Positive: P = .93 | Around 50% of the participants were diagnosed with a substance dependence disorder |
| Delusion: P = .79 | ||||||||
| Delusion: −0.09 [−0.74, 0.56] | ||||||||
| Acceptance: M = 4.31d, SD = 0.16d | ||||||||
| Moritz et al, 2013 | MCT (group): n = 76, vs CogPack n = 74 | Baseline, after 4 weeks | yes | yes | 10.0 | Positive: −0.10 [−0.44, 0.24] | Positive: P = .57 | |
| Delusion: P = .2 | ||||||||
| Acceptance: P < .01 | ||||||||
| Delusion: −0.22 [−0.56, 0.12] | ||||||||
| Acceptance: −0.49 [−0.83, −0.14] | ||||||||
| Moritz, Veckenstedt, et al, 2011 | MCT (group and individual): n = 24, vs CogPack: n = 24 | Baseline, after 4 weeks | yes | yes | 8.3, but ITT approach | Positive: −0.65 [−1.24, −0.07] | Positive: P = .03 | Knorring algorithm for PANSS positive was used |
| Delusion: P = .10 | ||||||||
| Acceptance: P < .01 | ||||||||
| Delusion: −0.48 [−1.05, 0.09] | ||||||||
| Acceptance: −1.71 [−2.38, −1.05] | ||||||||
| Moritz & Woodward, 2007 | MCT (group): n = 20, vs CogPack: n = 20 | Retrospective assessment after 4 weeks | yes | no, subjective assessment | 0 | Acceptance: −1.34 [−2.03, −0.65] | Acceptance: P < .01 | |
| Naughton et al, 2012 | MCT (group): n = 11, vs wait list control: n = 8 | Baseline, after 6 months | no | no | 0 | Positive: −0.36 [−1.28, 0.56] | Positive: P = .45 | |
| So et al, 2015 | MCT (short version, modules 2,3,5, and 7 in individual setting): n = 23, vs TAU: n = 21 | Baseline, after 4 weeks | yes | yes | 29.6 | Positive: −0.98 [−1.74, −0.23] | Positive: P = .01 | |
| Delusion: P = .01 | ||||||||
| Delusion: −1.33 [−2.12, −0.54] | ||||||||
| Acceptance: M = −4.06d, SD = 0.06d | ||||||||
| Van Oosterhout et al, 2014 | MCT (group): n = 75, vs TAU: n = 79 | Baseline, after 8 weeks | yes | yes | 16.9 but ITT approach | Delusion: 0.25 [−0.06, 0.57] | Delusion: P = .12 | Study included only moderately to severely deluded patients |
Note: n, number of participants; TAU, treatment as usual; RCT, randomized controlled trial; ITT, intention to treat; MCT, metacognitive training; PANSS, Positive and Negative Syndrome Scale; PDI-21, Peters et al Delusion Inventory.
aGiven values are the number of participants at the start of the study.
bOnly measurements relevant for this meta-analysis are stated.
cValues are given in percent.
dDescriptive results of acceptance for MCT group.
Effect Sizes
Positive Symptoms.
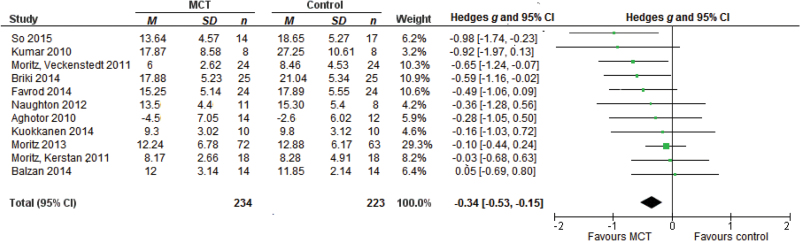
The effect size for 11 studies on positive symptoms was g = −0.34, 95% CI [−0.53, −0.15], P < .01 (negative sign favors MCT, see figure 2). The studies were homogeneous with Q = 10.28, P = .42, I 2 = 2.68, 95% CI [0.00, 68.70] and τ = 0.05, 95% CI [0.00, 0.48].
Fig. 2.
Forest plot of studies in the meta-analysis of positive symptoms. Effect sizes of metacognitive training (MCT) on positive symptoms.
Delusions.
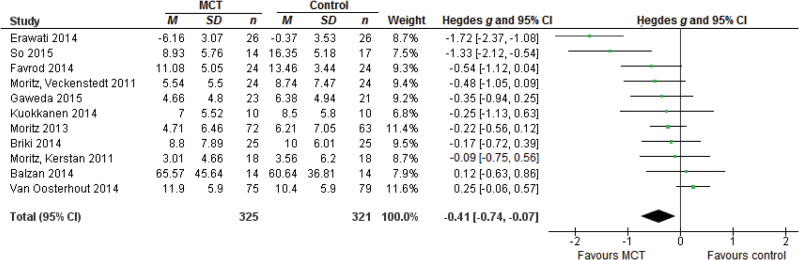
The effect size for 11 studies on delusions was g = −0.41, 95% CI [−0.74, −0.07], P = .02 (figure 3). The studies were heterogeneous with Q = 40.49, P < .01, I 2 = 75.30, 95% CI [49.13, 92.85] and τ = 0.48, 95% CI [0.27, 0.99].
Fig. 3.
Forest plot of studies in the meta-analysis of delusions. Effect sizes of metacognitive training (MCT) on delusions.
Acceptance of MCT.
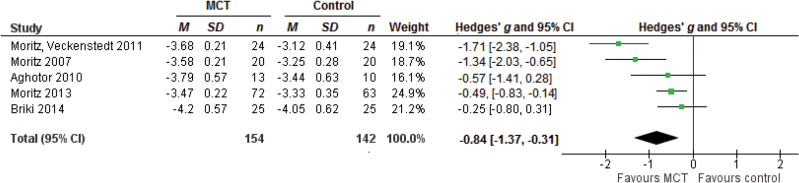
The effect size for 5 studies on acceptance of the intervention was g = −0.84, 95% CI [−1.37, −0.31], P < .01 (figure 4). The studies were heterogeneous with Q = 16.50, P < .01, I 2 = 75.75, 95% CI [33.36, 97.33] and τ = 0.52, 95% CI [0.21, 1.77].
Fig. 4.
Forest plot of studies in the meta-analysis of acceptance of the intervention. Effect sizes of acceptance of the intervention.
Analysis of Potential Biases
Risk of Bias.
No significant difference in effect sizes were observed between studies with high vs low risk of bias with respect to randomization, masking and completeness of outcome data (supplementary material S2).
Sensitivity Analyses.
Removing individual studies from the meta-analysis of positive symptoms made little difference to the findings (all gs between −0.29 to −0.44 with Ps < .01). Removing the study from Erawati et al22 in the meta-analysis of delusions considerably reduced the effect size to g = −0.25, 95% CI [−0.51, 0.00], P = .05, which remained significant. This also considerably reduced heterogeneity in the remaining studies with Q = 19.07, P = .02, I 2 = 52.80, 95% CI [17.74, 87.31] and τ = 0.29, 95% CI [0.01, 0.71]. A similar result was obtained if the So et al47 study was removed from the meta-analysis of delusions with g = −0.33, 95% CI [−0.65, 0.00], P = .05. In contrast, if the study by van Oosterhout et al50 was removed the effect size increased to g = −0.49, 95% CI [−0.81, −0.16], P < .01 and heterogeneity of the remaining studies dropped to Q = 26.76, P < .01, I 2 = 66.37, 95% CI [31.98, 91.67] and τ = 0.42, 95% CI [0.21, 1.00]. The effect size of acceptance of the intervention changed only slightly if individual studies were removed (all gs between −0.61 to −1.01with Ps ≤ .01).
Analysis of Baseline Differences Between Groups.
The effect size of pretest scores for positive symptoms was g = 0.01, 95% CI [−0.28, 0.30]. The effect size of pretest scores for delusions was g = 0.13, 95% CI [−0.03, 0.29], indicating that participants in the control groups had slightly less delusions than participants in the groups about to get MCT, although this effect was not significant.
Influence of the Use of an Active Control Intervention.
Effect sizes did not differ significantly according to the presence or absence of an active control group (supplementary material S3).
Difference Between Group and Individual MCT.
Effect sizes were higher in studies using Individual than Group MCT but differences were not significant (all Ps > .09; supplementary material S4).
Publication Bias.
Funnel plots for positive symptoms, delusions and acceptance of the intervention are presented in supplementary figures S5, S6 and S7, respectively. Using trim and fill procedures 2 studies were imputed in the meta-analysis of positive symptoms. If the asymmetry is due to publication bias, our analyses suggest that the true effect size on positive symptoms is g = −0.29, 95% CI [−0.50, −0.07], P = .01. Using trim and fill procedures no studies were imputed in the meta-analysis on delusions or acceptance of the intervention.
Discussion
This meta-analysis showed a significant small to medium effect of MCT on positive symptoms. Studies were somewhat more homogeneous, thus allowing generalization of the findings. Results are thus in accordance with a previous meta-analysis19 which also found a significant effect of MCT on positive symptoms, but considered fewer studies. A second meta-analysis20 showed a slightly smaller and nonsignificant effect of MCT on positive symptoms, but this study had used different inclusion criteria, excluding studies that did not provide complete outcome data for both pretest and posttest. Likewise, there was also a small to medium effect on delusions. The studies were heterogeneous, making it more difficult to generalize the findings. Effects were larger than in the second meta-analysis,20 again reflecting differences in inclusion criteria. There was a large effect for acceptance of MCT, indicating that acceptance of MCT was considerably better than the acceptance of control interventions. This is noteworthy in view of high rates of nonadherence in patients with schizophrenia for both pharmacological and psychological interventions.26,51,52 However, as 3 out of the 5 studies used to compare acceptance of the intervention used CogPack53 as the control intervention, the finding that MCT is better accepted than control interventions seems to be true for the comparison with CogPack, but must still be put to test for other psychological interventions. Heterogeneity of the included studies makes it difficult to generalize the findings.
Influences of Potential Biases
Influences on Effect Sizes for Positive Symptoms.
Sensitivity analyses as well as analyses examining a possible publication bias suggest robust findings with applies to both studies using active and passive control interventions. If nonrandomized group allocation, non-masked outcome assessment and incomplete outcome data were accounted for simultaneously, there was a larger effect on positive symptoms for studies being at high risk of bias than for studies being at low risk of bias. Yet, the small to medium effect on positive symptoms remained significant even for those studies being at low risk of bias, indicating that differences in methodological rigor were responsible for some, but not all of the effects.
Influences on Effect Sizes for Delusions.
When randomized group allocation, masked assessment of outcomes and missing outcome data were considered simultaneously, the effect on delusions differed considerably between studies being at low risk for bias and studies being at high risk for bias, indicating that differences in methodological rigor were partly responsible for the effect. Yet, there was still a nonsignificant effect on delusions when only studies being at low risk for bias were considered. The nonsignificant findings for studies being at low risk of bias were mainly driven by one study.50 It remains unclear if the nonsignificant findings for studies being at low risk for bias indicate that there was no effect above chance or if the results just remained insignificant due to low power, often a problem in subgroup analyses when there are few studies per subgroup.
There was considerable difference between studies using an active control intervention and studies using treatment as usual or a waiting group, indicating that some of the effects on delusions were due to lack of appropriate control interventions. However, even for the subgroup using active control interventions, there was a significant small effect on delusions, so some, but not all of the effect size on delusions can be attributed to differences regarding the control interventions used.
Sensitivity analyses showed that omitting individual studies changed the results considerably in some cases. If the study by Erawati et al22 was removed the effect size decreased. This study showed a very large effect of MCT on delusions. But effect size calculations could not be carried out using the original data, so change scores for individual items of the PSYRATS delusion subscale were summed up and used as the mean score of the posttest. Furthermore, neither standard deviations of change scores nor standard deviations of posttest scores were provided, so standard deviations from pretest scores had to be imputed. This might have led to an overestimation of the actual effect in this study. Removing the study by So et al47 also reduced the effect size considerably.
On the other hand, removing the study by van Oosterhout et al50 increased the effect size on delusions. It was one of 2 studies reporting lower posttest values of delusions for patients in the control condition than for patients in the condition receiving MCT. It was a high-quality study. However, patients with severe delusions were included, whereas the other studies in this meta-analysis tended to exclude severely deluded patients for the group intervention. It is possible that patients in this study50 were too disorganized or not attentive enough due to concurrent positive symptoms, which might explain the negative effect on delusions in this study. In addition, an earlier version of MCT was used, which failed to stress the importance of attenuating one’s level of confidence in the light of incomplete or ambiguous evidence. Newer versions of the MCT have added this feature as a core element of MCT. No publication bias was evident for the meta-analysis of delusions.
Influences on Effect Sizes for Acceptance of the Intervention.
The findings were robust to sensitivity analyses. One study44 that was considered to be at high risk of bias considering randomized group allocation and missing outcome data showed a considerably smaller effect on acceptance than the studies being classified as being at low risk of bias. The reason for this might be that this study used a different scale than the other studies for assessing acceptance and only 2 items (originally 3 items, but only 2 items were reported), whereas the other studies used 10 items. The items used were also formulated differently than in the other studies. Thus it may be difficult to compare the value for acceptance in this study with the other studies. No publication bias was evident for the meta-analysis of acceptance of the intervention.
Special Issues of Individual Studies.
One study54 included in this meta-analysis reported lower posttest scores on positive symptoms for the control group than for the MCT group. It also reported lower posttest scores of delusions for the control group. This was due to significant pretest group differences favoring the control group with regard to both positive symptoms and delusions.
Differences Between Individual and Group MCT
Effect sizes for individual MCT were considerably larger than effect sizes for group MCT for all outcome measures. Still it needs to be considered that except for one study55 that investigated a version of MCT that combined group and individual sessions, all studies investigating individual MCT either used nonrandomized group allocation and non-masked assessment of outcomes, or were at high risk for bias regarding incomplete outcome data. So one cannot be sure whether the larger effects of individual MCT really indicate an advantage of individual MCT over group MCT or were simply due to lower study quality of studies investigating individual MCT.
Limitations
One limitation of this meta-analysis is that it sometimes used different types of outcome data for calculating effect sizes. While most studies reported means and standard deviations for posttest values, one study23 reported only change scores from pretest to posttest with the corresponding standard deviations. One study22 also reported change scores only and failed to report standard deviations of change scores, making it necessary to impute pretest standard deviations. Pretest standard deviations had also to be imputed in another study.31 These inconsistent outcome measures might have influenced the effect size calculations. As studies investigating individual MCT were of lower methodological quality (except one study55), it cannot be determined if larger effects for individual MCT were the result of a decreased of methodological rigor. Therefore more high-quality studies investigating individual MCT should be conducted. Significance tests for subgroup analyses and tests for differences between subgroups had low power because the number of studies was relatively small and so the number of studies in subgroups was even smaller. Therefore it is hard to interpret tests of significance for subgroups.
In addition to these methodological limitations, these results are limited by few investigations into long-term effects of MCT. Due to the small number of studies reporting follow-up assessments, a meta-analysis of these data was not possible, but these 2 studies found significant positive results for both delusions and positive symptoms in patients reassessed 6 to 36 months after the end of MCT.56,57 Finally, over and above the significant effect of MCT on positive symptoms, its clinical relevance in terms of both daily life and social functioning has not been assessed to date, and future studies should include measures of global functioning and social cognition to better document these points.
Conclusions
The present meta-analysis showed small to moderate effect sizes for MCT on delusions and positive symptoms of schizophrenia. These were in similar range as those reported with cognitive-behavioral therapy of positive symptoms for schizophrenia.8,9,12 Acceptance of MCT was also high, and altogether, this evidence supports the dissemination of MCT in routine care. Clinicians should however be aware that individual MCT may be more effective than group MCT for patients with severe delusions, given the results of one study50 that did not found significant effects of group MCT on samples including severely delusional patients. Cognitive-behavioral therapy also represents a validated therapeutic option for patients with medication-resistant psychotic symptoms.12
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
This study did not receive financial support.
Supplementary Material
Acknowledgment
Authors declare no conflict of interest.
References
- 1. Freeman D, Garety P. Advances in understanding and treating persecutory delusions: a review. Soc Psychiatry Psychiatr Epidemiol. 2014;49:1179–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bell V, Halligan PW, Ellis HD. Explaining delusions: a cognitive perspective. Trends Cogn Sci. 2006;10:219–226. [DOI] [PubMed] [Google Scholar]
- 3. Van der Gaag M. A neuropsychiatric model of biological and psychological processes in the remission of delusions and auditory hallucinations. Schizophr Bull. 2006;32:S113–S122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Freeman D. Suspicious minds: the psychology of persecutory delusions. Clin Psychol Rev. 2007;27:425–457. [DOI] [PubMed] [Google Scholar]
- 5. Fine C, Gardner M, Craigie J, Gold I. Hopping, skipping or jumping to conclusions? Clarifying the role of the JTC bias in delusions. Cogn Neuropsychiatry. 2007;12:46–77. [DOI] [PubMed] [Google Scholar]
- 6. Dudley R, Taylor P, Wickham S, Hutton P. Psychosis, delusions and the “Jumping to Conclusions” reasoning bias: a systematic review and meta-analysis. Schizophr Bull. 2016;42:652–665. doi:10.1093/schbul/sbv150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garety PA, Freeman D. The past and future of delusions research: from the inexplicable to the treatable. Br J Psychiatry. 2013;203:327–333. [DOI] [PubMed] [Google Scholar]
- 8. Jauhar S, McKenna PJ, Radua J, Fung E, Salvador R, Laws KR. Cognitive-behavioural therapy for the symptoms of schizophrenia: systematic review and meta-analysis with examination of potential bias. Br J Psychiatry. 2014;204:20–29. [DOI] [PubMed] [Google Scholar]
- 9. Wykes T, Steel C, Everitt B, Tarrier N. Cognitive behavior therapy for schizophrenia: effect sizes, clinical models, and methodological rigor. Schizophr Bull. 2008;34:523–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xia J, Merinder LB, Belgamwar MR. Psychoeducation for schizophrenia. Schizophr Bull. 2011;37:21–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Turner DT, van der Gaag M, Karyotaki E, Cuijpers P. Psychological interventions for psychosis: a meta-analysis of comparative outcome studies. Am J Psychiatry. 2014;171:523–538. [DOI] [PubMed] [Google Scholar]
- 12. Burns AMN, Erickson DH, Brenner CA. Cognitive-behavioral therapy for medication-resistant psychosis: a meta-analytic review. Psychiatr Serv. 2014;65:874–880. [DOI] [PubMed] [Google Scholar]
- 13. Moritz S, Vitzthum F, Randjbar S, Veckenstedt R, Woodward TS. Detecting and defusing cognitive traps: metacognitive intervention in schizophrenia. Curr Opin Psychiatry. 2010;23:561–569. [DOI] [PubMed] [Google Scholar]
- 14. Garety PA, Hemsley DR, Wessely S. Reasoning in deluded schizophrenic and paranoid patients. Biases in performance on a probabilistic inference task. J Nerv Ment Dis. 1991;179:194–201. [DOI] [PubMed] [Google Scholar]
- 15. Lincoln TM, Ziegler M, Mehl S, Rief W. The jumping to conclusions bias in delusions: specificity and changeability. J Abnorm Psychol. 2010;119:40–49. [DOI] [PubMed] [Google Scholar]
- 16. Savla GN, Vella L, Armstrong CC, Penn DL, Twamley EW. Deficits in domains of social cognition in schizophrenia: a meta-analysis of the empirical evidence. Schizophr Bull. 2013;39:979–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moritz S, Werner R, von Collani G. The inferiority complex in paranoia readdressed: a study with the Implicit Association Test. Cogn Neuropsychiatry. 2006;11:402–415. [DOI] [PubMed] [Google Scholar]
- 18. Moritz S, Andreou C, Schneider BC, et al. Sowing the seeds of doubt: a narrative review on metacognitive training in schizophrenia. Clin Psychol Rev. 2014;34:358–366. [DOI] [PubMed] [Google Scholar]
- 19. Jiang J, Zhang L, Zhu Z, Li W, Li C. Metacognitive training for schizophrenia: a systematic review. Shanghai Arch Psychiatry. 2015;27:149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Van Oosterhout B, Smit F, Krabbendam L, Castelein S, Staring ABP, van der Gaag M. Metacognitive training for schizophrenia spectrum patients: a meta-analysis on outcome studies. Psychol Med. 2016;46:47–57. [DOI] [PubMed] [Google Scholar]
- 21. Moritz S, Werner D, Menon M, Balzan RP, Woodward TS. Jumping to negative conclusions - a case of study-gathering bias? Psychol Med. 2016;46:59–61. [DOI] [PubMed] [Google Scholar]
- 22. Erawati E, Keliat BA, Helena N, Hamid A. The influence of metacognitive training on delusion severity and metacognitive ability in schizophrenia. J Psychiatr Ment Health Nurs. 2014;21:841–847. [DOI] [PubMed] [Google Scholar]
- 23. Aghotor J, Pfueller U, Moritz S, Weisbrod M, Roesch-Ely D. Metacognitive training for patients with schizophrenia (MCT): feasibility and preliminary evidence for its efficacy. J Behav Ther Exp Psychiatry. 2010;41:207–211. [DOI] [PubMed] [Google Scholar]
- 24. Lipsey MW, Wilson DB. Practical Meta-Analysis. 1st ed. Thousand Oaks, CA: SAGE Publications; 2000. [Google Scholar]
- 25. Moritz S, Kerstan A, Veckenstedt R, et al. Further evidence for the efficacy of a metacognitive group training in schizophrenia. Behav Res Ther. 2011;49:151–157. [DOI] [PubMed] [Google Scholar]
- 26. Startup M, Wilding N, Startup S. Patient treatment adherence in cognitive behaviour therapy for acute psychosis: the role of recovery style and working alliance. Behav Cogn Psychother. 2006;34:191–199. [Google Scholar]
- 27. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Revised Fourth Edition (DSM-IV-TR). 4th ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 28. Ross K, Freeman D, Dunn G, Garety P. A randomized experimental investigation of reasoning training for people with delusions. Schizophr Bull. 2011;37:324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rocha NBF, Queirós C. Metacognitive and social cognition training (MSCT) in schizophrenia: a preliminary efficacy study. Schizophr Res. 2013;150:64–68. [DOI] [PubMed] [Google Scholar]
- 30. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 31. Kuokkanen R, Lappalainen R, Repo-Tiihonen E, Tiihonen J. Metacognitive group training for forensic and dangerous non-forensic patients with schizophrenia: a randomised controlled feasibility trial. Crim Behav Ment Health CBMH. 2014;24:345–357. [DOI] [PubMed] [Google Scholar]
- 32. Mass R, Schoemig T, Hitschfeld K, Wall E, Haasen C. Psychopathological syndromes of schizophrenia: evaluation of the dimensional structure of the positive and negative syndrome scale. Schizophr Bull. 2000;26:167–177. [DOI] [PubMed] [Google Scholar]
- 33. Haddock G, McCarron J, Tarrier N, Faragher EB. Scales to measure dimensions of hallucinations and delusions: the psychotic symptom rating scales (PSYRATS). Psychol Med. 1999;29:879–889. [DOI] [PubMed] [Google Scholar]
- 34. Drake R, Haddock G, Tarrier N, Bentall R, Lewis S. The Psychotic Symptom Rating Scales (PSYRATS): their usefulness and properties in first episode psychosis. Schizophr Res. 2007;89:119–122. [DOI] [PubMed] [Google Scholar]
- 35. Kronmüller K-T, von Bock A, Grupe S, et al. Psychometric evaluation of the Psychotic Symptom Rating Scales. Compr Psychiatry. 2011;52:102–108. [DOI] [PubMed] [Google Scholar]
- 36. Peters E, Joseph S, Day S, Garety P. Measuring delusional ideation: the 21-item Peters et al. Delusions Inventory (PDI). Schizophr Bull. 2004;30:1005–1022. [DOI] [PubMed] [Google Scholar]
- 37. Eisen JL, Phillips KA, Baer L, Beer DA, Atala KD, Rasmussen SA. The Brown Assessment of Beliefs Scale: reliability and validity. Am J Psychiatry. 1998;155:102–108. [DOI] [PubMed] [Google Scholar]
- 38. Buhlmann U. The German version of the Brown Assessment of Beliefs Scale (BABS): development and evaluation of its psychometric properties. Compr Psychiatry. 2014;55:1968–1971. [DOI] [PubMed] [Google Scholar]
- 39. Moritz S, Woodward TS. Metacognitive training for schizophrenia patients (MCT): a pilot study on feasibility, treatment adherence, and subjective efficacy. Ger J Psychiatry. 2007;10:69–78. [Google Scholar]
- 40. Borenstein M. Introduction to Meta-Analysis. 1st ed. Chichester, UK: Wiley; 2009. [Google Scholar]
- 41. Moritz S, Veckenstedt R, Bohn F, et al. Complementary group Metacognitive Training (MCT) reduces delusional ideation in schizophrenia. Schizophr Res. 2013;151:61–69. [DOI] [PubMed] [Google Scholar]
- 42. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. 1st ed. Chichester, UK: Wiley; 2011. [Google Scholar]
- 44. Briki M, Monnin J, Haffen E, et al. Metacognitive training for schizophrenia: a multicentre randomised controlled trial. Schizophr Res. 2014;157:99–106. [DOI] [PubMed] [Google Scholar]
- 45. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. [DOI] [PubMed] [Google Scholar]
- 46. Duval S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc. 2000;95:89–98. [Google Scholar]
- 47. So SH-W, Chan AP, Chong CS-Y, et al. Metacognitive training for delusions (MCTd): effectiveness on data-gathering and belief flexibility in a Chinese sample. Front Psychol. 2015;6:730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ussorio D, Giusti L, Wittekind CE, et al. Metacognitive training for young subjects (MCT young version) in the early stages of psychosis: Is the duration of untreated psychosis a limiting factor? [published online ahead of print March 20, 2015] Psychol Psychother . doi:10.1111/papt.12059. [DOI] [PubMed] [Google Scholar]
- 49. Lam KCK, Ho CPS, Wa JC, et al. Metacognitive training (MCT) for schizophrenia improves cognitive insight: a randomized controlled trial in a Chinese sample with schizophrenia spectrum disorders. Behav Res Ther. 2015;64:38–42. [DOI] [PubMed] [Google Scholar]
- 50. Van Oosterhout B, Krabbendam L, de Boer K, et al. Metacognitive group training for schizophrenia spectrum patients with delusions: a randomized controlled trial. Psychol Med. 2014;44:3025–3035. [DOI] [PubMed] [Google Scholar]
- 51. Lambert M, Conus P, Cotton S, Robinson J, McGorry PD, Schimmelmann BG. Prevalence, predictors, and consequences of long-term refusal of antipsychotic treatment in first-episode psychosis. J Clin Psychopharmacol. 2010;30:565–572. [DOI] [PubMed] [Google Scholar]
- 52. McCann TV, Clark E, Lu S. Subjective side effects of antipsychotics and medication adherence in people with schizophrenia. J Adv Nurs. 2009;65:534–543. [DOI] [PubMed] [Google Scholar]
- 53. Marker K. COGPACK Manual Version 5.9. Ladenburg, Germany: Marker Software; 2003. [Google Scholar]
- 54. Balzan RP, Delfabbro PH, Galletly CA, Woodward TS. Metacognitive training for patients with schizophrenia: preliminary evidence for a targeted, single-module programme. Aust N Z J Psychiatry. 2014;48:1126–1136. [DOI] [PubMed] [Google Scholar]
- 55. Moritz S, Veckenstedt R, Randjbar S, Vitzthum F, Woodward TS. Antipsychotic treatment beyond antipsychotics: metacognitive intervention for schizophrenia patients improves delusional symptoms. Psychol Med. 2011;41:1823–1832. [DOI] [PubMed] [Google Scholar]
- 56. Moritz S, Veckenstedt R, Andreou C, et al. Sustained and “sleeper” effects of group metacognitive training for schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2014;71:1103–1111. [DOI] [PubMed] [Google Scholar]
- 57. Favrod J, Rexhaj S, Bardy S, et al. Sustained antipsychotic effect of metacognitive training in psychosis: a randomized-controlled study. Eur Psychiatry. 2014;29:275–281. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.