Abstract
In the cortex of subjects with schizophrenia, expression of glutamic acid decarboxylase 67 (GAD67), the enzyme primarily responsible for cortical GABA synthesis, is reduced in the subset of GABA neurons that express parvalbumin (PV). This GAD67 deficit is accompanied by lower cortical levels of other GABA-associated transcripts, including GABA transporter-1, PV, brain-derived neurotrophic factor (BDNF), tropomyosin receptor kinase B, somatostatin, GABAA receptor α1 subunit, and KCNS3 potassium channel subunit mRNAs. In contrast, messenger RNA (mRNA) levels for glutamic acid decarboxylase 65 (GAD65), another enzyme for GABA synthesis, are not altered. We tested the hypothesis that this pattern of GABA-associated transcript levels is secondary to the GAD67 deficit in PV neurons by analyzing cortical levels of these GABA-associated mRNAs in mice with a PV neuron-specific GAD67 knockout. Using in situ hybridization, we found that none of the examined GABA-associated transcripts had lower cortical expression in the knockout mice. In contrast, PV, BDNF, KCNS3, and GAD65 mRNA levels were higher in the homozygous mice. In addition, our behavioral test battery failed to detect a change in sensorimotor gating or working memory, although the homozygous mice exhibited increased spontaneous activities. These findings suggest that reduced GAD67 expression in PV neurons is not an upstream cause of the lower levels of GABA-associated transcripts, or of the characteristic behaviors, in schizophrenia. In PV neuron-specific GAD67 knockout mice, increased levels of PV, BDNF, and KCNS3 mRNAs might be the consequence of increased neuronal activity secondary to lower GABA synthesis, whereas increased GAD65 mRNA might represent a compensatory response to increase GABA synthesis.
Key words: schizophrenia, cerebral cortex, mouse, in situ hybridization, prepulse inhibition, working memory
Introduction
In the cerebral cortex of subjects with schizophrenia, the 67kDa isoform of glutamic acid decarboxylase (GAD67), the enzyme principally responsible for cortical γ-amino butyric acid (GABA) synthesis, is markedly reduced in the subset of GABA neurons that express parvalbumin (PV), indicating altered GABA neurotransmission by these neurons.1,2 The reduced GAD67 expression in schizophrenia is accompanied by lower cortical expression of other genes associated with GABA neuron functions. For example, reduced messenger RNA (mRNA) and protein levels were observed for GABA transporter-1 (GAT-1) and PV in PV neurons.1,3–5 Reduced mRNA levels were also reported for 2 proteins that mediate trophic effects on GABA neurons, namely brain-derived neurotrophic factor (BDNF) and its receptor tropomyosin receptor kinase B (TrkB),6–8 for somatostatin (SST),9–11 a specific marker for another subset of cortical GABA neurons, for GABAA receptor (GABAAR) α1 subunit,12,13 and for KCNS3 voltage-gated potassium channel subunit,14,15 which appears to be important for electrophysiological properties of PV neurons.14,16,17 In contrast, cortical mRNA levels for glutamic acid decarboxylase 65 (GAD65), another enzyme for GABA synthesis, appear to be unaffected in schizophrenia.18–20
Reduced GAD67 expression in PV neurons could induce changes in other GABA-associated transcripts. For example, the resulting reduced synaptic availability of GABA could induce downregulation of GAT-1, PV, and KCNS3 expression which would serve as compensatory responses by reducing presynaptic GABA reuptake,21 increasing GABA release,22 and enhancing PV neuron excitability,16 respectively. Reduced GABA neurotransmission by PV neurons also might lead to excessive pyramidal neuron activity which could then trigger a compensatory reduction in dendritic spines during postnatal development.23,24 Following maturation, the resulting pyramidal neuron hypoactivity could lead to an activity-dependent downregulation of BDNF, TrkB, and GABAAR α1 subunit expression25–27 in these neurons. Finally, lower BDNF signaling could drive down SST mRNA expression,9,10,28 which might provide a compensatory disinhibition of PV neurons by SST neurons, as SST neurons suppress PV neuron activity.29,30 Consistent with this interpretation, the reduction in GAD67 mRNA levels in schizophrenia was significantly correlated with the reductions in GAT-1,31 PV,1 BDNF,7 TrkB,7 and SST9,13 mRNAs.
Based on these findings, we hypothesized that lower levels of certain GABA-associated transcripts in the cortex of schizophrenia subjects could be secondary to the GAD67 deficit in PV neurons. In order to test this hypothesis, we created a conditional knockout mouse line in which Gad1, the gene encoding GAD67, was selectively inactivated in PV neurons. In the cortex of these mice, we assessed mRNA levels for GAT-1, PV, KCNS3, BDNF, TrkB, GABAAR α1, SST, and GAD65. We also tested whether the GAD67 knockout in PV neurons resulted in behavioral alterations characteristic of patients with schizophrenia by subjecting the mutant mice to a battery of behavioral tests. As GAD67 mRNA expression is undetectable in a subset of GABA neurons,32,33 including ~50% of PV neurons,1 in schizophrenia, we were particularly interested in the homozygous knockout that recapitulates such profound GAD67 deficits in PV neurons.
Methods
PV Neuron-Specific Inactivation of the Gad1 Gene
In order to achieve a PV neuron-specific inactivation of Gad1, which codes for GAD67, we used 2 genetically engineered mouse lines. The first line contained an IRES-Cre-pA cassette inserted into the 3′-untranslated region of exon 5 of the Pvalb gene that encodes PV (PV-Cre mice).34 The second line contained floxed exon 1 of the Gad1 gene (floxed-GAD67 mice).35 Both lines had a mixed genetic background of 129/OlaHsd and C57BL/6. We first crossed homozygous PV-Cre mice (Pvalb Cre/Cre) and homozygous floxed-GAD67 mice (Gad1 loxp/loxp) to obtain heterozygous mice for both Pvalb and Gad1 genes (Pvalb Cre/+; Gad1 loxp/+). Mating of these heterozygous mice generated homozygous PV-Cre/heterozygous floxed-GAD67 mice (Pvalb Cre/Cre; Gad1 loxp/+) and heterozygous floxed-GAD67 mice (Pvalb +/+;Gad1 loxp/+). We then crossed these 2 genotypes and obtained littermates of 3 genotypes, Pvalb Cre/+;Gad1 +/+, Pvalb Cre/+;Gad1 loxp/+, and Pvalb Cre/+;Gad1 loxp/loxp, and subsequently referred to as control (PVGAD67+/+), heterozygous (PVGAD67+/−), and homozygous (PVGAD67−/−) mice, respectively. Three same sex littermates with different genotypes, PVGAD67+/+, PVGAD67+/−, and PVGAD67−/−, respectively, were designated as a triad. Five triads were used for in situ hybridization analysis at each of 8 and 15 weeks of age and for Western blot analysis at 8 weeks. Behavioral tests were performed between 11 and 15 weeks with 3 mice from 1 triad tested on the same day. Four triads were used for the rotarod test. Six triads were used in the open field, light–dark transition, prepulse inhibition, and Y-maze tests. The animal experiments were conducted according to the Fundamental Guidelines for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology of Japan, and approved by the Committee on Animal Experimentation of Kanazawa University.
Tissue Preparation and Single-Label in situ Hybridization With 35S-Labeled Riboprobes
Serial coronal sections (12 μm) were cut from +1.94mm to +1.54mm from the bregma, and 3 sections evenly spaced at ≈160 μm intervals were selected from each mouse and subjected to in situ hybridization (ISH) for each mRNA of interest. Riboprobe synthesis and hybridization were performed as described previously15 and in the supplementary material. Hybridized sections were exposed to BioMax MR film (Kodak) for 2–7 days depending on the abundance of transcripts. Throughout these ISH procedures, sections from each triad were processed together.
All antisense riboprobes revealed distinctive signal distributions in the mouse cortex that were consistent with previous ISH studies7,9,36–38 and Allen Brain Atlas (http://mouse.brain-map.org/). No signal beyond the background was detected with sense riboprobes for all mRNAs.
Quantification of mRNA Expression Levels
Trans-illuminated autoradiographic film images were captured by a video camera, digitized and analyzed using a Microcomputer Imaging Device (MCID) system (InterFocus Imaging Ltd). Images of adjacent sections stained with cresyl violet were also captured and superimposed onto the autoradiographic images to draw contours of the pia mater and gray matter/white matter border. Quantification of mRNA levels was done in 2 regions of interest: the prefrontal cortex (PFC), including the cingulate and prelimbic cortices, and the sensorimotor cortex (SMC), including the primary sensory and primary motor cortices.39 Optical densities in these regions were expressed as microcuries per gram of tissue (μCi/g) by reference to Carbon-14 radioactive standards (ARC Inc) exposed on the same film. The data were averaged across the 3 sections in each animal.
Dual-Label ISH and Grain Analysis
To directly verify reduced GAD67 mRNA expression in PV neurons in the knockout mice at 8 weeks of age, we performed dual-label ISH with 35S-labeled riboprobe for GAD67 mRNA and digoxigenin (DIG)-labeled riboprobe for PV mRNA as described previously15 and then carried out grain analysis to quantify relative GAD67 mRNA levels in PV neurons in the SMC of PVGAD67+/+, PVGAD67+/−, and PVGAD67−/− mice. Procedures for dual-label ISH and grain analysis are described in the supplementary material.
Western Blotting Analysis
GAD67 protein levels were quantified in the SMC by Western blotting analysis using anti-GAD67 and anti-β-tubulin antibodies. Protein extraction and subsequent Western blotting analysis were performed as described in the supplementary material.
Behavioral Tests
Influences of the PV neuron-specific GAD67 knockout on motor coordination and learning, general activity, anxiety, sensorimotor gating, and working memory were tested in a behavioral test battery that consists of rotarod, open field, light–dark transition, prepulse inhibition (PPI), and Y-maze tests. Procedures and equipment for each test are described in the supplementary material.
Statistical Analyses
Analyses were performed on SPSS (SPSS, Inc). Across the 3 genotypes (PVGAD67+/+, PVGAD67+/−, and PVGAD67−/−), differences in cortical mRNA and protein expression levels, as well as behavioral measures in the open field, light–dark transition, and Y-maze tests were assessed with single-factor ANOVA models. Genotype effects on behavioral data obtained from the rotarod and PPI tests were analyzed with 2-factor ANOVA models with repeated measures in 1 factor (trial for rotarod test and prepulse intensity for PPI test). If the ANOVA models detected significance, Tukey’s post hoc tests were used to identify statistically different genotypes. All statistical tests were conducted with an α-level = .05.
Results
GAD67 Knockout in Cortical PV Neurons
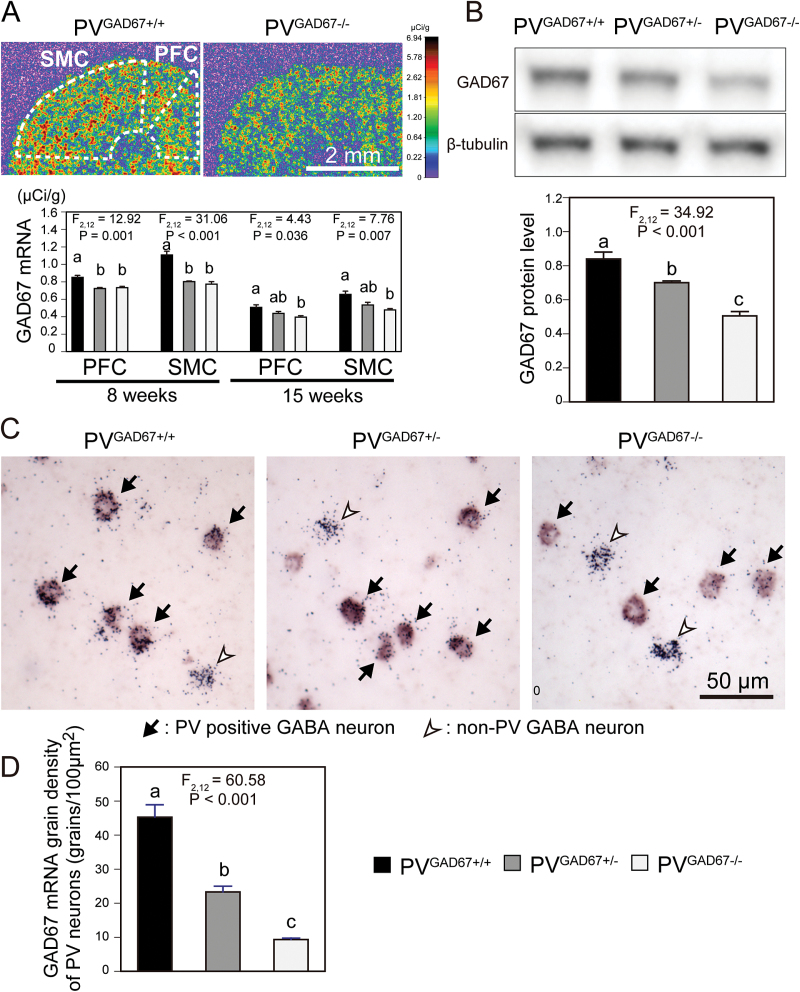
Single-label ISH with 35S-labeled riboprobe revealed reduced GAD67 mRNA expression in both the PFC and SMC of mice with a PV neuron-specific Gad1 inactivation compared with control mice at both 8 and 15 weeks of age (figure 1A). The ANOVA model detected significant effects of genotype on the mRNA levels in both regions at 8 weeks (F 2,12 = 12.92, P = .001 and F 2,12 = 31.06, P < .001 for the PFC and SMC, respectively) and at 15 weeks (F 2,12 = 4.43, P = .036 and F 2,12 = 7.76, P = .007 for the PFC and SMC, respectively; figure 1A). At 8 weeks, GAD67 mRNA levels in the homozygous (PVGAD67−/−) mice were significantly decreased by 13.8% and 30.2% in the PFC and SMC, respectively, compared with control (PVGAD67+/+) mice (figure 1A). At 15 weeks, the mRNA levels in PVGAD67−/− mice were significantly decreased by 22.0% and 27.1% in the PFC and SMC, respectively, compared with PVGAD67+/+ mice (figure 1A). In the heterozygous (PVGAD67+/−) mice, GAD67 mRNA levels were similar to PVGAD67−/− mice at 8 weeks and intermediate between PVGAD67−/− and PVGAD67+/+ mice at 15 weeks (figure 1A). In the Western blotting analysis, we detected a gene dose-dependent effect of the Gad1 inactivation on GAD67 protein levels (F 2,12 = 34.92, P < .001) in the SMC at 8 weeks (figure 1B). Compared with PVGAD67+/+ mice, GAD67 protein levels were significantly decreased by 16.6% and 39.8% in PVGAD67+/− and PVGAD67−/− mice, respectively (figure 1B). Finally, with dual-label ISH that detected GAD67 and PV mRNAs as silver grain accumulation and color reaction, respectively (figure 1C), we found that GAD67 mRNA levels in individual SMC PV neurons differed significantly by genotype (F 2,12 = 60.58, P < .001) at 8 weeks of age. Compared with PVGAD67+/+ mice, mean grain densities in PV neurons were significantly decreased by 48.5% and 79.4% in PVGAD67+/− and PVGAD67−/− mice, respectively (figure 1D). In contrast, grain density appeared similar across the 3 genotypes in non-PV GABA neurons (figure 1C). These findings indicate that reductions in GAD67 expression in PV neurons, especially in the homozygous mice, were robust and similar to those present in schizophrenia.1
Fig. 1.
Glutamic acid decarboxylase 67 (GAD67) knockout in cortical parvalbumin (PV) neurons. A: (Top) Film images of cortical GAD67 messenger RNA (mRNA) expression, detected by in situ hybridization (ISH), in a PVGAD67+/+ (left) and a PVGAD67−/− (right) mice from the same triad. The optical densities were presented in a pseudo-color manner according to the color bar on the right. Quantification was performed for the prefrontal cortex (PFC) and sensorimotor cortex (SMC) within the areas contoured by broken lines. (Bottom) Mean ± SEM GAD67 mRNA expression levels in the PFC and SMC of PVGAD67+/+ (black bars), PVGAD67+/− (gray bars), and PVGAD67−/− (white bars) mice at 8 and 15 weeks of age. Bars not sharing the same letter are statistically different. B: (Top) Immunoreactive bands for GAD67 and β-tubulin, detected by Western blotting, in the SMC of a triad of PVGAD67+/+, PVGAD67+/−, and PVGAD67−/− mice at 8 weeks of age. (Bottom) Mean ± SEM β-tubulin-normalized GAD67 protein levels in the SMC of PVGAD67+/+, PVGAD67+/−, and PVGAD67−/− mice at 8 weeks of age. C: Photomicrographs of dual-label ISH detecting GAD67 mRNA signals as silver grain clusters and PV mRNA signals as color reaction products. In the PFC of 8-week-old PVGAD67+/+ (left), PVGAD67+/− (center), and PVGAD67−/− (right) mice, GAD67 mRNA expression is detected in PV neurons (arrows), as well as in other GABA neurons that are indicated by silver grain clusters without color reaction (white arrowhead). D: Mean ± SEM grain densities of individual PV neurons in the SMC of PVGAD67+/+, PVGAD67+/−, and PVGAD67−/− mice at 8 weeks of age.
Expression of GABA-Associated Transcripts
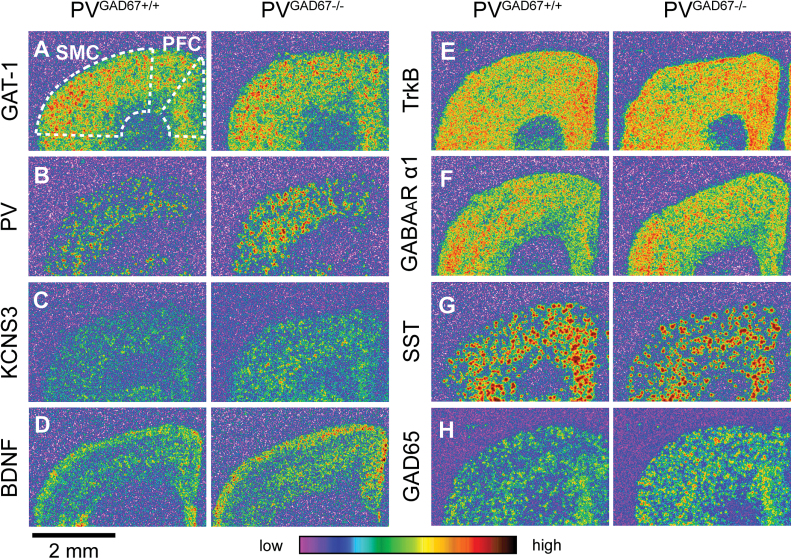
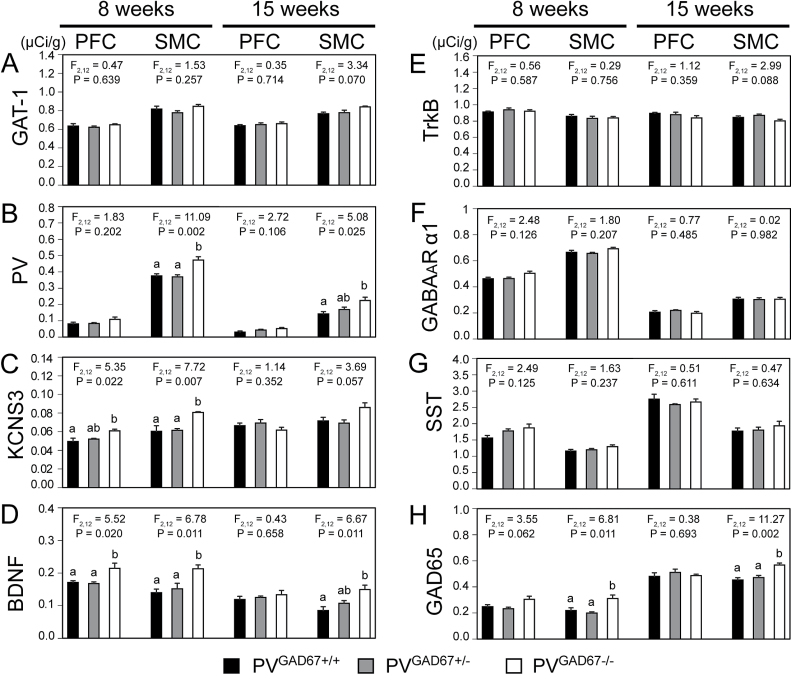
For each GABA-associated transcript examined, the cortical expression pattern did not differ across PVGAD67+/+, PVGAD67+/−, and PV GAD67−/− mice (figure 2). The ANOVA model and subsequent post-hoc tests revealed significant increases in cortical mRNA levels for PV, KCNS3, BDNF, and GAD65 in PVGAD67−/− mice, relative to age- and sex-matched PVGAD67+/+ littermates (figure 3, table 1). For both PV and GAD65 mRNAs, significant increases were detected in the SMC at 8 and 15 weeks (table 1, figures 3B and 3H). KCNS3 mRNA levels were significantly greater in the PFC and SMC at 8 weeks (table 1, figure 3C). BDNF mRNA levels were increased in the PFC and SMC at 8 weeks and in the SMC at 15 weeks (table 1, figure 3D). None of the other GABA-associated transcripts (ie, GAT-1, TrkB, GABAAR α1, or SST), whose cortical expression levels are lower in schizophrenia subjects, showed a significant reduction in their expression levels in the cortex of PV neuron-specific GAD67 knockout mice.
Fig. 2.
Expression of GABA-associated messenger RNAs (mRNAs) in the cortex of mice with glutamic acid decarboxylase 67 (GAD67) knockout in parvalbumin (PV) neurons. Film images of the cortical expression of GABA transporter-1 (GAT-1; A), PV (B), KCNS3 (C), brain-derived neurotrophic factor (BDNF; D), tropomyosin receptor kinase B (TrkB; E), GABAAR α1 (F), somatostatin (SST; G), and glutamic acid decarboxylase 65 (GAD65; H) mRNAs in PVGAD67+/+ and PVGAD67−/− mice from the same triad at 8 weeks of age. The optical densities are presented in a pseudo-color manner according to the color bar at the bottom. Regions of interests in the prefrontal cortex (PFC) and sensorimotor cortex (SMC) are indicated by broken lines (A).
Fig. 3.
Expression levels of GABA-associated mRNAs in the cortex of mice with glutamic acid decarboxylase 67 (GAD67) knockout in parvalbumin (PV) neurons. Mean ± SEM expression levels of GABA transporter-1 (GAT-1; A), PV (B), KCNS3 (C), brain-derived neurotrophic factor (BDNF; D), tropomyosin receptor kinase B (TrkB; E), GABAAR α1 (F), somatostatin (SST; G), and glutamic acid decarboxylase 65 (GAD65; H) mRNAs in the prefrontal cortex (PFC) and sensorimotor cortex (SMC) of PVGAD67+/+, PVGAD67+/−, and PVGAD67−/− mice at 8 and 15 weeks of age. Bars not sharing the same letter are statistically different.
Table 1.
Magnitude of Significant Increases in GABA-Associated Transcript Levels in the Homozygous (PVGAD67−/−) Mice
| mRNA | 8 Weeks | 15 Weeks | ||
|---|---|---|---|---|
| PFC | SMC | PFC | SMC | |
| PV | ns | +25.7% | ns | +57.9% |
| KCNS3 | +22.8% | +33.6% | ns | ns |
| BDNF | +25.3% | +52.4% | ns | +75.5% |
| GAD65 | ns | +42.7% | ns | +25.3% |
Note: mRNA, messenger RNA; PFC, prefrontal cortex; SMC, sensorimotor cortex; PV, parvalbumin; BDNF, brain-derived neurotrophic factor; GAD65, glutamic acid decarboxylase 65; ns, The effect of genotype on mRNA levels in ANOVA was not significant.
Behavioral Phenotype of Mice With GAD67 Knockout in PV Neurons
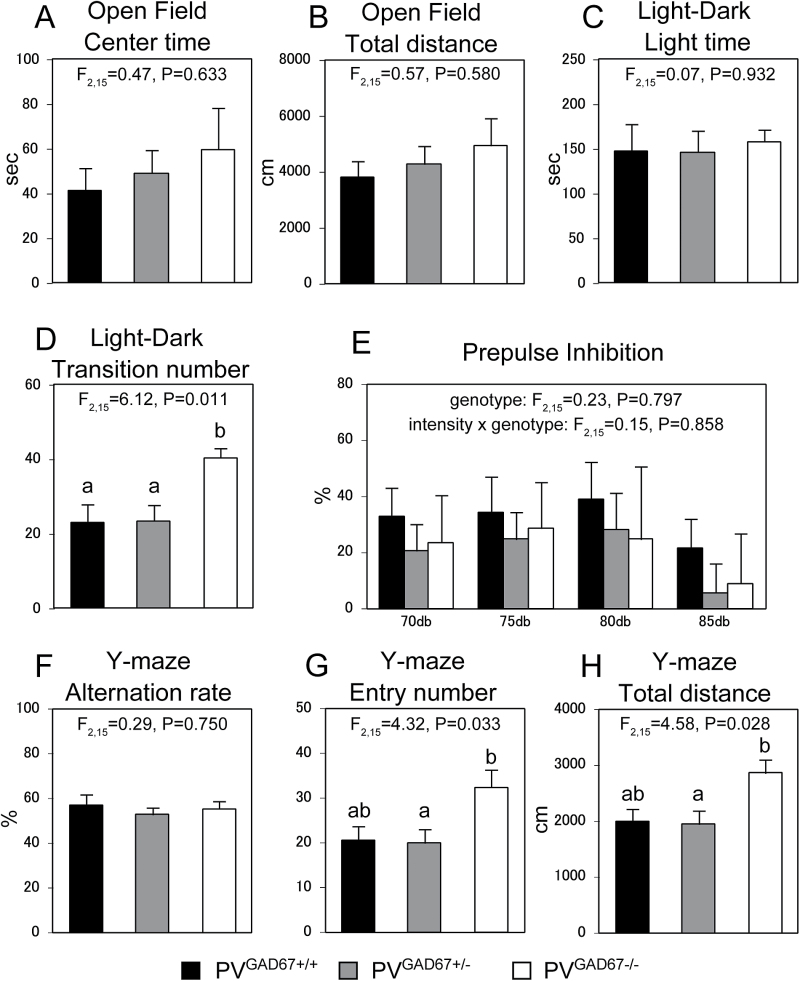
In the rotarod test, PVGAD67+/+, PVGAD67+/−, and PVGAD67−/− mice all exhibited similar motor coordination and learning. The repeated measures ANOVA model did not detect a significant effect of genotype (F 2,9 = 0.027, P = .973) or genotype × trial interaction (F 2,9 = 0.56, P = .590) on the latency to fall from the accelerating rotarod. The open field and light-dark transition tests indicated that the PV neuron-specific GAD67 knockout had little influence on anxiety, as genotype did not have a significant effect on total time spent in the center field (F 2,15 = 0.47, P = .633) or total time in the light chamber (F 2,15 = 0.07, P = .932), respectively (figures 4A and 4C). On the other hand, in the light-dark transition test, PVGAD67−/− mice exhibited significantly higher light-dark transition number than both PVGAD67+/− and PVGAD67+/+ mice (F 2,15 = 6.12, P = .011; figure 4D), indicating that the homozygous knockout is associated with higher spontaneous activity. PV neuron-specific GAD67 knockout mice did not exhibit deficits in either sensorimotor gating (PPI) or working memory (Y-maze), behavioral hallmarks of schizophrenia. The ANOVA models failed to detect a significant effect of genotype (F 2,15 = 0.23, P = .797) or genotype × prepulse intensity interaction (F 2,15 = 0.15, P = .858) on percent PPI (figure 4E), or a significant effect of genotype on alternation rate (F 2,15 = 0.29, P = .750, figure 4F) in the Y-maze test. However, we observed significant genotype effects on the entry number (F 2,15 = 4.32, P = .033) and total distance traveled (F 2,15 = 4.58, P = .028) in the Y-maze test (figures 4G and 4H). These measures were higher in PVGAD67−/− mice than in both PVGAD67+/− and PVGAD67+/+ mice, although statistical significances were detected only between PVGAD67−/− and PVGAD67+/− mice (figures 4G and 4H). These observations are consistent with higher spontaneous activity of PVGAD67−/− mice (figure 4D).
Fig. 4.
Effects of parvalbumin (PV) neuron-specific glutamic acid decarboxylase 67 (GAD67) knockout on mouse behavior. Mean ± SEM behavioral measures of PVGAD67+/+, PVGAD67+/−, and PVGAD67−/− mice (n = 6 for each genotype) in the open filed (A and B), light–dark transition (C and D), prepulse inhibition (PPI; E), and Y-maze (F–H) tests. Bars not sharing the same letter are statistically different.
Discussion
In order to test a causal relationship between reduced GAD67 expression in cortical PV neurons and lower expression levels of transcripts associated with GABA neuron functions, both of which are found in the cortex of subjects with schizophrenia,1–13,17 we created mice with a PV neuron-specific GAD67 knockout. Although multiple measures confirmed that the genetic manipulation produced the expected gene dose-related reductions of GAD67 in cortical PV neurons, we failed to detect the predicted lower cortical expression of GABA-associated transcripts or the expected disturbance in sensorimotor gating or working memory in our conditional knockout mice. These findings indicate that reduced GAD67 expression in PV neurons is not a sufficient upstream cause of the downregulated expression of other GABA-associated mRNAs or of the cognitive/behavioral impairments present in subjects with schizophrenia.
Reduced GAD67 and PV expression in cortical PV neurons was observed in animal models of N-methyl-D-aspartate glutamate receptor (NMDAR) hypofunction,40,41 which has been suggested to underlie cortical dysfunction in schizophrenia.42 In these models, the activity-dependent downregulation of GAD6743 and PV44 expression was the predicted consequence of reduced NMDAR signaling in PV neurons.45 However, because NMDAR hypofunction in PV neurons would also be predicted to cause disinhibition of pyramidal neurons, as seen following systemic administration of NMDAR antagonists and genetic inactivation of NMDAR in PV neurons,41,45 other activity-dependent GABA-associated transcripts (ie, BDNF, TrkB and GABAAR α1)25–27 would also be expected to be upregulated in pyramidal neurons. However, these predictions are contrary to the lower levels of these transcripts observed in schizophrenia. Our current data also indicate that the GAD67 deficit in PV neurons does not cause reduced expression of other GABA-associated transcripts. Interestingly, the contribution of NMDAR to excitatory synaptic transmission and action potential generation is much larger in pyramidal neurons than in PV neurons.46 Therefore, in schizophrenia, NMDAR hypofunction in pyramidal neurons could cause activity-dependent downregulation of BDNF, TrkB, and GABAAR α1 mRNAs. Such a state of pyramidal neuron NMDAR hypofunction could result from a deficit in pyramidal neuron dendritic spines, a major site of NMDAR localization, in schizophrenia.47,48
The following lines of information indicate that our analyses of the PV neuron-specific GAD67 knockout mice provided a robust means for proof-of-concept testing of the hypothesis that GAD67 deficits in PV neurons are responsible for lower GABA-associated transcript levels and behavioral abnormalities in schizophrenia. First, although our film-based quantification of transcript levels might have limited resolution and sensitivity to detect alterations that are restricted to a minor subpopulation of neurons, most postmortem studies of schizophrenia to date have examined tissue-level expression of GABA-associated transcripts. Thus, we used these measures in order to maximize comparisons to the human disease. Second, we confirmed at the cellular level marked deficits in GAD67 expression in our PVGAD67−/− mice (figure 1) which parallel the reduction of GAD67 mRNA to undetectable levels in a subset of cortical GABA neurons, including PV neurons, in schizophrenia.1,32,33 Third, we analyzed transcript levels at both 8 and 15 weeks of age and behaviors between 11 and 15 weeks; this age range corresponds to early adulthood in humans, a period when the diagnosis of schizophrenia is often made and characteristic behavioral and cognitive symptoms are evident. Thus, the age of mice examined in this study was appropriate for behavioral assessments. Fourth, although older mice might be better for testing GABA-associated transcript levels, as most postmortem studies of schizophrenia have used subjects with mean age greater than 40 years, cortical levels of KCNS3, BDNF, TrkB, and SST mRNAs, which showed negative correlations with age in both schizophrenia and control subjects, are lower in schizophrenia subjects compared with age-matched control subjects across a wide age range from 20 to 70 years of age.7,9,17 These findings indicate that the levels of some GABA-associated transcripts are lower early in the course of illness.
Despite the appropriateness of our model for hypothesis testing, PVGAD67−/− mice exhibited increased cortical mRNA levels for PV, KCNS3, BDNF, and GAD65 (table 1) in contrast to the deficits in these transcripts present in schizophrenia. These findings provide compelling evidence against a primary problem in PV neurons as the cause of GABA-associated transcript alterations in schizophrenia. At 8 weeks, these increases were more prominent in the SMC than the PFC, especially for PV and GAD65 mRNAs that showed a significant increase only in the SMC, but not in the PFC. In the mouse PFC, unlike the human PFC, PV mRNA expression is lower than in other cortical areas (figure 2B),7 reflecting the presence of fewer PV neurons.49 Therefore, the smaller mRNA changes in the PFC relative to the SMC might reflect a lesser impact of the GAD67 knockout on PFC circuity. At 15 weeks, we observed significantly increased expression of PV, BDNF, and GAD65 mRNAs only in the SMC of PVGAD67−/− mice, and none of these transcripts showed a significant change in the PFC (table 1), suggesting that the impact of PV neuron-specific GAD67 knockout was effectively compensated in the PFC at 15 weeks of age.
In our PV-Cre mice, Cre activity becomes detectable in the cortex at P12.50 Furthermore, in the previous study, the heterozygous GAD67 knockout in PV neurons resulted in a nonsignificant 9% decrease at P17 and a significant 18% decrease in GAD67 mRNA levels at P30.38 Thus, our GAD67 knockout is likely to have started at the end of the second postnatal week, after the shift in GABA actions from depolarization to hyperpolarization.51 In the mouse cortex, PV neurons strongly inhibit pyramidal neurons and PV neurons through perisomatic innervations with little inhibition to other GABA neuron subsets.29 Thus, the primary effect of GAD67 knockout in PV neurons should be disinhibition of both pyramidal neurons and PV neurons. Neuronal activity regulates expression of BDNF mRNA in pyramidal neurons26 and PV mRNA in PV neurons.44 KCNS3 mRNA expression, which is selective to cortical PV neurons,14,15,17 is also regulated by neuronal activity.52 Therefore, increased levels of BDNF, PV, and KCNS3 mRNAs appear to reflect hyperactivity of both pyramidal and PV neurons. Consistent with this interpretation, a reduction of inhibitory postsynaptic current (IPSC) charge from PV neurons to pyramidal neurons and an increase in excitability of both pyramidal neurons and PV neurons were observed in the PFC of mice with a heterozygous GAD67 knockout in PV neurons from P23–P30.50 Although this reduction in IPSCs was normalized by P50–P60 presumably through compensatory mechanisms,50 increased BDNF, PV, and KCNS3 mRNA expression in the SMC of PVGAD67−/− mice suggest that the greater GAD67 deficit in our homozygous mice was not fully compensated in the SMC and resulted in hyperactivity of pyramidal neurons and PV neurons even at 8 and 15 weeks of age.
Two studies have analyzed cortical expression of some GABA-associated transcripts and proteins in independently generated mouse lines with a GAD67 knockout in PV neurons.38,53 In one study, neither GAT-1 nor PV mRNA levels were significantly altered in the heterozygous mice compared with their wild type littermates,38 consistent with our observation in PVGAD67+/− mice. In another study,53 cortical GAD65 protein levels were unaltered in heterozygous mice and significantly increased in homozygous mice, corroborating our observation in the SMC of PV GAD67+/− and PV GAD67−/− mice. Increased GAD65 expression in homozygous mice might reflect a compensatory response to pyramidal neuron hyperactivity due to the extensive GAD67 deficit in PV neurons.
Studies of rodent models demonstrated that alterations of PFC GABA neurotransmission cause abnormalities of sensorimotor gating and working memory.41,54,55 However, our homozygous PVGAD67−/− mice did not exhibit an abnormality in percent PPI or Y-maze alternation rate during 11–15 weeks of age (figures 4E and 4F), despite the significant GAD67 deficit in the PFC. These findings, together with the pattern of expression changes in GABA-associated transcripts in the PFC at 8 and 15 weeks (table 1), indicate that the impact of our PV neuron-specific GAD67 knockout on PFC GABA neurotransmission was effectively compensated after the maturation. Fujihara et al53 reported impaired PPI in mice with a GAD67 knockout primarily induced in PV neurons. This could be due to an involvement other types of PFC GABA neurons, as PV neurons accounted for only 70% of cortical Cre recombinase-positive GABA neurons in their bacterial artificial chromosome (BAC) transgenic mice.53 In contrast, PV neurons corresponded to 97% of Cre recombinase-positive cortical neurons in our PV-Cre mice.56
A series of studies tested effects of GABA neuron subset-selective reduction of GAD67 expression using BAC transgenic mice that express a synthetic microRNA under the control of GABA neuron subset-specific promoters.57–60 These studies revealed that GAD67 deficits in different subsets of GABA neurons resulted in distinctive, yet partially overlapping, behavioral phenotypes. Recently, a deficit in PPI was reported in such BAC transgenic mice with reduced GAD67 expression in PV neurons.60 The discrepancy with unaltered PPI in our knockout mice might be due to differences in the cell-type specificity and timing of GAD67 reduction in the BAC-driven microRNA silencing of GAD67 mRNA vs the excision of the floxed Gad1 gene by Cre expressed from the Pvalb gene locus. Nonetheless, as a direct demonstration of altered GABA neurotransmission is not available in our PV neuron-specific GAD67 knockout mice, we cannot exclude the possibility that the absence of a PPI change could be due to an insufficient functional impact of our genetic manipulation on GABA neurotransmission by PV neurons. However, this scenario appears unlikely, especially in our homozygous mice, because we observed upregulations of BDNF, PV, KCNS3, and GAD65 mRNAs, which are associated with enhanced neuronal activity and a compensation in response to reduced GABA neurotransmission by PV neurons.
Given that the findings of the present study failed to support the hypothesis that reduced GAD67 expression in PV neurons is the upstream cause of other GABA-associated transcript alterations in schizophrenia, what other mechanisms might explain these abnormalities? In schizophrenia, excitatory inputs to pyramidal neurons appear to be reduced, as indicated by a reduced density of dendritic spines.47,48 This could cause pyramidal neuron hypoactivity and an activity-dependent downregulation of BDNF,26 TrkB,27 and GABAAR α1 subunit25 expression in these neurons. Furthermore, resultant weaker excitatory drive from pyramidal neurons to GABA neurons could drive down GAD67,43 PV,44 KCNS3,52 and SST61 mRNA levels. Therefore, the lower expression levels of GAD67 and other GABA-associated transcripts might be a consequence of pyramidal neuron hypoactivity in schizophrenia.62
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
This work was supported by Japan Society for the Promotion of Science (Grants-in-Aid 24791207 to D.G. and 25293247, 26670536, 15H01280 to T.H.) and National Institutes of Health (grants MH043784, MH084053 to D.A.L.).
Supplementary Material
Acknowledgment
We thank Junko Konishi for technical assistances. D.A.L. currently receives investigator-initiated research support from Pfizer and in 2012–2015 served as a consultant in the areas of target identification and validation and new compound development to Autifony, Bristol-Myers Squibb, Concert Pharmaceuticals and Sunovion.
References
- 1. Hashimoto T, Volk DW, Eggan SM, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Curley AA, Arion D, Volk DW, et al. Cortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: clinical, protein, and cell type-specific features. Am J Psychiatry. 2011;168:921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ohnuma T, Augood SJ, Arai H, McKenna PJ, Emson PC. Measurement of GABAergic parameters in the prefrontal cortex in schizophrenia: focus on GABA content, GABA(A) receptor alpha-1 subunit messenger RNA and human GABA transporter-1 (HGAT-1) messenger RNA expression. Neuroscience. 1999;93:441–448. [DOI] [PubMed] [Google Scholar]
- 4. Pierri JN, Chaudry AS, Woo TU, Lewis DA. Alterations in chandelier neuron axon terminals in the prefrontal cortex of schizophrenic subjects. Am J Psychiatry. 1999;156:1709–1719. [DOI] [PubMed] [Google Scholar]
- 5. Glausier JR, Fish KN, Lewis DA. Altered parvalbumin basket cell inputs in the dorsolateral prefrontal cortex of schizophrenia subjects. Mol Psychiatry. 2014;19:30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weickert CS, Ligons DL, Romanczyk T, et al. Reductions in neurotrophin receptor mRNAs in the prefrontal cortex of patients with schizophrenia. Mol Psychiatry. 2005;10:637–650. [DOI] [PubMed] [Google Scholar]
- 7. Hashimoto T, Bergen SE, Nguyen QL, et al. Relationship of brain-derived neurotrophic factor and its receptor TrkB to altered inhibitory prefrontal circuitry in schizophrenia. J Neurosci. 2005;25:372–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ray MT, Shannon Weickert C, Webster MJ. Decreased BDNF and TrkB mRNA expression in multiple cortical areas of patients with schizophrenia and mood disorders. Transl Psychiatry. 2014;4:e389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morris HM, Hashimoto T, Lewis DA. Alterations in somatostatin mRNA expression in the dorsolateral prefrontal cortex of subjects with schizophrenia or schizoaffective disorder. Cereb Cortex. 2008;18:1575–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mellios N, Huang HS, Baker SP, et al. Molecular determinants of dysregulated GABAergic gene expression in the prefrontal cortex of subjects with schizophrenia. Biol Psychiatry. 2009;65:1006–1014. [DOI] [PubMed] [Google Scholar]
- 11. Fung SJ, Webster MJ, Sivagnanasundaram S, et al. Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. Am J Psychiatry. 2010;167:1479–1488. [DOI] [PubMed] [Google Scholar]
- 12. Akbarian S, Huntsman MM, Kim JJ, et al. GABAA receptor subunit gene expression in human prefrontal cortex: comparison of schizophrenics and controls. Cereb Cortex. 1995;5:550–560. [DOI] [PubMed] [Google Scholar]
- 13. Hashimoto T, Arion D, Unger T, et al. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2008;13:147–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Okaty BW, Miller MN, Sugino K, Hempel CM, Nelson SB. Transcriptional and electrophysiological maturation of neocortical fast-spiking GABAergic interneurons. J Neurosci. 2009;29:7040–7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Georgiev D, Gonzalez-Burgos G, Kikuchi M, et al. Selective expression of KCNS3 potassium channel alpha-subunit in parvalbumin-containing GABA neurons in the human prefrontal cortex. PLoS One. 2012;7:e43904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Patel AJ, Lazdunski M, Honoré E. Kv2.1/Kv9.3, a novel ATP-dependent delayed-rectifier K+ channel in oxygen-sensitive pulmonary artery myocytes. EMBO J. 1997;16:6615–6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Georgiev D, Arion D, Enwright JF, et al. Lower gene expression for KCNS3 potassium channel subunit in parvalbumin-containing neurons in the prefrontal cortex in schizophrenia. Am J Psychiatry. 2014;171:62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guidotti A, Auta J, Davis JM, et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000;57:1061–1069. [DOI] [PubMed] [Google Scholar]
- 19. Huang HS, Matevossian A, Whittle C, et al. Prefrontal dysfunction in schizophrenia involves mixed-lineage leukemia 1-regulated histone methylation at GABAergic gene promoters. J Neurosci. 2007;27:11254–11262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Glausier JR, Kimoto S, Fish KN, Lewis DA. Lower glutamic acid decarboxylase 65-kDa isoform messenger RNA and protein levels in the prefrontal cortex in schizoaffective disorder but not schizophrenia. Biol Psychiatry. 2015;77:167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Overstreet LS, Westbrook GL. Synapse density regulates independence at unitary inhibitory synapses. J Neurosci. 2003;23:2618–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vreugdenhil M, Jefferys JG, Celio MR, Schwaller B. Parvalbumin-deficiency facilitates repetitive IPSCs and gamma oscillations in the hippocampus. J Neurophysiol. 2003;89:1414–1422. [DOI] [PubMed] [Google Scholar]
- 23. Segal M, Andersen P. Dendritic spines shaped by synaptic activity. Curr Opin Neurobiol. 2000;10:582–586. [DOI] [PubMed] [Google Scholar]
- 24. Yin DM, Sun XD, Bean JC, et al. Regulation of spine formation by ErbB4 in PV-positive interneurons. J Neurosci. 2013;33:19295–19303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hendry SH, Huntsman MM, Vinuela A, et al. GABAA receptor subunit immunoreactivity in primate visual cortex: distribution in macaques and humans and regulation by visual input in adulthood. J Neurosci. 1994;14:2383–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang ZJ, Kirkwood A, Pizzorusso T, et al. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. [DOI] [PubMed] [Google Scholar]
- 27. Kingsbury TJ, Murray PD, Bambrick LL, Krueger BK. Ca(2+)-dependent regulation of TrkB expression in neurons. J Biol Chem. 2003;278:40744–40748. [DOI] [PubMed] [Google Scholar]
- 28. Glorioso C, Sabatini M, Unger T, et al. Specificity and timing of neocortical transcriptome changes in response to BDNF gene ablation during embryogenesis or adulthood. Mol Psychiatry. 2006;11:633–648. [DOI] [PubMed] [Google Scholar]
- 29. Pfeffer CK, Xue M, He M, Huang ZJ, Scanziani M. Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat Neurosci. 2013;16:1068–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cottam JC, Smith SL, Häusser M. Target-specific effects of somatostatin-expressing interneurons on neocortical visual processing. J Neurosci. 2013;33:19567–19578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Volk D, Austin M, Pierri J, Sampson A, Lewis D. GABA transporter-1 mRNA in the prefrontal cortex in schizophrenia: decreased expression in a subset of neurons. Am J Psychiatry. 2001;158:256–265. [DOI] [PubMed] [Google Scholar]
- 32. Akbarian S, Kim JJ, Potkin SG, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. [DOI] [PubMed] [Google Scholar]
- 33. Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57:237–245. [DOI] [PubMed] [Google Scholar]
- 34. Hippenmeyer S, Vrieseling E, Sigrist M, et al. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 2005;3:e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Obata K, Hirono M, Kume N, et al. GABA and synaptic inhibition of mouse cerebellum lacking glutamate decarboxylase 67. Biochem Biophys Res Commun. 2008;370:429–433. [DOI] [PubMed] [Google Scholar]
- 36. Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Esclapez M, Tillakaratne NJ, Kaufman DL, Tobin AJ, Houser CR. Comparative localization of two forms of glutamic acid decarboxylase and their mRNAs in rat brain supports the concept of functional differences between the forms. J Neurosci. 1994;14:1834–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Curley AA, Eggan SM, Lazarus MS, et al. Role of glutamic acid decarboxylase 67 in regulating cortical parvalbumin and GABA membrane transporter 1 expression: implications for schizophrenia. Neurobiol Dis. 2013;50:179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. 3rd ed. San Diego, CA: Academic Press; 2008. [Google Scholar]
- 40. Behrens MM, Ali SS, Dao DN, et al. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318:1645–1647. [DOI] [PubMed] [Google Scholar]
- 41. Belforte JE, Zsiros V, Sklar ER, et al. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13:76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol. 2006;26:365–384. [DOI] [PubMed] [Google Scholar]
- 43. Luo Y, Lathia J, Mughal M, Mattson MP. SDF1alpha/CXCR4 signaling, via ERKs and the transcription factor Egr1, induces expression of a 67-kDa form of glutamic acid decarboxylase in embryonic hippocampal neurons. J Biol Chem. 2008;283:24789–24800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Patz S, Grabert J, Gorba T, Wirth MJ, Wahle P. Parvalbumin expression in visual cortical interneurons depends on neuronal activity and TrkB ligands during an Early period of postnatal development. Cereb Cortex. 2004;14:342–351. [DOI] [PubMed] [Google Scholar]
- 45. Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27:11496–11500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rotaru DC, Yoshino H, Lewis DA, Ermentrout GB, Gonzalez-Burgos G. Glutamate receptor subtypes mediating synaptic activation of prefrontal cortex neurons: relevance for schizophrenia. J Neurosci. 2011;31:142–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Garey LJ, Ong WY, Patel TS, et al. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neurosurg Psychiatry. 1998;65:446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. [DOI] [PubMed] [Google Scholar]
- 49. Fitzgerald ML, Lupica CR, Pickel VM. Decreased parvalbumin immunoreactivity in the cortex and striatum of mice lacking the CB1 receptor. Synapse. 2011;65:827–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lazarus MS, Krishnan K, Huang ZJ. GAD67 deficiency in parvalbumin interneurons produces deficits in inhibitory transmission and network disinhibition in mouse prefrontal cortex. Cereb Cortex. 2015;25:1290–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Deidda G, Allegra M, Cerri C, et al. Early depolarizing GABA controls critical-period plasticity in the rat visual cortex. Nat Neurosci. 2015;18:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lee KY, Royston SE, Vest MO, et al. N-methyl-D-aspartate receptors mediate activity-dependent down-regulation of potassium channel genes during the expression of homeostatic intrinsic plasticity. Mol Brain. 2015;8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fujihara K, Miwa H, Kakizaki T, et al. Glutamate decarboxylase 67 deficiency in a subset of GABAergic neurons induces schizophrenia-related phenotypes. Neuropsychopharmacology. 2015;40:2475–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Richetto J, Calabrese F, Riva MA, Meyer U. Prenatal immune activation induces maturation-dependent alterations in the prefrontal GABAergic transcriptome. Schizophr Bull. 2014;40:351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cabungcal JH, Counotte DS, Lewis EM, et al. Juvenile antioxidant treatment prevents adult deficits in a developmental model of schizophrenia. Neuron. 2014;83:1073–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kuhlman SJ, Huang ZJ. High-resolution labeling and functional manipulation of specific neuron types in mouse brain by Cre-activated viral gene expression. PLoS One. 2008;3:e2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Garbett KA, Horváth S, Ebert PJ, et al. Novel animal models for studying complex brain disorders: BAC-driven miRNA-mediated in vivo silencing of gene expression. Mol Psychiatry. 2010;15:987–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Brown JA, Horváth S, Garbett KA, et al. The role of cannabinoid 1 receptor expressing interneurons in behavior. Neurobiol Dis. 2014;63:210–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schmidt MJ, Horvath S, Ebert P, et al. Modulation of behavioral networks by selective interneuronal inactivation. Mol Psychiatry. 2014;19:580–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Brown JA, Ramikie TS, Schmidt MJ, et al. Inhibition of parvalbumin-expressing interneurons results in complex behavioral changes. Mol Psychiatry. 2015;20:1499–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Marty S, Onténiente B. The expression pattern of somatostatin and calretinin by postnatal hippocampal interneurons is regulated by activity-dependent and -independent determinants. Neuroscience. 1997;80:79–88. [DOI] [PubMed] [Google Scholar]
- 62. Glausier JR, Lewis DA. Dendritic spine pathology in schizophrenia. Neuroscience. 2013;251:90–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.