Abstract
Protein carbamylation may result from chronic exposure to elevated levels of urea in patients with chronic kidney disease. Carbamylation could cause conformational changes in proteins resulting in alterations in binding sites and disturbances in cellular functions. Elevated levels of carbamylated protein has been shown to be associated with increased risk of death from cardiac causes in patients with end-stage renal disease. The precise mechanism by which carbamylated proteins mediate toxicity in uremia needs further investigation.
Keywords: Uremic toxin, cardiovascular disease, protein energy wasting
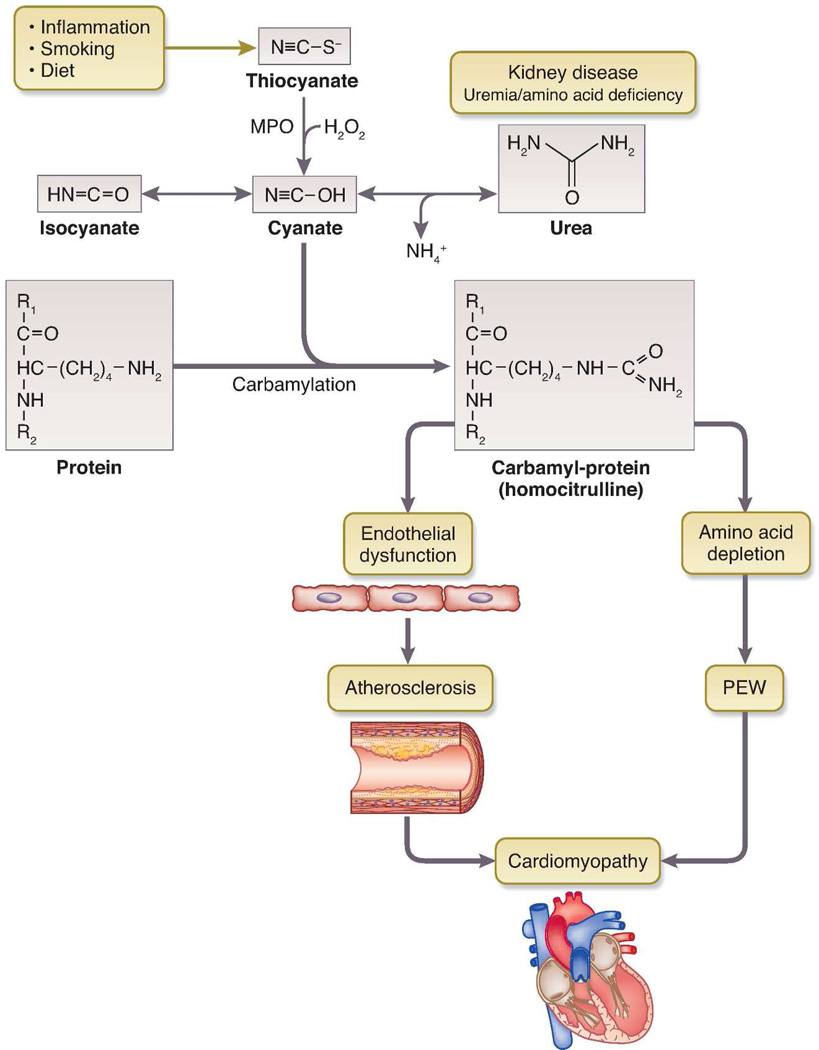
Since the identification of urea by Fourcroy and Vauquelin in 17th century, it has been shrouded with controversy. In Richard Bright's era, "morbid anatomy” was the doctrine and the possibility that urea could be a “toxin” was almost heretical. During subsequent years the term “uremia” came into general usage and urea became accepted as a marker for kidney failure, but still not accepted as a “uremic toxin”. This belief was supported by the observation that maintaining high concentration of blood urea level in end-stage renal disease (ESRD) patients by adding urea to the dialysate did not present any acute toxic consequences. Also, data from the Hemodialysis (HEMO) and ADEquacy of PD in MEXico (ADEMEX) studies showing no survival benefit from higher dialysis dose argue against urea being the uremic toxin. However, a growing body of evidence indicates that urea may mediate at least part of its toxic effects through carbamylation of serum or tissue proteins. (Figure)
Figure 1.
Carbamylation of protein and potential toxicity. PEW, protein energy wasting.
Urea and carbamylation: Old wine in a new bottle?
Urea is in equilibrium with electrophilic species, cyanate and isocynate, which can react with amino groups of free amino acids and with lysine residues of proteins, resulting in ε- amino – carbamoyl-lysine (homocitrulline). Carbamylation is an irreversible post-translational modification and occurs at multiple lysine sites within a protein, with accumulation over the life span of the protein. Recent evidence suggests that oxidation of thiocyanate catalyzed by myeloperoxidase is an important mechanism of cyanate formation and protein carbamylation, especially at sites of inflammation.[1] Carbamylation reaction could cause conformational changes in proteins resulting in inhibition of enzyme activity, alterations in binding sites and disturbances in other cellular functions. A number of studies have shown increased carbamylation of proteins in patients with chronic kidney disease (CKD).[2] Protein carbamylation has been shown to be associated with increased mortality in subjects with and without impaired kidney function.[1;3;4] Koeth et al. [3] quantified plasma level of protein-bound homocitrulline (PBHCit), a marker of carbamylation in a cohort of 347 patients undergoing maintenance hemodialysis with 5 years of follow-up and found that the risk for death among patients with PBHCit values in the highest tertile was more than two times the risk among those in lower tertiles.
Carbamylation, a novel biomarker or agent provocateur?
It is well recognized that patients with ESRD have a high risk of cardiovascular (CV) events. This excess mortality does not appear to be explained entirely by traditional CV risk factors. Increased attention has, therefore, been directed towards investigation of non-traditional risk factors, including among others, uremic toxins, oxidative stress, and systemic inflammation as potential mechanisms to explain the excess CV disease burden associated with CKD. In this issue of Kidney International, Berg and associates [5] report an association between elevated levels of carbamylated albumin (carb-Alb) and mortality in a secondary analysis of the data from the 'Deutsche Diabetes Dialyse Studie' (4D) study, which investigated the CV benefits of atorvastatin in maintenance hemodialysis patients with diabetes. The present study is a continuation of their previous work in which they showed that the proportion of albumin carbamylated on Lys-549 (%carb-Alb) is associated with increased risk of death at 12 months in 187 Accelerated Mortality in Renal Replacement (ArMORR) study and 1,161 4D study participants.[6] The exciting new findings in this study are that elevated carb-Alb levels were associated with risk for CV mortality, especially with short-term risk of sudden death and long-term risk of death from congestive heart failure (CHF). It is possible that those with more severe heart disease died from sudden cardiac death early and others with less severe disease developed and died from CHF later. The mechanisms for the association between elevated serum protein carbamylation with makers of cardiac damage, sudden cardiac death, and risk of CHF are unknown. The authors speculate that the risk of sudden cardiac death is consistent with the hypothesis that cardiac protein “hypercarbamylation” represents an acute insult to the cardiac conduction system, whereas the long-term risk of CHF may be a consequence of the chronic effects of carbamylation resulting in myocardial protein dysfunction due to uremic cardiomyopathy and fibrosis.
Lack of association with ischemic heart disease: Lost in translation?
Epidemiological studies have shown that about 64% of all cardiac deaths among hemodialysis patients are due to sudden cardiac death. Many triggering events for fatal arrhythmias and sudden cardiac death have been identified, but acute myocardial ischemia is believed to be the most common precipitating event. Substantial evidence from clinical and laboratory science suggest that carbamylation of proteins may promote atherosclerosis. Animal studies in a mouse model of CKD have shown that uremia increases carbamylation of plasma low density lipoproteins (cLDL) and high density lipoproteins (cHDL), and this modification inhibits receptor mediated uptake and instead promotes foam cell formation and accumulation in atherosclerotic tissues. In addition, cLDL exhibits distinct cytotoxic effects when tested in vitro on endothelial cells, induces the expression of adhesion molecules, and aggravates the monocyte adhesion to endothelial cells. LDL isolated from uremic patients and uremic aortic plaques exhibit higher degree of carbamylation than those from controls. cLDL binds to macrophage scavenger receptors facilitating foam cell formation and inflammatory signaling, endothelial cell apoptosis and vascular smooth muscle proliferation. In patients undergoing cardiac catheterization, those with elevated serum carbamylated protein were at increased risk of coronary artery disease, future myocardial infarction, stroke and death.[1] However, in the study by Berg and associates [5] no specific association between carb-Alb and risk for myocardial infarction was evident. Diabetics with advanced kidney disease have a large burden of traditional and novel CV risk factors. Thus, it is possible that carb-Alb may not be the most important mechanism for accelerated atherosclerosis in this patient population.
Does carbamylation mediate CHF through aberrant cardiac remodeling?
Elevated carb-Alb was associated with long term risk for death from CHF in the study by Berg and associates.[5] Although left ventricular hypertrophy begins as an adaptive response to pressure or volume overload, it often results in diastolic dysfunction, eventually leading to heart failure. In a small study involving 115 patients with chronic systolic heart failure, Tang et al. [4] showed that PBHCit levels were not related to indices of cardiac structure or function, but it predicted poor long term survival in these patients. In a recent study, plasma levels of carbamylated proteins increased about two fold in nephrectomized vs. control mice over the 20 weeks of the experiment.[7] The progressive accumulation of carbamylated proteins was evident in extracellular matrix proteins such as collagen in multiple organs including heart and the kidney.[7] Carbamylation could potentially alter interaction with inflammatory cells and susceptibility to proteolysis leading to aberrant tissue remodeling in target organs.[8] High resistance of electrical conduction pathways in the fibrotic tissue could contribute to intraventricular conduction disturbance and arrhythmia leading to sudden cardiac death. Future studies should examine the role of carbamylation in abnormal cardiac remodeling and contractile function in ESRD.
Carbamylation and cholesterol: One more twist in the paradox?
Serum cholesterol and CV disease exhibit a “U” shaped association in ESRD, with increased CV event rates noted at both ends of the spectrum. The 4D study demonstrated that lowering LDL cholesterol with atorvastatin had no statistically significant effect on mortality or CV events. An interesting finding in this study [5] is that patients with low carb-Alb did benefit from atorvastatin treatment. It is now well recognized that carbamylation alters the LDL and HDL function and the authors speculate that the beneficial effect of lipid lowering was lost because of excessive cabamylation. Given the complexity of the uremic state and cholesterol metabolism in ESRD, further clinical and experimental studies are warranted exploring this possibility.
Does carbamylation cause functional amino acid deficiency?
Protein carbamylation could be the potential link between inflammation, protein energy wasting and CV disease in ESRD patients. Carbamylation reaction can also occur on amino acids (AAs) and convert free AAs (F-AAs) to carbamoyl-amino acids (carb-AAs). Carb-AAs cannot form peptide bonds with the carboxyl group of other amino acids in protein synthesis, thus contributing to protein energy wasting (Figure). Taking advantage of the fact that F-AAs are potential scavengers for reactive isocyanate, this group of investigators is examining the effect of supplementation with specific amino acids on carbamylation in ESRD patients undergoing maintenance hemodialysis (NCT01612429).
Advances in analytical techniques and emergence of “omics” have identified a myriad of retention solutes in patients with ESRD, which is ever growing. The journey however started with urea, used as a surrogate marker primarily because of its abundance and not necessarily because of its toxicity, which is re-emerging as a strong contender as a uremic toxin in recent years. This study by Berg and associates [5] fortifies the notion that urea could be a potential uremic toxin that mediates its toxicity through carbamylation. However, given the complexity of uremic syndrome and the enormous number of solutes retained, generated and modified in uremia, protein carbamylation may be one of the toxins, but certainly is not the only uremic toxin. This should be considered as we move forward with targeted intervention to reduce protein carbamylation.
Acknowledgments
Dr. Raj is supported by the National Institute of Health Grants 1R01DK073665-01A1, 1U01DK099924-01 and 1U01DK099914-01.
Footnotes
Disclosures
None.
References
- 1.Wang Z, Nicholls SJ, Rodriguez ER, et al. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat Med. 2007;13:1176–1184. doi: 10.1038/nm1637. [DOI] [PubMed] [Google Scholar]
- 2.Kalim S, Karumanchi SA, Thadhani RI, Berg AH. Protein carbamylation in kidney disease: pathogenesis and clinical implications. Am J Kidney Dis. 2014;64:793–803. doi: 10.1053/j.ajkd.2014.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koeth RA, Kalantar-Zadeh K, Wang Z, et al. Protein carbamylation predicts mortality in ESRD. J Am Soc Nephrol. 2013;24:853–861. doi: 10.1681/ASN.2012030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang WH, Shrestha K, Wang Z, et al. Protein carbamylation in chronic systolic heart failure: relationship with renal impairment and adverse long-term outcomes. J Card Fail. 2013;19:219–224. doi: 10.1016/j.cardfail.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drechsler C, Kalim S, Wenger J, et al. Protein carbamylation is associated with heart failure and mortality in diabetic patients with end stage kidney disease. Kidney International. 2015 doi: 10.1038/ki.2014.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berg AH, Drechsler C, Wenger J, et al. Carbamylation of serum albumin as a risk factor for mortality in patients with kidney failure. Sci Transl Med. 2013;5:175ra29. doi: 10.1126/scitranslmed.3005218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pietrement C, Gorisse L, Jaisson S, Gillery P. Chronic increase of urea leads to carbamylated proteins accumulation in tissues in a mouse model of CKD. PLoS One. 2013;8:e82506. doi: 10.1371/journal.pone.0082506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaisson S, Lorimier S, Ricard-Blum S, et al. Impact of carbamylation on type I collagen conformational structure and its ability to activate human polymorphonuclear neutrophils. Chem Biol. 2006;13:149–159. doi: 10.1016/j.chembiol.2005.11.005. [DOI] [PubMed] [Google Scholar]