Abstract
Background
MicroRNAs (miRNAs) are non-coding RNA molecules that play important roles in the pathogenesis of various kidney diseases. We investigated whether patients with minimal change disease (MCD) and focal segmental glomerulosclerosis (FSGS) have distinct circulating and urinary miRNA expression profiles that could lead to potential development of noninvasive biomarkers of the disease.
Materials and methods
Exosome miRNAs were extracted from plasma and urine samples of patients with primary FSGS (n=16) or MCD (n=5) and healthy controls (n=5). Differences in miRNA abundance were examined using Affymetrix GeneChip miRNA 3.0 arrays. QRT-PCR was used to validate the findings from the array.
Results
Comparison analysis of FSGS versus MCD revealed 126 and 155 differentially expressed miRNAs in plasma and in urine, respectively. Only 38 of these miRNAs were previously cited, whereas the remaining miRNAs have not been described. Comparison analysis showed that a significant number of miRNAs were down-regulated in both plasma and urine samples of FSGS patients compared to those with MCD. Plasma levels of miR-30b, miR-30c, miR-34b, miR-34c, and miR-342, and urine levels of mir-1225-5p were up-regulated in MCD patients compared to FSGS patients and controls (p<0.001). Urinary levels of mir-1915 and miR-663 were down-regulated in FSGS patients compared to MCD and controls (p<0.001), whereas the urinary levels of miR-155 were up-regulated in FSGS patients when compared to MCD patients and controls (p<0.005).
Conclusions
Patients with FSGS and MCD have a unique circulating and urinary miRNA profile. The diagnostic and prognostic potential of miRNAs in FSGS and MCD warrants further studies.
Keywords: chronic kidney disease, circulatory miRNA, focal segmental glomerulosclerosis, minimal change disease, urinary miRNA
Introduction
Focal segmental glomerulosclerosis (FSGS) and minimal change disease (MCD) likely represent distinct forms of podocytopathies, which are glomerular diseases defined by primary lesions of the podocyte or the visceral epithelial cell [1]. Since primary FSGS and MCD are characterized by diffuse effacement of foot processes by electron microscopy, it was previously thought that these two conditions were part of a continuum of one disease. However, advances in molecular biology have led to increased consensus that these are two separate processes. The prognosis and treatments for FSGS and MCD are quite different, hence early diagnosis is essential [2]. Most children presenting with nephrotic syndrome due to MCD respond to corticosteroid therapy. In contrast, patients with FSGS have higher rates of corticosteroid resistance and progression to chronic kidney disease leading to end-stage renal disease [3].
It may be difficult to distinguish MCD from early FSGS when glomerular injury is in an early presclerotic stage or when biopsies are small and diagnostic segmental lesions are not adequately sampled. FSGS can be missed because of biopsy sampling error. Several studies have attempted to distinguish FSGS from MCD by focusing on individual podocyte-associated or profibrotic proteins, but they were inconclusive [4, 5]. More recent studies have shown altered proteomic signals which may distinguish MCD from FSGS [6]. Currently in the absence of a reliable biomarker, kidney biopsy remains the only precise approach to distinguish MCD from FSGS.
MicroRNAs (miRNAs) are small (18–22 nucleotides), non-coding RNA molecules that modulate differentiation, growth, apoptosis and proliferation of cells by negatively regulating gene expression at the post-transcriptional level [7]. Finding of tissue-restricted miRNA expression has led investigators to believe that miRNA expression may have organ-specific roles. For instance, miRNA-192, -194, -204, -215 and -216 are abundant in kidney compared to other tissues [8]. Many of the miRNAs have also been detected in extracellular fluids such as serum [9] and urine [10]. It is believed that urinary miRNAs may be derived from the kidney and urinary tract and are passively filtered through the glomerulus and or secreted by the renal tubules [11].
Exosomes are 50–90 nm membrane-derived vesicles found in body fluids including blood, saliva, and urine. They encapsulate proteins and mRNA as well as miRNA that may be exchanged as a signaling mechanism between cells. Encapsulated mRNA and miRNA are relatively stable because exosomes protect nucleic acids from extracellular degradation. It is possible that the urinary excretion of specific miRNAs might be a novel indicator for diagnosis and prognosis of kidney disease. Preliminary evidence indicates that miRNA may regulate the progression of glomerular and tubular disease [12]. There is also strong evidence that the process of epithelial mesenchymal transition (EMT) is regulated by miRNAs. EMT has been recognized as an integral part of glomerular and interstitial fibrosis [7].
In the present study, we investigated whether patients with FSGS and MCD have distinct circulating and urinary miRNA expression profiles that could lead to potential development of noninvasive biomarkers of the disease.
Methods
Subjects
Urine and plasma samples were obtained from cohorts of patients available from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Central Biorepository. Human samples were collected under research protocols approved in advance by the NIDDK Institutional Review Board and all subjects provided written informed consent. The selected individuals included 16 cases with primary FSGS, 5 with MCD and 5 healthy controls. Peripheral blood samples were obtained using EDTA tubes, placed on ice immediately and centrifuged at 4°C, 1,000 g for 15 minutes. Plasma supernatant was aliquoted and stored immediately at −80°C until analysis. Mid-day urine samples were obtained and processed within 2 hours. Processing involved centrifugation at 2000 g for 10 min. The supernatant was removed and stored at −80°C until analysis. The diagnoses of all cases had been confirmed by light and electron microscopy as well as immunofluorescence examination. Control samples were obtained from healthy volunteers; normal kidney function was confirmed by normal serum creatinine and urine protein/creatinine ratios.
Exosome RNA extraction and quantification
Plasma and urine RNAs (exosome/microvesicle) were isolated using exosome RNA Isolation kit (Norgen Biotek, Thorold, ON) according to the manufacturers’ protocol. In brief, RNA was extracted from 1 mL of urine and 0.5 ml of plasma that had been stored at −80°C. RNA extractions were eluted in 100 μl and stored at −80°C until use. The quantity and quality of the RNA extractions were determined by the Agilent Bioanalyzer 2100 with a small RNA Chip for exosomal miRNA (Agilent Technologies, Columbia, MD). RNA yields were normalized to ng RNA per ml of plasma or urine.
Microarray miRNA Analysis
MiRNA expression microarrays were performed using Affymetrix GeneChip miRNA 3.0 Arrays kit as described by the manufacturer (Affymetrix, Santa Clara, CA). With over 19,700 total mature miRNA probe sets (1,733 human mature miRNA probes), the GeneChip miRNA 3.0 Array interrogates all mature miRNA sequences in miRBase Release 17 and detects 94% of miRNA transcripts at 1.0 amol. The arrays were washed and stained on the Affymetrix Fluidics station 450, scanned with an Affymetrix gene chip scanner 3000 7G and analyzed using Affymetrix® miRNA QC tool 1.1.1.0 (Affymetrix, Santa Clara, CA) for data summarization, normalization and quality control. Expression values were analyzed using Affymetrix Expression Console Summarization probe-set algorithm for miRNA using the RMA and Detection Above the Background methods. The signal values were filtered based on absent/present calls and only miRNAs with present calls >10% were accepted for further analyses. A total of 5,070 probes were used for analysis. In this study we focused on only the miRNA probes (n = 1,342). Differences in miRNA abundance were studied using Affymetrix GeneChip miRNA 3.0 arrays and the miRNA targets were predicted by means of miRBase miRNA database (http://www.mirbase.org/) [13].
TaqMan miRNA qRT-PCR Analysis
MiR-30b/c, miR-34b/c, miR-155, miR-1225-5p and miR-1915 were selected for verification of the microarray data by qRT-PCR. These miRNAs were chosen based on expression level as well as biological significance. TaqMan MicroRNA Reverse Transcription kit and Universal Mater Mix II (Applied Biosystems, Foster City, CA) were used for quantitative reverse transcription-PCR (RT-PCR) assays of mature miRNA, as described by the manufacturer. For RT reactions, 10 ng of RNA, 1x target-specific RT primer, 3.33 U/ml reverse transcriptase, 3.8 units RNase inhibitor, 0.15 mM dNTPs, and 1x reaction buffer were run in a total reaction volume of 15 μl and incubated at 16°C for 30 min, 42°C for 30 min, and 85°C for 5 min in a thermocycler (Applied Biosystems). Real-time PCR was performed using an Applied Biosystems 7900HT Sequence Detection System in a 10-ul PCR mixture containing 4.5 μl of RT product, 2x TaqMan Universal PCR Master Mix II, 20x TaqMan Assay, and water to adjust the final volume to 10 μl. All reactions were incubated in a 96-well plate at 95°C for 10 min, followed by 40 cycles of 95°C for 15 seconds, and 60°C for 1 min; all were performed in triplicate. Relative miRNA expression levels were compared via the 2−ΔΔCt method [14]. U6B small nuclear RNA gene (RNU6B, Applied Biosystems) was used as an endogenous control [15].
Statistics
Partek Genomics Suite 6.5 was used for the statistics and data visualization analyses for differentially expressed miRNAs. For all microarray data, one-way ANOVA using covariates of disease status (FSGS, MCD, and control) and tissue type (plasma and urine) with a false discovery rate (FDR) set to 0.05 was used. However, we utilized significance of the comparative results with p ≤ 0.05 being considered significant for further analyses. Kruskal-Wallis test was used to compare miRNA expression levels between groups, and Spearman’s rank-order correlation was used to test associations between miRNA expression levels and clinical parameters. Student’s t test was used for two parametric groups. All probabilities were two-tailed.
Results
The demographic and baseline clinical data of the patients are summarized in Table 1. Samples were obtained from 16 patients with primary FSGS, 5 patients with MCD and 5 normal controls. There were significant differences in age, sex, serum creatinine and estimated glomerular filtration rate (GFR) as measured by the CKD-EPI equation or MDRD between the three groups.
Table 1.
Summary of clinical and pathological characteristics of patients
| FSGS | MCD | Control | P-value | |
|---|---|---|---|---|
| Number of patients | 16 | 5 | 5 | |
| Sex (M:F) | 10:6 | 3:2 | 4:1 | <0.001 |
| Age (years) | 46.8 ± 16.4 | 22.0 ± 22.5 | 24.2 ± 3.4 | <0.001 |
| Proteinuria (g/day) | 3.7 ± 2.3 | 2.3* | - | - |
| Serum creatinine (mg/dL) | 1.7 ± 0.7 | 0.5 ± 0.1 | - | 0.001 |
| Serum albumin (g/dL) | 3.1 ± 0.7 | 3.9 ± 0.6 | - | 0.05 |
| GFR (ml/min/1.73 m2) | 53.4 ± 22.8 | 151.2 ± 39.1 | - | <0.001 |
FSGS, focal segmental glomerulosclerosis; MCD, minimal change disease; GFR; glomerular filtration rate. P-values depict comparisons by Student’s t-test between diagnosis groups.
Data available from one patient only.
Comparison between groups
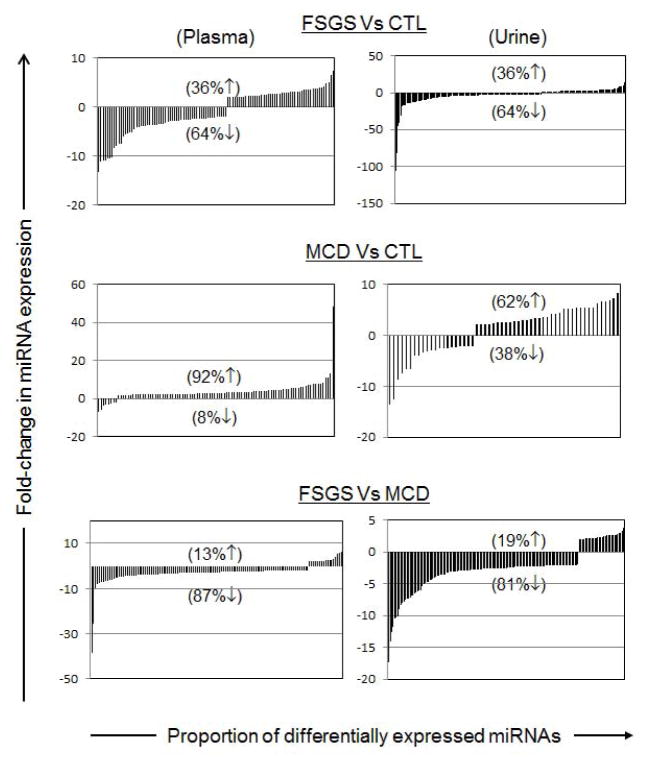
The plasma and urine levels of the 5,070 exosomal/microvesicle miRNAs tested were compared between the FSGS, MCD and normal control groups (Figure 1). A significantly higher number of miRNAs were down-regulated in both plasma and urine samples of FSGS patients compared to those with MCD. Seventeen miRNAs were up-regulated and 109 miRNAs were down-regulated in plasma of patients with FSGS compared to those with MCD. Similarly, when comparing urine samples from FSGS to MCD patients, 30 miRNAs were up-regulated and 125 miRNAs were down-regulated in FSGS patients. The lists of 10 most dysregulated miRNA with the highest fold-changes in plasma and urine samples are presented in Tables 2 and 3, respectively.
Figure 1.
Comparative analysis of miRNA expression levels in plasma and urine samples obtained from FSGS, MCD and control subjects. FSGS induces miRNA down-regulation in both plasma and urine. Significantly more miRNAs were down-regulated in both plasma and urine samples of FSGS patients compared to normal controls and MCD. On the other hand, miRNA expression was significantly up-regulated in both plasma and urine of MCD patients when compared to FSGS or controls. miRNAs with > 2-fold dysregulated expression are graphed for plasma and urine samples. The percentages of up- or down-regulated miRNAs are shown with corresponding up or down arrows on each graph.
Table 2.
List of 10 most dysregulated miRNA entities in FSGS and MCD plasma samples
| miRNA | Fold- Change | P-value | miRNA | Fold- Change | P-value | miRNA | Fold- Change | P-value |
|---|---|---|---|---|---|---|---|---|
| (FSGS vs. Normal) | (MCD vs. Normal) | (FSGS vs. MCD) | ||||||
| hsa-miR-3065-5p | 7.45 | 0.01 | hsa-miR-371-5p | 8.26 | 0.0004 | hsa-miR-455-3p | 5.86 | 0.001 |
| hsa-miR-495 | 4.91 | 0.04 | hsa-miR-625 | 7.20 | 0.002 | hsa-miR-3613-5p | 2.99 | 0.03 |
| hsa-miR-548d-3p | 5.03 | 0.04 | hsa-miR-4689 | 6.82 | 0.001 | hsa-miR-1323 | 2.49 | 0.01 |
| hsa-miR-455-3p | 6.53 | 0.0001 | hsa-miR-4327 | 6.75 | 4.69E-05 | hsa-miR-130a | 2.45 | 0.02 |
| hsa-miR-3065-5p | 7.45 | 0.005 | hsa-miR-4667-3p | 6.18 | 0.0004 | hsa-miR-4799-3p | 2.32 | 0.03 |
| hsa-miR-4270 | −10.43 | 0.01 | hsa-miR-4758-5p | −6.58 | 5.08E-06 | hsa-miR-4701-5p | −7.72 | 0.001 |
| hsa-miR-936 | −10.89 | 0.001 | hsa-miR-4640-5p | −6.62 | 0.003 | hsa-miR-574-3p | −8.07 | 0.003 |
| hsa-miR-4484 | −11.03 | 0.005 | hsa-miR-4530 | −7.54 | 0.002 | hsa-miR-574-5p | −9.76 | 0.001 |
| hsa-miR-4281 | −11.08 | 0.04 | hsa-miR-4484 | −12.67 | 0.004 | hsa-miR-4484 | −25.45 | 0.001 |
| hsa-miR-4728-5p | −13.29 | 0.004 | hsa-miR-4516 | −13.54 | 0.004 | hsa-miR-1234 | −38.48 | 0.001 |
Table 3.
List of 10 most dysregulated miRNA entities in FSGS and MCD urine samples
| miRNA | Fold- Change | P-value | miRNA | Fold- Change | P-value | miRNA | Fold- Change | P-value |
|---|---|---|---|---|---|---|---|---|
| (FSGS vs. Normal) | (MCD vs. Normal) | (FSGS vs. MCD) | ||||||
| hsa-miR-3145-5p | 16.79 | 0.003 | hsa-miR-1234 | 5.82 | 6.88E-06 | hsa-miR-3134 | 3.78 | 0.003 |
| hsa-miR-373 | 14.28 | 3.49E-05 | hsa-miR-4701-5p | 2.75 | 0.0006 | hsa-miR-19b | 2.98 | 0.009 |
| has-mir-559 | 10.03 | 0.002 | hsa-miR-3591-3p | 2.72 | 0.002 | hsa-miR-606 | 2.93 | 5.02E-07 |
| hsa-miR-548h-4 | 9.23 | 2.32E-05 | hsa-miR-138-1 | −7.12 | 0.0002 | hsa-miR-377 | 2.86 | 0.004 |
| hsa-miR-4801 | 9.16 | 0.002 | hsa-miR-574-5p | −8.00 | 0.0001 | hsa-miR-548c-3p | 2.70 | 0.0001 |
| hsa-miR-4466 | −30.98 | 5.69E-11 | hsa-miR-2115 | −2.98 | 5.08E-06 | hsa-miR-4270 | −10.45 | 2.64E-05 |
| hsa-miR-4728-5p | −40.31 | 1.14E-07 | hsa-miR-4799-3p | −3.38 | 0.003 | hsa-miR-1207-5p | −11.82 | 0.0003 |
| hsa-miR-3175 | −44.90 | 3.0E-08 | hsa-miR-4310 | −3.47 | 0.002 | hsa-miR-3175-5p | −12.63 | 1.39E-05 |
| hsa-miR-4484 | −81.71 | 2.14E-10 | hsa-miR-4713-3p | −5.90 | 0.004 | hsa-miR-1225-5p | −14.03 | 1.27E-05 |
| hsa-miR-4516 | −105.70 | 1.11E-11 | hsa-miR-936 | −6.93 | 0.004 | hsa-miR-4728-5p | −17.30 | 5.76E-06 |
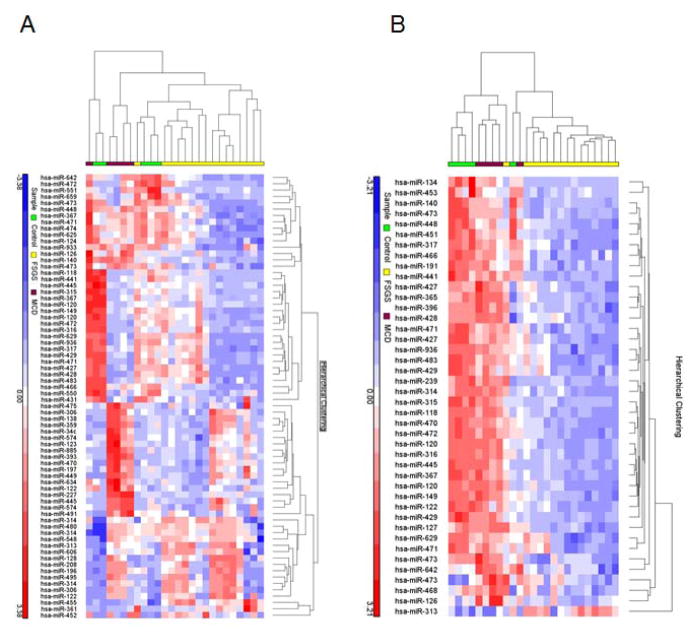
Figure 2 shows the heat map for altered miRNA expression in plasma and urine samples. Results from an unsupervised hierarchical clustering based on the identified differentially expressed miRNAs indicated that some of the significantly altered miRNA entities could distinguish between FSGS and MCD. Some of the individual clusters were clearly discerned by the clustering algorithms.
Figure 2.
Unsupervised hierarchical clustering over selected miRNAs (Pearson correlation, average linkage). Heatmap colors represent relative miRNA expression as indicated in the color key for each panel. Brackets on the top margins indicate samples from the same cohort. (A) Clustering of plasma samples classified as primary FSGS (n = 16, yellow), MCD (n = 5, magenta) and controls (n = 5, green) over 70 miRNAs. Samples are in columns, miRNAs in rows. Only miRNAs that survived multiple testing (FDR), and had a fold-change > 3 or < −3 and p < 0.05 are shown. (B) Clustering of urine samples classified as FSGS (n = 15, yellow), MCD (n = 5, magenta) and normal control (n = 5, green) over 42 miRNAs. Only miRNAs that survived multiple testing (FDR), and fold-change > 3 or < −3 and p < 0.05 are shown. (Not all of the differentially regulated miRNA are shown in the figure).
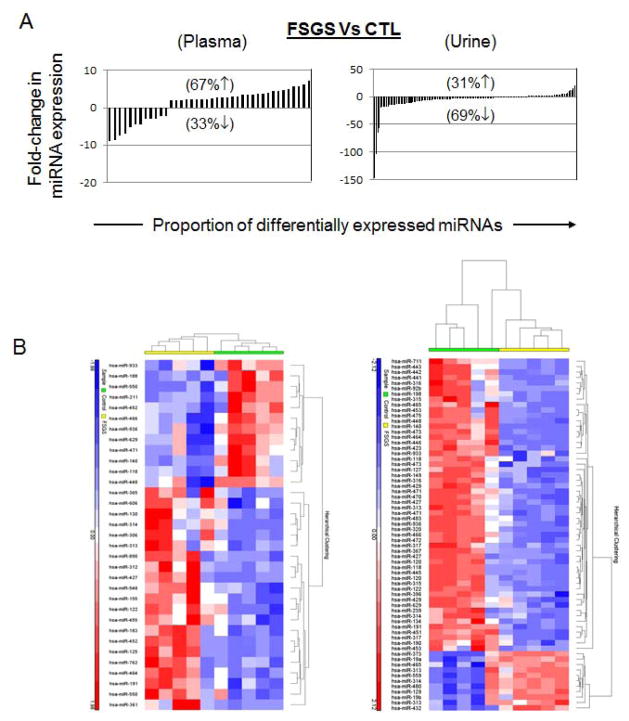
In order to correct for the changes in miRNA levels due to differences in sex and age, a secondary analysis was performed using a subgroup of primary FSGS patients (n=5) who were sex- and age-matched with the controls (n=5). Despite the small sample size, the secondary analysis also revealed a significantly different miRNA expression profile between FSGS and normal control group (Figure 3). Thirty nine serum miRNA and 135 urine miRNA were significantly dysregulated between sex- and age-matched primary FSGS and control groups.
Figure 3.
(A) Comparative analysis of miRNA expression levels in plasma and urine samples obtained from sex- and age-matched FSGS and control subjects. Significantly more miRNAs were down-regulated in urine samples of FSGS patients compared to normal controls. miRNAs with > 2-fold dysregulated expression are graphed for plasma and urine samples. The percentages of up- or down-regulated miRNAs are shown with corresponding up or down arrows on each graph. (B) Unsupervised hierarchical clustering over selected miRNAs (Pearson correlation, average linkage). Heatmap colors represent relative miRNA expression as indicated in the color key for each panel. Brackets on the top margins indicate samples from the same cohort. (Left) Clustering of plasma samples classified as primary FSGS (n = 16, yellow), MCD (n = 5, magenta) and controls (n = 5, green) over 33 miRNAs. Samples are in columns, miRNAs in rows. Only miRNAs that survived multiple testing (FDR), and had a fold-change > 3 or < −3 and p < 0.05 are shown. (Right) Clustering of urine samples classified as FSGS (n = 15, yellow), MCD (n = 5, magenta) and normal control (n = 5, green) over 65 miRNAs. Only miRNAs that survived multiple testing (FDR), and fold-change > 3 or < −3 and p < 0.05 are shown. (Not all of the differentially regulated miRNA are shown in the figure).
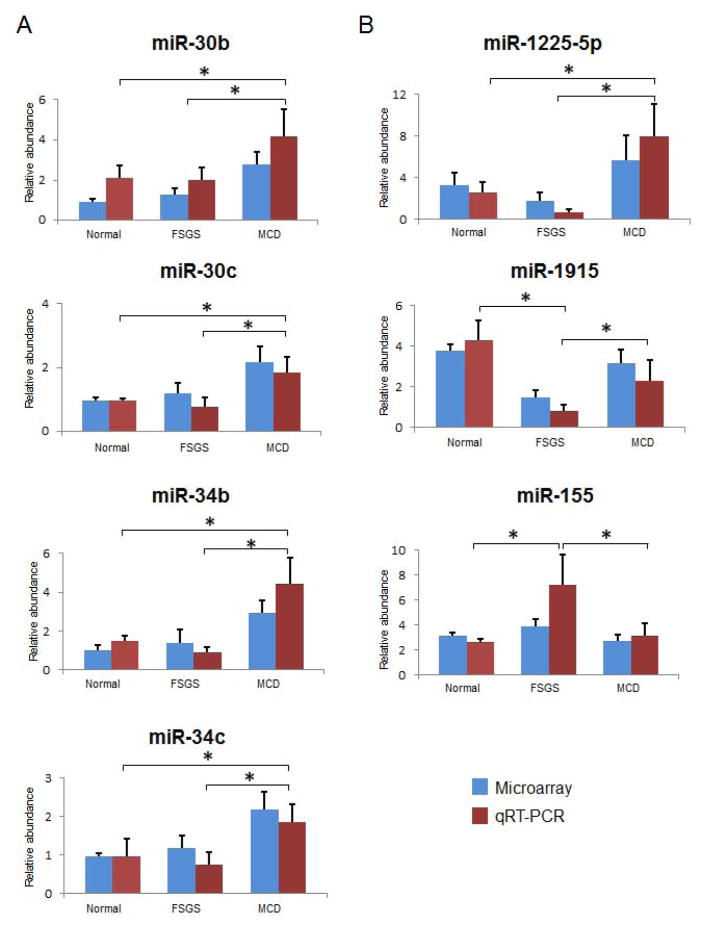
Verification by QRT-PCR
The plasma and urine levels of seven miRNAs were verified by qRT-PCR method. Only miRNA detected at cycle threshold (Ct) value 20–35 were included. The RNU6B reference gene was detected at Ct value 26 with no association with any of the disease groups. Among the differentially regulated plasma miRNA, the expression levels of two members of the miR-30 family, namely miR-30b and miR-30c were validated. QRT-PCR confirmed that, as compared to patients with FSGS and healthy controls, those with MCD had higher plasma levels of miR-30b (2.19-fold-change, p < 0.001) and miR-30c (2.0-fold-change, p < 0.001) (Figure 4A). Similarly, when compared to patients with FSGS and normal controls, patients with MCD also had higher plasma levels of miR-34b (4.0-fold-change, p < 0.0001) and miR-34c (2.2-fold-change, p < 0.001).
Figure 4.
QRT-PCR verification of (A) plasma and (B) urine miRNAs expression results from microarray data. Blue bars represent the results from microarray, while red bars indicate the results from qRT-PCR. The error bars are the standard error of mean (SEM) for each analysis. Four representative miRNAs (miR-30b/c and miR-34b/c) were observed up-regulated in MCD plasma compared to FSGS and controls. miR-1225 found to be up-regulated specifically in MCD urine compared to FSGS and normal control. miR-1915 was significantly down-regulated in FSGS urine compared to MCD and controls, whereas miR-155 was up-regulated in FSGS urine compared to MCD and controls. *P<0.01.
Among the differentially regulated urine miRNA, the expression levels of miR-1225-5p and miR-1915 were validated by qRT-PCR. As compared to patients with FSGS and healthy controls, patients with MCD had significantly higher urine levels of miR-1225-5p (2.7-fold-change, p < 0.0001) and miR-1915 (2.0-fold-change, p < 0.0001) (Figure 4B). Analysis of differentially regulated urine miRNA in urine samples also identified miR-155 and miR-663. QRT-PCR analysis of urine miR-155 confirmed that miR-155 levels in FSGS patients were significantly higher (2.3-fold, p<0.005) when compared to that of the MCD patients and normal controls. Overall, the correlative expression results from qRT-PCR analysis were consistent with the expression patterns by microarray assays.
Relation between miRNA expression levels and clinical parameters
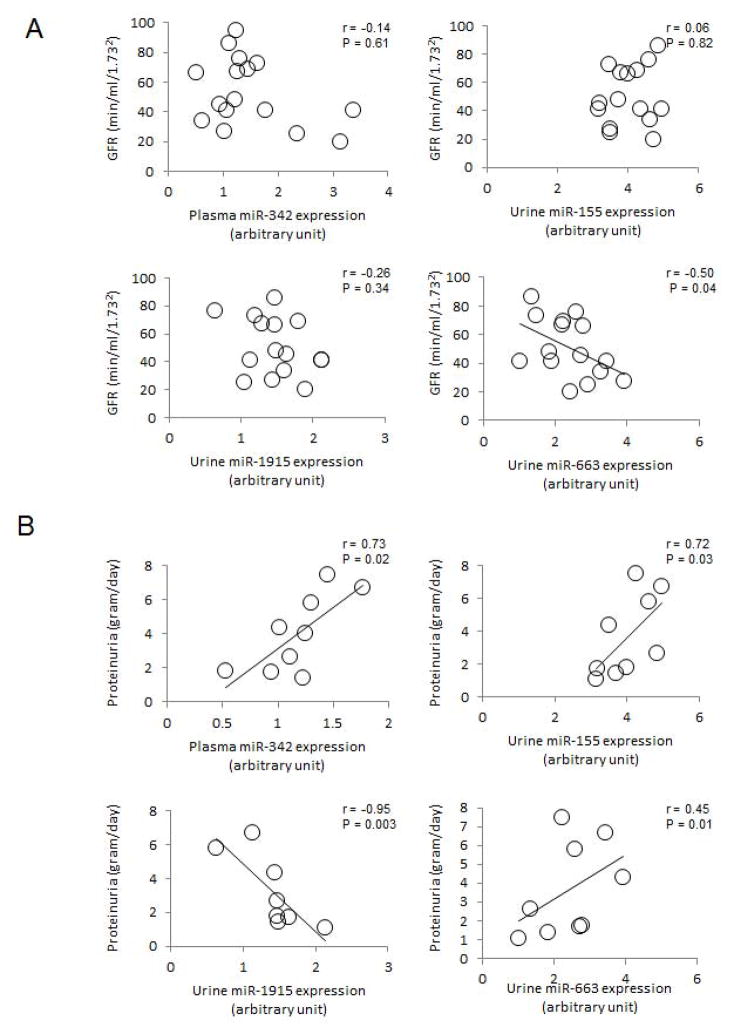
In FSGS patients, urinary levels of miR-663 are inversely correlated with GFR (r = −0.50, P = 0.04) (Figure 5A). In these patients, plasma levels of miR-342, and urine levels of miR-155 positively correlated with proteinuria (r = 0.73, P = 0.02 and r = 0.72, P = 0.03, respectively) (Figure. 5B). Urinary levels miR-1915 inversely correlated with proteinuria (r = 0.95, P = 0.003), whereas urinary levels of miR-663 positively correlated with proteinuria (r = 0.45, P = 0.04).
Figure 5.
Relation between levels of representative dysregulated plasma and urine miRNAs in samples from FSGS patients and: (A) glomerular filtration rate (GFR) and (B) proteinuria. Data are compared by Spearman’s rank correlation coefficient.
Discussion
In this study we compared the plasma and urinary miRNA profile in patients with FSGS and MCD using Affymetrix GeneChip miRNA 3.0 array and noted differential expression of 101 miRNAs in plasma and 148 miRNAs in urine. From these, only 38 have been previously reported in literature and the functional significance of all other miRNAs remains to be defined. These known miRNAs identified in our study have been previously shown to be involved in the regulation of biological processes of development, differentiation/morphogenesis, cell cycle regulation, cytoskeleton organization, signal transduction and inflammation (http://atlas.dmi.unict.it/mirandola/index.html). Among these, we found (a) plasma levels of miR-30b/c, miR34b/c and miR-342, and urine levels of mir-1225-5p to be significantly up-regulated in MCD patients compared to FSGS patients and normal controls; (b) urinary levels of mir-1915 and miR-663 were significantly down-regulated in FSGS patients compared to MCD patients and normal controls and; (c) the urinary level of miR-155 was significantly up-regulated in FSGS patients compared to MCD patients and normal controls.
In theory, the expression of miRNA may change with age, however, our post hoc analysis showed that FSGS patients continued to have a unique and significantly different circulatory and urinary miRNA profiles from healthy controls even when they were age- and sex-matched, suggesting that in our study the observed differences in urine and serum miRNA expression profile between FSGS and control samples were not simply due to differences in sex or age.
Expression and clearance of miRNA may be altered in the presence of impaired kidney function [16]. Wang et al. showed miR-192 and miR-205 were elevated in renal tissues of patients with hypertensive glomerulosclerosis [17]. These miRNAs were also shown to be up-regulated in patients with FSGS compared to MCD [18]. Putta et al. used a locked nucleic acid (LNA)–modified inhibitor of miR-192 in mouse models of diabetic nephropathy and showed that it significantly attenuated proteinuria, increased Zeb1/2 and decreased gene expression of collagen, TGF-β, and fibronectin in diabetic mice [19].
In our study, we noted that more miRNAs were down-regulated in both plasma and urine samples of FSGS patients compared to MCD, which is in accordance with an earlier observation [20]. The cause and consequence of such consistent reduction in miRNA expression in FSGS warrants further studies.
Identification of miRNAs with roles, or differential expression in EMT, fibrosis or activation of renal stem cells may be a relevant biomarker for renal disease [21–24]. Among the dysregulated miRNA in our study, miR-155 [20, 25–27], miR-223 [27], miR-320 [28], miR-302 [29], miR-193a [12], miR-34 family members [30, 31], mir-30 family members [32, 33], miR-1225-5p [34], and miR-1915 [34] have been shown previously to be expressed in kidney tissue or play important roles in the pathogenesis and progression of kidney diseases. Wu et al [32] found that expression of the miR-30 family is down-regulated in the podocytes of patients with FSGS and that miR-30s exert their protective roles by direct inhibition of Notch1 and p53 which mediate podocyte injury. Thus, miR-30s may be a marker of podocyte injury and probably glomerular disease. In another study, miR-30 family miRNAs were shown to target mesenchymal gene transcripts and maintain them in a translationally inactive state, preventing the EMT [33]. Down-regulation of miR-30 has been shown to lead to EMT [33], which could subsequently contribute to the renal fibrosis in patients with FSGS [35].
Analysis of differentially regulated plasma miRNA identified two members of the miR-34 family, namely miR-34b and miR-34c [30, 31]. As compared to patients with FSGS and normal controls, patients with MCD had significantly higher plasma levels of miR-34b and miR-34c. These miRNAs have also been shown to target the 3′ untranslated region (UTR) of Notch1, and altered Notch signaling has been associated with FSGS pathogenesis [36, 37]. Another significantly dysregulated plasma miRNA identified in this study was miR-342, which was found to be up-regulated in MCD patients compared to patients with FSGS and normal controls. mir-342, has previously been shown to be expressed in macrophages and be the most prominently up-regulated miRNA during early atherosclerosis [38, 39]. It has long been recognized that FSGS and atherosclerotic lesions seem to share certain common pathophysiologic mechanisms, including endothelial cell injury and macrophage infiltration [40].
In this study, we also found that urinary miR-1225-5p and miR-1915 levels are specifically reduced in FSGS compared to MCD patients. To our knowledge, this is the first time urinary expression of these miRNAs is reported. Both miR-1225-5p and miR-1915 have been shown to play specific roles in regulating genes involved in the maintaining adult renal stem/progenitor cells (ARPCs) [34]. These miRNAs were recently shown to be down-regulated in ARPCs isolated from the glomeruli and from tubular compartment [34]. Both miR-1225-5p and miR-1915 regulate genes involved in maintaining ARPCs, such as CD133 and renal embryonic transcription factors PAX2 and PAX8, which are markers of adult renal progenitors, the hyaluronic acid receptor CD44, the toll-like receptor 2 (TLR2), IL-8, HOXA1, several WNT genes, and other chemokines and growth factors [34, 41]. Investigators have identified a putative miR-1225-5p binding element in the 3′ UTR of TLR2 transcript, a key damage signaling gene found to be up-regulated in ARPCs, which can give rise to repair processes in acute renal tubular damage [34, 41, 42]. The further decrease in the miR1225-5p and miR-1915 levels observed in FSGS urine samples could potentially be a reflection of the loss of ARPCs which are the source of such miRNAs in FSGS kidneys as compared to MCD kidneys.
Dysregulated immune responses and the resultant inflammation are mediators and/or catalysts in the processes of progression of renal disease [43, 44]. We identified miR-155, an inflammation-related miRNA in the urine samples from patients with FSGS [25, 26]. Unlike most of the other miRNAs in our study, urine miR-155 levels in FSGS patients were significantly higher compared to those levels in MCD patients and controls. miR-155 has been reported to regulate inflammatory response in endothelial cells mediated by angiotensin II [45]. We also found urinary levels of miR-663 to be down-regulated in FSGS patients compared to MCD patients and controls. miR-663 targets the REN and APOE 3 untranslated regions and regulates REN and APOE mRNA levels [46]. The reduced expression of miR-663 in hypertensive kidneys has been correlated to the renin mRNA elevation seen in these patients.
This study has a number of strengths. It is the first study exploring the possible utility of circulating and urinary miRNA in distinguishing FSGS from MCD. Other strengths of the study include (a) simultaneous comparison of both plasma and urine exosomal miRNA profiles, (b) use of an agnostic approach to identify differential expression of miRNA using array technology and (c) validation of the findings using qRT-PCR. However, the study was limited by the small number of subject in the control and MCD groups, as well as a significant age difference between study groups. Since most patients had been on therapy at the time of sample collection, we could not rule out the possibility that some of the changes in miRNA expression levels reflect differences in therapy rather than differences in pathology. Furthermore, due to differences in clinical characteristics of the patients, one cannot rule out, that the identified differences are due to clinical and laboratory characteristics, and not entirely explained by the pathology. Although we did adjust for multiple comparisons in order to avoid type 1 statistical error, our result should be considered preliminary and hypothesis generating. In addition, this is a cross-sectional study, and therefore the levels of miRNAs may change with disease progression.
Conclusion
In this pilot study we identified significant differences in the plasma and urine miRNA profiles between patients with MCD and FSGS. These miRNAs could potentially serve as novel biological markers for distinguishing FSGS from MCD, reducing the need for kidney biopsy. Interfering with miRNAs in other organ systems has already been shown to alter pathophysiological processes [47–49]. Thus, exploring the in-depth mechanism by which these miRNAs accelerate the progressive process of FSGS may provide additional targets for therapeutic intervention.
Acknowledgments
This work is supported in part by grants R01 DK073665-01A1, 1U01DK099924-01 and 1U01DK099914-01 from the National Institute of Diabetes and Digestive and Kidney Diseases, and grant UL1TR000075 from the NIH National Center for Advancing Translational Sciences, awarded to Dominic Raj. The work was also supported by the NIDDK Intramural Research Program.
Footnotes
Disclosure statement
The authors declare that they have no conflict of interests.
Authors’ contributions
AR carried out the miRNA isolation and verification, participated in the design of the study and data analysis, and helped draft the manuscript. JMD, performed the miRNA microarray data analysis and performed the statistical analysis. SC, critically reviewed the manuscript. MW, participated in the data analysis and helped to draft the manuscript. RS, carried out the microarray experiments. SK, participated in its design and coordination and helped to draft the manuscript. RS, participated in performing miRNA isolation and verification. LH, obtained, processed and stored the patient urine and serum samples. JBK, provided the patient samples and helped to analyze the data and draft the manuscript. DSR, is the PI and conceived of the study, participated in its design and coordination and helped with data analysis and draft of the manuscript. All authors read and approved the final manuscript.
Contributor Information
Ali Ramezani, Email: ramezani@gwu.edu.
Joseph M. Devaney, Email: JDevaney@childrensnational.org.
Scott Cohen, Email: scohen@mfa.gwu.edu.
Maria R. Wing, Email: mwing@mfa.gwu.edu.
Richard Scott, Email: Richard.Scott@childrensnational.org.
Suzan Knoblach, Email: SKnoblach@childrensnational.org.
Rishi Singhal, Email: rsinghal@gwmail.gwu.edu.
Lilian Howard, Email: howardlv@mail.nih.gov.
Jeffrey B. Kopp, Email: jeffreyk@intra.niddk.nih.gov.
Dominic S. Raj, Email: draj@mfa.gwu.edu.
Reference List
- 1.Cravedi P, Kopp JB, Remuzzi G. Recent progress in the pathophysiology and treatment of FSGS recurrence. Am J Transplant. 2013;13:266–274. doi: 10.1111/ajt.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meyrier A. An update on the treatment options for focal segmental glomerulosclerosis. Expert Opin Pharmacother. 2009;10:615–628. doi: 10.1517/14656560902754029. [DOI] [PubMed] [Google Scholar]
- 3.Cattran DC, Rao P. Long-term outcome in children and adults with classic focal segmental glomerulosclerosis. Am J Kidney Dis. 1998;32:72–79. doi: 10.1053/ajkd.1998.v32.pm9669427. [DOI] [PubMed] [Google Scholar]
- 4.Patrakka J, Ruotsalainen V, Ketola I, et al. Expression of nephrin pediatric kidney diseases. J Am Soc Nephrol. 2001;12:289–296. doi: 10.1681/ASN.V122289. [DOI] [PubMed] [Google Scholar]
- 5.Horinouchi I, Nakazato H, Kawano T, et al. In situ evaluation of podocin in normal and glomerular diseases. Kidney Int. 2003;64:2092–2099. doi: 10.1046/j.1523-1755.2003.00303.x. [DOI] [PubMed] [Google Scholar]
- 6.Garin EH, Mu W, Arthur JM, et al. Urinary CD80 is elevated in minimal change disease but not in focal segmental glomerulosclerosis. Kidney Int. 2010;78:296–302. doi: 10.1038/ki.2010.143. [DOI] [PubMed] [Google Scholar]
- 7.Wing MR, Ramezani A, Gill HS, Devaney JM, Raj DS. Epigenetics of progression of chronic kidney disease: fact or fantasy? Semin Nephrol. 2013;33:363–374. doi: 10.1016/j.semnephrol.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tian Z, Greene AS, Pietrusz JL, Matus IR, Liang M. MicroRNA-target pairs in the rat kidney identified by microRNA microarray, proteomic, and bioinformatic analyis. Genome Res. 2008;18:404–411. doi: 10.1101/gr.6587008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cortez MA, Calin GA. MicroRNA identification in plasma and serum: a new tool to diagnose and monitor diseases. Expert Opin Biol Ther. 2009;9:703–711. doi: 10.1517/14712590902932889. [DOI] [PubMed] [Google Scholar]
- 10.Hanke M, Hoefig K, Merz H, et al. A robust methodology to study urine microRNA as tumor marker: microRNA-126 and microRNA-182 are related to urinary bladder cancer. Urol Oncol. 2010;28:655–661. doi: 10.1016/j.urolonc.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 11.Melkonyan HS, Feaver WJ, Meyer E, et al. Transrenal nucleic acids: from proof of principle to clinical tests. Ann NY Acad Sci. 2008;1137:73–81. doi: 10.1196/annals.1448.015. [DOI] [PubMed] [Google Scholar]
- 12.Gebeshuber CA, Kornauth C, Dong L, et al. Focal segmental glomerulosclerosis is induced by microRNA-193 and its downregulation of WT1. Nat Med. 2013;19:481–487. doi: 10.1038/nm.3142. [DOI] [PubMed] [Google Scholar]
- 13.Griffiths-Jones S. miRBase: the microRNA sequence database. Methods Mol Biol. 2006;342:129–138. doi: 10.1385/1-59745-123-1:129. [DOI] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C (T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Ng EK, Chong WW, Jin H, et al. Differential expression of microRNAs in plasma of patients with colorectal cancer: a potential marker for colorectal cancer screening. Gut. 2009;58:1375–1381. doi: 10.1136/gut.2008.167817. [DOI] [PubMed] [Google Scholar]
- 16.Zager RA, Johnson AC, Lund S. Uremia impacts renal inflammatory cytokine gene expression in the setting of experimental acute kidney injury. Am J Physiol Renal Physiol. 2009;297:F961–F970. doi: 10.1152/ajprenal.00381.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang G, Kwan BC, Lai FM, et al. Intrarenal expression of miRNAs in patients with hypertensive nephrosclerosis. Am J Hypertens. 2010;23:78–84. doi: 10.1038/ajh.2009.208. [DOI] [PubMed] [Google Scholar]
- 18.Cai X, Xia Z, Zhang C, et al. Serum microRNAs levels in primary focal segmental glomerulosclerosis. Pediatr Nephrol. 2013;28:1797–1801. doi: 10.1007/s00467-013-2434-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Putta S, Lanting L, Sun G, Lawson G, Kato M, Natarajan R. Inhibiting microRNA-192 ameliorates renal fibrosis in diabetic nephropathy. J Am Soc Nephrol. 2012;23:458–469. doi: 10.1681/ASN.2011050485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neal CS, Michael MZ, Pimlott LK, Yong TY, Li JY, Gleadle JM. Circulating microRNA expression is reduced in chronic kidney disease. Nephrol Dial Transplant. 2011;26:3794–3802. doi: 10.1093/ndt/gfr485. [DOI] [PubMed] [Google Scholar]
- 21.Humphreys BD, Bonventre JV. Mesenchymal stem cells in acute kidney injury. Annu Rev Med. 2008;59:311–325. doi: 10.1146/annurev.med.59.061506.154239. [DOI] [PubMed] [Google Scholar]
- 22.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato M, Arce L, Natarajan R. MicroRNAs and their role in progressive kidney diseases. Clin J Am Soc Nephrol. 2009;4:1255–1266. doi: 10.2215/CJN.00520109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saal S, Harvey SJ. MicroRNAs and the kidney: coming of age. Curr Opin Nephrol Hypertens. 2009;18:317–323. doi: 10.1097/MNH.0b013e32832c9da2. [DOI] [PubMed] [Google Scholar]
- 25.Wang G, Kwan BC, Lai FM, Chow KM, Li PK, Szeto CC. Elevated levels of miR-146a and miR-155 in kidney biopsy and urine from patients with IgA nephropathy. Dis Markers. 2011;30:171–179. doi: 10.3233/DMA-2011-0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H, Peng W, Shen X, Huang Y, Ouyang X, Dai Y. Circulating levels of inflammation-associated miR-155 and endothelial-enriched miR-126 in patients with end-stage renal disease. Braz J Med Biol Res. 2012;45:1308–1314. doi: 10.1590/S0100-879X2012007500165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anglicheau D, Sharma VK, Ding R, et al. MicroRNA expression profiles predictive of human renal allograft status. Proc Natl Acad Sci USA. 2009;106:5330–5335. doi: 10.1073/pnas.0813121106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lorenzen JM, Kielstein JT, Hafer C, et al. Circulating miR-210 predict survival in critically ill patients with acute kidney injury. Clin J Am Soc Nephrol. 2011;6:1540–1546. doi: 10.2215/CJN.00430111. [DOI] [PubMed] [Google Scholar]
- 29.Faherty N, Curran SP, O’Donovan H, et al. CCN2/CTGF increases expression of miR-302 microRNAs, which target the TGFbeta type II receptor with implications for nephropathic cell phenotypes. J Cell Sci. 2012;125:5621–5629. doi: 10.1242/jcs.105528. [DOI] [PubMed] [Google Scholar]
- 30.Shi S, Yu L, Chiu C, et al. Podocyte-selective deletion of dicer induces proteinuria and glomerulosclerosis. J Am Soc Nephrol. 2008;19:2159–2169. doi: 10.1681/ASN.2008030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bae Y, Yang T, Zeng HC, et al. miRNA-34c regulates Notch signaling during bone development. Hum Mol Genet. 2012;21:2991–3000. doi: 10.1093/hmg/dds129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu J, Zheng C, Fan Y, et al. Downregulation of microRNA-30 facilitates podocyte injury and is prevented by glucocorticoids. J Am Soc Nephrol. 2014;25:92–104. doi: 10.1681/ASN.2012111101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joglekar MV, Patil D, Joglekar VM, et al. The miR-30 family microRNAs confer epithelial phenotype to human pancreatic cells. Islets. 2009;1:137–147. doi: 10.4161/isl.1.2.9578. [DOI] [PubMed] [Google Scholar]
- 34.Sallustio F, Serino G, Costantino V, et al. miR-1915 and miR-1225-5p regulate the expression of CD133, PAX2 and TLR2 in adult renal progenitor cells. PLoS One. 2013;8:e68296. doi: 10.1371/journal.pone.0068296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rastaldi MP. Epithelial-mesenchymal transition and its implications for the development of renal tubulointerstitial fibrosis. J Nephrol. 2006;19:407–412. [PubMed] [Google Scholar]
- 36.Niranjan T, Bielesz B, Gruenwald A, et al. The Notch pathway in podocytes plays a role in the development of glomerular disease. Nat Med. 2008;14:290–298. doi: 10.1038/nm1731. [DOI] [PubMed] [Google Scholar]
- 37.Waters AM, Wu MY, Onay T, et al. Ectopic notch activation in developing podocytes causes glomerulosclerosis. J Am Soc Nephrol. 2008;19:1139–1157. doi: 10.1681/ASN.2007050596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Te JL, Dozmorov IM, Guthridge JM, et al. Identification of unique microRNA signature associated with lupus nephritis. PLoS One. 2010;5:e10344. doi: 10.1371/journal.pone.0010344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei Y, Nazari-Jahantigh M, Chan L, et al. The microRNA-342-5p fosters inflammatory macrophage activation through an Akt1- and microRNA-155-dependent pathway during atherosclerosis. Circulation. 2013;127:1609–1619. doi: 10.1161/CIRCULATIONAHA.112.000736. [DOI] [PubMed] [Google Scholar]
- 40.Diamond JR, Karnovsky MJ. Focal and segmental glomerulosclerosis: analogies to atherosclerosis. Kidney Int. 1988;33:917–924. doi: 10.1038/ki.1988.87. [DOI] [PubMed] [Google Scholar]
- 41.Sallustio F, Costantino V, Cox SN, et al. Human renal stem/progenitor cells repair tubular epithelial cell injury through TLR2-drive inhibin-A and microvesicle-shuttled decorin. Kidney Int. 2013;83:392–403. doi: 10.1038/ki.2012.413. [DOI] [PubMed] [Google Scholar]
- 42.Sallustio F, De BL, Castellano G, et al. TLR2 plays a role in the activation of human resident renal stem/progenitor cells. FASEB J. 2010;24:514–525. doi: 10.1096/fj.09-136481. [DOI] [PubMed] [Google Scholar]
- 43.Gupta J, Mitra N, Kanetsky PA, et al. Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin J Am Soc Nephrol. 2012;7:1938–1946. doi: 10.2215/CJN.03500412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wing MR, Devaney JM, Joffe MM, et al. DNA methylation profile associated with rapid decline in kidney function: findings from the CRIC Study. Nephrol Dial Transplant. 2014 doi: 10.1093/ndt/gft537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Staszel T, Zapala B, Polus A, et al. Role of microRNAs in endothelial cell pathophysiology. Pol Arch Med Wewn. 2011;121:361–366. [PubMed] [Google Scholar]
- 46.Marques FZ, Campain AE, Tomaszewski M, et al. Gene expression profiling reveals renin mRNA overexpression in human hypertensive kidneys and a role for microRNAs. Hypertension. 2011;58:1093–1098. doi: 10.1161/HYPERTENSIONAHA.111.180729. [DOI] [PubMed] [Google Scholar]
- 47.Small EM, Olson EN. Pervasive roles of microRNAs in cardiovascular biology. Nature. 2011;469:336–342. doi: 10.1038/nature09783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thum T, Gross C, Fiedler J, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–984. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 49.Thum T, Chau N, Bhat B, et al. Comparison of different miR-21 inhibitor chemistries in a cardiac disease model. J Clin Invest. 2011;121:461–462. doi: 10.1172/JCI45938. [DOI] [PMC free article] [PubMed] [Google Scholar]