Abstract
Background
Exposure to disinfection by-products (DBPs) during pregnancy was associated with reduced fetal growth. Genetic susceptibility might play a role, especially for genes encoding for the Cytochrome P450 (CYP2E1) and Glutathione S-Transferase (GST) enzymes, involved in metabolism and activation of DBPs. Few epidemiological studies evaluated these gene-environment interactions and their results were never replicated.
Objective
This study aims to examine interactions between trihalomethanes (THM) or haloacetic acids (HAA) exposure and genetic polymorphisms on small for gestational age (SGA) neonates by investigating single nucleotide polymorphisms (SNPs) in CYP2E1 gene and GSTM1 and GSTT1 deletions in mothers-children pairs.
Methods
A population-based case-control study of 1549 mothers and 1455 children was conducted on SGA and THM/HAA exposure. DNA was extracted from blood or saliva cells. Targeted SNPs and deletions were genotyped. Statistical interaction between SNPs/deletions and THMs or HAAs in utero exposure with regard to SGA occurrence was evaluated by unconditional logistic regression with control of potential confounders.
Results
Previously reported positive modification of the effect of THM uterine exposure by mothers or newborns CYP2E1 rs3813867 C allele or GSTM1 deletion was not replicated. However interactions with CYP2E1 rs117618383 and rs2515641 were observed but were not statistically significant after correction for multiple testing.
Conclusions
Previous positive interactions between THMs exposure and CYP2E1 and GSTM1 were not replicated but interactions with other CYP2E1 polymorphisms are reported.
Keywords: DBPs, intra-uterine-growth-restriction, gene polymorphisms, CYP2E1, GSTM1, GSTT1
1. Introduction
Disinfection of drinking water is an essential component of public health protection. However, chlorine, the main and more widespread disinfectant of drinking water, reacts with organic matter naturally present in water to form numerous “by-product” chemicals (Richardson et al. 2007); the main ones being trihalomethanes (THMs) and haloacetic acids (HAAs), which are omnipresent in chlorinated waters at concentrations easily measurable (10–100 μg/l). These compounds have a well established toxicity at high doses on animals (Amy and International Programme on Chemical Safety 2000). Although data are still limited, there is evidence of a possible effect of THM and HAA exposure during pregnancy on intra-uterine foetal growth (Grellier et al. 2010; Villanueva et al. 2015). Due to the importance of foetal growth restriction on infants and its long term consequences in adult life (Pallotto and Kilbride 2006; Varvarigou 2010), it is necessary to identify factors which might enhance or reduce this risk.
In 2004, in a hospital-based case-control study, Infante-Rivard (Infante-Rivard 2004) found that newborns whose mothers have been exposed at home during their whole pregnancy to an average water supply THM levels above 29.7μg/L (90th percentile of the distribution of concentrations in participants water supply systems) were at higher risk of SGA (<10th percentile birth weight) if they were carrying one or two C alleles of the CYP2E1 gene rs3813867 (G1295C) polymorphism (guanidine being replaced by cytosine in the allele). The CYP2E1 gene represents a target of choice for the study of genetic modification of potential toxic effects of several disinfection by-products because it encodes an isoenzyme which is part of the cytochrome P-450, and therefore might play a major role in phase-1 biological activation of such xenobiotics (Bolt et al. 2003).
More recently (Danileviciute et al. 2012), a population-based case-control study reported that women with the highest exposure to THMs and carrying a deletion of the Glutathione S-Transferase M1(GSTM1) gene were at higher risk of delivering low birth weight babies. However, no relationship was found with the SGA outcome, which is a better indicator of intrauterine growth retardation Deletion of the Glutathione S-Transferase Theta 1 (GSTT1) in mothers was also studied by Danilevicuite et al. (Danileviciute et al. 2012) but no significant statistical interaction was found. Glutathione S-Transferase (GST) enzymes family play an important role in phase-2 biotransformation of xenobiotics and in cellular detoxification (Hayes and Strange 2000). However, mutations in genes modulating the activity of enzymes such as GSTT1 and GSTM1 may also be responsible for enhancing toxic activities of chemicals (Bolt and Thier 2006).
The objective of this study was to revisit previously examined interactions between THM or HAA exposure and genetic polymorphisms with respect to foetal growth restriction by investigating single nucleotide polymorphisms (SNPs) capturing common genetic variation in CYP2E1 gene as well as GSTT1 and GSTM1 deletions in biological samples of mothers-children pairs.
2. Materials and methods
2.1. Study design and population
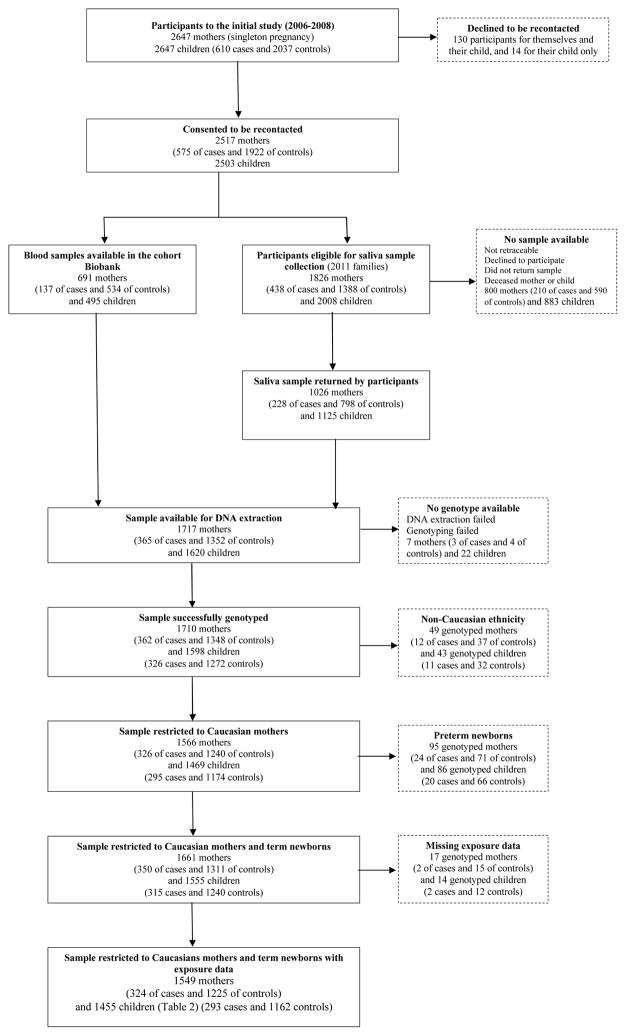
This is a population-based case-control study conducted prospectively, between August 2006 to April 2008, in the Québec City (Canada) metropolitan area, a region of about 650,000 inhabitants. Participants were from a previous study on the association between exposure to THMs and HAAs during pregnancy and the occurrence of SGA (Levallois et al. 2012). Among the 2647 women (and their child) participating to the original study, 2517 (95%) accepted to be contacted for a follow-up study. A total of 1717 mothers and 1620 children provided DNA samples either from blood (for participants to a previous cohort study (Forest et al. 2014)) or from saliva (for participants recontacted for this study). Details on participants are given on the flow diagram (Figure 1) and in Supplemental material (Methods S1).
Figure 1.
Diagram Flow Chart starting from the participants to the initial study (610 cases and 2037 controls) to the participants to this study (293 cases and 1162 controls)
To reduce the possibility of a population stratification bias, non Caucasian participants (about 3%) identified from a self-administered questionnaire were removed from the initial sample for this study. Also, because our focus was the effect of the DBPs exposure in the third trimester and in accordance to our previous study (Levallois et al. 2012), only term babies were considered for this study (see Figure 1).
Ethical considerations
The access to the birth certificates for the selection of cases and controls was allowed by the Commission d’accès à l’information of Québec. The initial case-control study and this follow-up gene-environment study were both approved by the Ethics committee of CHU de Québec. For this follow-up study, a consent form was sent by mail to potential participants and returned with signature to the researchers by those who had provided saliva samples. As for the subgroup of participants who had previously consented to the other cohort study, informed written consent had been given during the first perinatal visit, for their own blood sample as well as for cord blood, and included consent for genetic analyses. This study was also approved by the CHU de Québec Ethics Review Board.
2.2. Definition of cases and controls
Cases of SGA were all term singleton newborns with birth weight less than the sex-specific 10th percentile of weight for gestational age, according to the Canadian standards (Kramer et al. 2001). Controls were also term newborns but with birth weight at or above the same sex-specific standard for gestational age. About three controls per case were randomly selected among singletons born the same calendar week in the same geographical study area.
2.3. Interview of mothers
Mothers of cases of SGA and controls had been interviewed by telephone as part of the original study about two months after the birth to gather information on risk factors for SGA as well as socio-economic and lifestyles variables. Usual water consumption (number of glasses per day) and frequency of showers and baths per day or week were asked. Detailed information on the type of water consumed during the whole pregnancy as well as the use of water treatment home-devices and other water handling (boiling or letting stay in the fridge) was also collected.
2.4. DBPs Exposure assessment
The exposure assessment to disinfection by-products of participants was particularly improved over previous studies on this issue. Details are given in the original study (Levallois et al. 2012). In brief, THM and HAA were monitored monthly during the study at 53 sites within the 16 water distribution systems serving the residence of participants. The detection limits for THM species were 0.3 μg/L for chloroform, 0.3 μg/L for bromodichloromethane, 0.4 μg/L for chlorodibromomethane and 0.5 μg/L for bromoform. The detection limits for HAA species were 1.3 μg/L for monochloracetic, 0.9 μg/L for dichloroacetic, 0.4 μg/L for trichloracetic, 1.0 μg/L for monobromoacetic, 0.7 μg/L for dibromoacetic, 0.8 μg/L for bromochloroacetic, 4.6 μg/L for dibromochloroacetic, 4.2 μg/L for bromodichloroacetic and 6.4 μg/L for tribromoacetic.
Exposure assessment of mothers during the last trimester of their pregnancy was based on the estimation of concentrations of these chemicals in the tap water of participants’ residence (after correction for home water treatment devices and other handlings) during that period and on the amount of water consumed through ingestion and, for THMs, through the dermal and inhalation routes during home shower and bath during a typical day. (See Supplemental material Methods S1 for details)
2.5.Potential confounders
Variables of interest were collected during the interview of the mothers and considered as potential confounders: maternal age, maternal education, annual household income, working status, marital status, prepregnancy body mass index (BMI), parity, history of chronic disease, medical problems during pregnancy, active smoking during the third trimester and passive smoking throughout the pregnancy, coffee and alcohol consumption, and risky occupational exposure. In addition, since the proportion of subjects with DNA extracted from saliva vs. blood differed between cases and controls (see Figure 1), we included DNA source as potential confounder in our statistical analysis despite genotyping quality rates seem very comparable between the two sources (Abraham et al. 2012).
2.6. Biological samples and DNA extraction
Blood samples of participants to the cohort study (Giguère et al. 2015) were drawn at the first prenatal visit between 10 and 18 weeks, while cord blood was sampled after delivery. Saliva was sampled using the ORAGENE-DNA kits (OG-500 and OG-575; GENOTEK, Kanata, On, Canada) mailed to potential participants with directives for sampling according to the manufacturer, and returned back to the research team by mail. In total, DNA samples from members of 1719 families (1618 mothers-child pairs, 2 children and 99 mothers) were available for DNA extraction. Further details on biological samples and DNA extractions can be found in the Supplemental material (Methods S2).
2.7. Genotyping
2.7.1 SNP selection
Using the Tagger program (Broad Institute, Cambridge, MA), we used a systematic approach to determine the polymorphisms allowing to better capture of the genetic diversity of the locus of interest. To optimize cost-efficiency, we used a r2 threshold of 0.8 for tag SNPs selection with minor allele frequency of at least 5% (based on the Caucasian samples from Great Britain (GBR), Toscany (TSI) and Utah of Western, and Northern European origin (CEU) of the 1000 Genomes project (1000 Genomes Project).
In addition to the SNPs rs3813867 (G1295C) and rs2031920 (G1055C) analyzed in previous studies (Cantor et al. 2010; Infante-Rivard 2004), additional SNPs were selected for genotyping of common variants in CYP2E1 gene as described above. Fifteen SNPs within CYP2E1 (including 5 kb at both ends of the gene) were selected in total and twelve of them were successfully genotyped (see Table 1).
Table 1.
Frequency of SNPs and deletions genotypes in comparison with the European sub-populations of the (1000 Genomes Project)
| Chrom | Gene | Number rs | Positiona | Molecular consequenceb | Clinical significanceb | Allele | MAc | MAFCd | MAFe |
|---|---|---|---|---|---|---|---|---|---|
| 10 | CYP2E1 | ||||||||
| rs10857730 | 135326822 | intron | - | T/G | G | 0.13 | 0.17 | ||
| rs11101800 | 135327325 | intron | - | C/T | T | 0.06 | 0.08 | ||
| rs117618383 | 135328727 | 2KB upstream | - | A/T | T | 0.04 | 0.04 | ||
| rs11101807 | 135335647 | unknown | - | T/C | C | - | 0.09 | ||
| rs6537612 | 135336717 | unknown | - | A/T | T | 0.08 | 0.10 | ||
| rs41299398 | 135339122 | 2KB upstream | - | C/T | T | 0.09 | 0.09 | ||
| rs3813866 | 135339334 | 2KB upstream | - | T/A | A | 0.05 | 0.06 | ||
| rs3813867 | 135339605 | 2KB upstream | pathogenic | G/C | C | 0.03 | 0.04 | ||
| rs2031920 | 135339845 | 2KB upstream | pathogenic | C/T | T | 0.03 | 0.04 | ||
| rs6413421 | 135345811 | intron | - | T/C | C | 0.06 | 0.06 | ||
| rs915907 | 135346927 | intron | - | C/A | A | 0.16 | 0.18 | ||
| rs2011661 | 135348786 | Intron | - | C/T | T | - | 0.06 | ||
| rs2515641 | 135351362 | synonymous | -f | T/C | T | 0.11 | 0.13 | ||
| rs2480259 | 135352076 | intron | - | A/G | A | 0.19 | 0.23 | ||
| rs11101815 | 135355927 | unknown | - | A/G | G | - | 0.10 | ||
| 1 | GSTM1 | – | 110230230-110251661 | deletion | Ag mu1 | Nullh | 0.56 | – | |
| 22 | GSTT1 | – | 24376133-24384680 | deletion | Ag theta1 | Nullh | 0.21 | – |
Position in genome assembly GRCh37.p13
Terminology and data from the National Center for Biotechnology Information Variation Viewer, retrieved on March 9th, 2016 (www.ncbi.nlm.nih.gov)
MA: minor allele
MAFC: minor allele frequency computed in the sample of mothers of controls. A missing value indicates that genotyping of the SNP failed.
MAF: minor allele frequency of 1000 Genomes EUR sub-populations (1000 Genomes Project).
rs2515641 was recently associated with kidney transplant rejection (Kim et al. 2014)
A: Glutathione S-transferase
Null: double deletion.
2.7.2 SNPs and deletions genotyping
SNPs were genotyped by Sequenom Technology at the Plate-forme de génotypage du CHU de Québec-Université Laval. For DNA quantification, double stranded DNA concentration was assessed using the QuantiFluor dsDNA system (Promega Corporation, Madison, USA). Genotyping of GSTT1 and GSTM1 deletions was performed by a multiplex PCR approach (adapted from (Bauer et al. 2006)G. Details on both methods are given in Supplemental materials (Methods S2).
2.8. Statistical analysis
Multivariable unconditional logistic regression was used to calculate odds ratios (ORs) and their 95% confidence interval (CI). The concentrations in tap water and exposure doses of THMs and HAAs were categorized by quartiles based on the control group exposure, and associations with SGA were determined by comparing the fourth quartile (the exposed category) with the first three quartiles of exposure (the reference category). SNP genotypes were coded as variant allele counts (0, 1 or 2) or as variant allele carrier status (yes/no) when the variant allele had a frequency of less than 5%. Both mother and child genotypes were included in the same model, as well as product terms with the indicator variable for the exposed category. Statistical interaction on the multiplicative scale was assessed by Wald tests of each product term. ORs of SGA between high and low exposure levels were then computed for child carriers, mother carriers, and mother or child non-carriers (wild type) from the regression coefficients (note that the exposure OR was the same whether the mother or the child was non carrier, due to our assumption of no interaction between mother and child genotypes). Known SGA risk factors associated in univariate analysis with SGA (with p< 0.2) were added to the regression model: maternal age, maternal education, annual household income, prepregnancy BMI, parity, history of chronic disease, preeclampsia, active smoking during the third trimester, passive smoking throughout pregnancy, coffee and alcohol consumption during pregnancy and month of selection (due to the small number of cases and matched controls per week, dates of selection within the same month were combined to insure sufficient size of all selection strata). Missing values of prepregnancy BMI were imputed to the reference category.
In addition, in order to maximize the power to replicate the statistical interaction with total THM concentration in newborns carriers of the C allele of the CYP2E1 gene rs3813867 polymorphism (Infante-Rivard 2004), we re-estimated logistic regression coefficients using the semiparametric likelihood of Chen et al. (Chen et al. 2012) assuming that exposure and child genotypes are conditionally independent given mother’s genotype. A further gain in precision was obtained by including mother-child pairs with missing child genotypes, where maternal genotype provides partial information on child genotype (Nguile-Makao and Bureau 2015). We selected prepregnancy BMI and parity as covariates with the largest confounding effect, since the semiparametric estimation procedure converges only with a limited number of covariates.
Multiplicative interaction involving previously studied polymorphisms was declared statistically significant when p for interaction was <0.05. Multiple testing was considered for polymorphisms not previously studied using Bonferroni correction, i.e. dividing the significance level by the number of SNPs investigated (n=10).
3. Results
3.1. Characteristics of participants and exposure to DBPs
As expected, characteristics of mothers of cases were different from those of controls (Table 2). Mean concentrations of DBPs were slightly higher in drinking water serving the homes of cases than those of controls but these differences are very slight (Table 3). Mean and 75 percentiles of the distribution of DBPs concentration among controls (on which are based our highest exposure category for statistical analysis) are presented in supplemental materials (Table S1).
Table 2.
Distribution of maternal characteristics of cases (n=293) and controls (n=1162)
| Cases n (%) |
Controls n (%) |
|
|---|---|---|
| Maternal age (yrs) | ||
| < 25 | 25 (9) | 95 (8) |
| 25–29 | 112 (38) | 509 (44) |
| 30–34 | 122 (42) | 416 (36) |
| ≥ 35 | 34 (12) | 142 (12) |
| Highest education level obtained (yrs) | ||
| ≤ 12 | 55 (19) | 167 (15) |
| > 12 | 237 (81) | 983 (85) |
| Annual household income ($ Can) | ||
| <35,000 | 50 (17) | 111 (10) |
| 35,000–69,999 | 110 (38) | 502 (43) |
| ≥70,000 | 133 (45) | 549 (47) |
| Prepregnancy Body mass index (kg/m2) | ||
| <19.8 | 87 (29) | 191 (17) |
| 19.8–25.9 | 152 (52) | 699 (60) |
| 26.0–29.9 | 28 (9) | 143 (12) |
| >29.9 | 23 (8) | 120 (10) |
| Missing | 5 (2) | 9 (1) |
| Parity | ||
| Nulliparous | 201 (69) | 555 (48) |
| Parous | 91 (31) | 595 (51) |
| Maternal smoking* | ||
| Never or only before the 3rd trimester | 253 (86) | 1086 (93) |
| Ever in the 3rd trimester | 40 (14) | 76 (7) |
| Passive smoking at home* | ||
| Yes | 36 (12) | 78 (7) |
| No | 256 (88) | 1072 (93) |
| Coffee consumption* | ||
| Yes | 153 (52) | 526 (46) |
| No | 139 (48) | 624 (54) |
| Alcohol consumption* | ||
| Yes | 130 (45) | 431 (37) |
| No | 162 (55) | 719 (63) |
| History of chronic disease | ||
| Yes | 29 (10) | 84 (7) |
| No | 263 (90) | 1066 (93) |
| Preeclampsia* | ||
| Yes | 25 (9) | 52 (5) |
| No | 267 (91) | 1098 (95) |
During pregnancy
Table 3.
Exposure to DBPs of cases and controls during the last trimester of pregnancy
| Cases Mean (SD) |
Controls Mean (SD) |
p | |
|---|---|---|---|
| DBPs water concentration | |||
| Trihalomethanes (THMs), μg/L | |||
| Chloroform | 43.6 (40.6) | 41.9 (40.3) | 0.26 |
| Brominated THM | 6.2 (4.4) | 6.0 (3.9) | 0.47 |
| Total THMs | 49.8 (39.9) | 47.9 (39.4) | 0.20 |
| Haloacetic acids (HAAs), μg/L | |||
| Dichloroacetic acid | 15.7 (15.1) | 15.3 (15.0) | 0.59 |
| Trichloroacetic acid | 17.8 (21.2) | 17.0 (21.1) | 0.47 |
| Total AAH (5 species)a | 36.6 (36.8) | 35.3 (36.7) | 0.47 |
| Total AAH (9 species)b | 44.8 (37.4) | 43.5 (37.1) | 0.47 |
| DBPs internal dose | |||
| Trihalomethanes (THMs) multiexposure dose, μg/day | |||
| Chloroform | 134.9 (149.8) | 132.7 (151.6) | 0.66 |
| Brominated THM | 18.2 (16.9) | 18.4 (16.5) | 0.91 |
| Total THM | 153.1 (152.5) | 151.0 (153.1) | 0.72 |
| Haloacetic acids (HAAs) internal dose, μg/day | |||
| Dichloroacetic acid | 14.5 (20.6) | 12.1 (19.2) | 0.007 |
| Trichloroacetic acid | 16.2 (26.3) | 13.2 (25.6) | 0.005 |
| Total AAHs (5 species)a | 34.0 (50.0) | 28.0 (46.7) | 0.005 |
| Total AAHs (9 species)b | 41.2 (54.1) | 34.5 (50.7) | 0.006 |
HAA(5)=sum of the main five HAA species (DCAA, TCAA, monochloracetic acid, monobromacetic acid, dibromoacetic acid)
HAA(9) =sum of all HAA species
SD : Standard deviation
3.2 Multiple statistical interactions
We present successively the results of each gene with exposure assessed first by concentration of DBPs in participant’s residence followed by results for internal doses of DBPs in participant mothers. Also, only previously studied polymorphisms and polymorphisms with a nominal interactions p-value < 0.05 in CYP2E1 are presented in the full paper (all p-values are for interaction tests). Results of the association with DBPs exposure without considering genes polymorphisms and the complete results for CYP2E1 are presented in supplemental materials (Table S2 and S3 respectively).
3.2.1 CYP2E1
3.2.1.1. Tap water concentration of THMs
The modifying effect of newborns and mothers genotypes on the association of DBPs with SGA was analyzed for 12 CYP2E1 SNPs. No statistically significant interaction was found for either rs3813867 or rs2031920 for THMs concentration. However, for both SNPs, an OR above 3 was found for total THMs exposure above the 3rd quartile: OR=3.7 (95%CI: 0.7–19.1) and 3.4 (95%CI: 0.7–16.5) in newborn carriers of the C allele (variant with cytosine) respectively compared to about 1 for the wild types (Table 4a).
Table 4a.
Association between exposure to THMs concentration (within the fourth quartile in comparison to the first three quartiles) and SGA for newborns and mothers carriers of CYP2E1 SNP genotypes of wild type or with 1 or 2 variant alleles
| CYP2E1/SNPs | Cases | Controls | CHCL3a | THMBra | TTHMa | |||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| n | n | Adjusted ORb (95% CI) | p interaction | Adjusted ORb (95% CI) | p interaction | Adjusted ORb (95% CI) | p interaction | |
|
|
||||||||
| Newborns | ||||||||
| rs3813867 | ||||||||
| Wild type | 275 | 1071 | 1.1 (0.8 – 1.6) | 0.56 | 1.1 (0.8 – 1.6) | 0.26 | 1.2 (0.8 – 1.8) | 0.36 |
| 1 or 2 var.alc | 15 | 66 | 1.8 (0.4 – 8.9) | 0.4 (0.1 – 2.5) | 3.7 (0.7 – 19.1) | |||
| rs2031920 | ||||||||
| Wild type | 267 | 1042 | 1.1 (0.8 – 1.6) | 0.59 | 1.1 (0.8 – 1.6) | 0.15 | 1.1 (0.8 – 1.7) | 0.43 |
| 1 or 2 var.al | 17 | 65 | 1.7 (0.4 – 7.8) | 0.3 (0.0 – 1.9) | 3.4 (0.7 – 16.5) | |||
| rs117618383 | ||||||||
| Wild type | 258 | 1061 | 1.0 (0.7 – 1.5) | 0.03d | 1.2 (0.8 – 1.6) | 0.05d | 1.0 (0.7 – 1.5) | 0.03d |
| 1 or 2 var.al | 27 | 77 | 4.0 (1.2 – 13.7) | 0.3 (0.1 – 1.1) | 4.6 (1.2 – 17.6) | |||
| rs2515641 | ||||||||
| Wild type | 210 | 905 | 1.2 (0.8 – 1.7) | 0.85d | 1.1 (0.7 – 1.6) | 0.05d | 1.2 (0.8 – 1.9) | 0.98d |
| 1 or 2 var.al | 71 | 231 | 1.3 (0.6 – 2.7) | 2.5 (1.1 – 5.5) | 1.7 (0.7 – 4.0) | |||
| Mothers | ||||||||
| rs3813867 | ||||||||
| Wild type | 273 | 1061 | 1.1 (0.8 – 1.6) | 0.32 | 1.1 (0.8 – 1.6) | 0.84 | 1.2 (0.8 – 1.6) | 0.23 |
| 1 or 2 var.al | 17 | 76 | 0.5 (0.1 – 2.4) | 1.3 (0.3 – 7.1) | 0.4 (0.1 – 7.1) | |||
| rs2031920 | ||||||||
| Wild type | 267 | 1034 | 1.1 (0.8 – 1.6) | 0.31 | 1.1 (0.8 – 1.6) | 0.69 | 1.1 (0.8 – 1.6) | 0.26 |
| 1 or 2 var.al | 17 | 73 | 0.5 (0.1 – 2.4) | 1.6 (0.3 – 8.8) | 0.4 (0.1 – 8.8) | |||
| rs117618383 | ||||||||
| Wild type | 262 | 1054 | 1.0 (0.7 – 1.5) | 0.95d | 1.2 (0.8 – 1.6) | 0.58d | 1.0 (0.7 – 1.6) | 0.81d |
| 1 or 2 var.al | 23 | 84 | 1.1 (0.3 – 4.0) | 1.7 (0.5 – 6.1) | 1.0 (0.2 – 6.1) | |||
| rs2515641 | ||||||||
| Wild type | 216 | 910 | 1.2 (0.8 – 1.7) | 0.93d | 1.1 (0.7 – 1.6) | 0.01d | 1.2 (0.8 – 1.6) | 0.98d |
| 1 or 2 var.al | 65 | 226 | 1.2 (0.6 – 2.6) | 0.3 (0.1 – 0.8) | 1.0 (0.4 – 0.8) | |||
CHCl3=Chloroform, THMBr= Brominated THM, TTHM= Total Trihalomethanes
OR adjusted for the following variables: maternal age, maternal education, annual household income, prepregnancy BMI, parity, history of chronic disease, preeclampsia, active smoking during the third trimester, passive smoking throughout pregnancy, coffee and alcohol consumption during pregnancy and month of selection.
1 or var. al = 1 or 2 variant alleles.
The Bonferroni corrected significance level of 0.005 applies to these interaction tests.
Only one SNP (rs117618383) gave a statistically significant positive interaction between the variant T allele in children and concentration of total THMs above the third quartile: OR=4.6 (95%CI: 1.2–17.6) for 1 or 2 alleles compared to 1.0 (95%CI: 0.7–1.5) for the wild type (pinteraction : 0.03). Inversely, a negative interaction barely below the nominal 0.05 significance level (pinteraction : 0.049) was found in presence of this allele and exposure to brominated THMs above the third quartile, but did not result in a nominally significant negative association in the allele carriers (Table 4a). A negative interaction was also present for mothers carrying one or two T alleles of the SNP rs2515641 and exposed to higher levels of brominated THM: OR=0.3 (95% CI: 0.1–0.8) compared to 1.1 (95%CI: 0.7–1.6) for the wild type (pinteraction : 0.01) (Table 4.a). However, none of these interactions remained significant after correction for multiple testing.
3.2.1.2. Tap water concentration of HAAs
The presence of the rs117618383 T allele in children also resulted in a significant interaction with the exposure to total HAAs above the third quartile: OR=5.3 for HAA5 (95%CI: 1.5–18.6) compared to 1.4 (95%CI: 1.0–1.9) for the wild type (Table 4b, pinteraction : 0.04) but it was not statistically significant after correction for multiple testing.
Table 4b.
Association between exposure to HAAs concentration (within the fourth quartile in comparison to the first three quartiles) and SGA for newborns and mothers carriers of CYP2E1 SNP genotypes of wild type or with 1 or 2 variant alleles
| CYP2E1/SNPs | Cases | Controls | DCAAa | TCAAa | HAA5a | HAA9a | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| n | n | Adjusted ORb (95% CI) | p interaction | Adjusted ORb (95% CI) | p interaction | Adjusted ORb (95% CI) | p interaction | Adjusted ORb (95% CI) | p interaction | |
|
|
||||||||||
| Newborns | ||||||||||
| rs3813867 | ||||||||||
| Wild type | 275 | 1071 | 1.3 (0.9 – 1.7) | 0.38 | 1.4 (1.0 – 1.9) | 0.14 | 1.4 (1.0 – 2.0) | 0.15 | 1.5 (1.1 – 2.0) | 0.16 |
| 1 or 2 var.alc | 15 | 66 | 2.5 (0.6 – 11.2) | 4.5 (1.0 – 20.7) | 4.5 (1.0 –20.8) | 4.5 (1.0 – 21.0) | ||||
| rs2031920 | ||||||||||
| Wild type | 267 | 1042 | 1.2 (0.9 – 1.7) | 0.30 | 1.3 (1.0 – 1.9) | 0.16 | 1.4 (1.0 – 1.9) | 0.17 | 1.4 (1.0 – 2.0) | 0.18 |
| 1 or 2 var.al | 17 | 65 | 2.7 (0.6 – 11.9) | 3.9 (0.9 – 17.2) | 3.9 (0.9 –17.3) | 3.9 (0.9 –17.4) | ||||
| rs117618383 | ||||||||||
| Wild type | 258 | 1061 | 1.1 (0.8 – 1.6) | 0.03d | 1.4 (1.0 – 1.9) | 0.10d | 1.4 (1.0 – 1.9) | 0.04d | 1.4 (1.0 – 1.9) | 0.03d |
| 1 or 2 var.al | 27 | 77 | 4.9 (1.3 – 17.9) | 3.9 (1.1 – 13.5) | 5.3 (1.5 –18.6) | 5.8 (1.6 –20.7) | ||||
| rs2515641 | ||||||||||
| Wild type | 210 | 905 | 1.2 (0.8 – 1.7) | 0.96d | 1.3 (0.9 – 1.9) | 0.63d | 1.5 (1.0 – 2.2) | 0.81d | 1.5 (1.1 – 2.2) | 0.84d |
| 1 or 2 var.al | 71 | 231 | 1.2 (0.6 – 2.6) | 1.6 (0.8 – 3.4) | 1.4 (0.7 – 2.9) | 1.4 (0.7 – 3.0) | ||||
| Mothers | ||||||||||
| rs3813867 | ||||||||||
| Wild type | 273 | 1061 | 1.3 (0.9 – 1.7) | 0.19 | 1.4 (1.0 – 1.9) | 0.06 | 1.4 (1.0 – 1.9) | 0.05 | 1.5 (1.1 – 2.0) | 0.051 |
| 1 or 2 var.al | 17 | 76 | 0.5 (0.1 – 2.0) | 0.3 (0.1 – 1.5) | 0.3 (0.1 – 1.5) | 0.3 (0.1 – 1.5) | ||||
| rs2031920 | ||||||||||
| Wild type | 267 | 1034 | 1.2 (0.9 – 1.7) | 0.15 | 1.3 (1.0 – 1.9) | 0.06 | 1.4 (1.0 – 1.9) | 0.06 | 1.4 (1.0 – 2.0) | 0.057 |
| 1 or 2 var.al | 17 | 73 | 0.4 (0.1 – 1.9) | 0.3 (0.1 – 1.4) | 0.3 (0.1 – 1.4) | 0.3 (0.1 – 1.5) | ||||
| rs117618383 | ||||||||||
| Wild type | 262 | 1054 | 1.1 (0.8 – 1.6) | 0.84d | 1.4 (1.0 – 1.9) | 0.95d | 1.4 (1.0 – 1.9) | 0.77d | 1.4 (1.0 – 1.9) | 0.70d |
| 1 or 2 var.al | 23 | 84 | 1.0 (0.2 – 3.9) | 1.3 (0.4 – 4.8) | 1.1 (0.3 – 4.8) | 1.0 (0.3 – 4.1) | ||||
| rs2515641 | ||||||||||
| Wild type | 216 | 910 | 1.2 (0.8 – 1.7) | 0.40d | 1.3 (0.9 – 1.9) | 0.48d | 1.5 (1.0 – 1.9) | 0.69d | 1.5 (1.1 – 2.2) | 0.77d |
| 1 or 2 var.al | 65 | 226 | 1.6 (0.8 – 3.5) | 1.7 (0.8 – 3.6) | 1.7 (0.8 – 3.6) | 1.7 (0.8 – 3.6) | ||||
DCAA=Dichloracetic acid, TCAA=Trichloroacetic acid, HAA(5)=sum of the main five HAA species (DCAA, TCAA, monochloracetic acid, monobromacetic acid, dibromoacetic acid), HAA(9) =sum of all HAA species.
OR adjusted for the following variables: maternal age, maternal education, annual household income, prepregnancy BMI, parity, history of chronic disease, preeclampsia, active smoking during the third trimester, passive smoking throughout pregnancy, coffee and alcohol consumption during pregnancy and month of selection.
1 or var. al = 1 or 2 variant alleles.
The Bonferroni corrected significance level of 0.005 applies to these interaction tests.
3.2.1.3. Internal dose of DBPs
When the internal doses of THMs were taken into account, no interaction was found with either rs3813867 or rs2031920 for THM concentration. The interaction found previously for rs117618383 with TTHMs exposure persists but with a p-value > 0.05 (Table 4c). However, the negative interaction found in children for the same rs117618383 SNP with exposure to brominated THMs remained just below the nominal 0.05 significance level (pInteraction : 0.042) while the negative association to brominated THMs in T allele carriers remained non significant (Table 4c). The negative interaction found with mother carriers of the rs2515641 T allele (Table 4c) was reduced compared to the result with tap water concentration. When the internal dose of HAAs was taken into account, the association with exposure to total HAAs in newborn carriers of the rs117618383 T allele was slightly weaker and non significant (OR=4.2 for HAA5 (95%CI:1.2–14.5), pinteraction : 0.08) (Table 4d) than that observed with tap water concentration. A negative interaction was found for mothers with one or two alleles of the two target SNPs rs3813867 and rs2031920, especially for HAA9: OR=0.1 (95%CI: 0.0–1.0) and 0.1 (95%CI: 0.0–0.9) respectively compare to 1.7 (95%:1.2–2.3) for wild types, pinteraction : 0.01 for both interactions (Table 4d). None of these interactions remained significant after correction for multiple testing.
Table 4c.
Association between internal dose of THMs (within the fourth quartile in comparison to the first three quartiles) and SGA for newborns and mothers carriers of CYP2E1 SNP genotypes of wild type or 1 or 2 variant alleles
| CYP2E1/SNPs | Cases | Controls | CHCL3a | THMBra | TTHMa | |||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| n | n | Adjusted ORb (95% CI) | p interaction | Adjusted ORb (95% CI) | p interaction | Adjusted ORb (95% CI) | p interaction | |
|
|
||||||||
| Newborns | ||||||||
| rs3813867 | ||||||||
| Wild type | 275 | 1071 | 1.2 (0.9 – 1.7) | 0.69 | 1.1 (0.8 – 1.6) | 0.27 | 1.2 (0.9 – 1.7) | 0.52 |
| 1 or 2 var.alc | 15 | 66 | 1.7 (0.3 – 8.7) | 0.3 (0.0 – 3.2) | 2.1 (0.4 – 11.2) | |||
| rs2031920 | ||||||||
| Wild type | 267 | 1042 | 1.1 (0.8 – 1.6) | 0.47 | 1.1 (0.8 – 1.6) | 0.19 | 1.2 (0.9 – 1.7) | 0.52 |
| 1 or 2 var.al | 17 | 65 | 2.0 (0.4 – 9.8) | 0.2 (0.0 – 2.5) | 2.1 (0.4 – 10) | |||
| rs117618383 | ||||||||
| Wild type | 258 | 1061 | 1.1 (0.8 – 1.6) | 0.09d | 1.2 (0.8 – 1.6) | 0.04d | 1.1 (0.8 – 1.5) | 0.14d |
| 1 or 2 var.al | 27 | 77 | 3.4 (0.9 – 11.9) | 0.2 (0.0 – 1.1) | 2.8 (0.8 – 9.6) | |||
| rs2515641 | ||||||||
| Wild type | 210 | 905 | 1.2 (0.8 – 1.7) | 0.29d | 1.0 (0.7 – 1.5) | 0.12d | 1.2 (0.8 – 1.7) | 0.22d |
| 1 or 2 var.al | 71 | 231 | 1.8 (0.8 – 4.0) | 1.9 (0.9 – 4.3) | 1.9 (0.9 – 4.2) | |||
| Mothers | ||||||||
| rs3813867 | ||||||||
| Wild type | 273 | 1061 | 1.2 (0.9 – 1.7) | 0.16 | 1.1 (0.8 – 1.6) | 0.53 | 1.2 (0.9 – 1.6) | 0.13 |
| 1 or 2 var.al | 17 | 76 | 0.3 (0.1 – 2.0) | 0.7 (0.1 – 3.4) | 0.3 (0.1 – 3.4) | |||
| rs2031920 | ||||||||
| Wild type | 267 | 1034 | 1.1 (0.8 – 1.6) | 0.14 | 1.1 (0.8 – 1.6) | 0.66 | 1.2 (0.9 – 1.6) | 0.13 |
| 1 or 2 var.al | 17 | 73 | 0.3 (0.0 – 1.8) | 0.8 (0.1 – 4.0) | 0.3 (0.0 – 4.0) | |||
| rs117618383 | ||||||||
| Wild type | 262 | 1054 | 1.1 (0.8 – 1.6) | 0.67d | 1.2 (0.8 – 1.6) | 0.71d | 1.1 (0.8 – 1.6) | 0.97d |
| 1 or 2 var.al | 23 | 84 | 0.8 (0.2 – 3.2) | 0.9 (0.2 – 3.6) | 1.1 (0.3 – 3.6) | |||
| rs2515641 | ||||||||
| Wild type | 216 | 910 | 1.2 (0.8 – 1.7) | 0.39d | 1.0 (0.7 – 1.5) | 0.10d | 1.2 (0.8 – 1.5) | 0.20d |
| 1 or 2 var.al | 65 | 226 | 0.8 (0.4 – 1.8) | 0.5 (0.2 – 1.2) | 0.8 (0.4 – 1.2) | |||
CHCl3=Chloroform, THMBr= Brominated THM, TTHM= Total Trihalomethanes
OR adjusted for the following variables: maternal age, maternal education, annual household income, prepregnancy BMI, parity, history of chronic disease, preeclampsia, active smoking during the third trimester, passive smoking throughout pregnancy, coffee and alcohol consumption during pregnancy and month of selection.
1 or var. al = 1 or 2 variant alleles.
The Bonferroni corrected significance level of 0.005 applies to these interaction tests.
Table 4d.
Association between internal dose of HAAs (within the fourth quartile in comparison to the first three quartiles) and SGA for newborns and mothers carriers of CYP2E1 SNP genotypes of wild type or with 1 or 2 variant alleles
| CYP2E1/SNPs | Cases | Controls | DCAAa | TCAAa | HAA5a | HAA9a | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| n | n | Adjusted ORb (95% CI) | p interaction | Adjusted OR b (95% CI) | p interaction | Adjusted OR b (95% CI) | p interaction | Adjusted OR b (95% CI) | p interaction | |
|
|
||||||||||
| Newborns | ||||||||||
| rs3813867 | ||||||||||
| Wild type | 275 | 1071 | 1.7 (1.2 – 2.3) | 0.51 | 1.7 (1.2 – 2.3) | 0.55 | 1.6 (1.2 – 2.2) | 0.45 | 1.7 (1.2 – 2.3) | 0.23 |
| 1 or 2 var.alc | 15 | 66 | 2.9 (0.6 – 14.8) | 2.9 (0.5 – 15.1) | 3.0 (0.6 – 14.9) | 4.7 (0.9 – 23.7) | ||||
| rs2031920 | ||||||||||
| Wild type | 267 | 1042 | 1.6 (1.2 – 2.2) | 0.46 | 1.6 (1.2 – 2.2) | 0.51 | 1.6 (1.1 – 2.1) | 0.44 | 1.7 (1.2 – 2.3) | 0.23 |
| 1 or 2 var.al | 17 | 65 | 2.9 (0.6 – 13.4) | 2.7 (0.6 – 13.2) | 2.9 (0.6 – 13.5) | 4.4 (0.9 – 20.8) | ||||
| rs117618383 | ||||||||||
| Wild type | 258 | 1061 | 1.5 (1.0 – 2.0) | 0.07d | 1.5 (1.1 – 2.0) | 0.12d | 1.3 (1.0 – 1.9) | 0.08d | 1.4 (1.0 – 2.0) | 0.12d |
| 1 or 2 var.al | 27 | 77 | 4.7 (1.3 – 16.8) | 4.1 (1.1 – 14.8) | 4.2 (1.2 – 14.5) | 3.8 (1.1 – 13.2) | ||||
| rs2515641 | ||||||||||
| Wild type | 210 | 905 | 1.6 (1.1 – 2.2) | 0.52d | 1.5 (1.1 – 2.2) | 0.66d | 1.4 (1.0 – 2.0) | 0.71d | 1.6 (1.1 – 2.3) | 0.60d |
| 1 or 2 var.al | 71 | 231 | 1.2 (0.6 – 2.6) | 1.8 (0.9 – 3.8) | 1.2 (0.6 – 2.6) | 1.3 (0.6 – 2.8) | ||||
| Mothers | ||||||||||
| rs3813867 | ||||||||||
| Wild type | 273 | 1061 | 1.7 (1.2 – 2.3) | 0.098 | 1.7 (1.2 – 2.3) | 0.050 | 1.6 (1.2 – 2.3) | 0.086 | 1.7 (1.2 – 2.3) | 0.011 |
| 1 or 2 var.al | 17 | 76 | 0.4 (0.1 – 2.2) | 0.3 (0.1 – 1.7) | 0.4 (0.1 – 1.7) | 0.1 (0.0 – 1.0) | ||||
| rs2031920 | ||||||||||
| Wild type | 267 | 1034 | 1.6 (1.2 – 2.2) | 0.12 | 1.6 (1.2 – 2.2) | 0.054 | 1.6 (1.1 – 2.2) | 0.089 | 1.7 (1.2 – 2.3) | 0.010 |
| 1 or 2 var.al | 17 | 73 | 0.4 (0.1 – 2.2) | 0.3 (0 – 1.6) | 0.4 (0.1 – 1.6) | 0.1 (0.0 – 0.9) | ||||
| rs117618383 | ||||||||||
| Wild type | 262 | 1054 | 1.5 (1.0 – 2.0) | 0.97d | 1.5 (1.1 – 2) | 0.67d | 1.3 (1.0 – 2.0) | 0.79d | 1.4 (1.0 – 2.0) | 0.75d |
| 1 or 2 var.al | 23 | 84 | 1.5 (0.4 – 5.6) | 2 (0.5 – 7.4) | 1.6 (0.4 – 7.4) | 1.8 (0.5 – 6.5) | ||||
| rs2515641 | ||||||||||
| Wild type | 216 | 910 | 1.6 (1.1 – 2.2) | 0.45d | 1.5 (1.1 – 2.2) | 0.76d | 1.4 (1.0 – 2.2) | 0.39d | 1.6 (1.1 – 2.3) | 0.48d |
| 1 or 2 var.al | 65 | 226 | 2.1 (1.0 – 4.3) | 1.7 (0.8 – 3.5) | 1.9 (0.9 – 3.5) | 2.1 (1.0 – 4.3) | ||||
DCAA=Dichloracetic acid, TCAA=Trichloroacetic acid, HAA(5)=sum of the main five HAA species (DCAA, TCAA, monochloracetic acid, monobromacetic acid, dibromoacetic acid), HAA(9) =sum of all HAA species.
OR adjusted for the following variables: maternal age, maternal education, annual household income, prepregnancy BMI, parity, history of chronic disease, preeclampsia, active smoking during the third trimester, passive smoking throughout pregnancy, coffee and alcohol consumption during pregnancy and month of selection.
1 or var. al = 1 or 2 variant alleles.
The Bonferroni corrected significance level of 0.005 applies to these interaction tests.
3.2.2. GST deletions
Deletion of GSTT1 and GSTM1 were analyzed. Neither deletion was associated with a modification effect of DBPs (Table 5a, 5b, 5c, 5d).
Table 5a.
Association between exposure to THMs concentration (within the fourth quartile in comparison to the first three quartiles) and SGA for newborns and mothers GSTT1 or GSTM1 deletion status
| Gene/deletion | Cases | Controls | CHCL3a | THMBra | TTHMa | |||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| n | n | Adjusted ORb (95% CI) | p interaction | Adjusted ORb (95% CI) | p interaction | Adjusted ORb (95% CI) | p interaction | |
|
|
||||||||
| Newborns | ||||||||
| GSTT1 | ||||||||
| No or single deletion | 198 | 793 | 1.1 (0.8 – 1.7) | 0.86 | 0.9 (0.6 – 1.4) | 0.54 | 1.3 (0.9 – 2.0) | 0.47 |
| Double deletion | 73 | 300 | 1.2 (0.6 – 2.4) | 1.2 (0.6 – 2.3) | 1.7 (0.9 – 3.3) | |||
| GSTM1 | ||||||||
| No or single deletion | 112 | 491 | 1.6 (0.9 – 2.7) | 0.18 | 1.1 (0.6 – 2.0) | 0.77 | 1.6 (1.0 – 2.8) | 0.31 |
| Double deletion | 159 | 602 | 1.0 (0.5 – 2.0) | 1.3 (0.7 – 2.5) | 1.1 (0.6 – 2.3) | |||
| Mothers | ||||||||
| GSTT1 | ||||||||
| No or single deletion | 59 | 228 | 1.1 (0.8 – 1.7) | 0.57 | 0.9 (0.6 – 1.4) | 0.28 | 1.3 (0.9 – 1.4) | 0.77 |
| Double deletion | 212 | 865 | 1.4 (0.7 – 3.0) | 1.4 (0.7 – 3.0) | 1.5 (0.7 – 3.0) | |||
| GSTM1 | ||||||||
| No or single deletion | 146 | 610 | 1.6 (0.9 – 2.7) | 0.91 | 1.1 (0.6 – 2.0) | 0.53 | 1.6 (1.0 – 2.0) | 0.61 |
| Double deletion | 125 | 483 | 1.7 (0.8 – 3.4) | 0.9 (0.4 – 1.9) | 2.0 (1.0 – 1.9) | |||
CHCl3=Chloroform, THMBr= Brominated THM, TTHM= Total Trihalomethanes
OR adjusted for the following variables: maternal age, maternal education, annual household income, prepregnancy BMI, parity, history of chronic disease, preeclampsia, active smoking during the third trimester, passive smoking throughout pregnancy, coffee and alcohol consumption during pregnancy and month of selection.
Table 5b.
Association between exposure to HAAs concentration (within the fourth quartile in comparison to the first three quartiles) and SGA for newborns and mothers GSTT1 or GSTM1 deletion status
| Gene/deletion | Cases | Controls | DCAAa | TCAAa | HAA5a | HAA9a | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| n | n | Adjusted ORb (95% CI) | p interaction | Adjusted ORb (95% CI) | p interaction | Adjusted ORb (95% CI) | p interaction | Adjusted ORb (95% CI) | p interaction | |
|
|
||||||||||
| Newborns | ||||||||||
| GSTT1 | ||||||||||
| No or single deletion | 198 | 793 | 1.3 (0.9 – 2.0) | 0.87 | 1.6 (1.0 – 2.3) | 0.61 | 1.6 (1.1 – 2.3) | 0.80 | 1.6 (1.1 – 2.4) | 0.61 |
| Double deletion | 73 | 300 | 1.4 (0.7 – 2.7) | 1.3 (0.7 – 2.5) | 1.4 (0.8 – 2.7) | 1.3 (0.7 – 2.6) | ||||
| GSTM1 | ||||||||||
| No or single deletion | 112 | 491 | 1.4 (0.8 – 2.3) | 0.90 | 1.5 (0.9 – 2.5) | 0.69 | 1.5 (0.9 – 2.5) | 0.57 | 1.5 (0.9 – 2.5) | 0.57 |
| Double deletion | 159 | 602 | 1.4 (0.7 – 2.8) | 1.3 (0.7 – 2.5) | 1.2 (0.6 – 2.3) | 1.2 (0.6 – 2.4) | ||||
| Mothers | ||||||||||
| GSTT1 | ||||||||||
| No or single deletion | 212 | 865 | 1.3 (0.9 – 2.0) | 0.51 | 1.6 (1.0 – 2.3) | 0.99 | 1.6 (1.1 – 2.3) | 0.63 | 1.6 (1.1 – 2.4) | 0.91 |
| Double deletion | 59 | 228 | 1.0 (0.5 – 2.2) | 1.6 (0.8 – 3.3) | 1.3 (0.6 – 3.3) | 1.6 (0.7 – 3.2) | ||||
| GSTM1 | ||||||||||
| No or single deletion | 125 | 483 | 1.4 (0.8 – 2.3) | 0.61 | 1.5 (0.9 – 2.5) | 0.70 | 1.5 (0.9 – 2.5) | 0.53 | 1.5 (0.9 – 2.5) | 0.49 |
| Double deletion | 146 | 610 | 1.2 (0.6 – 2.3) | 1.7 (0.9 – 3.4) | 1.8 (0.9 – 3.4) | 1.9 (1.0 – 3.8) | ||||
DCAA=Dichloracetic acid, TCAA=Trichloroacetic acid, HAA(5)=sum of the main five HAA species (DCAA, TCAA, monochloracetic acid, monobromacetic acid, dibromoacetic acid), HAA(9) =sum of all HAA species.
OR adjusted for the following variables: maternal age, maternal education, annual household income, prepregnancy BMI, parity, history of chronic disease, preeclampsia, active smoking during the third trimester, passive smoking throughout pregnancy, coffee and alcohol consumption during pregnancy and month of selection.
Table 5c.
Association between internal dose of THMs (within the fourth quartile in comparison to the first three quartiles) and SGA for newborns and mothers GSTT1 or GSTM1 deletion status
| Gene/deletion | Cases | Controls | CHCL3a | THMBra | TTHMa | |||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| n | n | Adjusted ORb (95% CI) | p interaction | Adjusted ORb (95% CI) | p interaction | Adjusted ORb (95% CI) | p interaction | |
|
|
||||||||
| Newborns | ||||||||
| GSTT1 | ||||||||
| No or single deletion | 198 | 793 | 1.1 (0.7 – 1.7) | 0.61 | 0.9 (0.6 – 1.4) | 0.86 | 1.1 (0.7 – 1.6) | 0.44 |
| Double deletion | 73 | 300 | 1.4 (0.7 – 2.7) | 1.0 (0.5 – 2.0) | 1.4 (0.7 – 2.7) | |||
| GSTM1 | ||||||||
| No or single deletion | 112 | 491 | 1.4 (0.8 – 2.4) | 0.96 | 1.1 (0.6 – 1.8) | 0.67 | 1.4 (0.8 – 2.4) | 0.95 |
| Double deletion | 159 | 602 | 1.4 (0.7 – 2.8) | 1.2 (0.6 – 2.4) | 1.4 (0.7 – 2.8) | |||
| Mothers | ||||||||
| GSTT1 | ||||||||
| No or single deletion | 59 | 228 | 1.1 (0.7 – 1.7) | 0.48 | 0.9 (0.6 – 1.4) | 0.65 | 1.1 (0.7 – 1.4) | 0.18 |
| Double deletion | 212 | 865 | 1.5 (0.7 – 3.2) | 1.1 (0.5 – 2.3) | 1.8 (0.9 – 2.3) | |||
| GSTM1 | ||||||||
| No or single deletion | 146 | 610 | 1.4 (0.8 – 2.4) | 0.67 | 1.1 (0.6 – 1.8) | 0.38 | 1.4 (0.8 – 1.8) | 0.64 |
| Double deletion | 125 | 483 | 1.2 (0.6 – 2.4) | 0.8 (0.4 – 1.6) | 1.2 (0.6 – 1.6) | |||
CHCl3=Chloroform, THMBr= Brominated THM, TTHM= Total Trihalomethanes
OR adjusted for the following variables: maternal age, maternal education, annual household income, prepregnancy BMI, parity, history of chronic disease, preeclampsia, active smoking during the third trimester, passive smoking throughout pregnancy, coffee and alcohol consumption during pregnancy and month of selection.
Table 5d.
Association between internal dose of HAAs (within the fourth quartile in comparison to the first three quartiles) and SGA for newborns and mothers GSTT1 or GSTM1 deletion status
| Gene/deletion | Cases | Controls | DCAAa | TCAAa | HAA5a | HAA9a | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| n | n | Adjusted ORb (95% CI) | p interaction | Adjusted ORb (95% CI) | p interaction | Adjusted ORb (95% CI) | p interaction | Adjusted ORb (95% CI) | p interaction | |
|
|
||||||||||
| Newborns | ||||||||||
| GSTT1 | ||||||||||
| No or single deletion | 198 | 793 | 1.6 (1.1 – 2.3) | 0.35 | 1.7 (1.1 – 2.5) | 0.40 | 1.7 (1.1 – 2.5) | 0.41 | 1.7 (1.2 – 2.5) | 0.95 |
| Double deletion | 73 | 300 | 2.2 (1.2 – 4.1) | 2.3 (1.2 – 4.3) | 2.2 (1.2 – 4.2) | 1.7 (0.9 – 3.3) | ||||
| GSTM1 | ||||||||||
| No or single deletion | 112 | 491 | 1.6 (1.0 – 2.6) | 0.91 | 1.9 (1.1 – 3.1) | 0.84 | 1.5 (0.9 – 2.5) | 0.88 | 1.5 (0.9 – 2.5) | 0.42 |
| Double deletion | 159 | 602 | 1.5 (0.8 – 3.0) | 1.8 (0.9 – 3.4) | 1.6 (0.8 – 3.0) | 2.0 (1.0 – 3.9) | ||||
| Mothers | ||||||||||
| GSTT1 | ||||||||||
| No or single deletion | 212 | 865 | 1.6 (1.1 – 2.3) | 0.26 | 1.7 (1.1 – 2.5) | 0.14 | 1.7 (1.1 – 2.5) | 0.061 | 1.7 (1.2 – 2.5) | 0.27 |
| Double deletion | 59 | 228 | 1.0 (0.5 – 2.1) | 0.9 (0.4 – 2.0) | 0.8 (0.4 – 2.0) | 1.1 (0.5 – 2.3) | ||||
| GSTM1 | ||||||||||
| No or single deletion | 125 | 483 | 1.6 (1.0 – 2.6) | 0.95 | 1.9 (1.1 – 3.1) | 0.50 | 1.5 (0.9 – 3.1) | 0.94 | 1.5 (0.9 – 2.5) | 0.51 |
| Double deletion | 146 | 610 | 1.6 (0.8 – 3.3) | 1.5 (0.7 – 3.0) | 1.5 (0.8 –3.0) | 1.2 (0.6 – 2.4) | ||||
DCAA=Dichloracetic acid, TCAA=Trichloroacetic acid, HAA(5)=sum of the main five HAA species (DCAA, TCAA, monochloracetic acid, monobromacetic acid, dibromoacetic acid), HAA(9) =sum of all HAA species.
OR adjusted for the following variables: maternal age, maternal education, annual household income, prepregnancy BMI, parity, history of chronic disease, preeclampsia, active smoking during the third trimester, passive smoking throughout pregnancy, coffee and alcohol consumption during pregnancy and month of selection.
3.2.3. Semiparametric likelihood analysis of total THMs and rs3813867 SNP of the CYP2E1 gene
First, we confirmed that adjusting for prepregnancy BMI and parity only produced estimates of OR of SGA for exposure to total THMs in newborns carrier of the C allele roughly similar to the estimate from the fully adjusted logistic regression on the same sample (Supplemental materials, Table S4). We then reintroduced 106 mother-child pairs with missing child genotype but otherwise complete observations, for a total sample size of 1535 (321 cases and 1214 controls) and estimated the smaller model by maximizing the semiparametric model of Chen (Chen et al. 2012). The interaction p-values in mothers were lower but not significant at the 0.05 level (Supplemental materials, Table S4).
4. Discussion
Foetal growth restriction is both an environmental and a genetic disease (Infante-Rivard 2007; Johnston et al. 2002). The study of genetic polymorphisms is expected to shed light on the causal nature of previously found environmental associations. Because of the possibility of biased results and type 1 error, replication is considered a gold rule in genetic epidemiology (Ioannidis 2013). Our study aimed to replicate the results of the two previous gene-interaction studies on foetal growth restriction and DBP exposure (Danileviciute et al. 2012; Infante-Rivard 2004). However, despite efforts to use cutting edge environmental epidemiologic methods, we were not able to replicate the interactions previously reported. Effect modifications by other gene polymorphisms were observed with a nominal p-value < 0.05, but none of these interactions remained statistically significant after correction for multiple testing.
4.1 Replication of previous studies
The effect modification found by Infante-Rivard (Infante-Rivard 2004) was very striking with an OR of 13.2 for children carriers of one or two C alleles of CYP2E1 rs3813867 and exposed in utero to total THMs ≥30 μg/L, compared to 0.82 for carrier of the wild type (p : 0.027). However results were very imprecise (95%CI of the OR: 1.2–146.7) and the author stressed the need of confirmation. Our study evaluated effect modification with an exposure to total THMs≥58 μg/L (75th percentile of distribution of THMs in controls residence), and found a non-statistically interaction: OR=3.7 (95%CI: 0.7–19.1) for children with one or two C alleles compared to 1.2 (95%CI: 0.8–1.8) for carriers of the wild type (p interaction=0.36). Moreover, the association was weaker with the internal dose of THMs. Infante-Rivard also found a non-statistically significant association with SGA for mothers carriers of one or two alleles exposed to THMs (OR=6.5; 95%CI:0.6–71.5), while we found a non statistically significant protective effect for such mothers (OR=0.4; 95%CI:0.1–7.1). However, our understanding is that Infante-Rivard analyzed the two genotypes in separate logistic regression models instead of the same logistic regression model as we did, such that the association with SGA in mothers may be confounded by the effect of the newborn genotype.
CYP2E1 gene is known to encode part of the cytochromes P-450 which play an important role in phase-1 biological activation of chemicals (Bolt et al. 2003) and particularly chloroform (Gemma et al. 2003)(Meek et al. 2002). Because of the limited statistical power of our study and possible random misclassification of exposure, we cannot exclude a possible weak effect. Danileviciute et al. (Danileviciute et al. 2012) found an OR of low birth weight of 4.4 (95%CI:1.4–14.1) in women with a GSTM1 double deletion exposed to higher internal doses of THMs (levels in drinking water was reported to be 21.9μg/L in the highest exposed area), compared to an OR of 0.34 in women with the GSTM1 gene (p <0.05). The association was reduced to 1.8 (95%CI: 0.9–3.6) when SGA was considered as the issue under study, compared to an OR of 0.86 for non deletion (p >0.05). In our study, either using the concentrations of THMs at the residence or the internal dose estimated by our models, no interactions were found for mothers with deleted GSTM1. Moreover, we confirmed the absence of interaction with the deletion of GSTT1 that was reported in the same study (Danileviciute et al. 2012).
The rationale to study of GSTM1 and GSTT1 refers to the possible interaction with phase 2 metabolism of chemicals such as brominated THMs and HAAs. As a matter of fact, the GSTT1 enzyme is responsible for increasing toxic properties of some chemicals (Nakajima and Aoyama 2000) and essential in the activation of the mutagen activity of brominated THMs (Landi et al. 1999). Moreover, GSTT1 polymorphisms were involved in the increased associations between THM and bladder cancer in Spain (Cantor et al. 2010). The levels of HAAs found in our drinking water networks were important but the levels of our brominated THMs were low (Table 3). This could have reduced our capacity to study such interaction.
4.2. Systematic assessment of genetic variation in CYP2E1
We observed a positive interaction between THM exposure and rs117618383 but it was not statistically significant after correction for multiple testing. This is the first time to our knowledge such interaction is reported with this SNP. It was also found to interact with the exposure to HAAs, this has never been reported either. A negative interaction with brominated THMs was found for newborn carriers of rs117618383 with some consistency between concentration and internal dose exposure assessment. A negative interaction with brominated THM concentration was also found for mother carriers of rs2515641 without consistency when considering internal dose. These interactions also disappeared after correction for multiple testing
Examination of the squared correlation between SNP alleles in gene CYP2E1 showed little linkage disequilibrium, with the only exception of rs3813867 and rs2031920 which are strongly correlated (see Supplemental materials Figure S1), confirming that the selection procedure selected non-redundant polymorphisms. Therefore, the possible interactions involving rs117618383 and rs2515641 represent independent signals from the previously reported interactions in CYP2E1. In the 1000 Genomes samples of European ancestry, rs117618383 tags no other SNP with a r2 > 0.8, but exhibits weaker correlation with several SNPs (www.1000genomes.org).
Of note, the biological function of the SNPs selected using the Tagger program to capture as much genetic diversity as possible is unknown, and a positive association would suggest that the SNP is in linkage disequilibrium with another, yet to be identified SNP, biologically responsible for the interaction. However, we note that rs117618383 is in the upstream regulatory area, and that rs2515641 is a synonymous coding variant. Variant rs2515641 was recently associated with kidney transplant rejection in a Korean study (Kim et al. 2014). The direct causality of this synonymous substitution remains to be shown. Most likely, it is in linkage disequilibrium with another SNP biologically responsible for the association.
4.3. Strengths and limitations of the study
Our study has several strengths compared to previous studies. We conducted a population-based study which is less prone to selection bias (Rothman et al. 2008) than a previous hospital-based study (Infante-Rivard 2004). Our exposure assessment was optimized with monthly sampling of DBPs in the drinking water systems serving the residences of participants, high quality DBPs laboratory analysis and detailed description of water consumption as well as correction for the effectiveness of home treatment devices and other handling for DBP removal. All routes of exposure were also considered for THMs which are volatiles and lipophilic and internal dose was estimated using a PBPK model taking into consideration personal anthropometric characteristics of each participant (Levallois et al. 2012). Also, it is important to consider that our higher exposure levels to THMs (nearly the double than the levels evaluated in previous studies) are normally more favourable to detect an association. We also studied specifically the exposure to DBPs during the third trimester, the most important for foetal growth, and thus the optimal window for detecting an effect of growth parameters.
Moreover, an important strength of our study is the statistical analysis of the maternal and newborn genotypes which was done in the same regression model, mutually adjusting the effects involving one for the effects involving the other, as previously recommended to avoid confounding bias (Shi et al. 2008). Our sample size was comparable to the Infante-Rivard sample and larger than the Danileviciute study which evaluated only genotyped mothers. We were also able to minimize population stratification bias, limiting our study to Caucasians living in an area of Quebec with a large majority of French-Canadian ancestry.
Despite important strengths, our study has also some limitations. Even if the global sample size was important, our study has limited statistical power. For instance, the CYP2E1 rs3813867 was found in 9% of Infante-Rivard 412 controls (Infante-Rivard 2004) but it was only found in 6% of our 1137 controls for which this SNP was successfully genotyped. Also, because of the need for recontacting 73% of our 2500 potential participants with a subsequent moderate participation rate, our sample size was reduced to nearly 60% of the original sample. Even if it is unlikely that this has led to a selection bias, it has reduced our statistical power. As a matter of fact, a post-hoc statistical power analysis found that our power to detect an interaction relative risk (RR) of 3 was only 54% for CYP2E1 rs3813867. However, GSTM1 and GSTT1 deletions were more frequent in our sample which gave a better statistical power for these genetic polymorphisms (to detect an interaction RR of 3 our statistical power was 80% for GSTM1). Also, we studied only the effect of last trimester exposure and did not consider the exposure occurring in the first trimester of pregnancy. Since this exposure might differ from the last trimester, we were not able to evaluate the possible interaction of these polymorphism and deletions with THMs and HAAs on the early stage of development.
5. Conclusion
With an improved methodology and quite a large sample size, we did not replicate the previously reported positive interactions found with CYP2E1 rs3813867 and GSTT1 deletion with THM exposure, suggesting that any effect of these polymorphisms in our population must be small. However, we report a new positive gene-environment interaction with CYP2E1 rs117618383 and exposure to THMs and HAAs, on the occurrence of SGA, which needs to be replicated. Other reported negative interactions might also to be explored further.
Supplementary Material
Acknowledgments
This study was funded by the Canadian Institutes for Health Research (MOP 102708). We would like to thank Sylvie Marcoux, co-investigator of the initial study, Marion Bustamante for help in choosing the selected SNPs, Catherine Gonthier, coordinator of the data collection, Christelle Legay for help in drinking water sampling and DBPs analysis, Nathalie Bernard for extracting DNA and performing deletion analysis, Sylvie Desjardins of the genetic platform of CHU de Quebec, and research technicians and nurses who worked for the recruitment of participants and retrieval of data from the medical records.
Footnotes
Competing financial interests
The authors declare they have no financial conflict of interest.
Contributor Information
Patrick Levallois, Département de santé environnementale et toxicologie, Institut national de santé publique and Axe Santé des populations et pratiques optimales en santé, Centre de recherche du CHU de Québec, Québec (Québec), Canada G1V 7B3.
Yves Giguère, Département de biologie moléculaire, de biochimie médicale et de pathologie, Faculté de médecine, Université Laval and Axe Reproduction, santé de la mère et de l’enfant, Centre de recherche du CHU de Québec, Québec (Québec), Canada G1L 3L5.
Molière Nguile-Makao, Centre de Recherche Clinique et Évaluative en Oncologie. Hôtel-Dieu de Québec, CHU de Québec-Université Laval (Québec), Canada G1R 3S1.
Manuel Rodriguez, École supérieure d’aménagement du territoire and Chaire d’eau potable, Université Laval, Québec (Québec), Canada G1V 0A6.
Céline Campagna, Direction de la santé environnementale et toxicologie, Institut national de santé publique du Québec, and Département de médecine sociale et préventive, Faculté de médecine, Université Laval, Québec (Québec), Canada G1V 5B3.
Robert Tardif, Département de santé environnementale et santé au travail, École de santé publique, Université de Montréal, Montréal (Québec), Canada H3T 1J4.
Alexandre Bureau, Département de médecine sociale et préventive, Faculté de médecine, Université Laval et Axe de recherche neurosciences cliniques et cognitives, Institut universitaire en santé mentale de Québec du Centre intégré de santé et de services sociaux de la Capitale-Nationale, Québec (Québec), Canada G1V 0A6.
References
- 1000 Genomes Project. 1000 Genomes Deep Cat. [accessed 31 August 2015];Hum Genet Var. Available: http://www.1000genomes.org/data.
- Abraham JE, Maranian MJ, Spiteri I, Russell R, Ingle S, Luccarini C, et al. Saliva samples are a viable alternative to blood samples as a source of DNA for high throughput genotyping. BMC Med Genomics. 2012;5:19. doi: 10.1186/1755-8794-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amy G, editor. International Programme on Chemical Safety, editor. Disinfectants and disinfectant byproducts. World Health Organization; Geneva: 2000. [Google Scholar]
- Bauer M, Herbarth O, Aust G, Hengstler JG, Dotzauer A, Graebsch C, et al. Expression patterns and novel splicing variants of glutathione-S-transferase isoenzymes of human lung and hepatocyte cell lines. Cell Tissue Res. 2006;324:423–432. doi: 10.1007/s00441-005-0150-8. [DOI] [PubMed] [Google Scholar]
- Bolt HM, Roos PH, Thier R. The cytochrome P-450 isoenzyme CYP2E1 in the biological processing of industrial chemicals: consequences for occupational and environmental medicine. Int Arch Occup Environ Health. 2003;76:174–185. doi: 10.1007/s00420-002-0407-4. [DOI] [PubMed] [Google Scholar]
- Bolt HM, Thier R. Relevance of the deletion polymorphisms of the glutathione S-transferases GSTT1 and GSTM1 in pharmacology and toxicology. Curr Drug Metab. 2006;7:613–628. doi: 10.2174/138920006778017786. [DOI] [PubMed] [Google Scholar]
- Cantor KP, Villanueva CM, Silverman DT, Figueroa JD, Real FX, Garcia-Closas M, et al. Polymorphisms in GSTT1, GSTZ1, and CYP2E1, disinfection by-products, and risk of bladder cancer in Spain. Environ Health Perspect. 2010;118:1545–1550. doi: 10.1289/ehp.1002206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Lin D, Hochner H. Semiparametric maximum likelihood methods for analyzing genetic and environmental effects with case-control mother-child pair data. Biometrics. 2012;68:869–877. doi: 10.1111/j.1541-0420.2011.01728.x. [DOI] [PubMed] [Google Scholar]
- Danileviciute A, Grazuleviciene R, Vencloviene J, Paulauskas A, Nieuwenhuijsen MJ. Exposure to drinking water trihalomethanes and their association with low birth weight and small for gestational age in genetically susceptible women. Int J Environ Res Public Health. 2012;9:4470–4485. doi: 10.3390/ijerph9124470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forest J-C, Thériault S, Massé J, Bujold E, Giguère Y. Soluble Fms-like tyrosine kinase-1 to placental growth factor ratio in mid-pregnancy as a predictor of preterm preeclampsia in asymptomatic pregnant women. Clin Chem Lab Med CCLM FESCC. 2014;52:1169–1178. doi: 10.1515/cclm-2013-0955. [DOI] [PubMed] [Google Scholar]
- Gemma S, Vittozzi L, Testai E. Metabolism of chloroform in the human liver and identification of the competent P450s. Drug Metab Dispos Biol Fate Chem. 2003;31:266–274. doi: 10.1124/dmd.31.3.266. [DOI] [PubMed] [Google Scholar]
- Giguère Y, Massé J, Thériault S, Bujold E, Lafond J, Rousseau F, et al. Screening for pre-eclampsia early in pregnancy: performance of a multivariable model combining clinical characteristics and biochemical markers. BJOG Int J Obstet Gynaecol. 2015;122:402–410. doi: 10.1111/1471-0528.13050. [DOI] [PubMed] [Google Scholar]
- Grellier J, Bennett J, Patelarou E, Smith RB, Toledano MB, Rushton L, et al. Exposure to disinfection by-products, fetal growth, and prematurity: a systematic review and meta-analysis. Epidemiol Camb Mass. 2010;21:300–313. doi: 10.1097/EDE.0b013e3181d61ffd. [DOI] [PubMed] [Google Scholar]
- Hayes JD, Strange RC. Glutathione S-transferase polymorphisms and their biological consequences. Pharmacology. 2000;61:154–166. doi: 10.1159/000028396. 28396. [DOI] [PubMed] [Google Scholar]
- Infante-Rivard C. Drinking water contaminants, gene polymorphisms, and fetal growth. Environ Health Perspect. 2004;112:1213–1216. doi: 10.1289/ehp.7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante-Rivard C. Studying genetic predisposition among small-for-gestational-age newborns. Semin Perinatol. 2007;31:213–218. doi: 10.1053/j.semperi.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Ioannidis JPA. This I believe in genetics: discovery can be a nuisance, replication is science, implementation matters. Front Genet. 2013;4:33. doi: 10.3389/fgene.2013.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LB, Arends N, Dahlgren J, Leger J, Czernichow P, Albertsson-Wikland K, et al. Gene association studies in small for gestational age infants. J Pediatr Endocrinol Metab JPEM. 2002;15(Suppl 5):1459. [PubMed] [Google Scholar]
- Kim SK, Park HJ, Seok H, Jeon HS, Lee TW, Lee SH, et al. Association studies of cytochrome P450, family 2, subfamily E, polypeptide 1 (CYP2E1) gene polymorphisms with acute rejection in kidney transplantation recipients. Clin Transplant. 2014;28:707–712. doi: 10.1111/ctr.12369. [DOI] [PubMed] [Google Scholar]
- Kramer MS, Platt RW, Wen SW, Joseph KS, Allen A, Abrahamowicz M, et al. A new and improved population-based Canadian reference for birth weight for gestational age. Pediatrics. 2001;108:E35. doi: 10.1542/peds.108.2.e35. [DOI] [PubMed] [Google Scholar]
- Landi S, Hanley NM, Warren SH, Pegram RA, DeMarini DM. Induction of genetic damage in human lymphocytes and mutations in Salmonella by trihalomethanes: role of red blood cells and GSTT1-1 polymorphism. Mutagenesis. 1999;14:479–482. doi: 10.1093/mutage/14.5.479. [DOI] [PubMed] [Google Scholar]
- Levallois P, Gingras S, Marcoux S, Legay C, Catto C, Rodriguez M, et al. Maternal exposure to drinking-water chlorination by-products and small-for-gestational-age neonates. Epidemiol Camb Mass. 2012;23:267–276. doi: 10.1097/EDE.0b013e3182468569. [DOI] [PubMed] [Google Scholar]
- Meek ME, Beauchamp R, Long G, Moir D, Turner L, Walker M. Chloroform: exposure estimation, hazard characterization, and exposure-response analysis. J Toxicol Environ Health B Crit Rev. 2002;5:283–334. doi: 10.1080/10937400290070080. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Aoyama T. Polymorphism of drug-metabolizing enzymes in relation to individual susceptibility to industrial chemicals. Ind Health. 2000;38:143–152. doi: 10.2486/indhealth.38.143. [DOI] [PubMed] [Google Scholar]
- Nguile-Makao M, Bureau A. Semi-Parametric Maximum Likelihood Method for Interaction in Case-Mother Designs: Package SPmlficmcm. J Stat Sofware. 2015;60:1–17. [Google Scholar]
- Pallotto EK, Kilbride HW. Perinatal outcome and later implications of intrauterine growth restriction. Clin Obstet Gynecol. 2006;49:257–269. doi: 10.1097/00003081-200606000-00008. [DOI] [PubMed] [Google Scholar]
- Richardson SD, Plewa MJ, Wagner ED, Schoeny R, Demarini DM. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: a review and roadmap for research. Mutat Res. 2007;636:178–242. doi: 10.1016/j.mrrev.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Rothman KJ, Greenland S, Lash TL, editors. Wolters Kluwer, editor. Modern epidemiology. Vol. 3. Lippincott Williams & Wilkins; Philadelphia, Pa: 2008. [rev. and updated] ed. [Google Scholar]
- Shi M, Umbach DM, Vermeulen SH, Weinberg CR. Making the most of case-mother/control-mother studies. Am J Epidemiol. 2008;168:541–547. doi: 10.1093/aje/kwn149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varvarigou AA. Intrauterine growth restriction as a potential risk factor for disease onset in adulthood. J Pediatr Endocrinol Metab JPEM. 2010;23:215–224. doi: 10.1515/jpem.2010.23.3.215. [DOI] [PubMed] [Google Scholar]
- Villanueva CM, Cordier S, Font-Ribera L, Salas LA, Levallois P. Overview of Disinfection Byproducts and Associated Health Effects. Curr Environ Health Rep. 2015;2:107–115. doi: 10.1007/s40572-014-0032-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.