Abstract
The prevalence of obesity in the world is endemic with one rapidly growing health concern being maternal obesity. Obesity during pregnancy increases the risk of gestational diabetes, miscarriage, and preeclampsia, while rendering offspring susceptible to developmental anomalies and long-term metabolic complications including type 2 diabetes and cardiovascular disease. Several studies in humans and rodents demonstrate a correlation between the risks of maternal overnutrition and factors such as epigenetics, mitochondrial dysfunction, insulin resistance, ER stress, and immune system disruption. At present, the molecular mechanisms connecting these factors to maternal obesity are unknown. This review focuses on the use of Drosophila melanogaster to study human metabolic diseases, including obesity, and its emerging use to elucidate the mechanisms of maternal overnutrition and the impact on offspring.
Keywords: Obesity, drosophila, diabetes, mitochondria, pregnancy, nutritional programming
1. Introduction — Prevalence and clinical impact of maternal obesity
The prevalence of obesity among adults has become a global epidemic; in 2014 over 1.9 billion adults were overweight, with 600 million classified as obese (Organization, 2015). This trend is also impacting the young; among children under the age of 5, 42 million were classified as obese or overweight (Organization, 2015). Individuals are identified as overweight or obese based on their body mass index (BMI), with a BMI ≥ 25 denoting an overweight individual, and a BMI ≥ 30 considered obese. While the impact of obesity on human health has been widely studied, one rapidly growing health concern is the prevalence of obesity in pregnant women.
Between 25 to 30% of pregnant women in the United States are obese and are at greater risk for developing major health complications not only for themselves but also for their offspring (Vahratian, 2009; Yogev and Catalano, 2009)(Yogev and Catalano, 2009). In early pregnancy, maternal obesity increases the risk of single and recurrent miscarriages as well as fetal congenital anomalies that include neural tube defects, cardiovascular disorders, and orofacial clefts (Dokras et al., 2006; Metwally et al., 2008; Stothard et al., 2009). Later in pregnancy, obese mothers exhibit a higher incidence of preeclampsia, gestational diabetes mellitus, hypertension, preterm delivery, and have twice the risk for stillbirth compared to normal weight mothers (Leddy et al., 2008; O’Brien et al., 2003). Rates of caesarian delivery also double in obese mothers and the risk of operative morbidity increases (Leddy et al., 2008; Nohr et al., 2009). The offspring of obese females are at increased risk for fetal macrosomia, shoulder dystocia, and increased birth weight (Leddy et al., 2008). There is also evidence suggesting maternal overnutrition increases the offspring’s risk of developing type 2 diabetes, obesity, cardiovascular disease, and asthma in later childhood (Patrick M Catalano et al., 2009; Patrick M. Catalano et al., 2009; Clausen et al., 2009; Diesel et al., 2015; Forno et al., 2014; Lowe et al., 2011; Nohr et al., 2009). For the mother, maternal obesity also correlates with an increase in cardiovascular disease and premature death later in life (Lee et al., 2015).
In an effort to mitigate these health risks, physicians have amended prenatal testing practices in obese females to allow for early detection of complications (Leddy et al., 2008). At present the molecular mechanisms by which maternal obesity imposes these detrimental effects on mother and offspring are not known.
2. Impact of Maternal Nutrition on Offspring
The concept that maternal diet impacts offspring health was introduced by David Barker in 1990, and proposed that the manifestation of adult cardiovascular disease was a result of intrauterine and neonatal environments; a novel concept since later childhood influences such as diet, housing conditions and familial income were originally attributed to adult disease (Barker, 1990). This concept later gave rise to Hales and Barker’s “thrifty phenotype” hypothesis, which suggested that maternal under-nutrition forces an adaptive fetal response that impairs development of target organs (i.e., decreased pancreatic beta-cell mass and islet function) leading to disadvantaged fetal growth and permanently altered metabolism (Hales and Barker, 1992). Hales and Barker also suggested a contribution by infant malnutrition in their hypothesis. Poor fetal and infant nutrition would be advantageous in offspring who remained malnourished into adulthood; however, because these offspring are metabolically pre-adapted to famine-like conditions, overnutrition renders them susceptible to type 2 diabetes and insulin resistance (Hales and Barker, 2001).
While these studies focused on undernourished fetal environments, later work demonstrated a similar pre-disposition to metabolic disruptions in fetuses exposed to over-nourished environments, and proposes that maternal obesity impacts offspring metabolism well into adulthood. Maternal overnutrition is positively associated with increased offspring BMI and an increased occurrence of insulin resistance from birth through adulthood (Hochner et al., 2012; Reynolds et al., 2010). Children and adult offspring of obese mothers showed a higher incidence of hypertension, increased serum glucose and triglycerides, and decreased levels of HDL cholesterol (Clausen et al., 2009; Godfrey et al., 1994; Hochner et al., 2012; Laor et al., 1997). While the fetal impacts from maternal obesity are clearly apparent, the contributions of socioeconomic status, diet, genetics, and physical activity during childhood and adulthood in offspring of obese mothers may also contribute to these negative outcomes on offspring health (Davey Smith et al., 2009). However, studies in siblings from obese mothers suggested that the intrauterine environment does contribute to offspring metabolic health (Clausen et al., 2009; Kral et al., 2006).
There is also evidence demonstrating the transgenerational impact of maternal obesity, albeit from animal models. F2 and F3 offspring of obese female mice exhibit increased body weight, and in swine the F2 generation shows increased adiposity and altered lipid profiles (Dunn and Bale, 2011, 2009; Gonzalez-Bulnes et al., 2014). However, in the mouse model, insulin sensitivity is reduced in the F2 generation, but is normalized in the F3 offspring (Dunn and Bale, 2011, 2009).
Given the endemic increase in maternal obesity in Western society, several studies have focused on using animal models to define the modes by which a calorically excessive maternal diet impacts offspring development and health. Key factors that are thought to contribute to this phenomenon include epigenetics, mitochondrial dysfunction, insulin resistance, endoplasmic reticulum (ER) stress, and immune system disruption.
Epigenetics
Epigenetic control of gene expression is required for normal cellular functions and accounts for differences in gene expression between cell types. Epigenetic gene silencing can occur by the methylation of cytosine in CpG sites, histone modifications (either via acetylation or methylation) that can repress or activate gene expression, and RNA silencing that can promote the formation of heterochromatin, histone modifications, or DNA methylation (Egger et al., 2004).
In addition to regulating basic cellular functions, epigenetics also has a role in modifying gene expression in response to environmental factors such as diet. With regards to obesity, human studies have demonstrated a correlation between increased maternal BMI and offspring DNA methylation in neonates (cord blood) and in later childhood (Herbstman et al., 2013; Sharp et al., 2015). Interestingly, sites of DNA methylation in neonates from overnourished mothers were different from the modified sites of neonates from undernourished mothers, suggesting that differential gene regulation is dependent on specificities of the maternal environment (Sharp et al., 2015). A similar trend was also reported in rodent models of maternal obesity demonstrating an impact on methylation and expression of genes that regulate behavior (Vucetic et al., 2010), this is of particular interest because maternal obesity has been implicated in the occurrence of behavioral disorders (Buss et al., 2012).
Mitochondrial dysfunction
Mitochondria utilize oxidative phosphorylation to generate the majority of ATP in the cell, making them the main center of cellular energy metabolism. Unlike other organelles, mitochondria contain a functional genome (mtDNA) that encodes for a portion of the genes required for energy production. Because mitochondria are maternally inherited, maternal mutations in mtDNA or disruptions in mitochondrial function are passed along to the offspring. This characteristic of mitochondria makes it a prime vessel by which obese mothers may predispose their offspring to metabolic dysfunction.
Recent reports have demonstrated that female overnutrition contributes to mitochondrial disruption in mother and offspring (Szendroedi et al., 2012). Oocytes of obese females exhibited numerous mitochondrial defects including abnormal mitochondrial distribution, altered mitochondrial membrane potential, and increased swelling and vacuole number relative to lean controls (Igosheva et al., 2010; Luzzo et al., 2012; Wu et al., 2015; Wu and Brown, 2006). In rodent offspring of obese females, levels of hepatic electron transport chain complexes (ETC; I, II, III, and IV) and SIRT3 and peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α) mRNA, key regulators of fatty acid oxidation and mitochondrial biogenesis, are reduced (Borengasser et al., 2014; Bruce et al., 2009; Kong et al., 2010; Taylor et al., 2005). There are conflicting reports as to whether mtDNA levels are altered in oocytes from obese females and the exact impact of maternal obesity on mitochondrial membrane potential; however, these contrasting reports may be attributed to strain and diet differences (Igosheva et al., 2010; Luzzo et al., 2012; Wu et al., 2010). Though some data is conflicting, there is the consensus that maternal obesity negatively impacts oocyte mitochondria.
Studies using human umbilical cord blood have correlated maternal nutrition with offspring mitochondrial dysfunction. Cord blood of newborns from obese mothers exhibited significantly decreased levels of mtDNA content compared to offspring from healthy mothers; however, mtDNA content of offspring from undernourished mothers was also decreased, suggesting maternal malnutrition in general impacts mitochondrial function (Gemma et al., 2006). A subsequent study demonstrated a positive correlation between increasing maternal BMI and methylation of the offspring PGC-1α promoter, suggestive of an epigenetic mechanism (Gemma et al., 2009). There have also been reports demonstrating increased oxidative stress in offspring cord blood from obese mothers (Gallardo et al., 2015; Malti et al., 2014). Although oxidative stress is not a direct measure of mitochondrial function, because mitochondria are a major source of cellular reactive oxygen species generation, it is suggestive of an impact on the organelle. While many animal studies highlight an association between maternal obesity and offspring mitochondrial dysfunction, more research is needed to strengthen this correlation in humans.
Insulin resistance
Insulin serves as a major regulator of lipid and glucose homeostasis, controlling several downstream signaling pathways that allow cells to respond to changes in nutrient availability (Cheng et al., 2010; Saltiel and Kahn, 2001). Failure of cells to respond to insulin is a key feature of obesity and type 2 diabetes, resulting in hyperlipidemia, hyperglycemia, and hyperinsulinemia (Saltiel and Kahn, 2001).
Both rodent and human studies have suggested a link between maternal obesity and offspring insulin resistance. Human offspring of obese mothers are insulin resistant in utero and as adults (Patrick M. Catalano et al., 2009; Mingrone et al., 2008; Tan et al., 2015). In rodents, adult offspring of obese females exhibit increased adiposity and elevated fasting plasma insulin levels and develop β-cell exhaustion and hyperglycemia (Fernandez-Twinn et al., 2014; Samuelsson et al., 2008; Srinivasan et al., 2006). Exactly how maternal overnutrition contributes to offspring insulin resistance is not known. Interestingly, in obese rodents and humans, there is a strong correlation between insulin resistance and the occurrence of mitochondrial dysfunction; however, whether insulin resistance leads to mitochondrial disruption or vice versa remains up for debate (Cheng et al., 2009; Goo et al., 2012; Kelley et al., 2002; Morino et al., 2005; Petersen et al., 2004; Sleigh et al., 2011; Stump et al., 2003).
ER stress
ER stress is a key molecular characteristic of obesity and treatment with ER chaperones not only alleviates hepatic ER stress in rodents, but also restores systemic insulin sensitivity and glucose homeostasis (Ozcan et al., 2006, 2004). Interestingly, ER stress is also present in the oocytes and offspring of obese female rodents (Melo et al., 2014; Wu et al., 2015). Pharmacological inhibition of ER stress with either salubrinal or BGP-15, an inducer of sarcoplasmic reticulum Ca2+ ATPase activity, improved oocyte function, resulting in an increased rate of ovulation and improved oocyte viability and blastocyst development, oocyte mitochondrial membrane potential and mtDNA copy number were also normalized (Wu et al., 2015). Additionally, alleviating ER stress also normalized mtDNA copy number in the offspring kidney and liver, highlighting a potential link between ER stress and offspring mitochondrial dysfunction (Wu et al., 2015).
Immune system disruption
Inflammation is a characteristic of obesity and is implicated in obesity-related insulin resistance and other metabolic dysfunctions (Gregor and Hotamisligil, 2011). Maternal obesity causes increased circulating cytokines and chemokines in the mother and is associated with disruptions to the offspring immune system (Challier et al., 2008; Frias et al., 2011; Schmatz et al., 2010). Pregnant obese humans exhibit placental macrophage accumulation and increased levels of TNFα, IL-1, and IL-6 (Challier et al., 2008; Frias et al., 2011; Schmatz et al., 2010). The infant cord blood from obese mothers also has increased cytokines and chemokines as well as reduced immune cell populations (Wilson et al., 2015). Children of overnourished mothers exhibit increased levels of circulating pro-inflammatory factor C-reactive protein and have a higher risk of developing asthma (Forno et al., 2014; Leibowitz et al., 2012). Murine models of maternal obesity report offspring with increased susceptibility to and decreased survival from bacterial infections such as E. coli and Staphylococcus aureus (Myles et al., 2013).
3. Paternal obesity and offspring health
The impact of paternal traits such as diet, age, and smoking to offspring health has been demonstrated in several human studies (El-Helaly et al., 2011; Veenendaal et al., 2013). An appreciation for the contribution of paternal overnutrition to offspring health is emerging. A relationship between male BMI and sperm impairment has been shown, specifically higher BMIs correlated with increased sperm DNA damage and reduced mitochondrial activity (Fariello et al., 2012). Studies investigating the contribution of paternal obesity to offspring health demonstrated a correlation between increased paternal BMI and increased offspring weight (Chen et al., 2012; Danielzik et al., 2002; Patel et al., 2011). There is also evidence of an association between paternal obesity and methylation of metabolic genes in cord blood, suggestive of an epigenetic mechanism (Soubry et al., 2013). In rodents, several models of paternal obesity demonstrated a negative impact on offspring metabolic gene expression in adipose tissue and the pancreas, insulin disrupted embryonic development, mitochondrial activity, and reproductive function (Binder et al., 2012; Ng et al., 2014).
4. Drosophila as a model system for obesity
Much work has been achieved using rodent models to understand maternal obesity and its impact on offspring health; however, the fact remains that many studies, while informative, remain correlative and have not yet identified the molecular pathways at play. Recently, Drosophila melanogaster has emerged as a powerful model organism to identify and characterize the molecular mechanisms involved in obesity and other human metabolic disorders (Baker and Thummel, 2007; Birse et al., 2010; Fernández-Moreno et al., 2007; Musselman et al., 2011; Na et al., 2013; Sanchez-Martinez et al., 2006).
Many mammalian tissues important in lipid and carbohydrate metabolism can also be found in the fly (Liu and Huang, 2013; Akhila Rajan and Perrimon, 2013). The Drosophila midgut is the site of dietary nutrient absorption and digestion, mirroring functions of the mammalian stomach and small intestine. Carbohydrate and fat storage and lipid mobilization occur in the fat body – making this tissue analogous to mammalian liver and white adipose tissue. Secretion of adipokinetic hormone from the fly corpora cardiaca cells in the ring gland is equivalent to the secretion of glucagon from pancreatic α-cells (Liu and Huang, 2013; A Rajan and Perrimon, 2013). Production and secretion of insulin is also present in the fly; however, because Drosophila lack a pancreas insulin-like-peptides (dILPs 1–8) are secreted from insulin producing cells (IPCs) in the median neurosecretory region of the brain and from the fat body (Liu and Huang, 2013; A Rajan and Perrimon, 2013).
The presence of these analogous tissues in the fly underscores the fact that mammalian carbohydrate and lipid metabolic systems are highly conserved in Drosophila. Insulin signaling in the fly is stimulated during nutrient abundance by the leptin homolog Unpaired 2 (Upd2) and ablation of IPCs or Upd2 results in increased circulating sugars (Rajan and Perrimon, 2012; Rulifson et al., 2002). dILPs bind and activate the insulin receptor causing downstream activation of the PI3K-AKT/PKB pathway as in the mammalian system (Garofalo, 2002). The fat body stores triacylglycerides (TAG) in lipid droplets and serves as the site of de novo TAG biosynthesis utilizing enzymes homologous to mammalian glycerol-3-phosphate acyltransferase, 1-acylglycerol-3-phosphate-O-acyltransferase, phosphatidate phosphatase, and diacylglycerol acyltransferase (Liu and Huang, 2013). Lipid metabolism in the fly is also controlled by the highly conserved sterol regulatory element-binding protein (SREBP) pathway (Dobrosotskaya et al., 2002; Seegmiller et al., 2002). However, while mammalian SREBP responds to changes in cellular sterol levels, in the cholesterol auxotrophic fly, SREBP activity is instead regulated by phosphatidylethanolamine (Dobrosotskaya et al., 2002). The high conservation of both metabolic tissue and biosynthetic and signaling pathways in the fly, make Drosophila an ideal organism for modeling human metabolic diseases.
Drosophila offer well-developed genetics that allow for rapid whole-body, tissue-specific, and developmental stage-specific gene manipulation. Massive transgenic libraries exist for the fly including over 26,000 RNAi lines targeting 91% of the Drosophila protein-coding genome (Center, 2015; Dietzl et al., 2007). Overexpression lines covering a vast portion of the genome are also available, allowing for the expression of a plethora of various versions of a single gene. Additionally, generating new mutant fly lines with specific gene mutations is straightforward and rapid and can employ the use of transposons and CRISPR (Bassett et al., 2013). One prime example of employing the sophistication of fly genetics to better understand mammalian metabolism is a Drosophila RNAi obesity screen in which 11,594 transgenic RNAi lines targeting 10,489 open reading frames were used to identify 500 candidate genes of triglyceride regulation (Pospisilik et al., 2010). Interestingly, this approach uncovered a novel function for hedgehog signaling in fly fat body that extended to white and brown adipose determination in mammals (Pospisilik et al., 2010). Because many components of maternal obesity (i.e., ER stress, insulin signaling, immunity, and mitochondrial dysfunction) are intricately linked, being able to genetically manipulate one factor to address the impact on another in a disease setting, which the fly system affords, is invaluable in determining how each component contributes to a specific disorder (Gregor and Hotamisligil, 2011; Hotamisligil, 2010; Ozcan et al., 2004; Wu et al., 2015).
Pre-pregnancy obesity is associated with offspring cellular and organismal disruption; however, because mammalian embryogenesis occurs within the mother, determining the impact of pre-gestational versus gestational maternal obesity on offspring health would be highly invasive in humans, requiring in vitro fertilization (IVF). In mice, IVF has been used to specifically assess pre-gestational influences of maternal obesity, and has shown that embryos from obese mothers transplanted into non-obese females still exhibited increased fetal weight, decreased hepatic, cardiac, and renal mtDNA content, and increased hepatic mtDNA variants compared to embryos transplanted from lean littermates (Wu et al., 2015). Because fly embryogenesis occurs outside of the female physiologic milieu, the contribution of pre-gestational maternal obesity to offspring health can be specifically investigated without the use of invasive and time-consuming procedures. Additionally, the brief life cycle of the fly, 10 days from embryo to adult, is extremely beneficial for elucidating the mechanisms behind the transgenerational impact of maternal obesity since it allows studies on F3 and subsequent generations to be conducted in a short period of time.
The ease and speed of dietary manipulation in the fly also make it an ideal model for obesity research, primarily because fly diets can be produced within the lab. Concentrations of various fats and carbohydrates, as well as other dietary components, can be easily adjusted or omitted in fly diets and several methods exist to quantify the ingestion of food by the fly (Deshpande et al., 2014). Moreover, addition of specific compounds or pharmaceuticals can also be added to assess their therapeutic potential (Agrawal et al., 2005; Kang et al., 2002). With regards to immunity, measured amounts of bacteria can also be incorporated into diets to elicit an immune response (Neyen et al., 2014).
Obesity can be induced in the fly by either high-fat-diet (HFD) or high-sucrose-diet (HSD) feeding and mimics the pathophysiology of obesity observed in humans and rodents – insulin resistance, hyperglycemia, lipid accumulation, and increased expression of lipogenic and gluconeogenic genes (Birse et al., 2010; Musselman et al., 2011; Na et al., 2013; Pasco and Leopold, 2012). HSD feeding in larvae is known to cause increased hyperglycemia, systemic insulin resistance, developmental delays, and increased dILP2 secretion (Musselman et al., 2011). HFD feeding disrupted Drosophila cardiac function, resulting in cardiac lipid accumulation, reduced contractility, and structural damage, as has been reported in human and rodent obesity studies (Birse et al., 2010). Using the HFD model, it was discovered that disruption of the insulin-TOR pathway inhibits obesity-induced cardiac dysfunction (Birse et al., 2010). HSD feeding also disrupted cardiac function and was shown to be dependent on the hexosamine biosynthetic pathway (Na et al., 2013). New roles for other metabolic regulators such as SREBP, PGRC-1, and Retinol-Binding Protein 4 have also been identified using the Drosophila obesity model (Diop et al., 2015; Pasco and Leopold, 2012). These and other studies highlight the benefits of using Drosophila to identify novel regulators of obesity that have the potential to serve as therapeutic targets in humans.
5. Contributions of paternal obesity to offspring health in the fly
Given the conservation of metabolic pathways and ease of dietary manipulation, the fly has emerged as a valuable tool to uncover the molecular mechanisms whereby paternal diet influences offspring health (Ost et al., 2014; Valtonen et al., 2012). Male flies exposed to a protein-poor diet from the embryonic state to adulthood produced offspring with shortened developmental times and larger male offspring compared to males raised on standard diet (Valtonen et al., 2012).
Valtonen et al. also went on to investigate the impact of exposing both parents to a protein-poor diet and demonstrated that offspring of these flies exhibited extreme developmental delays relative to offspring from control parents and offspring with only one parent on a protein-poor diet (Valtonen et al., 2012).
On the other spectrum, offspring from parents exposed to a high-protein, albeit low-sucrose, diet from the first instar larval stage to adulthood had a shorter developmental time compared to offspring from low-protein, high-sucrose parents; however, there was no difference in survival between the two groups of offspring (Matzkin et al., 2013). The high-protein parental diet also produced female offspring with increased egg production. These studies analyzed parental diet and, thus the contribution of altering protein and sucrose levels solely of the paternal diet was not investigated. However, in light of findings by others who demonstrated that manipulation of the paternal fly diet impacts the offspring, these data suggest a paternal effect may have contributed to the results reported by Matzkin et al.
Alterations to the offspring genome are also dependent on paternal diet (Aldrich and Maggert, 2015; Ost et al., 2014). Male flies exposed to a protein-rich diet during adulthood sired females with decreased rDNA copy number, which was rescued by supplementing the paternal diet with the mTOR inhibitor rapamycin. Interestingly, the impact on offspring rDNA copy number was also observed in the F2 generation demonstrating the transgenerational impact of paternal diet in Drosophila (Aldrich and Maggert, 2015). The contribution of paternal-diet induced rDNA instability to offspring gene expression and therefore health is unknown; however rDNA integrity has been associated with global chromatin state changes as well as organismal longevity (Kobayashi, 2011; Kwan et al., 2013; Paredes and Maggert, 2009). These data highlight rDNA instability as a potential mechanism by which paternal-diet may impact offspring health.
An altered offspring chromatin state has been associated with changes in paternal-diet (Ost et al., 2014). Using adult male flies, Ost et al. demonstrated paternal TAG levels increased in a concentration dependent manner relative to dietary sucrose levels. This concentration dependent trend was transferred to the offspring since the body weight of adult progeny increased relative to paternal-diet sucrose concentrations. Moreover, when these offspring were challenged with a high-sucrose diet, body weight, TAG levels and lipid droplet size increased, suggesting these offspring are pre-sensitized to an obesogenic diet as a result of the paternal diet. Interestingly, only two days of high-sucrose paternal feeding was needed to induce this offspring obese phenotype. No changes were observed in offspring developmental timing or size relative to the paternal diet. Additionally, there was no indication of a transgenerational effect of the high-sucrose paternal diet. Analysis of adult offspring heterochromatin revealed progeny of high-sucrose fed males exhibited desilencing of peri-centric heterochromatin on the X chromosome, which correlated with an overall increase in gene expression (including several involved in energy metabolism and of unknown function) during the embryonic state of offspring from high-sucrose males. These observations suggest altered chromatin state as a potential mechanism of paternal-linked transgenerational inheritance. Interestingly, this trend of derepression was also evident in the sperm of high-sucrose fed fathers, implicating alterations in sperm gene expression as a vehicle for changes observed in offspring.
6. Maternal obesity in the fly
We and others have utilized the numerous genetic and developmental benefits of the fly to further understand the contribution of altered maternal diet on the health of the offspring and subsequent generations (Buescher et al., 2013; Matzkin et al., 2013; N.G. Prasad, Mallikarjun Shakarad, 2003; Valtonen et al., 2012; Vijendravarma et al., 2010). Most maternal diet studies have focused on undernourished conditions and highlight the fact that females reared on a protein- and sucrose-poor diet produced heavier eggs (Vijendravarma et al., 2010). When only dietary protein levels are altered, females reared on protein-poor conditions produced larger F1 offspring with longer developmental times relative to progeny of protein-rich females (Valtonen et al., 2012). With regards to offspring-survivorship, offspring of protein-poor females exhibited increased survival at the larval stage relative to offspring of protein-rich females, however, this advantage disappeared by the pupal stage (N.G. Prasad, Mallikarjun Shakarad, 2003). Interestingly, while offspring of protein-poor females are larger, actual egg production and ovary size are negatively correlated with dietary protein levels (Drummond-Barbosa and Spradling, 2001). The molecular mechanisms controlling female reproductive capacity and offspring health as a function of fly diet are not fully understood; however, several studies have demonstrated a requirement for Hedgehog and insulin signaling pathways in diet-induced ovarian stem cell proliferation (Drummond-Barbosa and Spradling, 2001; Hartman et al., 2013; Hsu and Drummond-Barbosa, 2009; Hsu et al., 2008).
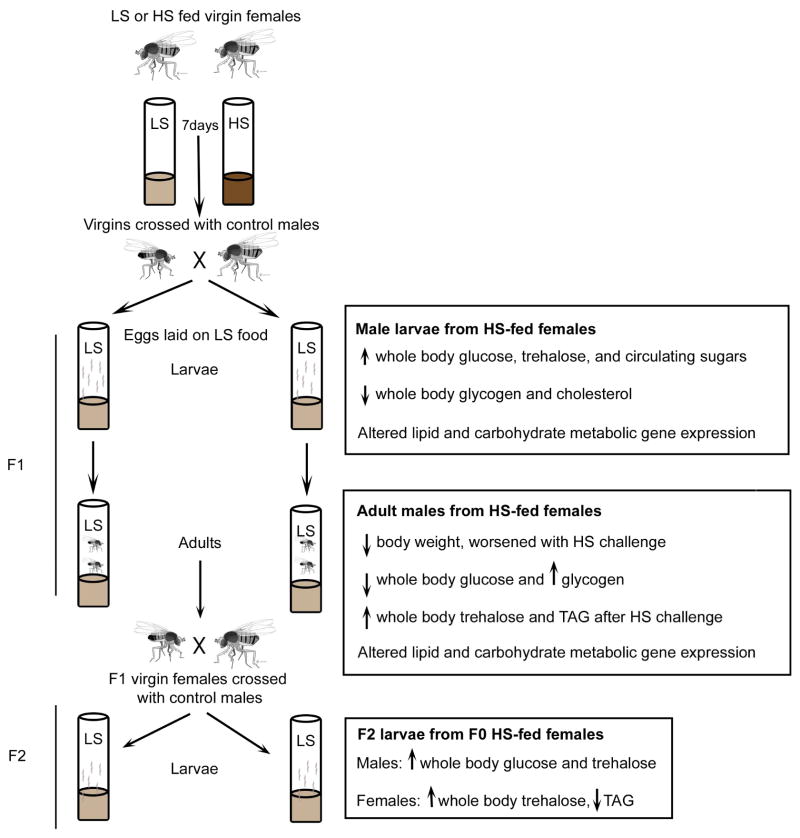
Studies of overnutrition in the fly have primarily focused on overall parental contributions to offspring health rather than specifically on the maternal dietary effect. To elucidate the molecular mechanisms that impact offspring health specifically as a result of maternal obesity, we developed a Drosophila model in which female adults were exposed to either high-sucrose or low-sucrose diets (HSD and LSD, respectively) prior to mating with standard diet-fed males (Buescher et al., 2013) (Figure 1). HSD females exhibited an obese-like phenotype characterized by increased levels of whole-body TAG, as seen in the paternal fly obesity model (Ost et al., 2014), glycogen, and the disaccharide trehalose as well as a failure to respond to insulin and elevated insulin-like peptide levels (Buescher et al., 2013, Duncan unpublished results).
Fig. 1. Drosophila model of maternal obesity.
Virgin w1118 female flies are fed LS or HS diet for 7 days and crossed with w1118 males fed stock food. All eggs for F1 and F2 generations are laid on LS food. Body composition changes and gene expression alterations are shown for each generation at the indicated developmental stage.
Male offspring of HSD females possessed metabolic disruptions at both larval and adult developmental stages, while larval female offspring displayed decreased cholesterol levels relative to offspring from LSD females (Buescher et al., 2013). In contrast to the paternal fly obesity model, no difference in whole-body TAG was observed in the offspring (Ost et al., 2014). Whole animal gene expression profiling using RNA sequencing of male larval offspring from HSD and LSD females revealed many differentially regulated genes between these two progeny groupings, which were later confirmed by qPCR. Of these genes, several were regulators of lipid and carbohydrate metabolic pathways including Cpt1, PDK, DHR38, and the SREBP target genes FAS and dACC (Buescher et al., 2013).
Adult offspring of HSD females challenged with an obesogenic diet exhibited increased levels of whole-body trehalose, glycogen, and TAG along with differential expression of carbohydrate and lipid metabolic genes relative to challenged control offspring, suggesting that maternal obesity in the fly predisposes offspring to adiposity and metabolic dysfunction, similar to reports in rodents (Buescher et al., 2013). Moreover, a transgenerational effect was observed wherein both male and female F2 larvae of HSD females had altered body composition, this is in contrast to studies of paternal fly obesity (Buescher et al., 2013; Ost et al., 2014). Similar to reports from maternal obesity rodent studies, HSD female ovaries exhibited impaired mitochondrial function and altered number (Duncan unpublished results), thus current studies in the lab are focused on addressing the contribution of mitochondrial inheritance to these phenomena of maternal overnutrition.
6. Conclusion
Maternal obesity is not only detrimental to maternal and offspring health, but it also threatens the health of subsequent generations. While current rodent models of maternal overnutrition have identified factors that contribute to maternal programming, the molecular mechanisms underlying these factors remain unknown. We review a Drosophila melanogaster model of maternal obesity that imparts tools unique to the fly – highly developed genetics, easy diet manipulation, and a rapid life cycle – that will aid in elucidating molecular pathways of maternal programming. Many of the known factors that contribute to maternal obesity are interconnected, thus being able to study these components individually will provide insight into their contributions to maternal overnutrition, a feat employable in the fly. For these reasons and the high conservation of metabolic pathways between humans and flies, Drosophila is an invaluable tool for understanding the complexity of maternal programming.
Highlights.
Maternal obesity is a rapidly growing health concern for both mother and offspring
Epigenetics, the ER, and mitochondria are avenues of potential impact on progeny
Molecular mechanisms that connect maternal overnutrition to these avenues are unknown
Drosophila has provided insight into obesity and other human metabolic diseases
A maternal obesity fly model mirrors findings in mammals and may aid in mechanistic insight
Acknowledgments
This work was supported by grants from the American Heart Association, 11IRG5450013 and 12GRNT12080056 (J.G.D), DRTC P30DK020579 (J.G.D) and the National Institutes of Health, K12HD001459 (R.T.B).
Abbreviations
- BMI
body mass index
- ER
endoplasmic reticulum
- ETC
electron transport chain
- PGC-1α
peroxisome proliferator-activated receptor-γ coactivator 1α
- dILPs
Drosophila insulin-like-peptides
- IPCs
insulin producing cells
- Upd2
Unpaired 2
- TAG
triacylglycerides
- SREBP
sterol regulatory element-binding protein
- IVF
in vitro fertilization
- HFD
high-fat-diet
- HSD
high-sucrose-diet
- LSD
low-sucrose-diet
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agrawal N, Pallos J, Slepko N, Apostol BL, Bodai L, Chang LW, Chiang AS, Thompson LM, Marsh JL. Identification of combinatorial drug regimens for treatment of Huntington’s disease using Drosophila. Proc Natl Acad Sci U S A. 2005;102:3777–3781. doi: 10.1073/pnas.0500055102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldrich JC, Maggert KA. Transgenerational inheritance of diet-induced genome rearrangements in Drosophila. PLoS Genet. 2015;11:e1005148. doi: 10.1371/journal.pgen.1005148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KD, Thummel CS. Diabetic larvae and obese flies-emerging studies of metabolism in Drosophila. Cell Metab. 2007;6:257–266. doi: 10.1016/j.cmet.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ. The fetal and infant origins of adult disease. BMJ. 1990;301:1111. doi: 10.1136/bmj.301.6761.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett AR, Tibbit C, Ponting CP, Liu J-L. Highly efficient targeted mutagenesis of Drosophila with the CRISPR/Cas9 system. Cell Rep. 2013;4:220–8. doi: 10.1016/j.celrep.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder NK, Hannan NJ, Gardner DK. Paternal diet-induced obesity retards early mouse embryo development, mitochondrial activity and pregnancy health. PLoS One. 2012;7:e52304. doi: 10.1371/journal.pone.0052304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birse RT, Choi J, Reardon K, Rodriguez J, Graham S, Diop S, Ocorr K, Bodmer R, Oldham S. High-fat-diet-induced obesity and heart dysfunction are regulated by the TOR pathway in Drosophila. Cell Metab. 2010;12:533–544. doi: 10.1016/j.cmet.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borengasser SJ, Faske J, Kang P, Blackburn ML, Badger TM, Shankar K. In utero exposure to prepregnancy maternal obesity and postweaning high-fat diet impair regulators of mitochondrial dynamics in rat placenta and offspring. Physiol Genomics. 2014;46:841–850. doi: 10.1152/physiolgenomics.00059.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce KD, Cagampang FR, Argenton M, Zhang J, Ethirajan PL, Burdge GC, Bateman AC, Clough GF, Poston L, Hanson Ma, McConnell JM, Byrne CD. Maternal high-fat feeding primes steatohepatitis in adult mice offspring, involving mitochondrial dysfunction and altered lipogenesis gene expression. Hepatology. 2009;50:1796–1808. doi: 10.1002/hep.23205. [DOI] [PubMed] [Google Scholar]
- Buescher JL, Musselman LP, Wilson Ca, Lang T, Keleher M, Baranski TJ, Duncan JG. Evidence for transgenerational metabolic programming in Drosophila. Dis Model Mech. 2013;6:1123–32. doi: 10.1242/dmm.011924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Entringer S, Davis EP, Hobel CJ, Swanson JM, Wadhwa PD, Sandman CA. Impaired executive function mediates the association between maternal pre-pregnancy body mass index and child ADHD symptoms. PLoS One. 2012;7:e37758. doi: 10.1371/journal.pone.0037758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano PM, Farrell K, Thomas A, Huston-Presley L, Mencin P, de Mouzon SH, Amini SB. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr. 2009;90:1303–13. doi: 10.3945/ajcn.2008.27416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano PM, Presley L, Minium J, De Mouzon SH, Hauguel-de Mouzon S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care. 2009;32:1076–80. doi: 10.2337/dc08-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center, V.D.R. VDRC Transgenic RNAi Libraries [WWW Document] 2015 URL http://stockcenter.vdrc.at/control/about_rnailibrary.
- Challier JC, Basu S, Bintein T, Minium J, Hotmire K, Catalano PM, Hauguel-de Mouzon S. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta. 2008;29:274–81. doi: 10.1016/j.placenta.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YP, Xiao XM, Li J, Reichetzeder C, Wang ZN, Hocher B. Paternal body mass index (BMI) is associated with offspring intrauterine growth in a gender dependent manner. PLoS One. 2012;7:e36329. doi: 10.1371/journal.pone.0036329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z, Guo S, Copps K, Dong X, Kollipara R, Rodgers JT, Depinho RA, Puigserver P, White MF. Foxo1 integrates insulin signaling with mitochondrial function in the liver. Nat Med. 2009;15:1307–11. doi: 10.1038/nm.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z, Tseng Y, White MF. Insulin signaling meets mitochondria in metabolism. Trends Endocrinol Metab. 2010;21:589–98. doi: 10.1016/j.tem.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, Lauenborg J, Schmidt L, Damm P. Overweight and the metabolic syndrome in adult offspring of women with diet-treated gestational diabetes mellitus or type 1 diabetes. J Clin Endocrinol Metab. 2009;94:2464–70. doi: 10.1210/jc.2009-0305. [DOI] [PubMed] [Google Scholar]
- Danielzik S, Langnäse K, Mast M, Spethmann C, Müller MJ. Impact of parental BMI on the manifestation of overweight 5–7 year old children. Eur J Nutr. 2002;41:132–8. doi: 10.1007/s00394-002-0367-1. [DOI] [PubMed] [Google Scholar]
- Davey Smith G, Leary S, Ness A, Lawlor DA. Challenges and novel approaches in the epidemiological study of early life influences on later disease. Adv Exp Med Biol. 2009;646:1–14. doi: 10.1007/978-1-4020-9173-5_1. [DOI] [PubMed] [Google Scholar]
- Deshpande SA, Carvalho GB, Amador A, Phillips AM, Hoxha S, Lizotte KJ, Ja WW. Quantifying Drosophila food intake: comparative analysis of current methodology. Nat Methods. 2014;11:535–40. doi: 10.1038/nmeth.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diesel JC, Eckhardt CL, Day NL, Brooks MM, Arslanian SA, Bodnar LM. Is gestational weight gain associated with offspring obesity at 36 months? Pediatr. Obes. 2015;10:305–10. doi: 10.1111/ijpo.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, Couto A, Marra V, Keleman K, Dickson BJ. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–6. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- Diop SBB, Bisharat-Kernizan J, Birse RTT, Oldham S, Ocorr K, Bodmer R. PGC-1/Spargel Counteracts High-Fat-Diet-Induced Obesity and Cardiac Lipotoxicity Downstream of TOR and Brummer ATGL Lipase. Cell Rep. 2015;10:1572–1584. doi: 10.1016/j.celrep.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrosotskaya IY, Seegmiller aC, Brown MS, Goldstein JL, Rawson RB. Regulation of SREBP processing and membrane lipid production by phospholipids in Drosophila. Science. 2002;296:879–883. doi: 10.1126/science.1071124. [DOI] [PubMed] [Google Scholar]
- Dokras A, Baredziak L, Blaine J, Syrop C, VanVoorhis BJ, Sparks A. Obstetric outcomes after in vitro fertilization in obese and morbidly obese women. Obstet Gynecol. 2006;108:61–69. doi: 10.1097/01.AOG.0000219768.08249.b6. [DOI] [PubMed] [Google Scholar]
- Drummond-Barbosa D, Spradling AC. Stem cells and their progeny respond to nutritional changes during Drosophila oogenesis. Dev Biol. 2001;231:265–78. doi: 10.1006/dbio.2000.0135. [DOI] [PubMed] [Google Scholar]
- Dunn GA, Bale TL. Maternal high-fat diet effects on third-generation female body size via the paternal lineage. Endocrinology. 2011;152:2228–36. doi: 10.1210/en.2010-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn GA, Bale TL. Maternal high-fat diet promotes body length increases and insulin insensitivity in second-generation mice. Endocrinology. 2009;150:4999–5009. doi: 10.1210/en.2009-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429:457–463. doi: 10.1038/nature02625. [DOI] [PubMed] [Google Scholar]
- El-Helaly M, Abdel-Elah K, Haussein A, Shalaby H. Paternal occupational exposures and the risk of congenital malformations--a case-control study. Int J Occup Med Environ Health. 2011;24:218–27. doi: 10.2478/s13382-011-0019-x. [DOI] [PubMed] [Google Scholar]
- Fariello RM, Pariz JR, Spaine DM, Cedenho AP, Bertolla RP, Fraietta R. Association between obesity and alteration of sperm DNA integrity and mitochondrial activity. BJU Int. 2012;110:863–7. doi: 10.1111/j.1464-410X.2011.10813.x. [DOI] [PubMed] [Google Scholar]
- Fernández-Moreno MA, Farr CL, Kaguni LS, Garesse R. Drosophila melanogaster as a model system to study mitochondrial biology. Methods Mol Biol. 2007;372:33–49. doi: 10.1007/978-1-59745-365-3_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Twinn DS, Alfaradhi MZ, Martin-Gronert MS, Duque-Guimaraes DE, Piekarz A, Ferland-McCollough D, Bushell M, Ozanne SE. Downregulation of IRS-1 in adipose tissue of offspring of obese mice is programmed cell-autonomously through post-transcriptional mechanisms. Mol Metab. 2014;3:325–333. doi: 10.1016/j.molmet.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forno E, Young OM, Kumar R, Simhan H, Celedon JC. Maternal Obesity in Pregnancy, Gestational Weight Gain, and Risk of Childhood Asthma. Pediatrics. 2014;134:e535–e546. doi: 10.1542/peds.2014-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frias AE, Morgan TK, Evans AE, Rasanen J, Oh KY, Thornburg KL, Grove KL. Maternal high-fat diet disturbs uteroplacental hemodynamics and increases the frequency of stillbirth in a nonhuman primate model of excess nutrition. Endocrinology. 2011;152:2456–64. doi: 10.1210/en.2010-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo JM, Gómez-López J, Medina-Bravo P, Juárez-Sánchez F, Contreras-Ramos A, Galicia-Esquivel M, Sánchez-Urbina R, Klünder-Klünder M. Maternal obesity increases oxidative stress in the newborn. Obesity (Silver Spring) 2015;23:1650–4. doi: 10.1002/oby.21159. [DOI] [PubMed] [Google Scholar]
- Garofalo RS. Genetic analysis of insulin signaling in Drosophila. Trends Endocrinol Metab. 2002;13:156–62. doi: 10.1016/s1043-2760(01)00548-3. [DOI] [PubMed] [Google Scholar]
- Gemma C, Sookoian S, Alvariñas J, García SI, Quintana L, Kanevsky D, González CD, Pirola CJ. Maternal pregestational BMI is associated with methylation of the PPARGC1A promoter in newborns. Obesity (Silver Spring) 2009;17:1032–9. doi: 10.1038/oby.2008.605. [DOI] [PubMed] [Google Scholar]
- Gemma C, Sookoian S, Alvariñas J, García SI, Quintana L, Kanevsky D, González CD, Pirola CJ. Mitochondrial DNA depletion in small- and large-for-gestational-age newborns. Obesity (Silver Spring) 2006;14:2193–9. doi: 10.1038/oby.2006.257. [DOI] [PubMed] [Google Scholar]
- Godfrey KM, Forrester T, Barker DJ, Jackson AA, Landman JP, Hall JS, Cox V, Osmond C. Maternal nutritional status in pregnancy and blood pressure in childhood. Br J Obstet Gynaecol. 1994;101:398–403. doi: 10.1111/j.1471-0528.1994.tb11911.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Bulnes a, Astiz S, Ovilo C, Lopez-Bote CJ, Sanchez-Sanchez R, Perez-Solana ML, Torres-Rovira L, Ayuso M, Gonzalez J. Early-postnatal changes in adiposity and lipids profile by transgenerational developmental programming in swine with obesity/leptin resistance. J Endocrinol. 2014;223:M17–M29. doi: 10.1530/JOE-14-0217. [DOI] [PubMed] [Google Scholar]
- Goo CK, Lim HY, Ho QS, Too HP, Clement MV, Wong KP. PTEN/Akt signaling controls mitochondrial respiratory capacity through 4E-BP1. PLoS One. 2012;7:e45806. doi: 10.1371/journal.pone.0045806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35:595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- Hartman TR, Strochlic TI, Ji Y, Zinshteyn D, O’Reilly AM. Diet controls Drosophila follicle stem cell proliferation via Hedgehog sequestration and release. J Cell Biol. 2013;201:741–757. doi: 10.1083/jcb.201212094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbstman JB, Wang S, Perera FP, Lederman SA, Vishnevetsky J, Rundle AG, Hoepner LA, Qu L, Tang D. Predictors and consequences of global DNA methylation in cord blood and at three years. PLoS One. 2013;8:e72824. doi: 10.1371/journal.pone.0072824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochner H, Friedlander Y, Calderon-Margalit R, Meiner V, Sagy Y, Avgil-Tsadok M, Burger A, Savitsky B, Siscovick DS, Manor O. Associations of maternal prepregnancy body mass index and gestational weight gain with adult offspring cardiometabolic risk factors: the Jerusalem Perinatal Family Follow-up Study. Circulation. 2012;125:1381–1389. doi: 10.1161/CIRCULATIONAHA.111.070060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H-J, Drummond-Barbosa D. Insulin levels control female germline stem cell maintenance via the niche in Drosophila. Proc Natl Acad Sci U S A. 2009;106:1117–21. doi: 10.1073/pnas.0809144106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H-J, LaFever L, Drummond-Barbosa D. Diet controls normal and tumorous germline stem cells via insulin-dependent and -independent mechanisms in Drosophila. Dev Biol. 2008;313:700–12. doi: 10.1016/j.ydbio.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igosheva N, Abramov AY, Poston L, Eckert JJ, Fleming TP, Duchen MR, McConnell J. Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PLoS One. 2010;5:e10074. doi: 10.1371/journal.pone.0010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HL, Benzer S, Min KT. Life extension in Drosophila by feeding a drug. Proc Natl Acad Sci U S A. 2002;99:838–843. doi: 10.1073/pnas.022631999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- Kobayashi T. How does genome instability affect lifespan? Genes to Cells. 2011;16:617–624. doi: 10.1111/j.1365-2443.2011.01519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X, Wang R, Xue Y, Liu X, Zhang H, Chen Y, Fang F, Chang Y. Sirtuin 3, a new target of PGC-1alpha, plays an important role in the suppression of ROS and mitochondrial biogenesis. PLoS One. 2010;5:e11707. doi: 10.1371/journal.pone.0011707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral JG, Biron S, Simard S, Hould FS, Lebel S, Marceau S, Marceau P. Large maternal weight loss from obesity surgery prevents transmission of obesity to children who were followed for 2 to 18 years. Pediatrics. 2006;118:e1644–9. doi: 10.1542/peds.2006-1379. [DOI] [PubMed] [Google Scholar]
- Kwan EX, Foss EJ, Tsuchiyama S, Alvino GM, Kruglyak L, Kaeberlein M, Raghuraman MK, Brewer BJ, Kennedy BK, Bedalov A. A natural polymorphism in rDNA replication origins links origin activation with calorie restriction and lifespan. PLoS Genet. 2013;9:e1003329. doi: 10.1371/journal.pgen.1003329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laor A, Stevenson DK, Shemer J, Gale R, Seidman DS. Size at birth, maternal nutritional status in pregnancy, and blood pressure at age 17: population based analysis. BMJ. 1997;315:449–453. doi: 10.1136/bmj.315.7106.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leddy MA, Power ML, Schulkin J. The impact of maternal obesity on maternal and fetal health. Rev Obs Gynecol. 2008;1:170–178. [PMC free article] [PubMed] [Google Scholar]
- Lee KK, Raja EA, Lee AJ, Bhattacharya S, Bhattacharya S, Norman JE, Reynolds RM. Maternal Obesity During Pregnancy Associates With Premature Mortality and Major Cardiovascular Events in Later Life. Hypertension. 2015 doi: 10.1161/HYPERTENSIONAHA.115.05920. [DOI] [PubMed] [Google Scholar]
- Leibowitz KL, Moore RH, Ahima RS, Stunkard AJ, Stallings VA, Berkowitz RI, Chittams JL, Faith MS, Stettler N. Maternal obesity associated with inflammation in their children. World J Pediatr. 2012;8:76–79. doi: 10.1007/s12519-011-0292-6. [DOI] [PubMed] [Google Scholar]
- Liu Z, Huang X. Lipid metabolism in Drosophila: development and disease. Acta Biochim Biophys Sin. 2013;45:44–50. doi: 10.1093/abbs/gms105. [DOI] [PubMed] [Google Scholar]
- Lowe A, Braback L, Ekeus C, Hjern A, Forsberg B. Maternal obesity during pregnancy as a risk for early-life asthma. J Allergy Clin Immunol. 2011;128:1102–1107. doi: 10.1016/j.jaci.2011.08.025. [DOI] [PubMed] [Google Scholar]
- Luzzo KM, Wang Q, Purcell SH, Chi M, Jimenez PT, Grindler N, Schedl T, Moley KH. High fat diet induced developmental defects in the mouse: oocyte meiotic aneuploidy and fetal growth retardation/brain defects. PLoS One. 2012;7:e49217. doi: 10.1371/journal.pone.0049217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malti N, Merzouk H, Merzouk SA, Loukidi B, Karaouzene N, Malti A, Narce M. Oxidative stress and maternal obesity: feto-placental unit interaction. Placenta. 2014;35:411–416. doi: 10.1016/j.placenta.2014.03.010. [DOI] [PubMed] [Google Scholar]
- Matzkin LM, Johnson S, Paight C, Markow Ta. Preadult parental diet affects offspring development and metabolism in Drosophila melanogaster. PLoS One. 2013;8:e59530. doi: 10.1371/journal.pone.0059530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo AM, Benatti RO, Ignacio-Souza LM, Okino C, Torsoni AS, Milanski M, Velloso LA, Torsoni MA. Hypothalamic endoplasmic reticulum stress and insulin resistance in offspring of mice dams fed high-fat diet during pregnancy and lactation. Metabolism. 2014;63:682–692. doi: 10.1016/j.metabol.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Metwally M, Ong KJ, Ledger WL, Li TC. Does high body mass index increase the risk of miscarriage after spontaneous and assisted conception? A meta-analysis of the evidence. Fertil Steril. 2008;90:714–726. doi: 10.1016/j.fertnstert.2007.07.1290. [DOI] [PubMed] [Google Scholar]
- Mingrone G, Manco M, Mora MEV, Guidone C, Iaconelli a, Gniuli D, Leccesi L, Chiellini C, Ghirlanda G. Influence of maternal obesity on insulin sensitivity and secretion in offspring. Diabetes Care. 2008;31:1872–1876. doi: 10.2337/dc08-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, Neschen S, White MF, Bilz S, Sono S, Pypaert M, Shulman GI. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest. 2005;115:3587–3593. doi: 10.1172/JCI25151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musselman LP, Fink JL, Narzinski K, Ramachandran PV, Hathiramani SS, Cagan RL, Baranski TJ. A high-sugar diet produces obesity and insulin resistance in wild-type Drosophila. Dis Model Mech. 2011;4:842–849. doi: 10.1242/dmm.007948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myles IA, Fontecilla NM, Janelsins BM, Vithayathil PJ, Segre JA, Datta SK. Parental dietary fat intake alters offspring microbiome and immunity. J Immunol. 2013;191:3200–3209. doi: 10.4049/jimmunol.1301057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad NG, Mallikarjun Shakarad MR, AJ Interaction between the effects of maternal and larval levels of nutrition on pre-adult survival in Drosophila melanogaster. Evol Ecol Res. 2003;5:903–911. [Google Scholar]
- Na J, Musselman LP, Pendse J, Baranski TJ, Bodmer R, Ocorr K, Cagan R. A Drosophila model of high sugar diet-induced cardiomyopathy. PLoS Genet. 2013;9:e1003175. doi: 10.1371/journal.pgen.1003175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyen C, Bretscher AJ, Binggeli O, Lemaitre B. Methods to study Drosophila immunity. Methods. 2014;68:116–128. doi: 10.1016/j.ymeth.2014.02.023. [DOI] [PubMed] [Google Scholar]
- Ng SF, Lin RC, Maloney CA, Youngson NA, Owens JA, Morris MJ. Paternal high-fat diet consumption induces common changes in the transcriptomes of retroperitoneal adipose and pancreatic islet tissues in female rat offspring. FASEB J. 2014;28:1830–1841. doi: 10.1096/fj.13-244046. [DOI] [PubMed] [Google Scholar]
- Nohr EA, Timpson NJ, Andersen CS, Davey Smith G, Olsen J, Sorensen TI. Severe obesity in young women and reproductive health: the Danish National Birth Cohort. PLoS One. 2009;4:e8444. doi: 10.1371/journal.pone.0008444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien TE, Ray JG, Chan WS. Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiology. 2003;14:368–74. doi: 10.1097/00001648-200305000-00020. [DOI] [PubMed] [Google Scholar]
- Organization, World Health. Obesity and Overweight [WWW Document] Fact Sheet N. 2015;311 http://www.who.int/mediacentre/factsheets/fs311/en/ [Google Scholar]
- Ost A, Lempradl A, Casas E, Weigert M, Tiko T, Deniz M, Pantano L, Boenisch U, Itskov PM, Stoeckius M, Ruf M, Rajewsky N, Reuter G, Iovino N, Ribeiro C, Alenius M, Heyne S, Vavouri T, Pospisilik JA. Paternal diet defines offspring chromatin state and intergenerational obesity. Cell. 2014;159:1352–1364. doi: 10.1016/j.cell.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science (80- ) 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science (80- ) 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes S, Maggert KA. Ribosomal DNA contributes to global chromatin regulation. Proc Natl Acad Sci U S A. 2009;106:17829–17834. doi: 10.1073/pnas.0906811106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasco MY, Leopold P. High sugar-induced insulin resistance in Drosophila relies on the lipocalin Neural Lazarillo. PLoS One. 2012;7:e36583. doi: 10.1371/journal.pone.0036583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R, Martin RM, Kramer MS, Oken E, Bogdanovich N, Matush L, Smith GD, Lawlor DA. Familial associations of adiposity: findings from a cross-sectional study of 12,181 parental-offspring trios from Belarus. PLoS One. 2011;6:e14607. doi: 10.1371/journal.pone.0014607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pospisilik JA, Schramek D, Schnidar H, Cronin SJF, Nehme NT, Zhang X, Knauf C, Cani PD, Aumayr K, Todoric J, Bayer M, Haschemi A, Puviindran V, Tar K, Orthofer M, Neely GG, Dietzl G, Manoukian A, Funovics M, Prager G, Wagner O, Ferrandon D, Aberger F, Hui CC, Esterbauer H, Penninger JM. Drosophila genome-wide obesity screen reveals hedgehog as a determinant of brown versus white adipose cell fate. Cell. 2010;140:148–160. doi: 10.1016/j.cell.2009.12.027. [DOI] [PubMed] [Google Scholar]
- Rajan A, Perrimon N. Of flies and men: insights on organismal metabolism from fruit flies. BMC Biol. 2013;11:38. doi: 10.1186/1741-7007-11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan A, Perrimon N. Of flies and men: insights on organismal metabolism from fruit flies. BMC Biol. 2013;11:38. doi: 10.1186/1741-7007-11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan A, Perrimon N. Drosophila cytokine unpaired 2 regulates physiological homeostasis by remotely controlling insulin secretion. Cell. 2012;151:123–137. doi: 10.1016/j.cell.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds RM, Osmond C, Phillips DI, Godfrey KM. Maternal BMI, parity, and pregnancy weight gain: influences on offspring adiposity in young adulthood. J Clin Endocrinol Metab. 2010;95:5365–5369. doi: 10.1210/jc.2010-0697. [DOI] [PubMed] [Google Scholar]
- Rulifson EJ, Kim SK, Nusse R. Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science (80- ) 2002;296:1118–1120. doi: 10.1126/science.1070058. [DOI] [PubMed] [Google Scholar]
- Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- Samuelsson a-MM, Matthews Pa, Argenton M, Christie MR, McConnell JM, Jansen EHJM, Piersma aH, Ozanne SE, Twinn DF, Remacle C, Rowlerson a, Poston L, Taylor PD. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension. 2008;51:383–392. doi: 10.1161/HYPERTENSIONAHA.107.101477. [DOI] [PubMed] [Google Scholar]
- Sanchez-Martinez A, Luo N, Clemente P, Adan C, Hernandez-Sierra R, Ochoa P, Fernandez-Moreno MA, Kaguni LS, Garesse R. Modeling human mitochondrial diseases in flies. Biochim Biophys Acta. 2006;1757:1190–1198. doi: 10.1016/j.bbabio.2006.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmatz M, Madan J, Marino T, Davis J. Maternal obesity: the interplay between inflammation, mother and fetus. J Perinatol. 2010;30:441–446. doi: 10.1038/jp.2009.182. [DOI] [PubMed] [Google Scholar]
- Seegmiller AC, Dobrosotskaya I, Goldstein JL, Ho YK, Brown MS, Rawson RB. The SREBP pathway in Drosophila: Regulation by palmitate, not sterols. Dev Cell. 2002;2:229–238. doi: 10.1016/S1534-5807(01)00119-8. [DOI] [PubMed] [Google Scholar]
- Sharp GC, Lawlor DA, Richmond RC, Fraser A, Simpkin A, Suderman M, Shihab HA, Lyttleton O, McArdle W, Ring SM, Gaunt TR, Davey Smith G, Relton CL. Maternal pre-pregnancy BMI and gestational weight gain, offspring DNA methylation and later offspring adiposity: findings from the Avon Longitudinal Study of Parents and Children. Int J Epidemiol. 2015 doi: 10.1093/ije/dyv042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleigh A, Raymond-Barker P, Thackray K, Porter D, Hatunic M, Vottero A, Burren C, Mitchell C, McIntyre M, Brage S, Carpenter TA, Murgatroyd PR, Brindle KM, Kemp GJ, O’Rahilly S, Semple RK, Savage DB. Mitochondrial dysfunction in patients with primary congenital insulin resistance. J Clin Invest. 2011;121:2457–2461. doi: 10.1172/JCI46405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubry A, Schildkraut JM, Murtha A, Wang F, Huang Z, Bernal A, Kurtzberg J, Jirtle RL, Murphy SK, Hoyo C. Paternal obesity is associated with IGF2 hypomethylation in newborns: results from a Newborn Epigenetics Study (NEST) cohort. BMC Med. 2013;11:29. doi: 10.1186/1741-7015-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan M, Aalinkeel R, Song F, Mitrani P, Pandya JD, Strutt B, Hill DJ, Patel MS. Maternal hyperinsulinemia predisposes rat fetuses for hyperinsulinemia, and adult-onset obesity and maternal mild food restriction reverses this phenotype. Am J Physiol Endocrinol Metab. 2006;290:E129–E134. doi: 10.1152/ajpendo.00248.2005. [DOI] [PubMed] [Google Scholar]
- Stothard KJ, Tennant PW, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA. 2009;301:636–650. doi: 10.1001/jama.2009.113. [DOI] [PubMed] [Google Scholar]
- Stump CS, Short KR, Bigelow ML, Schimke JM, Nair KS. Effect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proc Natl Acad Sci U S A. 2003;100:7996–8001. doi: 10.1073/pnas.1332551100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szendroedi J, Phielix E, Roden M. The role of mitochondria in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8:92–103. doi: 10.1038/nrendo.2011.138. [DOI] [PubMed] [Google Scholar]
- Tan HC, Roberts J, Catov J, Krishnamurthy R, Shypailo R, Bacha F. Mother’s pre-pregnancy BMI is an important determinant of adverse cardiometabolic risk in childhood. Pediatr Diabetes. 2015;16:419–426. doi: 10.1111/pedi.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PD, Mcconnell J, Khan IY, Holemans K, Lawrence KM, Asare-anane H, Persaud SJ, Jones PM, Petrie L, Hanson Ma, Poston L, Paul D, Mcconnell J, Khan IY, Lawrence KM, Asare-anane H, Shanta J, Jones PM, Petrie L, Hanson Ma. Impaired glucose homeostasis and mitochondrial abnormalities in offspring of rats fed a fat-rich diet in pregnancy. Am J Physiol Regul Integr Comp Physiol. 2005;288:R134–9. doi: 10.1152/ajpregu.00355.2004. [DOI] [PubMed] [Google Scholar]
- Vahratian A. Prevalence of overweight and obesity among women of childbearing age: results from the 2002 National Survey of Family Growth. Matern Child Heal J. 2009;13:268–273. doi: 10.1007/s10995-008-0340-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtonen TM, Kangassalo K, Polkki M, Rantala MJ. Transgenerational effects of parental larval diet on offspring development time, adult body size and pathogen resistance in Drosophila melanogaster. PLoS One. 2012;7:e31611. doi: 10.1371/journal.pone.0031611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenendaal MV, Painter RC, de Rooij SR, Bossuyt PM, van der Post JA, Gluckman PD, Hanson MA, Roseboom TJ. Transgenerational effects of prenatal exposure to the 1944–45 Dutch famine. BJOG. 2013;120:548–553. doi: 10.1111/1471-0528.12136. [DOI] [PubMed] [Google Scholar]
- Vijendravarma RK, Narasimha S, Kawecki TJ. Effects of parental larval diet on egg size and offspring traits in Drosophila. Biol Lett. 2010;6:238–241. doi: 10.1098/rsbl.2009.0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucetic Z, Kimmel J, Totoki K, Hollenbeck E, Reyes TM. Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology. 2010;151:4756–4764. doi: 10.1210/en.2010-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RM, Marshall NE, Jeske DR, Purnell JQ, Thornburg K, Messaoudi I. Maternal obesity alters immune cell frequencies and responses in umbilical cord blood samples. Pediatr Allergy Immunol. 2015;26:344–351. doi: 10.1111/pai.12387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LL, Dunning KR, Yang X, Russell DL, Lane M, Norman RJ, Robker RL. High-fat diet causes lipotoxicity responses in cumulus-oocyte complexes and decreased fertilization rates. Endocrinology. 2010;151:5438–5445. doi: 10.1210/en.2010-0551. [DOI] [PubMed] [Google Scholar]
- Wu LL, Russell DL, Wong SL, Chen M, Tsai TSS, St John JC, Norman RJ, Febbraio Ma, Carroll J, Robker RL. Mitochondrial dysfunction in oocytes of obese mothers: transmission to offspring and reversal by pharmacological endoplasmic reticulum stress inhibitors. Development. 2015;142:681–691. doi: 10.1242/dev.114850. [DOI] [PubMed] [Google Scholar]
- Wu Q, Brown MR. Signaling and function of insulin-like peptides in insects. Annu Rev Entomol. 2006;51:1–24. doi: 10.1146/annurev.ento.51.110104.151011. [DOI] [PubMed] [Google Scholar]
- Yogev Y, Catalano PM. Pregnancy and Obesity. Obstet Gynecol Clin North Am. 2009;36:285–300. doi: 10.1016/j.ogc.2009.03.003. [DOI] [PubMed] [Google Scholar]