Abstract
Treatment of Central Nervous System (CNS) disorders still remains a major clinical challenge. The Blood-brain-barrier (BBB), known as the major hindrance, greatly limits therapeutics penetration into the brain. Moreover, even though some therapeutics can cross BBB based on their intrinsic properties or via the use of proper nanoscale delivery vehicles, their therapeutic efficacy is still often limited without the specific uptake of drugs by the cancer or disease-associated cells. As more studies have started to elucidate the pathological roles of major cells in the CNS (for example, microglia, neurons, and astrocytes) for different disorders, nanomedicines that can enable targeting of specific cells in these diseases may provide great potential to boost efficacy. In this review, we aim to briefly cover the pathological roles of endothelial cells, microglia, tumor-associated microglia/macrophage, neurons, astrocytes, and glioma in CNS disorders and to highlight the recent advances in nanomedicines that can target specific disease-associated cells. Furthermore, we summarized some strategies employed in nanomedicine to achieve specific cell targeting or to enhance the drug neuroprotective effects in the CNS. The specific targeting at the cellular level by nanotherapy can be a more precise and effective means not only to enhance the drug availability but also to reduce side effects.
Keywords: Nanomedicine, CNS disorders, targeted therapy, BBB, microglia, neuron
Graphical abstract
1. Introduction
Achieving effective therapy for many diseases in the central nervous system (CNS) remains a great challenge. To date, clinical treatments for CNS diseases provide only limited improvement in outcomes and are often accompanied by severe side effects. For instance, patients diagnosed with glioblastoma only have a median survival period of 14 months following surgical resection, radiation, or concomitant chemotherapy [1]. The difficulty in achieving improved outcome for CNS diseases stems from the inability to deliver therapeutically relevant doses of the therapeutic to diseased cells or regions. The Blood-Brain Barrier (BBB) poses as the main obstacle that prevents most systematically administrated drugs from entering the CNS.
Nanomedicine offers great potential for improving the therapeutic efficacy or diagnosis efficiency in the clinical settings for many CNS disorders. The use of nanoparticles as drug delivery vehicles is not only widely reported in pre-clinical studies but is now also being implemented in clinical applications. While the entry of drugs to the brain is highly restricted, drug-carrying nanoparticles can significantly improve the CNS pharmacokinetics and biodistribution relative to free drugs [2]. Recent discoveries have shed more light on the CNS disease pathology at the cellular or even molecular level, and have greatly impacted the design of nanomedicine to target specific cell populations in the CNS. This review will describe the pathological roles of major CNS cells (such as neurons, microglia/macrophage, astrocytes, endothelial cells, and brain tumor cells) in CNS disorders. We will then focus on recent advances of nanomedicine design that have been implemented to target specific cell populations in the CNS.
2. Cells of Interest for Designing Nanomedicine for CNS Delivery and Their Functions in Pathology
2.1 Endothelium at BBB in Normal Physiology
The BBB is composed of endothelial cells adjoined with tight junctions which act as physical barriers and constrain the passage of most molecules through the barrier to transcellular trafficking. More than 98% of small molecular drugs and almost 100% of large-molecules (>500 Da) are simply excluded from CNS because they cannot cross the BBB [3]. It not only functions as a protective obstruction that blocks possible neurotoxic substances from CNS but also mediates several important functions, such as i) selectively transporting molecules that provides essential nutrients to the brain, ii) effluxing waste products, and iii) regulating homeostasis in the brain [4].
Small molecules are known to pass through endothelial cells by passive diffusion (for lipophilic molecules) or by active transport (as in the case of required nutrients into the brain, such as glucose, amino acids, nucleosides, or nucleobases) [4]. Small lipophilic molecules (<400 Da) exhibit certain abilities to pass the BBB by passive diffusion through the endothelial cells [5]. Unfortunately, many therapeutic molecules do not belong to this category. Most hydrophobic substances over 400 Da fail to enter the brain through this mechanism, often due to the efflux system regulated by ATP-binding cassette (ABC) transporters [5, 6]. These transporters are protective mediator that actively exclude possible neurotoxic substances from entering the CNS and are expressed on the endothelial cells. For instance, P-glycoprotein, an ATP-dependent transport protein localized in the blood luminal membrane, deters the entry of potentially toxic substances by pumping them back to the blood. While many small molecular drugs are substrates of ABC transporters, they possess fairly limited capabilities of entering the brain.
Large molecules, peptides or proteins, are incapable of passive diffusion across the BBB. Thus, their preferred mode of transport is through transcytosis, given that endothelial cells at the BBB exhibit much lower activity of internalization compared with other endothelia [4]. This mechanism involves either adsorptive-mediated (non-specific) or receptor-mediated (specific) transcytosis to allow macromolecules, captured in vesicles, to be internalized by endothelial cells and eventually exocytosed into the brain [4]. Adsorptive transcytosis involves endothelial internalization induced by non-specific binding caused by the interaction between cell surface and excess positive charges on the macromolecules. Some cationic proteins or peptides, such as cationic albumin, undergo this type of pathway to cross the endothelial cells [7]. On the other hand, internalization of substances can also occur by receptor-mediated transcytosis when ligands interact with their respective endothelial membrane receptors, such as transferrin receptor (TfR), melanotransferrin receptor (MTfR), lactoferrin (LfR), or LDL-receptor-related proteins [8-11]. Researchers that have designed nanomedicines in an attempt to increase drug delivery to the brain have heavily considered these two pathways, as discussed later.
2.2 BBB Dysfunction and Pathological Roles of Glia, Neurons in CNS disorders
2.2.1 BBB Dysfunction
Dysfunction of BBB endothelial cells is an important feature in many CNS pathologies [12]. This dysfunction is often associated with neuroinflammation which leads to alternation of permeability of the BBB. Under these conditions, the tight junctions between endothelial cells are disrupted, which enables additional substances to cross BBB or permits circulating macrophages to infiltrate into the CNS (Figures 1 and 2). This permeability change can be observed in neuroinflammation initiated by bacterial lipopolysaccharides (LPS), or in disease pathologies for stroke, traumatic brain injury, MS, and brain tumors [4, 13]. Such dysfunction can also affect the efflux system in endothelial cells. In some diseases such as AD or PD, the BBB permeability is altered due to decreased level of P-glycoprotein expression, which lowers the efficiency of efflux pumps in transporting neurotoxic substances back to the blood [14-18].
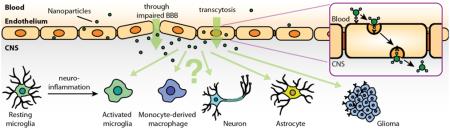
Figure 1. Common features in neuroinflammation.
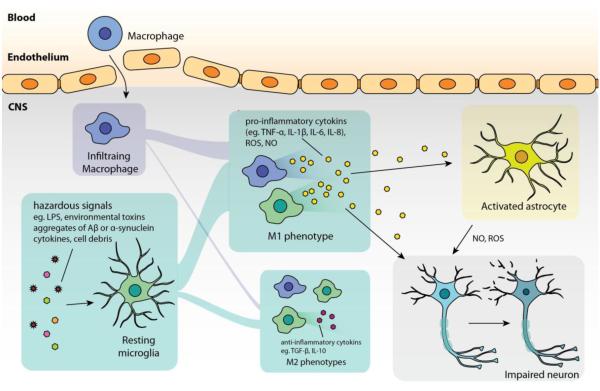
In general, microglial cells are initially activated by hazardous signals, such as damage-associated molecular pattern molecules and cell debris from damaged cells in acute inflammation, aggregates of Aβ in Alzheimer’s disease, α-synuclein aggregates from abnormal dopaminergic neurons in Parkinson’s disease, SOD1 aggregates from diseased neuron in amyotrophic lateral sclerosis. In such pathologies, microglia (together with infiltrating macrophages) tend to polarize to M1 phenotype that amplifies neuroinflammation by producing pro-inflammatory cytokines (TNF-α, IL-1β, IL-6) as well as ROS and NO which lead to neuronal injury. Some pro-inflammatory cytokines (such as TNF-α, IL-1β) can also activate astrocytes to induce over-production of ROS and NO that exacerbate the injury. The impaired neurons are then susceptible to undergo apoptosis or necrosis, which, in turn, release molecules that further activate microglia.
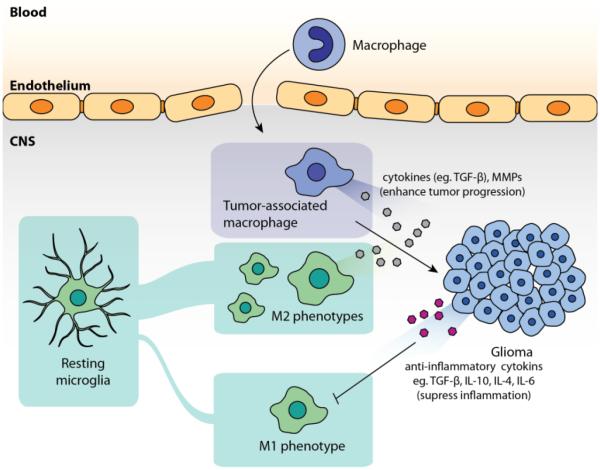
Figure 2. Role of tumor-associated microglia/macrophages and tumor cells in tumor microenvironment.
Glioma cells generally create an environment that suppresses classic inflammation (or, the functions of M1 phenotypes of microglia/infiltrating macrophage) by cytokines such as TGF-β, IL-10, IL-4, IL-6.
2.2.2 Microglia in Neurodegenerative Diseases
The CNS possesses a distinctive immune system since circulating immune cells cannot access to the CNS in physiological conditions. Microglial cells serve as the major resident immune cells in the CNS where they constantly monitor their surroundings and act as the mediator in innate immunity for early control of infections [19, 20]. They serve as key therapeutic targets because their functions are closely associated with the pathologies of many CNS disorders, including ischemic stroke, traumatic brain injury, spinal cord injury, AD, Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), and most types of brain tumors [19-25]. Under normal conditions, quiescent microglial cells constantly survey their surroundings, providing a defense mechanism for the brain. Upon sensing pathogen invasion or tissue damage, they rapidly switch to the activated phenotype that secretes cytokines to initiate secondary inflammatory responses. (Figure 1). This phenotype change of microglia, characterized by a morphological change from ramified form to amoeboid form, is often induced by the recognition of pathogen-associated moieties by toll-like receptors (TLRs), a family of receptors that plays a role in both innate and adaptive immunity [19, 20]. When recognizing stimuli by such receptors, microglia can produce cytokines to stimulate either pro-inflammatory or anti-inflammatory responses. It should be noted that the termed “activation” could indicate a range of activated states for microglia, broadly divided into two main phenotypes, M1 and M2, as indicated in other reviews [26, 27]. Activated microglia in the M1 state, or also called “classically activated”, promote the production of inflammatory cytokines, such as TNF-α, IL-1β, or IL-6 [19, 20]. On the other hand, M2 microglia contain phenotypes which suppress inflammation by cytokines TGF-β or IL-10 (M2a phenotype), or assist tissue remodeling and matrix deposition (M2c phenotype) [26]. The dynamic between M1 and M2 phenotypes of microglia enables the response to early stages of infection and is responsible for the clearance of cellular debris. However, in many pathological conditions, pro-inflammatory effects can persistently dominate without effective anti-inflammatory responses. For example, M1 microglia may release cytotoxic nitric oxide (NO), reactive oxygen species (ROS), or excessive cytokines (such as TNF-α) that cause neurotoxicity and lead to the injury/death of neurons, oligodendrocytes, and/or damage extracellular matrix [27].
Dysfunction of microglial responses has been observed in CNS diseases associated with both acute and chronic neuroinflammation. In acute inflammation, such as ischemic stroke, spinal cord injury, or traumatic brain injury, microglia activation is triggered by mechanisms that cope with damage-associated molecular pattern molecules (DAMPs) released from injured cells or cellular necrosis processes [28, 29]. Though this is a crucial step for tissue repair, a failure of follow-up anti-inflammation responses in these instances can result into more damages. As stated before, this activation (dominated by M1 phenotype) could lead to over-production of cytotoxic NO and ROS, which exacerbate the cellular death, or cytokines (for example, IL-1, IL-6, and TNF-α) that amplify inflammation response [30]. Chronic inflammation, in contrast to acute inflammation, is a self-propagating inflammatory response initiated from certain stimuli. This process generally starts from an initial microglia activation, which further develops into increased inflammation and glial activation when M2 responses occur and fail to exert their neuroprotective roles [26]. M1 microglia participates in the chronic inflammation of many neurodegenerative diseases, including AD, PD, ALS, or MS [25, 31-33]. In these instances, microglia respond to stimuli, such as aggregation of Aβ in AD and α-synuclein in PD, motor neuronal death in ALS, or signals from infiltrating lymphocytes in MS; and further produce more NO, ROS, proinflammatory cytokines (such as TNF-α, IL-1β, IL-6) that eventually cause progressive injury to other neurons/astrocytes without appropriate neuroprotective effects from M2 phenotypes [25-27, 34-37]. Since this cascade is often accompanied with BBB impairment in pathology, circulating macrophages can possibly infiltrate into the CNS and function similarly to microglial cells to amplify the inflammatory responses.
The production of proinflammatory cytokines and neurotoxic substances from microglia/infiltrating macrophages are linked to several pathways, including, but not limited to, the activation of NOX2 complex (an NADPH oxidase), mitogen-activated protein kinase (MAPK), and sphingosine kinase 1 (Sphk1) as detailed elsewhere [38-40]. In general, these pathways can be stimulated by variety of disease proteins (Aβ precursor protein, α-synuclein, myelin), LPS, cytokines (TNF-α, IL-1β, IL-4, IL-13), or environmental toxins. Since these pathways account for the production of neurotoxic molecules, inhibition of these pathways in microglia can possibly promote neuroprotective effects. For example, inhibition of NOX2 or p38 MAPK by treating dexamethasone or minocycline could attenuate the activation of microglia, as well as the production level of NO and IL-1β [39, 41, 42]. Dimethylsphingosine, an inhibitor of Sphk1, reportedly suppresses the expression of proinflammatory cytokines including TNF-α, IL-1β, and the production of NO of microglial cells [40]. But to optimize the therapeutic effects, these potent drugs require specific, effective delivery to microglia/infiltrating macrophages.
2.2.3 Tumor-Associated Microglia/Macrophages in Tumor Microenvironments
Tumor-associated microglia and infiltrating macrophages (TAMs) pose as important mediators in CNS cancer (Figure 2). Various studies regarding the synergic effects between TAMs and gliomas that allow them to co-exist have been reviewed previously [21, 43]. TAMs can be recruited by gliomas by chemokines, cytokines, or matrix metalloproteinases (MMPs)[21]. Although TAMs may polarize to M1 phenotype to give rise to pro-inflammatory effects (for initiating the clearance of potential hazards in CNS), their inflammatory functions are often suppressed in glioma pathology [23, 43-46]. Gliomas produce anti-inflammatory cytokines such as IL-10, IL-4, IL-6, and TGF-β [21], where tumor cells are able to establish an immunosuppressive microenvironment after recruiting TAMs. Under such condition, TAMs are instead polarized to the M2 phenotype that reduces classic neuroinflammation, and allows further tumor progression [21, 43]. In addition, TAMs themselves can also secrete cytokines (TGF-β) and MMPs (MMP-2, MMP-9, and MT1-MMP) that promote tumor growth and invasion [47-50]. These findings suggest TAMs can serve as a crucial target, as their pathological functions are strongly associated with tumor cells. Delivering therapeutics to alter the functions of TAMs or using TAMs as delivery vehicles could potentially provide an effective approach for the treatment of brain tumors.
Microglia/macrophages, under such circumstance, are polarized to M2 phenotypes and produce cytokines (TGF-β) or MMPs that are associated with tumor progression.
2.2.4 Astrocytes
Astrocyte is another major glial cells in the brain which perform a majority of functions related to maintenance of extracellular microenvironment and cell-cell communications in the CNS. They participate in the metabolism of amino acids, nutrients, and ions in the brain, communication with neurons and BBB endothelium, as well as the modulation of excitatory synaptic transmission [51, 52]. Abnormalities and dysfunctions of astrocytes are also linked with pathologies of CNS diseases, including stroke, neuroinfection, epilepsy, AD, PD and ALS [53, 54]. Changes of astrocyte functions in response to potent perturbations or injury, termed “astrogliosis”, is characterized by the increase in number, size, motility of astrocytes, and upregulation of pro- or anti-inflammatory cytokines. In addition, increased level of glial fibrillary acidic protein (GFAP) expression by reactive astrocytes is a common pathological observation of astrogliosis in various CNS diseases. However, the pathological functions of this upregulation of GFAP remain unclear [51]. Activated astrocytes are often found in malignant regions in neurodegenerative diseases, producing ROS and NO when neuroinflammation occurs [24]. In spite of the limited understanding of the pathological mechanisms of astrocytes, medicine that targets these activated astrocytes could potentially provide neuroprotective effects within local malignant tissues.
2.2.5 Neurons
Neuronal cells are the major cells that process and transmit information in the brain. They are also considered an important therapeutic target because they are often impaired in the pathologies of many CNS diseases. Ischemia related CNS diseases, such as stroke, spinal cord injury and traumatic brain injury, excitotoxicity often leads to subsequent neuronal death [55-58]. This process involves the neuronal up-regulation of excitatory neurotransmitter glutamate as well as glutamate receptors (such as NMDA and AMPA receptors) on neurons [58]. This cascade not only causes neuron damages, but also initiates microglia activation, which often exacerbates the injury as discussed previously [59]. On the other hand, neuronal cells can also undergo degeneration or apoptosis in chronic neuroinflammation. When neuroinflammation persists, neurons can possibly initiate apoptosis upon sensing cytokines (TNF-α) from activated microglia, due to ROS and NO from both activated microglia and astrocyte, or via interacting with neurotoxic substances (such as Aβ) [60]. These pathological roles of neurons in CNS diseases suggest that the effective delivery of therapeutics to neurons may alleviate the damage.
2.2.6 Brain Tumor Cells
Brain tumors are highly heterogeneous and present with a variety of phenotypes. Gliomas are the most common invasive tumors in the CNS and are derived from glial cells. For the treatment of CNS cancers, tumor cells could be the therapeutic targets for delivering chemotherapeutics. Chemotherapeutic agents such as carmustine, lomustine, and temozolomide (all approved by the FDA for the treatment of brain tumors) often damage the DNA and trigger the death of tumor cells. However, these agents normally do not carry targeting properties, and therefore do not distinguish between malignant cells and healthy cells, further causing severe side effects. As a result, surface receptors that over-expressed on glioma cells could serve as molecules of interest for active targeting when designing nanomedicine, which will be discussed in later context.
3.1 Targeting endothelial cells in the CNS
3.1.1 Targeting Endothelial Cells for Disease Diagnosis
Diagnostic tools aiming to target specific cell population are often engineered according to the pathology of diseases. In neuroinflammatory disorders (such as stroke, cerebral ischemia, and AD), endothelial activation plays a critical role at the initial stage in recruiting immune cells to the site of inflammation [61]. Such activation is marked by over-expression of some adhesion molecules—such as vascular cell adhesion molecule-1 (VCAM-1)—on the membrane of endothelial cells [62] (Table 1). In some reported work, VACM-1 was chosen as the targeting molecule for the early detection of vascular damages. Grafting VCAM-1 targeting peptides to ultrasmall superparamagnetic iron oxide allowed the designed nanoparticles being used for the detection of cerebrovascular cell activation in multiple models (such as stroke and ischemia), serving as a valuable strategy for non-invasive and quantitative evaluation for neuroinflammation in pre-clinical models [63-65]. However, VCAM-1 expression varies at different stages after injury in different models. One should note that successful targeting of activated endothelial cells is highly associated with the time course of disease progression.
Table 1.
A summary of endothelial cell-targeting platforms reported in recent studies.
| Targeted cells | Platform | Targeting ligand | Route of administration | Delivered therapeutics? | Model of
evaluation/Application |
Effects on targeted cells |
|---|---|---|---|---|---|---|
|
Activated endothelial
cells |
Iron oxide microparticles [63] |
Vascular cell adhesion molecule-1 binding peptide |
Intravenous | No | Mouse model of cerebral
ischemia |
Imaging endothelial cell
activation |
|
| ||||||
|
Activated endothelial
cells |
Iron oxide microparticles [64] |
Vascular cell adhesion molecule-1 binding peptide |
Intravenous | No | Mouse model of acute brain inflammation, vascular dementia, Alzheimer's disease (AD) and multiple sclerosis |
Imaging endothelial cell
activation |
|
| ||||||
|
Activated endothelial
cells |
Iron oxide microparticles [65] |
Vascular cell adhesion molecule-1 binding peptide |
Intravenous | No | Mouse model of Stroke | Imaging endothelial cell
activation |
|
| ||||||
| Endothelial cells | Poly(butylcyanoacrylate)
nanoparticles (PBCN) [66] |
Polysorbate 80 | Intravenous | No | Healthy mouse, mouse model of AD |
Enhance BBB interaction |
|
| ||||||
| Endothelial cells | Poly(butylcyanoacrylate)
nanoparticles (PBCN) [67] |
Polysorbate 80 | Intravenous | Puerarin | Rat model of ischemia
reperfusion |
Enhance BBB interaction |
|
| ||||||
| Endothelial cells | Monovalent molecular shuttle (modified anti- TfR monoclonal antibody )[68-70] |
Anti-TfR monoclonal antibody |
Intravenous | Antibody | Mouse model of Alzheimer disease |
Enhance BBB penetration |
|
| ||||||
|
Endothelial cells
(BBTB) |
Liposome [71] | Cyclic Arg-Gly-Asp (RGD) peptide |
Intravenous | No | Mouse model of C6 glioma |
Enhance BBB penetration |
|
| ||||||
|
Endothelial cells
(BBTB) |
Liposome [72] | Folate and transferrin (Tf) |
Intravenous | Doxorubicin | Rat model of C6 glioma |
Enhance BBB penetration |
|
| ||||||
|
Endothelial cells
(BBTB) |
Liposome [73] | Cell penetration peptide TAT and transferrin (Tf) |
Intravenous | Doxorubicin | Mouse model of glioma | Enhance BBB penetration |
|
| ||||||
| Endothelial cells | Chitosan nanoparticle [74] |
Transferrin (Tf) | Intravenous | Peptide | Mouse model of stroke | Enhance BBB penetration |
|
| ||||||
| Endothelial cells | Angiopep2-neurotensin conjugates [75] |
Angiopep-2 | Intravenous | Neurotensin | Rat model of pain | Enhance BBB penetration |
|
| ||||||
|
Endothelial cells
(BBTB) |
Paclitaxel–Angiopep-2
peptide drug conjugate (GRN1005) [76] |
Angiopep-2 | Intravenous | Paclitaxel | Glioma patients (Phase I) | Enhance BBB penetration |
|
| ||||||
|
Endothelial cells
(BBTB) |
Paclitaxel–Angiopep-2
peptide drug conjugate (GRN1005) [77] |
Angiopep-2 | Intravenous | Paclitaxel | Mouse human glioma cell xerograph
model |
Enhance BBB penetration |
|
| ||||||
|
Endothelial cells
(BBTB) |
Paclitaxel–Angiopep-2
peptide drug conjugate (GRN1005) [78] |
Angiopep-2 | Intravenous | Paclitaxel | Mouse orthotopic glioma model |
Enhance BBB penetration |
|
| ||||||
|
Endothelial cells
(BBTB) |
Paclitaxel–Angiopep-2
peptide drug conjugate (GRN1005) [79] |
Angiopep-2 | Intravenous | Paclitaxel | Mouse model of brain metastases of breast cancer |
Enhance BBB penetration |
|
| ||||||
|
Endothelial cells
(BBTB) |
Doxorubicin–Angiopep-2
peptide drug conjugate (ANG1007 and ANG1009) [80] |
Angiopep-2 | Intravenous | Doxorubicin | Mouse model of glioma | Enhance BBB penetration |
|
| ||||||
| Endothelial cells | Fused protein [81] | Monoclonal antibody (mAb) against human insulin receptor (HIRMAb) |
Intravenous | Iduronate 2-sulfatase (IDS) |
Rhesus monkey | Enhance BBB penetration |
|
| ||||||
| Endothelial cells | Gold nanoparticles [82] | Insulin | Intravenous | No | Healthy mouse | Enhance BBB penetration |
|
| ||||||
|
Endothelial cells
(BBTB) |
Trail [83] | Choline derivate | Intravenous | Trail | Mouse model of glioma | Enhance BBB penetration |
|
| ||||||
| Endothelial cells (BBB) | PEG-dendrigraft poly-l- lysine (DGL)/DNA nanoparticles [83] |
Dermorphin | Intravenous | DNA | Cerebral ischemia reperfusion injury |
Enhance BBB penetration |
For each platform, targeting ligand; delivered therapeutics; model of evaluation/application and effects on targeted cells are described.
3.1.2 Targeting Endothelial Cells for Increasing the Brain Uptake
Targeting endothelial cells in the CNS could be used as a means to increase the efficiency of drug across BBB. Various strategies with this feature have been applied in the treatments of diverse CNS disorders such as AD [68-70], primary or secondary malignant glioma [72, 73, 76-80, 84], stroke [74], and cerebral ischemia [83]. These applications are summarized according to their platforms, targeting ligands, and models of evaluation in Table 1. Currently, this strategy has led to profound results in large animal models such in primates [81] and are being evaluated in clinical trials (NCT01480583) [76]. In these instances, ligands that were involved in the specific interactions with endothelial transporters were often incorporated into the therapeutic agents/cargos for the initiation of receptor-mediated transcytosis at BBB endothelium (Figure 3). Potential receptors of interest in recent studies include the choline transporter [85], the transferrin receptor (TfR) [68, 72-74], integrin αvβ3 (the RGD receptor) [71], the insulin receptor [81, 82], and the low-density lipoprotein receptor-related protein-1 (LRP1) [75, 76, 84].
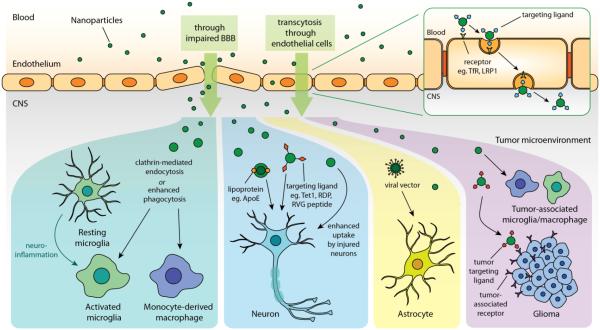
Figure 3. Schematic representation of the strategies for targeted drug delivery to different cell phenotypes in CNS.
First, nanomedicine can either enter CNS through impaired BBB or cross BBB endothelium via receptor-mediated transcytosis when the drug/cargo circulates systematically. Nanomedicine can potentially be uptake by activated microglia, infiltrating macrophage, or tumor-associated microglia/macrophage through clathrin-mediated endocytosis or phagocytosis. The drug/cargo can also possess cell-targeting properties when a targeting moiety is incorporated. Vesicles with lipoprotein ApoE or nanoparticles with ligands (eg. Tet1, RDP, RVG peptides) can be particularly uptake by neurons. Viral vector homing to astrocyte could be an effective cargo to deliver therapeutics. Nanoparticles functionalized with tumor targeting ligand can also be introduced to target glioma with enhanced accumulation.
To bring this to light, surface modification of large nanoparticles (>700 nm) with TfR targeting peptides could enhance the particle accumulation in the brain by the specific interactions with TfR on the endothelial cells as applied in a stroke model [74]. However, the successful delivery of TfR targeting devices to the CNS requires careful control of the binding affinity between the targeting device and TfR. Recent studies have shown that reducing TfR affinity is crucial for successful transcytosis and increased brain exposure of therapeutics. Therapeutic antibody with bivalent binding showed higher binding affinity with TfR, but resulted in incomplete transcellular trafficking and less brain exposure due to the higher binding affinity. Reducing the binding affinity to TfR by 5-8 fold, by using vehicles with monovalent binding, greatly increased the efficacy of this therapeutic antibody by more than 50-fold compared with the parent antibody [68-70].
Low-density lipoprotein receptor-related protein-1(LRP1) is another successful example that has been extensively considered as the targeting receptor on endothelial cells. Nanomedicines that target LRP1 have been shown to enhance the brain accumulation of chemotherapeutic agents, siRNA, and proteins in glioma models [76-80, 84, 86]. In these studies, angiopeps-2 (An2), known as the LRP1 targeting peptide, was incorporated into the nanodevices or drugs to enhance the therapeutic efficacy. It has been shown in in vivo human xenograft models that incorporating paclitaxel with An2 significantly improved the brain uptake of paclitaxel by 5-fold [77]. Moreover, an An2-conjugate of paclitaxel (ANG1005) has reached clinical trials and demonstrated encouraging preliminary efficacy in Phase I/II for the treatment of primary and secondary brain tumors (NCT01480583) [76].
Dual targeting may be an effective strategy to enhance the specific cellular uptake of therapeutics after the nanodevices pass the BBB. In addition to incorporating ligands that bind to endothelial transporter receptors, adding a targeting ligand that interacts with another cell in the CNS can potentially increase the accumulation of nanomedicine in targeted cell population after the entry to the CNS. For example, the combination of a folate receptor (tumor cells) and a TfR (BBB endothelial cell)[72], or a TAT receptor (tumor cells) and a TfR (BBB endothelial cells) [73], both demonstrated the increased brain tumor uptake of therapeutics in vitro and extended survival compared to single targeting therapy. Still, it should be noted that this strategy would only be effective when both targeting ligands could maintain their respective targeting activity or provide synergic effects. A thorough understanding of the mechanism of how two targeting ligands synergically affect each other when integrated into one single system will provide more insight.
In addition to targeting ligands or antibodies, surfactants such as polysorbate 80 can also be chosen as surface moieties to enhance the interactions of nanoparticles with BBB. Coating polysorbate 80 to the surface of nanoparticles has been shown to enhance the efficiency for nanoparticles to interact with BBB through the following mechanisms: (1) binding with apolipoprotein E in the blood and the follow-up endocytosis by endothelial cells, and (2) suppressing the function of P-glycoprotein (P-gp), consequently reducing the efflux of nanoparticles from brain parenchyma [67]. Recent studies have successfully applied this concept both in therapeutics and diagnostics [66, 67]. These polysorbate 80 coated nanoparticles could significantly increase the brain uptake of doxorubicin at 1 hour post administration (nanoparticle with doxorubicin: undetectable amount, polysorbate 80 coated nanoparticle with doxorubicin: 2 µg/g) [87]. Another study has employed clinical relevant MRI imaging technologies and further demonstrated higher accumulation levels of gadolinium (5.34% injected dose at 2.5 hours post injection) when formulated with polysorbate 80 compared with free gadolinium (0.009% injected dose), which accordingly resulted into a 1.5-fold increase in signals [66]. However, as polysorbate 80 was passively adsorbed to the surface of nanoparticles in these studies, the in vivo stability of these formulations should be tested before moving polysorbate 80 based therapies into clinical studies.
3.2 Targeting microglia/macrophages and TAMs in the CNS
As stated in previous paragraphs, neuroinflammation, a hallmark of many neurodegenerative diseases and CNS cancer, is mediated by microglia and macrophages in the CNS. They are considered as the main candidates to function as “Trojan horses” in targeted drug delivery, because of their inherent phagocytic nature and disease homing properties in pathology [88]. Many nanoparticle platforms have been designed to target microglia/macrophage so that they can bring the therapeutics to the diseased regions for the treatments of neurodegenerative disorders (such as AD/PD) or glioblastoma [89-102]. Based on the size, surface chemistry (cationic/anionic/neutral, hydrophilic/lipophilic) and chemical structures, the inherent properties of nanoparticles can accordingly impact the targeting ability and pharmacokinetics. The following paragraph will depict different strategies related to microglia/macrophage targeting by nanomedicine for therapeutic purposes and diagnosis.
3.2.1 echanism of Nanoparticles Uptake by Microglia/Macrophage
Understanding the mechanistic internalization of nanoparticles by microglia/macrophage can provide great insights towards designing effective platforms with improved targeting efficiency. In vitro studies have suggested that clathrin-mediated endocytosis serves as the major pathway for microglia to uptake quantum dots and polymeric nanoparticles, regardless of the activation status of microglia [89, 92, 95]. These results echoed well with the findings from another study on how nanoparticle geometry affects their uptake by microglia [103]. Since clathrin-mediated endocytosis involves the binding of clathrin receptor with the surface of nanoparticles, the geometries with larger surface area to volume ratio can have higher uptake efficiency. This was confirmed by Maysinger et al., where they discovered that gold nanoparticles with “urchin-mimicking” geometry (10 times larger surface area than the rod and sphere nanoparticle) were taken up more readily (detectable under confocal microscopy) by microglial cells when compared with spherical and rod-like gold nanoparticles (undetectable under confocal microscopy) [103]. In addition to clathrin mediated endocytosis, mannose receptors and macrophage scavenger receptors have also been implicated in playing a role in nanoparticle uptake in non-activated microglia [89].
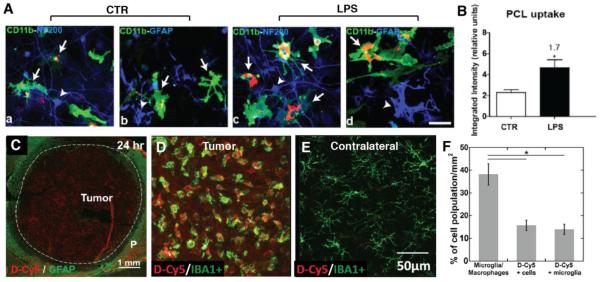
Several studies have demonstrated that activated microglia have a greater ability to internalize nanoparticles than non-activated microglia [90, 92, 95, 101, 104]. However, future studies focused on determining the differences in uptake mechanisms between activated and non-activated microglia are necessary to address this crucial knowledge gap. Specifically, under in vitro condition, LPS-activated microglial cells displayed a 1.7-fold higher uptake of poly-ε-caprolactone and polyethylene glycol (PLC) and 1.4-fold higher uptake of QD665 than non-activated microglial cells (Figure 4 A,B) [92]. In a rodent model of glioblastoma, activated TAMs accounted for most of the internalization of G4 hydroxyl-terminated PAMAM dendrimers (~4 nm) in the brain tumor microenvironments after systematic administration, as revealed from the strong co-localization of TAMs with fluorescence-labeled nanoparticles that were uniformly distributed in the tumor mass (Figure 4C–F) [101]. The enhanced nanoparticle uptake by activated microglia could be demonstrated by comparing the nanoparticle accumulation in the contralateral hemisphere of tumor bearing rats or in healthy rats, in which there was no apparent internalization of the dendrimers by non-activated microglial cells.
Figure 4. Specific uptake of nanoparticles by microglial cells and tumor-associated microglia/macrophage.
(A) Representative confocal image of PCL nanoparticles uptake by control (non-activated) microglia LPS stimulated (activated) microglia in vitro, (B) Semi-quantification based on integrated fluorescence intensity demonstrated higher 1.7-fold higher uptake of particles by LPS stimulated microglia than control microglia. Reprinted (adapted) with permission from Ref [92]. Copyright (2015) American Chemical Society. (C–F) Distribution of fluorescent-labeled generation 4 hydroxyl terminated PAMAM dendrimers (D-Cy5) in a rat brain bearing 9L glioblastoma. D-Cy5 was administrated systemically at day 9 after tumor inoculation. Confocal images of tumor region suggested that D-Cy5 (~4 nm in size) uniformly distributed across the 6 mm tumor after 24 hr post administration. (C), and was co-localized with Iba1+ TAM in tumor but not in the 'resting' microglia in the contralateral hemisphere of the same rat brain after 4 hr of D-Cy5 administration (D and E). Imaging analysis showed almost all D-Cy5+ cells were Iba1+ cells, indicating the high efficiency of TAMs taking up dendrimers (F) [101]. Green: Iba1+ microglia/macrophages; red: D-Cy5; blue: DAPI; bar: 100 μm. Reprinted from Biomaterials, Vol 52, F Zhang, et al., Uniform brain tumor distribution and tumor associated macrophage targeting of systemically administered dendrimers, 507-516, Copyright (2015), with permission from Elsevier.
Along with the nanoparticles surface geometry, nanoparticle surface functionalities also affect the amount and rate of nanoparticle uptake by microglia. For instance, Veglianese et, al. tested the uptake of poly(methyl methacrylate) nanoparticles (PMMA-NPs) with different surface charges by activated microglia; in this study, the surface properties of PMMA-NP varied between positively charged (PMMA-pos NPs, ζ-potentia: 21.5 mV), negatively charged (PMMA-NPs, ζ-potentia: −18.0 mV), and PEGylated (PMMA-PEG NPs, ζ-potentia: −2.52 mV, the size of nanoparticles did not vary significantly). After LPS-activated microglial cells were incubated with nanoparticles for 3 hours, it was found that the internalization of PMMA-pos was significantly higher (4.83 ± 5.3 mean integrated density ± SD) than that of PMMA-NPs (3.46 ± 4.53 mean integrated density ± SD). It was also found that the PEGylation reduced the nanoparticle uptake by 1.5 fold and delayed the rate of NPs uptake for 45 hours compare to non-PEGylated nanoparticles [95].
3.2.2 rategies of Manipulating Microglia/Macrophages
Recently, it was demonstrated that the manipulation of microglia/macrophages could provide beneficial effects in attenuating inflammation and reducing subsequent tissue damages [90-94, 104]. Most cases utilized two pathways: (I) the modulation of microglia/macrophage behavior, such as the activation status and the release of inflammatory signals, and (II) the control of microglia/macrophage population through microglia depletion or the prevention of infiltrating monocytes/macrophages (Table 2).
Table 2.
A summary of microglia/macrophage-targeting platforms reported in recent studies.
| Targeted cells | Platform | Route of administration | Delivered therapeutics? | Model of
evaluation/Application |
Effects on targeted cells |
|---|---|---|---|---|---|
| Microglia | Quantum dots (QD) [89] | Intracranial | No | Mixed primary glia culture, healthy mouse |
N/A |
|
| |||||
| Microglia | Gold Nanoparticle [103] | Intranasal | No | Mixed primary glia culture, healthy mouse |
N/A |
|
| |||||
|
Microglia, Astrocytes,
Oligodendrocytes |
Hydroxyl terminated G4 PAMAM Dendrimer [90] |
Intravenous | N-acetyl cysteine (NAC) | Mouse model of ischemia- induced neonatal white matter injury |
Control of microglial/macrophage activation and inflammatory cytokine release |
|
| |||||
| Microglia, Astrocytes | Hydroxyl terminated G4 PAMAM Dendrimer [104] |
Intravenous | N-acetyl cysteine (NAC) | Rabbit model of Cerebral Palsy |
Control of microglial/macrophage activation and inflammatory cytokine release |
|
| |||||
| Microglia | Liposome [91] | Intravenous | Inhibitor of macrophage migration inhibitory factor (MIF):Chicago sky blue |
Rat model of Spinal Cord
injury |
Control of microglial/macrophage activation and inflammatory cytokine release |
|
| |||||
| Microglia | Poly-ε-caprolactone (PCL)
nanoparticles [92] |
Intraspinal | Minocycline | Mouse model of Spinal Cord
injury |
Control of microglial/macrophage activation and inflammatory cytokine release |
|
| |||||
|
Microglia, Astrocytes,
Oligodendrocytes |
Carboxymethylchitosan/polya
midoamine (CMCht/PAMAM) dendrimer [93] |
- | Methylprednisolone | Mixed primary glia culture | N/A |
|
| |||||
| Microglia | PLGA nanoparticles [94] | Intraspinal | Methylprednisolone | Rat model of Spinal Cord
injury |
Reduce activated
microglia/macrophage population |
|
| |||||
| Microglia | poly(methyl methacrylate) (PMMA) nanoparticles [95] |
Intraspinal | No | Mouse model Spinal Cord injury |
N/A |
|
| |||||
| Microglia | Carbon Nanotube (CNT) [105] | - | Dexamethasone | in vitro microglia cell culture | Control of microglial/macrophage activation and inflammatory cytokine release |
|
| |||||
| Microglia | Iron oxide nanoparticle [106] | - | No | in vitro microglia cell culture | Control of microglial/macrophage activation and inflammatory cytokine release |
|
| |||||
| Circulating macrophage | Mannose modified liposome [96, 97] |
Intravenous | Dichloromethylene diphosphonate (Cl2MDP) |
Rat model of Experimental Allergic Encephalomyelitis (EAE) |
Control infiltrating
macrophages |
|
| |||||
| M2 Macrophage | Nanozyme [98] | Intravenous | Catalase | Mouse model of Parkinson’s
disease |
Attenuates oxidative stress |
|
| |||||
|
Tumor associated
macrophage |
Gold nanoshell [100, 107] | - | Photothermal therapy | in vitro microglia cell culture in vitro glioma spheroids culture |
Control of microglia/macrophage population |
|
| |||||
|
Tumor associated
macrophage |
Hydroxyl terminated G4 PAMAM Dendrimer [101] |
Intravenous | No | Rat model of Glioblastoma
(GBM) |
N/A |
|
| |||||
|
Tumor associated
macrophage |
Carbon Nanotube (CNT) [108, 109] |
Intratumoral | CpG oligodeoxynucleotides | Mouse model of GBM | Increase infiltration of anti-
tumor inflammatory cells |
|
| |||||
| Microglia | Iron oxide nanoparticle [110] | - | CpG oligodeoxynucleotides | in vitro microglia cell culture | Control microglia migration |
|
| |||||
| Microglia | G4 PAMAM Dendrimer, hydroxyl terminated (G4-OH) and amine terminated (G4- NH2) [111] |
- | Celastrol | in vitro microglia cell culture | Control of microglial/macrophage activation and inflammatory cytokine release |
|
| |||||
| Microglia | Carboxymethyldextran-block-
poly(ethylene glycol) [112] |
- | Minocycline | in vitro microglia cell culture | Control of microglial/macrophage activation and inflammatory cytokine release |
|
| |||||
| Microglia | Multivalent niacin nanoconjugates [113] |
- | No | in vitro microglia cell culture | Target lipid droplet in
microglia |
|
| |||||
| Microglia | Dendritic polyglycerol sulfate
(dPGS) [114] |
- | No | in vitro microglia cell culture and ex vivo hippocampus slice culture |
Control of microglial/macrophage activation and inflammatory cytokine release |
|
| |||||
| Microglia | Quantum Dots (QD) [115] | - | No | in vitro microglia cell culture | Study inflammation in molecular level |
|
| |||||
| Macrophage | Phosphorus Dendrimer [116] | - | No | in vitro macrophage cell
culture |
Imaging macrophage
polarization |
For each platform, delivered therapeutics; model of evaluation/application and effects on targeted cells are described.
(I) Modulation of microglial activation and control of cytokine release
Microglia/macrophage modulation has been well-documented in in vitro studies (see Table 2). Successful modulation of LPS-induced cytokine release from microglial cells was achieved through the inhibition of p38 MAPK pathway with the treatment of celastrol-encapsulated hydroxyl terminated generation 4 PAMAM dendrimers [111]. Similarly, anti-inflammatory effects were also shown through the use of minocycline-encapsulated polymeric micelles [112]. Methylprednisolone (MP)-loaded dendrimer nanoparticles have shown to affect the microglial cell viability as a function of released MP [93]. Nanoparticles with the ability to target inflammation-associated subcellular organelles such as lipid droplets in microglial cells were even realized in in vitro studies [113].
Nevertheless, successful application of these strategies in specific disease animal models is highly dependent with the type and status of inflammation, suggesting that effective treatments may differ from disease to disease. In general, the successful resolution of inflammation requires considering the stage of inflammation, physiochemical properties, toxicity of delivery vehicles and the mechanism of therapeutics. As examples in Table 2 have shown, treating inflammation at different stages of diseases may result in differing outcomes. In a recent study, Papa et al. locally injected minocycline encapsulated polymeric nanoparticles in a spinal cord injury mice model, and discovered that there were decreased levels of pro-inflammatory cytokines and reduced microglial activation in vivo [92]. However, treatments with anti-inflammatory agents may not always result in a reduction of pro-inflammatory cytokines, especially during later stages of inflammation. In another study of spinal cord injury model, Saxena et al. systemically injected liposomal formulation of macrophage migration inhibitory factor at 48 hours post injury, when inflammation is considered well established. In this case, the treatment in turn resulted in a significant increase of pro-inflammatory cytokines transcripts (such as CCL2, IL1-β, iNOS), and a simultaneous increase of anti-inflammatory cytokines transcript (such as Arginase-1 and TGF-β) that are believed to assist wound healing [91].
Properties of nanocarriers also affect the results of microglial/macrophage modulation. Sizes of nanoparticles determine the amount and the retention period of nanoparticles that stay in microglia/macrophages. Nanoparticles with larger sizes provide longer systemic circulation, providing additional temporal opportunities for microglia/macrophage uptake. Yet, their size needs to be ‘appropriate’ to achieve sufficient brain extravasation at the injured site before the nanoparticles are finally internalized by microglia/macrophages. In cases of spinal cord injury, the optimal size for systemic therapy was found to be around 200 nm, at which the treatment elongated the therapeutic window up to 90 hours [91, 92]. In a rabbit model of cerebral palsy, the systemic injection of single dose of PAMAM dendrimer-N-acetylcysteine conjugates (with ~4 nm diameter in size) at postnatal day 1 resulted in a long intracellular retention of this therapy in activated microglia and consequently elongated the therapeutic window of this disease [104]. It should be noted that the vehicles themselves may affect the activities of microglia/macrophage as well. For instance, dendritic polyglycerol sulfate (dPGS), as a polyglycerol scaffold, has been proved to be able to inhibit microglia activation in chronic inflammation [114]. Iron oxide nanoparticles could reportedly inhibit the production of IL1-β when microglial cells were activated with LPS in vitro [106]. Single wall carbon nanohorns were also shown to inhibit microglial activation, leading to reduction proliferation, delay mitotic entry, and increased apoptosis of activated mice microglial cells [92]. Hence, the intrinsic properties of the vehicle should also be taken into consideration when designing new formulations of anti-inflammatory drugs.
(II) Control of microglia/macrophage population
Regulation of microglia/macrophage population in CNS can modulate the pathological environments mediated by microglia/macrophage, although the effects can be either beneficial [117, 118] or detrimental [119, 120] according to disease models. Depletion of microglia/macrophage and inhibition of infiltrating monocytes/macrophages are the most commonly employed strategies to regulate microglia/macrophage population. Cytotoxic drugs, such as clodronate, glucocortoicoids, doxorubicin, and methotrexate were usually applied for microglia/macrophage depletion therapies, inducing the reduction of local microglia or global macrophage population [88]. For example, clodronate encapsulated in liposome was shown to successfully eliminate circulating macrophages but not the resident microglia, with concomitant reduction in clinical signs of experimental autoimmune encephalomyelitis (EAE) [96, 97]. In another case, locally delivered methylprednisolone encapsulated into PLGA nanoparticles dramatically reduced the number of macrophages/reactive microglia [94]. The other type of microglia/macrophage depletion approach is through photothermal therapy, which utilizes photoabsorbers such as organic dyes to generate localized hyperthermia upon illumination by visible-NIR light [121]. Based on this concept, Baek et al. synthesized gold nanoshell which can convert near-infrared light (NIR) to heat. Microphage can efficiently take up these nanoshells and infiltrate into the in vitro glioma sphere. NIR laser irradiation of spheroids incorporating NS loaded macrophages resulted in complete glioma growth inhibition in an irradiance dependent manner [99, 100]. However, microglia/macrophage depletion therapies are inherently aggressive, and can potentially cause significant side effects such as global immune suppression. Therefore, this approach is more valuable in life-threatening diseases such as cancer.
On the other hand, increasing the immune cell population is needed in some diseases such as glioma. The tumor microenvironment is known to be immunosuppressive; therefore, delivering immunostimulatory agents to TAMs can provide beneficial outcomes. Badie et al. showed that carbon nanotubes could target TAMs in glioma through intratumoral administration [108]. Based on this finding, they formulated CNT-cPG therapy and successfully exerted its anti-tumor activity by promoting the influx of inflammatory cells such as macrophages, nature killer cells, CD8 and CD4 cells [109]. Their recent study has even shown that, through the use of iron oxide nanoparticles, they could control the migration of targeted microglia in vitro under an external magnetic field and could further increase the infiltration of inflammatory macrophage to the brain tumor in vivo [110].
3.2.3 Imaging microglia/macrophage activity
Nanotechnology can also be employed to monitor microglia or macrophage activities (examples are listed in Table 2). Caspase-1 is a key participant in inflammation, yet there is currently no available assay for assessing its activity. Surface-modified quantum dots as nanosensors were developed for caspase-1 ratiometric measurements, and this platform has been applied in in vivo studies [115]. In another study, phosphorus dendrimers was used as a spectral nano-sensor for macrophage polarization and fate tracking in spinal cord injury [116]. Since these studies are still in early stages, the successful translation into clinics will need further optimization on instrumentation to improve the detection sensitivities.
3.3 Targeting neuronal cells
3.3.1 anoparticles intrinsically target neurons
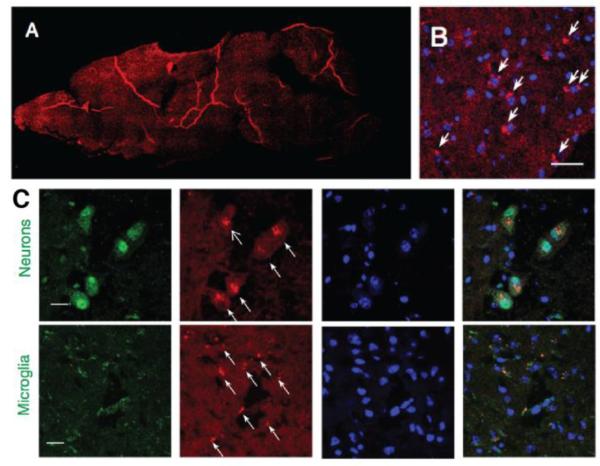
Neuron-specific delivery is challenging because neurons have a non-phagocytic nature relative to glial cells, and are highly outnumbered by the glial cell population in the brain. In some studies, neuron-specific targeting was achieved by taking advantage of the vehicle’s intrinsic ability to interact with neurons (Table 3) [122, 123]. For example, apolipoprotein E (ApoE) is a dominant lipoprotein in the brain produced by astrocytes. It delivers essential lipids to neurons via binding of the cell membrane. MacVicar et al. utilized the nature of lipid nanoparticles (LPNs) to deliver siRNA to the neuron cells through the absorption of ApoE to LPNs and the subsequent endocytosis into neurons mediated by ApoE receptor [122]. Batrakova et al. used the property of exosome as a mechanism for intercellular communication and delivered exosomes based therapeutics to CNS neurons in a mouse model of PD [123]. In this study, the exosomes (~100-200 nm) were administrated intranasally and mainly accumulated in neurons and microglia (Figure 5). This platform was then used to deliver catalase (a potent drug that could deactivate free radicals) to yield neuroprotective effects in the PD model.
Table 3.
A summary of neuron-targeting platforms reported in recent studies.
| Targeted cells | Platform | Targeting ligand | Route of administration | Delivered therapeutics? |
Model of
evaluation/Application |
Effects on targeted cells |
|---|---|---|---|---|---|---|
| Neuron | Magnetic nanoparticles [131] |
N/A | Intracranial | No | Healthy mouse | Neuron stimulation |
|
| ||||||
| Neuron | Gold nanoparticles [132] | Ts1 neurotoxin based synthetic molecule |
- | No | ex vivo hippocampus slice | Neuron stimulation |
|
| ||||||
| Neuron | Cationic block copolymer [124] |
Tet-1 | - | Plasmid DNA | in vitro neuronal cell culture | Transfection |
|
| ||||||
| Neural progenitor cells | PEGylated PEI nanoparticles [125] |
Tet-1 | Intraventricular | Plasmid DNA | Healthy mouse | Transfection |
|
| ||||||
| Neuron | HPMA-co-oligolysine copolymer nanoparticles [85] |
Tet-1 | - | Plasmid DNA | in vitro neuronal cell culture | Transfection |
|
| ||||||
| Neuron | RVG-siRNA conjugate [127] |
RVG | Intravenous | siRNA | Mouse model of encephalitis | Silence pathological gene |
|
| ||||||
| Neuron | Endosome [128] | RVG | Intravenous | siRNA | Healthy mouse | RNA and protein knockdown |
|
| ||||||
| Neuron | Gold nanoparticles [126] | RDG | Intravenous | No | Healthy mouse | N/A |
|
| ||||||
| Neuron | Organically modified silica nanoparticles [133] |
N/A | - | No | Healthy larvae | N/A |
|
| ||||||
| Injured neuron | Hydroxyl terminated G4 PAMAM Dendrimer[134] |
N/A | Intravenous, Intraventricular |
N-acetyl cysteine (NAC); Valproic acid (VPA) |
Canine model of hypothermic circulatory arrest (HCA) |
Neuroprotection |
|
| ||||||
| Neuron | Silica nanoparticles [126] | Dopamine | - | No | in vitro neuronal cell culture | N/A |
|
| ||||||
|
Neurons, Microglia,
Endothelial cells |
Exosome [123] | N/A | Intranasal | No | Mouse model of Parkinson's
disease |
N/A |
|
| ||||||
| Neuron | Lipid nanoparticles [122] | N/A | Intracortical,
Intraventricular |
siRNA | Healthy rat | |
For each platform, targeting ligand; delivered therapeutics; model of evaluation/application and effects on targeted cells are described.
Figure 5. Exosomes as vehicles for CNS drug delivery that targets neurons and microglia.
(A–B) Distribution of florescence labeled exosomes (DIL-exosomes) in a mouse brain at 4 hr after the dose was given. The exosomes were administrated intranasally to mice with 6-hydroxydopamine-induced neuroinflammation. Red: DIL-exosomes; blue: DAPI; bar: 40 μm (C) Exosomes were localized mostly in neurons and microglia in this mouse model of neuroinflammation according to confocal microscopy [123]. Green: neuron/CD11b+ microglia; red: DIL-exosomes; blue: DAPI; bar: 10 μm. Reprinted from Journal of Controlled Release, Vol 207, MJ Haney, et al., Exosomes as drug delivery vehicles for Parkinson's disease therapy, 18-30, Copyright (2015), with permission from Elsevier.
3.3.2 rategies of actively targeting neurons
To enhance the neuronal targeting, active targeting strategies, utilizing ligands such as Tet1 [85, 124, 125], dopamine [126] and rabies virus glycoprotein peptide (RVG and RDP peptide) [126-128] are being pursued (Table 3). Active targeting requires the specific expression of the target molecule on the cell of interest. GT1b gangliosides, sphingophospholipids are such molecules highly expressed on the neuronal cell types. Therefore, Tet1, a 12-amino acid peptide that binds to these molecules, was used in many studies to modify the nanoparticles surface to increase targeting efficiency [85, 124, 125]. Another type of active targeting is well adapted for CNS entry. A 29-amino-acid sequence of the RVG was identified to be critical for the entry and transport of virus in the CNS, [129] and therefore it was incorporated on the surface of delivery vehicles or directly used to deliver therapy for CNS diseases. Examples of RVG based delivery include: transvascular delivery of small interfering RNA to the CNS neurons through RVG peptide. This delivery efficiently silenced the specific gene and afforded robust protection against fatal viral encephalitis in mice [127]. Further studies have demonstrated that systemic delivery of siRNA using RVG peptide modified exosomes result in gene knockdown in neurons, microglia, oligodendrocytes [128].
However, modifying the surface of nanoparticles with excessive targeting ligands could reduce selectivity, due to enhanced binding to both target and non-target cells and organs [130]. Therefore, the balance between enhanced targeting efficiency and selectivity would be critical. Pun et al. developed an approach to precisely control over ligands density, orientation of display, and architectural display in the final polymeric construct. They found that the optimized Tet1 ligands density was around 3-5% (mol% by feed) in their in vivo model to provide neuron specific selectivity [85].
3.3.3 Targeting neurons based on pathology of disease
Neuron-specific targeting can also be achieved by taking advantage of the disease pathology. A recent example, reported by Kannan and coworkers, has indicated that hydroxyl-terminated generation-4 PAMAM dendrimers (size ~ 4 nm) could cross the impaired BBB and only be localized in injured neurons in the superficial dentate granule cell layer without accumulation in un-injured neurons via systematic administration in a canine model of hypothermic circulatory arrest induced brain injury, where neurons in the hippocampus and cerebellum region underwent apoptosis due to glutamate excitotoxicity [134]. This study further demonstrated excitotoxicity associated neuronal uptake of dendrimer can be utilized for neurological and neurobehavioral benefits by delivering dendrimer based therapy to the injured neuron [134].
3.3.4 Targeting neurons for stimulation
Neuron-targeting nanoparticles can also be used as tools to stimulate neurons. Gold nanoparticles modified with neuron-targeting ligands have been demonstrated to transduce millisecond pulses of light into heat, which changes neuron membrane capacitance, depolarizing the cell and eliciting action potentials [132]. Magnetic nanoparticles delivered to the brain can dissipate heat, triggering widespread and reversible firing in specific neurons [131]. These studies extended the use of neuron-targeting nanoparticles from therapeutics application to non-invasive methods for the study of neuronal activities or brain circuits.
3.3 Targeting Astrocytes
Although the contribution of astrocytes in pathological conditions was discussed earlier, drug delivery systems designed to specifically target these cells is still limited. Several viral vectors have been described to inherently target astrocytes in vivo (Table 4). Lentiviral vectors (LV) were found to have pseudotype-dependent neuron and glia tropism. Based on such vectors, it is possible to target astrocytes in vivo through modifying LV pseudotype, integrating cell-specific promoters, and using miRNA post-transcriptional regulatory elements [135-137]. However, more studies are needed to take the heterogeneity of astrocytes into consideration, especially when astrocytes are in pathological conditions. Another promising type of viral vectors, adeno-associated virus (AAV) vectors, has already been studied in clinical trials for the treatment of PD. Recently AAV vector-based therapy was found to target astrocytic CN/NFAT signaling in a mouse model of AD, resulting in reduced glial activation, lower amyloid levels, and subsequent improvement of cognitive and synaptic functions [138]. Nevertheless, there are concerns regarding the safety, tolerance, and immunogenicity of viral vector-based therapies, which has limited their translational potential.
Table 4.
A summary of astrocytes-targeting platforms reported in recent studies.
| Targeted cells | Platform | Route of administration | Delivered therapeutics? | Model of
evaluation/Application |
Effects on targeted cells |
|---|---|---|---|---|---|
| Astrocytes | Lentiviral Vector [135] | Intracranial | Gene | Healthy mouse | Transfection |
|
| |||||
| Astrocytes | Lentiviral Vector [136] | Intracranial | Gene | Healthy rat | Transfection |
|
| |||||
| Astrocytes | Adeno-Associated Virus (AAV) [138] |
Intracranial | Astrocyte-specific Gfa2
promoter |
Mouse Model of Alzheimer’s
Disease |
Reduced glia activation |
|
| |||||
| Astrocytes | Iron oxide, silver nanoparticles [139] |
- | No | in vitro | N/A |
|
| |||||
|
Microglia, Astrocytes,
Oligodendrocytes |
Carboxymethylchitosan/polya
midoamine (CMCht/PAMAM) dendrimer [140] |
- | No | in vitro | N/A |
|
| |||||
| Astrocytes, neurons | PLGA nanoparticles[141] | Intraspinal | Neurotrophic factor (GDNF) | Spinal cord injury | N/A |
|
| |||||
| Astrocytes, microglia | Hydroxyl terminated G4 PAMAM Dendrimer [104] |
Intravenous | NAC | CP | Reduced glia activation |
|
| |||||
| Astrocytes, Oligodendrocytes | Hydroxyl terminated G4 PAMAM Dendrimer [90] |
Intravenous | NAC | Mouse model of Ischemia- induced neonatal white matter injury |
N/A |
For each platform; delivered therapeutics; model of evaluation/application and effects on targeted cells are described.
Instead of utilizing viral vectors as therapeutic vehicles, synthetic nanoparticles such as iron oxide nanoparticles, silver nanoparticles, and some polymeric nanoparticles can be potential candidates for astrocyte specific targeting, even though many related studies remain in the in vitro stage [139, 140]. A few in vivo studies have shown that the internalization of nanoparticle by astrocyte was always accompanied by a more dominant nanoparticle uptake by other CNS cell types such as microglia [104, 142] or neurons [141]. In a study where PAMAM dendrimer was used for the treatment of ischemia-induced neonatal white matter injury, systemically administrated dendrimers were first found in astrocytes and oligodendrocytes at the initial stage of the injury. However, at later stage of neuroinflammation, dendrimer uptake switched from astrocytes into activated microglia, indicating that pathological environments in the brain might play a major role in the uptake of nanoparticles by different cells [90].
3.4 Targeting brain cancer cells
Brain cancer can be classified into different types according to the tissue of origin. Current studies have focused on developing therapies for glioblastoma, the most common and aggressive type of brain cancer which derives from astrocytes. Delivering drugs specifically to the tumor mass is considered as an effective means to increase the specific uptake by cancer cells. To achieve this, both passive and active strategies have been employed.
A study that utilized non-targeting polyglutamate-paclitaxel (CT2103, Xyotax) has reached clinical trials for the treatments of brain cancer, where it was tested in combination with temozolomide and radiotherapy [143, 144]. As no targeting ligand was used in this case, passive diffusion is major pathway for drug accumulation in the brain tumor.
In terms of active targeting strategies, a recent study has shown that CD44 receptor is highly expressed in GBM cell lines and primary glioma cells [145]. Grafting lipid-based nanoparticles (LNPs) with hyaluronan (HA), which specifically target CD44 receptor significantly increase the binding of LNPs to these GBM cell lines and primary glioma cells. When HA-grated LNPs were complexed with therapeutic siRNA, this increase in binding efficiency could translate into an effective glioma cell death (90%) in vitro, and 60% survival at day 95 post-tumor inoculation (the median survival of Mock-treated mice was 33 days) after 4 systemic doses treatment in vivo.
In many cases, both endothelial cells at the blood-tumor brain barrier (BBTB) and the glioma cells overexpress similar types of receptors (such as LPR1), ligands that actively target the BBTB often possess intrinsic targeting to glioma cells as well [70, 72, 73]. This included ANG1005 (now in clinical trial), a paclitaxel-angiopep conjugate where angiopep was a peptide designed to target LPR1 expressed on both BBTB and glioma cells [76-80]. However, it is worth noting that even when targeting ligands were incorporated, the total amount of injected dose that is delivered to the brain was only ~1% or less [144].
Recently, some other studies suggested a new strategy for the treatment of glioma by using polymer nanoparticle-delivered gene therapy [146, 147]. Although the polymer was not conjugated with any targeting ligand, but the possibility to recombine the building blocks of nanoparticle made it possible to select a combination with highest targeting and transfection efficiency.
4. Translation of CNS cell-targeting nanomedicine to the clinics
In the previous sections, we have discussed several novel strategies that have been used in recent years to enhance the cell-specific targeting of nanomedicine for different CNS diseases. It should be noted that most of these studies are still in pre-clinical stage. Drugs for CNS disorders are less than one-half as likely to achieve marketing approval than other drugs, and it also takes more than a year longer for the development [November/December Tufts CSDD Impact Report]. In addition to the widely-acknowledged barriers such as the BBB [148] and the extracellular space of brain parenchyma (both known for preventing nanoparticles from reaching the targeting site), [149] there are a few additional factors that need to be considered during the translation of cell-targeting nanomedicine.
In most studies, targeting effects are tightly associated with disease pathologies in the tested models. Therefore, it is crucial to select a clinically relevant disease model for the development and evaluation of nanomedicine for translational purposes. For instance, to develop a therapy that targets the endothelial cells for the treatment of glioblastoma, an orthotopic brain tumor model is a better representative of the tumor microenvironment than a subcutaneous tumor model. To develop therapies that are based on active target mechanisms, it will be essential to use cell lines that possess similar expression to the target cells in the patients as opposed to those do not carry similar patterns.
Secondly, many studies have reported that less than 1% of total injected dose eventually reach in the brain by intravenous administration [150]. Though it could be of interest to enhance the overall brain uptake of therapeutics by using other convective administration routes (such as intracerebral or intraventricular injection), even less than 1% of total systematic-injected dose in the targeted cells could provide significant effects if a majority of the drug accumulation is contributed by the uptake in targeted cells [104]. Therefore, maintaining a high targeting efficiency to the key cells can also greatly enhance the therapeutic efficacy.
Lastly, the toxicity of nanomedicine should be addressed. It is undoubtedly crucial to select a drug-delivering vehicle that is intrinsically safe at the therapeutic relevant dose for the clinical translation. Many drug delivery platforms were discovered to cause CNS toxicity. For example, intra-nasally administrated generation 5 cationic PAMAM dendrimers can modulate gene expression of brain-delivered neurotrophic pathway at a dose of 15 µg per mouse [151]. This dendrimer can also cause neurotoxicity by inducing autophagic flux and upregulation of ROS in the glioma cell lines in a concentration dependent manner (concentration ranging from 0 to 100 µg/mL) [152]. Similarly, many non-biodegradable vehicles such as carbon nanotubes and gold NPs can also cause immunogenicity in the brain: a single Intracortical dose (500 ng/mouse) of multi-walled carbon nanotubes was found to cause glial activation in the mouse brain at 30 days post injection [153]; intranasal administration of rod shape gold nanoparticles (5 μL at a concentration of 109 particle/mL) could induce biphasic activation of microglia at one day and a week post administration [103] The toxic properties of drug delivery vehicles need to be cautiously scrutinized in clinically relevant animal models before being translated into clinical study stage.
5. Conclusions
Nanomedicine offers significant opportunities to enhance drug delivery across the BBB, and for targeting specific cells. These advancements have shown various possibilities for effective treatments of many CNS disorders. The improved understanding of the pathologies of CNS disorders, from the cellular and molecular levels in recent years, has been embraced when it comes to design smart nanomaterials. Developing nanotherapies that could target the pathological cells in the CNS has attracted significant recent interest, and has led to promising results in animal models. In this review, we have systematically introduced the functions of several CNS cell types: endothelial cells, microglia/macrophages, TAMs, neurons, astrocytes, brain tumor cells, and their pathological roles in CNS disorders. We then connected the recent studies utilizing nanomedicine with targeting properties in accordance with the pathologies in these cells, and the key strategies being considered for engineering an appropriate delivery platform. This provides the general guidelines for designing of cell-specific targeting nanotherapies for CNS disorders.
Abbreviations
- ABC
ATP-binding cassette
- AD
Alzheimer’s disease
- ALS
amyotrophic lateral sclerosis
- BBB
blood brain barrier
- BBTB
blood brain tumor barrier
- CCL
chemokine ligand
- CNS
central nervous system
- dPGS
dendritic polyglycerol sulfate
- GFAP
glial fibrillary acidic protein
- IL
interleukin
- LFR
lactoferrin
- LPS
lipopolysaccarides
- MMP
matrix metalloproteinase
- MS
multiple sclerosis
- MTFR
melanotransferrin receptor
- NO
nitro oxide
- PAMAM
polyamidoamine
- PD
Parkinson’s disease
- ROS
reactive oxygen species
- TAM
tumor-associated microglia/macrophage
- TfR
transferrin receptor
- TNF
tumor necrosis factor
- VCAM
vascular adhesion molecule
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Krex D, Klink B, Hartmann C, von Deimling A, Pietsch T, Simon M, Sabel M, Steinbach JP, Heese O, Reifenberger G, Weller M, Schackert G, German Glioma N. Long-term survival with glioblastoma multiforme. Brain. 2007;130:2596–2606. doi: 10.1093/brain/awm204. [DOI] [PubMed] [Google Scholar]
- [2].Kreuter J. Drug delivery to the central nervous system by polymeric nanoparticles: What do we know? Advanced Drug Delivery Reviews. 2014;71:2–14. doi: 10.1016/j.addr.2013.08.008. [DOI] [PubMed] [Google Scholar]
- [3].Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx : the journal of the American Society for Experimental NeuroTherapeutics. 2005;2:3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Abbott NJ, Patabendige AAK, Dolman DEM, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiology of Disease. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- [5].Schinkel AH. P-glycoprotein, a gatekeeper in the blood-brain barrier. Advanced Drug Delivery Reviews. 1999;36:179–194. doi: 10.1016/s0169-409x(98)00085-4. [DOI] [PubMed] [Google Scholar]
- [6].Begley DJ. ABC transporters and the blood-brain barrier. Current Pharmaceutical Design. 2004;10:1295–1312. doi: 10.2174/1381612043384844. [DOI] [PubMed] [Google Scholar]
- [7].Chuang VTG, Kragh-Hansen U, Otagiri M. Pharmaceutical strategies utilizing recombinant human serum albumin. Pharmaceutical Research. 2002;19:569–577. doi: 10.1023/a:1015396825274. [DOI] [PubMed] [Google Scholar]
- [8].Jefferies WA, Brandon MR, Hunt SV, Williams AF, Gatter KC, Mason DY. TRANSFERRIN RECEPTOR ON ENDOTHELIUM OF BRAIN CAPILLARIES. Nature. 1984;312:162–163. doi: 10.1038/312162a0. [DOI] [PubMed] [Google Scholar]
- [9].Demeule M, Poirier J, Jodoin J, Bertrand Y, Desrosiers RR, Dagenais C, Nguyen T, Lanthier J, Gabathuler R, Kennard M, Jefferies WA, Karkan D, Tsai S, Fenart L, Cecchelli R, Beliveau R. High transcytosis of melanotransferrin (P97) across the blood brain barrier. Journal of Neurochemistry. 2002;83:924–933. doi: 10.1046/j.1471-4159.2002.01201.x. [DOI] [PubMed] [Google Scholar]
- [10].Talukder MJR, Takeuchi T, Harada E. Receptor-mediated transport of lactoferrin into the cerebrospinal fluid via plasma in young calves. Journal of Veterinary Medical Science. 2003;65:957–964. doi: 10.1292/jvms.65.957. [DOI] [PubMed] [Google Scholar]
- [11].Dehouck B, Fenart L, Dehouck MP, Pierce A, Torpier G, Cecchelli R. A new function for the LDL receptor: Transcytosis of LDL across the blood-brain barrier. Journal of Cell Biology. 1997;138:877–889. doi: 10.1083/jcb.138.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- [13].Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacological Reviews. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- [14].Cirrito JR, Deane R, Fagan AM, Spinner ML, Parsadanian M, Finn MB, Jiang H, Prior JL, Sagare A, Bales KR, Paul SM, Zlokovic BV, Piwnica-Worms D, Holtzman DM. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. Journal of Clinical Investigation. 2005;115:3285–3290. doi: 10.1172/JCI25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Vogelgesang S, Cascorbi I, Schroeder E, Pahnke J, Kroemer HK, Siegmund W, Kunert-Keil C, Walker LC, Warzok RW. Deposition of Alzheimer's beta-amyloid is inversely correlated with P-glycoprotein expression in the brains of elderly non-demented humans. Pharmacogenetics. 2002;12:535–541. doi: 10.1097/00008571-200210000-00005. [DOI] [PubMed] [Google Scholar]
- [16].Deane R, Zlokovic BV. Role of the blood-brain barrier in the pathogenesis of Alzheimer's disease. Current Alzheimer Research. 2007;4:191–197. doi: 10.2174/156720507780362245. [DOI] [PubMed] [Google Scholar]
- [17].Bartels AL, Willemsen ATM, Kortekaas R, de Jong BM, de Vries R, de Klerk O, van Oostrom JCH, Portman A, Leenders KL. Decreased blood-brain barrier P-glycoprotein function in the progression of Parkinson's disease, PSP and MSA. Journal of Neural Transmission. 2008;115:1001–1009. doi: 10.1007/s00702-008-0030-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kortekaas R, Leenders KL, van Oostrom JCH, Vaalburg W, Bart J, Willemsen ATM, Hendrikse NH. Blood-brain barrier dysfunction in Parkinsonian midbrain in vivo. Annals of Neurology. 2005;57:176–179. doi: 10.1002/ana.20369. [DOI] [PubMed] [Google Scholar]
- [19].Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nature Neuroscience. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- [20].Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of Microglia. Physiological Reviews. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- [21].Charles NA, Holland EC, Gilbertson R, Glass R, Kettenmann H. The Brain Tumor Microenvironment. Glia. 2011;59:1169–1180. doi: 10.1002/glia.21136. [DOI] [PubMed] [Google Scholar]
- [22].Jack C, Ruffini F, Bar-Or A, Antel JP. Microglia and multiple sclerosis. Journal of Neuroscience Research. 2005;81:363–373. doi: 10.1002/jnr.20482. [DOI] [PubMed] [Google Scholar]
- [23].Watters JJ, Schartner JM, Badie B. Microglia function in brain tumors. Journal of Neuroscience Research. 2005;81:447–455. doi: 10.1002/jnr.20485. [DOI] [PubMed] [Google Scholar]
- [24].Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms Underlying Inflammation in Neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].McGeer PL, McGeer EG. Targeting microglia for the treatment of Alzheimer's disease. Expert Opinion on Therapeutic Targets. 2015;19:497–506. doi: 10.1517/14728222.2014.988707. [DOI] [PubMed] [Google Scholar]
- [26].Cherry JD, Olschowka JA, O'Banion MK. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. Journal of Neuroinflammation. 2014;11 doi: 10.1186/1742-2094-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Boche D, Perry VH, Nicoll JAR. Review: Activation patterns of microglia and their identification in the human brain. Neuropathology and Applied Neurobiology. 2013;39:3–18. doi: 10.1111/nan.12011. [DOI] [PubMed] [Google Scholar]
- [28].Liu T, Gao YJ, Ji RR. Emerging role of Toll-like receptors in the control of pain and itch. Neuroscience Bulletin. 2012;28:131–144. doi: 10.1007/s12264-012-1219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Moskowitz MA, Lo EH, Iadecola C. The Science of Stroke: Mechanisms in Search of Treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hu XM, Li PY, Guo YL, Wang HY, Leak RK, Chen SE, Gao YQ, Chen J. Microglia/Macrophage Polarization Dynamics Reveal Novel Mechanism of Injury Expansion After Focal Cerebral Ischemia. Stroke. 2012;43:3063–U3474. doi: 10.1161/STROKEAHA.112.659656. [DOI] [PubMed] [Google Scholar]
- [31].Evans MC, Couch Y, Sibson N, Turner MR. Inflammation and neurovascular changes in amyotrophic lateral sclerosis. Molecular and Cellular Neuroscience. 2013;53:34–41. doi: 10.1016/j.mcn.2012.10.008. [DOI] [PubMed] [Google Scholar]
- [32].Blandini F. Neural and Immune Mechanisms in the Pathogenesis of Parkinson's Disease. Journal of Neuroimmune Pharmacology. 2013;8:189–201. doi: 10.1007/s11481-013-9435-y. [DOI] [PubMed] [Google Scholar]
- [33].Tannahill GM, Iraci N, Gaude E, Frezza C, Pluchino S. Metabolic reprograming of mononuclear phagocytes in progressive multiple sclerosis. Frontiers in Immunology. 2015;6:7. doi: 10.3389/fimmu.2015.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hwang O. Role of oxidative stress in Parkinson's disease. Experimental neurobiology. 2013;22:11–17. doi: 10.5607/en.2013.22.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Michelucci A, Heurtaux T, Grandbarbe L, Morga E, Heuschling P. Characterization of the microglial phenotype under specific pro-inflammatory and anti-inflammatory conditions: Effects of oligomeric and fibrillar amyloid-beta. Journal of Neuroimmunology. 2009;210:3–12. doi: 10.1016/j.jneuroim.2009.02.003. [DOI] [PubMed] [Google Scholar]
- [36].Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol. 2010;6:193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- [37].Breza T JS, Magro CM. Lichenoid and granulomatous dermatitis associated with atypical mycobacterium infections. J Cutan Pathol. 2006;33:512–515. doi: 10.1111/j.1600-0560.2006.00474.x. [DOI] [PubMed] [Google Scholar]
- [38].Surace MJ, Block ML. Targeting microglia-mediated neurotoxicity: the potential of NOX2 inhibitors. Cellular and Molecular Life Sciences. 2012;69:2409–2427. doi: 10.1007/s00018-012-1015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Chen H, Kim GS, Okami N, Narasimhan P, Chan PH. NADPH oxidase is involved in post-ischemic brain inflammation. Neurobiology of Disease. 2011;42:341–348. doi: 10.1016/j.nbd.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Nayak D, Huo Y, Kwang WXT, Pushparaj PN, Kumar SD, Ling EA, Dheen ST. SPHINGOSINE KINASE 1 REGULATES THE EXPRESSION OF PROINFLAMMATORY CYTOKINES AND NITRIC OXIDE IN ACTIVATED MICROGLIA. Neuroscience. 2010;166:132–144. doi: 10.1016/j.neuroscience.2009.12.020. [DOI] [PubMed] [Google Scholar]
- [41].Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. Journal of Neuroscience. 2001;21:2580–2588. doi: 10.1523/JNEUROSCI.21-08-02580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhou Y, Ling E-A, Dheen ST. Dexamethasone suppresses monocyte chemoattractant protein-1 production via mitogen activated protein kinase phosphatase-1 dependent inhibition of Jun N-terminal kinase and p38 mitogen-activated protein kinase in activated rat microglia. Journal of Neurochemistry. 2007;102:667–678. doi: 10.1111/j.1471-4159.2007.04535.x. [DOI] [PubMed] [Google Scholar]
- [43].da Fonseca ACC, Badie B. Microglia and Macrophages in Malignant Gliomas: Recent Discoveries and Implications for Promising Therapies. Clinical & Developmental Immunology. 2013:5. doi: 10.1155/2013/264124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Komohara Y, Ohnishi K, Kuratsu J, Takeya M. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. Journal of Pathology. 2008;216:15–24. doi: 10.1002/path.2370. [DOI] [PubMed] [Google Scholar]
- [45].Wu A, Wei J, Kong LY, Wang YT, Priebe W, Qiao W, Sawaya R, Heimberger AB. Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro-Oncology. 2010;12:1113–1125. doi: 10.1093/neuonc/noq082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wesolowska A, Kwiatkowska A, Slomnicki L, Dembinski M, Master A, Sliwa M, Franciszkiewicz K, Chouaib S, Kaminska B. Microglia-derived TGF-beta as an important regulator of glioblastoma invasion - an inhibition of TGF-beta-dependent effects by shRNA against human TGF-beta type II receptor. Oncogene. 2008;27:918–930. doi: 10.1038/sj.onc.1210683. [DOI] [PubMed] [Google Scholar]
- [48].Markovic DS, Vinnakota K, Chirasani S, Synowitz M, Raguet H, Stock K, Sliwa M, Lehmann S, Kalin R, van Rooijeng N, Holmbeck K, Heppner FL, Kiwit J, Matyash V, Lehnardt S, Kaminska B, Glass R, Kettenmann H. Gliomas induce and exploit microglial MT1-MMP expression for tumor expansion. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:12530–12535. doi: 10.1073/pnas.0804273106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Held-Feindt J, Hattermann K, Mueerkoester SS, Wedderkopp H, Knerlich-Lukoschus F, Ungefroren H, Mehdorn HM, Mentlein R. CX3CR1 promotes recruitment of human glioma-infiltrating microglia/macrophages (GIMs) Experimental Cell Research. 2010;316:1553–1566. doi: 10.1016/j.yexcr.2010.02.018. [DOI] [PubMed] [Google Scholar]
- [50].Zhai HY, Heppner FL, Tsirka SE. Microglia/Macrophages Promote Glioma Progression. Glia. 2011;59:472–485. doi: 10.1002/glia.21117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Maragakis NJ, Rothstein JD. Mechanisms of disease: astrocytes in neurodegenerative disease. Nature Clinical Practice Neurology. 2006;2:679–689. doi: 10.1038/ncpneuro0355. [DOI] [PubMed] [Google Scholar]
- [52].Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathologica. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Seifert G, Schilling K, Steinhauser C. Astrocyte dysfunction in neurological disorders: a molecular perspective. Nature Reviews Neuroscience. 2006;7:194–206. doi: 10.1038/nrn1870. [DOI] [PubMed] [Google Scholar]
- [54].Binder DK, Steinhauser C. Functional changes in astroglial cells in epilepsy. Glia. 2006;54:358–368. doi: 10.1002/glia.20394. [DOI] [PubMed] [Google Scholar]
- [55].Choi DW. Ischemia-induced neuronal apoptosis. Current Opinion in Neurobiology. 1996;6:667–672. doi: 10.1016/s0959-4388(96)80101-2. [DOI] [PubMed] [Google Scholar]
- [56].Park E, Velumian AA, Fehlings MG. The role of excitotoxicity in secondary mechanisms of spinal cord injury: A review with an emphasis on the implications for white matter degeneration. Journal of Neurotrauma. 2004;21:754–774. doi: 10.1089/0897715041269641. [DOI] [PubMed] [Google Scholar]
- [57].Morganti-Kossmann MC, Satgunaseelan L, Bye N, Kossmann T. Modulation of immune response by head injury. Injury-International Journal of the Care of the Injured. 2007;38:1392–1400. doi: 10.1016/j.injury.2007.10.005. [DOI] [PubMed] [Google Scholar]
- [58].Arundine M, Tymianski M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium. 2003;34:325–337. doi: 10.1016/s0143-4160(03)00141-6. [DOI] [PubMed] [Google Scholar]
- [59].Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nature Reviews Neuroscience. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- [60].Yuan JY, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407:802–809. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]
- [61].Bell E. INNATE IMMUNITY Endothelial cells as sentinels. Nat Rev Immunol. 2009;9:532–532. [Google Scholar]
- [62].Frijns CJ, Kappelle LJ, van Gijn J, Nieuwenhuis HK, Sixma JJ, Fijnheer R. Soluble adhesion molecules reflect endothelial cell activation in ischemic stroke and in carotid atherosclerosis. Stroke. 1997;28:2214–2218. doi: 10.1161/01.str.28.11.2214. [DOI] [PubMed] [Google Scholar]
- [63].Frechou M, Beray-Berthat V, Raynaud JS, Meriaux S, Gombert F, Lancelot E, Plotkine M, Marchand-Leroux C, Ballet S, Robert P, Louin G, Margaill I. Detection of vascular cell adhesion molecule-1 expression with USPIO-enhanced molecular MRI in a mouse model of cerebral ischemia. Contrast Media Mol Imaging. 2013;8:157–164. doi: 10.1002/cmmi.1512. [DOI] [PubMed] [Google Scholar]
- [64].Montagne A, Gauberti M, Macrez R, Jullienne A, Briens A, Raynaud JS, Louin G, Buisson A, Haelewyn B, Docagne F, Defer G, Vivien D, Maubert E. Ultra-sensitive molecular MRI of cerebrovascular cell activation enables early detection of chronic central nervous system disorders. Neuroimage. 2012;63:760–770. doi: 10.1016/j.neuroimage.2012.07.018. [DOI] [PubMed] [Google Scholar]
- [65].Gauberti M, Montagne A, Marcos-Contreras OA, Le Behot A, Maubert E, Vivien D. Ultra-sensitive molecular MRI of vascular cell adhesion molecule-1 reveals a dynamic inflammatory penumbra after strokes. Stroke. 2013;44:1988–1996. doi: 10.1161/STROKEAHA.111.000544. [DOI] [PubMed] [Google Scholar]
- [66].Koffie RM, Farrar CT, Saidi LJ, William CM, Hyman BT, Spires-Jones TL. Nanoparticles enhance brain delivery of blood-brain barrier-impermeable probes for in vivo optical and magnetic resonance imaging. Proc Natl Acad Sci U S A. 2011;108:18837–18842. doi: 10.1073/pnas.1111405108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Zhao LX, Liu AC, Yu SW, Wang ZX, Lin XQ, Zhai GX, Zhang QZ. The permeability of puerarin loaded poly(butylcyanoacrylate) nanoparticles coated with polysorbate 80 on the blood-brain barrier and its protective effect against cerebral ischemia/reperfusion injury. Biol Pharm Bull. 2013;36:1263–1270. doi: 10.1248/bpb.b12-00769. [DOI] [PubMed] [Google Scholar]
- [68].Niewoehner J, Bohrmann B, Collin L, Urich E, Sade H, Maier P, Rueger P, Stracke JO, Lau W, Tissot AC, Loetscher H, Ghosh A, Freskgard PO. Increased Brain Penetration and Potency of a Therapeutic Antibody Using a Monovalent Molecular Shuttle. Neuron. 2014;81:49–60. doi: 10.1016/j.neuron.2013.10.061. [DOI] [PubMed] [Google Scholar]
- [69].Bell RD, Ehlers MD. Breaching the blood-brain barrier for drug delivery. Neuron. 2014;81:1–3. doi: 10.1016/j.neuron.2013.12.023. [DOI] [PubMed] [Google Scholar]
- [70].Couch JA, Yu YJ, Zhang Y, Tarrant JM, Fuji RN, Meilandt WJ, Solanoy H, Tong RK, Hoyte K, Luk W, Lu YM, Gadkar K, Prabhu S, Ordonia BA, Nguyen Q, Lin YW, Lin ZH, Balazs M, Scearce-Levie K, Ernst JA, Dennis MS, Watts RJ. Addressing Safety Liabilities of TfR Bispecific Antibodies That Cross the Blood-Brain Barrier. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3005338. [DOI] [PubMed] [Google Scholar]
- [71].Liu YY, Ran R, Chen JT, Kuang QF, Tang J, Mei L, Zhang QY, Gao HL, Zhang ZR, He Q. Paclitaxel loaded liposomes decorated with a multifunctional tandem peptide for glioma targeting. Biomaterials. 2014;35:4835–4847. doi: 10.1016/j.biomaterials.2014.02.031. [DOI] [PubMed] [Google Scholar]
- [72].Gao JQ, Lv Q, Li LM, Tang XJ, Li FZ, Hu YL, Han M. Glioma targeting and blood-brain barrier penetration by dual-targeting doxorubincin liposomes. Biomaterials. 2013;34:5628–5639. doi: 10.1016/j.biomaterials.2013.03.097. [DOI] [PubMed] [Google Scholar]
- [73].Zong T, Mei L, Gao H, Cai W, Zhu P, Shi K, Chen J, Wang Y, Gao F, He Q. Synergistic dual-ligand doxorubicin liposomes improve targeting and therapeutic efficacy of brain glioma in animals. Mol Pharm. 2014;11:2346–2357. doi: 10.1021/mp500057n. [DOI] [PubMed] [Google Scholar]
- [74].Yemisci M, Caban S, Gursoy-Ozdemir Y, Lule S, Novoa-Carballal R, Riguera R, Fernandez-Megia E, Andrieux K, Couvreur P, Capan Y, Dalkara T. Systemically administered brain-targeted nanoparticles transport peptides across the blood-brain barrier and provide neuroprotection. J Cerebr Blood F Met. 2015;35:469–475. doi: 10.1038/jcbfm.2014.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Demeule M, Beaudet N, Regina A, Besserer-Offroy E, Murza A, Tetreault P, Belleville K, Che C, Larocque A, Thiot C, Beliveau R, Longpre JM, Marsault E, Leduc R, Lachowicz JE, Gonias SL, Castaigne JP, Sarret P. Conjugation of a brain-penetrant peptide with neurotensin provides antinociceptive properties. Journal of Clinical Investigation. 2014;124:1199–1213. doi: 10.1172/JCI70647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Drappatz J, Brenner A, Wong ET, Eichler A, Schiff D, Groves MD, Mikkelsen T, Rosenfeld S, Sarantopoulos J, Meyers CA, Fielding RM, Elian K, Wang X, Lawrence B, Shing M, Kelsey S, Castaigne JP, Wen PY. Phase I study of GRN1005 in recurrent malignant glioma. Clin Cancer Res. 2013;19:1567–1576. doi: 10.1158/1078-0432.CCR-12-2481. [DOI] [PubMed] [Google Scholar]
- [77].Regina A, Demeule M, Che C, Lavallee I, Poirier J, Gabathuler R, Beliveau R, Castaigne JP. Antitumour activity of ANG1005, a conjugate between paclitaxel and the new brain delivery vector Angiopep-2. Brit J Pharmacol. 2008;155:185–197. doi: 10.1038/bjp.2008.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Bertrand Y, Currie JC, Poirier J, Demeule M, Abulrob A, Fatehi D, Stanimirovic D, Sartelet H, Castaigne JP, Beliveau R. Influence of glioma tumour microenvironment on the transport of ANG1005 via low-density lipoprotein receptor-related protein 1. Br J Cancer. 2011;105:1697–1707. doi: 10.1038/bjc.2011.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Thomas FC, Taskar K, Rudraraju V, Goda S, Thorsheim HR, Gaasch JA, Mittapalli RK, Palmieri D, Steeg PS, Lockman PR, Smith QR. Uptake of ANG1005, A Novel Paclitaxel Derivative, Through the Blood-Brain Barrier into Brain and Experimental Brain Metastases of Breast Cancer. Pharmaceutical Research. 2009;26:2486–2494. doi: 10.1007/s11095-009-9964-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Che C, Yang GQ, Thiot C, Lacoste MC, Currie JC, Demeule M, Regina A, Beliveau R, Castaigne JP. New Angiopep-Modified Doxorubicin (ANG1007) and Etoposide (ANG1009) Chemotherapeutics With Increased Brain Penetration. J Med Chem. 2010;53:2814–2824. doi: 10.1021/jm9016637. [DOI] [PubMed] [Google Scholar]
- [81].Boado RJ, Hui EKW, Lu JZ, Sumbria RK, Pardridge WM. Blood-Brain Barrier Molecular Trojan Horse Enables Imaging of Brain Uptake of Radioiodinated Recombinant Protein in the Rhesus Monkey. Bioconjugate Chem. 2013;24:1741–1749. doi: 10.1021/bc400319d. [DOI] [PubMed] [Google Scholar]
- [82].Shilo M, Motiei M, Hana P, Popovtzer R. Transport of nanoparticles through the blood-brain barrier for imaging and therapeutic applications. Nanoscale. 2014;6:2146–2152. doi: 10.1039/c3nr04878k. [DOI] [PubMed] [Google Scholar]
- [83].An S, Kuang Y, Shen T, Li J, Ma H, Guo Y, He X, Jiang C. Brain-targeting delivery for RNAi neuroprotection against cerebral ischemia reperfusion injury. Biomaterials. 2013;34:8949–8959. doi: 10.1016/j.biomaterials.2013.07.060. [DOI] [PubMed] [Google Scholar]
- [84].Yang ZZ, Li JQ, Wang ZZ, Dong DW, Qi XR. Tumor-targeting dual peptides-modified cationic liposomes for delivery of siRNA and docetaxel to gliomas. Biomaterials. 2014;35:5226–5239. doi: 10.1016/j.biomaterials.2014.03.017. [DOI] [PubMed] [Google Scholar]
- [85].Chu DS, Schellinger JG, Bocek MJ, Johnson RN, Pun SH. Optimization of Tet1 ligand density in HPMA-co-oligolysine copolymers for targeted neuronal gene delivery. Biomaterials. 2013;34:9632–9637. doi: 10.1016/j.biomaterials.2013.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Kurzrock R, Gabrail N, Chandhasin C, Moulder S, Smith C, Brenner A, Sankhala K, Mita A, Elian K, Bouchard D, Sarantopoulos J. Safety, pharmacokinetics, and activity of GRN1005, a novel conjugate of angiopep-2, a peptide facilitating brain penetration, and paclitaxel, in patients with advanced solid tumors. Mol Cancer Ther. 2012;11:308–316. doi: 10.1158/1535-7163.MCT-11-0566. [DOI] [PubMed] [Google Scholar]
- [87].Gulyaev AE, Gelperina SE, Skidan IN, Antropov AS, Kivman GY, Kreuter J. Significant transport of doxorubicin into the brain with polysorbate 80-coated nanoparticles. Pharmaceutical Research. 1999;16:1564–1569. doi: 10.1023/a:1018983904537. [DOI] [PubMed] [Google Scholar]
- [88].Patel SK, Janjic JM. Macrophage Targeted Theranostics as Personalized Nanomedicine Strategies for Inflammatory Diseases. Theranostics. 2015;5:150–172. doi: 10.7150/thno.9476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Minami SS, Sun B, Popat K, Kauppinen T, Pleiss M, Zhou Y, Ward ME, Floreancig P, Mucke L, Desai T, Gan L. Selective targeting of microglia by quantum dots. J Neuroinflammation. 2012;9:22. doi: 10.1186/1742-2094-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Nance E, Porambo M, Zhang F, Mishra MK, Buelow M, Getzenberg R, Johnston M, Kannan RM, Fatemi A, Kannan S. Systemic dendrimer-drug treatment of ischemia-induced neonatal white matter injury. J Control Release. 2015;214:112–120. doi: 10.1016/j.jconrel.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Saxena T, Loomis KH, Pai SB, Karumbaiah L, Gaupp E, Patil K, Patkar R, Bellamkonda RV. Nanocarrier-mediated inhibition of macrophage migration inhibitory factor attenuates secondary injury after spinal cord injury. ACS Nano. 2015;9:1492–1505. doi: 10.1021/nn505980z. [DOI] [PubMed] [Google Scholar]
- [92].Papa S, Rossi F, Ferrari R, Mariani A, De Paola M, Caron I, Fiordaliso F, Bisighini C, Sammali E, Colombo C, Gobbi M, Canovi M, Lucchetti J, Peviani M, Morbidelli M, Forloni G, Perale G, Moscatelli D, Veglianese P. Selective nanovector mediated treatment of activated proinflammatory microglia/macrophages in spinal cord injury. ACS Nano. 2013;7:9881–9895. doi: 10.1021/nn4036014. [DOI] [PubMed] [Google Scholar]
- [93].Cerqueira SR, Oliveira JM, Silva NA, Leite-Almeida H, Ribeiro-Samy S, Almeida A, Mano JF, Sousa N, Salgado AJ, Reis RL. Microglia response and in vivo therapeutic potential of methylprednisolone-loaded dendrimer nanoparticles in spinal cord injury. Small. 2013;9:738–749. doi: 10.1002/smll.201201888. [DOI] [PubMed] [Google Scholar]
- [94].Kim YT, Caldwell JM, Bellamkonda RV. Nanoparticle-mediated local delivery of Methylprednisolone after spinal cord injury. Biomaterials. 2009;30:2582–2590. doi: 10.1016/j.biomaterials.2008.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Papa S, Ferrari R, De Paola M, Rossi F, Mariani A, Caron I, Sammali E, Peviani M, Dell'Oro V, Colombo C, Morbidelli M, Forloni G, Perale G, Moscatelli D, Veglianese P. Polymeric nanoparticle system to target activated microglia/macrophages in spinal cord injury. J Control Release. 2014;174:15–26. doi: 10.1016/j.jconrel.2013.11.001. [DOI] [PubMed] [Google Scholar]
- [96].Huitinga I, van Rooijen N, de Groot CJ, Uitdehaag BM, Dijkstra CD. Suppression of experimental allergic encephalomyelitis in Lewis rats after elimination of macrophages. J Exp Med. 1990;172:1025–1033. doi: 10.1084/jem.172.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Bauer J, Huitinga I, Zhao W, Lassmann H, Hickey WF, Dijkstra CD. The role of macrophages, perivascular cells, and microglial cells in the pathogenesis of experimental autoimmune encephalomyelitis. Glia. 1995;15:437–446. doi: 10.1002/glia.440150407. [DOI] [PubMed] [Google Scholar]
- [98].Klyachko NL, Haney MJ, Zhao Y, Manickam DS, Mahajan V, Suresh P, Hingtgen SD, Mosley RL, Gendelman HE, Kabanov AV, Batrakova EV. Macrophages offer a paradigm switch for CNS delivery of therapeutic proteins. Nanomedicine (Lond) 2014;9:1403–1422. doi: 10.2217/nnm.13.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Baek SK, Makkouk AR, Krasieva T, Sun CH, Madsen SJ, Hirschberg H. Photothermal treatment of glioma; an in vitro study of macrophage-mediated delivery of gold nanoshells. J Neurooncol. 2011;104:439–448. doi: 10.1007/s11060-010-0511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Madsen SJ, Christie C, Hong SJ, Trinidad A, Peng Q, Uzal FA, Hirschberg H. Nanoparticle-loaded macrophage-mediated photothermal therapy: potential for glioma treatment. Lasers Med Sci. 2015;30:1357–1365. doi: 10.1007/s10103-015-1742-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Zhang F, Mastorakos P, Mishra MK, Mangraviti A, Hwang L, Zhou J, Hanes J, Brem H, Olivi A, Tyler B, Kannan RM. Uniform brain tumor distribution and tumor associated macrophage targeting of systemically administered dendrimers. Biomaterials. 2015;52:507–516. doi: 10.1016/j.biomaterials.2015.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Weinstein JS, Varallyay CG, Dosa E, Gahramanov S, Hamilton B, Rooney WD, Muldoon LL, Neuwelt EA. Superparamagnetic iron oxide nanoparticles: diagnostic magnetic resonance imaging and potential therapeutic applications in neurooncology and central nervous system inflammatory pathologies, a review. J Cereb Blood Flow Metab. 2010;30:15–35. doi: 10.1038/jcbfm.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Hutter E, Boridy S, Labrecque S, Lalancette-Hebert M, Kriz J, Winnik FM, Maysinger D. Microglial response to gold nanoparticles. ACS Nano. 2010;4:2595–2606. doi: 10.1021/nn901869f. [DOI] [PubMed] [Google Scholar]
- [104].Kannan S, Dai H, Navath RS, Balakrishnan B, Jyoti A, Janisse J, Romero R, Kannan RM. Dendrimer-based postnatal therapy for neuroinflammation and cerebral palsy in a rabbit model. Sci Transl Med. 2012;4:130. doi: 10.1126/scitranslmed.3003162. ra146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Luo X, Matranga C, Tan S, Alba N, Cui XT. Carbon nanotube nanoreservior for controlled release of anti-inflammatory dexamethasone. Biomaterials. 2011;32:6316–6323. doi: 10.1016/j.biomaterials.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Wu HY, Chung MC, Wang CC, Huang CH, Liang HJ, Jan TR. Iron oxide nanoparticles suppress the production of IL-1beta via the secretory lysosomal pathway in murine microglial cells. Part Fibre Toxicol. 2013;10:46. doi: 10.1186/1743-8977-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Baek SK, Makkouk AR, Krasieva T, Sun CH, Madsen SJ, Hirschberg H. Photothermal treatment of glioma; an in vitro study of macrophage-mediated delivery of gold nanoshells. J Neuro-Oncol. 2011;104:439–448. doi: 10.1007/s11060-010-0511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].VanHandel M, Alizadeh D, Zhang L, Kateb B, Bronikowski M, Manohara H, Badie B. Selective uptake of multi-walled carbon nanotubes by tumor macrophages in a murine glioma model. J Neuroimmunol. 2009;208:3–9. doi: 10.1016/j.jneuroim.2008.12.006. [DOI] [PubMed] [Google Scholar]
- [109].Fan H, Zhang I, Chen X, Zhang L, Wang H, Da Fonseca A, Manuel ER, Diamond DJ, Raubitschek A, Badie B. Intracerebral CpG immunotherapy with carbon nanotubes abrogates growth of subcutaneous melanomas in mice. Clin Cancer Res. 2012;18:5628–5638. doi: 10.1158/1078-0432.CCR-12-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].White EE, Pai A, Weng Y, Suresh AK, Van Haute D, Pailevanian T, Alizadeh D, Hajimiri A, Badie B, Berlin JM. Functionalized iron oxide nanoparticles for controlling the movement of immune cells. Nanoscale. 2015;7:7780–7789. doi: 10.1039/c3nr04421a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Boridy S, Soliman GM, Maysinger D. Modulation of inflammatory signaling and cytokine release from microglia by celastrol incorporated into dendrimer nanocarriers. Nanomedicine (Lond) 2012;7:1149–1165. doi: 10.2217/nnm.12.16. [DOI] [PubMed] [Google Scholar]
- [112].Soliman GM, Choi AO, Maysinger D, Winnik FM. Minocycline block copolymer micelles and their anti-inflammatory effects on microglia. Macromol Biosci. 2010;10:278–288. doi: 10.1002/mabi.200900259. [DOI] [PubMed] [Google Scholar]
- [113].Sharma A, Khatchadourian A, Khanna K, Sharma R, Kakkar A, Maysinger D. Multivalent niacin nanoconjugates for delivery to cytoplasmic lipid droplets. Biomaterials. 2011;32:1419–1429. doi: 10.1016/j.biomaterials.2010.10.025. [DOI] [PubMed] [Google Scholar]
- [114].Maysinger D, Groger D, Lake A, Licha K, Weinhart M, Chang PK, Mulvey R, Haag R, McKinney RA. Dendritic Polyglycerol Sulfate Inhibits Microglial Activation and Reduces Hippocampal CA1 Dendritic Spine Morphology Deficits. Biomacromolecules. 2015 doi: 10.1021/acs.biomac.5b00999. [DOI] [PubMed] [Google Scholar]
- [115].Moquin A, Hutter E, Choi AO, Khatchadourian A, Castonguay A, Winnik FM, Maysinger D. Caspase-1 activity in microglia stimulated by pro-inflammagen nanocrystals. ACS Nano. 2013;7:9585–9598. doi: 10.1021/nn404473g. [DOI] [PubMed] [Google Scholar]
- [116].Shakhbazau A, Mishra M, Chu TH, Brideau C, Cummins K, Tsutsui S, Shcharbin D, Majoral JP, Mignani S, Blanchard-Desce M, Bryszewska M, Yong VW, Stys PK, van Minnen J. Fluorescent Phosphorus Dendrimer as a Spectral Nanosensor for Macrophage Polarization and Fate Tracking in Spinal Cord Injury. Macromol Biosci. 2015 doi: 10.1002/mabi.201500150. [DOI] [PubMed] [Google Scholar]
- [117].Cusick MF, Libbey JE, Patel DC, Doty DJ, Fujinami RS. Infiltrating macrophages are key to the development of seizures following virus infection. J Virol. 2013;87:1849–1860. doi: 10.1128/JVI.02747-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Gomez-Nicola D, Fransen NL, Suzzi S, Perry VH. Regulation of microglial proliferation during chronic neurodegeneration. J Neurosci. 2013;33:2481–2493. doi: 10.1523/JNEUROSCI.4440-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Lalancette-Hebert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci. 2007;27:2596–2605. doi: 10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Mirrione MM, Konomos DK, Gravanis I, Dewey SL, Aguzzi A, Heppner FL, Tsirka SE. Microglial ablation and lipopolysaccharide preconditioning affects pilocarpine-induced seizures in mice. Neurobiol Dis. 2010;39:85–97. doi: 10.1016/j.nbd.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Rai P, Mallidi S, Zheng X, Rahmanzadeh R, Mir Y, Elrington S, Khurshid A, Hasan T. Development and applications of photo-triggered theranostic agents. Adv Drug Deliv Rev. 2010;62:1094–1124. doi: 10.1016/j.addr.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Rungta RL, Choi HB, Lin PJ, Ko RW, Ashby D, Nair J, Manoharan M, Cullis PR, Macvicar BA. Lipid Nanoparticle Delivery of siRNA to Silence Neuronal Gene Expression in the Brain. Mol Ther Nucleic Acids. 2013;2:e136. doi: 10.1038/mtna.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Haney MJ, Klyachko NL, Zhaoa YL, Gupta R, Plotnikova EG, He ZJ, Patel T, Piroyan A, Sokolsky M, Kabanov AV, Batrakova EV. Exosomes as drug delivery vehicles for Parkinson's disease therapy. Journal of Controlled Release. 2015;207:18–30. doi: 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Wei H, Schellinger JG, Chu DSH, Pun SH. Neuron-Targeted Copolymers with Sheddable Shielding Blocks Synthesized Using a Reducible, RAFT-ATRP Double-Head Agent. J Am Chem Soc. 2012;134:16554–16557. doi: 10.1021/ja3085803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Kwon EJ, Lasiene J, Jacobson BE, Park IK, Horner PJ, Pun SH. Targeted nonviral delivery vehicles to neural progenitor cells in the mouse subventricular zone. Biomaterials. 2010;31:2417–2424. doi: 10.1016/j.biomaterials.2009.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Zhang HL, Jiang YH, Zhao SG, Jiang LQ, Meng Y, Liu P, Kim MO, Li SP. Selective neuronal targeting, protection and signaling network analysis via dopamine-mediated mesoporous silica nanoparticles. Medchemcomm. 2015;6:1117–1129. [Google Scholar]
- [127].Kumar P, Wu HQ, McBride JL, Jung KE, Kim MH, Davidson BL, Lee SK, Shankar P, Manjunath N. Transvascular delivery of small interfering RNA to the central nervous system. Nature. 2007;448:39–43. doi: 10.1038/nature05901. [DOI] [PubMed] [Google Scholar]
- [128].Alvarez-Erviti L, Seow YQ, Yin HF, Betts C, Lakhal S, Wood MJA. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–U179. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- [129].Lafon M. Rabies virus receptors. J Neurovirol. 2005;11:82–87. doi: 10.1080/13550280590900427. [DOI] [PubMed] [Google Scholar]
- [130].Zern BJ, Chacko AM, Liu J, Greineder CF, Blankemeyer ER, Radhakrishnan R, Muzykantov V. Reduction of nanoparticle avidity enhances the selectivity of vascular targeting and PET detection of pulmonary inflammation. ACS Nano. 2013;7:2461–2469. doi: 10.1021/nn305773f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Chen R, Romero G, Christiansen MG, Mohr A, Anikeeva P. Wireless magnetothermal deep brain stimulation. Science. 2015;347:1477–1480. doi: 10.1126/science.1261821. [DOI] [PubMed] [Google Scholar]
- [132].Carvalho-de-Souza JL, Treger JS, Dang B, Kent SB, Pepperberg DR, Bezanilla F. Photosensitivity of neurons enabled by cell-targeted gold nanoparticles. Neuron. 2015;86:207–217. doi: 10.1016/j.neuron.2015.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Barandeh F, Nguyen PL, Kumar R, Iacobucci GJ, Kuznicki ML, Kosterman A, Bergey EJ, Prasad PN, Gunawardena S. Organically Modified Silica Nanoparticles Are Biocompatible and Can Be Targeted to Neurons In Vivo. Plos One. 2012;7 doi: 10.1371/journal.pone.0029424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Mishra MK, Beaty CA, Lesniak WG, Kambhampati SP, Zhang F, Wilson MA, Blue ME, Troncoso JC, Kannan S, Johnston MV, Baumgartner WA, Kannan RM. Dendrimer brain uptake and targeted therapy for brain injury in a large animal model of hypothermic circulatory arrest. ACS Nano. 2014;8:2134–2147. doi: 10.1021/nn404872e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Colin A, Faideau M, Dufour N, Auregan G, Hassig R, Andrieu T, Brouillet E, Hantraye P, Bonvento G, Deglon N. Engineered lentiviral vector targeting astrocytes in vivo. Glia. 2009;57:667–679. doi: 10.1002/glia.20795. [DOI] [PubMed] [Google Scholar]
- [136].Cannon JR, Sew T, Montero L, Burton EA, Greenamyre JT. Pseudotype-dependent lentiviral transduction of astrocytes or neurons in the rat substantia nigra. Exp Neurol. 2011;228:41–52. doi: 10.1016/j.expneurol.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Delzor A, Escartin C, Deglon N. Lentiviral vectors: a powerful tool to target astrocytes in vivo. Curr Drug Targets. 2013;14:1336–1346. doi: 10.2174/13894501113146660213. [DOI] [PubMed] [Google Scholar]
- [138].Furman JL, Sama DM, Gant JC, Beckett TL, Murphy MP, Bachstetter AD, Van Eldik LJ, Norris CM. Targeting astrocytes ameliorates neurologic changes in a mouse model of Alzheimer's disease. J Neurosci. 2012;32:16129–16140. doi: 10.1523/JNEUROSCI.2323-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Hohnholt MC, Geppert M, Luther EM, Petters C, Bulcke F, Dringen R. Handling of iron oxide and silver nanoparticles by astrocytes. Neurochem Res. 2013;38:227–239. doi: 10.1007/s11064-012-0930-y. [DOI] [PubMed] [Google Scholar]
- [140].Cerqueira SR, Silva BL, Oliveira JM, Mano JF, Sousa N, Salgado AJ, Reis RL. Multifunctionalized CMCht/PAMAM dendrimer nanoparticles modulate the cellular uptake by astrocytes and oligodendrocytes in primary cultures of glial cells. Macromol Biosci. 2012;12:591–597. doi: 10.1002/mabi.201100294. [DOI] [PubMed] [Google Scholar]
- [141].Wang YC, Wu YT, Huang HY, Lin HI, Lo LW, Tzeng SF, Yang CS. Sustained intraspinal delivery of neurotrophic factor encapsulated in biodegradable nanoparticles following contusive spinal cord injury. Biomaterials. 2008;29:4546–4553. doi: 10.1016/j.biomaterials.2008.07.050. [DOI] [PubMed] [Google Scholar]
- [142].Balakrishnan B, Nance E, Johnston MV, Kannan R, Kannan S. Nanomedicine in cerebral palsy. Int J Nanomed. 2013;8:4183–4195. doi: 10.2147/IJN.S35979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Jeyapalan SA, Goldmann M, Donahue J, Elinzano H, Evans DL, O'Connor BM, Puthawala MA, Oyelese A, Cielo D, Blitstein M, Dargush M, Santaniello A, Constantinou M, Dipetrillo T, Safran H, Univ B. A phase II study of paclitaxel poliglumex (PPX), temozolamide (TMZ), and radiation (RT) for newly diagnosed high-grade gliomas. J Clin Oncol. 2011;29 [Google Scholar]
- [144].Costantino L, Boraschi D. Is there a clinical future for polymeric nanoparticles as brain-targeting drug delivery agents? Drug Discov Today. 2012;17:367–378. doi: 10.1016/j.drudis.2011.10.028. [DOI] [PubMed] [Google Scholar]
- [145].Cohen ZR, Ramishetti S, Peshes-Yaloz N, Goldsmith M, Wohl A, Zibly Z, Peer D. Localized RNAi Therapeutics of Chemoresistant Grade IV Glioma Using Hyaluronan-Grafted Lipid-Based Nanoparticles. Acs Nano. 2015;9:1581–1591. doi: 10.1021/nn506248s. [DOI] [PubMed] [Google Scholar]
- [146].Mangraviti A, Tzeng SY, Kozielski KL, Wang Y, Jin YK, Gullotti D, Pedone M, Buaron N, Liu A, Wilson DR, Hansen SK, Rodriguez FJ, Gao GD, DiMeco F, Brem H, Olivi A, Tyler B, Green JJ. Polymeric Nanoparticles for Nonviral Gene Therapy Extend Brain Tumor Survival in Vivo. Acs Nano. 2015;9:1236–1249. doi: 10.1021/nn504905q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Guerrero-Cazares H, Tzeng SY, Young NP, Abutaleb AO, Quinones-Hinojosa A, Green JJ. Biodegradable Polymeric Nanoparticles Show High Efficacy and Specificity at DNA Delivery to Human Glioblastoma in Vitro and in Vivo. Acs Nano. 2014;8:5141–5153. doi: 10.1021/nn501197v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Pardridge WM. Drug transport across the blood-brain barrier. J Cereb Blood Flow Metab. 2012;32:1959–1972. doi: 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Nance EA, Woodworth GF, Sailor KA, Shih TY, Xu QG, Swaminathan G, Xiang D, Eberhart C, Hanes J. A Dense Poly(Ethylene Glycol) Coating Improves Penetration of Large Polymeric Nanoparticles Within Brain Tissue. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3003594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].van Rooy I, Cakir-Tascioglu S, Hennink WE, Storm G, Schiffelers RM, Mastrobattista E. In vivo methods to study uptake of nanoparticles into the brain. Pharm Res. 2011;28:456–471. doi: 10.1007/s11095-010-0291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Win-Shwe TT, Sone H, Kurokawa Y, Zeng Y, Zeng Q, Nitta H, Hirano S. Effects of PAMAM dendrimers in the mouse brain after a single intranasal instillation. Toxicol Lett. 2014;228:207–215. doi: 10.1016/j.toxlet.2014.04.020. [DOI] [PubMed] [Google Scholar]
- [152].Wang S, Li Y, Fan J, Wang Z, Zeng X, Sun Y, Song P, Ju D. The role of autophagy in the neurotoxicity of cationic PAMAM dendrimers. Biomaterials. 2014;35:7588–7597. doi: 10.1016/j.biomaterials.2014.05.029. [DOI] [PubMed] [Google Scholar]
- [153].Bardi G, Nunes A, Gherardini L, Bates K, Al-Jamal KT, Gaillard C, Prato M, Bianco A, Pizzorusso T, Kostarelos K. Functionalized carbon nanotubes in the brain: cellular internalization and neuroinflammatory responses. Plos One. 2013;8:e80964. doi: 10.1371/journal.pone.0080964. [DOI] [PMC free article] [PubMed] [Google Scholar]