Abstract
Background
In the HF-ACTION trial, exercise training improved functional capacity in heart failure with reduced ejection fraction (HFrEF). Previous studies have suggested that diabetes mellitus (DM) may be associated with an attenuated response to exercise. We explored whether DM attenuated the improvement in functional capacity with exercise.
Methods/Results
HF-ACTION randomized 2,331 patients with HFrEF to medical therapy with or without exercise training over a median follow-up of 2.5 years. We examined the interaction between DM and exercise response measured by change in 6-minute walk distance (6MWD) and peak VO2. We also examined outcomes by DM status. In HF-ACTION, 748 (32%) patients had DM. DM patients had lower functional capacity at baseline and had lower exercise volumes at 3 months. There was a significant interaction between DM status and exercise training for change in peak VO2 (interaction p=0.02), but not 6MWD. In the exercise arm, DM patients had a smaller mean increase in peak VO2 than non-DM patients (p=0.03). There was no interaction between DM and exercise on clinical outcomes. After risk adjustment, DM was associated with increased all-cause mortality/hospitalization (p=0.03).
Conclusions
In HF-ACTION, DM was associated with lower baseline functional capacity, an attenuated improvement in peak VO2 and increased hospitalizations.
Keywords: Diabetes Mellitus, Chronic Heart Failure, Functional Capacity
Introduction
Despite effective pharmacologic therapies for patients with heart failure with reduced ejection (HFrEF), these patients continue to have high rates of morbidity and mortality. Fifty percent of HFrEF patients die within 5 years of diagnosis; thus, there is a need for individual and population-based interventions to improve outcomes (1). A treatment strategy that influences comorbidities, such as diabetes mellitus (DM), chronic obstructive pulmonary disease, and chronic kidney disease, may represent an effective approach to improve outcomes in HF (2).
DM is a common comorbidity, seen in approximately 40–45% of patients with HFrEF (3,4). Prior studies suggest that DM is associated with an increased incidence of HF, more comorbidities and worse outcomes (4–7). However, the data on outcomes in HF patients with DM is conflicting. Several HF registries demonstrate an association between DM and increased all-cause mortality (8,9), while others demonstrate an association with increased risk of hospitalization but similar mortality (4,5). In addition, most prior studies had relatively short-term follow up, with a median duration of less than 10 months.
Thus, there is an unmet need to determine long-term clinical outcomes and assess interventions to reduce events in HF patients with DM. Heart Failure: a Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) was a randomized trial of 2,331 ambulatory HFrEF patients with long-term follow up, high usage of evidence-based HF therapies, and a large population of DM patients (10). In addition, HF-ACTION was the largest randomized trial to date of exercise in patients with HFrEF, which enables us to describe the baseline functional capacity of patients with HFrEF and DM, as well as their response to exercise training. In HF-ACTION, 3 months of exercise training was associated with a modest increase in 6-minute walk distance (6MWD) (median of 15 meters) and peak oxygen uptake (VO2) (median 0.4 mL/kg/min) compared with usual care (both p<0.001). Previous studies in the general population suggest that type 1 or 2 DM patients experience an attenuated physiologic response to exercise (10,11). Despite this attenuated physiologic response, a previous study of cardiac rehabilitation in patients with coronary artery disease demonstrated that both DM and non-DM patients benefitted from exercise training with similar improvements in exercise capacity observed in both groups (12). However, no large-scale study to our knowledge has examined the efficacy of exercise training in HFrEF patients with DM.
We, therefore, explored whether DM attenuated the benefit of exercise in patients with HFrEF. We hypothesized that improvement in functional capacity, as measured by change 6MWD and change peak VO2 after 3 months, would be attenuated in the exercise group with DM. We also evaluated long-term medical outcomes stratified by DM status, and assessed whether there is an interaction between DM status and exercise training on clinical outcomes.
Methods
The design and analysis of the HF-ACTION study has been previously published (clinicaltrials.gov, NCT00047437) (13,14). HF-ACTION randomized 2,331 patients with HFrEF (EF≤35%) and New York Heart Association (NYHA) class II–IV symptoms to aerobic exercise training vs. usual care with a median follow-up of 2.5 years. DM status was prospectively recorded at study enrollment by self-report and confirmed by the clinician-investigator based on clinical evidence and knowledge of past medical history by chart review. In addition, the use of insulin and oral hyperglycemic agents were documented at the time of enrollment. HF-ACTION was approved by local institutional review boards, and all patients provided informed consent.
Patients completed a cardiopulmonary exercise (CPX) test, 6-minute walk, and health status surveys at baseline and were subsequently randomized to exercise training vs. usual care. Patients randomized to exercise were scheduled to participate in 3 supervised exercise sessions/week for 3 months. Patients exercised using a treadmill or stationary cycle ergometer as their training mode. Patients were encouraged to begin home-based exercise after 18 supervised sessions and to fully transition to home exercise after 36 supervised sessions. Adherence was defined as ≥90 min/wk of exercise during months 1–3 and ≥120 min/wk during subsequent months. MET-HRs/week data were recorded to assess exercise volume based on the supervised exercise sessions and self-reported home activity logs. Patients were instructed to continue home-based exercise training, along with one supervised session every 3 months. Exercise (CPX testing and 6- minute walk) and health status measures were repeated at 3 and 12 months after baseline. (13,14)
Statistical Methods
Baseline characteristics were assessed including DM status and stratification by insulin vs. non-insulin dependent DM. Continuous variables were described with the median and interquartile ranges (25th and 75th percentile) and compared for DM vs. Non-DM using the Wilcoxon rank-sum statistic. Discrete variables are presented as percentages and compared for DM vs. non-DM using the Pearson Chi-Squared statistic or Fisher’s exact test. Exercise volume (MET-HRs/week) was measured after 3 months to assess adherence and compared between DM and non-DM patients using the Wilcoxon rank-sum test.
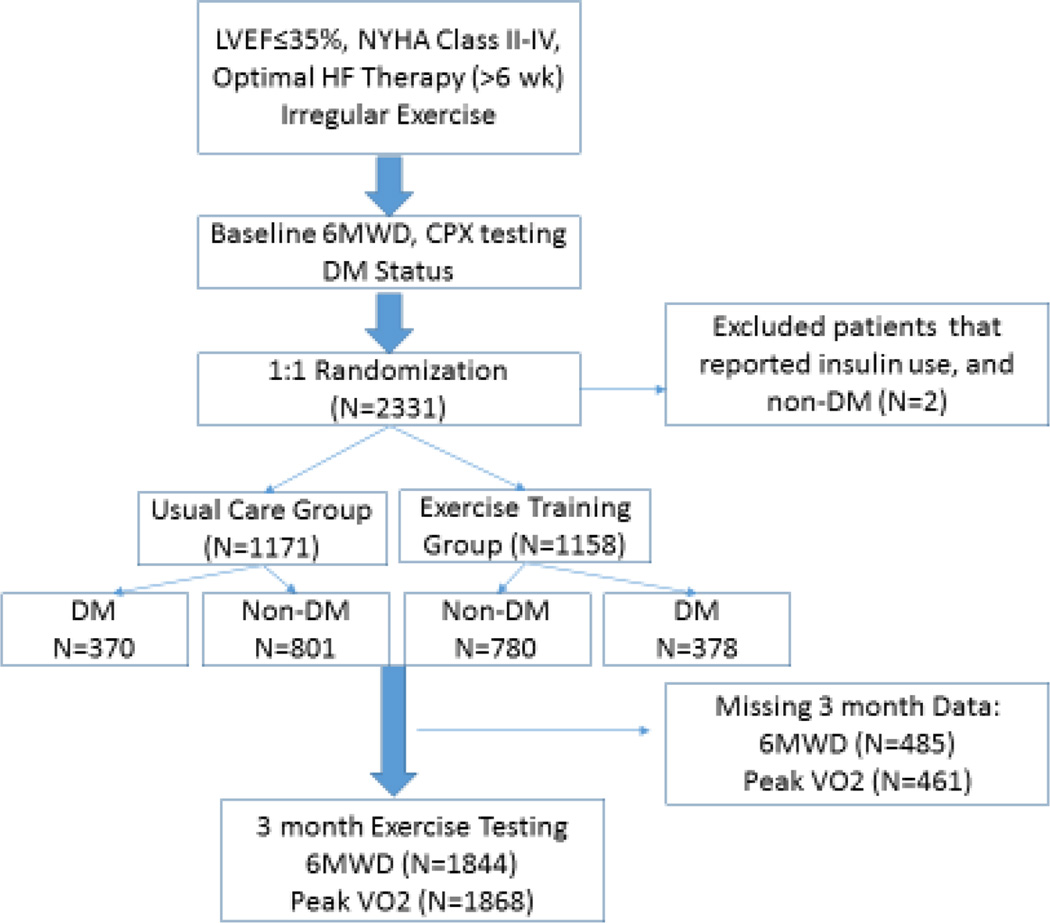
The co-primary outcomes for the present study were change in 6MWD and peak VO2 after 3 months of training. We also evaluated the secondary outcomes of change in health status (i.e., Kansas City Cardiomyopathy Questionnaire) and time to clinical outcomes (all-cause mortality/hospitalization, all-cause mortality, cardiovascular (CV) mortality/HF hospitalization). We used linear regression to examine the interaction between DM and exercise training as a predictor for change in 6MWD and peak VO2 from baseline to 3 months adjusted only for baseline 6MWD and peak VO2, respectively. Inverse weighted averages were used to account for patients with missing exercise data at 3 months. Figure 1 presents the study population. If the interaction P-value was significant (P<0.05), then the mean difference and 95% confidence interval for change in the exercise variable were reported. As a sub-analysis, we repeated the above statistical analysis comparing insulin- and non-insulin-dependent DM.
Figure 1. Study Flow Chart.
Study Flow Chart showing flow of patients to 3-month CPX testing for our primary analysis.
Adjusted Cox proportional hazard models were used to assess an interaction between DM status and exercise training for clinical outcomes. If the interaction P-value was significant, then the hazard ratio and 95% confidence interval were reported. The relationship between DM status and clinical outcomes was investigated, irrespective of treatment assignment, using unadjusted and adjusted Cox proportional hazard models. The hazard models were adjusted for a comprehensive set of covariates that have previously been identified for the HF-ACTION cohort using a stepwise variable selection based on a bootstrap-backward selection process (15).
A two-tailed P-value of <0.05 was considered to be statistically significant. There was no correction for multiple comparisons. All statistical analyses were performed by the Duke Clinical Research Institute (Durham, NC, USA) using SAS version 9.2 (Cary, NC, USA). The authors are solely responsible for the design and conduct of this study, all statistical analyses, and the drafting and editing of the manuscript.
Results
In HF-ACTION, 748 (32%) patients had DM. Table 1 presents the baseline characteristics stratified by DM status. DM patients were older, more likely to be African-American, had higher BMIs and worse NYHA class symptoms at baseline compared to non-DM patients. DM patients were more likely to be hypertensive and had higher creatinine and blood urea nitrogen at baseline. Guideline-directed medical- therapy for HF were high in both patient groups.
Table 1.
Baseline characteristics of the study cohort based on DM Status
| Variable | DM Status | P-value | |
|---|---|---|---|
| DM (n=748) |
Non-DM (n=1583) |
||
| Age, years | 61 (54,68) | 59(50,68) | <0.001 |
| Female Sex, % | 25.9 | 29.5 | 0.075 |
| Race, % | 0.008 | ||
| Black or African American | 36.6 | 30.8 | |
| White | 57.5 | 64.3 | |
| Other | 5.9 | 5.0 | |
| New York Heart Classification, % III/IV | 41.8 | 34.2 | <0.001 |
| BMI | 33.1 | 30.1 | <0.001 |
| Ischemic cardiomyopathy, % | 61.4 | 46.6 | <0.001 |
| Severe Mitral Regurgitation, % | 8.2 | 12.3 | 0.003 |
| Beck Depression Score at Baseline | 8.0 (5.0–16.0) | 8.0 (4.0, 15.0) | 0.052 |
| Hypertension, % | 75.9 | 52.3 | <0.001 |
| Atrial Fibrillation or Flutter, % | 21.7 | 20.6 | 0.545 |
| Hemoglobin (g/dL) | 13.3 (12.1, 14.3) | 13.6 (12.4, 14.7) | <0.001 |
| Creatinine, mg/dL | 1.3 (1.0, 1.6) | 1.2 (1.0, 1.4) | <0.001 |
| Blood urea nitrogen, mg/dL | 23.0 (17.0, 33.5) | 19.0 (14.0, 26.0) | <0.001 |
| Baseline Glomerular Filtration Rate (mL/hr) | 61.5 (45.8, 79.3) | 68.5 (54.2, 82.0) | <0.001 |
| ACE-Inhibitor or Angiotensin II Receptor Blocker | 93.0 | 94.9 | 0.064 |
| Beta-blocker | 94.8 | 94.4 | 0.686 |
| Dose, mg/day carvedilol equivalent | 50 (19.0, 50.0) | 36.9 (13.0, 50.0) | <0.001 |
| Aldosterone antagonist | 43.4 | 45.9 | 0.274 |
| Loop diuretic | 85.3 | 74.4 | <0.001 |
| Implantable Cardioverter Defibrillator | 41.8 | 39.5 | 0.277 |
Expressed as median interquartile range(IQR) or %.
Table 2 presents exercise capacity and health status at baseline in patients stratified by DM and insulin use. Mean baseline peak VO2, 6MWD and KCCQ clinical summary scores were lower in patients with DM. After adjustment for age, race, BMI, CKD, hypertension, and NYHA class the mean baseline peak VO2 was still significantly lower in patients with DM. For comparison, the minimal clinically importance differences (MCID) for 6MWD, peak VO2 and KCCQ clinical summary score have previously been estimated to be approximately 30 meters (16,17), 1 mL/kg/min or a 6% change from baseline (18,19) and 5 points, respectively (20). For the sub-analysis comparing insulin-dependent DM patients vs. non-insulin-dependent DM patients, the mean baseline functional capacity was significantly lower for the insulin-dependent patients as measured by 6MWD and peak VO2 (Table 2). Heart rate (HR) at peak exercise and HR reserve (HR at peak exercise minus resting HR) were also significantly lower in the DM versus non-DM patients and in the insulin-dependent versus non-insulin dependent DM patients. However, this may be at least partially driven by an increased mean beta-blocker dose in the DM patients. During the first 3 months following randomization, exercise volume was lower in DM versus non-DM for patients in the exercise arm (2.5 MET-HRs/week IQR (0.1–4.7) versus 3.3 (0.6–5.9), p <0.001). This difference in adherence may be partially driven by the older age, higher BMI, and higher NYHA class within the DM group.
Table 2.
Baseline Health Status and Exercise Parameters by DM Status and insulin use.
| Variable | Non-DM (n=1581) |
DM | P-Value for DM vs. Non-DM |
P-Value for Insulin vs. Non- Insulin DM |
||
|---|---|---|---|---|---|---|
| Overall (n=748) |
No Insulin (n=417) |
Insulin (n=331) |
||||
| 6-Minute Walk Distance, meters | 384 (272, 410) | 344 (311, 443) | 356 (280, 426) | 327 (259, 397) | <0.001 | 0.004 |
| Peak VO2, mL/kg/min | 15.2 (12.3, 18.3) | 13.0 (10.3, 16.3) | 13.7 (10.9, 16.8) | 12.0 (9.8, 15.6) | <0.001 | <0.001 |
| KCCQ Clinical Summary Score | 76.0 (59.4, 87.5) | 70.8 (53.6, 84.3) | 73.8 (57.1, 87.5) | 66.7 (49.0, 81.3) | <0.001 | <0.001 |
| Heart Rate at Peak Exercise (BPM) | 121 (107, 136) | 115 (100, 130) | 120 (105, 133) | 110 (96, 123) | <0.0001 | <0.0001 |
Expressed as median (IQR).
There was a statistically significant interaction between DM status and exercise training for change in peak VO2 (interaction p=0.02), but not 6MWD (interaction p=0.53) after adjustment for baseline peak VO2 and 6MWD, respectively. Table 3 displays the median baseline and 3-month values for the explored exercise variables. The mean increase in 6MWD and peak VO2 after 3 months can be seen in Table 4 for the exercise and usual care arm. In the exercise arm, DM patients had a smaller mean increase in peak VO2 than non-DM patients (0.5 ± 2.4 vs. 0.9 ± 2.6 mL/kg/min, p=0.03). Despite this attenuated exercise response in the DM patients compared to non-DM patients, the DM patients in the exercise arm of HF-ACTION still had a statistically significant improvement in both peak VO2 (0.5 vs. 0.3 ml/kg/min, p <0.001) and 6MWD (17.4 vs. 5.8 meters, p<0.001) after 3 months of exercise training as compared to usual care. Since exercise volume was significantly lower in the DM patients, we retrospectively adjusted our primary analysis for exercise volume (MET-HRs/week). After adjustment for exercise volume the interaction between DM status and exercise training for peak VO2 remained significant (interaction p=0.04). The DM patients within HF-ACTION were also older, more likely to be African-American, had more CKD and hypertension, with higher BMIs and worse heart failure symptoms at baseline. However, after adjustment for age, race, BMI, CKD, hypertension, and NYHA class, the interaction between DM status and exercise training for change in peak VO2 demonstrated a trend for significance (interaction p=0.05). There was no interaction between DM status and exercise training on health status, as measured by KCCQ clinical summary score. There was no interaction between insulin status and exercise training on functional capacity, as measured by 6MWD (interaction p=0.43) or peak VO2 (interaction p=0.41).
Table 3.
Baseline and 3 month Health Status and Exercise Parameters by DM Status and Exercise group.
| Exercise | Usual care | |||
|---|---|---|---|---|
| Variables | DM | Non-DM | DM | Non-DM |
| 6-Minute Walk Distance, meters | ||||
| • Baseline 6MWD | 344 | 384 | 344 | 384 |
| • 3-Month 6MWD | 357 | 409 | 350 | 389 |
| Peak VO2, mL/kg/min | ||||
| • Baseline Peak VO2 | 12.7 | 15.2 | 13.4 | 15.2 |
| • 3-month Peak VO2 | 13.6 | 16.2 | 13.8 | 15.4 |
| KCCQ Clinical Summary Score | ||||
| • Baseline KCCQ | 71.9 | 75.1 | 70.1 | 76.3 |
| • 3-month KCCQ | 72.3 | 76.0 | 71.3 | 74.5 |
Expressed as median.
Table 4.
Interaction between DM Status, Exercise Training, and Functional Capacity
| Outcome | Exercise training | Usual care | P-value for Interaction between DM Status and Treatment Assignment* |
|---|---|---|---|
| Change after 3 months Mean (SD) [Confidence Interval] | |||
| Variables | |||
| 6-Minute Walk Distance, meters | |||
| • DM | 17.4 (69.2) | 5.8 (67.7) | 0.53 |
| • Non-DM | 25.2 (74.0) | 2.9 (69.5) | |
| Peak VO2, mL/kg/min | |||
| • DM | 0.5 (2.4) [0.23–0.77] |
0.3 (2.4) [0.02–0.58] |
0.02 |
| • Non-DM | 0.9 (2.6) [0.70–1.10] |
0.2 (2.6) [−0.01–0.41] |
|
| KCCQ Clinical Summary Score | |||
| • DM | 5.0 (14.6) | 2.6 (15.6) | 0.23 |
| • Non-DM | 5.4 (14.5) | 2.6 (13.6) | |
Adjusted only for Baseline 6MWD, Peak VO2, and KCCQ Clinical Summary Score, respectively. Confidence intervals are listed for exercise variables with statistically significant interaction p-values.
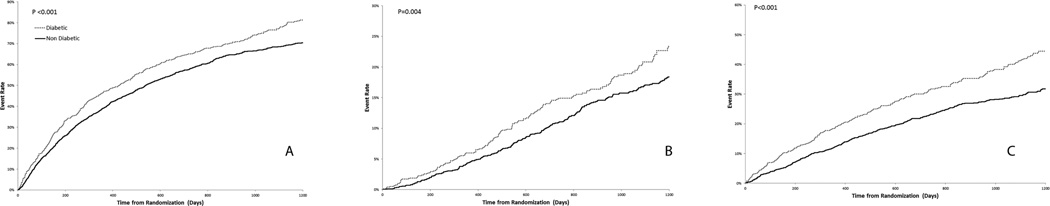
After a median follow-up of 2.5 years, DM was associated with increased all-cause mortality/hospitalization, all-cause mortality and CV mortality/HF hospitalization (Table 5). Kaplan-Meier event curves for all-cause mortality/hospitalization, all-cause mortality and CV mortality/HF hospitalization are displayed in Figure 2. After adjustment for the HF-ACTION risk model covariates, DM was associated with a significant increase in all-cause mortality/hospitalizations, but similar all-cause mortality and CV mortality/HF hospitalization (Table 5). Hospitalization was retrospectively evaluated as a separate clinical outcome. By censoring at death, the cause-specific hazard for hospitalization identified a significant association between DM and hospitalization (Wald Chi-Square- 4.02, p=0.05). The composite outcome of CV mortality/HF hospitalization was not significantly different between the DM and non-DM patients. These observations suggest that the between-group difference in DM vs. non-DM patients was specifically due to an increase in non-HF hospitalizations. There was no evidence of an interaction between DM and exercise training on any of the clinical outcomes.
Table 5.
Association between DM Status and Clinical Outcomes
| Outcome | Unadjusted | Adjusted* | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) |
P-value | Hazard Ratio (95% CI) |
P-value | |
| Mortality or Hospitalization | 1.27 (1.14, 1.41) | <.001 | 1.14 (1.01, 1.30) | 0.033 |
| All-cause Mortality | 1.35 (1.10, 1.66) | 0.004 | 0.97 (0.78, 1.2) | 0.80 |
| CV Mortality or HF Hospitalization | 1.52 (1.31, 1.77) | <0.001 | 1.08 (0.90, 1.30) | 0.41 |
Adjustment variables:
All-Cause Mortality/Hospitalization: peak VO2, KCCQ stability score, BUN, country, ejection fraction, sex, beta blocker dose, mitral regurgitation grade, ventricular conduction.
All-Cause Mortality: Baseline CPX test duration, creatinine, body mass index, sex, loop diuretic dose, left ventricular ejection fraction, canadian cardiovascular society anginal score, ventricular conduction.
CV Mortality/HF Hospitalization: Loop diuretic dose, ejection fraction, mitral regurgitation grade, ventricular conduction, KCCQ symptom stability score, BUN, race, sex, age.
Figure 2. Kaplan-Meier event curves.
Kaplan-Meier event curves for (A) All-Cause Mortality/Hospitalization (B) All-Cause mortality and (C) CV Mortality/HF Hospitalization prior to adjustment.
Discussion
In HF-ACTION, approximately one third of HFrEF patients had concomitant DM. DM was associated with reduced baseline exercise capacity and functional status, as well as lower adherence to exercise training. Insulin-dependent DM patients had even lower exercise and functional capacity, as compared to the non-insulin dependent DM patients. Our primary hypothesis was supported and DM was associated with an attenuated improvement in peak VO2 after 3 months of exercise. However, DM patients had similar improvement in 6MWD as compared to non-DM patients. After covariate adjustment, DM was associated with increased all-cause mortality/hospitalization due to an increased risk of hospitalization, but similar risk for other endpoints. Thus, DM was associated with lower baseline exercise capacity, lower adherence to exercise training, and an increased risk of all-cause mortality/hospitalization. Importantly, there was insufficient evidence to suggest a differential association between exercise training and clinical outcomes.
In previous studies, the association between DM status and clinical outcomes has been inconsistent. Several registries have shown an association between DM and increased mortality compared to non-DM patients (8,9), while others have shown increased rates of hospitalization with similar risk for mortality (4,5). In the unadjusted analysis of HF-ACTION, comorbid DM was associated with increased all-cause mortality/hospitalization, all-cause mortality, and CV mortality/HF hospitalization. After adjustment, comorbid DM was only associated with increased hospitalization. These findings are consistent with those prior studies suggesting that increased hospitalization is the primary difference in outcomes between DM and non-DM patients (4,9).
The effect of exercise on the change in functional capacity by DM status in HFrEF patients has not previously been investigated. In HF-ACTION, there was a statistically significant interaction between DM status and exercise on change in functional capacity as measured by peak VO2, but not 6MWD. In the exercise training arm, DM patients had a smaller mean increase in peak VO2 compared with non-DM patients, while in the usual care arm, the DM vs. non-DM changes for peak VO2 and 6MWD were similar. This difference was modest and less than the previously recognized MCID for change in peak VO2 of 1 ml/kg/min. Notably, this difference was after 3 months of exercise training; the long-term association between DM status and exercise training response in HFrEF patients is unknown. We did not assess the change in peak VO2 or 6MWD at the 12 month time point because of greater missing CPX testing and 6MWD data at this time point, which could lead to increased confounding. In addition, after 3 months, adherence rates with the exercise training regimen decreased when HF-ACTION patients were transitioned from supervised exercise training to a home-based exercise regimen. Thus, the 3 month result, following supervised exercise training, provides the least confounded result.
The attenuated increase in exercise capacity in the DM patients within HF-ACTION is likely driven by a combination of lower adherence, higher BMI and physiologic maladaptations in patients with DM. The attenuated benefit of exercise in this cohort is likely due in part to reduced adherence, as a previous analysis of HF-ACTION has demonstrated that higher exercise volume (MET-HRs/week) is associated with larger improvements in exercise capacity and improved outcomes (21). However, even after adjustment for exercise volume the exercise training benefit as quantified by change in peak VO2 was attenuated in DM vs. non-DM patients. Several previously described physiologic maladaptations contribute to an attenuated benefit of exercise training in HF patients with DM. DM patients have cardiac, autonomic and peripheral dysfunction that contribute to decreased exercise tolerance. From a cardiac perspective, insulin resistance leads to increased uptake and utilization of fatty acids by myocytes. Increased fatty acid utilization causes increased oxidative stress in the myocardium leading to both myocyte apoptosis and interstitial fibrosis (11,22). As a result, myocardial contractility and relaxation are impaired in DM. This decreased myocardial reserve is exacerbated further by an attenuated autonomic response leading to an impaired exercise reserve for HR, contractility and relaxation (23). This is consistent with the attenuated peak exercise HR in the DM patients within our study. Finally, DM patients have impaired peripheral arterial dilation of both small and large vessels and reduced skeletal muscle capillary density. Several studies have demonstrated reduced cardiac output and oxygen utilization at exercise in DM patients due to peripheral dysfunction, independent of myocardial factors (24,25). These physiologic factors likely contributed to the reduction in exercise capacity and response in the DM patients in HF-ACTION. In addition, the DM patients had significantly higher BMIs compared to non-DM patients. Obesity is associated with reduced exercise capacity and may be partially responsible for the attenuated exercise training response in DM (26). However, after adjustment for comorbidities and symptom burden (age, BMI, CKD, hypertension, race, and NYHA class) the DM patients in HF-ACTION still exhibited a reduced training response.
The current data suggests that the clinical benefit of exercise is reduced in DM by physiologic maladaptations and reduced adherence in HF patients with DM. The DM patients within HF-ACTION did receive some attenuated benefit in functional capacity and at this point physicians should continue to promote aerobic exercise in HF patients with DM. However, future research needs to target different modalities and intensities of training in DM patients to improve both adherence and physiologic response to exercise.
This study should be interpreted in the context of several limitations. This was a retrospective analysis from a randomized controlled trial of exercise training. While there was adjustment for previously identified covariates, there are likely additional measured and unmeasured variables within the cohort that may have influenced our results. The use of self-reported exercise logs limits the reliability of our exercise volume data. In addition, measures of glycemic control, such as hemoglobin A1c, fasting glucose, or duration of DM, were not recorded in the trial dataset. However, DM status was self-reported and confirmed by clinician-investigators using available clinical data at the time of enrollment. In addition, use of insulin and oral hyperglycemic agents were recorded at baseline, which enabled us to explore characteristics of insulin vs. non-insulin dependent DM patients. HF-ACTION had strict exclusion and inclusion criteria to enroll an ambulatory cohort of chronic HFrEF patients. As a result, this cohort of patients was younger and had higher usage of evidence-based HF therapies than the general HF population (13,14).
Conclusions
In HF-ACTION, DM was associated with older age, higher BMI, an increased prevalence of hypertension, worse HF symptoms, reduced health status, lower adherence to exercise training, and reduced peak VO2 and 6MWD at baseline. These differences were of greater magnitude in insulin-dependent DM. DM was associated with an attenuated improvement in peak VO2, but similar improvement in 6MWD with exercise training. After risk adjustment, DM was associated with increased hospitalization, but similar all-cause mortality and CV mortality/HF hospitalization. These results suggest that DM patients have a differential functional response to exercise training, independent of reduced adherence, and may need to be considered as a separate cohort in the design and analysis of future randomized controlled trials of exercise training in HF patients. Future trials should explore whether response to exercise training in DM patients can be improved by different modalities or intensities of exercise training.
Highlights.
DM was associated with lower adherence to exercise and reduced peak VO2 and 6MWD at baseline
DM patients have an attenuated response to exercise, independent of reduced adherence
DM patients have cardiac and peripheral dysfunction that result in attenuated exercise tolerance
DM was associated with increased hospitalization, driven by non-HF hospitalizations
Clinical Perspective
DM patients have cardiac, autonomic and peripheral dysfunction that contribute to decreased exercise capacity and tolerance. These physiologic maladaptations seem to be more pronounced in diabetic patients on insulin. In addition, HF patient with DM are at increased risk of non-adherence and hospitalization. They may benefit from more frequent outpatient follow up with an emphasis on a multidisciplinary approach including primary care, nutrition, endocrinology, and cardiology.
Translational Outlook
These results suggest that diabetic patients have a differential functional response to exercise training, independent of reduced adherence. DM status will need to be considered carefully in the design and analysis of future randomized controlled trials of exercise training in HF patients.
Acknowledgments
Funding: No extramural funding was used for the present analysis. The HF-ACTION trial was funded by the National Heart, Lung and Blood Institute.
Abbreviations
- HFrEF
Heart Failure with Reduced Ejection Fraction
- DM
Diabetes Mellitus
- 6MWD
6-minute walk distance
- HRs
Hours
- HF-ACTION
Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training
- VO2
oxygen uptake
- NYHA
New York Heart Association
- CPX
Cardiopulmonary Exercise
- KCCQ
Kansas City Cardiomyopathy Questionnaire
- CV
Cardiovascular
- MCID
Minimally Clinical Important Difference
Footnotes
Disclosures: The other authors report no relevant conflicts of interest or relationships with industry.
Disclaimer: The content of this manuscript is solely the responsibility of the authors and does not necessarily reflect the views of the National Institutes of Health or the Department of Health and Human Services.
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Executive summary: heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- 2.Mentz RJ, Felker GM. Noncardiac comorbidities and acute heart failure patients. Heart failure clinics. 2013;9:359–367. vii. doi: 10.1016/j.hfc.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams KF, Jr, Fonarow GC, Emerman CL, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) American heart journal. 2005;149:209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Greenberg BH, Abraham WT, Albert NM, et al. Influence of diabetes on characteristics and outcomes in patients hospitalized with heart failure: a report from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) American heart journal. 2007;154:277.e1–277.e8. doi: 10.1016/j.ahj.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Sarma S, Mentz RJ, Kwasny MJ, et al. Association between diabetes mellitus and post-discharge outcomes in patients hospitalized with heart failure: findings from the EVEREST trial. European journal of heart failure. 2013;15:194–202. doi: 10.1093/eurjhf/hfs153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shindler DM, Kostis JB, Yusuf S, et al. Diabetes mellitus, a predictor of morbidity and mortality in the Studies of Left Ventricular Dysfunction (SOLVD) Trials and Registry. The American journal of cardiology. 1996;77:1017–1020. doi: 10.1016/s0002-9149(97)89163-1. [DOI] [PubMed] [Google Scholar]
- 7.Smooke S, Horwich TB, Fonarow GC. Insulin-treated diabetes is associated with a marked increase in mortality in patients with advanced heart failure. American heart journal. 2005;149:168–174. doi: 10.1016/j.ahj.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 8.De Groote P, Lamblin N, Mouquet F, et al. Impact of diabetes mellitus on long-term survival in patients with congestive heart failure. European heart journal. 2004;25:656–662. doi: 10.1016/j.ehj.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 9.MacDonald MR, Petrie MC, Varyani F, et al. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. European heart journal. 2008;29:1377–1385. doi: 10.1093/eurheartj/ehn153. [DOI] [PubMed] [Google Scholar]
- 10.Baldi JC, Cassuto NA, Foxx-Lupo WT, Wheatley CM, Snyder EM. Glycemic status affects cardiopulmonary exercise response in athletes with type I diabetes. Medicine and science in sports and exercise. 2010;42:1454–1459. doi: 10.1249/MSS.0b013e3181d1fdb3. [DOI] [PubMed] [Google Scholar]
- 11.Gusso S, Hofman P, Lalande S, Cutfield W, Robinson E, Baldi JC. Impaired stroke volume and aerobic capacity in female adolescents with type 1 and type 2 diabetes mellitus. Diabetologia. 2008;51:1317–1320. doi: 10.1007/s00125-008-1012-1. [DOI] [PubMed] [Google Scholar]
- 12.Mourot L, Boussuges A, Maunier S, et al. Cardiovascular rehabilitation in patients with diabetes. Journal of cardiopulmonary rehabilitation and prevention. 2010;30:157–164. doi: 10.1097/HCR.0b013e3181c565fe. [DOI] [PubMed] [Google Scholar]
- 13.O'Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. Jama. 2009;301:1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whellan DJ, O'Connor CM, Lee KL, et al. Heart failure and a controlled trial investigating outcomes of exercise training (HF-ACTION): design and rationale. American heart journal. 2007;153:201–211. doi: 10.1016/j.ahj.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 15.O'Connor CM, Whellan DJ, Wojdyla D, et al. Factors related to morbidity and mortality in patients with chronic heart failure with systolic dysfunction: the HF-ACTION predictive risk score model. Circulation Heart failure. 2012;5:63–71. doi: 10.1161/CIRCHEARTFAILURE.111.963462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shoemaker MJ, Curtis AB, Vangsnes E, Dickinson MG. Triangulating Clinically Meaningful Change in the Six-minute Walk Test in Individuals with Chronic Heart Failure: A Systematic Review. Cardiopulmonary physical therapy journal. 2012;23:5–15. [PMC free article] [PubMed] [Google Scholar]
- 17.Shoemaker MJ, Curtis AB, Vangsnes E, Dickinson MG. Clinically meaningful change estimates for the six-minute walk test and daily activity in individuals with chronic heart failure. Cardiopulmonary physical therapy journal. 2013;24:21–29. [PMC free article] [PubMed] [Google Scholar]
- 18.Corra U, Mezzani A, Bosimini E, Giannuzzi P. Prognostic value of time-related changes of cardiopulmonary exercise testing indices in stable chronic heart failure: a pragmatic and operative scheme. European journal of cardiovascular prevention and rehabilitation : official journal of the European Society of Cardiology, Working Groups on Epidemiology & Prevention and Cardiac Rehabilitation and Exercise Physiology. 2006;13:186–192. doi: 10.1097/01.hjr.0000189807.22224.54. [DOI] [PubMed] [Google Scholar]
- 19.Keteyian SJ, Brawner CA, Ehrman JK, Ivanhoe R, Boehmer JP, Abraham WT. Reproducibility of peak oxygen uptake and other cardiopulmonary exercise parameters: implications for clinical trials and clinical practice. Chest. 2010;138:950–955. doi: 10.1378/chest.09-2624. [DOI] [PubMed] [Google Scholar]
- 20.Spertus J, Peterson E, Conard MW, et al. Monitoring clinical changes in patients with heart failure: a comparison of methods. American heart journal. 2005;150:707–715. doi: 10.1016/j.ahj.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 21.Keteyian SJ, Leifer ES, Houston-Miller N, et al. Relation between volume of exercise and clinical outcomes in patients with heart failure. Journal of the American College of Cardiology. 2012;60:1899–1905. doi: 10.1016/j.jacc.2012.08.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peterson LR, Herrero P, Schechtman KB, et al. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation. 2004;109:2191–2196. doi: 10.1161/01.CIR.0000127959.28627.F8. [DOI] [PubMed] [Google Scholar]
- 23.Izawa K, Tanabe K, Omiya K, et al. Impaired chronotropic response to exercise in acute myocardial infarction patients with type 2 diabetes mellitus. Japanese heart journal. 2003;44:187–199. doi: 10.1536/jhj.44.187. [DOI] [PubMed] [Google Scholar]
- 24.Lalande S, Gusso S, Hofman PL, Baldi JC. Reduced leg blood flow during submaximal exercise in type 2 diabetes. Medicine and science in sports and exercise. 2008;40:612–617. doi: 10.1249/MSS.0b013e318161aa99. [DOI] [PubMed] [Google Scholar]
- 25.Rissanen AP, Tikkanen HO, Koponen AS, Aho JM, Peltonen JE. Central and peripheral cardiovascular impairments limit v o2peak in type 1 diabetes. Medicine and science in sports and exercise. 2015;47:223–230. doi: 10.1249/MSS.0000000000000419. [DOI] [PubMed] [Google Scholar]
- 26.Horwich TB, Leifer ES, Brawner CA, Fitz-Gerald MB, Fonarow GC. The relationship between body mass index and cardiopulmonary exercise testing in chronic systolic heart failure. American heart journal. 2009;158:S31–S36. doi: 10.1016/j.ahj.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]