Abstract
Patients with inflammatory bowel disease (IBD) or irritable bowel syndrome (IBS) often experience increased sensory responsiveness in the urinary bladder reflecting neurogenic bladder overactivity. Here we demonstrate that colitis-induced up-regulation of the phospholipase C gamma (PLCγ) pathway downstream of brain-derived neurotrophic factor (BDNF) in bladder afferent neurons in the dorsal root ganglia (DRG) plays essential roles in activating these neurons thereby leading to bladder hyperactivity. Upon induction of colitis with 2,4,6-trinitrobenzenesulfonic acid (TNBS) in rats, we found that the phosphorylation (activation) level of cAMP responsive element-binding (p-CREB) protein, a molecular switch of neuronal plasticity, was increased in specifically labeled bladder afferent neurons in the thoracolumbar and lumbosacral DRGs. In rats having reduced levels of BDNF (BDNF+/−), colitis failed to elevate CREB protein activity in bladder afferent neurons. Physiological examination also demonstrated that colitis-induced urinary frequency was not shown in BDNF+/− rats, implicating an essential role of BDNF in mediating colon-to-bladder sensory cross-sensitization. We further implemented in vivo and in vitro studies and demonstrated that BDNF-mediated colon-to-bladder sensory cross-activation involved the TrkB - PLCγ-calcium/calmodulin-dependent protein kinase II (CaMKII) cascade. In contrast, the PI3K/Akt pathway was not activated in bladder afferent neurons during colitis and was not involved in BDNF action in the DRG. Our results suggest that colon-to-bladder sensory cross-sensitization is regulated by specific signal transduction initiated by the up-regulation of BDNF in the DRG.
Keywords: colon, bladder, cross-sensitization, BDNF, signal transduction
INTRODUCTION
Patients with inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS) often show evidence of bladder hypersensitivity such as detrusor instability, nocturia, frequency and some forms of urinary urge incontinence, back pain and, in women, dyspareunia, leading to significant problems in diagnosis and treatment (Ben-Ami et al., 2002; Whorwell et al., 1986). Several theories have been formed to explain the cross-organ sensitization or the referred pain suggestive of the involvement of spinal segments receiving inputs from different organs (Brumovsky and Gebhart, 2010). It is described that convergent neural input via dichotomizing primary afferents to the sensory limb including dorsal root ganglia (DRG) and spinal dorsal horn occurs between the heart and the stomach or the gallbladder (Ammons and Foreman, 1984; Qin et al., 2007), the colon and the urethra (Peng et al., 2010), as well as the distal colon and the urinary bladder (Qiao and Grider, 2007; Qin et al., 2005). However, the majority of the colonic afferent neurons do not overlap with bladder afferent neurons in the DRG (Chaban, 2008; Christianson et al., 2007; Qiao and Grider, 2007). It is not certain whether the few number of dichotomizing sensory neurons is sufficient to be principal players in the generation and maintenance of cross-organ sensitization. Emerging evidence suggests other peripheral mechanisms such as intraganglionic neurochemical coupling within the DRG thereby mediating sensory cross-activation of the neighboring DRG neurons innervating different visceral organs (Amir and Devor, 1996; Ulrich-Lai et al., 2001; Xia et al., 2012).
Brain-derived neurotrophic factor (BDNF) possesses neurotransmitter-like properties and can release locally in an extrasynaptic fashion (Swanwick et al., 2004) thereby acting in a paracrine manner on TrkB cells (Apfel et al., 1996). Among the neurotrophin family of growth factors, BDNF is important to memory formation and long-term potentiation (LTP) (Bekinschtein et al., 2008). BDNF synthesized in primary afferent neurons has profound effects on DRG/spinal cord neurons and peripheral tissues, and participates in sensory plasticity and pain (Obata and Noguchi, 2006). In IBS patients, an increase in the expression of BDNF and nerve fibers is found in colonic biopsies, which correlates significantly with the severity and frequency of abdominal pain/discomfort in these patients (Yu et al., 2012b). In animals, inhibition of BDNF activity with a neutralizing antibody or using BDNF+/− mice also attenuates colonic hypersensitivity in response to colonic distention (Delafoy et al., 2006; Yu et al., 2012b). In a recent study by us, intrathecal inhibition of BDNF activity reverses colonic inflammation-associated bladder hyperactivity (Xia et al., 2012), implicating a paracrine action of endogenous BDNF in visceral hypersensitivity and cross-organ sensitization.
The cellular biological function of BDNF is mediated by high affinity receptor TrkB and modulated by low affinity receptor p75NTR. In general, TrkB transduces BDNF signals via the Ras (a GTPase)-ERK (extracellular signal-regulated kinase), PI3K (phosphatidylinositol 3-kinase)/Akt (protein kinase B), and/or PLCγ (phospholipase C-gamma) cascades. The Ras-ERK and PI3K/Akt pathways are expressed in neuronal and non-neuronal cells, promoting growth and survival (Manning and Cantley, 2007; McCubrey et al., 2007). Activation of PLCγ hydrolyses PtdIns(4,5)P2 to produce the secondary messenger inositol trisphosphate (IP3) to facilitate Ca2+ release from the intracellular store, and leads to Ca2+ influx through activation of ion channels, thereby increasing the intracellular Ca2+ ([Ca2+]i) level and the activity of Ca2+-dependent pathways (Mizoguchi et al., 2002; Numakawa et al., 2001). One of those pathways regulated by Ca2+ is the Ca2+/calmodulin-dependent protein kinase II (CaMKII) (Mizoguchi et al., 2002; Numakawa et al., 2001). The transcription factor cAMP-responsive element binding (CREB) protein functions as a molecular switch by regulating approximately 4,000 genes in human tissues (Zhang et al., 2005). Signaling upstream of CREB is very complex and more than 300 stimuli are reported to activate CREB by up-regulating Ca2+ pathways and a list of kinases that phosphorylate CREB on serine 133 (Johannessen et al., 2004).
Here we assessed endogenous BDNF in bladder afferent neuronal cross-activation and investigated the signaling pathways that turned on CREB in bladder afferent neurons post colonic inflammation. We show that downstream of BDNF and TrkB, the PLCγ pathway is involved in CREB activation in the DRG. CaMKII is an intermediator in CREB activation. The PI3K/Akt pathway is reported to activate CREB elsewhere (Johannessen et al., 2004) but is not involved in bladder afferent cross-activation post colitis. The PLCγ/CaMKII cascade is well documented in the central nervous system and is important to LTP; however, less information is known for their roles in the peripheral nervous system.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats of the age 6–8 weeks consisting of wildtype (BDNF+/+: Harlan Laboratories, Indianapolis, IN) and BDNF heterozygous knockout (BDNF+/−: SAGE® Labs, Boyertown, PA) were treated with intracolonic instillation of 2, 4, 6-trinitrobenzenesulfonic acid (TNBS) at a dose of 90 mg/kg (60 mg/mL solution in 50 % EtOH) or vehicles (50 % EtOH) as described previously by us (Xia et al., 2011). All experimental protocols involving animal use were approved by Institutional Animal Care and Use Committee.
Retrograde labeling of bladder afferent neurons
Bladder afferent neurons were specifically labeled by conventional neuronal tracing dye Fast Blue (FB, 4 %, weight/volume; Polysciences, Inc. Warrington, PA) in live animals through surgery as described previously (Qiao and Grider, 2007). Briefly, the rat urinary bladder was exposed with a lower abdominal incision under anesthesia (2.5 % isoflurane). FB was injected into 10 sites (4 μL per site) in the muscular wall of the bladder to label bladder afferent neurons in the DRG. Sterilized Hamilton syringe (10 μL size) was used for injection. To prevent leakage and labeling of adjacent tissues, a cotton swab was used, close to the injection site, to wipe off any excess dye that might leak during needle withdrawal after each injection. We also avoided to inject dyes into the lumen, major blood vessels, or overlying fascial layers. The incision was closed with 4–0 sutures. The surgeries were done 7 days prior to harvest of tissues. We also checked the leakage of the dyes by examining the bladder and surrounding tissues after euthanasia of the rats. No contamination was found.
Immunohistochemistry
Animals used for immunohistochemistry were perfused intracardiacally with oxygenated Krebs buffer (pH 7.4) (95 % O2, 5 % CO2) followed by 4 % paraformaldehyde. DRG segments were identified and isolated for cryosectioning. The on-slide immunostaining technique was used. Briefly, DRG sections (20 μm thickness) were incubated for 1 h at room temperature with blocking solution containing 3 % normal donkey serum (Jackson ImmunoResearch, West Grove, PA) in PBST (0.3 % Triton X-100 in 0.1 M PBS, pH 7.4). Samples were then incubated in primary antibodies diluted in PBST containing 5 % normal donkey serum overnight at 4 °C. The primary antibodies used in this study were rabbit anti-phospho (p) -CREB (1:1000, Cell Signaling Technology, Inc.), mouse anti-p-CREB (1:400, Cell Signaling Technology, Inc.), rabbit anti-PLCγ (1:1500, Santa Cruz Biotechnology, Inc.), rabbit anti-p-Akt (1:500, Cell Signaling Technology, Inc.), and rabbit anti-p-CaMKII (1:1000, Cell Signaling Technology, Inc.). After rinse (3 × 10 min with 0.1 M PBS), tissues were incubated with fluorescence-conjugated species-specific secondary antibody (1:500, Molecular Probes, Eugene, OR) for 2 h at room temperature. Slides were coverslipped for analysis. The specificity of the antibodies was assessed by western blot shown in the present study. We chose every third section for one specific antibody to avoid double counting.
DRG cells exhibiting visible nucleus were counted with a Zeiss fluorescent photomicroscope. 6 to 10 sections from each DRG were randomly chosen for analysis. We focused on L1 and S1 DRGs. The results were presented as number cells per mm2 area, or percentage of bladder afferent neurons expressing immunoreactivity. For the former, the area of section containing cells (excluding the area containing fibers) was selected using free-line tools integrated with the AxioVision measurement software (Carl Zeiss, Inc.) and was measured as mm2 for normalization. For the latter, the number of positive stain of protein of interest was counted in FB-labeled bladder afferent neurons and was normalized against the number of FB cells.
Western Blot
The protein extracts from pooled L1–L2 and L6–S1 DRGs were subject to centrifugation at 20,200 g for 10 min at 4 °C, and the supernatant was removed to a fresh tube. The protein concentration was determined using Bio-Rad DC protein assay kit. Proteins were then separated on a 10 % SDS-PAGE gel and transferred to a nitrocellulose membrane. The membrane was blocked with 5 % milk in Tris-buffered saline for 1 h and then incubated with a specific primary antibody followed by HRP-conjugated secondary antibody. For internal loading control, the same membrane was stripped and re-probed with anti-β-actin antiserum or antibody to a non-phosphorylated form of the protein examined. The concentrations of antibodies used for p-CREB, CREB, p-Akt, Akt, PLCγ and p-CaMKII were 1:1000, and for β-actin was 1:3000 (Sigma-Aldrich, St. Louis, MO). The bands were visualized by enhanced chemiluminescence (ECL). Densitometric quantification of the immunoreactive bands was performed using the software FluorChem 8800 (Alpha Innotech, San Leandro, CA).
DRG explant culture
The segment-matched DRG pairs were used for comparison. DRGs were freshly dissected out and acutely cultured in Dulbecco’s Modified Eagle Medium (DMEM). To study the effect of BDNF, one DRG explant was treated with BDNF (50 ng/mL) and the counterpart served as control. To study the effect of a specific inhibitor, one DRG was treated with BDNF plus inhibitor and the counterpart served as control by receiving treatment of BDNF plus vehicle.
Examination of voiding behavior
Void stain on paper (VSOP) method (Sugino et al., 2008) was used to assess bladder activity. This non-invasive procedure allowed characterizing spontaneous urination patterns without surgical manipulation. We have also used this method in our previous studies (Xia et al., 2012; Yu et al., 2012a). Briefly, each animal was placed in a meshed cage and comparison groups were examined simultaneously. Experiments were conducted in the morning. The urine was collected naturally onto an underneath filter paper placed 20 cm below the mesh cage that contained the tested animal. We used a cage with a dimension of 25×15×15 cm3 (L×W×H). The number of urine drops from each animal in a 2-h window was counted. The diameters of the urine drops were measured at 4 directions (two cross-overs and two diagonal) and averaged for each drop.
Data analysis
The results from each study were presented as mean ± SEM. Comparison between control and experimental groups was made by using Kruskal-Wallis nonparametric one-way ANOVA, or by student t-test. Differences between means at a level of p ≤ 0.05 were considered to be significant.
RESULTS
Activation of CREB in bladder afferent neurons post colitis was regulated by endogenous BDNF
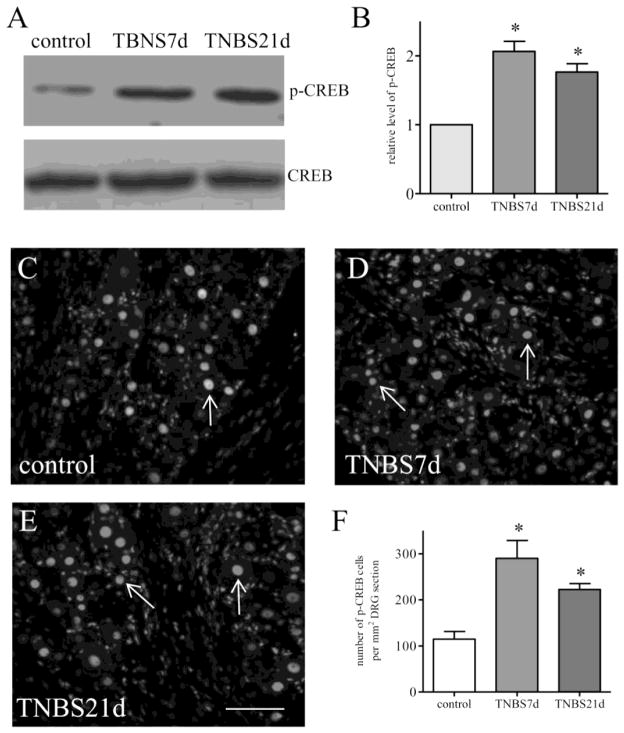
Bladder afferent activity was examined by the expression level of p-CREB in specifically labeled primary afferent neurons that innervated the urinary bladder. The L1–L2 and L6–S1 DRGs were identified and extracted from control rats and rats that received TNBS treatment and survived for 7 days and 21 days. Western blot of CREB phosphorylation on serine 133 was conducted in DRG samples from control animals and animals at 7 days and 21 days post colitis induction. Although the degree of colonic inflammation was subsided at day 7 and significantly recovered by day 21 (Xia et al., 2011), the levels of p-CREB in the DRGs were sustained at both 7 days and 21 days (Figure 1A). There were significant 2-fold increases in the level of p-CREB at both 7 days and 21 days post colitis induction (Figure 1B). We then performed immunostaining of the DRG cryosections with a specific antibody against p-CREB on serine 133. We found that p-CREB was expressed in the nucleus of DRG neurons (Figure 1C–1E, arrows) in all DRGs examined. The p-CREB immunoreactivity in the neurons was examined and showed increases post colitis induction (Figure 1F), confirming the western blot results (Figure 1B).
Figure 1. CREB activity was increased in the DRG post colitis.
Western blot of phospho-CREB (A) was performed to identify CREB activity level in the whole DRG (pooled L1–L2 and L6–S1) showing increases (B) at both 7 days and 21 days post colitis induction (animal numbers: control, 6; TNBS 7 days, 6; TNBS 21 days, 5). Immunostaining of phospho-CREB (C–E) demonstrated that phospho-CREB was expressed in the nucleus of DRG neurons (arrows). Number counting of phospho-CREB in DRG neurons (F) showed significant increases at 7 days and 21 days post colitis induction (animal numbers: control, 4; TNBS 7 days, 4; TNBS 21 days, 3), confirming the western blot results above. *, p<0.05. Calibration bar = 40 μm. Representative microphotographs (C–E) are from L1 DRGs.
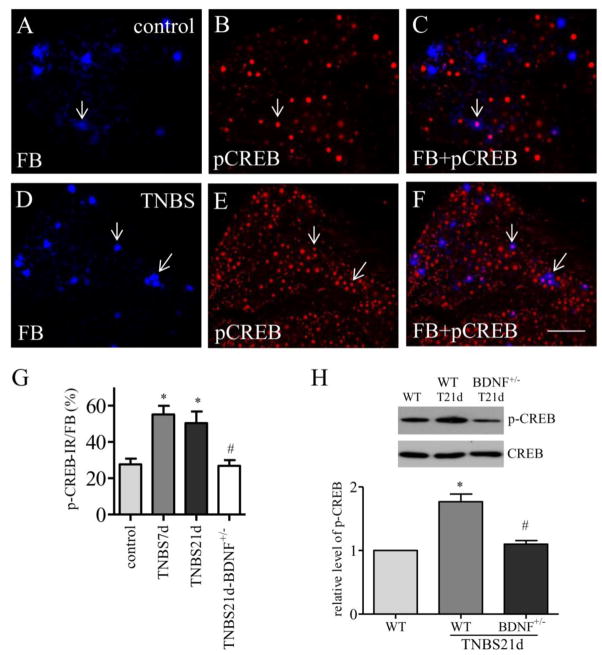
In FB labeled bladder afferent neurons (Figure 2A and 2D, blue cells), we also observed p-CREB expression (Figure 2B and 2E, red nuclear stain). Colitis did not cause changes in the number of FB labeling in the DRG (Qiao and Grider, 2007). In control rat (Figure 2A–2C), baseline of p-CREB expression in bladder afferent neurons (Figure 2A, FB cells) was evident (Figure 2A–2C, arrows). Post colitis induction, the number of bladder afferent neurons expressing p-CREB was significantly increased in the DRG (Figure 2D–2F; Figure 2G). The percentage of bladder afferent neurons expressing p-CREB was 27.6 ± 3.1 in control rats. At 7 days after TNBS treatment, the percentage of bladder afferent neurons expressing p-CREB was increased to 55.2 ± 4.7 (p<0.05). A similar trend was seen at 21 days after TNBS treatment yielding 50.3 ± 6.5 % of bladder afferent neurons expressing p-CREB (p<0.05).
Figure 2. CREB activity was increased in bladder afferent neurons and was regulated by endogenous BDNF post colitis.
Immunostaining of DRG segments containing bladder afferent neurons (A and D, blue cells) showed that CREB phosphoryaltion (B and E, red nuclear stain) was expressed in bladder afferent neurons (C and F, purple cells indicated by arrows) in both control animals (A–C) and animals treated with TNBS (D–F). CREB phosphorylation was increased in bladder afferent neurons at 7 days and 21 days post colitis induction (G, animal numbers: control: 5, TNBS 7 days, 4; TNBS 21 days, 4), which was reduced in BDNF+/− rats (G, open bar, n=4). The level of CREB phosphorylation in the DRG (L1–L2 and L6–S1) was also compared between wildtype and BDNF+/− rats by western blot (H, n=3) showing attenuation of CREB phosphorylation by BDNF partial deletion in vivo. *, p<0.05 vs. control; #, p<0.05 vs TNBS 21 days. Calibration bar = 80 μm.
The effect of endogenous BDNF on CREB phosphorylation in the DRG was tested in a genetically modified rat model containing a monoallelic deletion of the bdnf gene. In these animals, the level of endogenous BDNF is 50 % lower than that in wildtype. We performed both the immunostaining and western blot and confirmed that the expression levels of p-CREB in bladder afferent neurons (Figure 2G, open bar) and in the whole DRG (Figure 2H) were reduced in BDNF+/− rats when compared to wild type counterparts post colitis induction. The number of FB labeled bladder afferent neurons in wildtype and BDNF+/− rats post colitis stayed the same, which were similar to those in healthy animals reported by us previously (Qiao and Grider, 2007).
The PLCγ but not the PI3K/Akt pathway was increased in bladder afferent neurons post colitis
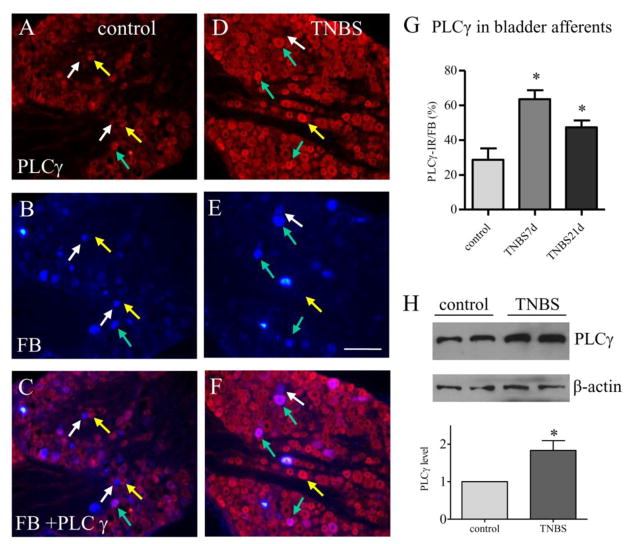
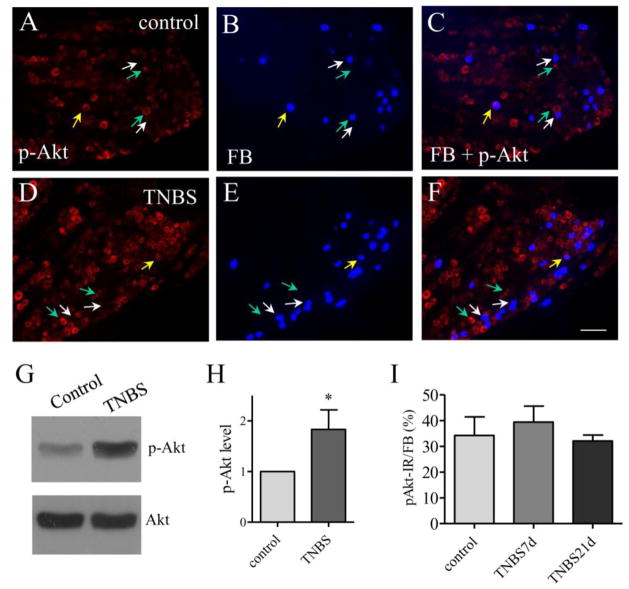
The PLCγ pathway is one of the major pathways that transduce the BDNF signaling in neurons and is important in neuronal plasticity and LTP in the central nervous system (Gartner et al., 2006). In the peripheral nervous system, a subpopulation of the PLCγ immunoreactivity (Figure 3A and 3D, red cells) was also expressed in bladder afferent neurons labeled by FB (Figure 3B and 3E, blue cells) in the DRG. The expression level of PLCγ was increased in bladder afferent neurons at 7 days and 21 days post colitis induction (Figure 3D–3F) when compared to control (Figure 3A–3C; Figure 3G). The increases in PLCγ expression in the DRG post colitis were confirmed by western blot (Figure 3H). It was noted that in control animals a large portion of bladder afferent neurons did not express PLCγ (Figure 3A–3C, white arrow). In rats treated with TNBS, only few bladder afferent neurons did not have PLCγ (Figure 3D–3F, white arrows). We also compared the Akt activity (Figure 4A, 4D) in bladder afferent neurons (Figure 4B, 4E) before (Figure 4A–4C) and post colitis induction (Figure 4D–4F). Although the phosphorylation level of Akt was increased in the DRG during colitis (Figure 4G and 4H), there were few bladder afferent neurons expressing phospho-Akt immunoreactivity (Figure 4C, 4F, purple cells indicated by yellow arrows). There was no significant change in the number of bladder afferent neurons expressing Akt phosphorylation post colitis when compared to control (Figure 4I).
Figure 3. PLCγ expression was increased in bladder afferent neurons post colitis.
Immunostaining showed that PLCγ expression (A and D, red cells) was expressed in bladder afferent neurons (B and E, blue cells) in both control animals (A–C) and animals treated with TNBS (D–F). PLCγ expression was increased in bladder afferent neurons (compare F to C, purple cells indicated by green arrows) at 7 days and 21 days post colitis induction (G, animal numbers: control: 5, TNBS 7 days, 4; TNBS 21 days, 4). Western blot confirmed that PLCγ expression was up-regulated in the DRG by colitis (H, n=3). *, p<0.05. Calibration bar = 80 μm.
Figure 4. The expression level of phospho-Akt was not changed in bladder afferent neurons post colitis.
Immunostaining showed that a small population of phospho-Akt immunoreactivity (A and D, red cells) was expressed in bladder afferent neurons (B and E, blue cells) in both control animals and animals treated with TNBS (compare F to C). Western blot showed an up-regulation of Akt phosphorylation (activation) in the DRG post colitis (G and H, n=3). However, the number of bladder afferent neuron expressing phospho-Akt expression was not changed at 7 days and 21 days post colitis induction (I, animal numbers: control: 5, TNBS 7 days, 4; TNBS 21 days, 4). Calibration bar = 60 μm. *, p<0.05.
TrkB, PLCγ, and CaMKII mediated BDNF-induced CREB activation in the DRG
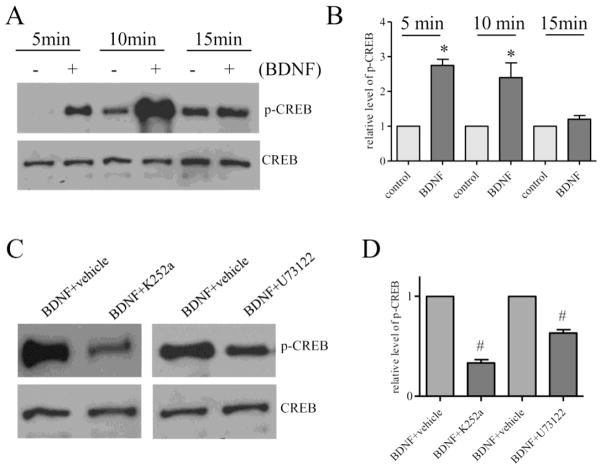
To examine the signaling pathways that mediated BDNF-induced CREB activation in the DRG, we treated the DRG explants culture with BDNF (50 ng/mL) and found that BDNF elicited a 2–3 folds increase in CREB activity at 5 and 10 min post treatment (Figure 5A and 5B). BDNF-induced CREB phosphorylation was blocked by inhibition of TrkB with K252a (5 μM) and by inhibition of PLCγ by U73122 (5 μM) (Figure 5C and 5D), suggesting that the high affinity receptor-mediated PLCγ pathway was involved in BDNF action in the DRG.
Figure 5. BDNF-induced CREB activity was mediated by TrkB and PLCγ.
BDNF stimulation of cultured DRG explants for 5 or 10 min (n=5) resulted in an increase in CREB phosphorylation (A and B). Treatment of DRG explants with TrkB inhibitor K252a (C and D) blocked BDNF-elicited CREB phosphorylation (n=3). Treatment of DRG explants with PLCγ inhibitor U73122 (C and D) also blocked BDNF-elicited CREB phosphorylation (n=3). The inhibition experiments were done with BDNF treatment for 5 min. Segment-matched DRG explants were used as comparison. *, p<0.05 vs control. #, p<0.05 vs BDNF treatment.
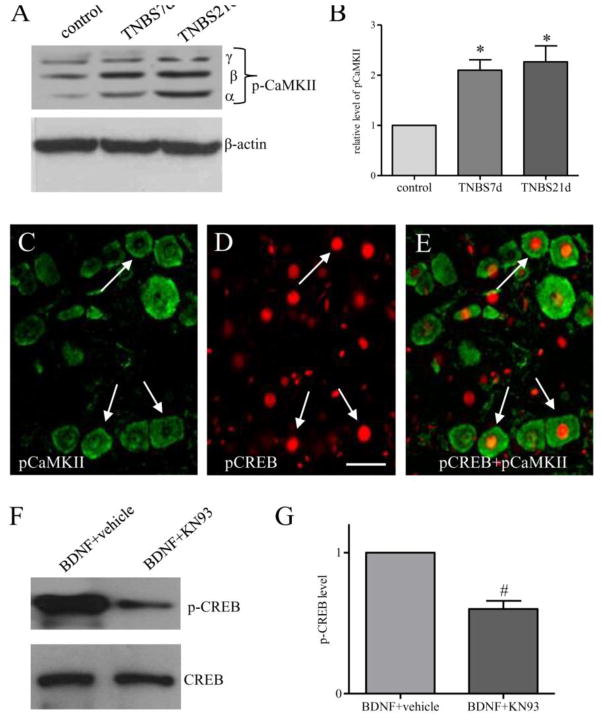
CaMKII is one of those kinases whose activity is regulated by intracellular Ca2+ levels. In culture CaMKII is also able to phosphorylate CREB on serine 133 and lead to CREB activation (Johannessen et al., 2004). To examine whether CaMKII was involved in CREB activation in the DRG in vivo post colitis, we performed western blot and showed an increase in the phosphorylation level of CaMKII in the DRGs at 7 days and 21 days of colitis (Figure 6A–6B). A subpopulation of p-CaMKII (Figure 6C, green cells) was also co-localized with p-CREB (Figure 6D, red nuclear stain) in the DRG. To examine whether CaMKII was upstream of CREB activated by BDNF, we treated DRG explants culture with CaMKII specific inhibitor KN-93 (5 μM) prior to BDNF stimulation and found that BDNF-induced CREB activation was blocked by inhibition of CaMKII activity (Figure 6F–6G).
Figure 6. Colitis increased CaMKII phosphorylation in the DRG which was associated with phospho-CREB.
Western blot of phospho-CaMKII (A) was performed to identify CaMKII activity level in the whole DRG. Three CaMKII isoforms, namely α, β, and γ were detected (A). Summary data in B showed increases in p-CaMKII in the DRG at both 7 days and 21 days post colitis induction (animal numbers: control, 6; TNBS 7 days, 6, TNBS 21 days, 5). Double immunostaining of p-CaMKII (C, green cells) and p-CREB (D, red nuclear stain) from colitis animals (7 days post colitis induction) showed co-localization of these two phospho-proteins (E, green cells having red nuclear stain indicated by arrows). In cultured DRG explants, CaMKII inhibitor KN-93 treatment reduced BDNF-induced CREB activity (F and G, n=3). The inhibition experiments were done with BDNF treatment for 5 min. Segment-matched DRG explants were used as comparison. *, p<0.05 vs control. #, p<0.05 vs BDNF treatment. Calibration bar = 30 μm.
BDNF+/− rat demonstrated reduced bladder activity associated with colitis
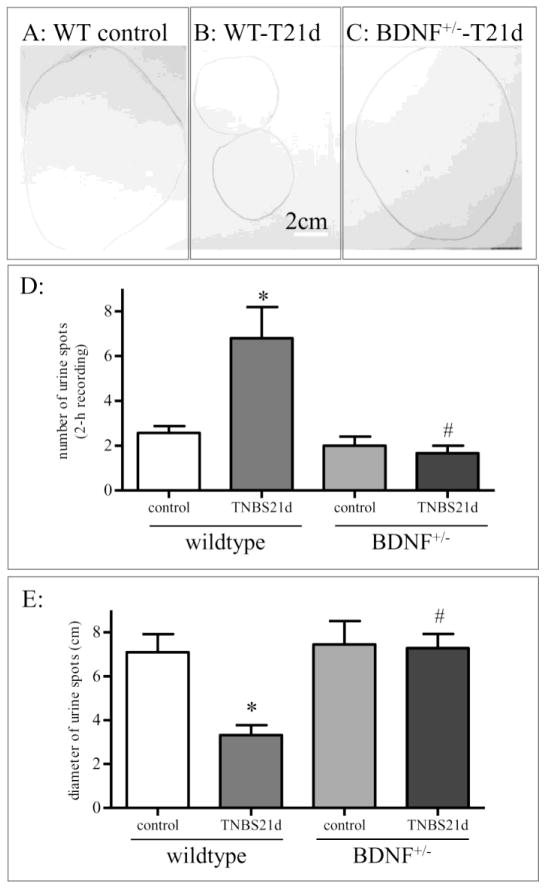
We previously showed that intrathecal inhibition of endogenous BDNF action with a specific BDNF neutralizing antibody reversed colitis-induced bladder hyperactivity that was examined by cystometry at an acute stage within 3 days post TNBS treatment (Xia et al., 2012). Neurogenic bladder activity and bladder pain were also evident at 10 days to 1 month post colitis induction (Pan et al., 2010; Yoshikawa et al., 2015). Using the non-invasive methodology to record bladder function via void stain on paper (VSOP), we examined that the control rats excreted fewer times (i.e., lower number of urine drops) with larger volumes per drop (i.e., bigger urine spots) (Figure 7A, 7D, and 7E). At 21 days post TNBS treatment, the rats voided more frequently (i.e., higher number of urine drops) with smaller quantities of urine per voiding (i.e., smaller urine spots) (Figure 7B, 7D, and 7E), suggesting bladder hyperactivity. The BDNF+/− rats that had 21 days of colitis demonstrated reduced number of urination with relatively larger urine spots when compared to wild type colitis (Figure 7C, 7D, and 7E).
Figure 7. Bladder hyperactivity was present at 21 days post colitis induction which was reduced in BDNF+/− rats.
VSOP (A–C) showed that control rats excreted fewer times with larger volumes per drop (A). TNBS treatment increased voiding frequency and reduced urine amount per drop (B), reflecting bladder hyperactivity. Bladder hyperactivity was reduced in BDNF+/− rats receiving TNBS treatment (C). The number of urine drops were recorded in a 2-h window (D). The average diameters of the urine spots were measured and calculated (E). Animal numbers used: for wildtype: control, 6; TNBS 21 days, 5; for BDNF+/− rats: total 8 animals were analyzed. Two pairs (control and TNBS 21 days) were analyzed simultaneously in a pair-matched manner. Additional 4 animals were examined before and post TNBS treatment. There was no significant difference within each treatment group thus data were pooled). *, p<0.05 vs control. #, p<0.05 vs TNBS in wildtype.
DISCUSSION
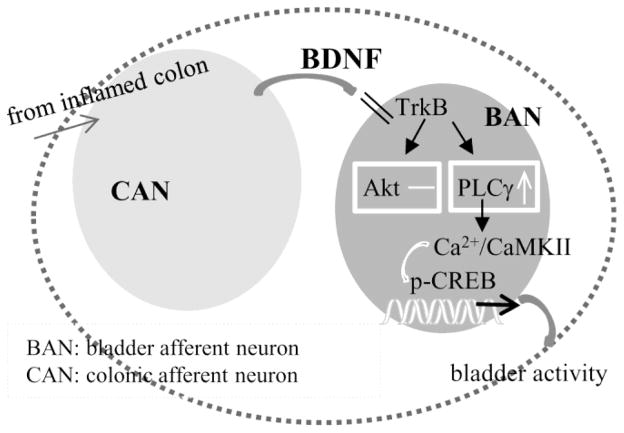
The spinal reflex participating in cross-organ sensitization was documented in the late 19th century (see review by (Brumovsky and Gebhart, 2010). In the past decade the underlying mechanisms began to be investigated in experimental animal models (Lei and Malykhina, 2012; Xia et al., 2012; Yoshikawa et al., 2015). The present study extends from previous findings by us and others (Lei and Malykhina, 2012; Xia et al., 2012; Yoshikawa et al., 2015) to illustrate the signal transduction by endogenous BDNF in regulating bladder afferent neuronal activity resulting from colitis. In our earlier studies we blocked endogenous BDNF action in the DRG by intrathecal neutralization and found that BDNF inhibition reversed colitis-induced bladder hyperactivity, suggesting a paracrine mechanism of BDNF in colon-to-bladder cross-sensitization (Xia et al., 2012). Here we show that bladder afferent neuronal activation demonstrated as an up-regulation of CREB phosphorylation in these neurons during colitis is reduced in BDNF+/− rats, further implicating an essential role of BDNF in mediating intraganglionic neuronal cross-activation in colon-to-bladder cross-organ sensory sensitization. Concordantly, exogenous BDNF is also able to activate CREB in the DRG. BDNF-induced CREB activation is attenuated by inhibition of PLCγ and CaMKII. During colitis, the levels of PLCγ and CaMKII phosphorylation are increased in the thoracolumbar and lumbosacral DRGs, and is up-regulated in bladder afferent neurons or co-expressed with phospho-CREB. In contrast, the PI3K/Akt pathway is not involved in bladder afferent neuronal activation during colitis. These studies confirm that BDNF plays essential roles in the regulation of colon-to-bladder sensory cross-activation, and also reveal a unique signaling pathway involving PLCγ-CaMKII-CREB axis in mediating BDNF action in vivo and in culture (Figure 8). The PLCγ/CaMKII pathway is traditionally known for their roles in memory and LTP (Gartner et al., 2006; Yamauchi, 2005). The present study provides evidence implicating their roles in the peripheral neuronal plasticity in response to visceral inflammation.
Figure 8.
Schematic diagram illustrating the putative mechanism for a paracrine role of BDNF in the regulation of colon-to-bladder cross-sensitization which is mediated by the PLCγ-CaMKII-CREB axis in bladder afferent neurons.
Nociceptive primary afferent neurons housed in the DRG are pseudounipolar which give rise to a single main process splitting into a peripheral (distal) branch to the organ and a central (proximal) branch to the spinal cord. The contribution of primary afferent reflex pathway in colon-to-bladder cross-sensitization has been investigated and well documented. Several anatomic pathways have been suggested including sensory convergence projection to DRG neurons and the spinal cord dorsal horn, which denotes input from both organs onto the same sensory neurons named dichotomizing neurons (Christianson et al., 2007; Qiao and Grider, 2007; Qin et al., 2007). Neuronal tracing studies from independent laboratories reveal that colonic and bladder afferent neurons locate in the same spinal segments, however, the number of dichotomizing sensory neurons project to both colon and urinary bladder ranges 5–27 %, in rat, and 21 %, in mouse, of traced neurons (Christianson et al., 2007; Qiao and Grider, 2007). Approximate 80 % of specific bladder afferent neurons do not innervate the distal colon but are also activated during colitis (Lei and Malykhina, 2012; Qiao and Grider, 2007). It is likely that paracrine mediators that are released from the activated primary afferent neurons upon irritation of the innervating organ (i.e. distal colon in this study) can act on nearby neurons thus facilitating intraganglionic neuronal cross-sensitization. Chemical coupling within the DRG is found to be attributable to sensory neuron cross-excitation in culture (Amir and Devor, 1996).
In the acute phase of colonic inflammation (3-days post TNBS), there is an increased amplitude of total Na+ current in bladder afferent neurons (Lei and Malykhina, 2012). In the intermediate phase of colonic inflammation (7-days post TNBS), more excitatory neurotransmitters are found to be produced by bladder afferent neurons (Qiao and Grider, 2007), suggesting an increase in transcriptional and translational regulation in the neurons. CREB functions in neurons as a molecular switch that turns on or shuts off many genes resulting in long-term neuronal plasticity. At 7–21 days post colitis induction, CREB activity is increased in bladder afferent neurons which may participate in cAMP-responsive element (CRE)-dependent transcription and induce prolonged neuronal activation. Recent update on spinal LTP in facilitating nociception is extensively reviewed by (Sandkuhler and Gruber-Schoffnegger, 2012). In this study, we show that the key players in LTP in hippocampus, the PLCγ and CaMKII (Gartner et al., 2006; Yamauchi, 2005), are also associated with bladder afferent activation post colitis. Although Akt serves as a convergent point in multiple signaling networks and is involved in neuronal growth and survival with a role recently discovered in mediating nerve injury pain (Sun et al., 2006; Xu et al., 2007), Akt, in contrast, is not activated in bladder afferent neurons post colitis. Bladder afferent neuronal activation examined by CREB activity is prolonged at 21 days when the colonic inflammation has already recovered (Xia et al., 2011). At 1 month post colitis induction, there is an increase in calcitonin gene-related peptide (CGRP) and Substance P (SP) release into the urinary bladder (Pan et al., 2010). CGRP promoter contains a CRE and activation of CREB can lead to CGRP up-regulation (Lanigan and Russo, 1997).
BDNF is a member of the neurotrophin family of growth factors consisting of nerve growth factor (NGF), neurotrophin-3 (NT-3) and NT-4. It has long been implicated that BDNF plays a significant role in neuronal plasticity especially the LTP (Bekinschtein et al., 2008). Recent studies suggest that BDNF also participates in modulating sensory activity in the peripheral nervous system and acts as a pain modulator (Merighi et al., 2008; Obata and Noguchi, 2006). Peripheral and visceral inflammation elicits BDNF synthesis in the primary sensory neurons in the DRG where it facilitates intracellular signal transduction and gene expression (Mannion et al., 1999; Qiao et al., 2008). BDNF regulates spinal central sensitization via releasing anterogradely to the superficial dorsal horn of the spinal cord where TrkB is expressed (Papadia and Hardingham, 2007; Zhou and Rush, 1996). Paracrine secretion of BDNF within the DRG has also been documented (Valdes-Sanchez et al., 2010). However, the signal transduction of BDNF within the DRG has not been examined. During colitis, the level of TrkB expression is up-regulated in bladder afferent neurons (Qiao and Grider, 2007). The accumulation of TrkB in bladder afferent neurons may enhance the responsiveness of these neurons to BDNF, thus lead to changes in the plasticity of these neurons. In DRG neuron culture, BDNF increases the expression level of CGRP, an excitatory neurotransmitter that is up-regulated in bladder afferent neurons (Qiao and Grider, 2007) and releases to the urinary bladder during colitis (Pan et al., 2010). Demonstrated in the present study, BDNF induces CREB activation in vitro and also regulates CREB activation in bladder afferent neurons in vivo. Intrathecal inhibition of BDNF with a specific neutralizing antibody reverses colitis-induced bladder hyperactivity (Xia et al., 2012). In these studies, BDNF antibody is unable to enter into the plasma thus supporting a paracrine role of BDNF in cross-neuron activation. In BDNF+/− rat, colitis-induced CREB activation in bladder afferent neurons is also reduced, which is correlated to the decreased bladder activity, again supporting a role of endogenous BDNF in colon-to-bladder cross-organ sensitization. To speculate, other possible mechanisms underlying endogenous BDNF-regulated cross-organ sensitization may also involve the regulation of mast cell activity. It is shown that mast cells are activated in the urinary bladder during colitis contributing to cross-organ sensitization (Fitzgerald et al., 2013). In the periphery, TrkB/BDNF binding can acutely sensitize nocireceptive pathway that require the presence of mast cells (Huang and Reichardt, 2001). Additional pathways may involve ion channel activation. The transient receptor potential ion channel (TRPV)1 and tetrodotoxin-resistant (TTX-R) sodium channels in the DRG are involved in viscera-visceral cross-organ sensitization (Chaban, 2008; Lei and Malykhina, 2012; Malykhina et al., 2006; Pan et al., 2010). The ability of BDNF in regulating the activity of these channels (Zhang et al., 2008) may also underlie the mechanisms of DRG neuronal cross-activation.
PLCγ and CaMKII are key mediators in BDNF-induced LTP in central nervous system. A few recent studies also suggest that CaMKII may also have a role in sensory sensitization (Ferhatovic et al., 2014; Kostic et al., 2014). The present study demonstrates that PLCγ and CaMKII are unique intracellular mediators in visceral organ cross-sensitization that is regulated by endogenous BDNF through high affinity receptor TrkB. Discovery of this unique pathway provides specific therapeutic target in the treatment of cross-organ sensitization.
Highlights.
Colon-to-bladder sensory cross-sensitization is mediated by BDNF.
The PLCγ pathway is essential in BDNF-regulated TrkB-mediated sensory neuronal activation
The PLCγ but no the PI3K/Akt pathway is activated in bladder afferent neurons and is regulated by endogenous BDNF in colitis.
Acknowledgments
This work was supported by grant NIH/NIDDK DK077917 to LYQ
ABBREVIATIONS
- BDNF
brain-derived neurotrophic factor
- CaMKII
calcium/calmodulin-dependent protein kinase II
- CREB
cAMP response element-binding
- DMEM
Dulbecco’s Modified Eagle Medium
- DRG
dorsal root ganglia
- ERK
extracellular signal-regulated kinase
- IBD
inflammatory bowel disease
- IBS
irritable bowel syndrome
- IP3
inositol trisphosphate
- LTP
long-term potentiation
- PI3K
phosphatidylinositol 3-kinase
- PLCγ
phospholipase C gamma
- TNBS
2,4,6-Trinitrobenzenesulfonic acid
- VSOP
void stain on paper
Footnotes
Conflicts of Interest: The Authors disclose no conflicts
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amir R, Devor M. Chemically mediated cross-excitation in rat dorsal root ganglia. J Neurosci. 1996;16:4733–4741. doi: 10.1523/JNEUROSCI.16-15-04733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammons WS, Foreman RD. Cardiovascular and T2–T4 dorsal horn cell responses to gallbladder distention in the cat. Brain research. 1984;321:267–277. doi: 10.1016/0006-8993(84)90179-3. [DOI] [PubMed] [Google Scholar]
- Apfel SC, Wright DE, Wiideman AM, Dormia C, Snider WD, Kessler JA. Nerve growth factor regulates the expression of brain-derived neurotrophic factor mRNA in the peripheral nervous system. Mol Cell Neurosci. 1996;7:134–142. doi: 10.1006/mcne.1996.0010. [DOI] [PubMed] [Google Scholar]
- Bekinschtein P, Cammarota M, Katche C, Slipczuk L, Rossato JI, Goldin A, Lzquierdo I, Medina JH. BDNF is essential to promote persistence of long-term memory storage. P Natl Acad Sci USA. 2008;105:2711–2716. doi: 10.1073/pnas.0711863105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ami H, Ginesin Y, Behar DM, Fischer D, Edoute Y, Lavy A. Diagnosis and treatment of urinary tract complications in Crohn’s disease: An experience over 15 years. Can J Gastroenterol. 2002;16:225–229. doi: 10.1155/2002/204614. [DOI] [PubMed] [Google Scholar]
- Brumovsky PR, Gebhart GF. Visceral organ cross-sensitization - an integrated perspective. Auton Neurosci. 2010;153:106–115. doi: 10.1016/j.autneu.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaban VV. Visceral sensory neurons that innervate both uterus and colon express nociceptive TRPv1 and P2X3 receptors in rats. Ethn Dis. 2008;18:S2-20–24. [PubMed] [Google Scholar]
- Christianson JA, Liang RM, Ustinova EE, Davis BM, Fraser MO, Pezzone MA. Convergence of bladder and colon sensory innervation occurs at the primary afferent level. Pain. 2007;128:235–243. doi: 10.1016/j.pain.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delafoy L, Gelot A, Ardid D, Eschalier A, Bertrand C, Doherty AM, Diop L. Interactive involvement of brain derived neurotrophic factor, nerve growth factor, and calcitonin gene related peptide in colonic hypersensitivity in the rat. Gut. 2006;55:940–945. doi: 10.1136/gut.2005.064063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferhatovic L, Kadic AJ, Boric M, Puljak L. Changes of calcium/calmodulin-dependent protein kinase II expression in dorsal root ganglia during maturation in long-term diabetes. Histol Histopathol. 2014;29:649–658. doi: 10.14670/HH-29.10.649. [DOI] [PubMed] [Google Scholar]
- Fitzgerald JJ, Ustinova E, Koronowski KB, de Groat WC, Pezzone MA. Evidence for the role of mast cells in colon-bladder cross organ sensitization. Auton Neurosci-Basic. 2013;173:6–13. doi: 10.1016/j.autneu.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner A, Polnau DG, Staiger V, Sciarretta C, Minichiello L, Thoenen H, Bonhoeffer T, Korte M. Hippocampal long-term potentiation is supported by presynaptic and postsynaptic tyrosine receptor kinase B-mediated phospholipase C gamma signaling. J Neurosci. 2006;26:3496–3504. doi: 10.1523/JNEUROSCI.3792-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannessen M, Delghandi MP, Moens U. What turns CREB on? Cellular Signalling. 2004;16:1211–1227. doi: 10.1016/j.cellsig.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Kostic S, Pan B, Guo Y, Yu HW, Sapunar D, Kwok WM, Hudmon A, Wu HE, Hogan QH. Regulation of voltage-gated Ca2+ currents by Ca2+/calmodulin-dependent protein kinase II in resting sensory neurons. Mol Cell Neurosci. 2014;62:10–18. doi: 10.1016/j.mcn.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanigan TM, Russo AF. Binding of upstream stimulatory factor and a cell-specific activator to the calcitonin/calcitonin gene-related peptide enhancer. J Biol Chem. 1997;272:18316–18324. doi: 10.1074/jbc.272.29.18316. [DOI] [PubMed] [Google Scholar]
- Lei Q, Malykhina AP. Colonic inflammation up-regulates voltage-gated sodium channels in bladder sensory neurons via activation of peripheral transient potential vanilloid 1 receptors. Neurogastroent Motil. 2012;24:575-+. doi: 10.1111/j.1365-2982.2012.01910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malykhina AP, Qin C, Greenwood-Van Meerveld B, Foreman RD, Lupu F, Akbarali HI. Hyperexcitability of convergent colon and bladder dorsal root ganglion neurons after colonic inflammation: mechanism for pelvic organ cross-talk. Neurogastroent Motil. 2006;18:936–948. doi: 10.1111/j.1365-2982.2006.00807.x. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannion RJ, Costigan M, Decosterd I, Amaya F, Ma QP, Holstege JC, Ji RR, Acheson A, Lindsay RM, Wilkinson GA, Woolf CJ. Neurotrophins: peripherally and centrally acting modulators of tactile stimulus-induced inflammatory pain hypersensitivity. Proc Natl Acad Sci U S A. 1999;96:9385–9390. doi: 10.1073/pnas.96.16.9385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EWT, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A, Stivala F, Libra M, Basecke J, Evangelisti C, Martelli AM, Franklin RA. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Bba-Mol Cell Res. 2007;1773:1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merighi A, Salio C, Ghirri A, Lossi L, Ferrini F, Betelli C, Bardoni R. BDNF as a pain modulator. Prog Neurobiol. 2008;85:297–317. doi: 10.1016/j.pneurobio.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Mizoguchi Y, Monji A, Nabekura J. Brain-derived neurotrophic factor induces long-lasting Ca2+-activated K+ currents in rat visual cortex neurons. Eur J Neurosci. 2002;16:1417–1424. doi: 10.1046/j.1460-9568.2002.02198.x. [DOI] [PubMed] [Google Scholar]
- Numakawa T, Matsumoto T, Adachi N, Yokomaku D, Kojima M, Takei N, Hatanaka H. Brain-derived neurotrophic factor triggers a rapid glutamate release through increase of intracellular Ca2+ and Na+ in cultured cerebellar neurons. Journal of Neuroscience Research. 2001;66:96–108. doi: 10.1002/jnr.1201. [DOI] [PubMed] [Google Scholar]
- Obata K, Noguchi K. BDNF in sensory neurons and chronic pain. Neurosci Res. 2006;55:1–10. doi: 10.1016/j.neures.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Pan XQ, Gonzalez JA, Chang SH, Chacko S, Wein AJ, Malykhina AP. Experimental colitis triggers the release of substance P and calcitonin gene-related peptide in the urinary bladder via TRPV1 signaling pathways. Experimental Neurology. 2010;225:262–273. doi: 10.1016/j.expneurol.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadia S, Hardingham GE. The dichotomy of NMDA receptor signaling. Neuroscientist. 2007;13:572–579. doi: 10.1177/10738584070130060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng HY, Chen GD, Lai CH, Tung KC, Chang JL, Lin TB. Endogenous ephrinB2 mediates colon-urethra cross-organ sensitization via Src kinase-dependent tyrosine phosphorylation of NR2B. American journal of physiology. Renal physiology. 2010;298:F109–117. doi: 10.1152/ajprenal.00287.2009. [DOI] [PubMed] [Google Scholar]
- Qiao LY, Grider JR. Up-regulation of calcitonin gene-related peptide and receptor tyrosine kinase TrkB in rat bladder afferent neurons following TNBS colitis. Exp Neurol. 2007;204:667–679. doi: 10.1016/j.expneurol.2006.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao LY, Gulick MA, Bowers J, Kuemmerle JF, Grider JR. Differential changes in brain-derived neurotrophic factor and extracellular signal-regulated kinase in rat primary afferent pathways with colitis. Neurogastroenterol Motil. 2008;20:928–938. doi: 10.1111/j.1365-2982.2008.01119.x. [DOI] [PubMed] [Google Scholar]
- Qin C, Farber JP, Foreman RD. Gastrocardiac afferent convergence in upper thoracic spinal neurons: a central mechanism of postprandial angina pectoris. The journal of pain: official journal of the American Pain Society. 2007;8:522–529. doi: 10.1016/j.jpain.2007.02.428. [DOI] [PubMed] [Google Scholar]
- Qin C, Malykhina AP, Akbarali HI, Foreman RD. Cross-organ sensitization of lumbosacral spinal neurons receiving urinary bladder input in rats with inflamed colon. Gastroenterology. 2005;129:1967–1978. doi: 10.1053/j.gastro.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Sandkuhler J, Gruber-Schoffnegger D. Hyperalgesia by synaptic long-term potentiation (LTP): an update. Current opinion in pharmacology. 2012;12:18–27. doi: 10.1016/j.coph.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino Y, Kanematsu A, Hayashi Y, Haga H, Yoshimura N, Yoshimura K, Ogawa O. Voided stain on paper method for analysis of mouse urination. Neurourol Urodynam. 2008;27:548–552. doi: 10.1002/nau.20552. [DOI] [PubMed] [Google Scholar]
- Sun RQ, Tu YJ, Yan JY, Willis WD. Activation of protein kinase B/Akt signaling pathway contributes to mechanical hypersensitivity induced by capsaicin. Pain. 2006;120:86–96. doi: 10.1016/j.pain.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Swanwick CC, Harrison MB, Kapur J. Synaptic and extrasynaptic localization of brain-derived neurotrophic factor and the tyrosine kinase B receptor in cultured hippocampal neurons. J Comp Neurol. 2004;478:405–417. doi: 10.1002/cne.20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Flores CM, Harding-Rose CA, Goodis HE, Hargreaves KM. Capsaicin-evoked release of immunoreactive calcitonin gene-related peptide from rat trigeminal ganglion: evidence for intraganglionic neurotransmission. Pain. 2001;91:219–226. doi: 10.1016/S0304-3959(00)00439-5. [DOI] [PubMed] [Google Scholar]
- Valdes-Sanchez T, Kirstein M, Perez-Villalba A, Vega JA, Farinas I. BDNF is essentially required for the early postnatal survival of nociceptors. Dev Biol. 2010;339:465–476. doi: 10.1016/j.ydbio.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Whorwell PJ, Lupton EW, Erduran D, Wilson K. Bladder Smooth-Muscle Dysfunction in Patients with Irritable-Bowel-Syndrome. Gut. 1986;27:1014–1017. doi: 10.1136/gut.27.9.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia CM, Colomb DG, Jr, Akbarali HI, Qiao LY. Prolonged sympathetic innervation of sensory neurons in rat thoracolumbar dorsal root ganglia during chronic colitis. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2011;23:801–e339. doi: 10.1111/j.1365-2982.2011.01728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia CM, Gulick MA, Yu SJ, Grider JR, Murthy KS, Kuemmerle JF, Akbarali HI, Qiao LY. Up-regulation of brain-derived neurotrophic factor in primary afferent pathway regulates colon-to-bladder cross-sensitization in rat. Journal of Neuroinflammation. 2012:9. doi: 10.1186/1742-2094-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu JT, Tu HY, Xin WJ, Liu XG, Zhang GH, Zhai CH. Activation of phosphatidylinositol 3-kinase and protein kinase B/Akt in dorsal root ganglia and spinal cord contributes to the neuropathic pain induced by spinal nerve ligation in rats. Exp Neurol. 2007;206:269–279. doi: 10.1016/j.expneurol.2007.05.029. [DOI] [PubMed] [Google Scholar]
- Yamauchi T. Neuronal Ca2+/calmodulin-dependent protein kinase II - Discovery, progress in a quarter of a century, and perspective: Implication for learning and memory. Biol Pharm Bull. 2005;28:1342–1354. doi: 10.1248/bpb.28.1342. [DOI] [PubMed] [Google Scholar]
- Yoshikawa S, Kawamorita N, Oguchi T, Funahashi Y, Tyagi P, Chancellor MB, Yoshimura N. Pelvic organ cross-sensitization to enhance bladder and urethral pain behaviors in rats with experimental colitis. Neuroscience. 2015;284:422–429. doi: 10.1016/j.neuroscience.2014.08.064. [DOI] [PubMed] [Google Scholar]
- Yu SJ, Xia CM, Kay JC, Qiao LY. Activation of extracellular signal-regulated protein kinase 5 is essential for cystitis- and nerve growth factor-induced calcitonin gene-related peptide expression in sensory neurons. Mol Pain. 2012a:8. doi: 10.1186/1744-8069-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YB, Zuo XL, Zhao QJ, Chen FX, Yang J, Dong YY, Wang P, Li YQ. Brain-derived neurotrophic factor contributes to abdominal pain in irritable bowel syndrome. Gut. 2012b;61:685–694. doi: 10.1136/gutjnl-2011-300265. [DOI] [PubMed] [Google Scholar]
- Zhang XM, Odom DT, Koo SH, Conkright MD, Canettieri G, Best J, Chen HM, Jenner R, Herbolsheimer E, Jacobsen E, Kadam S, Ecker JR, Emerson B, Hogenesch JB, Unterman T, Young RA, Montminy M. Genome-wide analysis of cAMP-response element binding protein occupancy, phosphorylation, and target gene activation in human tissues. P Natl Acad Sci USA. 2005;102:4459–4464. doi: 10.1073/pnas.0501076102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Chi XX, Nicol GD. Brain-derived neurotrophic factor enhances the excitability of rat sensory neurons through activation of the p75 neurotrophin receptor and the sphingomyelin pathway. J Physiol-London. 2008;586:3113–3127. doi: 10.1113/jphysiol.2008.152439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XF, Rush RA. Endogenous brain-derived neurotrophic factor is anterogradely transported in primary sensory neurons. Neuroscience. 1996;74:945–951. doi: 10.1016/0306-4522(96)00237-0. [DOI] [PubMed] [Google Scholar]