Abstract
Recombinant human BMP-2 (rhBMP-2) is a potent osteoinductive agent, but has been associated not only with bone formation, but also osteoclastogenesis and bone resorption. Osteoprotegerin (OPG) is a RANKL inhibitor that blocks differentiation and function of osteoclasts. We hypothesized that the combination of local BMP-2 (recombinant protein or a product of gene therapy) plus systemic OPG-Fc is more effective than BMP-2 alone in promoting bone repair. To test this hypothesis we used a mouse critical-sized femoral defect model. Col2.3eGFP (osteoblastic marker) male mice were treated with rhBMP-2 (group I), rhBMP-2 and systemic OPG (group II), rhBMP-2 and delayed administration of OPG (group III), mouse BM cells transduced with a lentiviral vector containing the BMP-2 gene (LV-BMP-2; group IV), LV-BMP-2 and systemic OPG (group V), a carrier alone (group VI) and administration of OPG alone (group VII). All bone defects treated with BMP-2 (alone or combined with OPG) healed, whereas minimal bone formation was noted in animals treated with the carrier alone or OPG alone. MicroCT analysis showed that bone volume (BV) in rhBMP-2 + OPG and LV-BMP-2 + OPG groups was significantly higher compared to rhBMP-2 alone (p < 0.01) and LV-BMP-2 alone (p < 0.001). Similar results were observed in histomorphometry, with rhBMP-2 alone defects exhibiting significantly lower bone area (B.Ar) compared to rhBMP-2 + OPG defects (p < 0.005) and LV-BMP-2 defects having a significantly lower B.Ar compared to all BMP-2 + OPG treated groups (p ≤ 0.01). TRAP staining demonstrated a major osteoclast response in the groups that did not receive OPG (rhBMP-2, LV-BMP-2 and sponge alone) beginning as early as 7 days post-operatively. In conclusion, we demonstrated that locally delivered BMP-2 (recombinant protein or gene therapy) in combination with systemically administered OPG improved bone healing compared to BMP-2 alone in a mouse critical-sized bone defect. These data indicate that osteoclasts can diminish healing responses to BMP-2 and that RANKL inhibition may thus accentuate BMP-2 efficacy.
Keywords: Bone healing, BMP, Antiresorptives, Osteoclasts, Bone histomorphometry
1. Introduction
RhBMP-2 is a potent osteoinductive agent that has been extensively investigated in animal and clinical studies and is FDA approved for clinical use for anterior lumbar spinal fusions and treatment of open tibia fractures [1]. However, the supraphysiologic doses of rhBMP used in clinical practice and the kinetics of BMP release from the carrier [2] are associated with inconsistent clinical results and several complications [3,4,5]. Previous animal studies using a mouse critical-sized femoral defect model in our laboratory have demonstrated that treatment with rhBMP-2 leads to healing of the defect, with the bony bridge gradually becoming thinner at later time points (28 and 56 days after treatment) [6]. It has been hypothesized that this thin cortical bone repair associated with rhBMP-2 treatment is secondary to the rapid release of the protein from the collagen sponge [6,7], leading to osteoclast stimulation and subsequent bone resorption. Another potential option for BMP protein delivery is ex vivo regional gene therapy [8]. Regional gene therapy may have some advantage as it allows the delivery of osteoprogenitor cells transduced with osteoinductive factors to a specific anatomic site where they induce bone formation. Hsu et al. and Virk et al. have used a BMP-2 containing lentivirus to transduce bone marrow stromal cells (BMSCs) [9,10]. These transduced BMSCs overexpressed BMP-2 and were able to heal a rat critical sized bone defect, with the bone healing being more robust compared to rhBMP-2 alone treated animals. This may be due to the fact that lentiviral gene therapy is characterized by a sustained production of BMP-2 (up to 12 weeks with a lentiviral vector) which leads to a prolonged osteoinductive signal and thus increased new bone formation compared to rhBMP-2 [11]. However, BMP not only induces bone formation, but also stimulates both osteoclastogenesis and premature bone resorption [2,6,12–14]. Thus, in order to optimize the biologic healing potential of BMP-2 it may be necessary to inhibit this osteoclast activation.
Osteoprotegerin (OPG) is a member of the TNF receptor superfamily that is produced by osteoblasts and other cell types in various tissues. OPG acts by binding RANKL, thus preventing it from binding and activating its receptor RANK, a key regulator of osteoclast formation, activation and survival [15]. The important role of the OPG/RANKL/RANK system in bone resorption has been confirmed in several in vitro and in vivo studies. RANKL knockout mice completely lacked osteoclasts and showed severe osteopetrosis and defective tooth eruption [16]. On the other hand, mice lacking the gene for OPG presented with generalized osteoporosis due to excessive RANKL activity and increased bone resorption [17]. Administration of recombinant OPG to normal mice induced an increase in bone mineral density in the metaphysis of the tibia and distal femur [18].
In the present study we hypothesized that the combination of systemic OPG-Fc and local BMP-2 would lead to enhanced bone repair in a femoral defect by preventing the premature resorption of newly formed bone. It has also been shown that cells transduced with a lentivirus expressing BMP-2 cDNA exhibit a sustained (up to 12 weeks) low-level release of BMP-2 [11]. We used this construct to test the secondary hypothesis that the prolonged BMP release associated with gene therapy would blunt the osteoclastic activation, such that the positive impact of OPG on bone repair would be less significant in the gene therapy group than the recombinant BMP group.
2. Material and methods
2.1. Cell culture and transduction with lentiviral vector
Mouse BMSC (MBMSC) were isolated from the content of the intramedullary canal of femora and tibiae of 8-week-old male C57BL/6 mice. The whole bone marrow cell pellet was re-suspended with Iscove’s modified Dulbecco’s media (IMDM) (Life Technologies, Grand Island, NY, USA) supplemented with 15% fetal bovine serum (Omega Scientific, Tarzana, CA, USA), penicillin (100 U/ml) and streptomycin (100 mg/ml), and plated on a 10-cm dish. MBMSCs were cultured for 4–5 days at 5% CO2, 37 °C until passage 1. Non-adherent cells were removed by aspiration when changing media. Adherent MBMSCs were split and plated at a concentration of 2 × 106 cells per 10 cm-dish one day before the viral transduction. Transfection with a lentiviral vector carrying the gene for BMP-2 (LV-BMP-2) was carried out overnight, in 5 ml media containing 0.8 μg/ml polybrene (Sigma, St. Louis, MO, USA) at a multiplicity of infection (MOI) of 25 as previously described [19]. At the end of the transduction, the MBMSCs were washed to remove any free virus and then incubated for an additional 24 h in fresh media.
2.2. Quantification of in vitro BMP-2 production
Transduced MBMSCs were incubated with fresh media for a 24 hour (24 h) period following transduction. The culture medium was then harvested for evaluation of in vitro BMP-2 production. The BMP-2 levels in culture supernatant were quantified by a commercial enzyme-linked immunosorbent assay (ELISA) kit (BMP-2 Quantikine ELISA Kit, R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. The assay results were standardized by cell number and reported as nanograms of BMP-2/day per 1 × 106 cells.
2.3. Animal studies
IACUC reviewed and approved the animal protocol before the commencement of any animal studies. We used a total of 156 male, 12–16 week old, Bl/6 lineage-specific transgenic mice expressing GFP under the control of the 2.3 Col1a1 promoter fragment (Col2.3eGFP). This mouse strain allows for imaging and tracking of differentiated osteoblasts and osteocytes (Col2.3eGFP positive cells). A critical-sized full-thickness transverse femoral defect (2 mm) was created unilaterally using a well-established model [6,19]. Briefly, after administration of inhalational anesthesia (2% isoflurane), the left limbs of mice were shaved and prepared with three alternating scrubs. Using aseptic surgical technique, an incision was made on the anterolateral aspect of the femur, extending from the greater trochanter to the region immediately above the knee. The muscles were dissected off the femur and the periosteum was incised along the length of the diaphysis. An external fixator was secured on the femur (proximally and distally) with a 0.01 ligature wire. A 2-mm mid-diaphyseal defect was then created with a small bone saw. A collagen sponge alone or loaded with 5 μg of rhBMP-2 or 2 × 106 LV-BMP-2 transduced MBMSC was implanted in the defect. A careful multi-layer closure with absorbable sutures was performed. Post-operatively the mice received buprenorphine subcutaneously for 2 days and antibiotics in drinking water for 5 days. Animals were allowed to bear weight immediately and to eat and drink ad libitum. The animals were randomly assigned to one of the following groups: rhBMP-2 (group I); rhBMP-2 and systemic OPG (group II); rhBMP-2 and delayed administration of OPG (group III); LV-BMP-2 transduced MBMSCs (group IV); LV-BMP-2 transduced MBMSCs and systemic OPG (group V); a collagen sponge alone (group VI); and OPG alone (group VII) (Supplementary Table 1). Both the rhBMP-2 and the LV-BMP-2 transduced MBMSCs were delivered on a collagen sponge. All of the animals received weekly subcutaneous injections of OPG (15 mg/kg) or 0.9% NaCl (groups I, IV and VI), until euthanasia at 56 days. In the continuous administration groups, weekly OPG injections began on the first post-operative day. In the delayed administration group, mice started receiving weekly OPG injections 2 weeks after the surgical procedure.
The animals were injected i.p. with xylenol orange (3 μl/g, concentration: 30 mg/ml) one day prior to euthanasia to mark the areas of recently mineralized bone matrix in association with type I collagen expressed by the Col2.3GFP+ cells. The mice were euthanized at various time points post-operatively (7, 14, 28, and 56 days) and their limbs were harvested for further analysis (radiographic evaluation, microCT, histologic analysis). Mice in group III were euthanized only at 28 and 56 days because of the delayed commencement of the OPG administration.
2.4. Radiographic evaluation
The healing of the bone defect was evaluated qualitatively with radiographs performed post mortem at 7, 14, 28 and 56 days postoperatively using a Faxitron X-ray device (Faxitron Bioptics, Tucson, AZ). A defect was considered completely healed when a bony bridge across both cortices of the defect was formed, restoring the osseous continuity of the femur. The 56-day radiographs were assessed by 3 blinded independent observers, who scored the bone healing within the area of interest using a previously established protocol [20]. Briefly, a score of 0 indicated no healing, a score of 1, 0–25% healing, a score of 2, 25–50% healing, a score of 3, 50–75% healing, a score of 4 75–99% healing and a 5 indicated complete healing. The three independent scores were converted to an average score for each defect and used for statistical analysis. Inter-observer agreement was measured with the kappa statistic.
2.5. Micro-CT imaging and analysis
Thirty two femora harvested at the 56-day time point were imaged using microCT to measure bone formation within the defect (μCT40, Scanco Medical, Bassersdorf, Switzerland). Six femora were imaged from each of groups I–V and one from groups VI and VII.
The external fixator and the wires were removed prior to imaging. Serial tomographic images were acquired within a 12.3 mm diameter field of view at 55 kV and 145 μA, collecting 2000 projections per rotation at 300 ms integration time. Three-dimensional 16-bit grayscale images of the entire femur were reconstructed using standard convolution back projection algorithms with Shepp and Logan filtering and rendered at a discrete density of 4,629,630 voxels/mm3 (isometric 6 μm voxels). Bone formation was segmented from the marrow and soft tissue at a hydroxyapatite equivalent mineral density threshold of 575 mg/cm3, allowing calculation of bone volume (BV), total volume (TV), and bone volume fraction (BVF:BV/TV) within the defect area.
2.6. Histologic analysis
After limb harvesting and removal of the external fixator and adjacent soft tissue, the specimens were processed for either standard decalcified or frozen non-decalcified histology.
For the standard histologic and subsequent quantitative histomorphometric analyses the femora were fixed in 10% formalin for 24 h followed by decalcification in 10% EDTA for 2 weeks at room temperature. The specimens were then embedded in paraffin and cut longitudinally. Sections were stained with H&E and/or tartrate-resistant acid phosphatase (TRAP) and then imaged using a Nikon’s AZ100 Multizoom microscope (Nikon Instruments Inc., Melville, NY) and analyzed with Bioquant analysis software (Bioquant Image Analysis, Nashville, TN).
Bone formation in the operated femora was quantified in longitudinal H&E sections (6 slides per group, 56-day time point) at 2× magnification according to a previously established protocol [6,21]. The ROI was selected to include any newly formed bone, by marking a rectangular area comprising the defect area, superior and inferior bony bridges. The surface area of the ROI represented the tissue area (T.Ar). The amount of new bone was quantified in the defined ROI (bone area; B.Ar). The ratio of B.Ar/T.Ar was then calculated.
The number of osteoclasts per mm2 of bone (OcN/BS) was determined by examining TRAP stained sections (6 slides per group per time point) at 20× magnification. Four representative rectangular areas were selected at each corner of the defect. TRAP positive cells in close association with the bone surface appearing as multinucleated, mononuclear or without any nuclei were regarded as osteoclasts [22–24] and included in the counts as described before [6,25]. The mean of the four quadrants was taken and used for statistical analysis.
2.7. Non-decalcified frozen histology and fluorescent imaging
The Kawamoto method was used to perform the frozen sections of full-length non-decalcified femora [26]. In brief, the specimens were frozen in −80 °C, immersed into cooled SCEM embedding medium and then frozen quickly together in hexane to form a frozen block. Non-decalcified frozen sections were cut with a cryostat (Leica, Nussloch, Germany), mounted on adhesive film (Cryofilm, Section-lab Co. Ltd., Hiroshima, Japan) and then on glass slides. The sections were kept at −20 °C until use. Before fluorescent imaging, the sections were thawed, washed with PBS for 30 min at room temperature and then mounted with 50% glycerin on new glass slides. The slides were imaged with both phase contrast and fluorescent illumination using a Zeiss Axio Observer.Z1 equipped with a 10×/0.3 Ph1 EC Plan-NEOFLUAR objective lens (Carl Zeiss Microscopy, Thornwood, NY) and AxioVision software. Following a previously established protocol [27] each fluorophore (red and green) and phase contrast were imaged separately using a different combination of excitation and emission filters. The images were captured sequentially using an AxioCam MRm camera, then overlaid to give a multichannel image of the entire femur.
2.8. Statistical analysis
Statistical analysis was done with IBM SPSS Statistics 21 with the significance level set at 0.05. One way ANOVA and post hoc analysis with Tukey’s range test were used for ELISA results (in vitro BMP-2 production), microCT data (BV & BVF), H&E quantitative histomorphometry (B.Ar and B.Ar/T.Ar) and TRAP histomorphometry (within-group analysis). Between-group analysis for TRAP histomorphometry and radiographic score analysis were done with Kruskal–Wallis test and Dunn’s post hoc test.
3. Results
3.1. In vitro BMP-2 production by LV-BMP-2 transduced cells
Total BMP-2 production over a 24 h period was quantified for study groups IV and V. Non-transduced cells were used as negative control. At MOI of 25, the 1 × 106 transduced MBMSCs used for group IV implantations produced 1.93 ± 0.51 ng of BMP-2 in a 24 h period whereas MBMSCs used for group V implantations produced 2.10 ± 0.51 ng. The BMP-2 production between groups IV and V was not statistically significant (p > 0.05). Non-transduced cells did not produce any BMP-2 (0.00 ng) (Fig. 1).
Fig. 1.

In vitro BMP-2 production by MBMSCs transduced with LV-BMP-2 at an MOI of 25. The results are presented as ng of BMP-2/24 h per 1 × 106 cells. Non-transduced cells were used as negative control. Data expressed as mean ± SD, *p < 0.05 compared with non transduced cells.
3.2. Radiographic evaluation of bone healing
At 7 days no new bone formation was seen in the bone defect area in any of the groups. A bony bridge across both cortices of the defect was noted at 14 days in animals treated with rhBMP-2 (6/6 animals), rhBMP-2 + OPG (6/6), LV-BMP-2 (5/6) and LV-BMP-2 + OPG (6/6). However, none of the animals in the sponge alone and OPG alone groups showed any evidence of new bone formation around the defect area at this time point.
All bone defects in the BMP-2 treated groups were considered completely healed at 28 and 56 days (6/6 at both time points) (Fig. 2). The callus was generally more robust in the BMP-2 + OPG treated groups. It was also noted that the callus was thinner in groups I (Fig. 2, a, b) and IV (Fig. 2, g, h) and thicker in groups II (Fig. 2, c, d), III (Fig. 2, e, f) and V (Fig. 2, i, j) at 56 days compared to the 28-days radiographs. In contrast, the majority of the animals in the sponge alone and OPG alone groups exhibited minimal bone formation even at the later time points, mostly as bony caps formed at the distal and proximal ends of the defect (Fig. 2, k–n). Only 1/12 femora in the sponge alone group and 2/12 in the OPG alone group showed some bone formation in the defect.
Fig. 2.
Representative radiographic images of the bone defects at the 28- (left image in each panel) and 56-day time points (right image in each panel). Complete healing of the femoral defect is seen in group I (a, b; rhBMP), group II (c, d; rhBMP + OPG), group III (e, f; rhBMP + delOPG), group IV (g, h; LV-BMP-2) and group V (i, j; LV-BMP-2 + OPG) in both time-points, with the callus looking less robust in the BMP-2 alone treated groups. Little bone formation and lack of bridging of the bone defect are evident in groups VI (k, l; collagen sponge alone) and VII (m, n; OPG).
At day 56, radiographic healing scores were significantly higher (p < 0.001) in the BMP-2 treated femora (mean score of 5 ± 0 for groups I, II, III and V and 4.95 ± 0.12 for group IV) compared to the control defects (sponge alone: 1.1 ± 0.56 and OPG alone: 1.06 ± 0.30). No significant differences were observed between the various BMP-2 treatment groups (rhBMP-2 and gene therapy, alone or combined with OPG). Furthermore, there was no significant difference in the radiographic scores between the carrier alone and the OPG alone groups. Inter-observer agreement for day 56 radiographic healing scores was strong, with a kappa statistic of κ = 0.97.
3.3. Bone formation on microCT
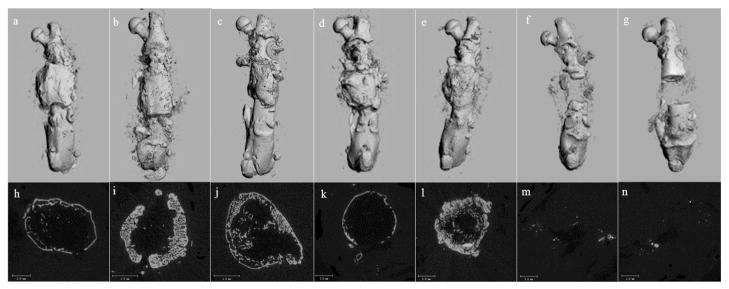
MicroCT demonstrated bone healing at the area of the bone defect in all BMP-exposed groups (I–V), with formation of continuous bony cortex and reconstitution of the medullary canal (Fig. 3, a–e). Mid-defect axial views showed a thicker cortex in BMP-exposed groups co-treated with OPG-Fc (groups II, III, V), compared to the thinner shell-like cortex observed in groups exposed to BMP-2 alone (I and IV) (Fig. 3, f–j). In contrast, groups treated with the carrier alone or OPG alone exhibited no bone formation within the femoral defect.
Fig. 3.
Representative micro-CT images obtained in the longitudinal and axial planes showing the healing of the femoral defect at the 56-day time point. Complete healing of the defect is evident in the longitudinal view for the BMP-2 treated groups (a. rhBMP-2, b. rhBMP-2 + OPG, c. rhBMP-2 + delOPG, d. LV-BMP-2, e. LV-BMP-2 + OPG). The axial view (h–l) indicates the formation of new cortices and reconstitution of the medullary canal across the femoral defect. A thicker cortex is observed in BMP-2 + OPG treated groups (i, j, l) compared to the groups treated with BMP alone (h and k). Minimal bone formation within the femoral defect is seen in the carrier alone (f and m) and OPG alone (g and n) groups.
Post-imaging volumetric analysis revealed that the rhBMP-2 alone group had a significantly lower BV compared to the rhBMP-2 + OPG (p = 0.005) and LV-BMP-2 + OPG (p = 0.008) groups and a lower BVF compared to rhBMP-2 + OPG (p = 0.003). BV and BVF in the LV-BMP-2 alone group were significantly lower compared to rhBMP-2 + OPG (p < 0.001), rhBMP-2 + delOPG (p < 0.02) and LV-BMP-2 + OPG (p < 0.001) (Table 1). No significant differences with respect to BV and BVF were observed between the various BMP-2 + OPG groups (groups II, III, V). Since none of the control groups healed, only one sample from the carrier alone (group VI) and OPG alone (group VII) groups was imaged and therefore the results from the post-scanning analysis were not included in the statistical analysis.
Table 1.
Post-imaging (microCT) volumetric analysis, including calculation of BV and BVF within the defect area, for groups I–V.
| Groups | Bone volume (BV, mm3) | Bone volume fraction (BVF, %) |
|---|---|---|
| Group I: rhBMP-2 | 1.71 ± 0.63* | 15.65 ± 12.17♭ |
| Group II: rhBMP-2 + OPG | 3.47 ± 1.37 | 34.42 ± 11.28 |
| Group III: rhBMP-2 + delOPG | 2.59 ± 0.63 | 25.42 ± 4.99 |
| Group IV: LV-BMP-2 | 1.08 ± 0.27† | 6.92 ± 2.08† |
| Group V: LV-BMP-2 + OPG | 3.40 ± 0.52 | 27.48 ± 2.44 |
p < 0.01 versus groups II and V, p > 0.05 vs groups III and IV.
p < 0.01 versus group II, p > 0.05 vs groups III, IV, and V.
p < 0.02 versus groups II, III and V, p > 0.05 vs group I.
3.4. Histologic and histomorphometric analysis
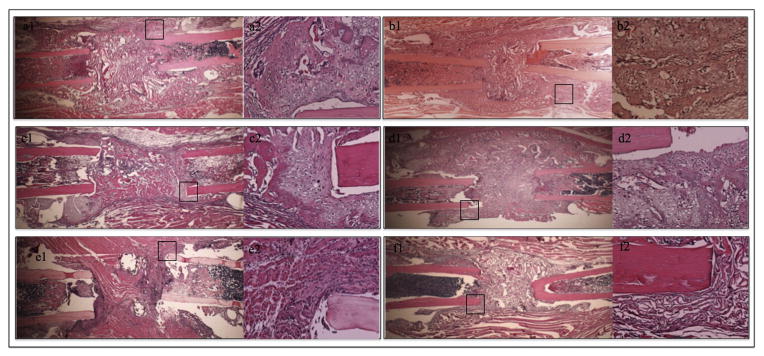
Histological analysis of the bone defects in all groups during the 7-day time point revealed a hypercellular area inside and around the bone defect, consistent with acute inflammation and early stages of bone repair. In the BMP-2 treated groups (I, II, IV, V) fibrocartilaginous tissue was also present (Fig. 4). New bone formation with bridging of the defect was evident in all groups exposed to BMP-2 as early as 14 days post-operatively (Fig. 5, a, b, c, d). The bone present at 14 days was woven with few remnants of cartilage. No bone or cartilage intermediate formation was observed at this time point in carrier alone and OPG alone treated femora (Fig. 5, e, f). At 28 days, there was continuous bony cortex spanning the length of the defect in all of the BMP-2 groups (groups I–V). The cortex was thinner in BMP-2 alone (groups I and IV) compared to the BMP-2 + OPG-Fc groups (groups II, III, V) (Fig. 6, a–e). Thinning of the bony bridge was observed in groups I and IV at 28 and 56 days compared to the 14-day time point (Figs. 5, a, b, 6, a, d, h, k). In contrast to the healed defects in BMP-2 groups, in the control groups (VI and VII, 28 and 56 days time points) fibrous and adipose tissues were present in the bone defect and only minimal bone formation at the proximal and distal ends of the defect (Fig. 6, f, g, m, n).
Fig. 4.
Representative H&E stained sections at the 7-day time point in the BMP-2 treated groups (groups I, II, IV and V). Within each panel, the left image is a longitudinal section of the femoral defect (2× magnification) and the image on the right is a 20× magnification of the black rectangle showing hypercellular areas of acute inflammation and cartilaginous tissue. This tissue forms a superior bridge over the bone ends/bone defect in the area where the bony bridge will later be formed in groups I, II, IV and V.
Fig. 5.
Representative 2× (left image in each panel) and 20× (right image) histological images of the defect area at 14 days. The femora in groups I, II, IV and V demonstrate new bone formation spanning the length of the defect. The callus at this time point consists mostly of woven bone with few remnants of cartilage mostly evident at the host defect interface. Femora in the control groups (groups VI and VII) did not exhibit any bone or cartilage intermediate formation.
Fig. 6.
Histologic evaluation of bone healing at 28 and 56 days post-operatively. Representative longitudinal H&E stained sections (2× magnification) of the femora in group I (a, h), group II (b, i), group III (c, j), group IV (d, k) and group V (e, l) demonstrate new periosteal and endosteal bone formation adjacent to the host cortical bone with bridging of the defect. The newly formed cortex is significantly thinner in BMP-2 alone groups (groups I and IV) compared to groups treated with a combination of BMP-2 and OPG (groups II, III and V). In groups VI (f, m) and VII (g, n), fibrous and adipose tissues are evident within the defect, with minimal bone formation at the proximal and distal ends.
Quantitative histomorphometric analysis of the H&E stained slides at the 56-day time point confirmed significant differences in bone formation demonstrated by microCT and qualitative histologic analyses. Mean B.Ar and B.Ar/T.Ar were lower in groups treated with OPG-Fc alone and sponge alone defects compared to the remaining groups. However this difference between controls (groups VI and VII) and experimental groups (groups I–V) was statistically significant (p ≤ 0.001) only versus BMP-2 + OPG groups (II, III and V). Although the rhBMP-2 and LV-BMP-2 treated defects had a higher B.Ar than the controls (groups VI and VII), no statistically significant difference was found (vs carrier alone, p = 0.053 and p = 0.79 respectively and vs OPG alone, p = 0.11 and p = 0.94 respectively). Furthermore, no significant difference in respect with B.Ar and B.Ar/T.Ar was shown between the sponge alone and OPG alone groups. RhBMP-2 alone defects had a significantly lower B.Ar and B.Ar/T.Ar compared to rhBMP-2 + OPG treated defects. There was a trend towards lower B.Ar and B.Ar/T.Ar in the rhBMP-2 group compared to rhBMP-2 + delOPG and LV-BMP-2 + OPG treated defects, but the difference was not statistically significant. LV-BMP-2 defects had a significantly lower B.Ar and B.Ar/T.Ar compared to all BMP-2 + OPG treated groups (groups II, III and V, p ≤ 0.01). On the other hand no significant differences in B.Ar or B.Ar/T.Ar were observed between the BMP-2 + OPG-Fc groups (Table 2).
Table 2.
Histomorphometric analysis at 56 days. RhBMP-2 alone defects had a significantly lower (p < 0.005) B.Ar and B.Ar/T.Ar compared to rhBMP + OPG treated defects. LV-BMP-2, OPG alone and collagen sponge alone defects had a significantly lower B.Ar and B.Ar/T.Ar compared to all BMP-2 + OPG treated groups (II, III and V). No difference was observed with respect to B.Ar and B.Ar/T.Ar between the BMP-2 + OPG treated groups.
| Groups | Bone area (B.Ar, mm2) | Bone areal fraction (B.Ar/T.Ar, %) |
|---|---|---|
| Group I: rhBMP | 1.77 ± 0.49† | 12.1 ± 3.25† |
| Group II: rhBMP + OPG | 3.72 ± 1.62 | 25.55 ± 11.27 |
| Group III: rhBMP + delOPG | 3.01 ± 1.09 | 20.79 ± 7.6 |
| Group IV: LV-BMP | 1 ± 0.32* | 7.03 ± 2.71* |
| Group V: LV-BMP + OPG | 2.71 ± 0.31 | 18.74 ± 2.3 |
| Group VI: CTRL | 0.42 ± 0.23 | 3.45 ± 1.54 |
| Group VII: OPG | 1.12 ± 0.15 | 7.39 ± 0.74 |
p < 0.005 vs group II, p > 0.05 vs groups III–VII.
p ≤ 0.01 vs groups II, III and V, p > 0.05 vs groups I, VI and VII.
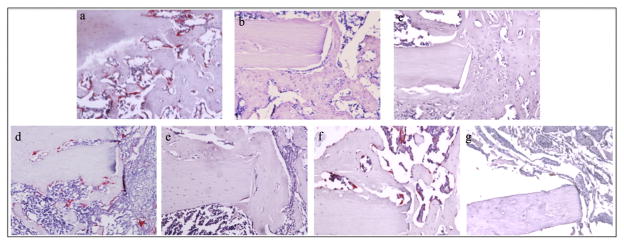
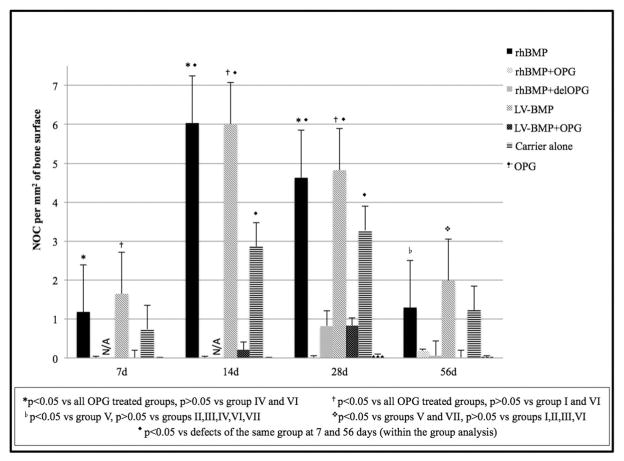
Trap staining verified the presence of TRAP+ cells as early as 7 days postoperatively in the groups that did not receive OPG-Fc (rhBMP-2, LV-BMP-2 and sponge alone). Most of the TRAP+ cells at this time point were mononuclear and located at the periphery of the bone defect. The groups not treated with OPG (I, IV and VI) exhibited significant increases in the numbers of osteoclasts surrounding the resorbing collagen sponge, bone ends and newly formed callus at the 14-day and 28-day time points (Fig. 7). By 56 days the number of osteoclasts was significantly reduced (p < 0.05 vs 14- and 28-day time points for groups I, IV and VI), to levels similar to those observed at 7 days (Fig. 8).
Fig. 7.
Representative 20× TRAP stained sections for all experimental groups at the 28-day time point. TRAP+ cells (red) adjacent to the bone were classed as osteoclasts. Abundant osteoclasts were observed in groups I (rhBMP-2; a), IV (LV-BMP-2; d) and VI (carrier alone; f). No osteoclasts were seen in animals treated with OPG (group II; b, group III; c, group V; e, group VII; g).
Fig. 8.
Average number of osteoclasts (NOC) per mm2 of bone surface at the bone defect area for the 7 groups at different time points is presented. Between the groups analysis revealed a higher NOC in defects treated with rhBMP-2 or LV-BMP-2 compared to OPG treated defects (groups II, II, V, and VII). No difference was seen between rhBMP-2 and LV-BMP-2. There was also no difference between the OPG treated groups. Within the group analysis showed a significant difference in the NOC at the 7- and 56-day time points compared to the 14- and 28-day time points for rhBMP-2, LV-BMP-2 and sponge alone defects. N/A: not applicable since rhBMP-2 + delOPG animals were not euthanized at days 7 or 14.
In contrast, few if any TRAP+ cells were detected at all time points in the OPG-Fc groups. Between-group analysis revealed a higher OcN/BS in defects treated with rhBMP-2 or LV-BMP-2 compared to any of the OPG-Fc groups at days 7, 14 and 28. At 56 days a difference in OcN/BS (p < 0.05) was seen only between rhBMP-2 alone versus LV-BMP-2 + OPG and between LV-BMP-2 alone versus LV-BMP-2 + OPG or OPG alone. No difference in OcN/BS was observed between rhBMP-2, LV-BMP-2 and carrier alone groups. There was also no difference between the OPG treated groups at all time points (Fig. 8).
3.5. Evaluation of frozen sections
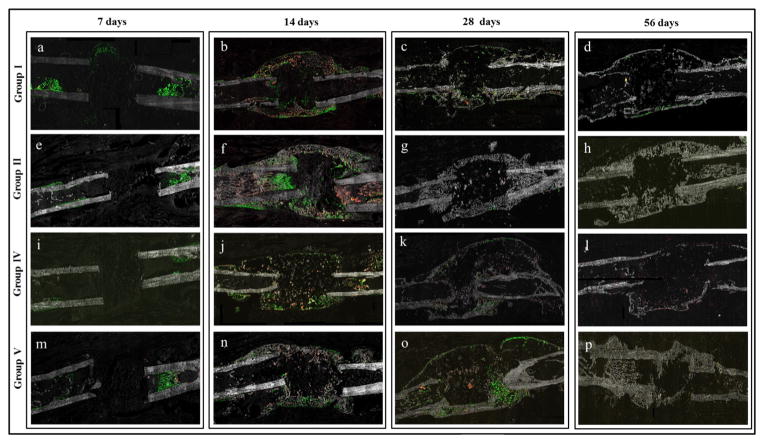
At 7 days, GFP+ cells (differentiated osteoblasts and osteocytes) started to appear at the defect region of rhBMP2 treated animals, forming a superior bridge over the defect in an area where the bony bridge would later be formed. The remaining groups showed a minimal osteogenic cell response, limited to the endosteum and intramedullary canal (Fig. 9, a, e, i, m).
Fig. 9.
Fluorescent imaging of frozen sections. GFP expression indicates the presence of differentiated osteoblasts and osteocytes. Red color represents newly mineralized bone matrix stained with xylenol orange. The area of the bone defect is depicted for experimental groups I (a–d), II (e–h), IV (i–l) and V (m–p) from the 7-day through the 56-day time point. At the earliest time point, prior to visible evidence of new bone formation, GFP+ cells were apparent in the marrow of all 4 groups, but only the rhBMP-2 group exhibited GFP+ cells within the defect region (a, e, i, m). A mineralized bony bridge with a high number of GFP+ cells first appeared at 14 days in all of the groups (b, f, j, n). At the later time points the bridge became significantly thinner in the BMP-2 alone treated groups (c–d, k–l) and thicker in the BMP-2 + OPG treated groups (g–h, o–p), with fewer Col2.3 cells.
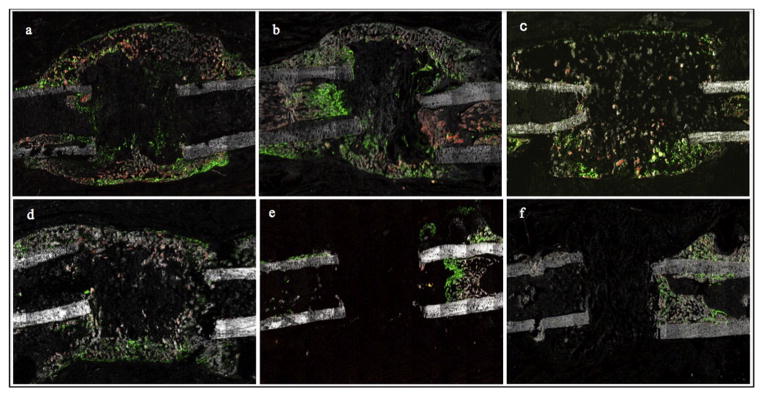
A disorganized, mineralized (obvious by xylenol orange staining) bony bridge containing significant numbers of mature osteoblasts (GFP+ cells) was present at 14 days in BMP-2 groups (groups I, II, IV and V) (Fig. 10). At 28 and 56 days the bony bridge gradually became thinner in BMP-2 alone groups (I and IV) and thicker in BMP-2 + OPG groups. A prominent Col2.3 cell response was evident in the bony bridge at the 14 and 28-day time points. The bony bridge consisted mostly of cells and less of bone in the rhBMP-2 and LV-BMP-2 alone groups at the 14-day time point. An apparently higher bone to cells ratio was noted in the bony bridge in the BMP-2 + OPG treated groups. For the 56-day samples, bone dominated and the GFP+ cells decreased noticeably (Fig. 9).
Fig. 10.
Representative fluorescent images at 14 days postoperatively. Mineralized woven bone spanning the length of the defect is evident in the rhBMP-2 (a), rhBMP-2 + OPG (b), LV-BMP-2 (c) and LV-BMP-2 + OPG groups (d), with the bony bridge being more robust in the BMP-2 + OPG treated femora (b, d). A prominent cellular response, with numerous mature osteoblasts present in the defect site, was observed for all BMP-2 treated groups (a–d). In the two BMP-2 alone groups, the bony bridge consisted mostly of GFP+ cells with more modest amounts of bone in comparison with the corresponding BMP-2 + OPG groups. The carrier alone (e) and OPG alone (f) controls exhibited minimal bone formation, with GFP+ cells limited to the endosteum, intramedullary canal and within the newly formed bone on top of the original cortex (periosteal reaction).
Minimal cell activation, predominantly around the endosteum and periosteum, was noted at 7 days in the negative control groups (VI and VII). Bony caps at the proximal and/or distal ends of the defect were seen at the 28- and 56-day time points. However, no bony bridge spanning the length of the defect was observed in any samples from the two control groups at any time point. GFP+ cells were present in the area of the bony caps and bone formed on top of the original cortex as a result of periosteal reaction (close to the defect and/or around the wires) at the 14- (Fig. 9, e–f) and 28-day samples. Fewer Col2.3 cells were detected at 56 days in all groups.
4. Discussion
To date rhBMP-2 is the most potent osteoinductive agent available for clinical use, but the quality of bone formation is variable. A local osteoclastic activation in response to rhBMP-2 treatment is associated with bone resorption and has been noted in both animal studies and in humans [12]. Toth et al. showed that rhBMP-2 delivered on a collagen sponge in a sheep corticocancellous femoral defect can lead to a concentration-dependent osteoclastic response and peri-implant bone resorption 1–4 weeks post-operatively [13]. Alaee et al. noted thinning of the bone in a mouse critical-sized femoral defect at 28 and 56 days after treatment with rhBMP-2 [6]. This osteoclast response may resorb away newly-formed bone, thereby diminishing the total volume of bone induced via BMP-2 stimulation. Furthermore, evidence suggests that rhBMP-2 may also stimulate bone resorption prior to the bone healing response, as was reported in a non-human primate model of metaphyseal core defect healing at the proximal femur [2]. In that study, resorption of the carrier and the trabecular bone surrounding the region of the bone defect by multinucleated osteoclasts was observed as early as 7 days and reached a peak at 14 days post-operatively, followed by new bone formation [2]. Osteolysis leading to graft subsidence and migration or loss of fixation has also been reported in clinical studies, following the use of rhBMP-2 delivered on a collagen sponge for spinal fusions [3,14,28,29].
In our study TRAP staining revealed a considerable osteoclast response in rhBMP-2 alone and LV-BMP-2 alone groups beginning as early as 7 days post-operatively, reaching a peak at 14 to 28 days and declining at 56 days. This osteoclastic response was associated with bone resorption and thinning of the bony bridge in the BMP-2 alone groups at the later time points. In contrast, minimal or no TRAP+ cells were detected in or around the defect area and/or bony bridge at all time points in the OPG treated groups, an effect that was consistently associated with relative improvements in the volume of, and bridging by, newly formed bone. These findings suggest that local BMP-2 delivery (recombinant protein or gene therapy) can activate bone resorption thus attenuating its efficacy. By inhibiting the RANK/RANKL pathway, and therefore osteoclastogenesis and osteoclast differentiation and function, BMP-2’s osteoinductive potential is highly enhanced.
Our results demonstrated that the combination of locally delivered BMP-2, in the form of either recombinant protein or gene therapy, with systemically administered OPG can improve bone healing compared to rhBMP-2 alone or gene therapy alone in a mouse critical-sized bone defect. The microCT imaging and histologic/histomorphometric analysis demonstrated that BMP-2 + OPG defects (group II; rhBMP-2 + OPG, group III; rhBMP-2 + delOPG and group V; LV-BMP-2 + OPG) were associated with a more robust bone formation than BMP-2 alone femora (group I; rhBMP-2 and group IV; LV-BMP-2). No difference in bone volume/bone area was observed between the three BMP-2 + OPG groups with respect to microCT and histomorphometry. This important and novel observation suggests that very early osteoclast responses that may arise from local BMP-2 do not necessarily impair overall healing, provided osteoclasts are subsequently inhibited. This suggests that in clinical situations, RANKL inhibition therapy may not be commenced at the time of BMP-2 treatment, but still enhance bone repair.
The osteoclastic response to rhBMP-2 has been attributed to both the supraphysiologic dose (milligrams to induce an adequate biological effect in humans) and the rapid release profile of the protein from the carrier, as rhBMP-2 deposited on a collagen sponge is released with an early burst and diffuses rapidly from the implantation site. More specifically it has been demonstrated that approximately half of the drug is typically eluted in the first hour and two thirds in the first four days following implantation [2]. On the other hand BMP-2 production by lentiviral-mediated gene therapy is characterized by very different kinetics, with a sustained low level (100–150 ng/ml/day/mg of protein) protein release lasting up to 12 weeks after implantation [11]. However in our study no difference was observed with respect to osteoclast activation and subsequent osteolysis between rhBMP-2 on a collagen sponge and gene therapy with a lentiviral vector carrying the gene for BMP-2, despite the difference in the release profile between the two. This suggests that the presence of BMP-2 alone, regardless of the pattern of exposure, can lead to stimulation of osteoclastogenesis and bone resorption at the site of implantation, and thus is a possible explanation for the inconsistent treatment results with rhBMP-2 in humans [1,3,5,30].
In vitro studies have demonstrated a direct stimulatory effect of BMP-2 on osteoclast differentiation and function. BMP-2 enhanced osteoclastogenesis was noted in murine bone marrow cell cultures in a time- and concentration-dependent manner. [31,32] In addition, BMP-2 stimulated both osteoclast formation and bone resorption activity in rabbit mature osteoclasts [33] and in co-cultures with myoblastic C2C12 cells and mouse spleen cells [34]. This effect has been attributed to the type IA and II BMP receptors (BMPR-IA and BMPR-II) and their downstream signal transduction molecules, Smad1 and Smad5, expressed in osteoclasts. In addition, BMP treatment was associated with a significant increase in mRNA expression of cathepsin K and carbonic anhydrase II, enzymes produced by osteoclasts [34]. Itoh et al. demonstrated that BMP-2 can stimulate osteoclastic differentiation of mouse progenitor cells when cultured with RANKL and M-CSF. They also noted that BMP-2, RANKL and BMPR-IA mRNAs were expressed both in osteoclast progenitors and in mature osteoclasts, suggesting that endogenous production of BMP-2 by osteoclast progenitors is involved in their differentiation into osteoclasts in the presence of RANKL and that a cross-communication between BMP receptor-mediated signals and RANK-mediated signals exists [35].
Investigators have tested the hypothesis that an antiresorptive agent can be used to further enhance BMP efficacy. A combination of local rhBMP-7 and systemically administered bisphosphonates (BPs) has been examined in rat osteotomy [36,37] and femoral bone defect models [38]. These studies demonstrated that treatment with zolendronic acid enhances BMP-7 induced bone healing and leads to a significant increase in callus volume and mechanical strength. However, in BMP + BP studies inhibition of bone resorption was not readily evaluated histomorphometrically.
In contrast with bisphosphonates that inactivate osteoclasts without reducing their numbers, making the evaluation of bone resorption inhibition extremely difficult to measure, RANKL inhibitors uniquely reduce osteoclast numbers to barely-detectable levels. This allows histomorphometric analysis to succinctly indicate the degree of resorption inhibition (no osteoclasts = no resorption). Thus OPG-Fc was the perfect tool to evaluate the potential role of osteoclasts in limiting overall BMP-2 efficacy in our model. OPG was not tested in late-stage clinical trials, in part because of the potential formation of anti-OPG antibodies that may also interfere with endogenous OPG activity [39]. While OPG is the natural RANKL inhibitor, other recombinant RANKL inhibitors, including Fc-OPG, OPG-Fc, RANK-Fc and anti-RANKL blocking antibodies have been developed and tested in several bone loss scenarios [40]. Denosumab is the only agent approved for clinical use. Denosumab is a human monoclonal RANKL antibody that has been shown to decrease bone resorption, leading to increased bone mineral density and reduced risk of osteoporotic fractures in postmenopausal women [41,42], osteoporotic men [43] and men receiving androgen-deprivation therapy for prostate cancer [44]. Denosumab has been shown to increase callus size, strength and stiffness in a mouse closed femoral fracture model [45].
It may be true that the results with OPG are somewhat predictable based on the effects of bisphosphonates described in the literature, but our study is still novel, since we have provided preclinical proof of concept for a new clinical approach. OPG-Fc reduced osteoclast numbers dramatically, thus effectively inhibiting bone resorption and clearly improving the efficacy of BMP-2. What we think we learned from BMP + BP studies, regarding the role of osteoclast inhibition in enhancing bone healing, has now been confirmed by our BMP + OPG study. Another advantage of our study is that we compared two different local BMP-2 delivery systems (recombinant protein and gene therapy), proving that the presence of BMP-2, regardless of the pattern of exposure, can lead to stimulation of osteoclastogenesis. Finally, we evaluated the effect of continuous versus delayed systemic OPG-Fc administration in combination with local BMP-2 and showed that both administration plans reduced osteoclast numbers dramatically, thus equally inhibiting bone resorption and increasing BMP-2 efficacy.
There are several limitations to our study. First, the animals were euthanized at 56 days and therefore we were unable to determine the long-term influence of the OPG on bone remodeling and the quality of bone repair enhancement. Another limitation is that the animal model we used was a femoral defect stabilized with an external fixator in a single plane. The quality of bone repair may have been different if we had used a different construct that provided more rigid fixation in two planes.
Finally, the lack of biomechanical testing is another limitation of the present study. Our choice of experimental design placed a priority on imaging and histology to observe and quantify the formation/resorption responses, knowingly at the expense of performing mechanical tests.
However, the images seen in Figs. 3, 6 and 9 show very clearly that BMP-2 alone results in a very thin cortical shell that integrates nearly point-wise with the original diaphyseal cortex (Fig. 9, panel d), whereas BMP-2 + OPG shows bone formation filling the entire diaphyseal buttress (the entire triangular area at periosteal base, Fig. 9, panel h). It is this point of integration that dictates outcome of the overall quality of the bone repair. The inference from these images is that the bone in the BMP + OPG groups would likely be stronger and stiffer, unless that entire, filled buttress is not integrated to diaphyseal cortex. In this case there is no evidence to suggest a lack of integration/continuity (Fig. 6 i, l).
In conclusion, this study demonstrates that the combination of locally delivered BMP-2, in the form of either recombinant protein or gene therapy, and systemically administered OPG leads to enhanced bone healing compared to rhBMP-2 alone or gene therapy with BMP-2 producing cells alone in a mouse critical-sized femoral defect. RANKL inhibitors could be potentially used as adjuvants in clinical situations with challenging bone healing problems as they can be easily administered via a subcutaneous injection, in an outpatient setting following surgical intervention.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.bone.2015.12.052.
Supplementary Material
Acknowledgments
This work was supported by a research grant from the National Institute of Health (R01AR057076-05) to J.R.L. OPG was provided by Amgen, Inc. The authors would like to thank Dr. Esteban Fernandez of Children’s Hospital Los Angeles for his invaluable assistance with fluorescent imaging of frozen sections.
References
- 1.Garrison KR, Donell S, Ryder J, et al. Clinical effectiveness and cost-effectiveness of bone morphogenetic proteins in the non-healing of fractures and spinal fusion: a systematic review. Health Technol Assess. 2007;11:1–150. doi: 10.3310/hta11300. [DOI] [PubMed] [Google Scholar]
- 2.Seeherman HJ, Li XJ, Bouxsein ML, Wozney JM. RhBMP-2 induces transient bone resorption followed by bone formation in a nonhuman primate core-defect model. J Bone Joint Surg Am. 2010;92:411–426. doi: 10.2106/JBJS.H.01732. [DOI] [PubMed] [Google Scholar]
- 3.Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11:471–491. doi: 10.1016/j.spinee.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 4.Chan DS, Garland J, Infante A, Sanders RW, Sagi HC. Wound complications associated with BMP-2 in orthopaedic trauma surgery. J Orthop Trauma. 2014;28:599–604. doi: 10.1097/BOT.0000000000000117. [DOI] [PubMed] [Google Scholar]
- 5.Woo EJ. Adverse events after recombinant human BMP2 in nonspinal orthopaedic procedures. Clin Orthop Relat Res. 2013;471:1707–1711. doi: 10.1007/s11999-012-2684-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alaee F, Hong SH, Dukas AG, Pensak MJ, Rowe DW, Lieberman JR. Evaluation of osteogenic cell differentiation in response to bone morphogenetic protein or demineralized bone matrix in a critical sized defect model using GFP reporter mice. J Orthop Res. 2014;32:1120–1128. doi: 10.1002/jor.22657. [DOI] [PubMed] [Google Scholar]
- 7.Lieberman JR, Daluiski A, Einhorn TA. The role of growth factors in the repair of bone. Biology and clinical applications. J Bone Joint Surg Am. 2002;84:1032–1044. doi: 10.2106/00004623-200206000-00022. [DOI] [PubMed] [Google Scholar]
- 8.Carofino BC, Lieberman JR. Gene therapy applications for fracture healing. J Bone Joint Surg Am. 2008;14(Suppl 1):99–110. doi: 10.2106/JBJS.G.01546. [DOI] [PubMed] [Google Scholar]
- 9.Hsu WK, Sugiyama O, Park SH, et al. Lentiviral-mediated BMP-2 gene transfer enhances healing of segmental femoral defects in rats. Bone. 2007;40:931–938. doi: 10.1016/j.bone.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 10.Virk MS, Sugiyama O, Park SH, et al. “Same day” ex-vivo regional gene therapy: a novel strategy to enhance bone repair. Mol Ther. 2011;19:960–968. doi: 10.1038/mt.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feeley BT, Conduah AH, Sugiyama O, Krenek L, Chen IS, Lieberman JR. In vivo molecular imaging of adenoviral versus lentiviral gene therapy in two bone formation models. J Orthop Res. 2006;24:1709–1721. doi: 10.1002/jor.20229. [DOI] [PubMed] [Google Scholar]
- 12.Giannoudis PV, Kanakaris NK, Einhorn TA. Interaction of bone morphogenetic proteins with cells of the osteoclast lineage: review of the existing evidence. Osteoporos Int. 2007;18:1565–1581. doi: 10.1007/s00198-007-0441-x. [DOI] [PubMed] [Google Scholar]
- 13.Toth JM, Boden SD, Burkus JK, Badura JM, Peckham SM, McKay WF. Short-term osteoclastic activity induced by locally high concentrations of recombinant human bone morphogenetic protein-2 in a cancellous bone environment. Spine. 2009;34:539–550. doi: 10.1097/BRS.0b013e3181952695. [DOI] [PubMed] [Google Scholar]
- 14.Pradhan BB, Bae HW, Dawson EG, Patel VV, Delamarter RB. Graft resorption with the use of bone morphogenetic protein: lessons from anterior lumbar interbody fusion using femoral ring allografts and recombinant human bone morphogenetic protein-2. Spine. 2006;31:E277–E284. doi: 10.1097/01.brs.0000216442.12092.01. [DOI] [PubMed] [Google Scholar]
- 15.Brendan BF, Xing L. Biology of RANK, RANKL and osteoprotegerin. Arthritis Res Ther. 2007;9(Suppl 1):S1. doi: 10.1186/ar2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kong YY, Yoshida H, Sarosi I, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 17.Mizuno A, Amizuka N, Irie K, et al. Severe osteoporosis in mice lacking osteoclastogenesis inhibitory factor/osteoprotegerin. Biochem Biophys Res Commun. 1998;247:610–615. doi: 10.1006/bbrc.1998.8697. [DOI] [PubMed] [Google Scholar]
- 18.Simonet WS, Lacey DL, Dunstan CR, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 19.Alaee F, Sugiyama O, Virk MS, et al. Suicide gene approach using a dual-expression lentiviral vector to enhance the safety of ex vivo gene therapy for bone repair. Gene Ther. 2014;21:139–147. doi: 10.1038/gt.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lieberman JR, Daluiski A, Stevenson S, et al. The effect of regional gene therapy with bone morphogenetic protein-2-producing bone-marrow cells on the repair of segmental femoral defects in rats. J Bone Joint Surg Am. 1999;81:905–917. doi: 10.2106/00004623-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Alaee F, Virk MS, Tang H, et al. Evaluation of the effects of systemic treatment with a sclerostin neutralizing antibody on bone repair in a rat femoral defect model. J Orthop Res. 2014;32:197–203. doi: 10.1002/jor.22498. [DOI] [PubMed] [Google Scholar]
- 22.Ballanti P, Minisola S, Pacitti MT, et al. Tartrate-resistant acid phosphate activity as osteoclastic marker: sensitivity of cytochemical assessment and serum assay in comparison with standardized osteoclast histomorphometry. Osteoporos Int. 1997;7(1):39–43. doi: 10.1007/BF01623458. [DOI] [PubMed] [Google Scholar]
- 23.Kaye M. When is it an osteoclast? J Clin Pathol. 1984:398–400. doi: 10.1136/jcp.37.4.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whang PG, Schwarz EM, Gamradt SC, Dougall WC, Lieberman JR. The effects of RANK blockade and osteoclast depletion in a model of pure osteoblastic prostate cancer metastasis in bone. J Orthop Res. 2005 Nov;23:1475–1483. doi: 10.1016/j.orthres.2005.05.004.1100230634. [DOI] [PubMed] [Google Scholar]
- 25.Feeley BT, Gamradt SC, Hsu WK, et al. Influence of BMPs on the formation of osteoblastic lesions in metastatic prostate cancer. J Bone Miner Res. 2005;20:2189–2199. doi: 10.1359/JBMR.050802. [DOI] [PubMed] [Google Scholar]
- 26.Kawamoto T, Kawamoto K. Preparation of thin frozen sections from nonfixed and undecalcified hard tissues using Kawamoto’s film method. Methods Mol Biol. 2014;1130:149–164. doi: 10.1007/978-1-62703-989-5_11. [DOI] [PubMed] [Google Scholar]
- 27.Jiang X, Kalajzic Z, Maye P, et al. Histological analysis of GFP expression in murine bone. J Histochem Cytochem. 2005;53:593–602. doi: 10.1369/jhc.4A6401.2005. [DOI] [PubMed] [Google Scholar]
- 28.McClellan JW, Mulconrey DS, Forbes RJ, Fullmer N. Vertebral bone resorption after transforaminal lumbar interbody fusion with bone morphogenetic protein. J Spinal Disord Tech. 2006 Oct;19(7):483–486. doi: 10.1097/01.bsd.0000211231.83716.4b. [DOI] [PubMed] [Google Scholar]
- 29.Helgeson MD, Lehman RA, Jr, Patzkowski JC, et al. Adjacent vertebral body osteolysis with bone morphogenetic protein usein transforaminal lumbar. Spine J. 2011 Jun;11(6):507–510. doi: 10.1016/j.spinee.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 30.Garrison KR, Shemilt I, Donell S, et al. Bone morphogenetic protein (BMP) for fracture healing in adults. Cochrane Database Syst Rev. 2010 Jun 16;6:CD006950. doi: 10.1002/14651858.CD006950.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wutzl A, Rauner M, Seemann R, et al. Bone morphogenetic proteins 2, 5, and 6 in combination stimulate osteoblasts but not osteoclasts in vitro. J Orthop Res. 2010;28:1431–1439. doi: 10.1002/jor.21144. [DOI] [PubMed] [Google Scholar]
- 32.Paul S, Lee JC, Yeh LC. A comparative study on BMP-induced osteoclastogenesis and osteoblastogenesis in primary cultures of adult rat bone marrow cells. Growth Factors. 2009;27:121–131. doi: 10.1080/08977190802707324. [DOI] [PubMed] [Google Scholar]
- 33.Kaneko H, Arakawa T, Mano H, et al. Direct stimulation of osteoclastic bone resorption by bone morphogenetic protein (BMP)-2 and expression of BMP receptors in mature osteoclasts. Bone. 2000;27:479–486. doi: 10.1016/s8756-3282(00)00358-6. [DOI] [PubMed] [Google Scholar]
- 34.Otsuka E, Notoya M, Hagiwara H. Treatment of myoblastic C2C12 cells with BMP-2 stimulates vitamin D induced formation of osteoclasts. Calcif Tissue Int. 2003;73:72–77. doi: 10.1007/s00223-002-1071-0. [DOI] [PubMed] [Google Scholar]
- 35.Itoh K, Udagawa N, Katagiri T, et al. Bone morphogenetic protein 2 stimulates osteoclast differentiation and survival supported by receptor activator of nuclear factor-kappaB ligand. Endocrinology. 2001;142:3656–3662. doi: 10.1210/endo.142.8.8300. [DOI] [PubMed] [Google Scholar]
- 36.Mathavan N, Bosemark P, Isaksson H, Tägil M. Investigating the synergistic efficacy of BMP-7 and zoledronate on bone allografts using an open rat osteotomy model. Bone. 2013;56:440–448. doi: 10.1016/j.bone.2013.06.030. [DOI] [PubMed] [Google Scholar]
- 37.Bosemark P, Isaksson H, McDonald MM, Little DG, Tägil M. Augmentation of autologous bone graft by a combination of bone morphogenic protein and bisphosphonate increased both callus volume and strength. Acta Orthop. 2013;84:106–111. doi: 10.3109/17453674.2013.773123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Little DG, McDonald M, Bransford R, Godfrey CB, Amanat N. Manipulation of the anabolic and catabolic responses with OP-1 and zoledronic acid in a rat critical defect model. J Bone Miner Res. 2005;20:2044–2052. doi: 10.1359/JBMR.050712. [DOI] [PubMed] [Google Scholar]
- 39.Bekker PJ, Holloway DL, Rasmussen AS, et al. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res. 2004;19:1059–1066. doi: 10.1359/JBMR.040305. [DOI] [PubMed] [Google Scholar]
- 40.Lacey DL, Boyle WJ, Simonet WS, et al. Bench to bedside: elucidation of the OPG–RANK–RANKL pathway and the development of denosumab. Nat Rev Drug Discov. 2012;11:401–419. doi: 10.1038/nrd3705. [DOI] [PubMed] [Google Scholar]
- 41.Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–776. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 42.Bone HG, Chapurlat R, Brandi ML, et al. The effect of three or six years of denosumab exposure in women with postmenopausal osteoporosis: results from the FREEDOM extension. J Clin Endocrinol Metab. 2013;98:4483–4492. doi: 10.1210/jc.2013-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Langdahl BL, Teglbjaerg CS, Ho PR, et al. A 24-month study evaluating the efficacy and safety of denosumab for the treatment of men with low bone mineral density: results from the ADAMO Trial. J Clin Endocrinol Metab. 2015;100:1335–1342. doi: 10.1210/jc.2014-4079. [DOI] [PubMed] [Google Scholar]
- 44.Smith MR, Egerdie B, Hernandez Toriz N, et al. Denosumab Halt Prostate Cancer Study Group. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361:745–755. doi: 10.1056/NEJMoa0809003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerstenfeld LC, Sacks DJ, Pelis M, et al. Comparison of effects of the bisphosphonate alendronate versus the RANKL inhibitor denosumab on murine fracture healing. J Bone Miner Res. 2009;24:196–208. doi: 10.1359/jbmr.081113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.