Abstract
The aim of our study is to investigate patient selection for the 21-gene recurrence score assay (RS) for breast cancer (BC) and the RS impact on chemotherapy administration (Chemo) in clinical practice across the United States through the retrospective observational study of National Cancer Data Base (NCDB) patients from 2010 to 2012. NCDB captures ~70 % of all newly diagnosed malignancies in the USA annually. The 2010–2012 period depicts data from the beginning of the NCDB that required recording of molecular assays and their data release in April 2015. De-identified demographic and clinical variables of patients that had RS results were analyzed. 513,080 patients had BC; 406,525 were estrogen receptor-positive (ER+). 74,334/91,651 patients with RS recorded as a numerical value (0–100) were analyzed (18.2 % of ER+). Patients’ ages ranged from 18 to 90 (mean = 58.8, median = 59); 99.1 % were females. Patients of Caucasian race, from regions with <7 % having no high school education, and >$63,000 median household income were more likely to be tested than patients of other races, education, or income (p < 0.001). 58.1 % of tests were performed in ER+/lymph node-negative/>1 cm tumors; 16.4 % included ≥N1 disease; 9.9 % included T1a, T3, Stage III and IV, or HER2-positive cancers. Low-risk RS result had 92.2 % negative predictive value for no Chemo. Intermediate-risk RS result had 40.1 % positive predictive value (PPV); high-risk RS had 81.2 % PPV for Chemo. RS is obtained in ~1/5 of ER + BC patients across the USA. Further studies investigating influence and implementation of the newest evidence-based management guidelines regarding patients’ selection for RS test and chemotherapy administration upon obtaining of test results are warranted.
Keywords: 21-gene recurrence score assay, Oncotype DX, Breast cancer, Clinical practice
Introduction
The 21-gene recurrence score (RS) assay for breast cancer, commercially available since January 2005 (Oncotype DX, Genomic Health Inc, Redwood City, CA), is designed for use in early-stage estrogen receptor-positive (ER+), human epidermal growth factor receptor-2 (HER2)-negative, node-negative invasive breast cancers to predict disease recurrence of tamoxifen-treated patients [1]. The test provides a low, intermediate, or high 10-year risk RS for breast cancer [1]. Based on the score, the addition of adjuvant chemotherapy to endocrine therapy is recommended for high-risk RS, while no benefit is seen for low-risk RS [2]; the benefits of adjuvant chemotherapy in addition to endocrine therapy for intermediate-risk RS are unclear [2].
The payment for Oncotype DX (OncoDX) test was approved by the Centers for Medicare & Medicaid Services (CMS) in 2006 [3]. Since 2010, the test has also been used for ER+/HER2- and 1–3 lymph node-positive postmenopausal women with breast cancer and provides low-, intermediate-, and high-risk 5-year RS and mortality score in these patients [4].
Since inception, patient selection for the OncoDX test has been evolving, reaching the level of category 2A evidence in the National Comprehensive Cancer Network (NCCN) guidelines for breast cancer version 2.2015 [5]. The NCCN panel suggested the OncoDX assay as “an option when evaluating patients with primary tumors characterized as 0.6–1 cm with unfavorable features or >1 cm, and node-negative, hormone receptor-positive, and HER2-negative”.
However, the most recent version of the NCCN breast cancer guidelines 1.2016 [6] as well as just published American Society of Clinical Oncology (ASCO) Clinical Practice Guideline [7] endorsed OncoDX assay as both a prognostic and predictive test for breast cancer recurrence and response to chemotherapy. This update coincided with Trial Assigning Individualized Options for Treatment (TAILORx) interim findings published in the New England Journal of Medicine [8] which claimed prospective prognostic and predictive validation of OncoDX assay. This trial, however, utilized different cut-off values for the low-, intermediate-, and high-risk RS from the commercial test values. Interestingly though, the traditional commercial test cut-off values are referenced by the newest NCCN guidelines [6].
Data on utilization and impact of the OncoDX breast cancer assay in clinical practice so far are based on small single or multi-institutional studies or meta-analysis [9–13], as well as impact of the test in countries other than the United States [14, 15]. Only a few recent publications studied utilization and impact of the test in limited population groups in the United States [16–19].
Since the data on appropriate utilization and impact of the OncoDX assay in everyday clinical practice across the entire United States population are still lacking, we investigated the impact of the test across the United States in a retrospective observational study of National Cancer Data Base (NCDB) patients from 2010 to 2012. We examined racial and socioeconomic factors in test utilization, practices used for ordering the test, and the impact of test results on adjuvant chemotherapy use. We also compared chemotherapy utilization and vital status of patients when applying commercial OncoDx cut-off values [2] versus the new TAILORx trial-defined cut-off values [8].
Methods
National Cancer Data Base (NCDB) and study approval
NCDB is a clinical oncology database, acquiring data from hospital tumor registries, gathered from more than 1500 Commission on Cancer (CoC)-accredited facilities. NCDB, jointly sponsored by the American College of Surgeons (ACoS) and the American Cancer Society (ACS), currently captures ~70 % of all newly diagnosed malignancies in the USA annually [20].
The 2010–2012 study period captures NCDB data from the beginning of the NCDB required recording of molecular assay test results in different types of cancers and the NCDB data released in April 2015. For breast cancer, NCDB captured data on genomic tests with only three separate codes which identified “Oncotype DX test,” “MammaPrint test,” and “Other” as separate tests. In 2010–2012 time period, 97,510 genomic tests for breast cancer were captured by NCDB, with Oncotype DX test dominating by the number of tests performed (91,651 tests = 94 %). MammaPrint test was performed in 2518 cases (2.5 %), and other tests, including the ones performed but of unknown type, were done in 3341 patients (3.4 %).
Data regarding patients’ and institutions’ names were de-identified by the NCDB prior to the release of the file and therefore met the criteria of 45 CFR 46.102 d research. Since the information received was not individually identifiable, the research was not a deemed research with human subjects; therefore, our Institutional Review Board was not required.
Patients’ selection
Demographic and clinicopathologic variables of patients with OncoDX results expressed as a numerical value (0–100) were analyzed. Those with performed OncoDX assay but unknown numerical results were excluded.
Analysis
Tables 1 and 2 define the demographic, clinical, and pathologic characteristics of this study population.
Table 1.
Race and socioeconomic characteristics of a 21-gene recurrence score assay for breast cancer (Oncotype DX) in patients tested compared to patients eligible for testing (ER-positive, lymph node-negative, HER2-negative patients with tumor size between 0.6 cm and 5 cm)
| Characteristics | Patients eligible for Oncotype DX test | Oncotype DX test performed | p value* | Odds ratio | 95 % CI |
|---|---|---|---|---|---|
| Race | 174,079 | 63,392 | |||
| White | 151,560 | 55,712 (36.8 %) | <0.001 | Referent | |
| Black | 13,958 | 4653 (33.3 %) | <0.001 | 0.860 | 0.829–0.892 |
| Other | 6945 | 2461 (35.4 %) | 0.025 | 0.944 | 0.898–0.993 |
| Unknown | 1616 | 566 (35.0 %) | 0.150 | 0.927 | 0.837–1.028 |
| Median income quartiles 2008–2012 | 172,730 | 62,948 | |||
| >$63000 | 66,219 | 25,913 (39.1 %) | <0.001 | Referent | |
| <$38000 | 23,096 | 7450 (32.3 %) | <0.001 | 0.741 | 0.718–0.764 |
| $38000–$47999 | 36,569 | 12,564 (34.4 %) | <0.001 | 0.814 | 0.793–0.836 |
| $48000–$62999 | 46,846 | 17,021 (36.3 %) | <0.001 | 0.888 | 0.866–.910 |
| Percent no high school degree 2008–2012 | 172,792 | 62,978 | |||
| <7 % | 52,617 | 20,978 (39.9 %) | <0.001 | Referent | |
| ≥21 % | 22,475 | 6857 (30.5 %) | <0.001 | 0.662 | 0.640–0.685 |
| 13–20.9 % | 39,273 | 13,325 (33.9 %) | <0.001 | 0.775 | 0.754–0.796 |
| 7–12.9 % | 58,427 | 21,818 (37.3 %) | <0.001 | 0.899 | 0.877–0.921 |
| US geographic location | 174,079 | 63,392 | |||
| New England (CT, MA, ME, NH, RI, VT) | 10,652 | 4013 (37.7 %) | <0.001 | Referent | |
| Middle Atlantic (NJ, NY, PA) | 26,294 | 11,810 (44.9 %) | <0.001 | 1.349 | 1.288–1.413 |
| South Atlantic (DC, DE, FL, GA, MD, NC, SC, VA, WV) | 38,319 | 13,858 (36.2 %) | 0.004 | 0.937 | 0.897–0.980 |
| East North Central (IL, IN, MI, OH, WI) | 30,617 | 12,298 (40.2 %) | <0.001 | 1.111 | 1.061–1.162 |
| East South Central (AL, KY, MS, TN) | 10,010 | 3308 (33.0 %) | <0.001 | 0.817 | 0.771–0.865 |
| West North Central (IA, KS, MN, MO, ND, NE, SD) | 13,344 | 5218 (39.1 %) | 0.024 | 1.062 | 1.008–1.119 |
| West South Central (AR, LA, OK, TX) | 12,384 | 3065 (24.7 %) | <0.001 | 0.544 | 0.514–0.576 |
| Mountain (AZ, CO, ID, MT, NM, NV, UT, WY) | 9110 | 3424 (37.6 %) | 0.898 | 0.996 | 0.940–1.055 |
| Pacific (AK, CA, HI, OR, WA) | 23,349 | 6398 (27.4 %) | <0.001 | 0.624 | 0.595–0.656 |
| Urban vs rural | 169,207 | 61,748 | |||
| Urban | 166,495 | 60,748 (36.5 %) | Referent | ||
| Rural | 2712 | 1000 (36.9 %) | 0.678 | 1.017 | 0.940–1.100 |
* Patients of Caucasian race, from regions with <7 % having no high school education, and >$63,000 median household income were more likely to be tested with Oncotype DX test than patients of other races, education, or income (p < 0.001). Urban versus rural location did not have a significant impact on Oncotype DX test utilization
Table 2.
Clinicopathologic characteristics of invasive breast carcinomas tested with a 21-gene recurrence score assay for breast cancer (Oncotype DX)
| Oncotype DX risk recurrence scores | ||||
|---|---|---|---|---|
| Low-risk score 0–17 | Intermediate-risk score 18–30 | High-risk score 31–100 | Total for all scores 0–100 | |
| # and % of analyzed patients per score | 41,682 (56.1 %) | 24,965 (33.6 %) | 7687 (10.3 %) | 74,334* (100 %) |
| Age | ||||
| Median (interquartile range) year | 59.0 (51-59) | 59.0 (51-66) | 59.0 (51–67) | 59.0 (51–66) |
| Mean (year) ± SD | 58.91 ± 10.42 | 58.59 ± 10.63 | 58.89 ± 11.41 | 58.8 ± 10.6 |
| Comorbidities # and % per score | ||||
| 0 | 35,649 (85.5 %) | 21,407 (85.7 %) | 6484 (84.4 %) | 63,540 (85.5 %) |
| 1 | 5118 (12.3 %) | 3.063 (12.3 %) | 1005 (13.1 %) | 9186 (12.4 %) |
| ≥2 | 915 (2.2 %) | 495 (2.0 %) | 198 (2.6 %) | 1608 (2.1 %) |
| Tumor size (greatest dimension) | ||||
| Median (interquartile range) (cm) | 1.5 (1.0–2.0) | 1.5 (1.1–2.1) | 1.7 (1.2–2.5) | 1.5 (1.1–2.1) |
| Mean (cm) | 2.4 ± 8.6 | 2.4 ± 8.1 | 2.67 ± 8.4 | 2.45 ± 8.4 |
| Tumor distribution—# and % per score | ||||
| 0.1–0.5 cm | 1424 (3.4 %) | 712 (2.9 %) | 238 (3.1 %) | 2374 (3.2 %) |
| 0.6–1.0 cm | 9,223 (22.1 %) | 5,217 (20.9 %) | 1184 (15.4 %) | 15,624 (21.0 %) |
| 1.1–2.0 cm | 21,113 (50.7 %) | 12,620 (50.6 %) | 3518 (45.8 %) | 37,251 (50.2 %) |
| 2.1–3.0 cm | 6898 (16.6 %) | 4480 (18.0 %) | 1843 (24 %) | 13,221 (17.8 %) |
| 3.1–4.0 cm | 1664 (4.0 %) | 1091 (4.4 %) | 564 (7.3 %) | 3319 (4.5 %) |
| 4.1–5.0 cm | 595 (1.4 %) | 403 (1.6 %) | 196 (2.6 %) | 1194 (1.6 %) |
| ≥5.1 cm | 656 (1.6 %) | 384 (1.5 %) | 123 (1.6 %) | 1163 (1.6 %) |
| Unknown | 67 (0.2 %) | 39 (0.2 %) | 13 (0.2 %) | 119 (0.2 %) |
| Histologic grade of tumor—# and % per score | ||||
| Low | 14,013 (33.6 %) | 5473 (21.9 %) | 351 (4.6 %) | 19,837 (26.7 %) |
| Intermediate | 22,200 (53.3 %) | 13,537 (54.2 %) | 2572 (33.5 %) | 38,309 (51.5 %) |
| High | 3355 (8 %) | 4826 (19.3 %) | 4,479 (58.3 %) | 12,660 (17 %) |
| Unknown | 2114 (5.1 %) | 1,129 (4.5 %) | 285 (3.7 %) | 3528 (4.7 %) |
| Number of positive lymph nodes # and % per score | Total # = 72,568 | |||
| 0 | 33,615 (82.5 %) | 20,332 (83.4 %) | 6430 (86.4 %) | 60,377 (81.2 %) |
| 1 | 5258 (12.9 %) | 2946 (12.1 %) | 709 (9.5 %) | 8913 (12 %) |
| 2–3 | 1597 (3.9 %) | 912 (3.7 %) | 217 (2.9 %) | 2726 (3.7 %) |
| ≥4 | 287 (0.7 %) | 175 (0.7 %) | 90 (1.2 %) | 552 (0.7 %) |
| Cancer stage # and % per score | Total # = 74,191 | |||
| I | 28,742 (69.1 %) | 17,000 (68.2 %) | 4569 (59.7 %) | 50,311 (67.8 %) |
| II | 12,078 (29.0 %) | 7453 (29.9 %) | 2871 (37.5 %) | 22,402 (30.21 %) |
| III | 519 (1.2 %) | 315 (1.3 %) | 131 (1.7 %) | 965 (1.3 %) |
| IV | 47 (0.1 %) | 34 (0.1 %) | 33 (0.4 %) | 114 (0.2 %) |
| Unknown | 224 (0.5 %) | 121 (0.5 %) | 54 (0.7 %) | 399 (0.5 %) |
| Estrogen receptor expression—# and % per score | ||||
| Negative | 145 (0.3 %) | 149 (0.6 %) | 614 (8 %) | 908 (1.2 %) |
| Positive | 41,445 (99.4 % | 24,773 (99.2 %) | 7040 (91.6 %) | 73,258 (98.6 %) |
| Borderline | 2 (< 0.01 %) | 1 (< 0.01 %) | 14 (0.2 %) | 17 (< 0.01) |
| Unknown | 90 (0.2 %) | 42 (0.2 %) | 19 (0.2 %) | 151 (0.2 %) |
| Progesterone receptor expression—# and % per score | ||||
| Negative | 1578 (3.8 %) | 3528 (14.2 %) | 3126 (40.7 %) | 8242 (11.1 %) |
| Positive | 39,997 (95.9 %) | 21,337 (85.5 %) | 4508 (58.6 %) | 65,822 (88.5 %) |
| Borderline | 20 (< 0.01 %) | 38 (0.2 %) | 26 (0.3 %) | 84 (0.1 %) |
| Unknown | 107 (0.3 %) | 52 (0.2 %) | 27 (0.4 %) | 186 (0.3 %) |
| HER2 expression—# and % per score | ||||
| Negative | 39,686 (95.2 %) | 23,304 (93.3 %) | 6415 (83.5 %) | 69,405 (93.4 %) |
| Positive | 573 (1.4 %) | 647 (2.6 %) | 916 (11.9 %) | 2,136 (2.9 %) |
| Borderline | 601 (1.4 %) | 536 (2.1 %) | 210 (2.7 %) | 1347 (1.8 %) |
| Unknown | 822 (2 %) | 478 (1.9 %) | 146 (1.9 %) | 1446 (1.9 %) |
* From 91,651 patients that had Oncotype DX test performed from 2010 to 2012, 74,334 patients (81.1 %) had known Oncotype DX test score recorded as a numerical value (0–100) and were analyzed. 74,344 is the total number of patients, if different, it is stated in the column “total for all scores 0–100”
Chemotherapy administration (Table 3), recorded as “chemotherapy at any CoC facility” was used as a variable for analysis of chemotherapy administration. Negative predictive value for no chemotherapy administration for low-risk RS and positive predictive value for chemotherapy administration for intermediate- and high-risk RS were calculated.
Table 3.
A 21-gene recurrence score breast cancer assay (Oncotype DX) score and chemotherapy administration
| Oncotype DX score | Chemotherapy | |||
|---|---|---|---|---|
| No | Yes | Unknown | Total for chemotherapy values | |
| Low-risk recurrence score (0–17) | 38,057 (91.3 %) | 3218 (7.7 %) | 407 (1 %) | 41,682 (100 %) |
| Intermediate-risk recurrence score (18–30) | 14,827 (59.4 %) | 9942 (38.8 %) | 196 (0.8 %) | 24,965 (100 %) |
| High-risk recurrence score (31–100) | 1430 (18.6 %) | 6214 (80.8 %) | 43 (0.6 %) | 7687 (100 %) |
| Total for all risk recurrence score values | 54,314 (73.1 %) | 19,374 (26.1 %) | 646 (0.9 %) | 74,334 (100 %) |
Upon obtaining of the Oncotype DX recurrence risk score results, 73,688/74,334 (99.1 %) of patients had known chemotherapy administration results
Chemotherapy utilization and vital status of patients tested with OncoDX (Table 4) were compared using traditional, commercial OncoDX cut-off values for low-, intermediate-, and high-risk RS versus TAILORx trial new cut-off values for the test.
Table 4.
Chemotherapy administration and vital status of the patients with invasive breast carcinoma tested with a 21-gene recurrence score assay for breast cancer (Oncotype DX): National Cancer Data Base analysis from 2010 to 2012 comparing commercial Oncotype Dx cut-off values [2] to the new TAILORx trial-defined cut-off values [8] for low-, intermediate-, and high-risk recurrence scores
| Commercial Oncotype DX cut-off values | New TAILORx trial-defined cut-off values | |||||
|---|---|---|---|---|---|---|
| Low-risk score 0–17 | Intermediate-risk score 18–30 | High- risk score 31–100 | Low- risk score 0–10 | Intermediate-risk score 11–25 | High- risk score 26–100 | |
| # and % of analyzed patients per score; total = 74,334* | 41,682 (56.1 %) | 24,965 (33.6 %) | 7687 (10.3 %) | 15,887 (21.4 %) | 45,549 (61.3 %) | 12,898 (17.4 %) |
| Chemotherapy (# and % of analyzed patients per score; total = 74,334) | ||||||
| Chemotherapy not received | 38,057 (91.3 %) | 14,827 (59.4 %) | 1430 (18.6 %) | 14,888 (93.7 %) | 36,073 (79.2 %) | 3353 (26 %) |
| Chemotherapy received | 3218 (7.7 %) | 9942 (39.8 %) | 6214 (80.8 %) | 844 (5.3 %) | 9069 (19.9 %) | 9461 (73.4 %) |
| Chemotherapy unknown | 407 (1 %) | 196 (0.8 %) | 43 (0.6 %) | 155 (1 %) | 407 (0.9 %) | 84 (0.7 %) |
| Vital status—# and % per score; total = 46,245 | ||||||
| Dead | 280 (1.1 %) | 246 (1.5 %) | 151 (3 %) | 113 (1.2 %) | 349 (1.2 %) | 215 (2.6 %) |
| Alive | 24,813 (98.9 %) | 15,912 (98.5 %) | 4843 (97 %) | 9056 (98.8 %) | 28,298 (98.8 %) | 8214 (97.4 %) |
* From 91,651 patients that had Oncotype DX test performed from 2010 to 2012, 74,334 patients (81.1 %) had known Oncotype DX test score recorded as a numerical value (0–100) and were analyzed here. 677/46,245 patients died in this study period (1.5 % overall mortality)
Patients with intermediate- and high-risk recurrence score were 1.37–2.76 times more likely to die, respectively, than patients with low-risk recurrence score (95 % CI 1.15–1.62 and 2.26–3.37, respectively) for commercial test cut-off values. With TAILORx cut-off values, patients with high-risk recurrence score were 2.1 times more likely to die than patients with low-risk recurrence score (95 % CI 1.66–2.63), but there was no significant difference between low-risk recurrence score and intermediate-risk recurrence score
Statistical analysis
Normality of continuous variables was assured using skewness and kurtosis statistics. Any skewness or kurtosis statistic above an absolute value of 2.0 was considered normal. Levene’s Test of Equality of Variances was used to test for homogeneity of variance in between-subjects comparisons of continuous outcomes. Frequency statistics were used to analyze categorical variables. Unadjusted odds ratios with 95 % confidence intervals were used to test associations with categorical outcomes. Logistic regression was used to generate adjusted odds ratios with 95 % confidence intervals. Residual analysis was conducted to assess the model fit. Normality and homoscedasticity were also assessed using plots of standardized residuals.
Statistical significance was assumed at an alpha value of 0.05, and all analyses were conducted using SPSS Version 23 (Armonk, NY; IBM Corp).
Results
The NCDB registered 513,080 patients with invasive breast carcinoma from 1/1/2010 to 12/31/2012 with 406,525 patients recorded as ER-positive. Of note, the NCDB records ER assay results from pathology reports or separate clinical laboratory report sources based on reported immunohistochemical results or much less frequently based on the amount of cytosol protein in the tumor sample measured in femtomoles of cytosol protein per milligram (fmol/mg) and not based on mRNA results from Genomic Health. However, there is no way to know which test was used by NCDB for reported ER results available for our analysis.
The OncoDX test was performed in 91,651 patients; 86,409 patients had known OncoDX results, and 74,334 patients had tests recorded as a numerical value from 0 to 100.
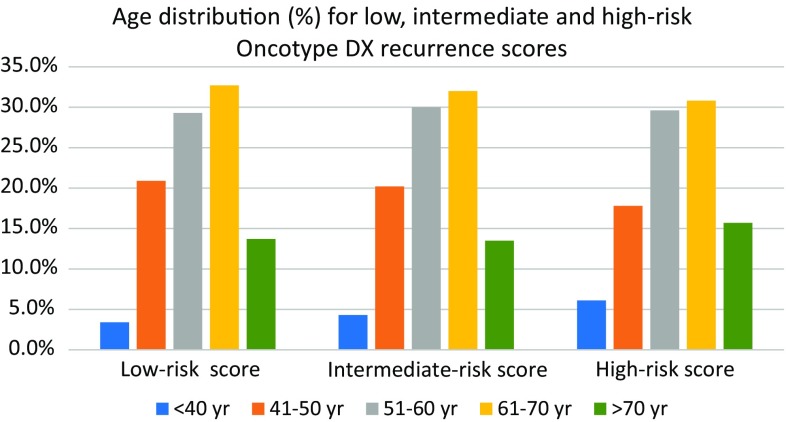
Ages ranged from 18 to 90; 99.1 % were females. Percent of breast cancer patients per age group was similar for low-, intermediate-, and high-risk score in patients 41–70 years old, but there was an increasing trend toward high-risk score in patients younger than 40 and older than 70 years of age. While this was expected in younger patients, it was unexpected in elderly patients (Fig. 1).
Fig. 1.
Percent of breast cancer patients per age group was similar for low-, intermediate-, and high-risk score in patients 41–70 years old, but there was an increasing trend towards high-risk score in patients younger than 40 and older than 70 years of age. While this was expected in younger patients, it was unexpected in elderly patients
Race and socioeconomic disparity analysis showed that patients of Caucasian race, from regions with <7 % having no high school education, and >$63,000 median household income were more likely to be tested with OncoDX than patients of other races, lower levels of education, or lower incomes (p < 0.001; Table 1).
US geographic location (geographic regions defined by NCDB), also revealed significant impact on test utilization (New England used as a referent in calculating odds ratio for being tested with OncoDX, Table 1).
The NCCN-defined intermediate-risk guidelines valid at the time of the study (ER+/lymph node-negative/>1.0 cm tumors) were followed in 58.1 % of tested patients; 16.4 % tests were performed in patients with ≥N1 disease. The majority of tests were performed on patients with T1c tumors, followed by T1b tumors (Table 2).
Interestingly, 24 % of ordered tests did not follow the guidelines applicable at the time of the study: 2.9 % of tested patients were HER2-positive; 3.2 % had T1a tumors; 16.4 % had positive lymph nodes, and 1.5 % had advanced III&IV cancer stage (Table 2).
Low-risk RS had 92.2 % negative predictive value for no adjuvant chemotherapy administration. Intermediate-risk RS had 40.1 % positive predictive value and high-risk RS had 81.2 % positive predictive value for adjuvant chemotherapy administration (Table 3).
Comparison of commercial OncoDX cut-off values for low-, intermediate-, and high-risk RS (0–17, 18–30, and 31–100, respectively) with the new TAILORx trial-defined cut-off values (0–10, 11–25, and 26–100, respectively) (Table 4) revealed that the majority of patients tested fell into the low-risk RS (0–17) with commercial OncoDX cut-off values (56.1 %), while the majority of patients (61.3 %) with TAILORx trial-defined values fell into the intermediate-risk RS (11–25). Chemotherapy for high-risk OncoDX RS was not administered in almost 20 % of patients (26 % for TAILORx values) and was administered to 7.7 % of patients with low-risk RS (5.3 % for TAILORx values).
Vital status was known in 62.2 % of cohort’s patients; 677/46245 (1.5 %) patients were expired at the end of 3-year study period (Table 4). This statistic represents overall mortality since NCDB does not record cancer-related mortality.
RS was a significant predictor of vital status in both commercial and TAILORx trial-defined cut-off values (p < 0.001; Table 4): Patients with intermediate- and high-risk RS were 1.37–2.76 times more likely to die, respectively, than patients with low-risk RS (95 % CI 1.15–1.62 and 2.26–3.37, respectively) (Table 4) for commercial test cut-off values.
Discussion
To our knowledge, this is the first study that describes utilization and impact of 21-gene recurrence score assay for breast cancer (OncoDX) in clinical practices across the entire United States.
This study revealed that there were significant racial and socioeconomic disparities in test utilization: patients of Caucasian race, from regions with <7 % having no high school education, and with >$63,000 median household income were more likely to be tested with OncoDX test than patients of other races, lower levels of education, or lower incomes. Racial disparity regarding OncoDX utilization similar to our data was also observed by Roberts et al. in 1468 breast cancer patients from the phase III Carolina Breast Cancer Study [18], and by Lund et al. [21] in 2186 breast cancer patients from tumor registries in three Atlanta area hospitals. In both studies, breast cancer patients of black race were less likely to undergo OncoDX testing than Caucasian patients. However, the racial disparity was not observed in Roberts’ study [18] if the test utilization was measured only in lymph node-negative patients. In addition, no disparity in test utilization was seen regarding the level of education or income in lymph node-negative patients [18], which is dissimilar to the data seen in our study. Guth et al. published results [11] from 374 breast cancer patients in New York City revealed that Caucasian patients and patients with higher income were more likely to be tested with OncoDX, similar to the data in our study. That study also showed that type of facility in which patients were treated also influenced the utilization of OncoDX assay, such as treatment in a tertiary center, findings consistent with our study (data not shown).
US geographic location showed a significant impact on OncoDX test utilization in our study: patients from Middle Atlantic location and West North Central location were 35 and 6 %, respectively, more likely to be tested with OncoDX than patients from New England. Patients from West South Central, Pacific, East South Central, and South Atlantic were 46 to 7 % less likely to be tested with OncoDX, while there was no difference in test utilization for patients living in Mountain geographic locations when compared to the patients from New England. To our knowledge, we are the first to describe US geographic location differences in OncoDX test ordering. Reasons for these observed differences will be explored in future studies.
Practices used for ordering of the OncoDX test were not optimally followed as per the NCCN guidelines applicable at the time of the study [22], with 24 % of tests ordered in a non-guideline-concordant fashion, such as HER2-positive cancers, T1a tumors, lymph node-positive, and stage III&IV cancers. Several other studies [18, 23, 24] revealed non-guideline-concordant ordering of the test similar to the results in our study. The test was ordered in guideline-concordant fashion when the test was paid for by CMS [11, 16], or when the test was being prospectively validated for chemotherapy decision impact or economic impact [25, 26].
The impact of the OncoDX test results on adjuvant chemotherapy use was reasonable for low- and high-risk RS (7.7 % negative predictive value and 80.8 % positive predictive value, respectively), but was far from satisfactory in the intermediate-risk score group (40.1 % positive predictive value for chemotherapy administration—Table 3). The impact of the OncoDX test results on adjuvant chemotherapy use was improved for low- and high-risk scores when our analysis was performed on patients on whom the test was performed in a guideline-concordant fashion for the 2010–2012 study time period (ER-positive, lymph node-negative, HER2-negative, with tumor size between 0.6 and 5 cm). The latter analysis revealed a 4.6 % negative predictive value for low-risk score (1306/28,582 patients) and 88.6 % positive predictive value for high-risk score (3850/4343 patients). The impact was still far from predictive in the intermediate-risk score group (42.4 % positive predictive value for chemotherapy administration, 6655/15,685).
The predictive value of OncoDX test results for chemotherapy administration benefits led to the endorsement of this test in the newest version (1.2016) of the NCCN guidelines [6] which notes the test to be superior to other available molecular tests. Similarly, recently published ASCO Clinical Practice Guideline for use of biomarkers to guide clinical decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer [7] found OncoDX test to be evidence based, with high evidence quality and strong strength of recommendation to guide decisions on the need for adjuvant chemotherapy in node-negative patients. Our NCDB data analysis revealed that 7.7 % of patients in the low-risk OncoDX RS category received chemotherapy. Conversely, almost 20 % of patients with high-risk OncoDX RS category did not receive chemotherapy. The impact of a high-risk RS results on chemotherapy administration in our study population was similar to results obtained in a study of prospective evaluation of OncoDX for breast cancer decision making in Ontario [25]. In the study from Ontario, 81 % of patients that were recommended to receive chemotherapy based on their OncoDX risk RS, received chemotherapy, however, only 2 % of patients with low-risk RS received chemotherapy. So far, no clear evidence-based guidelines have been established for chemotherapy administration for patients with intermediate-risk RS.
Availability of the final results of the TAILORx trial in 2020 will hopefully give more clarity to definitive evidence-based guidelines for all 21-gene recurrence score breast cancer assay test results including intermediate-risk scores. Requesting an expensive test, obtaining an intermediate-risk score result, and then not having clear guidelines for treatment recommendations is an expensive process, which appears to be leading to non-actionable information. Going forward, as a health care financial policy, this is especially concerning since up to 2/3 of the tested patients may fall into this intermediate-risk group. It is imperative that health care providers continually revisit guidelines and treatment recommendations and address appropriateness of intervention and cost in order to expend our resources prudently.
The TAILORx trial interim report findings [8] noted prospective validation of OncoDX 21-gene RS assay for breast cancer although notably introduced an entirely different range of cut-off values for the low-, intermediate-, and high-risk RS from the traditional commercial test values. Interestingly, the TAILORx trial revealed that the overall survival in patients with really low-risk RS was 98 % at 5 years of follow-up; our NCDB data analysis revealed similar overall survival for all groups at 98.5 %, however, with only 3 years of follow-up. Noteworthy, our data analysis revealed that high-risk RS was a significant predictor of worse overall survival in comparison with a low-risk RS, suggesting that the test, if appropriately interpreted and applied, may have not only have prognostic relevance for recurrence but also for overall survival.
Limitations as well as strengths of our study both lie in the methodology of data collection by the NCDB. The NCDB does not record recurrences of breast cancer at a distant or locoregional site, and does not record breast cancer-specific mortality, making follow-up studies of these patients for population-based validation of OncoDX 10-year RS results impossible. Another limitation includes the lack of record on the type of comorbidities encountered, which could potentially influence chemotherapy administration decisions. In addition, the NCDB neither identify the type of physician who orders OncoDX test (surgeon vs oncologist), nor can identify the balance between the patient and the clinician in decision making for or against chemotherapy use once the OncoDX test results are known, both of which greatly influence chemotherapy administration decisions. We also acknowledge the accepted NCDB process of recording data which allows inclusion of records that are only partially complete. The OncoDX test was performed in 91,651 patients from 2010 to 2012, but the test results were unknown in 5242 (5.7 %) patients, or recorded only as low-, intermediate-, and high-risk RS, without associated numerical values in 12,075 (13.2 %) patients, rendering almost 20 % of results unusable for some parts of our study.
A significant strength of this study is that data are derived from a very large number of patients across the United States in a database that includes more than 1500 CoC accredited facilities and approximately 70 % of all newly diagnosed malignancies.
Conclusions
This NCDB analysis reveals that race, socioeconomic status, and US geographic location impact utilization of the OncoDX test result in clinical practices across the United States. These data also reveal that at least 10 % of tests are ordered in a non-guideline-concordant fashion (up to 24 % if guidelines applicable during the time of the study are applied). Compliance with treatment recommendations based on OncoDX test results is reasonable for low- and high-risk RS. The impact of an intermediate-risk RS resulting in only a 40.1 % positive predictive value for chemotherapy administration suggests the need for clearer, evidence-based guidelines.
Acknowledgments
Disclaimers
The NCDB is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The CoC’s NCDB and the hospitals participating in the CoC NCDB are the source of the de-identified data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
Abbreviations
- RS
Recurrence score
- Chemo
Chemotherapy administration
- OncoDX
Oncotype DX
- NCDB
National Cancer Data Base
Compliance with ethical standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Standards
The experiments (data analyses) comply with the current laws of the country in which they were performed (United States of America).
Ethical Approval
“All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.” For retrospective studies (applies to our study): “For this type of study formal consent is not required”.
References
- 1.Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T, Hiller W, Fisher ER, Wickerham DL, Bryant J, Wolmark N. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 2.Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, Cronin M, Baehner FL, Watson D, Bryant J, Costantino JP, Geyer CE, Jr, Wickerham DL, Wolmark N. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24(23):3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 3.Barthelemy P, Heitz D, Mathelin C, Polesi H, Asmane I, Litique V, Rob L, Bergerat JP, Kurtz JE. Adjuvant chemotherapy in elderly patients with early breast cancer. Impact of age and comprehensive geriatric assessment on tumor board proposals. Crit Rev Oncol/Hematol. 2011;79(2):196–204. doi: 10.1016/j.critrevonc.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Albain KS, Barlow WE, Shak S, Hortobagyi GN, Livingston RB, Yeh IT, Ravdin P, Bugarini R, Baehner FL, Davidson NE, Sledge GW, Winer EP, Hudis C, Ingle JN, Perez EA, Pritchard KI, Shepherd L, Gralow JR, Yoshizawa C, Allred DC, Osborne CK, Hayes DF. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11(1):55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breast Cancer Version 2.2015 (2015) National Comprehensive Cancer Network. www.nccn.org. Accessed 20 Dec 2015
- 6.Breast cancer Version 1.2016 NCCN Clinical Practice Guidelines in Oncology (2015) National Comprehensive Cancer Network (NCCN). www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed 20 Dec 2015
- 7.Harris LN, Ismaila N, McShane LM, Andre F, Collyar DE, Gonzalez-Angulo AM, Hammond EH, Kuderer NM, Liu MC, Mennel RG, van Poznak C, Bast RC, Hayes DF. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2016 doi: 10.1200/JCO.2015.65.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, Geyer CE, Jr, Dees EC, Perez EA, Olson JA, Jr, Zujewski J, Lively T, Badve SS, Saphner TJ, Wagner LI, Whelan TJ, Ellis MJ, Paik S, Wood WC, Ravdin P, Keane MM, Gomez Moreno HL, Reddy PS, Goggins TF, Mayer IA, Brufsky AM, Toppmeyer DL, Kaklamani VG, Atkins JN, Berenberg JL, Sledge GW. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med. 2015;373(21):2005–2014. doi: 10.1056/NEJMoa1510764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Augustovski F, Soto N, Caporale J, Gonzalez L, Gibbons L, Ciapponi A. Decision-making impact on adjuvant chemotherapy allocation in early node-negative breast cancer with a 21-gene assay: systematic review and meta-analysis. Breast Cancer Res Treat. 2015;152(3):611–625. doi: 10.1007/s10549-015-3483-3. [DOI] [PubMed] [Google Scholar]
- 10.Carlson JJ, Roth JA. The impact of the Oncotype Dx breast cancer assay in clinical practice: a systematic review and meta-analysis. Breast Cancer Res Treat. 2013;141(1):13–22. doi: 10.1007/s10549-013-2666-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guth AA, Fineberg S, Fei K, Franco R, Bickell NA. Utilization of Oncotype DX in an inner city population: race or place? Int J Breast Cancer. 2013;2013:653805. doi: 10.1155/2013/653805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sparano JA, Paik S. Development of the 21-gene assay and its application in clinical practice and clinical trials. J Clin Oncol. 2008;26(5):721–728. doi: 10.1200/JCO.2007.15.1068. [DOI] [PubMed] [Google Scholar]
- 13.Hassett MJ, Silver SM, Hughes ME, Blayney DW, Edge SB, Herman JG, Hudis CA, Marcom PK, Pettinga JE, Share D, Theriault R, Wong YN, Vandergrift JL, Niland JC, Weeks JC. Adoption of gene expression profile testing and association with use of chemotherapy among women with breast cancer. J Clin Oncol. 2012;30(18):2218–2226. doi: 10.1200/JCO.2011.38.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katz G, Romano O, Foa C, Vataire AL, Chantelard JV, Herve R, Barletta H, Durieux A, Martin JP, Salmon R. Economic impact of gene expression profiling in patients with early-stage breast cancer in France. PLoS ONE. 2015;10(6):e0128880. doi: 10.1371/journal.pone.0128880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kip M, Monteban H, Steuten L. Long-term cost-effectiveness of Oncotype DX((R)) versus current clinical practice from a Dutch cost perspective. J Comp Eff Res. 2015;4(5):433–445. doi: 10.2217/cer.15.18. [DOI] [PubMed] [Google Scholar]
- 16.Dinan MA, Mi X, Reed SD, Hirsch BR, Lyman GH, Curtis LH. Initial trends in the use of the 21-gene recurrence score assay for patients with breast cancer in the medicare population, 2005–2009. JAMA Oncol. 2015;1(2):158–166. doi: 10.1001/jamaoncol.2015.43. [DOI] [PubMed] [Google Scholar]
- 17.Roberts MC, Weinberger M, Dusetzina SB, Dinan MA, Reeder-Hayes KE, Troester MA, Carey LA, Wheeler SB. Racial variation in adjuvant chemotherapy initiation among breast cancer patients receiving Oncotype DX testing. Breast Cancer Res Treat. 2015;153(1):191–200. doi: 10.1007/s10549-015-3518-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts MC, Weinberger M, Dusetzina SB, Dinan MA, Reeder-Hayes KE, Carey LA, Troester MA, Wheeler SB. Racial variation in the uptake of Oncotype DX testing for early-stage breast cancer. J Clin Oncol. 2016;34(2):130–138. doi: 10.1200/JCO.2015.63.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enewold L, Geiger AM, Zujewski J, Harlan LC. Oncotype Dx assay and breast cancer in the United States: usage and concordance with chemotherapy. Breast Cancer Res Treat. 2015;151(1):149–156. doi: 10.1007/s10549-015-3366-7. [DOI] [PubMed] [Google Scholar]
- 20.About National Cancer Data Base. American College of Surgeons. https://www.facs.org/quality-programs/cancer/ncdb/about. Accessed 6 Dec 2015
- 21.Lund MJ, Mosunjac M, Davis KM, Gabram-Mendola S, Rizzo M, Bumpers HL, Hearn S, Zelnak A, Styblo T, O’Regan RM. 21-Gene recurrence scores: racial differences in testing, scores, treatment, and outcome. Cancer. 2012;118(3):788–796. doi: 10.1002/cncr.26180. [DOI] [PubMed] [Google Scholar]
- 22.Carlson RW, Allred DC, Anderson BO, Burstein HJ, Carter WB, Edge SB, Erban JK, Farrar WB, Forero A, Giordano SH, Goldstein LJ, Gradishar WJ, Hayes DF, Hudis CA, Ljung BM, Mankoff DA, Marcom PK, Mayer IA, McCormick B, Pierce LJ, Reed EC, Sachdev J, Smith ML, Somlo G, Ward JH, Wolff AC, Zellars R. Invasive breast cancer. J Natl Compr Canc Netw. 2011;9(2):136–222. doi: 10.6004/jnccn.2011.0016. [DOI] [PubMed] [Google Scholar]
- 23.Swain SM, Nunes R, Yoshizawa C, Rothney M, Sing AP. Quantitative gene expression by recurrence score in ER-positive breast cancer, by age. Adv Ther. 2015;32(12):1222–1236. doi: 10.1007/s12325-015-0268-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldstein LJ, Gray R, Badve S, Childs BH, Yoshizawa C, Rowley S, Shak S, Baehner FL, Ravdin PM, Davidson NE, Sledge GW, Jr, Perez EA, Shulman LN, Martino S, Sparano JA. Prognostic utility of the 21-gene assay in hormone receptor-positive operable breast cancer compared with classical clinicopathologic features. J Clin Oncol. 2008;26(25):4063–4071. doi: 10.1200/JCO.2007.14.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levine MN, Julian JA, Bedard PL, Eisen A, Trudeau ME, Higgins B, Bordeleau L, Pritchard KI. Prospective evaluation of the 21-gene recurrence score assay for breast cancer decision-making in Ontario. J Clin Oncol. 2015 doi: 10.1200/JCO.2015.62.8503. [DOI] [PubMed] [Google Scholar]
- 26.Smyth L, Watson G, Walsh EM, Kelly CM, Keane M, Kennedy MJ, Grogan L, Hennessy BT, O’Reilly S, Coate LE, O’Connor M, Quinn C, Verleger K, Schoeman O, O’Reilly S, Walshe JM. Economic impact of 21-gene recurrence score testing on early-stage breast cancer in Ireland. Breast Cancer Res Treat. 2015;153(3):573–582. doi: 10.1007/s10549-015-3555-4. [DOI] [PubMed] [Google Scholar]