Abstract
Purpose
Recent evidence suggests that red cell distribution width (RDW) is associated with mortality in mixed cohorts of critically ill patients. Our goal was to investigate whether elevated RDW at initiation of critical care in the intensive care unit (ICU) is associated with 90-day mortality in surgical patients.
Methods
We performed a retrospective, single-center cohort study. Normal RDW was defined as 11.5%–14.5%. To investigate the association of admission RDW with 90-day mortality, we performed a logistic regression analysis, controlling for age, sex, race, body mass index, Nutrition Risk Screening 2002 score, Acute Physiology and Chronic Health Evaluation II score, hospital length of stay, as well as levels of creatinine, albumin, and mean corpuscular volume.
Results
500 patients comprised the analytic cohort; 47% patients had elevated RDW and overall 90-day mortality was 28%. Logistic regression analysis demonstrated that patients with elevated RDW had a greater than two-fold increased odds of mortality (OR 2.28: 95%CI 1.20–4.33) compared to patients with normal RDW.
Conclusions
Elevated RDW at initiation of care is associated with increased odds of 90-day mortality in surgical ICU patients. These data support the need for prospective studies to determine whether RDW can improve risk stratification in surgical ICU patients.
Keywords: RDW, red cell distribution width, mortality, ICU, critical care
INTRODUCTION
In the United States, nearly 25% of healthcare resources are spent on 6% of people who die in a given year, with roughly 20% of all deaths occurring in the ICU or shortly thereafter. [1–3] As such, critical care is an expensive, yet important, setting that is a target for cost containment. [4,5] One approach to strategically improve the cost-effectiveness of ICU care has been the use of mortality prediction scores, such as the Acute Physiologic and Chronic Health Evaluation (APACHE) II, [6] the Simplified Acute Physiology Score (SAPS), [7] and the Sequential-Related Organ Failure Assessment (SOFA), [8] to guide the utility of often resource-heavy interventions. These scoring tools attempt to quantify the degree of baseline comorbidities and acute organ dysfunction to create an individualized, objective assessment of mortality risk. [9,10] Given their potential to influence medical decision-making, the addition of variables or biomarkers to improve upon these predictive scores is of great interest to clinicians. [11,12] One such variable, which has lately received increasing attention, is red cell distribution width (RDW). [13,14]
RDW is widely available to clinicians, given that it is routinely reported as a part of the complete blood count (CBC). [15] Typically only utilized either in the differential diagnosis of anemia, [16,17] or thought of as a surrogate marker for systemic inflammation, [15,18–20] elevated RDW has recently been shown to be associated with all-cause mortality in both community-dwelling individuals as well as hospitalized patients. [15,19–21] Furthermore, recent evidence suggests that RDW is associated with mortality in critically ill patients; however, these studies focused on either medical or mixed (surgical and medical) cohorts of intensive care unit (ICU) patients. While medical ICU patients typically have underlying chronic diseases that may confound the observed relationship between RDW and mortality (and which may be difficult to adequately adjust for in multivariable regression analyses), data on surgical patients, who generally have a lower burden of chronic illness, is sparse. Therefore, our goal was to investigate the association between RDW on ICU admission and 90-day mortality in a cohort of critically ill surgical patients.
METHODS
Data Source
We performed a retrospective analysis of data from an ongoing Partners Human Research Committee (local Institutional Review Board) approved prospective study of nutrition in critical illness. Our cohort was comprised of patients from two, 18-bed surgical ICUs at the Massachusetts General Hospital (MGH) from 2012 to 2014. MGH is a 1,054-bed, teaching hospital, which is a major referral center, providing tertiary care for the residents of eastern Massachusetts and the surrounding areas.
Inclusion and exclusion criteria
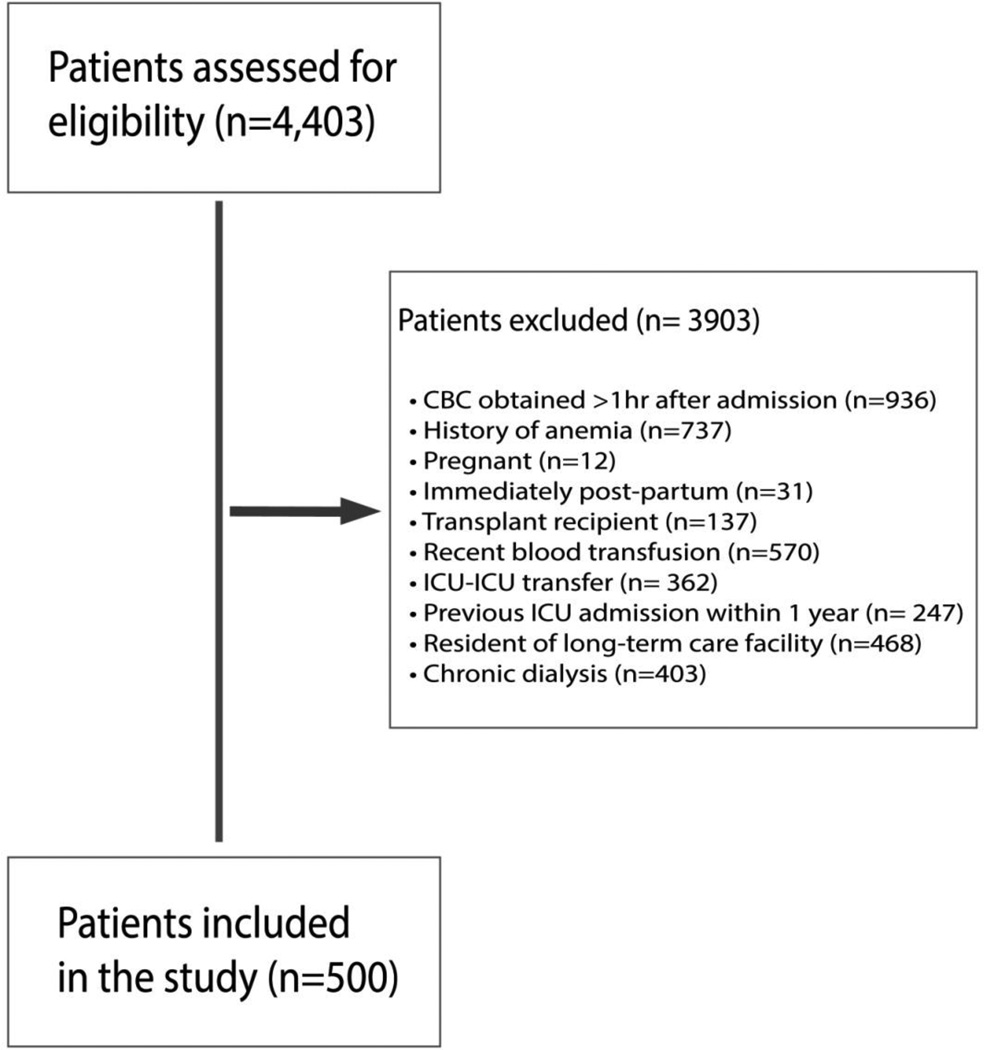
For the parent cohort, we included all adult patients (>17 years) admitted to the surgical ICU, who were expected to require >48 hours of critical care, and in whom the use of enteral and/or parenteral nutrition was anticipated. For the present study, we limited our analysis to include patients between October 01, 2012 through September 30, 2014, in whom a complete CBC was obtained within one hour of ICU admission (Figure 1). All admissions originated either from the MGH operating rooms, emergency department, or were transferred from non-ICU floors. Only patients with RDW assessed within one hour of ICU admission were considered for study inclusion. Patients with a known history of chronic anemia (defined as either documented serial hematocrits <25% during outpatient care or a formal diagnosis of chronic anemia, iron deficiency anemia, or vitamin b12 deficiency anemia on the admission problem list) were excluded, given these common forms of anemia are associated with elevations in RDW. [22,23] We further excluded patients who were either pregnant, immediately post-partum, immediate post-transplant recipients, or had received any units of packed red blood cells (PRBCs) up to four hours before and after the time of ICU admission. Moreover, to minimize confounding from chronic illness or ongoing care in a healthcare facility, we excluded patients who had been transferred from another ICU, had another ICU admission within one year of the current hospitalization, were residents of a long-term care facility, or had been on chronic dialysis.
Figure 1.
Patient exclusion flow diagram. CBC = complete blood count, RDW = red cell distribution width, ICU = intensive care unit
Primary exposure and outcomes
Our primary exposure of interest was RDW, measured within one hour of admission to the surgical ICU. Normal RDW was defined as 11.5%–14.5%. [24] Our primary outcome of interest was 90-day mortality after ICU admission, and was verified by review of individual medical records and cross-referencing each record with the Social Security Death Master File.
Covariates
Each electronic medical record was reviewed to abstract information for the following covariates: 1) age; 2) sex; 3) race; 4) body mass index (BMI); 5) Nutrition Risk Screening 2002 (NRS2002) score; 6) Acute Physiologic and Chronic Health Evaluation II (APACHE II) score; 7) hospital length of stay (LOS); 8) creatinine; 9) albumin; 10) mean corpuscular volume (MCV); and 11) C-reactive protein (CRP).
Statistical analysis
Descriptive statistics were calculated for patients with elevated RDW (>14.5%) vs. those with RDW within normal limits (11.5%–14.5%). Continuous variables are reported as means with standard deviations (SDs) or medians with interquartile ranges (IQRs), and compared using t-tests of log-rank tests, respectively. Categorical variables are expressed as proportions and compared using chi-squared tests.
To investigate the association of admission RDW with 90-day mortality, we performed a logistic regression analysis, while controlling for biologically plausible covariates, which included: age, sex, race, BMI, NRS 2002 score, APACHE II score, hospital LOS, as well as admission levels of creatinine, albumin, MCV, and CRP (in the subset of patients who had levels drawn within one hour of ICU admission). For the analyses, RDW (elevated vs. normal), 90-day mortality (dead vs. alive), sex (female vs. male), and race (non-white vs. white) were considered as dichotomous variables, while all others were considered as continuous variables. Model calibration was assessed for goodness of fit using the Hosmer-Lemshow test. Furthermore, we used locally weighted scatterplot smoothing (LOWESS), to graphically depict the relationship between RDW and 90-day mortality. LOWESS is a type of nonparametric regression that can be used to summarize the association between two variables utilizing relatively few assumptions regarding the strength or form of the relationship. [25,26]
Based on previous studies on the relationship between RDW and mortality in mixed (surgical and medical) ICU cohorts, we assumed that 90-day mortality in patients with elevated RDW at initiation of critical care would be 45% and 25% in those with normal RDW. [14] With alpha set at 0.05, a minimum of 118 patients would be required in each group to detect this difference with a power of 90% in the present study. We performed all analyses using STATA 13.0 (stataCorp LP, College Station, TX). A 2-tailed P value <0.05 and a 95% confidence interval (CI) that did not span 1 for odds ratio (OR) was considered statistically significant.
RESULTS
Our analytic cohort was comprised of 500 patients. On admission to the surgical ICU, 53% (n=263) had an RDW within normal range, while 47% (n=237) had elevated RDW. The overall mortality in our cohort was 28% (n=140). The main characteristics of the study cohort are shown in Table 1.
Table 1.
Baseline characteristics of adult surgical intensive care unit patients (n=500) to investigate the association of red cell distribution width at initiation of critical care and 90-day mortality.
| RDW: >14.5% (n = 237) |
RDW: 11.5%–14.5% (n = 263) |
P- value |
|
|---|---|---|---|
| Age (years) | 65 ± 16 | 60 ± 18 | 0.001 |
| Sex (%) | 0.28 | ||
| Female | 20 | 17 | |
| Male | 27 | 36 | |
| Race (%) | 0.78 | ||
| Non-white | 20 | 24 | |
| White | 27 | 29 | |
| BMI (kg/m2) | 28 ± 7 | 27 ± 6 | 0.09 |
| NRS 2002 | 5 ± 1 | 4 ± 1 | <0.001 |
| DCCI | 3 ± 3 | 3 ± 3 | - |
| APACHE II | 19 ± 9 | 16 ± 8 | <0.001 |
| Creatinine (mg/dL) | 1.6 ± 1.5 | 1.2 ± 2.1 | 0.01 |
| Albumin (g/dL) | 3.0 ± 0.7 | 3.3 ± 0.7 | <0.001 |
| MCV (fL) | 90 ± 9 | 90 ± 7 | - |
| CRP* (mg/L) | 135 ± 94 | 132 ± 90 | 0.78 |
| RDW (%) | 17.1 ± 4.3 | 13.5 ± 0.7 | <0.001 |
| Hospital LOS (days) | 23 (14–40) | 21 (13–33) | 0.07 |
| 90-day mortality (%) | 0.01 | ||
| Alive | 28 | 44 | |
| Dead | 19 | 9 |
RDW = red cell distribution width; BMI = body mass index; NRS = Nutrition Risk Screening; DCCI = Deyo-Charlson Comorbidity Index; APACHE = acute physiologic and chronic health evaluation; MCV = mean corpuscular volume; CRP = C-reactive protein; LOS = length of stay. Data are presented as either mean ± standard deviation, median (interquartile range), or proportions, and compared using t-tests, log-rank tests, and chi-square tests, respectively. Statistically significant p-values are shown in bold.
(*) Data for CRP is based on the 300 patients in whom levels were measured at initiation of critical care
Logistic regression analysis was used to investigate the association of admission RDW with 90-day mortality, while controlling for age, sex, race, BMI, NRS 2002 score, APACHE II score, hospital LOS, creatinine, albumin, and MCV. Using this model, we demonstrated a greater than two-fold odds of mortality in patients with RDW >14.5% compared to patients with RDW between 11.5%–14.5% (OR 2.28: 95%CI 1.20–4.33; p=0.012). Additional covariates found to be independently associated with 90-day mortality in this model were age (OR 1.05: 95%CI 1.02–1.08; p<0.001) and APACHE II score (OR 1.08: 95%CI 1.04–1.12; p<0.001). The adjusted model demonstrated good calibration (Hosmer-Lemeshow; p=0.64). Furthermore, admission CRP levels were available in 300 out of the 500 patients in the analytic cohort. Logistic regression analysis to investigate the association of admission RDW with 90-day mortality in these 300 patients using the same model as described above, with the addition of CRP as a covariate, demonstrated a twenty fold greater odds of mortality in patients with elevated RDW compared to patients with normal RDW (OR 19.65: 95%CI 1.58–244.21; p=0.021). The fully adjusted model continued to demonstrate acceptable calibration (Hosmer-Lemeshow; p=0.12).
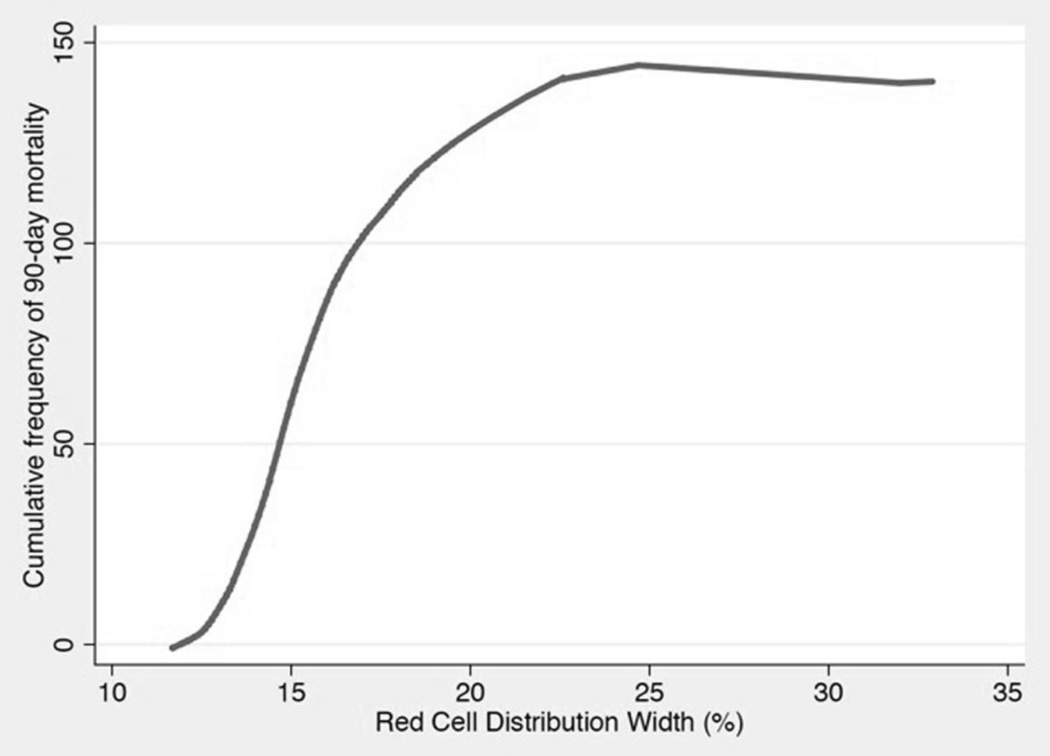
LOWESS curve analysis, shown in Figure 2, demonstrated a near linear relationship between RDW and the cumulative frequency of 90-day mortality between RDW levels of 12.5%–17.5%. On either side of this range, the curve appears significantly flatter, with virtually no additional cases of mortality at RDW values of 25% and higher.
Figure 2.
Locally weighted scatterplot smoothing curve analysis demonstrated a near-linear relationship between red cell distribution (RDW) and cumulative frequency of 90-day mortality over a RDW range of 12.5%–17.5% in critically ill surgical patients (n=500).
DISCUSSION
In this study, we investigated whether RDW at initiation of care was associated with mortality in critically ill surgical patients. Our data suggests that elevated admission RDW is associated with an increased risk of 90-day mortality in surgical ICU patients. Moreover, our data suggest that this relationship exists independent of the systemic inflammation that typically accompanies critical illness. However, given the retrospective nature of our study, these results should be considered as hypothesis generating in that they do not establish causation. Nonetheless, the biological plausibility of this relationship is undeniable.
RDW represents the size variance in circulating erythrocytes and becomes elevated during any physiologic process that upregulates erythropoiesis, causing an increased release of immature RBCs into circulation. [15,20] Increased systemic inflammation is the major theorized mechanism that leads to higher cell size heterogeneity. [15,17] Inflammation not only hinders the survival of erythrocytes, but also deforms RBC membranes. [15,20] In turn, this impedes the ability of erythrocytes to mature, leading to enhanced reticulocytosis. [15,17,27] RDW then increases as a result of naïve reticulocyte disbursement into the periphery. [15,17,27] Inflammation, however, is not the only theorized mechanism behind elevations in RDW in the acute care setting. Stress erythropoiesis, a response to acute physiologic strain mediated by hypoxia and endogenous stress-related compounds such as glucocorticoids and stem cell factor, results in increased RBC production. [28–30] During acute physiologic stress, endogenous release of glucocorticoids is amplified, which in turn stimulates erythropoietin receptors (EpoR) to augment RBC production. [31,32] Glucocorticoids also directly interact with c-kit, the receptor for stem cell factor, a cytokine that directly attenuates the differentiation of erythroid progenitors. [29,31] Altered differentiation of RBCs likely leads to sustained proliferation of naïve erythrocytes, which may further compound derangements in RDW. As such, the ability to maintain RDW within normal limits or to rapidly normalize levels after acute critical illness may be a reflection of host resilience, thereby indicating the ability to respond and adapt appropriately to physiologic stress.
Recent studies have shown that elevated RDW is associated with mortality in patients suffering from a variety of underlying ailments, such as respiratory illnesses, [33–35] cardiovascular disorders, [18,36] and septic shock [27,37] compared to those with RDW within normal limits. Specific to general critical illness, a number of studies have investigated the association of RDW and mortality in this cohort of patients. Loveday et al. (n=708), [13] and Meynaar et al. (n=2915), [38] both retrospectively investigated the association of RDW at the outset of acute illness with mortality in mixed (surgical and medical) ICU cohorts, and demonstrated that the highest elevations in RDW (>15.8% and >49.7 fl, respectively) had the most significant increase in odds of mortality compared to patients with RDW within normal limits (OR 2.62: CI 95% 2.39–2.88, p<0.0001 and OR 3.73: CI 95% 2.79–5.18, p<0.001, respectively). Furthermore, a large, multicenter, retrospective observational study in a mixed ICU cohort (n=51,413), confirmed these results, demonstrating a nearly 3-fold higher odds of 30-day mortality in those in the highest RDW quintile (adjusted OR 2.61: 95% CI 2.37–2.86, p<0.001) compared to those in the lowest quintile. [20] To add to this body of evidence, Purtle et al., investigated the association of RDW at hospital discharge after surviving critical illness and mortality in a large, multi-center retrospective analysis (n=43,212), and demonstrated a near 5-fold higher odds of 1-year mortality in patients with the highest RDW quintile (OR 4.86: 95% CI, 4.36–5.41, p < 0.001) compared to those in the lowest quintile. [39] And finally, Wang et al. prospectively investigated the association between elevated RDW and ICU mortality in a medical ICU cohort (n=602), which demonstrated that individuals with an RDW one standard deviation above normal had a 78% higher odds of mortality compared to those with RDW within the normal range (OR 1.78: 95% CI 1.475–2.158, p<0.001). [15] The results of our study build upon the current literature, and highlight a strong association between admission RDW and mortality specifically in surgical ICU patients, despite controlling for systemic inflammation and other relevant covariates.
Although our results are interesting, it is important to discuss the limitations of our analyses. Observational studies are inherently limited given that they may not be able to control for reverse causation, bias, or a lack of randomly distributed exposure. Moreover, our study cohort was derived from a single, large teaching hospital, which serves as a referral center for medically complex cases, thereby potentially limiting the generalizability of our findings. Although our analyses controlled for several clinically and biologically relevant confounders, there may remain unaccounted variables for which we are unable to fully adjust. Specifically, we were unable to adjust for transferrin saturation levels, as iron deficiency anemia is a common cause of elevated RDW. We were however able to control for MCV in our analysis, which enabled us to indirectly adjust for iron deficiency (microcytic anemia) [22] and vitamin B12 deficiency (macrocytic anemia). [23] In addition to this, although we were able to include the NRS 2002 scores, BMI, and albumin levels in our analysis, we may have been unable to fully adjust for nutritional status. Moreover, elevations in RDW may have resulted from several other factors, for which we were also unable to control, such as bone marrow dysfunction, exogenous corticosteroids use, smoking status, and the expression of various inflammatory mediators (e.g. cytokines). Likewise, we were unable to include reticulocyte count in our analyses, since it was not readily available for the majority of our patients. Furthermore, we were unable to adjust for functional status at hospital discharge, the variability in care patients received after discharge from the ICU as well as from acute care hospitalization, and compliance to medications and rehabilitation after hospital discharge. Finally, our enrollment criteria did not necessarily exclude patients that may have received PRBCs more than 4-hours before ICU admission. However, in patients who require a high volume of blood product transfusions, a 4-hour transfusion free window prior to ICU admission is unlikely. Nonetheless, these and other issues will need to be addressed in future studies to verify and build upon our results.
CONCLUSION
In our cohort of surgical ICU patients, elevated RDW at initiation of critical care was associated with increased risk of 90-day mortality, a relationship that persisted even after controlling for systemic inflammation. Although inflammation has largely been accepted as the mechanism for elevated RDW in chronic disease states, acute changes in RDW may be attributed to stress erythropoiesis, a physiologic response that is analogous to white blood cell demargination. Our data supports the need for future studies to verify the biological processes that lead to elevations in RDW in early critical illness and to determine whether changes in RDW may serve as a proxy for host resilience. A more comprehensive understanding of this routine laboratory value may influence future clinical decision-making and may help to improve the quality of ICU care.
Acknowledgments
We thank Kenneth B. Christopher, MD for his insights and guidance in conducting this work.
Funding Disclosure: Tiffany M.N. Otero received support from the National Center for Advancing Translational Sciences, National Institutes of Health, Award Number TL1TR001062. D. Dante Yeh received support from the Nestle Foundation, Award Number 226280. Sadeq A. Quraishi received support from the National Institutes of Health grants T32 GM007592, UL1 RR025758, L30 GM102903, and L30 TR00125.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Institution where work was performed: Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital, Boston, MA, USA
Conflict of Interest: Sadeq A. Quraishi has received consulting fees from Lungpacer, Inc and Trevena, Inc. In addition to this, Dr. Quraishi serves as an uncompensated Board member for both the Vitamin D Council and the C. Diff Foundation
Author Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Contributor Information
Tiffany M.N. Otero, Email: totero@partners.org.
Cecilia Canales, Email: canalesc@uci.edu.
D. Dante Yeh, Email: dyeh2@mgh.harvard.edu.
Peter C. Hou, Email: phou@bwh.harvard.edu.
Donna Belcher, Email: dmbelcher@partners.org.
REFERENCES
- 1.Angus DC, Barnato AE, Linde-Zwirble WT, Weissfeld LA, Watson RS, Rickert T, et al. Use of intensive care at the end of life in the United States: an epidemiologic study. Crit. Care Med. 2004 Mar;32(3):638–643. doi: 10.1097/01.ccm.0000114816.62331.08. [DOI] [PubMed] [Google Scholar]
- 2.Riley GF, Lubitz JD. Long-term trends in Medicare payments in the last year of life. Health Serv. Res. 2010 Apr;45(2):565–576. doi: 10.1111/j.1475-6773.2010.01082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seshamani M, Gray AM. A longitudinal study of the effects of age and time to death on hospital costs. J. Health Econ. 2004 Mar;23(2):217–235. doi: 10.1016/j.jhealeco.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Curtis JR, Engelberg RA, Bensink ME, Ramsey SD. End-of-life care in the intensive care unit: can we simultaneously increase quality and reduce costs? Am. J. Respir. Crit. Care Med. 2012 Oct 1;186(7):587–592. doi: 10.1164/rccm.201206-1020CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khandelwal N, Curtis JR. Economic implications of end-of-life care in the ICU. Curr. Opin. Crit. Care. 2014 Dec;20(6):656–661. doi: 10.1097/MCC.0000000000000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II. a severity of disease classification system. Crit. Care Med. 1985 Oct;13(10):818–829. [PubMed] [Google Scholar]
- 7.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993 Dec 22–29;270(24):2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 8.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996 Jul;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 9.Higgins TL. Quantifying risk and benchmarking performance in the adult intensive care unit. J. Intensive Care Med. 2007 May-Jun;22(3):141–156. doi: 10.1177/0885066607299520. [DOI] [PubMed] [Google Scholar]
- 10.Power GS, Harrison DA. Why try to predict ICU outcomes? Curr. Opin. Crit. Care. 2014 Oct;20(5):544–549. doi: 10.1097/MCC.0000000000000136. [DOI] [PubMed] [Google Scholar]
- 11.Pearce CB, Gunn SR, Ahmed A, Johnson CD. Machine learning can improve prediction of severity in acute pancreatitis using admission values of APACHE II score and C-reactive protein. Pancreatology : official journal of the International Association of Pancreatology (IAP) … [et al.] 2006;6(1–2):123–131. doi: 10.1159/000090032. [DOI] [PubMed] [Google Scholar]
- 12.Wang F, Pan W, Pan S, Wang S, Ge Q, Ge J. Usefulness of N-terminal pro-brain natriuretic peptide and C-reactive protein to predict ICU mortality in unselected medical ICU patients: a prospective, observational study. Crit. Care. 2011;15(1):R42. doi: 10.1186/cc10004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loveday S, Sinclair L, Badrick T. Does the addition of RDW improve current ICU scoring systems? Clin. Biochem. 2015 Jun;48(9):569–574. doi: 10.1016/j.clinbiochem.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Hunziker S, Celi LA, Lee J, Howell MD. Red cell distribution width improves the simplified acute physiology score for risk prediction in unselected critically ill patients. Crit. Care. 2012;16(3):R89. doi: 10.1186/cc11351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang F, Pan W, Pan S, Ge J, Wang S, Chen M. Red cell distribution width as a novel predictor of mortality in ICU patients. Ann. Med. 2011 Feb;43(1):40–46. doi: 10.3109/07853890.2010.521766. [DOI] [PubMed] [Google Scholar]
- 16.Forhecz Z, Gombos T, Borgulya G, Pozsonyi Z, Prohaszka Z, Janoskuti L. Red cell distribution width in heart failure: prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. Am. Heart J. 2009 Oct;158(4):659–666. doi: 10.1016/j.ahj.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 17.Fujita B, Franz M, Figulla HR, Pfeifer R, Kabisch B, Fritzenwanger M, et al. Red cell distribution width and survival in patients hospitalized on a medical ICU. Clin. Biochem. 2015 Jul 10; doi: 10.1016/j.clinbiochem.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 18.Felker GM, Allen LA, Pocock SJ, Shaw LK, McMurray JJ, Pfeffer MA, et al. Red cell distribution width as a novel prognostic marker in heart failure: data from the CHARM Program and the Duke Databank. J. Am. Coll. Cardiol. 2007 Jul 3;50(1):40–47. doi: 10.1016/j.jacc.2007.02.067. [DOI] [PubMed] [Google Scholar]
- 19.Patel KV, Ferrucci L, Ershler WB, Longo DL, Guralnik JM. Red blood cell distribution width and the risk of death in middle-aged and older adults. Arch. Intern. Med. 2009 Mar 9;169(5):515–523. doi: 10.1001/archinternmed.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bazick HS, Chang D, Mahadevappa K, Gibbons FK, Christopher KB. Red cell distribution width and all-cause mortality in critically ill patients. Crit. Care Med. 2011 Aug;39(8):1913–1921. doi: 10.1097/CCM.0b013e31821b85c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perlstein TS, Weuve J, Pfeffer MA, Beckman JA. Red blood cell distribution width and mortality risk in a community-based prospective cohort. Arch. Intern. Med. 2009 Mar 23;169(6):588–594. doi: 10.1001/archinternmed.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eldibany MM, Totonchi KF, Joseph NJ, Rhone D. Usefulness of certain red blood cell indices in diagnosing and differentiating thalassemia trait from iron-deficiency anemia. Am. J. Clin. Pathol. 1999 May;111(5):676–682. doi: 10.1093/ajcp/111.5.676. [DOI] [PubMed] [Google Scholar]
- 23.Davenport J. Macrocytic anemia. Am. Fam. Physician. 1996 Jan;53(1):155–162. [PubMed] [Google Scholar]
- 24.Walker HK, Hall WD, Hurst JW. Clinical methods : the history, physical, and laboratory examinations. Boston: Butterworths; 1990. [PubMed] [Google Scholar]
- 25.Cleveland WS. Robust Locally Weighted Regression and Smoothing Scatterplots. [1979/12/01];Journal of the American Statistical Association. 1979 74(368):829–836. [Google Scholar]
- 26.Quraishi SA, Bittner EA, Blum L, Hutter MM, Camargo CA., Jr Association between preoperative 25-hydroxyvitamin D level and hospital-acquired infections following Roux-en-Y gastric bypass surgery. JAMA surgery. 2014 Feb;149(2):112–118. doi: 10.1001/jamasurg.2013.3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sadaka F, O'Brien J, Prakash S. Red cell distribution width and outcome in patients with septic shock. J. Intensive Care Med. 2013 Sep-Oct;28(5):307–313. doi: 10.1177/0885066612452838. [DOI] [PubMed] [Google Scholar]
- 28.Socolovsky M. Molecular insights into stress erythropoiesis. Curr. Opin. Hematol. 2007 May;14(3):215–224. doi: 10.1097/MOH.0b013e3280de2bf1. [DOI] [PubMed] [Google Scholar]
- 29.Broudy VC, Lin NL, Priestley GV, Nocka K, Wolf NS. Interaction of stem cell factor and its receptor c-kit mediates lodgment and acute expansion of hematopoietic cells in the murine spleen. Blood. 1996 Jul 1;88(1):75–81. [PubMed] [Google Scholar]
- 30.Paulson RF, Shi L, Wu DC. Stress erythropoiesis: new signals and new stress progenitor cells. Curr. Opin. Hematol. 2011 May;18(3):139–145. doi: 10.1097/MOH.0b013e32834521c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Lindern M, Zauner W, Mellitzer G, Steinlein P, Fritsch G, Huber K, et al. The glucocorticoid receptor cooperates with the erythropoietin receptor and c-Kit to enhance and sustain proliferation of erythroid progenitors in vitro. Blood. 1999 Jul 15;94(2):550–559. [PubMed] [Google Scholar]
- 32.Bauer A, Tronche F, Wessely O, Kellendonk C, Reichardt HM, Steinlein P, et al. The glucocorticoid receptor is required for stress erythropoiesis. Genes Dev. 1999 Nov 15;13(22):2996–3002. doi: 10.1101/gad.13.22.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seyhan EC, Ozgul MA, Tutar N, Omur I, Uysal A, Altin S. Red blood cell distribution and survival in patients with chronic obstructive pulmonary disease. Copd. 2013 Aug;10(4):416–424. doi: 10.3109/15412555.2012.758697. [DOI] [PubMed] [Google Scholar]
- 34.Braun E, Kheir J, Mashiach T, Naffaa M, Azzam ZS. Is elevated red cell distribution width a prognostic predictor in adult patients with community acquired pneumonia? BMC Infect. Dis. 2014;14:129. doi: 10.1186/1471-2334-14-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grant BJ, Kudalkar DP, Muti P, McCann SE, Trevisan M, Freudenheim JL, et al. Relation between lung function and RBC distribution width in a population-based study. Chest. 2003 Aug;124(2):494–500. doi: 10.1378/chest.124.2.494. [DOI] [PubMed] [Google Scholar]
- 36.Jung C, Fujita B, Lauten A, Kiehntopf M, Kuthe F, Ferrari M, et al. Red blood cell distribution width as useful tool to predict long-term mortality in patients with chronic heart failure. Int. J. Cardiol. 2011 Nov 3;152(3):417–418. doi: 10.1016/j.ijcard.2011.08.052. [DOI] [PubMed] [Google Scholar]
- 37.Kim CH, Park JT, Kim EJ, Han JH, Han JS, Choi JY, et al. An increase in red blood cell distribution width from baseline predicts mortality in patients with severe sepsis or septic shock. Crit. Care. 2013;17(6):R282. doi: 10.1186/cc13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meynaar IA, Knook AH, Coolen S, Le H, Bos MM, van der Dijs F, et al. Red cell distribution width as predictor for mortality in critically ill patients. Neth. J. Med. 2013 Nov;71(9):488–493. [PubMed] [Google Scholar]
- 39.Purtle SW, Moromizato T, McKane CK, Gibbons FK, Christopher KB. The association of red cell distribution width at hospital discharge and out-of-hospital mortality following critical illness*. Crit. Care Med. 2014 Apr;42(4):918–929. doi: 10.1097/CCM.0000000000000118. [DOI] [PubMed] [Google Scholar]